Introduction
Recent statistics from the Centers for Disease
Control, the National Institutes of Health and the World Health
Organization report that obesity has more than doubled since 1980,
and almost two billion adults worldwide are overweight or obese
(1). Obesity is a preventable
disease; however, it is evident that current educational efforts
have failed to counteract its upward trend. The growing epidemic of
obesity in the world is a major factor in reducing life expectancy,
and represents an economic burden and a serious health problem. It
is largely responsible for the growing incidence and prevalence of
diabetes, as well as cardiovascular and renal disease. Evidence
indicates that obesity is a risk factor for the development of
renal inflammation and dysfunction, that may progress towards
chronic kidney disease (CKD) (2,3).
Considering that obesity is associated with a
chronic low-grade inflammatory condition, and that studies have
demonstrated that obesity-induced inflammation develops in the
renal tubularinterstitium (4),
exploring the mechanisms of the renal tubulointerstitial
inflammatory response in obesity may be of particular importance.
Multiple lines of evidence suggest that the direct action of
monocyte chemoattractant protein-1 (MCP-1) is a powerful instigator
of tubulointerstitial inflammation (5,6).
Excessive MCP-1 in the renal interstitium may recruit macrophages
and generate inflammation, contributing to tubulointerstitial
injury and the progression of CKD. Initially, circulatory MCP-1,
produced and secreted by adipose tissue, had been the focus of
attention as the root of tubulointerstitial inflammation. However,
it has been reported that the concentrations of serum and urinary
MCP-1 are not associated with each other in obesity, indicating a
role for locally produced MCP-1 in the kidney (7). Therefore, research into the
mechanisms of renal MCP-1 generation may be a useful step in
understanding and developing novel treatments for human renal
tubulointerstitial inflammation.
A previous study demonstrated that MCP-1 expression
in the vascular smooth muscle cells of diabetic mice is negatively
regulated by the transcription factor forkhead boxO1 (FOXO1)
(8). Other reports have indicated
that FOXO1 is able to regulate certain biological processes,
including cell cycle arrest, DNA damage repair and oxidative stress
inhibition (9–11). It has beneficial effects on renal
injury by attenuating oxidative stress and inhibiting the
activation of transforming growth factor beta-1 (TGF-β1) signaling
in diabetic rats (12,13). However, whether FOXO1 is able to
regulate MCP-1 production, thus mediating renal tubulointerstitial
inflammation during obesity, is poorly understood. Furthermore,
evidence shows that FOXO1 is a downstream target of mammalian
target of rapamycin (mTOR) in human tracheal smooth muscle cells.
When mTOR is activated by phosphorylation (p-mTOR), it stimulates
FOXO1 phosphorylation (p-FOXO1) and translocation from the nucleus
to the cytosol. In this case, FOXO1 is dissociated from the
promoter of the target gene, enabling the gene to be expressed
(14). Recent evidence
demonstrates that mTOR serves a key role in the generation and
development of renal inflammation, through functions such as NLRP3
inflammasome activation (15), and
increasing autophagy in macrophages and renal cells (16), which mediates renal inflammatory
cytokine release. Since mTOR is activated in obesity (17–19),
the authors propose a possible scenario in which activated mTOR may
enhance the phosphorylation of FOXO1, with p-FOXO1 subsequently
dissociating from the MCP-1 promoter, followed by the increased
expression of MCP-1, ultimately leading to a renal
tubulointerstitial inflammatory response.
In the present study, renal function was initially
evaluated in the participants with obesity, and the pathomorphology
and associated mechanisms of tubulointerstitial inflammation were
assessed in the high fat diet (HFD)-induced obese rat model and a
cultured human renal tubular epithelial cell line (HK-2). The
purpose of the current study was to provide insights into the onset
of obesity-associated renal tubulointerstitial inflammation and to
identify a potential novel approach to treating renal injury.
Materials and methods
Clinical study
A total of 35 subjects with obesity (19 males and 16
females) were recruited consecutively at the First Affiliated
Hospital of Soochow University (Suzhou, China). The inclusion
criteria were as follows: i) Obesity (BMI≥27 kg/m2); and
ii) Chinese adults (including mainland China, Taiwan, and
individuals from Hong Kong living in Jiangsu) aged 18–65. The
exclusion criteria were as follows: i) Diabetes mellitus; ii)
currently taking drugs that might interfere with the study, such as
statins, fibrates, steroids, antihypertensive or anti-diabetic
agents; iii) interstitial pneumonia; iv) severe renal or liver
disease; v) malignant tumors; and vi) a history of acute coronary
syndrome, stroke or acute pancreatitis within 3 months prior to
enrollment. In addition, 27 normal weight, healthy subjects (15
males and 12 females) with a BMI<24 kg/m2 served as a
control group. The Ethics Committee of the First Affiliated
Hospital of Soochow University approved this study, and all
subjects provided written informed consent. During the physical
examination, body weight (BW), height and waist circumference (WC)
were determined. Blood pressure (BP) was measured on the right arm
using a standard mercury sphygmomanometer with the subject in a
seated position after 10 min of rest. Venous blood samples were
collected from overnight fasted subjects. Blood urea nitrogen
(BUN), creatinine (Cr), total triglycerides (TG), total cholesterol
(TC) and free fatty acid (FFA) were measured using a chemistry
analyzer (Hitachi 7600; Hitachi, Ltd., Tokyo, Japan) at the central
laboratory of the hospital. Urinary MCP-1 (u-MCP) and urinary
neutrophil gelatinase-associated lipocalin (u-NGAL) concentrations
were determined using ELISA kits (cat. no. ab179886, Abcam,
Cambridge, UK; and cat. nos. SCB388Hu and SEB388Ra, Cusabio, Wuhan,
China) according to the manufacturer's instructions. Urinary
albumin was measured via the Coomassie brilliant blue method (cat.
no. CO35-2; Nanjing Jiancheng Bioengineering Institute, Nanjing,
China). The urinary albumin to creatinine ratio (UACR) was
calculated from first voided spot samples using clean-catch
techniques and sterile containers; ratios <30 mg/g creatinine
were defined as normoalbuminuria, those between 30 and 300 mg/g
creatinine as microalbuminuria, and those ≥300 mg/g creatinine as
macroalbuminuria.
Animal model
Male Sprague-Dawley (SD) rats (5 weeks old; SIPPR-Bk
Laboratory Animals Co., Ltd., Shanghai, China) weighing
approximately 160 g were housed in polypropylene cages and
maintained under controlled room temperature (22±2°C) and humidity
(60±5%) with a 12 h light/dark cycle. Housing and animal
experiments were approved by the Committee for Animal Subjects at
Soochow University according to institutional and national animal
welfare guidelines. After one week of adaptation, rats were
randomly assigned to receive a normal chow diet (NCD; D12102C;
Research Diets, Inc., New Brunswick, NJ, USA) or a HFD (60% kcal in
fat; D12492; Research Diets, Inc.) or HFD plus 1 mg/kg/day of
specific mTOR inhibitor rapamycin (Rap; Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) by intraperitoneal injection (HFD+Rap; n=5 rats
per group) for 8 weeks (20,21).
A 24 h urine sample was collected from the rats after 3 days of
adaptation in individual metabolic cages to measure 24 h urine
protein and u-NGAL levels. The rats were then weighed and
sacrificed. Blood was collected to test Cr, BUN, TG, TC and
FFA.
Renal histology
The kidneys were perfused with saline solution and
then removed and weighed. Kidneys were fixed in 10% neutral
formalin for 24 h at 4°C and embedded in paraffin. Sequential
paraffin-embedded tissue sections from the renal cortex were cut.
Cross-sections (3 µm) were placed on gelatin-coated slides and used
for hematoxylin-eosin (HE) staining, periodic acid-silver
methenamine (PASM) staining and immunohistochemical staining for
CD68 (a macrophage marker). In each kidney, at least 20
non-overlapping cortical areas of tubulointerstitial section were
analyzed. The remaining kidney tissue was snap-frozen in liquid
nitrogen and stored at −80°C for western blot analysis.
HE staining
The paraffin sections were stained using hematoxylin
(cat. no. D006; Nanjing Jiancheng Bioengineering Institute) at room
temperature for a total of 10 min, followed by washing under
running water for 30–60 sec. The sections were then placed in eosin
(cat. no. D006; Nanjing Jiancheng Bioengineering Institute) at room
temperature for 1 min, followed by dehydration in a gradient of
ethanol and clearing in xylene I and xylene II (cat. no.
GD-RY1215-12; Shanghai Guduo Science and Technology Co., Ltd.,
Shanghai, China) twice for 1 min. Stained sections were observed
and digital images were captured with a light microscope
(magnification, ×400; Zeiss GmbH, Jena, Germany).
PASM staining
Paraffin sections of each group were dewaxed in
water, oxidized with a 0.5% periodic acid (cat. no. SBJ-0404;
Nanjing SenBeiJia Biological Technology Co., Ltd, Nanjing, China)
for 30 min, and washed under tap and distilled water. Following
oxidization with 10% chromic acid (cat. no. 06; BaSiFu Co., Ltd,
Nanjing, China) for 20 min, the sections were rinsed with distilled
water, fixed in a 1% sodium sulfite solution (cat. no. SBJ-0628;
Nanjing SenBeiJia Biological Technology Co., Ltd.) for 30 sec,
washed with distilled water, and immersed under a hexamine-silver
solution (cat. no. SBJ-0600; Nanjing SenBeiJia Biological
Technology Co., Ltd.) for 30 min. The sections were left to stand
at a temperature of 65°C for a total of 4 min in a water bath,
following which they were removed and washed with distilled water
and measured under a microscope until the black precipitate
appeared in the glomerular capillary basement membrane. Following
the appearance of the black precipitate, the sections were colored
using a 0.1% auric chloride solution (cat. no. G810493-5 g;
Shanghai Chaoyan Biotechnology Co., Ltd., Shanghai, China) for 30
sec, washed under running water for 10 min, and stained with
hematoxylin (cat. no. D006; Nanjing Jiancheng Bioengineering
Institute) and 0.5% eosin for a total of 3 min. Upon completing a
conventional dehydration cycle, the sections were cleared, sealed
and observed, and digital images were captured with a light
microscope (magnification, ×400; Zeiss GmbH).
Immunohistochemical staining
Paraffin sections were routinely dewaxed into
solution, blocked with 3% H2O2 methanol
endogenous peroxidases (cat. no. AR1022; Wuhan Boster Biological
Technology, Ltd., Wuhan, China), incubated with rabbit anti-rat
CD68 monoclonal antibody (cat. no. ab125212; 1:50; Abcam) at 4°C
overnight, subsequently incubated with goat anti-rabbit secondary
antibodies (cat. no. ab6721; 1:200; Abcam) at room temperature for
30 min, stained with DAB (cat. no. AR1022; Wuhan Boster Biological
Technology, Ltd.), counterstained with hematoxylin (cat. no. D006;
Nanjing Jiancheng Bioengineering Institute), and dried. Stained
sections were observed and digital images were captured with a
light microscope (magnification, ×200; Zeiss GmbH).
Cell culture
HK-2 cells and a human monocyte cell line (THP-1)
were obtained from the American Type Culture Collection (Manassas,
VA, USA). HK-2 and THP-1 cells were cultured at 37°C in RPMI 1640
medium (Lonza Group, Ltd., Basel, Switzerland) containing 10% fetal
calf serum (HyClone; GE Healthcare Life Sciences, Logan, UT, USA),
2 mM L-glutamine solution, 100 U/ml penicillin and 100 µg/ml
streptomycin (Sigma-Aldrich; Merck KGaA), in a cell culture
incubator with 95% air and 5% CO2 for 24 h. All
experiments were performed in serum-free RPMI 1640 medium
containing 0.2% bovine serum albumin (BSA; Sigma-Aldrich; Merck
KGaA). Since palmitic acid (PA; Sigma-Aldrich; Merck KGaA) is a
major constituent of FFA, it was used to treat the cells and
simulate a high fat environment in the in vitro study
(22,23).
Transwell filter migration assay and
MCP-1 concentration detection
HK-2 cells were seeded in a 6-well plate at a
density of 5×105 cells/well and treated with the
experimental medium in the absence or presence of 0.32 mM PA or 10
ng/ml Rap plus PA. After a 24-h incubation at 37°C, culture
supernatants were harvested via centrifugation at 1,000 × g at 37°C
for 10 min. Microporous membrane (pore size, 8 µm) Transwell
inserts (Merck KGaA) were used for the chemotaxis assay. THP-1
cells (5×104) in 200 µl serum-free RPMI were added to
the upper chamber, with 500 µl of the above culture supernatants in
the lower chamber (HK-2 cells were treated with media containing
0.32 mM PA, 10 ng/ml Rap, or PA plus Rap; media without HK-2 cell
treatment were used for a control experiment). THP-1 cells were
allowed to migrate during a 2-h incubation at 37°C with 5%
CO2, and then the inserts were fixed with methanol (cat.
no. N168; Shanghai Chaoyan Biotechnology Co., Ltd.) for 20 min at
room temperature and stained with 0.1% crystal violet for 10 min at
room temperature (cat. no. C0775; Sigma-Aldrich; Merck KGaA). The
non-migratory cells were removed prior to mounting of the membrane,
and the number of migratory cells was observed under a light
microscope (magnification, ×200; Zeiss GmbH). MCP-1 in the
supernatant was detected with an MCP-1 ELISA kit (cat. no.
ab179886; Abcam), according to the manufacturer's protocol.
Small interfering (si)RNA
transfection
HK-2 cells were cultured in six-well plates at a
density of 5×105 cells/well and transiently transfected
with mTOR siRNA (sense, 5′-CCACCCGAAUUGGCAGAUUTT-3′; antisense,
5′-AAUCUGCCAAUUCGGGUGGTT-3′), FOXO1 siRNA (sense,
5′-GGAGGUAUGAGUCAGUAUATT-3′; antisense,
5′-UAUACUGACUCAUACCUCCTT-3′) or empty vector siRNA (cat. nos.
sc-35409 and sc-35382; Santa Cruz Biotechnology, Inc., Dallas, TX,
USA) using Lipofectamine® 2000 (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) following the
manufacturer's protocol. Briefly, 1 µg siRNA was mixed with 6 µl
Lipofectamine® 2000 and applied to the cells. The
transfected cells were incubated at room temperature for 30 min and
then seeded (5×105 cells) in a 6-well plate containing
900 µl Opti-MEM (Invitrogen; Thermo Fisher Scientific, Inc.). After
a 24-h treatment with the experimental medium, the cells were
harvested and examined by western blotting.
Protein extraction and western blot
analysis
Cellular protein was extracted using a protein
extraction kit (cat. no. W034; Nanjing SenBeiJia Biological
Technology, Co., Ltd.) and total protein was quantified via a
protein assay kit (bicinchoninic acid; cat. no. A045-4; Nanjing
SenBeiJia Biological Technology Co., Ltd.). Total protein (30–80
µg) was subjected to electrophoresis on 6–15% SDS polyacrylamide
gels. Proteins were transferred to polyvinylidene fluoride
membranes, which were blocked for 1 h at room temperature with 5%
BSA in Tris-buffered saline containing 0.05% Tween-20 (TBST).
Subsequently, blots were washed and incubated overnight at 4°C in
TBST containing 5% bovine serum albumin with antibodies (1:1,000
dilution) against interleukin (IL)-1β (cat. no. ab9722; 1:1,000;
Abcam), MCP-1 (cat. no. ab25124; 1:1,000; Abcam), p-FOXO1 (cat. no.
ab131339; 1:1,000; Abcam), p-mTOR (cat. no. ab137133; 1:1,000;
Abcam) and β-actin (cat. no. ab8227; 1:2,000; Abcam). Membranes
were washed three times with TBST, incubated with a horseradish
peroxidase-conjugated secondary antibody (cat. no. ab6721; 1:5,000;
Abcam) for 1 h at room temperature and then washed three times
again with TBST. Finally, detection procedures were performed using
an enhanced chemiluminescence (ECL) Advance Western Blotting
Detection kit and autoradiography was performed on Hyperfilm ECL
(both GE Healthcare, Chicago, IL, USA). For quantitative analysis,
bands were detected and evaluated by densitometry with LabWorks
software 7.0 (UVP, LLC, Upland, CA, USA) and normalized to
β-actin.
Statistical analysis
Statistical analyses were conducted using SPSS 18.0
(SPSS, Inc., Chicago, IL, USA). The χ2 test was applied
to assess the distribution of sex between participants with obesity
and the controls. Normally distributed variables were analyzed with
one-way analysis of variance (ANOVA) or Student's t-test. For
variables with non-normal distribution, Kruskal-Wallis' test was
used. Tukey's and Dunn's post hoc tests were used after the ANOVA
and Kruskal-Wallis' test, respectively. Pearson's correlation
analyses were used for continuous variables, and Spearman's
correlation analyses were used for ranked variables. P<0.05 was
considered to indicate a statistically significant difference.
Results
Baseline characteristics of obese and
non-obese subjects
Table I presents
the anthropometric and biochemical characteristics of the obese and
non-obese subjects. Compared with non-obese controls, the
participants with obesity had increased BMI, SBP and DBP, elevated
TC, TG and FFA, as well as a larger WC. The Cr, UACR and u-NGAL
were increased in the participants with obesity, while there was no
significant difference in the BUN between the two groups (Table I). In addition, the participants
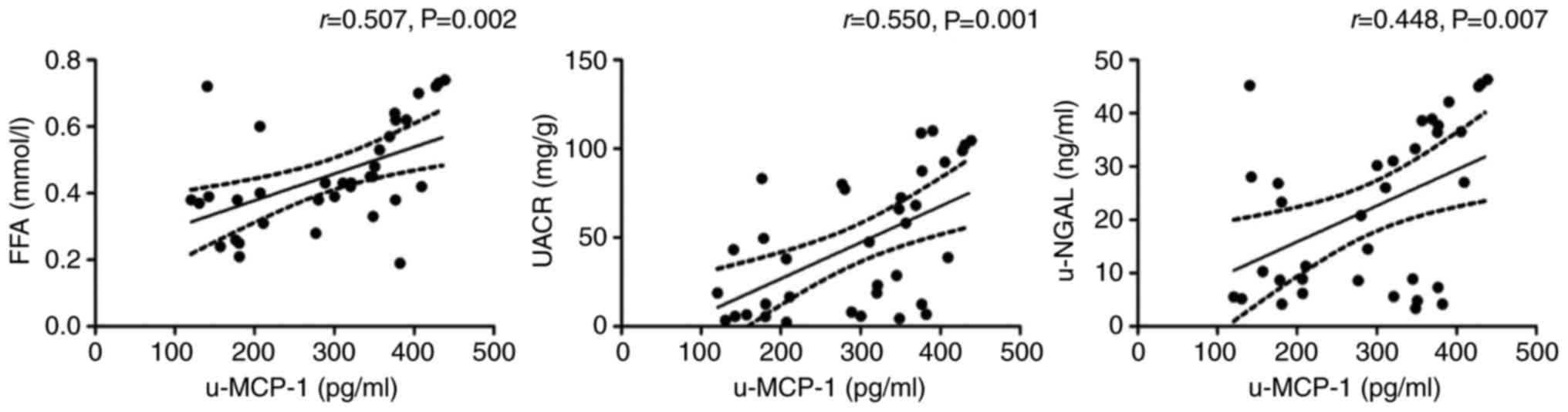
with obesity had higher concentrations of u-MCP-1 (Table I), and the level of u-MCP-1 was
positively correlated with FFA (r=0.507, P=0.002), UACR (r=0.550,
P=0.001) and u-NGAL (r=0.448, P=0.007) in the participants with
obesity (Fig. 1).
 | Table I.Baseline characteristics of obese and
non-obese subjects. |
Table I.
Baseline characteristics of obese and
non-obese subjects.
| Variables | Non-obese (n=27) | Obese (n=35) | P-value |
|---|
| Sex
(male/female) | 15/12 | 19/16 |
0.921 |
| Age (years) | 36±9 | 38±10 |
0.355 |
| BMI
(kg/m2) | 22.06±1.45 |
30.84±2.15a | <0.001 |
| WC (cm) | 77.33±6.76 |
117.80±5.03a | <0.001 |
| SBP (mmHg) | 110±4 | 123±15a | <0.001 |
| DBP (mmHg) | 72±7 | 82±9a | <0.001 |
| TC (mmol/l) | 3.37 (2.89,
4.02) | 5.48 (3.25,
6.56)a | <0.001 |
| TG (mmol/l) | 1.51±0.47 |
3.29±1.47a | <0.001 |
| FFA (mmol/l) | 0.24±0.05 |
0.45±0.16a | <0.001 |
| BUN (mmol/l) | 4.13 (2.62,
5.43) | 4.53 (2.56,
5.58) |
0.452 |
| Cr (µmol/l) | 67.22±10.59 |
75.69±14.13a |
0.012 |
| UACR (mg/g) | 4.67±2.35 |
45.88±37.15a | <0.001 |
| u-NGAL (ng/ml) | 6.65±2.25 |
22.18±15.14a | <0.001 |
| u-MCP-1
(pg/ml) | 196.23±61.54 |
293.02±100.19a | <0.001 |
Biochemical characteristics of the
rats
BW and the ratio of kidney weight (KW) to BW (KW/BW)
was significantly increased in the HFD group, indicating that an
obese rat model was successfully established by HFD feeding.
Moreover, the levels of serum TG, TC and FFA were markedly higher
in HFD-fed rats compared with normal rats. Rap treatment reduced
the KW/BW, although it did not notably affect the BW or serum
lipids (Table II).
 | Table II.Biochemical parameters of the NCD,
HFD and HFD+Rap groups at the end of the intervention study
(n=5). |
Table II.
Biochemical parameters of the NCD,
HFD and HFD+Rap groups at the end of the intervention study
(n=5).
|
|
|
|
| P-value |
|---|
|
|
|
|
|
|
|---|
| Group | NCD | HFD | HFD+Rap | HFD vs. NCD | HFD+Rap vs.
HFD |
|---|
| BW (g) | 387.33±8.74 |
462.67±11.37a | 459.67±4.51 | 0.001 | 0.693 |
| KW/BW (%) | 0.85±0.05 |
1.50±0.08a |
1.22±0.10b | <0.001 | 0.016 |
| TG (mmol/l) | 0.35±0.05 |
1.81±0.03a | 1.75±0.11 | <0.001 | 0.405 |
| TC (mmol/l) | 1.60±0.10 |
10.97±0.75a | 11.13±0.90 | <0.001 | 0.818 |
| FFA (mmol/l) | 1.02±0.08 |
1.63±0.06a | 1.63±0.08 | <0.001 | 1.000 |
| BUN (mmol/l) | 5.23±0.35 |
10.33±0.61a |
7.77±0.25b | <0.001 | 0.003 |
| Cr (µmol/l) | 34.67±1.53 |
68.33±1.53a |
37.67±4.04b | <0.001 | <0.001 |
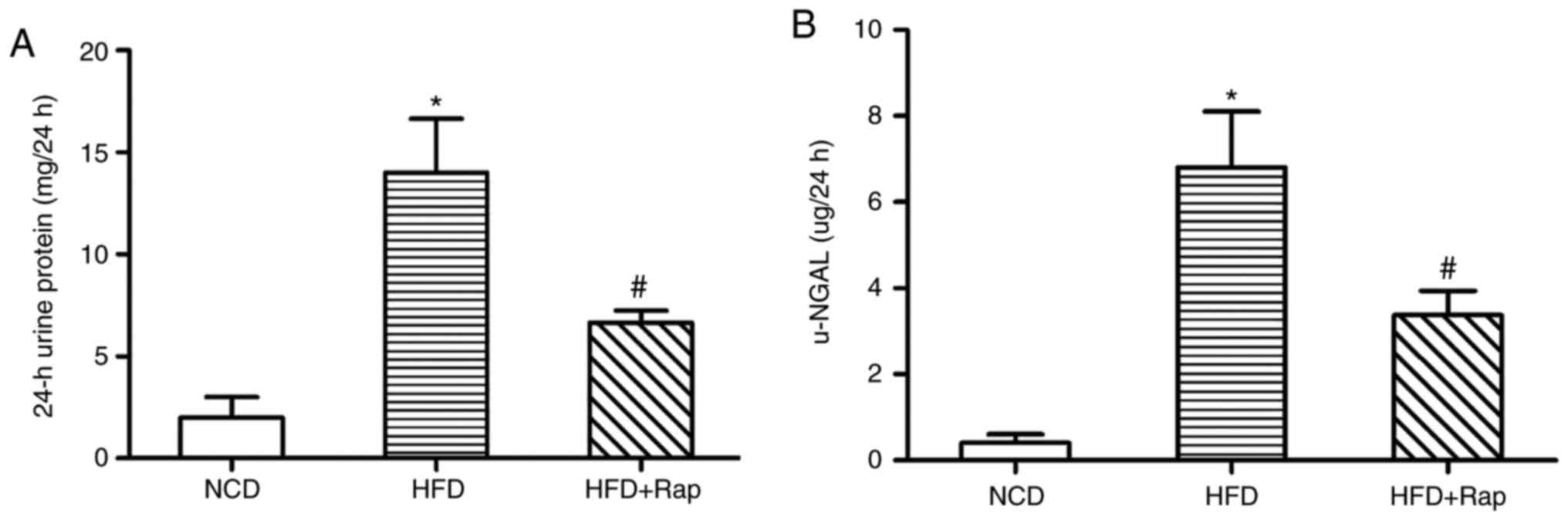
Renal function of the rats
The Cr, BUN, 24 h urine protein and u-NGAL in the
HFD group was increased compared with the NCD group, indicating
impaired renal function in these obese rats. By contrast, all the
above effects were alleviated in the HFD+Rap group, suggesting that
Rap improved the renal function of HFD-induced obese rats (Table II and Fig. 2).
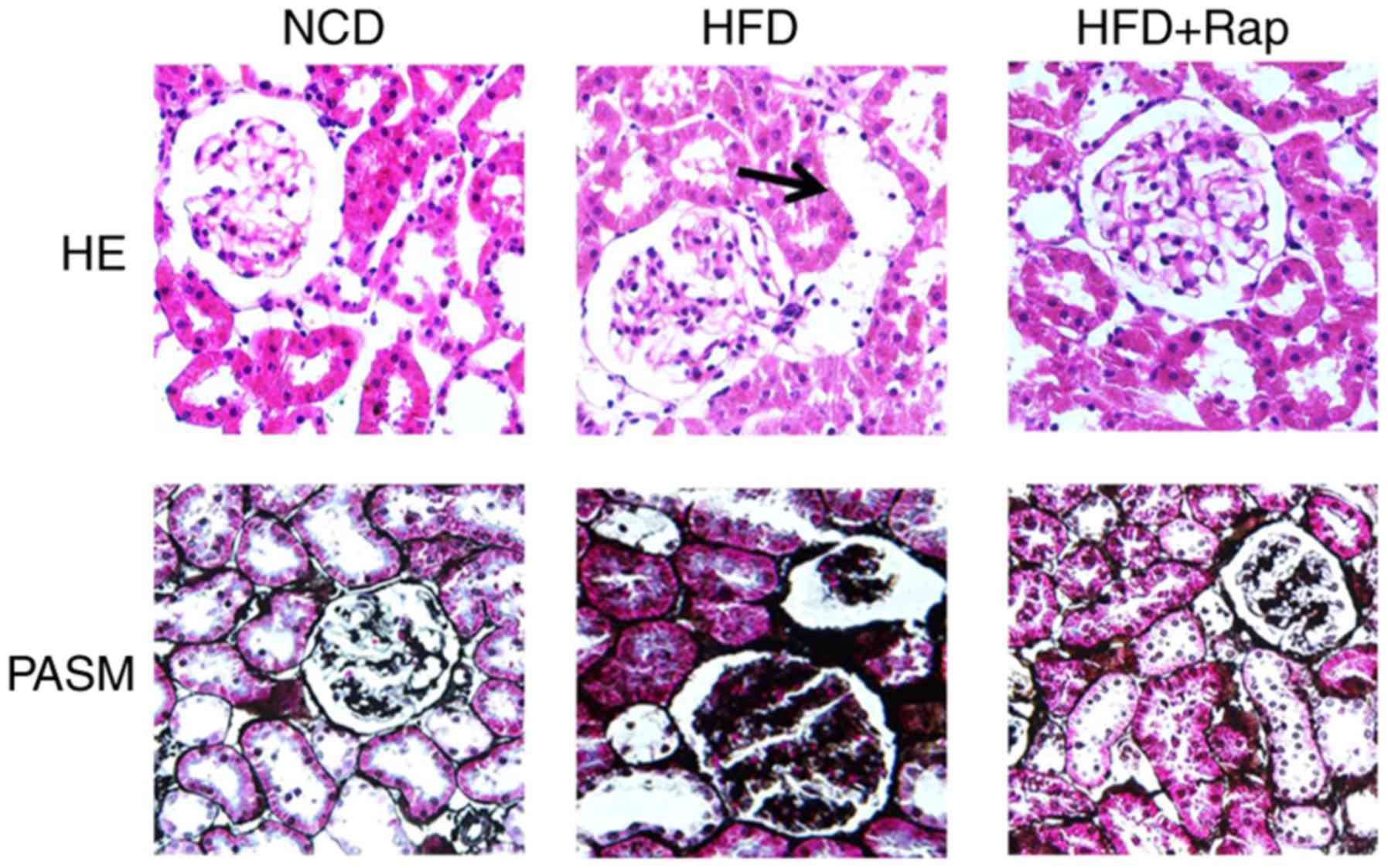
Renal morphology and IL-1β expression
in the rats
HE staining demonstrated vacuolar degeneration in
the renal tubular epithelial cells of the HFD group, and PASM
staining highlighted basement membrane thickening in the glomeruli
and tubules of HFD-fed rats, compared with the NCD group. These
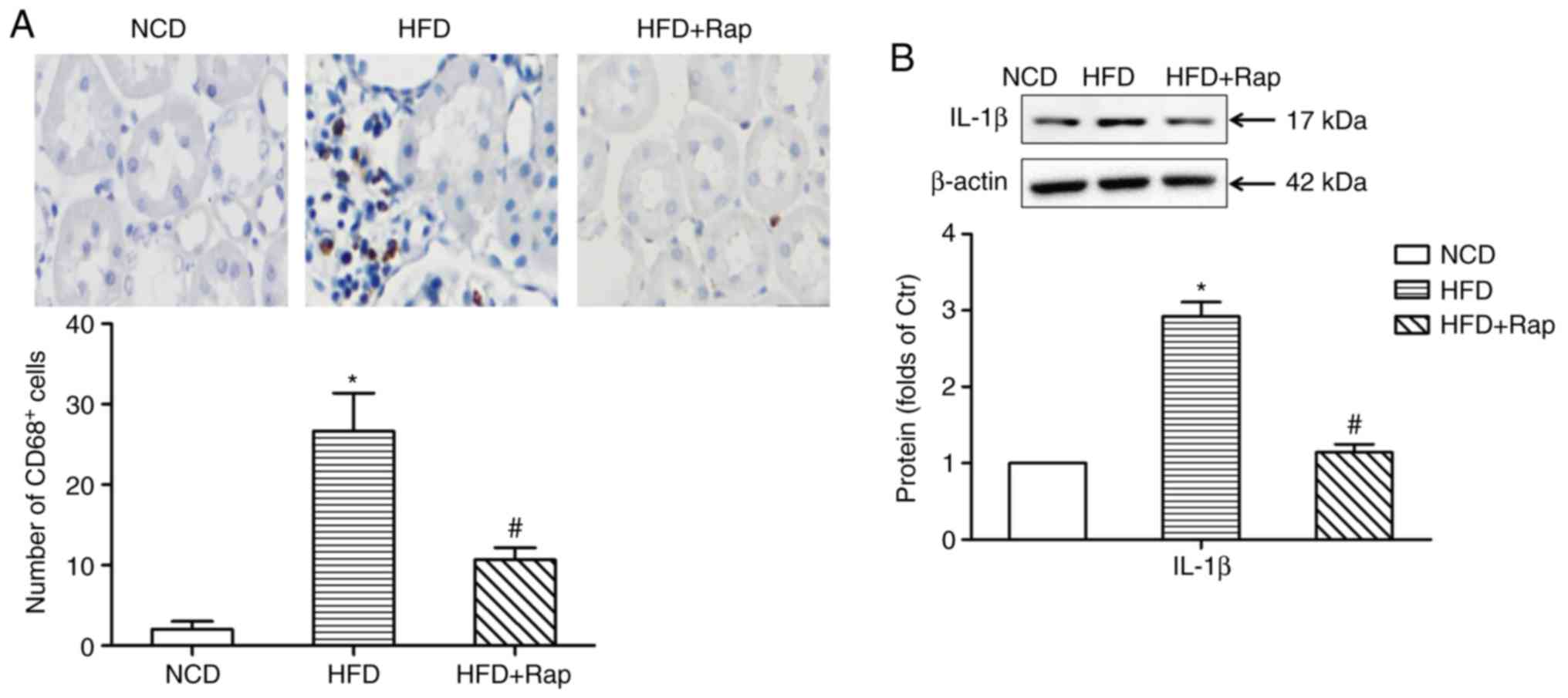
effects were alleviated by Rap treatment (Fig. 3). Immunohistochemical staining
showed that there was prominent infiltration of CD68+
cells (macrophages) into the renal interstitial area in the HFD
group. The tubulointerstitial infiltration of inflammatory cells
was significantly decreased by Rap treatment (Fig. 4A). Furthermore, the protein
expression of IL-1β was increased in the kidneys of obese rats,
compared with the NCD group, while Rap treatment inhibited the
expression of IL-1β (Fig. 4B).
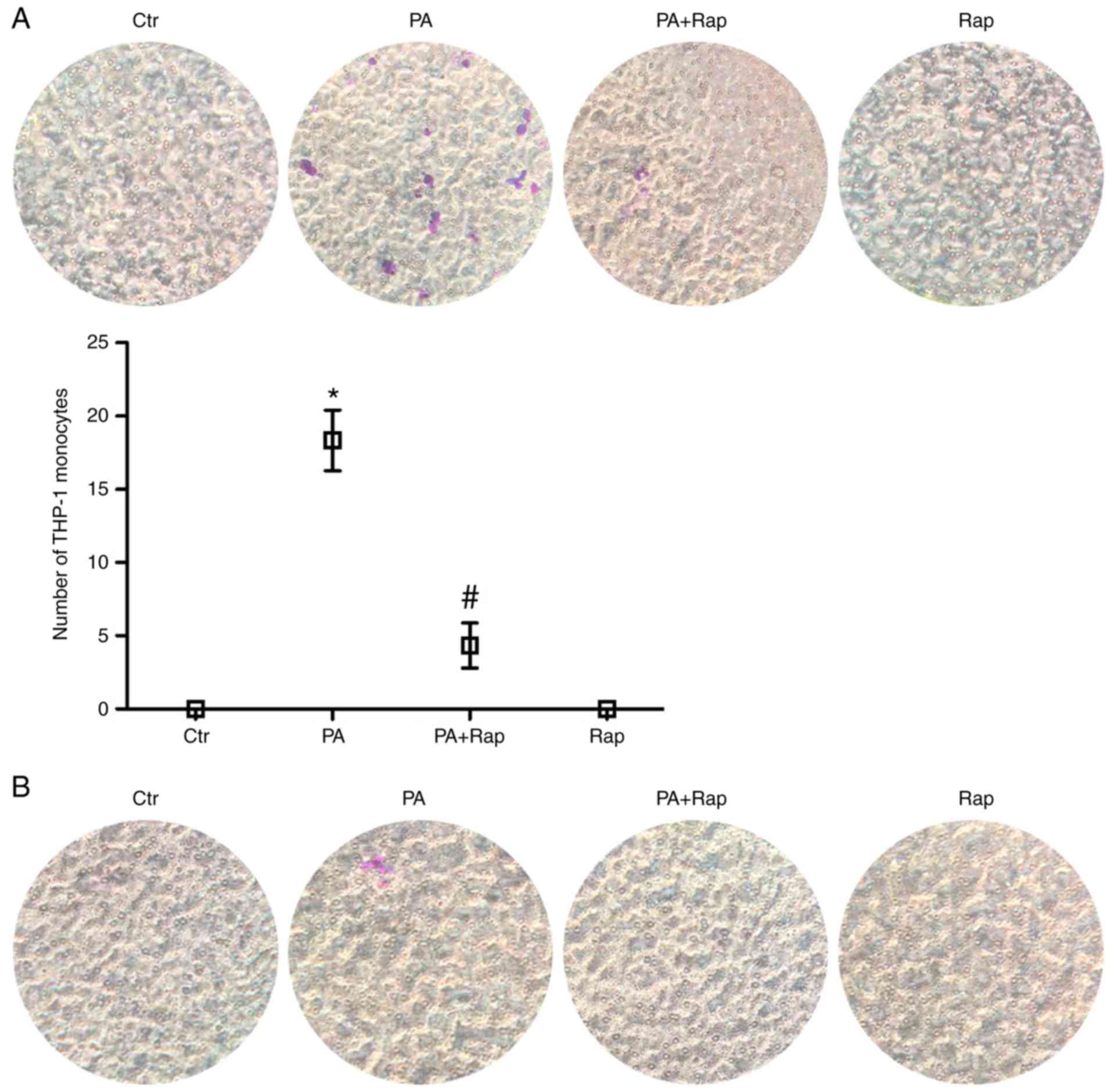
THP-1 monocyte migration
To assess whether PA and Rap were capable of
affecting the migration of monocytes, supernatants from HK-2 cells
treated with PA, PA plus Rap or Rap alone were tested for their
ability to induce monocyte migration using a Transwell migration
assay. The supernatants from HK-2 cells treated with PA stimulated
THP-1 monocyte migration, and compared with PA, the PA+Rap group
stimulated less monocyte migration (Fig. 5A). However, media containing PA,
Rap or PA plus Rap that had not been exposed to HK-2 cells had no
effect on THP-1 monocyte migration (Fig. 5B), suggesting that PA may mediate
monocyte migration by stimulating the secretion of a certain type
of chemokine from HK-2 cells, which is potentially inhibited by
Rap.
Protein expression levels of MCP-1,
p-FOXO1 and p-mTOR
To test the above hypothesis, the present study
detected the expression of MCP-1 in the kidneys of HFD-induced
obese rats and in HK-2 cells. The results demonstrated that the
protein expression of MCP-1 was significantly increased in the
kidneys of obese rats, while Rap treatment inhibited the expression
of MCP-1 (Fig. 6A). In addition,
PA induced the secretion of MCP-1 from the HK-2 cells, and this was
inhibited by Rap, which was consistent with the induction of
chemotaxis by the supernatants (Fig.
6B).
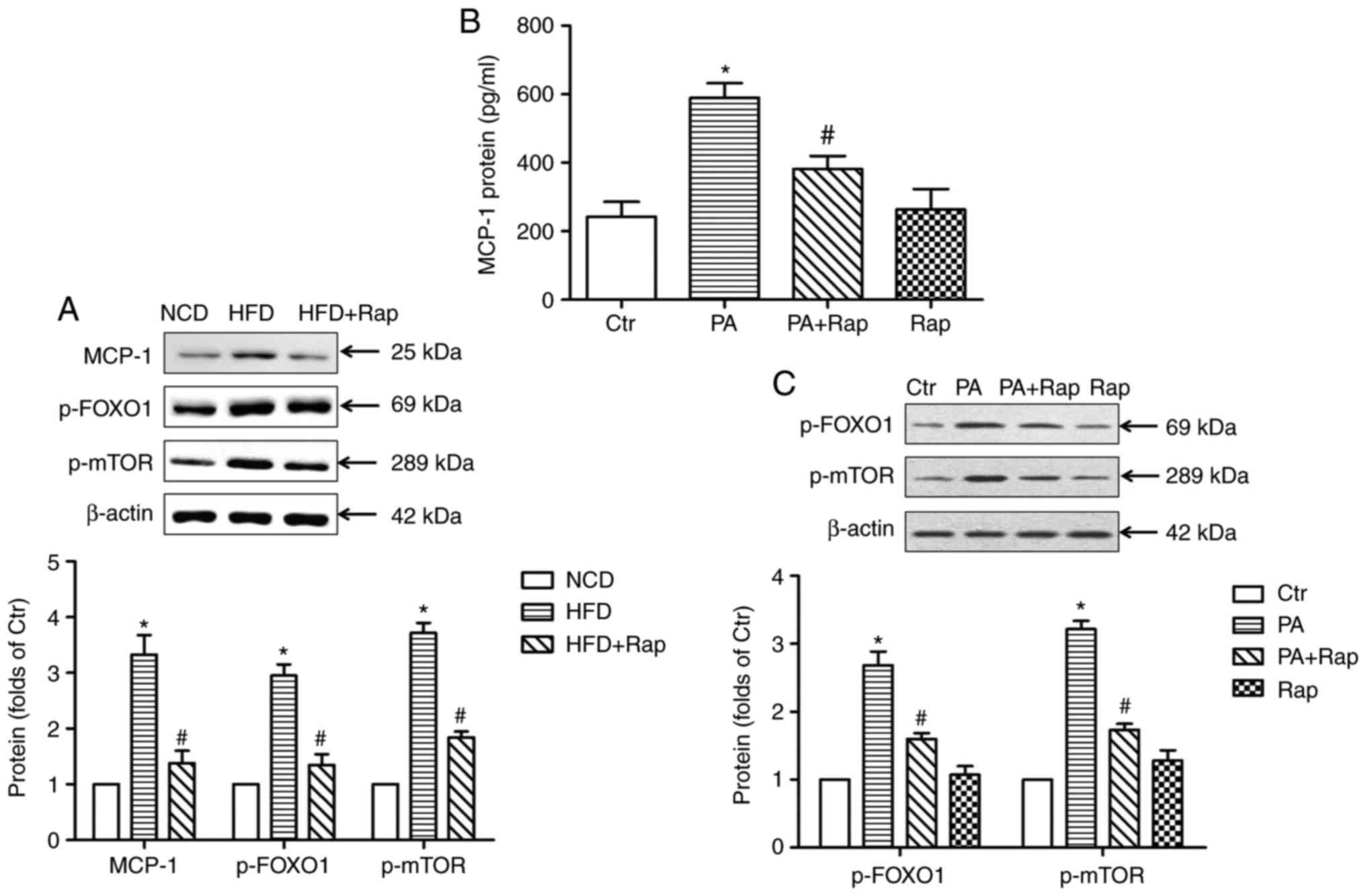 | Figure 6.MCP-1, p-FOXO1 and p-mTOR expression
in the kidneys of rats. (A) Protein expression was examined by
western blotting, normalized by comparison with β-actin and
expressed as a percentage of control. *P<0.05 vs. NCD group;
#P<0.05 vs. HFD group. (B) The release of MCP-1
protein from HK-2 cells. MCP-1 protein content in the supernatant
was detected with an ELISA kit. *P<0.05 vs. Ctr;
#P<0.05 vs. PA. (C) The protein expression of p-FOXO1
and p-mTOR in HK-2 cells. Protein expression was examined by
western blotting. *P<0.05 vs. Ctr; #P<0.05 vs. PA.
Data are presented as the mean ± standard deviation of three
individual experiments; statistical significance was determined by
one-way analysis of variance. NCD, normal chow diet; HFD, high fat
diet; Rap, rapamycin; MCP-1, monocyte chemoattractant protein-1;
p-FOXO1, phosphorylated forkhead boxO1; p-mTOR, phosphorylated
mammalian target of rapamycin; Ctr, control; PA, palmitic acid. |
To investigate the potential mechanisms underlying
this phenomenon, the protein expression of p-FOXO1 and p-mTOR was
assessed in vivo and in vitro. It was found that
p-FOXO1 and p-mTOR were upregulated at the protein level (Fig. 6A) in the kidneys of obese rats,
while Rap treatment inhibited this change. The in vitro
study showed that PA elevated the protein expression of p-FOXO1 and
p-mTOR in HK-2 cells, which was inhibited by Rap. Compared with the
control, Rap alone had no effect on the expression of these two
molecules in HK-2 cells (Fig. 6C).
mTOR siRNA or FOXO1 siRNA-transfected HK-2 cells were used to
further confirm the above results. The data indicated that mTOR
siRNA transfection downregulated the protein expression level of
p-FOXO1 (Fig. 7A) and reduced the
release of MCP-1 (Fig. 7B) in
PA-treated HK-2 cells. Additionally, FOXO1 siRNA transfection
increased the secretion of MCP-1 by the HK-2 cells, although not in
the PA-treated HK-2 cells (Fig.
7B). Reduced protein expression of mTOR was also observed in
the FOXO1 siRNA-transfected HK-2 cells with PA treatment (Fig. 7A).
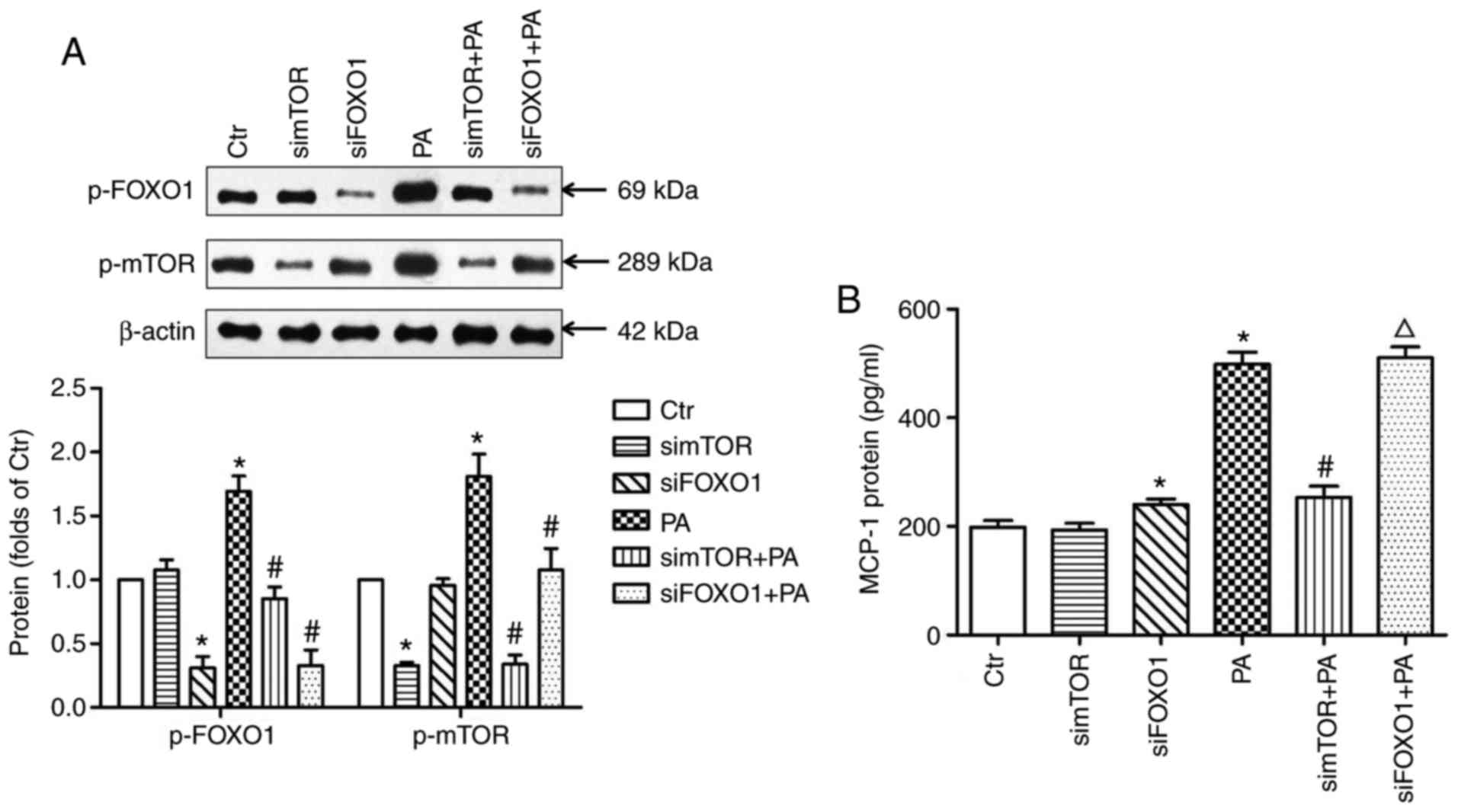 | Figure 7.MCP-1, p-FOXO1 and p-mTOR expression
in HK-2 cells transfected with mTOR siRNA or FOXO1 siRNA. (A) The
protein expression of p-FOXO1 and p-mTOR in HK-2 cells transfected
with mTOR siRNA or FOXO1 siRNA. Protein expression was examined by
western blotting. *P<0.05 vs. Ctr; #P<0.05 vs. PA.
(B) The release of MCP-1 from HK-2 cells transfected with mTOR
siRNA or FOXO1 siRNA. MCP-1 protein content in the supernatant was
detected with an ELISA kit. *P<0.05 vs. Ctr;
#P<0.05 vs. PA; ΔP<0.05 vs. siFOXO1.
Data are presented as the mean ± standard deviation of three
individual experiments; statistical significance was determined by
one-way analysis of variance. p-FOXO1, phosphorylated forkhead
boxO1; p-mTOR, phosphorylated mammalian target of rapamycin; siRNA,
small interfering RNA; Ctr, control; PA, palmitic acid; simTOR,
siRNA targeting mammalian target of rapamycin; siFOXO1, siRNA
targeting forkhead boxO1; HK-2, human renal tubular epithelial cell
line; MCP-1, monocyte chemoattractant protein-1. |
Discussion
Obesity, a chronic low-grade inflammatory condition,
is associated with the development of numerous comorbidities,
including renal disease. As more is understood about obesity, the
perturbation of renal physiology in the context of obesity has
become more apparent (24). In the
present clinical study, dyslipidemia was observed in the
participants with obesity, and these participants had higher Cr,
UACR and u-NGAL, although not BUN, indicating that they had poorer
renal function compared with those who were not obese, though not
to a severe degree. In addition, the high level of u-MCP-1 was
positively correlated with UACR and u-NGAL in the participants with
obesity, suggesting that obesity-induced inflammatory responses may
contribute to renal injury and dysfunction.
As it would be difficult to obtain kidney samples
from the patients with simple obesity, an obese rat model was used
to further investigate renal physiology and pathology. It was
demonstrated that a HFD provoked overt obesity that was
characterized by increased body weight, kidney weight gain and
dyslipidemia. Early renal damage was also observed in the
HFD-induced obese rats, as evidenced by elevated serum Cr and BUN
levels, increased secretion of u-NGAL, proteinuria, vacuolar
degeneration in the renal tubules, and glomerular and tubular
basement membrane thickening. Additionally, infiltration of
CD68+ cells (macrophages) into the renal interstitial
area and increased production of inflammatory factors (IL-1β) were
found in the HFD-induced obese rats. Further in vitro study
showed that supernatants from HK-2 cells treated with PA induced
THP-1 monocyte migration. The above results indicated that the
renal tubular cells may have been stimulated to release a
particular type of chemokine. To explore this hypothesis, the
expression of MCP-1 was assessed in vivo and in
vitro, since MCP-1 serves an important role in renal
inflammatory disease (25,26). The data illustrated that MCP-1
expression was significantly increased in the kidneys of
HFD-induced obese rats and in the PA-treated HK-2 cells, which
further confirmed the notable correlation between FFA and u-MCP-1
in the participants with obesity. Next, mTOR-FOXO1 signaling
pathway protein expression was detected to gain an insight into the
mechanism of MCP-1 secretion. The results demonstrated that the
expression of p-FOXO1 was elevated in the kidneys of HFD-induced
obese rats and in PA-treated HK-2 cells, suggesting that the
increased p-FOXO1 had dissociated from the MCP-1 gene promoter and
translocated from the nucleus to the cytosol, enabling MCP-1 to be
expressed. In addition, the expression of p-mTOR was upregulated in
the kidneys of HFD-induced obese rats and in PA-treated HK-2 cells.
However, Rap inhibited the expression of MCP-1, p-FOXO1 and p-mTOR,
and reduced the inflammatory cell infiltration and IL-1β release in
the renal interstitium. Further data revealed that mTOR siRNA
transfection downregulated the protein expression level of p-FOXO1
and reduced the release of MCP-1 from PA-treated HK-2 cells. These
results suggested that the activated mTOR may have exerted adverse
effects in obesity-associated renal tubulointerstitial
inflammation. Furthermore, 8 weeks of Rap treatment lowered the Cr
and BUN levels, reduced the secretion of urine protein and u-NGAL,
improved vacuolar degeneration in the renal tubules and alleviated
the glomerular and tubular basement membrane thickening in obese
rats, suggesting that inhibition of mTOR may have exerted an
anti-inflammatory effect to prevent obesity-associated renal
tubulointerstitial inflammation. In the in vitro study, it
was also identified that FOXO1 siRNA transfection increased MCP-1
release, indicating that FOXO1 silencing may have reduced MCP-1
promoter inhibition, and MCP-1 was consequently synthesized and
secreted. Notably, FOXO1 siRNA transfection reduced the protein
expression of p-mTOR in PA-treated HK-2 cells, which may explain
the finding that no more MCP-1 was expressed in the PA+FOXO1 siRNA
group compared with the PA group. Recently, certain reports have
suggested a role for FOXO1 in renal protection (27,28).
However, further investigation is required to explore whether FOXO1
is beneficial or detrimental in kidney diseases.
There are a number of limitations to the present
study. First, the sample size of this hospital-based
cross-sectional study was small, and larger samples are required in
the future. Second, the obese animal model does not fully reflect
the clinicopathological alterations in participants with obesity,
and renal biopsy is necessary to investigate the pathological
changes in the kidneys of patients with obesity. Third, other
pro-inflammatory factors that have been associated with lower
kidney function, such as TNF-α, were not evaluated in the present
study (29).
In conclusion, the present findings provided novel
evidence, to the best of our knowledge, that activated mTOR induced
FOXO1 phosphorylation, which mediated renal MCP-1 release, caused
tubulointerstitial inflammation and ultimately lead to renal
pathological changes and dysfunction. The inhibition of mTOR may
serve a renoprotective role during the progression of
obesity-associated renal tubulointerstitial inflammation.
Acknowledgements
Not applicable.
Funding
This work was supported by the National Natural
Science Youth Foundation of China (grant no. 81700632 to HS), the
Natural Science Youth Foundation of Jiangsu Province (grant no.
BK20170366 to HS), and the China Scholarship Council (CSC) (no.
201406090317).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HS and BS designed the research; HS performed the
research, analyzed the data and wrote the paper. XS, JH, MG and BS
analyzed the data and revised the paper. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
The clinical study protocol was approved by the
Institutional Review Board of The First Affiliated Hospital of
Soochow University and written informed consent was obtained from
each subject. All human rights were observed in keeping with the
Helsinki Declaration of 1975, as revised in 2008. The animal study
was conducted in accordance with the ethical guidelines of the
Declaration of Helsinki, the National Institutes of Health Guide
for the Care and Use of Laboratory Animals and was approved by the
Institutional Review Board of The First Affiliated Hospital of
Soochow University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Chade AR and Hall JE: Role of the renal
microcirculation in progression of chronic kidney injury in
obesity. Am J Nephrol. 44:354–367. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Declèves AE and Sharma K: Obesity and
kidney disease: Differential effects of obesity on adipose tissue
and kidney inflammation and fibrosis. Curr Opin Nephrol Hypertens.
24:28–36. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Redon J and Lurbe E: The kidney in
obesity. Curr Hypertens Rep. 17:5552015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Li LC, Yang JL, Lee WC, Chen JB, Lee CT,
Wang PW, Vaghese Z and Chen WY: Palmitate aggravates
proteinuria-induced cell death and inflammation via
CD36-inflammasome axis in the proximal tubular cells of obese mice.
Am J Physiol Renal Physiol. Sep 19–2018.(Epub ahead of print). doi:
10.1152/ajprenal.00536.2017. View Article : Google Scholar
|
|
5
|
Li A, Wang J, Zhu D, Zhang X, Pan R and
Wang R: Arctigenin suppresses transforming growth factor-β1-induced
expression of monocyte chemoattractant protein-1 and the subsequent
epithelial-mesenchymal transition through reactive oxygen
species-dependent ERK/NF-κB signaling pathway in renal tubular
epithelial cells. Free radical research. 49:1095–1113. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lv LL, Feng Y, Wen Y, Wu WJ, Ni HF, Li ZL,
Zhou LT, Wang B, Zhang JD, Crowley SD and Liu BC: Exosomal CCL2
from tubular epithelial cells is critical for albumin-induced
tubulointerstitial inflammation. J Am Soc Nephrol. 29:919–935.
2018.PubMed/NCBI
|
|
7
|
Fu CP, Lee IT, Sheu WH, Lee WJ, Liang KW,
Lee WL and Lin SY: The levels of circulating and urinary monocyte
chemoattractant protein-1 are associated with chronic renal injury
in obese men. Clin Chim Acta. 413:1647–1651. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu X, Yin D, Zhou B and Li T: miR-135a
promotes inflammatory responses of vascular smooth muscle cells
from db/db mice via downregulation of FOXO1. Int Heart J.
59:170–179. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xing YQ, Li A, Yang Y, Li XX, Zhang LN and
Guo HC: The regulation of FOXO1 and its role in disease
progression. Life Sci. 193:124–131. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Puthanveetil P, Wan A and Rodrigues B:
FoxO1 is crucial for sustaining cardiomyocyte metabolism and cell
survival. Cardiovasc Res. 97:393–403. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Maiese K, Chong ZZ, Hou J and Shang YC:
The ‘O’ class: Crafting clinical care with FoxO transcription
factors. Adv Exp Med Biol. 665:242–260. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Qin G, Zhou Y, Guo F, Ren L, Wu L, Zhang
Y, Ma X and Wang Q: Overexpression of the FoxO1 ameliorates
mesangial cell dysfunction in male diabetic rats. Mol Endocrinol.
29:1080–1091. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Du M, Wang Q, Li W, Ma X, Wu L, Guo F,
Zhao S, Huang F, Wang H and Qin G: Overexpression of FOXO1
ameliorates the podocyte epithelial-mesenchymal transition induced
by high glucose in vitro and in vivo. Biochem Biophys Res Commun.
471:416–422. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hsu CK, Lin CC, Hsiao LD and Yang CM:
Mevastatin ameliorates sphingosine 1-phosphate-induced
COX-2/PGE2-dependent cell migration via FoxO1 and CREB
phosphorylation and translocation. Br J Pharmacol. 172:5360–5376.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li X, Zhang X, Pan Y, Shi G, Ren J, Fan H,
Dou H and Hou Y: mTOR regulates NLRP3 inflammasome activation via
reactive oxygen species in murine lupus. Acta Biochim Biophys Sin
(Shanghai). 50:888–896. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang X, Howell GM, Guo L, Collage RD,
Loughran PA, Zuckerbraun BS and Rosengart MR: CaMKIV-dependent
preservation of mTOR expression is required for autophagy during
lipopolysaccharide-induced inflammation and acute kidney injury. J
Immunol. 193:2405–2415. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jiang H, Westerterp M, Wang C, Zhu Y and
Ai D: Macrophage mTORC1 disruption reduces inflammation and insulin
resistance in obese mice. Diabetologia. 57:2393–2404. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Paschoal VA, Amano MT, Belchior T,
Magdalon J, Chimin P, Andrade ML, Ortiz-Silva M, Castro É,
Yamashita AS, Rosa Neto JC, et al: mTORC1 inhibition with rapamycin
exacerbates adipose tissue inflammation in obese mice and
dissociates macrophage phenotype from function. Immunobiology.
222:261–271. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Perl A: mTOR activation is a biomarker and
a central pathway to autoimmune disorders, cancer, obesity, and
aging. Ann N Y Acad Sci. 1346:33–44. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang P, Xiao Y, Luo X, Zhao Y, Zhao L,
Wang Y, Wu T, Wei L and Chen Y: Inflammatory stress promotes the
development of obesity-related chronic kidney disease via CD36 in
mice. J Lipid Res. 58:1417–1427. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang B, Ding W, Zhang M, Li H and Gu Y:
Rapamycin attenuates aldosterone-induced tubulointerstitial
inflammation and fibrosis. Cell Physiol Biochem. 35:116–125. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lu Y, Cheng J, Chen L, Li C, Chen G, Gui
L, Shen B and Zhang Q: Endoplasmic reticulum stress involved in
high-fat diet and palmitic acid-induced vascular damages and
fenofibrate intervention. Biochem Biophys Res Commun. 458:1–7.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Mu Y, Yin TL, Yin L, Hu X and Yang J:
CTRP3 attenuates high-fat diet-induced male reproductive
dysfunction in mice. Clin Sci (Lond). 132:883–899. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ting SM, Nair H, Ching I, Taheri S and
Dasgupta I: Overweight, obesity and chronic kidney disease. Nephron
Clin Pract. 112:C121–C127. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Haller H, Bertram A, Nadrowitz F and Menne
J: Monocyte chemoattractant protein-1 and the kidney. Curr Opin
Nephrol Hypertens. 25:42–49. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lv W, Booz GW, Wang Y, Fan F and Roman RJ:
Inflammation and renal fibrosis: Recent developments on key
signaling molecules as potential therapeutic targets. Eur J
Pharmacol. 820:65–76. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen P, Shi X, Xu X, Lin Y, Shao Z, Wu R
and Huang L: Liraglutide ameliorates early renal injury by the
activation of renal FoxO1 in a type 2 diabetic kidney disease rat
model. Diabetes Res Clin Pract. 137:173–182. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hong YA, Bae SY, Ahn SY, Kim J, Kwon YJ,
Jung WY and Ko GJ: Resveratrol ameliorates contrast induced
nephropathy through the activation of SIRT1-PGC-1α-Foxo1 signaling
in mice. Kidney Blood Press Res. 42:641–653. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mehaffey E and Majid DSA: Tumor necrosis
factor-α, kidney function, and hypertension. Am J Physiol Renal
Physiol. 313:F1005–F1008. 2017. View Article : Google Scholar : PubMed/NCBI
|