Introduction
Hepatitis C virus (HCV) infects approximately 185
million people worldwide and is a leading cause of liver cirrhosis
and hepatocellular carcinoma (1).
Yet, no effective HCV vaccine is available. Although the
introduction of new direct-acting antiviral agents (DAAs) is highly
effective in the treatment of all HCV genotype infections, their
use is limited by high cost, treatment accessibility and potential
drug resistance (2). Furthermore,
patients cured following DAA use are susceptible to reinfection
(3). Therefore, an effective
prophylactic vaccine is necessary to protect against HCV
infection.
HCV, a positive-stranded RNA virus, exists as seven
major genotypes and numerous subtypes. However, the high genetic
variability of the HCV genome presents many challenges for vaccine
development (4). Previous studies
have shown that the T-cell immune response plays a crucial role in
HCV clearance (5,6). It is now known that timely production
of cross-neutralizing antibodies (NAbs) are associated with viral
resolution (7).
HCV envelope glycoproteins E1 and E2 form a
heterodimer on the surface of HCV. Various types of E1E2-based
vaccine candidates have been tested. However, a phase I study with
recombinant HCV E1/E2 envelope glycoprotein as a candidate vaccine
did not induce a strong immune response in volunteers (8,9). E2,
the larger one of the two envelope proteins, interacts directly
with cellular receptors CD81 and scavenger receptor class B member
1 (SR-B1) to mediate viral entry (10). E2 is an optimal antigen candidate
for HCV vaccination because it possesses most NAb-recognized
epitopes (11–13). To date, a number of HCV vaccine
candidates based on E2 have been explored. A DNA vaccine encoding
HCV E2 has been shown to induce specific antibody responses in mice
(14). Prime-boost immunization
with the virus-like particles (VLPs) containing E2, E1, or both
elicited NAbs in non-human primates (15). A subunit vaccine based on soluble
E2 (sE2) of the Con1 strain (GT1b) induces NAbs in mice and rhesus
monkeys (16,17). A recent study also demonstrated
that a trivalent HCV vaccine containing sE2 from genotypes 1a, 1b
and 3a elicited a broad, synergistic polyclonal antibody response
in mice and rhesus monkeys (18).
Viral vector vaccines have been demonstrated to
induce strong cellular and humoral immune responses. A variety of
viral vectors, such as adenovirus, poxvirus and measles, have been
tested as platforms for HCV vaccination (19,20).
In recent years, the adeno-associated virus (AAV) vector, a
single-stranded DNA virus from the Parvoviridae family, has emerged
as an attractive agent for vaccine development owing to its
long-term persistence, high efficiency, low immunogenicity and lack
of pathogenicity in gene delivery studies. Moreover, AAVs are also
able to infect both non-dividing and dividing cells in the liver,
muscle and brain (21–23). A remarkable feature of AAV vector
vaccines is their capacity to induce strong and long-lasting
antibody responses. Several studies have documented that the
induction of humoral responses could last for many months (24–27).
Such prominent antibody response may be relevant to the high and
sustained expression of the immunogen by most AAV serotypes
(27–29). AAV8 has shown remarkable potential
as a gene delivery vector in vivo (30). AAVrh32.33, a novel vector isolated
from rhesus macaques, has relatively low seroprevalence in humans
compared to AAV2 and AAV8 (31,32).
Genetic vaccines based on AAV8 and AAVrh32.33 vectors encoding
truncated dengue virus envelope proteins have been shown to elicit
a long-lasting humoral responses in mice (33).
Previously, we constructed an HCV vaccine based on
AAVrh32.33 expressing NS3/4 protein, which exhibits immunogenic
properties superior to those of an NS3-protein-based vaccine in
C57BL/6 mice (34). In the present
study, we continued to focus on AAV vectors and generated AAV2/8
and AAV2/rh32.33 vectors expressing HCV E2 protein. After
purification and titration of the two recombinant vectors, we
evaluated their humoral immunity induced in C57BL/6 mice.
Materials and methods
Plasmid construction
Serum samples of HCV GT1b were collected from six
patients (four females and two males, aged 30–50 years) diagnosed
at the Affiliated Hospital of Jining Medical University (Shandong,
China) between April and August 2017 after obtaining written
informed consent from the HCV-infected patients. The study was
approved by the Ethics Committee of Jining Medical University.
Total RNA was obtained using a viral RNA Mini kit (Qiagen,
Duesseldorf, Germany) according to the manufacturer's protocol.
cDNA was synthesized using the PrimeScript II First-Strand cDNA
Synthesis kit (Takara Bio Inc., Tokyo, Japan). Next, the HCV E2
gene was amplified by PCR with Pyrobest DNA polymerase (Takara Bio
Inc.) and specific primer. Two primer sequences were used: forward,
5′-GGAAGATCTCGCCGCCACCATGGTGGGGAACTGGGC-3′ and reverse,
5′-GTCTAGCGGCCATTAAACTCACGCCTCCGCTTGGGAT-3′. NotI and
BglII sites were used to clone the amplicons into the AAV
cis-plasmid (a kind gift from Dr Wilson, Gene Therapy
Program, Department of Pathology and Laboratory Medicine,
University of Pennsylvania, Philadelphia, PA, USA). The
identification of AAV recombinants was confirmed by restriction
enzyme digestion and sequencing. The recombinant plasmid was named
pAAV.CMV.HCV.E2.
Detection of HCV E2 expression in 293
cells
293 cells were plated at a density of
1×106 cells in a 6-well plate with Dulbecco's modified
Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) containing 10% fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc.) and then incubated at 37°C in a 5%
CO2 incubator. After 24 h, they were transfected with 4
mg AAV plasmids encoding eGFP (pAAV.CMV.eGFP) or HCV E2
(pAAV.CMV.HCV E2) by Lipofectamine 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) following the manufacturer's instructions.
Seventy-two hours after the transfection, eGFP gene expression was
confirmed using a direct fluorescence microscopy (Micro Publisher
3.3 RTV; Olympus Corp., Tokyo, Japan) and HCV E2 gene expression
was detected by western blot analysis. The transfected cells were
harvested and lysed as samples. After separation using 10% sodium
dodecyl-sulfate polyacrylamide gel electrophoresis gels (SDS-PAGE),
samples were transferred onto a polyvinylidene difluoride (PVDF)
membrane. The membrane was blocked for 2 h with 5% non-fat milk,
incubated overnight at 4°C with an anti-HCV E2 monoclonal antibody
(cat. no. 1876-E2; 1:100; Virostat; Bio-Lab Laboratories Ltd.,
Beijing, China), washed three times in Tris-buffered saline with
Tween-20 (TBST), and then incubated with the horseradish peroxidase
(HRP)-conjugated goat anti-mouse secondary antibody (cat. no.
ab6789; 1:10,000; Abcam, Cambridge, MA, USA) for 1 h at room
temperature. Finally, protein expression was visualized using
Pierce ECL Western Blotting substrate (Pierce Biotechnology, Inc.;
Thermo Fisher Scientific, Inc.), quantified using densitometry, and
analyzed using Gel-Pro software version 3.2 (Media Cybernetics,
Inc., Rockville, MD, USA). Glyceraldehyde 3-phosphate ehydrogenase
(GAPDH) was used an internal control in western blot analyses.
Production of rAAV vectors
All rAAV vectors were packaged by triple plasmid
transfection in 293 cells, as previously described (35,36).
The triple plasmid system is comprised of an AAV cis-plasmid
containing HCV E2 cDNA (pAAV.CMV.HCV E2), an adenovirus helper
plasmid (pAd.F6), and a chimeric packaging plasmid that contains
the AAV2 rep gene and the AAV8 (or AAVrh32.33) cap
gene (pAAV2/8 or pAAV2/rh32.33). All plasmids were extracted using
a Plasmid Maxi kit (Qiagen) following the manufacturer's
instructions. Briefly, 2 h before transfection, at the point when
the 293 cells were cultured in 15-cm culture dishes reaching high
confluence (70–80%), they were treated with 20 ml DMEM supplemented
with 10% FBS without antibiotics. Equimolar plasmids were dissolved
in 650 µl of CaCl2 (2.5 M) and 5.9 ml of Milli-Q water,
then mixed rapidly with 12.5 ml of 2X
4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid-buffered saline
(pH 7.05) to prepare the transfection solution. A 2.5 ml aliquot of
the above transfection solution was gently added to each dish.
After slowly swirling the contents to mix, the cells were incubated
at 37°C in a 5% CO2 incubator continuously. At 16 h
post-transfection, the medium was replaced with fresh DMEM
containing 10% FBS and 100 mg/ml streptomycin and penicillin.
Another 72 h after medium replacement [at which time eGFP signals
were visualized as distinctly shaped foci on fluorescence
microscopy (Thermo Fisher Scientific, Inc.)], cells were collected
and resuspended with 10 ml of NaCl (150 mM) and Tris (20 mM, pH
8.0). Next, benzonase nuclease (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) was added to a final concentration of 50 U/ml
to remove nucleic acid contamination. rAAV was obtained from lysed
293 cells by means of three consecutive freeze-thaw-cycles (−80°C
and 37°C). The rAAV vectors were purified by three rounds of cesium
chloride gradient centrifugation, and then concentrated using
Amicon Ultra-15 centrifugal filter devices (100K; Merck Millipore,
Billerica, MA, USA). The AAV genome titers [genome copies (GC) per
ml] were detected by real-time PCR using Premix Ex Taq (Takara Bio
Inc.) corresponding to the polyA region of the AAV vector.
Immunization of mice
All animal procedures were performed in accordance
with the protocols approved by the Ethics Committee of Jining
Medical University. Female C57BL/6 mice (4–6 weeks of age, weight
15.00±0.31 g) were purchased from SLAC Laboratory Animal Company
(Shanghai, China) and housed at a constant temperature with a 12 h
dark/light cycle. Food and water were available ad libitum.
The mice were randomly divided into 4 groups of 10 mice each. After
being anesthetized by inhalation of 1.5% isoflurane in oxygen, two
groups of mice were immunized intramuscularly (tibialis anterior
muscle of the hind leg) with rAAV2/8.HCV E2 vaccine or
rAAV2/rh32.33.HCV E2 vaccine (1×1011 GC per mouse). The
other two groups were immunized with pAAV.CMV.HCV.E2 plasmid (100
µg per mouse) or phosphate-buffered saline (PBS) (100 ml per mouse)
as controls. At weeks 12 and 16 post-immunization, two mice in each
group were sacrificed by inhalant isoflurane overdose (1.5%), for
skeletal muscle harvesting from the vaccine injection area. The
tissue samples were stored at −80°C for subsequent western blot
analysis. Blood samples of mice were collected via retro-orbital
puncture under anesthesia as above at weeks 0, 4, 8, 12 and 16
post-immunization and stored at −20°C.
Evaluation of HCV E2-specific antibody
reactivity
To measure HCV E2-specific antibody reactivity,
mouse sera were evaluated by enzyme-linked immunosorbent assay
(ELISA). Briefly, 96-well plates were coated with GST-E2 peptide
and incubated overnight at 4°C. Plates were then washed with
phosphate-buffered solution (PBST) and blocked with 1% bovine serum
albumin (BSA) for 2 h at room temperature. A 1:1,000 dilution
anti-mouse IgG HRP-conjugated antibody (cat. no. A5278;
Sigma-Aldrich; Merck KGaA) was used as a secondary antibody. Mouse
serum samples were diluted to 1:2 and 1:20 for ELISA analysis.
After washing with PBST, 3,3′,5,5′-tetramethylbenzidine
(Invitrogen; Thermo Fisher Scientific, Shanghai, China) was added
to each well. Finally, the plates were read at a wavelength of 450
nm (OD450 nm; ELx800; BioTek Instruments, Inc.,
Winooski, VT, USA). IgG titers are presented as the reciprocal of
the highest serum dilution at which the OD450 nm was
higher than twice that of the control.
Evaluation of neutralizing antibody
response to hepatitis C virus
To evaluate the neutralizing ability of HCV-specific
antibodies, infectious pseudo-particles expressing HCV envelope
glycoproteins (HCVpp) with E2 protein of five genotypes [1a (H77),
1b (Hebei), 2a (JFH1), 3a (S52) and 5a (SA13)] were produced to
evaluate NAb induction (37,38).
After purification in a protein G column, serum samples were
serially diluted and incubated with HCVpp for 1 h at 37°C, at which
time the mixtures were used to infect Huh7.5 cells. After 48 h,
luciferase activity in the infected Huh7.5 cells was detected with
a Bright-Glo luciferase assay (Promega, Madison, WI, USA). Data
were reported as relative luminescence units. The positive control,
AP33, is a monoclonal anti-E2 antibody with broad
cross-neutralization ability. Neutralizing capacity was evaluated
by comparing the luciferase activity in cells infected with HCVpp
that had been incubated with serum from vaccinated animals to those
that had been incubated with pre-vaccinated serum from the same
mice.
Evaluation of neutralizing antibody
response against the AAV vector
Serum samples collected from 20 naive mice and 20
humans were diluted to determine anti-AAV2/rh32.33 and -AAV2/8
vector NAb levels. Briefly, serum samples were inactivated at 56°C
for 35 min. The AAV2/8.CMV.eGFP and AAV2/rh32.33.CMV.eGFP vectors
were diluted in serum-free DMEM and incubated with serial dilutions
(1:20 and 1:80) of serum samples at 37°C for 1 h. Next, 100 µl of
the serum-vector mixture was added to the 293 cells that had been
infected 2 h earlier with wild-type adenovirus 5. After incubation
at 37°C under 5% CO2 for 24 h, eGFP protein expression
was evaluated for each group with fluorescence microscopy (Thermo
Fisher Scientific, Inc.). NAb titer was determined as the highest
serum dilution that inhibited AAV transduction (eGFP expression) by
50%, relative to the mouse serum control.
Statistical analysis
Mean values are reported with standard deviations
(SDs). GraphPad Prism 5 (GraphPad Software, Inc., La Jolla, CA,
USA) and SPSS software (version 17.0; SPSS, Inc., Chicago, IL, USA)
were used for statistical analyses. Differences between groups were
examined by Student's t-test for two groups and one-way analysis of
variance (ANOVA) followed by a least significant difference test
for multiple comparisons. A P-value of <0.05 was considered
statistically significant.
Results
Expression of the AAV plasmid encoding
the HCV E2 gene in vitro
In vitro expression of HCV E2 protein was
evaluated by western blot analysis in recombinant AAV
plasmid-transfected 293 cell lysates 72 h after transfection. 293
cells transfected with pAAV.CMV.HCV E2 plasmid had a band of ~70
kDa, whereas those transfected with pAAV.CMV.eGFP had no detectable
band (Fig. 1).
Comparison of HCV E2 expression among
the different vaccines
The rAAV final yield ranged from
5.53×1011 GC to 1.02×1012 GC. HCV E2
expression was compared between HepG2 cells infected with
rAAV2/8.HCV E2 vaccine or rAAV2/rh32.33.HCV E2 vaccine,
respectively (MOI=3×104 GC). HepG2 cells transfected
with pAAV.CMV.HCV.E2 plasmid or pAAV.CMV.eGFP plasmid served as
positive and negative controls, respectively. After 72 h, the
70-kDa antigen was effectively expressed in cell lysates (data not
shown). Western blot analysis of E2 expression in injection-area
skeletal muscle (2 mice/group) showed that AAV plasmid (control
group) and the two rAAV vaccines (AAV2/rh32.33 and AAV2/8)
expressed HCV E2 protein in immunized mice for at least 16 weeks
post-immunization (Fig. 2). In
addition, the HCV E2 expression level did not differ between the
rAAV and the AAV plasmid.
HCV E2-specific antibody induced by
AAV vaccine
To assess AAV immunogenicity, HCV E2-specific IgG
antibody in sera from mice vaccinated with AAV was measured by
ELISA. Four groups of mice were immunized with rAAV2/rh32.33.HCV E2
vaccine, rAAV2/8.HCV E2 vaccine, pAAV.CMV.HCV E2 plasmid, or PBS
(control). The result showed that antigen expressed by the AAV
vaccine elicited HCV E2-specific antibody production that persisted
for more than 16 weeks post-vaccination (time course shown in
Fig. 3A). All animals immunized
with rAAV vaccines or AAV plasmid produced anti-HCV E2 antibody.
Furthermore, the antibody level in the rAAV2/rh32.33 vaccinated
group was significantly higher than that of the PBS control group
at week 12 (P=0.012) and 16 post-immunization (P=0.006). For each
group, the finding that antibody levels were higher at week 16
compared to week 4 post-immunization (Fig. 3B-D) indicates that the immunologic
effect of each vaccine is time-dependent over a specified time
frame. When the serum was diluted out, it was also found that the
HCV E2-specific antibody level induced by rAAV2/rh32.33 vaccination
was higher than that induced by rAAV2/8 vaccination or AAV plasmid
administration, while the HCV E2-specific antibody response induced
by rAAV2/8 vaccine was similar to that induced by the AAV plasmid
(Fig. 3B-D).
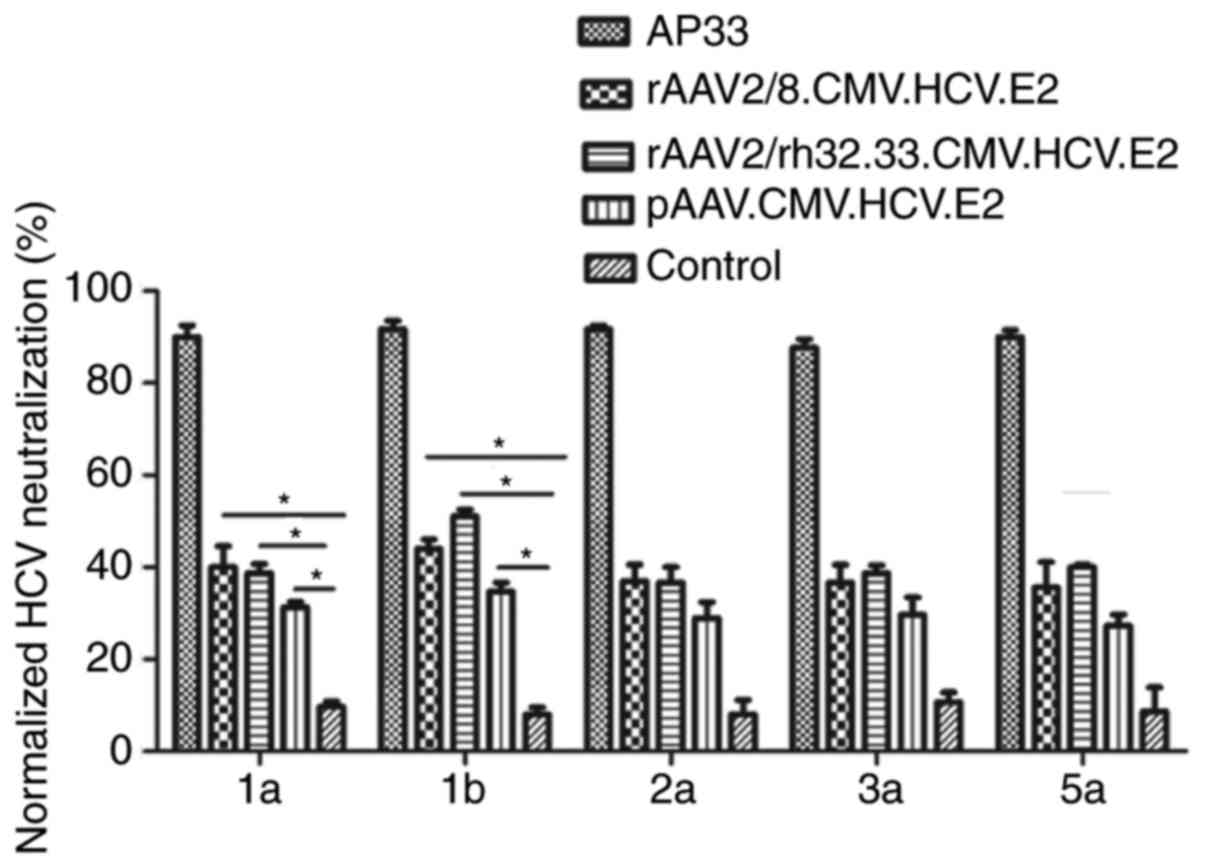
Neutralizing antibody against HCV
induced by AAV vaccine
To assess the cross-neutralization ability of
HCV-specific antibodies, a luciferase reporter HCVpp with E2
protein of five genotypes (1a, 1b, 2a, 3a and 5a) was generated.
Serum samples were collected at weeks 12 and 1:50 diluted antisera
were tested for neutralization against HCVpp of five genotypes. As
shown in Fig. 4, both AAV vaccines
demonstrated significant neutralization of HCVpp derived from HCV
genotypes 1a and 1b at week 12.
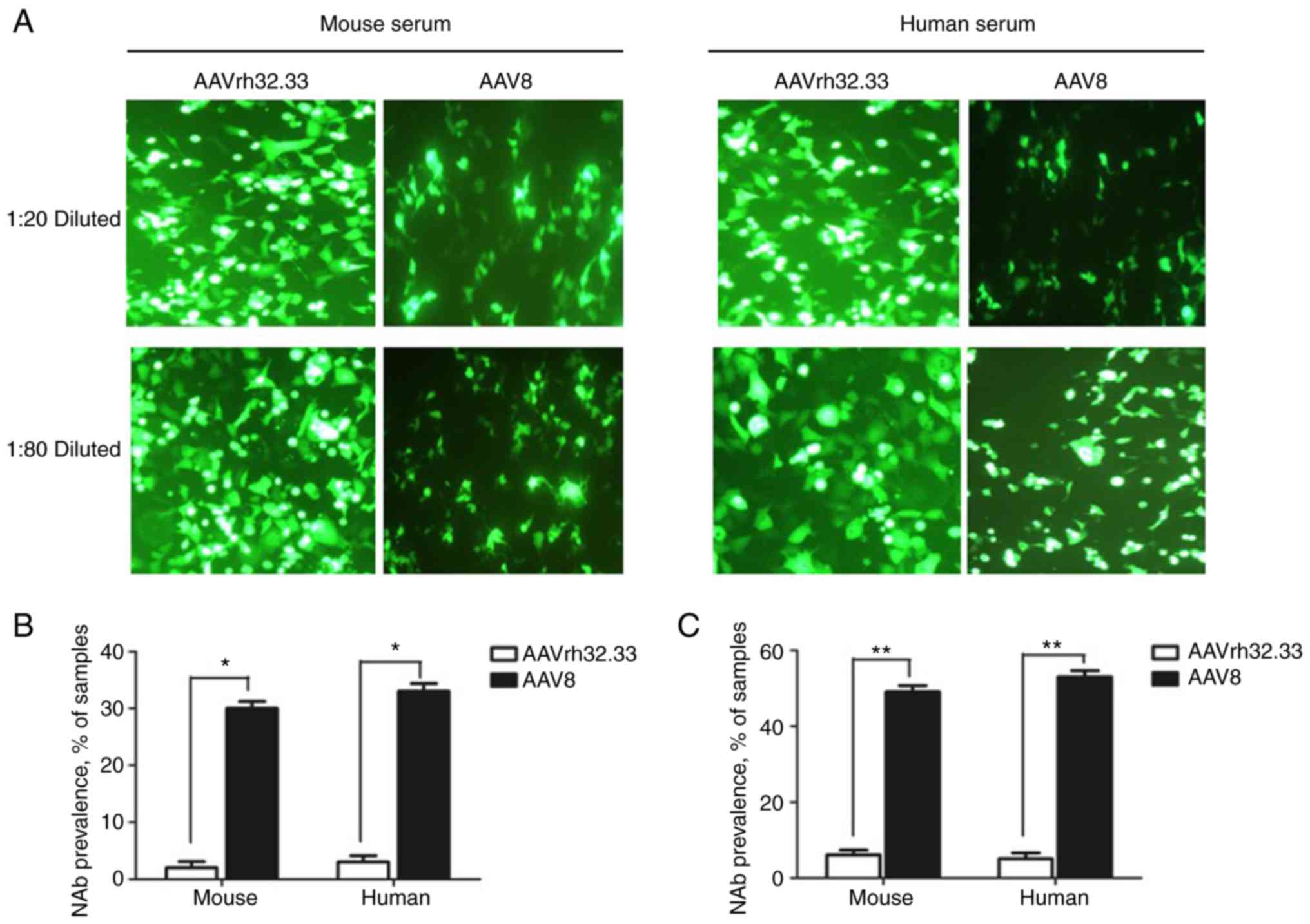
Neutralizing antibody against AAV in
human and mouse sera
To evaluate the NAbs to AAV vectors, serum samples
from human and naive mice were collected and diluted to detect the
levels of NAbs directed against AAV2/rh32.33 and AAV2/8 vectors.
The NAb titer was determined as the highest serum dilution that
inhibited AAV transduction (eGFP expression) by 50% compared to
mouse control serum. As shown in Fig.
5, the eGFP protein expression levels in the AAV2/8 group were
inhibited >50%, which is more than that observed for the
AAV2/rh32.33 group both at serum dilutions of 1:20 and 1:80. Thus,
NAb titers to AAV2/rh32.3 were lower than NAb titers to AAV2/8.
Discussion
There are many obstacles to develop a prophylactic
hepatitis C virus (HCV) vaccine, such as the extensive variability
of the HCV genome and the absence of a suitable small animal model.
However, several candidate HCV vaccines have been created,
including DNA vaccines, recombinant viral vectors, proteins and
virus-like particles (VLPs) (39,40).
Vector comparison is valuable to illustrate factors influencing
vaccine immunogenicity and to improve genetic vaccination.
HCV vaccines based on recombinant viral vectors have
generated promising results in preclinical experiments. To date,
four vector vaccines have been able to induce T-cell and B-cell
responses in rhesus macaques in prime-boost regimens: DNA, SFV,
human serotype 5 adenovirus (HuAd5) and modified vaccinia ankara
(MVA) poxvirus expressing HCV core, E1, E2 and NS3 (41). Simian adenoviral vaccine vectors
(ChAdOx) encoding genetically conserved gene segments from HCV
primed broad, cross-reactive T-cell responses successfully in
C57BL/6 mice (42). Relative to
these commonly used viral vectors, rAAV vectors have potential
advantages for vaccine and gene transfer applications owing to
their lower inflammatory potential, availability of viral serotypes
with different tissue tropisms, and possible long-lasting gene
expression. Additionally, AAV-based genetic vaccines encoding the
antigen genes of interest can elicit both humoral and cellular
immune responses to the transgene. A genetic HIV vaccine based on
AAV vectors induced anti-HIV T-cell responses in mice (43). The use of AAV vectors for delivery
of broadly neutralizing HIV antibodies could stimulate long-term,
systemic broadly neutralizing antibody in the absence of
immunization to prevent HIV infection (28). However, AAV vaccines have been
limited by factors such as high sero-prevalence in humans. To date,
more than 120 AAV serotypes and variants have been identified;
several serotypes, such as AAV8 and AAVrh32.33, show high
transduction efficiency but low sero-prevalence (44). Genetic vaccines based on AAV8 and
AAVrh32.33 vectors encoding truncated dengue virus envelope
proteins have been shown to elicit long-lasting humoral responses
in mice (33). Hence, we chose
AAV8 and AAVrh32.33 vectors to create HCV vaccines and compared
their immunogenicity in C57BL/6 mice by intramuscular
injection.
The present study is the first study to show that
AAV vectors can express HCV E2 antigen and induce humoral immune
responses in mice. In this study, both AAV vaccines produced high
absolute levels of HCV E2 antigen in mice. Antigen synthesis lasted
for several weeks, and the synthesized antigen provided continuous
stimulation to the mouse immune system. However, the HCV E2
expression level did not differ between the rAAV and the DNA. There
are a few possible explanations for this finding. Firstly, our AAV
vaccine was of low purity and quality. Secondly, the small sample
size of immunized mice may have underpowered the study to detect a
true difference. We will conduct further experiments to clarify
whether a difference does exist. Furthermore, the HCV E2-specific
antibody assay showed that the AAV2/rh32.33-based HCV vaccine
induced higher levels of E2-specific antibodies than did the AAV2/8
vaccine. Thus, the AAV2/rh32.33-based HCV vaccine appears to
possess superior immunogenic properties compared to the
AAV2/8-based vaccine. Moreover, there were fewer NAbs to AAVrh32.33
than to AAV8 in mice and human sera, indicating that AAVrh32.33 may
be safer than AAV8.
Previously, we developed HCV vaccines based on an
AAVrh32.33 vector expressing HCV NS3 or NS3/4b that could elicit
strong and persistent T-cell immunity in mice (34). Moreover, there have been studies
indicating that an AAVrh32.33 vector encoding antigens of HIV-1
would stimulate stronger immune responses than an AAV8 vector in
non-human primates (31). Above
all, we speculate that, beyond safety, AAVrh32.33 could stimulate
potent transgene production more efficiently. Thus, AAV vectors,
especially AAVrh32.33, are attractive potential vectors for HCV
vaccine design.
Since humoral immunity and NAbs play a pivotal role
in HCV clearance, the identification of conserved epitopes
associated with viral neutralization is a crucial factor in the
development of an effective prophylactic HCV vaccine. The envelop
glycoprotein E2 is the primary target of the anti-HCV NAb response.
In past decades, several studies have helped to elucidate the
structural features of HCV glycoprotein E2, showing that key
epitopes targeted by NAbs on the ‘front layer’ of E2 exhibit
structural heterogeneity. Thus, E2 appears to be an ideal antigen
candidate for HCV vaccine design (45,46).
In this study, we developed HCV vaccines based on AAV vectors
expressing E2 protein from GT1b, which is the predominant genotype
in the Chinese population, accounting for 56.8% (47). Firstly, we investigated and
compared HCV E2 protein expression levels for three different
designs. Western blot analysis showed that all constructs produced
the 70-kDa E2 protein. We then determined that AAV vaccines
encoding HCV E2 could induce high-level production of HCV
E2-specific antibodies and NAbs in C57BL/6 mice. Additionally,
cross protection against genotypes 1a and 1b were superior to that
directed against genotypes 2a, 3a and 5a HCVpp. Our finding is
consistent with that of previous research showing that genotype 1
antigen induced weaker neutralization against HCV genotypes 2, 3
and 5 than genotype 1 (48).
However, efficient T-cell responses were not detected in immunized
mice in this study (data not shown). One possible explanation is
that T-cell-specific epitopes are not properly exposed in whole HCV
E2 protein. Therefore, further exploration of truncated E2 proteins
possessing T-cell-specific epitopes is needed.
In conclusion, we constructed novel AAV-based
vaccines encoding E2 protein directed against HCV and confirmed
their ability to elicit E2-specific antibody and NAb responses in
C57BL/6 mice. We plan to further evaluate the immunity generated by
AAV vaccines in immune-competent humanized mice. Moreover, we will
explore other optimal antigens to induce both humoral and cell
responses against HCV, with the goal of developing an efficient
prophylactic HCV vaccine.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Natural
Science Foundation of Shandong Province, China (grant no.
ZR2017LH009) and the PhD Research Startup Foundation of the
Affiliated Hospital of Jining Medical University (grant no.
2016-BS-015).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
HH and FZ designed the study. FZ, YW, ZX, HQ, HZ,
LN, HX and DJ performed the experiments. YW, ZX, HQ, HZ, LN, HX and
DJ analyzed the data. FZ prepared the manuscript. HH revised the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All patients provided written informed consent to
participate. All experimental procedures performed with human and
mice were approved by the Ethics Committee of Jining Medical
University (Shandong, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li D, Huang Z and Zhong J: Hepatitis C
virus vaccine development: Old challenges and new opportunities.
Natl Sci Rev. 2:285–295. 2015. View Article : Google Scholar
|
|
2
|
Pawlotsky JM: Hepatitis C virus resistance
to direct-acting antiviral drugs in interferonfree regimens.
Gastroenterology. 151:70–86. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gardiner DF, Rodriguez-Torres M, Reddy KR,
Hassanein T, Jacobson I, Lawitz E, Lok AS, Hinestrosa F, Thuluvath
PJ, Schwartz H, et al: Daclatasvir plus sofosbuvir for previously
treated or untreated chronic HCV infection. N Engl J Med.
370:211–221. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Humphreys I, Flaxman A, Brown A, Cooke GS,
Pybus OG and Barnes E: Global distribution and prevalence of
hepatitis C virus genotypes. Hepatology. 61:77–87. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bassett SE, Guerra B, Brasky K, Miskovsky
E, Houghton M, Klimpel GR and Lanford RE: Protective immune
response to hepatitis C virus in chimpanzees rechallenged following
clearance of primary infection. Hepatology. 33:1479–1487. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rollier C, Depla E, Drexhage JA, Verschoor
EJ, Verstrepen BE, Fatmi A, Brinster C, Fournillier A, Whelan JA,
Whelan M, et al: Control of heterologous hepatitis C virus
infection in chimpanzees is associated with the quality of
vaccine-induced peripheral T-helper immune response. J Virol.
78:187–196. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Osburn WO, Snider AE, Wells BL, Latanich
R, Bailey JR, Thomas DL, Cox AL and Ray SC: Clearance of hepatitis
C infection is associated with the early appearance of broad
neutralizing antibody responses. Hepatology. 59:2140–2151. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Frey SE, Houghton M, Coates S, Abrignani
S, Chien D, Rosa D, Pileri P, Ray R, Di Bisceglie AM, Rinella P, et
al: Safety and immunogenicity of HCV E1E2 vaccine adjuvanted with
MF59 administered to healthy adults. Vaccine. 28:6367–6373. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ray R, Meyer K, Banerjee A, Basu A, Coates
S, Abrignani S, Houghton M, Frey SE and Belshe RB: Characterization
of antibodies induced by vaccination with hepatitis C virus
envelope glycoproteins. J Infect Dis. 202:862–866. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kong L, Giang E, Nieusma T, Kadam RU,
Cogburn KE, Hua Y, Dai X, Stanfield RL, Burton DR, Ward AB, et al:
Hepatitis C virus E2 envelope glycoprotein core structure. Science.
342:1090–1094. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Deng L, Zhong L, Struble E, Duan H, Ma L,
Harman C, Yan H, Virata-Theimer ML, Zhao Z, Feinstone S, et al:
Structural evidence for a bifurcated mode of action in the
antibody-mediated neutralization of hepatitis C virus. Proc Natl
Acad Sci USA. 110:7418–7422. 2010. View Article : Google Scholar
|
|
12
|
Kong L, Lee DE, Kadam RU, Liu T, Giang E,
Nieusma T, Garces F, Tzarum N, Woods VL Jr, Ward AB, et al:
Structural flexibility at a major conserved antibody target on
hepatitis C virus E2 antigen. Proc Natl Acad Sci USA.
113:12768–12773. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Das S, Mullick R, Kumar A, Tandon H, Bose
M, Gouthamchandra K, Chandra M, Ravishankar B, Khaja MN, Srinivasan
N, et al: Identification of a novel epitope in the C terminus of
hepatitis C virus-E2 protein that induces potent and cross-reactive
neutralizing antibodies. J Gen Virol. 98:962–976. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tedeschi V, Akatsuka T, Shih JW, Battegay
M and Feinstone SM: A specific antibody response to HCV E2 elicited
in mice by intramuscular inoculation of plasmid DNA containing
coding sequences for E2. Hepatology. 25:459–462. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Garrone P, Fluckiger AC, Mangeot PE,
Gauthier E, Dupeyrot-Lacas P, Mancip J, Cangialosi A, Du Chéné I,
LeGrand R, Mangeot I, et al: A prime-boost strategy using
virus-like particles pseudotyped for HCV proteins triggers broadly
neutralizing antibodies in macaques. Sci Transl Med. 3:94ra712011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li D, von Schaewen M, Wang X, Tao W, Zhang
Y, Li L, Heller B, Hrebikova G, Deng Q, Ploss A, et al: Altered
glycosylation patterns increase immunogenicity of a subunit
hepatitis C virus vaccine, inducing neutralizing antibodies which
confer protection in mice. J Virol. 90:10486–10498. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li D, Wang X, von Schaewen M, Tao W, Zhang
Y, Heller B, Hrebikova G, Deng Q, Sun Q, Ploss A, et al:
Immunization with a subunit hepatitis C virus vaccine elicits
pan-genotypic neutralizing antibodies and intrahepatic T-cell
responses in nonhuman primates. J Infect Dis. 215:1824–1831. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang X, Yan Y, Gan T, Yang X, Li D, Zhou
D, Sun Q, Huang Z and Zhong J: A trivalent HCV vaccine elicits
broad and synergistic polyclonal antibody response in mice and
rhesus monkey. Gut. Nov 27–2017.(Epub ahead of print). doi:
10.1136/gutjnl-2017-314870.
|
|
19
|
Draper SJ and Heeney JL: Viruses as
vaccine vectors for infectious diseases and cancer. Nat Rev
Microbiol. 8:62–73. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guo J, Mondal M and Zhou D: Development of
novel vaccine vectors: Chimpanzee adenoviral vectors. Hum Vaccin
Immunother. 14:1679–1685. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mak KY, Rajapaksha IG, Angus PW and Herath
CB: The adeno-associated virus-A safe and promising vehicle for
liverspecific gene therapy of inherited and non-inherited
disorders. Curr Gene Ther. 17:4–16. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen Q, Zhai H, Li X, Ma Y, Chen B, Liu F,
Lai H, Xie J, He C, Luo J, et al: Recombinant adeno-associated
virus serotype 9 in a mouse model of atherosclerosis: Determination
of the optimal expression time in vivo. Mol Med Rep. 15:2090–2096.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cabral-Miranda F, Nicoloso-Simões E,
Adão-Novaes J, Chiodo V, Hauswirth WW, Linden R, Chiarini LB and
Petrs-Silva H: rAAV8-733-mediated gene transfer of CHIP/Stub-1
prevents hippocampal neuronal death in experimental brain ischemia.
Mol Ther. 25:392–400. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Manning WC, Paliard X, Zhou S, Pat Bland
M, Lee AY, Hong K, Walker CM, Escobedo JA and Dwarki V: Genetic
immunization with adeno-associated virus vectors expressing herpes
simplex virus type 2 glycoproteins B and D. J Virol. 71:7960–7962.
1997.PubMed/NCBI
|
|
25
|
Kuck D, Lau T, Leuchs B, Kern A, Müller M,
Gissmann L and Kleinschmidt JA: Intranasal vaccination with
recombinant adeno-associated virus type 5 against human
papillomavirus type 16 L1. J Virol. 80:2621–2630. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nieto K, Kern A, Leuchs B, Gissmann L,
Müller M and Kleinschmidt JA: Combined prophylactic and therapeutic
intranasal vaccination against human papillomavirus type-16 using
different adeno-associated virus serotype vectors. Antivir Ther.
14:1125–1137. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ploquin A, Szécsi J, Mathieu C, Guillaume
V, Barateau V, Ong KC, Wong KT, Cosset FL, Horvat B and Salvetti A:
Protection against henipavirus infection by use of recombinant
adeno-associated virus-vector vaccines. J Infect Dis. 207:469–478.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lin J, Zhi Y, Mays L and Wilson JM:
Vaccines based on novel adeno-associated virus vectors elicit
aberrant CD8+ T-cell responses in mice. J Virol.
8:11840–11849. 2007. View Article : Google Scholar
|
|
29
|
Lin SW, Hensley SE, Tatsis N, Lasaro MO
and Ertl HC: Recombinant adeno-associated virus vectors induce
functionally impaired transgene product-specific CD8+ T
cells in mice. J Clin Invest. 117:3958–3970. 2007.PubMed/NCBI
|
|
30
|
Qiu Y, Tao L, Zheng S, Lin R, Fu X, Chen
Z, Lei C, Wang J, Li H, Li Q and Lei B: AAV8-mediated
angiotensin-converting enzyme 2 gene delivery prevents experimental
autoimmune uveitis by regulating MAPK, NF-κB and STAT3 pathways.
Sci Rep. 6:319122016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lin J, Calcedo R, Vandenberghe LH, Bell P,
Somanathan and Wilson JM: A new genetic vaccine platform based on
an adeno-associated virus isolated from a rhesus macaque. J Virol.
83:12738–12750. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mays LE, Vandenberghe LH, Xiao R, Bell P,
Nam HJ, Agbandje-McKenna M and Wilson JM: Adeno-associated virus
capsid structure drives CD4-dependent CD8+ T cell response to
vector encoded proteins. J Immunol. 182:6051–6060. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li X, Cao H, Wang Q, Di B, Wang M, Lu J,
Pan L, Yang L, Mei M, Pan X, et al: Novel AAV-based genetic
vaccines encoding truncated dengue virus envelope proteins elicit
humoral immune responses in mice. Microbes Infect. 14:1000–1007.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhu F, Chen T, Zhang Y, Sun H, Cao H, Lu
J, Zhao L and Li G: A novel adeno-associated virus-based genetic
vaccine encoding the hepatitis C virus NS3/4 protein exhibits
immunogenic properties in mice superior to those of an
NS3-protein-based vaccine. PLoS One. 10:e01423492015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Xiao X, Li J and Samulski RJ: Production
of high-titer recombinant adeno-associated virus vectors in the
absence of helper adenovirus. J Virol. 72:2224–2232.
1998.PubMed/NCBI
|
|
36
|
Wang L, Calcedo R, Nichols TC, Bellinger
DA, Dillow A, Verma IM and Wilson JM: Sustained correction of
disease in naive and AAV2-pretreated hemophilia B dogs:
AAV2/8-mediated, liver-directed gene therapy. Blood. 105:3079–3786.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Dreux M and Cosset FL: Detection of
neutralizing antibodies with HCV pseudoparticles (HCVpp). Methods
Mol Biol. 510:427–438. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Guan J, Wen B, Deng Y, Zhang K, Chen H, Wu
X, Ruan L and Tan W: Effect of route of delivery on heterologous
protection against HCV induced by an adenovirus vector carrying HCV
structural genes. Virol J. 8:5062011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Roohvand F and Kossari N: Advances in
hepatitis C virus vaccines, part two: Advances in hepatitis C virus
vaccine formulations and modalities. Expert Opin Ther Pat.
22:391–415. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Liang TJ: Current progress in development
of hepatitis C virus vaccines. Nat Med. 19:869–878. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rollier CS, Verschoor EJ, Verstrepen BE,
Drexhage JA, Paranhos-Baccala G, Liljeström P, Sutter G,
Arribillaga L, Lasarte JJ, Bartosch B, et al: T- and B-cell
responses to multivalent prime-boost DNA and viral vectored vaccine
combinations against hepatitis C virus in non-human primates. Gene
Ther. 23:753–759. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
von Delft A, Donnison TA, Lourenço J,
Hutchings C, Mullarkey CE, Brown A, Pybus OG, Klenerman P,
Chinnakannan S and Barnes E: The generation of a simian adenoviral
vectored HCV vaccine encoding genetically conserved gene segments
to target multiple HCV genotypes. Vaccine. 36:313–321. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Brady JM, Baltimore D and Balazs AB:
Antibody gene transfer with adeno-associated viral vectors as a
method for HIV prevention. Immunol Rev. 275:324–333. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Gao GP, Alvira MR, Wang L, Calcedo R,
Johnston J and Wilson JM: Novel adeno-associated viruses from
rhesus monkeys as vectors for human gene therapy. Proc Natl Acad
Sci USA. 99:11854–11859. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Meola A, Tarr AW, England P, Meredith LW,
McClure CP, Foung SK, McKeating JA, Ball JK, Rey FA and Krey T:
Structural flexibility of a conserved antigenic region in hepatitis
C virus glycoprotein E2 recognized by broadly neutralizing
antibodies. J Virol. 89:2170–2181. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Vasiliauskaite I, Owsianka A, England P,
Khan AG, Cole S, Bankwitz D, Foung SKH, Pietschmann T,
Marcotrigiano J, Rey FA, et al: Conformational flexibility in the
immunoglobulin-like domain of the hepatitis C virus glycoprotein
E2. MBio. 8:e00382–17. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Yuan G, Liu J, Hu C, Huang H, Qi M, Wu T,
Liang W, Li YP, Zhang YY and Zhou Y: Genotype distribution and
molecular epidemiology of hepatitis C virus in Guangzhou, China:
Predominance of genotype 1b and increasing incidence of genotype
6a. Cell Physiol Biochem. 43:775–787. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Law JL, Chen C, Wong J, Hockman D, Santer
DM, Frey SE, Belshe RB, Wakita T, Bukh J, Jones CT, et al: A
hepatitis C virus (HCV) vaccine comprising envelope glycoproteins
gpE1/gpE2 derived from a single isolate elicits broad
cross-genotype neutralizing antibodies in humans. PLoS One.
8:e597762013. View Article : Google Scholar : PubMed/NCBI
|