Introduction
Pancreatic cancer is a commonly diagnosed cancer and
one of the main causes of cancer-associated mortality (1,2). The
incidence of pancreatic cancer increases yearly, and 95% of
pancreatic cancer cases involve adenocarcinomas (3–5). It
is reported that the 5-year survival rate of pancreatic cancer
patients is only 20% following surgical resection, chemotherapy and
radiation treatment (6,7). The majority of patients are diagnosed
at a late stage with regional invasion or distant metastasis, due
to the lack of recognizable clinical symptoms or signs (8–10).
Therefore, it is urgent to screen effective biomarkers for the
prediction of tumor progression.
Ras-Related Protein Rab-38 (RAB38) is a member of
the RAB small G protein family (11), which is involved in regulating
signal transduction and cellular processes, including membrane
trafficking, cell growth and differentiation (12–14).
It is documented that the point mutations of RAB38 at the GTP
binding domain can result in the Hermansky-Pudlak syndrome in
humans (15–17). Previous studies reported that RAB38
was associated with the synthesis, storage and transport of melanin
pigments in the biogenesis of melanosomes (18,19).
In addition, the RAB38 protein was overexpressed at the RNA level
in melanoma cancer (20,21). It has also been indicated that the
high expression of RAB38 increased the progression of gliomas, and
was associated with poorer prognosis (17). However, the clinical significance
and potential function of RAB38 in the progression of pancreatic
cancer remain unclear.
In the present study, the RAB38 expression level was
analyzed in pancreatic cancer specimens from 82 patients, and its
correlation with the clinicopathological characteristics and
survival rate of these patients was examined. The study then
established RAB38 knockdown PANC-1 and BxPC-3 cell lines by RNA
interference (RNAi) technology, followed by assessing the effect of
RAB38 silencing on the proliferation, migration and invasion of
pancreatic cancer cells. In addition, the effect of low RAB38
expression was further investigated in PANC-1 ×enograft
tumor-bearing mice.
Materials and methods
Specimen collection and ethics
statement
Procedures involving human samples and animals used
in the present study were approved by the Ethics Committee of the
Secondary Hospital of Tianjin Medical University (Tianjin, China).
Written informed consent was provided by all the participants of
the present study. Subsequent to obtaining the Institutional Review
Board approval, the current study identified 82 patients undergoing
standard radical resection of pancreatic cancer in the Secondary
Hospital of Tianjin Medical University between March 2012 and
December 2015. All the patients had no history of preoperative
radiotherapy, chemotherapy or neoadjuvant chemotherapy. All the
patients did not manifest signs of tumor metastasis, as evidenced
by cross-sectional imaging. Normal para-carcinoma tissues adjacent
to carcinoma were obtained. All specimens were stored embedded in
paraffin, at room temperature. The clinicopathological characters
(such as age, sex, pTMN stage, tumor grade, tumor size, lymph
metastasis, and vascular invasion) and outcome information of
patient were recorded.
Immunohistochemical (IHC)
analysis
IHC analysis was performed as described previously
(22). Briefly, specimens were
fixed in 4% paraformaldehyde, embedded in paraffin and cut into
4-µm sections. Following deparaffinization and rehydration, the
sections were subjected to antigen retrieval. Anti-RAB38 antibody
(bs-11244R; BIOSS, Beijing, China) at 4°C overnight (12 h) was used
to detect the RAB38 expression in the specimens and horseradish
peroxidase (HRP; PV-6000; OriGene Technologies, Inc., Beijing,
China)-labeled secondary antibody at room temperature for 20 min.
Color development was then performed by incubating with DAB
provided in the kit (ZLI-9018; OriGene Technologies, Inc., Beijing,
China), and the sections counterstained with 0.5% hematoxylin at
room temperature for 20 sec for cell nuclei and mounted. Images of
the sections were subsequently captured with a light microscope
(Olympus BX-51; Olympus Corporation, Tokyo, Japan).
All specimens were reviewed and scored by
investigators blinded to the clinical characteristics of patients.
The expression of RAB38 in tumor cells was scored as follows: 0, no
positive expression in tumor cells; 1, <25% positive expression
in tumor cells; 2, 25–50% positive expression; and 3, >50%
positive expression. The staining intensity was also determined
according to the following scores: 0 (no staining), 1 (weak, light
yellow staining), 2 (moderate, yellowish brown staining) and 3
(strong, brown staining). The staining index was calculated as
follows: Staining index = (staining intensity score) × (tumor cell
staining grade). A staining index score of ≥4 was defined as high
RAB38 expression, while a staining index of <4 represented low
expression of RAB38.
Cells and animals
PANC-1 and BxPC-3 human pancreatic adenocarcinoma
cell lines were purchased from the American Type Culture Collection
(Manassas, VA, USA). PANC-1 cells were cultured in Dulbecco's
modified Eagle's medium, and BxPC-3 cells were cultured in Roswell
Park Memorial Institute 1640 medium from The Cell Resource Center
of Peking Union Medical College (Beijing, China). All cells were
incubated in medium containing 10% fetal bovine serum and 1%
penicillin combined with streptomycin at 37°C in 5%
CO2.
Female BALB/c nude mice (n=30; 6–8 weeks old; 20–25
g) were purchased from the Academy of Military Science (Beijing,
China). The mice were maintained under a germ-free environment in
individual ventilated cages, and at 20°C, humidity 40–60%, 12-h
light/dark cycle and ad libitum access to standard lab chow and
water.
Small hairpin RNA (shRNA)
transfection
Pancreatic cancer cells were seeded in 6-well plates
at a concentration of 3×104 cells/well and cultured
overnight. The plasmid TRCN0000048183 and negative vector were
purchased from Open Biosystems, Inc. (Huntsville, AL, USA) and
designated as sh-RAB38 (23).
Diluted shRNAs were added to Lipofectamine® 2000
(11668027; Thermo Fisher Scientific, Inc., Waltham, MA, USA) for 20
min, and then the cells were transfected using the mixture and
incubated at 4°C for 6 h. Subsequently, the transfection effect was
evaluated by reverse transcription-quantitative polymerase chain
reaction (RT-qPCR) and western blot analysis.
RT-qPCR assay
Total RNA in human cells was isolated by TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.). The
OD260/OD280 of the RNA was 2.0. RNA was then reverse-transcribed
into cDNA using PrimeScript RT Reagent kit (Takara Bio, Inc.,
Shiga, Japan). Next, qPCR was performed by Primer Mix Taq II
(Takara Biotechnology Co., Ltd., Dalian, China) with the following
conditions (24): 95°C for 2 min
(1 cycle), 95°C for 5 sec, 55°C for 30 sec, and 72°C for 30 sec (35
cycles) and final extension at 72°C for 6 min. Using the
2−∆∆Cq method (25),
the relative expression level was normalized by the internal
standard β-actin. The primer sequences used were: RAB38,
5′-GTAATCGGCGACCTAGGTG-3′ (forward) and 5′-TCCATTCCCGGAACCTTCAC-3′
(reverse); β-actin, 5′-CAGCTCACCATGGATGATGATATC-3′ (forward) and
5′-AAGCCGGCCTTGCACAT-3′ (reverse).
Western blot analysis
The nuclear and cytoplasmic protein was extracted by
protein lysate, and the concentration of protein was measured by a
protein assay kit (Beyotime Institute of Biotechnology, Haimen,
China). Immunoblotting was then performed as previously described
(6). Briefly, protein (30 µg) was
separated by 10% SDS-PAGE and transferred onto polyvinylidene
difluoride membranes. The membranes were blocked by 1% BSA at 4°C
for 2 h and then incubated with appropriate primary antibodies at
4°C overnight, followed by incubation with secondary antibodies at
37°C for 2 h. The primary antibodies used were as follows: Rabbit
anti-RAB38 (1:400; bs-11244R; BIOSS), rabbit anti-β-actin (1:1,000;
ab5694; Abcam, Cambridge, UK), rabbit anti-Ki67 (1:1,000; ab16667;
Abcam), rabbit anti-proliferating cell nuclear antigen (PCNA;
1:500; ab18197; Abcam), rabbit anti-matrix metalloproteinase 2
(MMP2; 1:1,000; ab37150; Abcam) and rabbit anti-MMP9 (1:1,000;
ab38898; Abcam). The secondary antibodies used in the assay were
polyclonal goat anti-rabbit IgG antibodies (1:10,000; 711–1122;
Rockland Immunochemicals, Inc., Limerick, PA, USA). Subsequently,
the protein was visualized by Immobilon Western Chemiluminescent
HRP Substrate (EMD Millipore, Billerica, MA, USA) and detected by
Tanon-5500 Chemiluminescent Imaging System (Tanon Science &
Technology Co., Ltd., Shanghai, China). Western blot data were
quantified by densitometric analysis with ImageJ software version
LSM880 (National Institutes of Health, Bethesda, MD, USA), and the
relative expression level was normalized by the internal standard
β-actin.
Cell proliferation
Colony formation assays were used to test the
proliferation ability of cells. Briefly, cells were seeded on
96-well plates at a concentration of 300 cells per well. The cells
were fixed using methanol for 10 min, and then the colonies were
stained by crystal violet stain solution at room temperature
(C0121; Beyotime Institute of Biotechnology) for 2 min and counted
by an optical microscope.
MTT assay
Cells were grown for 12 h in 96-well plates at a
concentration of 5×103 cells/well. Next, 0.5 mg/ml MTT
was added to the medium of each well and incubated for another 4 h
at 37°C. The supernatant was removed directly by pipettor and 100
µl dimethyl sulfoxide was added. A microplate reader (EXL-800;
BioTek Instruments, Inc., Winooski, VT, USA) was used to measure
the absorbance at 570 nm.
Scratch migration assays
The cells (1×106) were seeded on 6-well
plates overnight. Next, a sterile pipette tip was used to introduce
a scratch in the middle of the well. The migration of cells towards
the center of the wound was measured at 48 h.
Transwell invasion assay
A Transwell assay was conducted to assess the cell
invasion ability in response to RAB38 silencing. The upper surface
of the Transwell filter used in the assay was coated with Matrigel.
Cells (1×105) suspended in 150 µl serum-free medium were
added to the Transwell chamber, which was then placed into a
24-well plate containing complete medium. After 24 h of incubation
at 37°C, the filter was extracted, and Matrigel on the upper
surface of the filter was removed by cotton swabs. Cells on the
underside of the Transwell filter were then fixed with 4%
paraformaldehyde for 25 min and stained with 0.1% crystal violet
for 15 min, following which images were captured and the result
quantified by the number of cells.
Tumor challenge
The nude mice were divided into two groups (n=10),
and then subcutaneously inoculated in the right groin with
1×106 PANC-1/control or PANC-1/sh-RAB38 cells,
respectively. All mice were monitored daily, and tumor growth and
body weight were measured on the days 14, 17, 21, 23, 26 and 29.
The tumor size was calculated according to the following formula:
Volume = length × width × width/2.
Furthermore, in order to investigate the association
between RAB38 and metastasis of pancreatic cancer cells, PANC-1
cells transfected with RAB38 shRNA and controls were used to
establish the two kinds of stable cell lines, which were injected
into the tail vein of mice, respectively. The metastatic lung
tumors were detected and, after 4 weeks, the mice were euthanized
and tumors were harvested.
Statistical analysis
The continuous variables data were presented as the
mean ± standard deviation. The Student's t-test for paired data was
used to compare mean values. Categorical data were analyzed by the
χ2 method. Statistical analyses were performed with IBM
SPSS version 22.0 software (IBM Corporation, Armonk, NY, USA).
P<0.05 was considered to denote a statistically significant
difference.
Results
RAB38 protein expression is associated
with clinicopathological features in patients with pancreatic
cancer
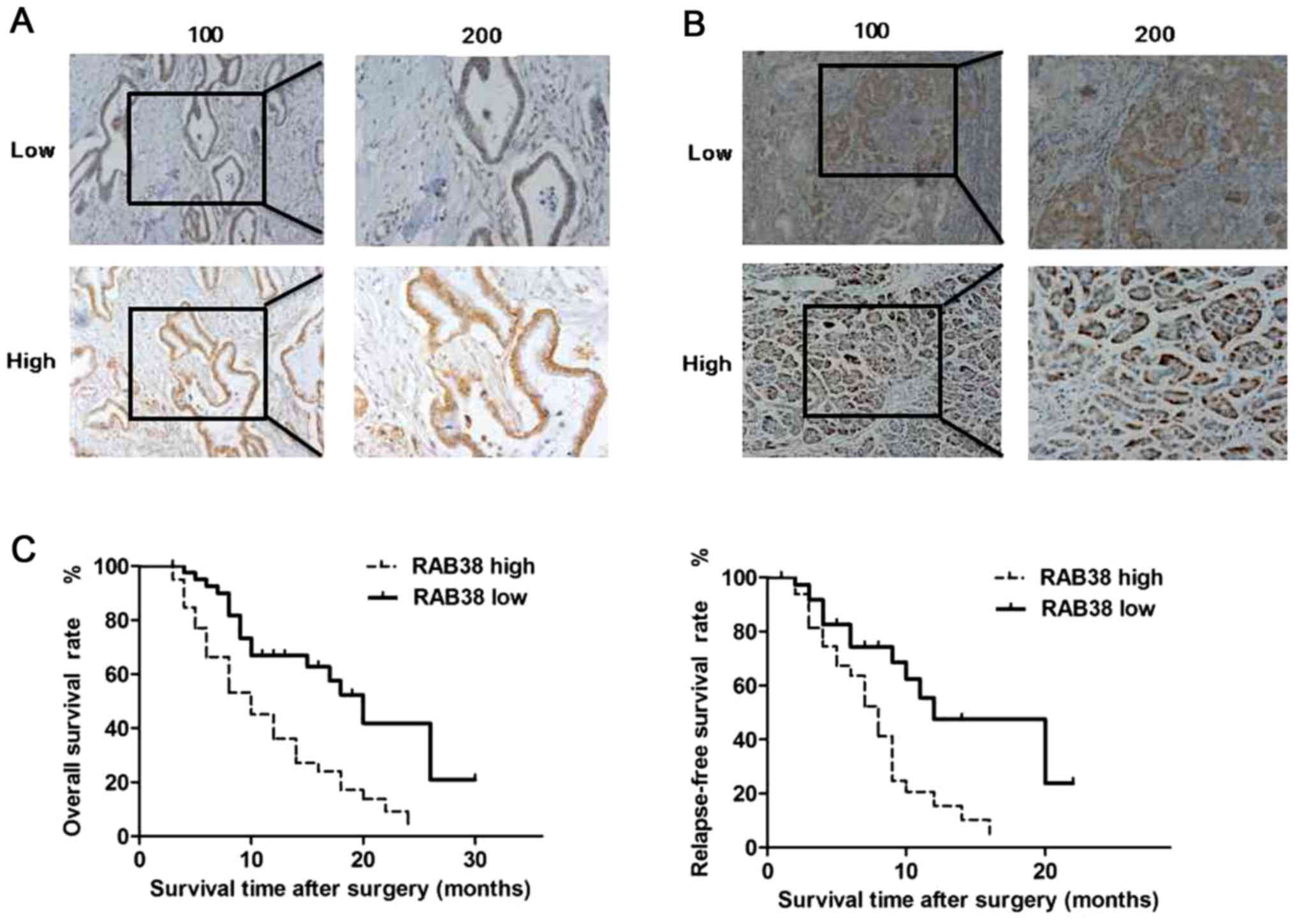
A total of 82 pancreatic ductal adenocarcinoma
(Fig. 1A) or para-carcinoma
tissues (Fig. 1B) were used to
detect the protein expression levels of RAB38 by IHC analysis.
Positive RAB38 expression was detected in 48.8% (40/82) and 30.5%
(25/82) of pancreatic ductal adenocarcinoma and para-carcinoma
tissues. The positive rate in pancreatic ductal adenocarcinoma
tissues was significantly higher in comparison with that in
para-carcinoma tissues (χ2=5.734; P=0.017). The
pancreatic ductal adenocarcinoma specimens included 40 samples with
high RAB38 positive expression and 42 samples with low RAB38
positive expression (Fig. 1A).
To further evaluate the association between the
expression of RAB38 and clinical outcome, the cancer relapse-free
survival rate (RFS) and overall survival rate (OS) in patients
according to their RAB38 expression was also examined. The results
demonstrated that pancreatic ductal adenocarcinoma patients with
high RAB38 expression exhibited a significantly shorter survival
time as compared with those with low expression (P=0.042<0.05
and P=0.046<0.05). Therefore, high RAB38 expression was
significantly associated with much shorter RFS and OS in 82
patients with pancreatic ductal adenocarcinoma (Fig. 1C).
As shown in Table
I, the results indicated that the expression of RAB38 protein
changed from low to high according to the grade progression of
pancreatic ductal adenocarcinoma. Furthermore, high RAB38
expression was significantly associated with a higher pTNM stage,
higher tumor grade and presence of vascular invasion. There were no
significant association between RAB38 expression and other
pathological factors, such as age, sex, tumor stage and lymph node
metastasis (Table I). However,
unlike pTNM stage or vascular invasion, RAB38 expression was not an
independent clinical prognostic factor for pancreatic ductal
adenocarcinoma (Table II).
 | Table I.Correlation of RAB38 with
clinicopathological characteristics in 82 patients with pancreatic
ductal adenocarcinoma. |
Table I.
Correlation of RAB38 with
clinicopathological characteristics in 82 patients with pancreatic
ductal adenocarcinoma.
|
|
| RAB38
expression |
|
|
|---|
|
|
|
|
|
|
|---|
| Feature | No. | High (n=40) | Low (n=42) | χ2 | P-value |
|---|
| Age (years) |
|
|
| 0.424 | 0.515 |
|
<65 | 64 | 30 | 34 |
|
|
|
≥65 | 18 | 10 | 8 |
|
|
| Sex |
|
|
| 0.402 | 0.526 |
|
Male | 48 | 22 | 26 |
|
|
|
Female | 34 | 18 | 16 |
|
|
| pTNM stage |
|
|
| 7.680 | 0.006a |
| I | 24 | 6 | 18 |
|
|
|
II–III | 58 | 34 | 24 |
|
|
| Tumor grade |
|
|
| 4.767 | 0.029a |
|
Low | 62 | 26 | 36 |
|
|
|
High | 20 | 14 | 6 |
|
|
| Tumor size
(cm) |
|
|
| 1.239 | 0.266 |
|
<5 | 58 | 26 | 32 |
|
|
| ≥5 | 24 | 14 | 10 |
|
|
| Lymph node
metastasis |
|
|
| 2.656 | 0.103 |
| No | 60 | 26 | 34 |
|
|
|
Yes | 22 | 14 | 8 |
|
|
| Vascular
invasion |
|
|
| 4.091 | 0.043a |
| No | 54 | 22 | 32 |
|
|
|
Yes | 28 | 18 | 10 |
|
|
 | Table II.Univariate and multivariate analyses
of RFS and OS for RAB38 in pancreatic cancer. |
Table II.
Univariate and multivariate analyses
of RFS and OS for RAB38 in pancreatic cancer.
|
| RFS | OS |
|---|
|
|
|
|
|---|
|
|
| Multivariate
survival analysis |
| Multivariate
survival analysis |
|---|
|
|
|
|
|
|
|---|
| Clinicopathological
characteristic | Univariate
P-value | HR | 95% CI | P-value | Univariate
P-value | HR | 95% CI | P-value |
|---|
| Age | 0.292 |
|
|
| 0.375 |
|
|
|
| Sex | 0.436 |
|
|
| 0.528 |
|
|
|
| pTNM stage | 0.010 | 1.856 | 1.203–3.436 | 0.047 | 0.014 | 1.895 | 1.005–3.574 | 0.045 |
| Tumor grade | 0.021 | 1.725 | 0.936–3.006 | 0.080 | 0.026 | 1.659 | 0.860–3.118 | 0.121 |
| Tumor size | 0.319 |
|
|
| 0.428 |
|
|
|
| Lymph node
metastasis | 0.082 |
|
|
| 0.113 |
|
|
|
| Vascular
invasion | 0.011 | 1.845 | 1.335–3.605 | 0.041 | 0.012 | 1.190 | 0.597–2.370 | 0.044 |
| RAB38
expression | 0.018 | 1.656 | 1.012–2.836 | 0.069 | 0.014 | 0.676 | 0.322–1.255 | 0.401 |
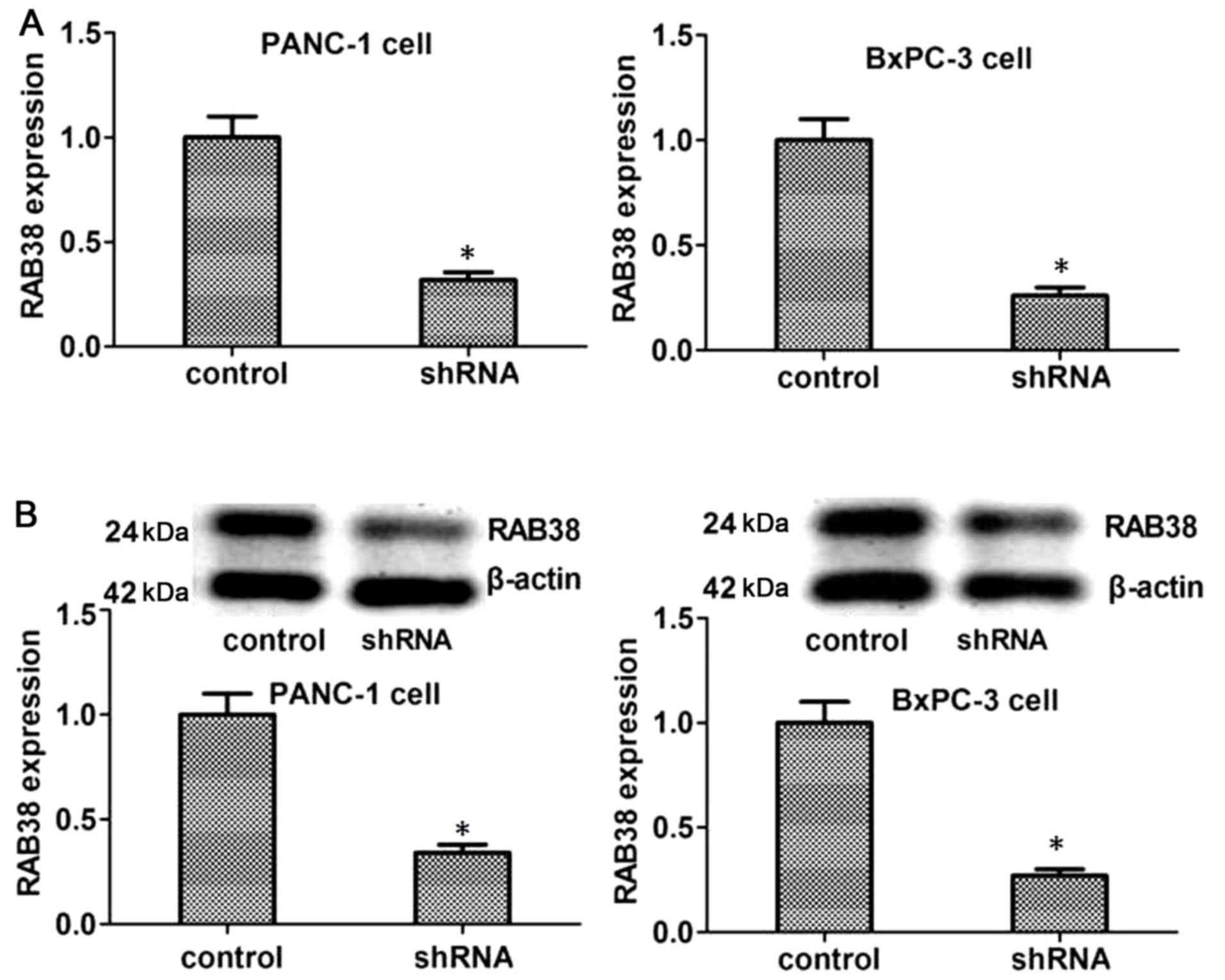
ShRNA transfection of PANC-1 or BxPC-3
cells
To substantiate the role of RAB38 in pancreatic
ductal adenocarcinoma, transfection with RAB38 shRNA was used to
silence RAB38 in PANC-1 and BxPC-3 cells. Next, the RAB38
expression level was detected by RT-qPCR and western blot analysis
in vitro. The results demonstrated that RAB38 expression in
shRNA-transfected cells decreased significantly at the mRNA level
as compared with that in the control cells. Furthermore, the
expression of RAB38 proteins identified by western blot analysis
was in accordance with the mRNA expression (Fig. 2). Therefore, RAB38 silencing in the
cancer cells was successfully established by shRNA
transfection.
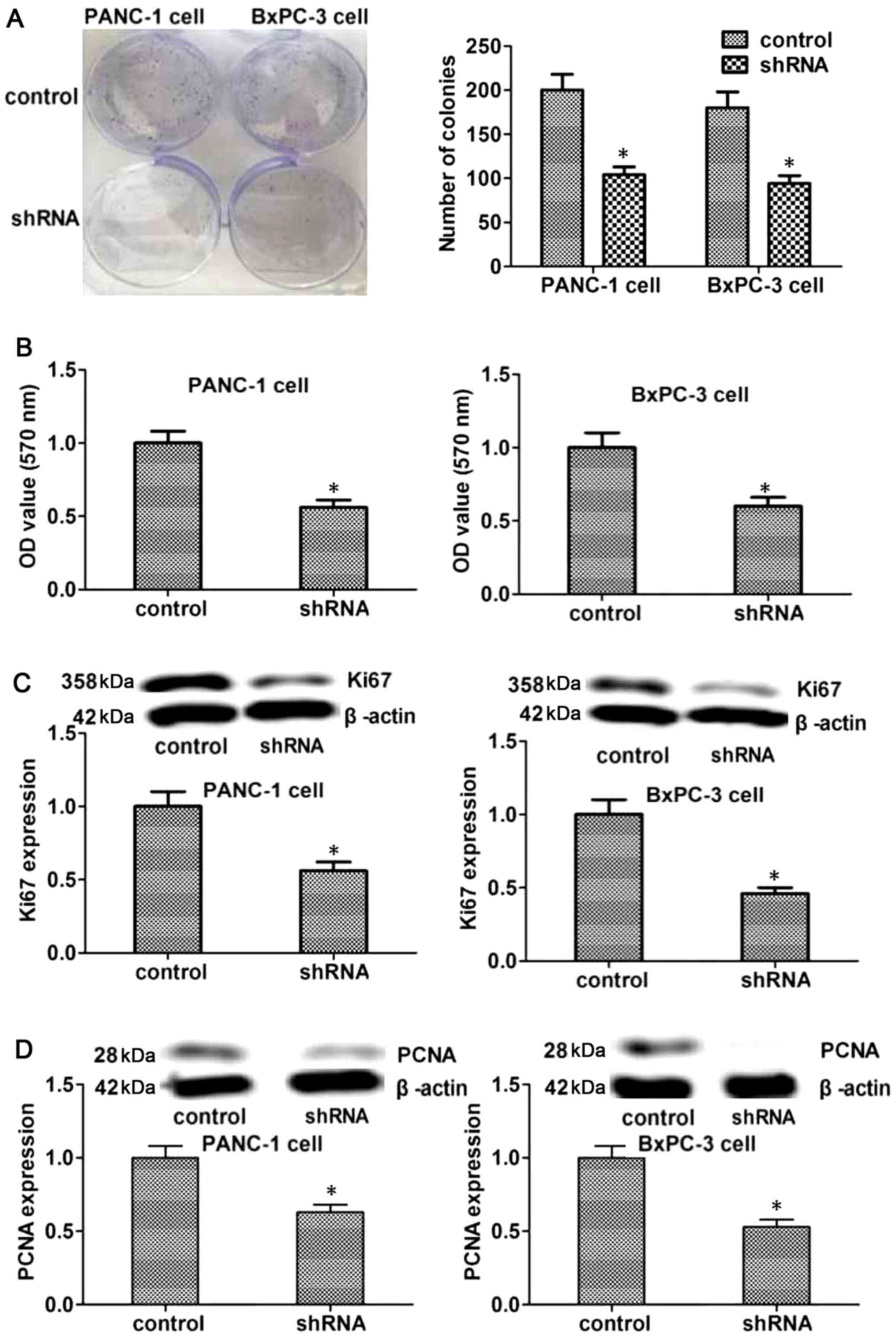
RAB38 silencing suppresses the
proliferation of cancer cells in vitro
The proliferation of tumor cells is important for
tumorigenesis, while the proliferation of endothelial cells is a
main factor influencing the tumor angiogenesis, which is associated
with tumor development. Therefore, the current study evaluated the
effects of RAB38 silencing on the proliferation of PANC-1 and
BxPC-3 cells using colony formation and MTT assays. As shown in
Fig. 3A and B, the results
revealed that RAB38 silencing in PANC-1 and BxPC-3 cells suppressed
their proliferation ability when compared with the control cells.
To further explore the mechanism underlying this effect, the
expression levels of Ki67 and PCNA were detected, which are
proteins associated with proliferation. The results demonstrated
that the expression levels of Ki67 and PCNA proteins decreased in
the RAB38 shRNA-transfected group as compared with the control
group (Fig. 3C and D). Taken
together, these results suggested that RAB38 silencing was able to
reduce cell proliferation by regulating associated proteins, such
as Ki67 and PCNA, in vitro.
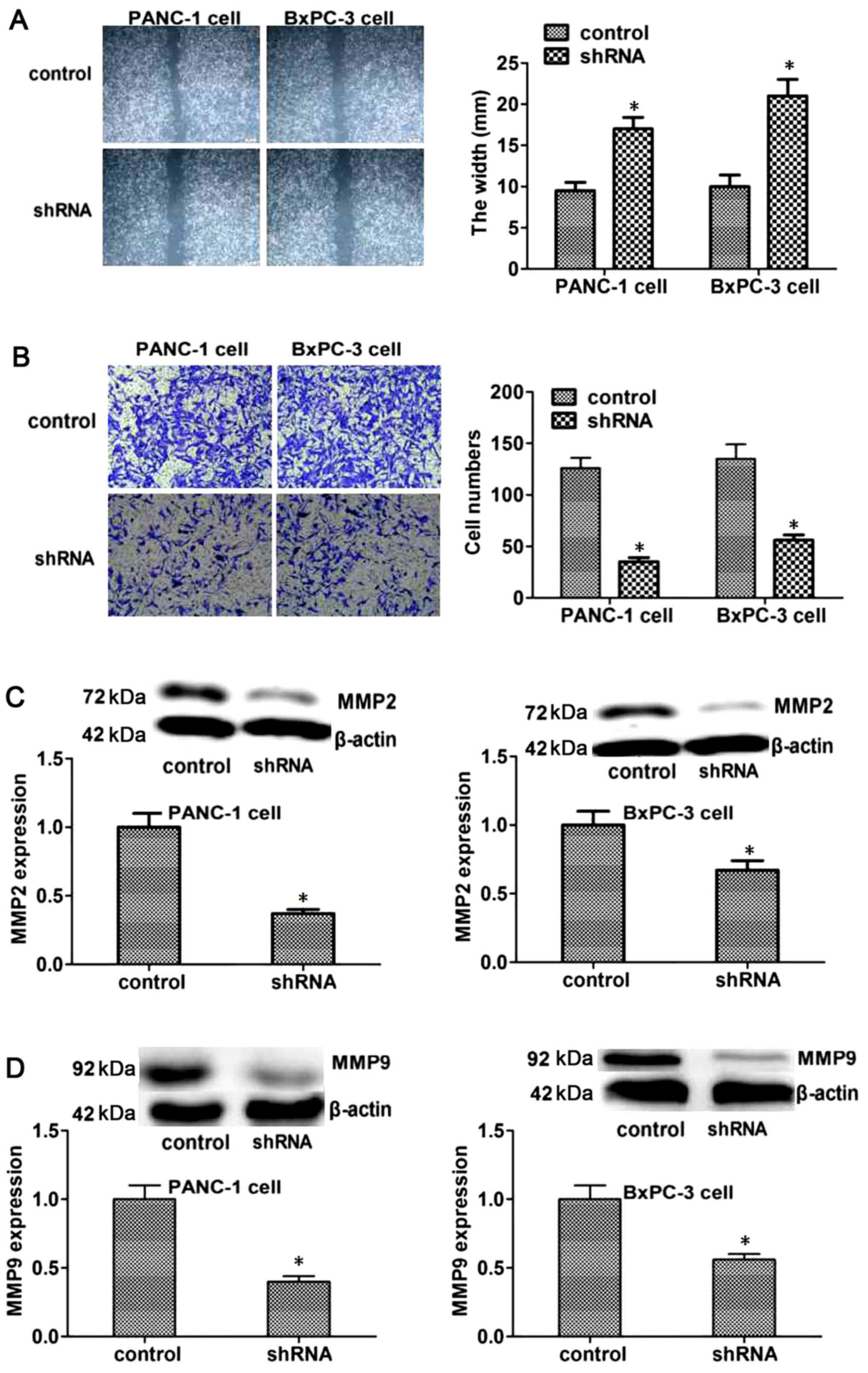
RAB38 silencing suppresses in vitro
migration and invasion in PANC-1 and BxPC-3 cells
To further explore the roles of RAB38 in pancreatic
cancer, the study investigated the effect of RAB38 on the migration
of tumor cells, which is a key step in the development of cancer.
The results demonstrated that the scratch width in RAB38
shRNA-transfected PANC-1 and BxPC-3 cells was significantly bigger,
suggesting reduced migration (Fig.
4A). In addition, the possible effect of RAB38 knockdown on
regulating the invasive capacity of cancer cells was detected by a
Transwell invasion assay. The data indicated that the invasive
capacity of RAB38-silenced PANC-1 and BxPC-3 cells was markedly
decreased compared with that of the control cells after 48 h of
culture (Fig. 4B).
Furthermore, to explore the mechanism underlying the
effect of RAB38 silencing on the migration and invasion of cancer
cells, the expression levels of the proteins that reflect invasion
and metastasis, such as MMP2 and MMP9, were measured. The results
suggested that the expression levels of MMP2 and MMP9 decreased
significantly in the RAB38 shRNA-transfected group compared with
the control group (Fig. 4C and D).
These results suggested that RAB38 silencing reduced the invasive
capacity of cancer cells by regulating the levels of associated
proteins, including MMP2 and MMP9.
RAB38 silencing suppresses the growth
of PANC-1 cancer cells in vivo
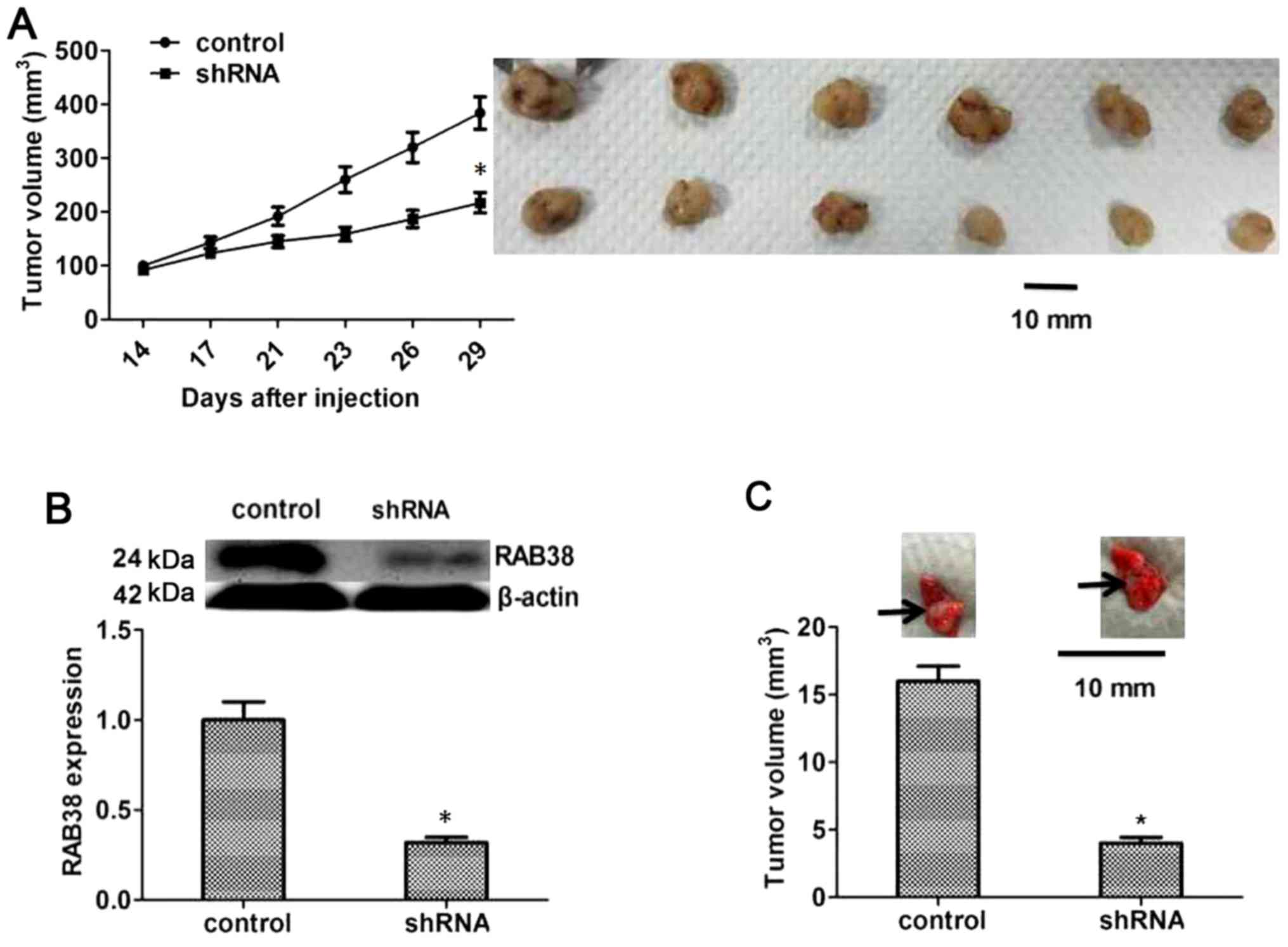
To further confirm that RAB38 silencing was able to
suppress the proliferation, migration and invasion of PANC-1 cancer
cells, the influence of RAB38 silencing on the growth of PANC-1
cancer cells was also detected in vivo. Nude mice were
inoculated with RAB38-silenced PANC-1 cancer cells in the
experimental group and with normal PANC-1 cancer cells in the
control group, and the tumor growth was monitored for 3 weeks. The
results demonstrated that RAB38 silencing significantly inhibited
the growth of tumor cells compared with the control group (Fig. 5A). Furthermore, RAB38 expression in
the tumors of these mice was detected by western blot analysis, and
the results revealed reduced RAB38 protein levels as a result of
knockdown by shRNA (P<0.05, Fig.
5B). Besides, PANC-1 cells in the two groups were also injected
into the tail vein of mice to observe the differences in lung
metastasis. The results suggested that metastatic tumors in the
RAB38 shRNA group were evidently smaller in comparison with those
in the control group (P<0.05, Fig.
5C). These results confirmed the inhibitory effect of RAB38
shRNA on tumor growth in vivo.
Discussion
Pancreatic cancer is a highly invasive malignancy
with resistance to the majority of chemotherapy strategies and no
effective approaches for early diagnosis due to the atypical
symptoms or signs in the initial stages of the disease in most
patients (26–28). Although great efforts have been
made to treat pancreatic cancer in the past decades, the outcome
remains poor. Thus, it is necessary to identify potential
biomarkers and therapeutic targets for diagnosis and treatment of
pancreatic cancer.
To the best of our knowledge, the present study is
the first to investigate RAB38 protein expression, as well as its
prognostic value, in pancreatic cancer. RAB38 belongs to the RAB
superfamily and is implicated in the biogenesis of melanosomes,
which helps in the synthesis, storage and transport of melanin
pigments (16,29). Mutation of RAB38 is associated with
platelet storage pool disease (11,30),
and RAB38 is overexpressed at the mRNA level in melanoma cancer
(21,31,32)
and glioma (17). It has been
demonstrated that a tendency of increased RAB38 expression was
observed in high grade glioma at the mRNA and protein levels, and
glioma patients with higher expression of RAB38 displayed a
markedly worse prognosis (17).
It is known that tumor grade progression and
survival rate are vital for the clinical assessment (33,34).
In the current study, the expression of RAB38 was higher in
pancreatic cancer with high grade as compared with that in
low-grade tumors (Fig. 1), which
is similar to the observations reported in the study by Wang and
Jiang, where a higher RAB38 expression was more prevalent in
high-grade than low-grade gliomas (17). Furthermore, the present study
identified that RAB38 expression was associated with pTNM stage,
tumor grade and vascular invasion; however, unlike for pTNM stage
or vascular invasion, RAB38 was not an independent prognostic
factor for pancreatic cancer, which was not consistent with
previous studies (17–21). In addition, the current results
demonstrated that pancreatic cancer patients with high expression
of RAB38 had a much shorter OS and RFS, and more advanced tumor
stage as compared with those in patients with low RAB38
expression.
Chemotherapeutic resistance of pancreatic cancer is
the main challenge in prolonging survival (35). In the present study, knockdown of
RAB38 was demonstrated to significantly inhibit the proliferation,
migration and invasion abilities of pancreatic cancer cells, and
reduce the expression of metastasis-associated proteins, including
MMP2 and MMP9. Further investigations in animals also revealed that
RAB38 silencing inhibited the growth of cancer cells. Therefore, it
is presumed that RAB38 may be a new therapeutic target in
pancreatic cancer. However, it is necessary to further investigate
the mechanisms of RAB38 in pancreatic cancer biology in future
studies. Owing to the interaction of the different signaling
pathways, the upstream and downstream genes and proteins of RAB38
in pancreatic cancer, which are directly associated with RAB38 in
the signaling pathway, should be identified in further studies.
In conclusion, the present study demonstrated high
expression of RAB38 protein in pancreatic cancer samples, as
detected by IHC assay. Prognostic analysis confirmed that high
expression of RAB38 indicates a shorter survival time in pancreatic
cancer. Furthermore, the current investigation provided an insight
into the molecular mechanisms of RAB38 that are involved in its
proliferation and metastatic potential. Therefore, RAB38 may be a
novel biomarker for pancreatic cancer.
Acknowledgements
Not applicable.
Funding
This study was funded by the Tianjin Health Bureau
Science and Technology Fund (grant no. 2015KZ098).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
BYL and LJH conceived and designed the experiments.
BYL, XLZ, HL and BL performed the experiments. XLZ, HL and BL
assisted in obtaining the reagents, materials and analytical tools.
All authors wrote and approved the manuscript.
Ethics approval and consent to
participate
All experimental procedures involving human
participants were performed in accordance with the ethical
standards of the institutional and/or national research committee,
and the 1964 Helsinki Declaration and its later amendments or
comparable ethical standards. Informed consent was obtained from
all individual participants included in the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Liggett T, Melnikov A, Yi QL, Replogle C,
Brand R, Kaul K, Talamonti M, Abrams RA and Levenson V:
Differential methylation of cell-free circulating DNA among
patients with pancreatic cancer versus chronic pancreatitis.
Cancer. 116:1674–1680. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kahlert C, Weber H, Mogler C, Bergmann F,
Schirmacher P, Kenngott HG, Matterne U, Mollberg N, Rahbari NN,
Hinz U, et al: Increased expression of ALCAM/CD166 in pancreatic
cancer is an independent prognostic marker for poor survival and
early tumour relapse. Br J Cancer. 101:457–464. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Arcidiacono PG, Bhutani MS and Giovannini
M: EURO-EUS 2003: Pancreatic tumor: Impact of endoscopic
ultrasonography on diagnosis, staging and treatment. Cancer Biol
Ther. 3:477–481. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Mcgough N and Cummings JH: Coeliac
disease: A diverse clinical syndrome caused by intolerance of
wheat, barley and rye. Proc Nut Soc. 64:434–450. 2005. View Article : Google Scholar
|
|
5
|
Garcea G, Dennison AR, Pattenden CJ, Neal
CP, Sutton CD and Berry DP: Survival following curative resection
for pancreatic ductal adenocarcinoma. A systematic review of the
literature. JOP. 9:99–132. 2008.PubMed/NCBI
|
|
6
|
Martin RC II, Kwon D, Chalikonda S,
Sellers M, Kotz E, Scoggins C, McMasters KM and Watkins K:
Treatment of 200 locally advanced (stage III) pancreatic
adenocarcinoma patients with irreversible electroporation: Safety
and efficacy. Ann Surg. 262:486–494. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yu J and Chen Q: Antitumor activities of
rauwolfia vomitoria extract and potentiation of gemcitabine effects
against pancreatic cancer. Integr Cancer Ther. 13:217–225. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mccarroll J, Teo J, Boyer C, Goldstein D,
Kavallaris M and Phillips PA: Potential applications of
nanotechnology for the diagnosis and treatment of pancreatic
cancer. Front Physiol. 5:22014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wolfgang CL, Herman JM, Laheru DA, Klein
AP, Erdek MA, Fishman EK and Hruban RH: Recent progress in
pancreatic cancer. CA Cancer J Clini. 63:318–348. 2013. View Article : Google Scholar
|
|
10
|
Hussain SP: Pancreatic cancer: Current
progress and future challenges. Int J Biol Sci. 12:270–272. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wasmeier C, Romao M, Plowright L, Bennett
DC, Raposo G and Seabra MC: Rab38 and Rab32 control post-Golgi
trafficking of melanogenic enzymes. J Cell Biol. 175:271–281. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chan AM and Weber T: A putative link
between exocytosis and tumor development. Cancer Cell. 2:427–428.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hutagalung AH and Novick PJ: Role of Rab
GTPases in membrane traffic and cell physiology. Physiol Rev.
91:119–149. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Grosshans BL, Ortiz D and Novick P: Rabs
and their effectors: Achieving specificity in membrane traffic.
Proc Nati Acad Sci USA. 103:11821. 2006. View Article : Google Scholar
|
|
15
|
Osanai K, Higuchi J, Oikawa R, Kobayashi
M, Tsuchihara K, Iguchi M, Huang J, Voelker DR and Toga H: Altered
lung surfactant system in a Rab38-deficient rat model of
Hermansky-Pudlak syndrome. Am J Physiol Lung Cell Mol Physiol.
298:243–251. 2010. View Article : Google Scholar
|
|
16
|
Osanai K, Takahashi K, Nakamura K,
Takahashi M, Ishigaki M, Sakuma T, Toga H, Suzuki T and Voelker DR:
Expression and characterization of Rab38, a new member of the Rab
small G protein family. Biol Chem. 386:143–153. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang H and Jiang C: Rab38 confers a poor
prognosis, associated with malignant progression and subtype
preference in glioma. Oncol Rep. 30:2350–2356. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Bultema JJ and Di PSM: Cell type-specific
Rab32 and Rab38 cooperate with the ubiquitous lysosome biogenesis
machinery to synthesize specialized lysosome-related organelles.
Small Gtpases. 4:16–21. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Lopes VS, Wasmeier C, Seabra MC and Futter
CE: Melanosome maturation defect in Rab38-deficient retinal pigment
epithelium results in instability of immature melanosomes during
transient melanogenesis. Mol Biol Cell. 18:3914–3927. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Jäger D, Stockert E, Jäger E, Güre AO,
Scanlan MJ, Knuth A, Old LJ and Chen YT: Serological cloning of a
melanocyte rab guanosine 5′-triphosphate-binding protein and a
chromosome condensation protein from a melanoma complementary DNA
library. Cancer Res. 60:3584–3591. 2000.PubMed/NCBI
|
|
21
|
Abdelmonsef AH, Dulapalli R, Dasari T,
Padmarao LS, Mukkera T and Vuruputuri U: Structure based drug
discovery of Rab38 protein-identification of antagonists as cancer
drug candidates. Comb Chem High Throughput Screen. 19:875–892.
2016.PubMed/NCBI
|
|
22
|
Na L, Tang B, Zhu ED, Li BS, Zhuang Y, Yu
S, Lu DS, Zou QM, Xiao B and Mao XH: Increased miR-222 in H.
pylori-associated gastric cancer correlated with tumor progression
by promoting cancer cell proliferation and targeting RECK. FEBS
Lett. 586:722–728. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Moffat J, Grueneberg DA, Yang X, Kim SY,
Kloepfer AM, Hinkle G, Piqani B, Eisenhaure TM, Luo B, Grenier JK,
et al: A lentiviral RNAi library for human and mouse genes applied
to an arrayed viral high-content screen. Cell. 124:1283–1298. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhu E, Wang X, Zheng B, Wang Q, Hao J,
Chen S, Zhao Q, Zhao L, Wu Z and Yin Z: miR-20b suppresses Th17
differentiation and the pathogenesis of experimental autoimmune
encephalomyelitis by targeting RORgammat and STAT3. J Immunol.
192:5599–5609. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and and Schmittgen TD: Analysis
of relative gene expression data using real-time quantitative PCR
and the 2−ΔΔCT method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Han S, Jin G, Wang L, Li M, He C, Guo X
and Zhu Q: The role of PAM4 in the management of pancreatic cancer:
Diagnosis, radioimmunodetection, and radioimmunotherapy. J Immunol
Res. 2014:2684792014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Buscarini E, Pezzilli R, Cannizzaro R, De
Angelis C, Gion M, Morana G, Zamboni G, Arcidiacono P, Balzano G,
Barresi L, et al: Italian consensus guidelines for the diagnostic
work-up and follow-up of cystic pancreatic neoplasms. Dig Liver
Dis. 46:479–493. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Herrmann P, Huber S, Hesler CV, Tischer A,
Luckner M, Bruns C and Heeschen C: Identification and
characterization of highly metastatic and therapy resistant tumor
stem cells in pancreatic cancer. Cancer Res. 67:1292. 2007.
|
|
29
|
Osanai K, Iguchi M, Takahashi K, Nambu Y,
Sakuma T, Toga H, Ohya N, Shimizu H, Fisher JH and Voelker DR:
Expression and localization of a novel Rab small G protein (Rab38)
in the rat lung. Am J Pathol. 158:1665–1675. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ninkovic I, White JG, Rangelfilho A and
Datta YH: The role of Rab38 in platelet dense granule defects. J
Thromb Haemost. 6:2143–2151. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Walton SM, Gerlinger M, de la Rosa O,
Nuber N, Knights A, Gati A, Laumer M, Strauss L, Exner C, Schäfer
N, et al: Spontaneous CD8 T cell responses against the melanocyte
differentiation antigen RAB38/NY-MEL-1 in melanoma patients. J
Immunol. 177:8212–8218. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Abdelmonsef AH, Dulapalli R, Dasari T,
Padmarao LS, Mukkera T and Vuruputuri U: Identification of novel
antagonists for Rab38 protein by homology modeling and virtual
screening. Comb Chem High Throughput Screen. 19:875–892. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
He AR and Goldenberg AS: Treating
hepatocellular carcinoma progression following first-line
sorafenib: Therapeutic options and clinical observations. Therap
Adv Gastroenterol. 6:447–458. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hicks J, Krasnitz A, Lakshmi B, Navin NE,
Riggs M, Leibu E, Esposito D, Alexander J, Troge J, Grubor V, et
al: Novel patterns of genome rearrangement and their association
with survival in breast cancer. Genome Res. 16:1465–1479. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Borowa-Mazgaj B: Pancreatic
cancer-mechanisms of chemoresistance. Postepy Hig Med Dosw
(Online). 70:169–179. 2016.(In Polish). View Article : Google Scholar : PubMed/NCBI
|