Introduction
High-density lipoprotein (HDL) is known to exert
anti-atherosclerotic effects, which are mediated by the HDL reverse
cholesterol transportation system in vivo (1). Furthermore, it has been demonstrated
that the anti-atherosclerotic effects of HDL may be associated with
its anti-inflammatory, antioxidant and endothelial protective
properties (2). Apolipoprotein M
(ApoM) is a major HDL apolipoprotein (3), which has been suggested to serve an
important role in the anti-atherosclerotic effects of HDL (4), possibly via the
ApoM/sphingosine-1-phosphate (S1P) axis (5). In addition, it has been reported that
the ApoM/S1P axis may have an important role in normal lipoprotein
metabolism, lipid disorders and atherosclerosis (5). S1P activates five G-protein-coupled
receptors, known as S1P-receptors 1–5 (6), and affects lymphocyte trafficking,
angiogenesis, wound repair, virus suppression and possibly cancer
progression (7). S1P, combined
with S1P receptor-1 (S1PR1), serves an important role in vascular
protection (8); impaired pulmonary
vascular endothelial cell permeability has been observed in
apoM-deficient mice in an S1P/S1PR1-dependent manner
(9). In vivo, ApoM acts as
a carrier of S1P (9); therefore,
it may be hypothesized that ApoM has a critical role in vascular
protection through the S1PR1 pathway.
In mice, 3β-hydroxysterol Δ-24-reductase (DHCR24) is
predominantly expressed in the medulla oblongata, mesencephalon,
liver, heart, ovaries and intestinal tissues. DHCR24 is a
cholesterol synthetase that is composed of 516 amino acids, which
catalyzes the final step in cholesterol biosynthesis, the
conversion of desmosterol to cholesterol (10). McGrath et al reported that
recombinant HDL could inhibit tumor necrosis factor-α
(TNF-α)-induced inflammation in human coronary artery endothelial
cells (HCAECs) by increasing DHCR24 gene expression (11). A previous study also demonstrated
that estrogen can significantly increase apoM and DHCR24
expression in vivo and in vitro (12,13).
However, whether ApoM regulates DHCR24 expression remains
unclear.
Scavenger receptor class B type I (SR-B1) is a
specific receptor for HDL in several cells and tissue types,
including liver parenchyma, adrenal cortical cells, platelets,
endothelial cells, smooth muscle cells and macrophages (14). It serves crucial roles in
cholesterol homeostasis, lipoprotein metabolism, inflammation and
atherosclerosis (15–18). SR-B1 also mediates the reverse
cholesterol transport of HDL and thus has an anti-atherosclerotic
effect (19). Notably, HDL induces
cyclooxygenase-2 expression and prostacylin production through the
SR-B1-mediated phosphoinositide 3-kinase (PI3K)-protein kinase B
(Akt)-endothelial nitric oxide synthase (eNOS) pathway in
endothelial cells (20). In
addition, SR-B1 signaling helps limit inflammation in
atherosclerotic lesions, thereby preventing plaque formation
(17). S1PR1 and SR-B1 are
co-localized in the caveolae within the plasma membrane (20,21).
Lee et al (22)
demonstrated that HDL-associated S1P can stimulate the molecular
interaction of S1PR1 and SR-BI proteins, in order to affect
S1P-mediated alterations in cell metabolism. HDL not only exerts
anti-inflammatory effects via S1PR1, but also through SR-B1. ApoM
is a major HDL apolipoprotein that acts as a carrier of S1P. In
addition, SR-BI is expressed in endothelial cells, where it can be
combined with ApoM. However, whether it is possible to have
two-fold anti-inflammatory action, or if there is an association
between the two receptors involved in ApoM-induced inhibition of
the inflammatory response requires further exploration Therefore,
the present study further investigated the importance of ApoM in
the inhibitory effects of HDL on inflammation.
Materials and methods
Animals
A total of 48 male C57BL/6 apoM
gene-deficient (apoM−/−) mice (age, 8–10 weeks;
weight, 23–25 g) and 48 age- and body weight-matched male wild-type
(apoM+/+) C57BL/6 mice were used in the present
study. The apoM−/− mice were generated by
homologous recombination at the Model Animal Research Center of
Nanjing University (Nanjing, China), as previously described
(23). All animals were bred under
laminar flow at the specific pathogen-free animal laboratory of
Soochow University (Changzhou, China), where the conditions were
maintained as follows: Interior temperature, 19–24°C; noise, <60
dB; humidity, 50–60%; 12-h light/dark cycle. Mouse feed (ad
libitum) and bedding materials underwent high temperature
disinfection, and drinking water (ad libitum) underwent high
temperature sterilization. The present study was conducted in
accordance with the National Institutes of Health guidelines for
the use of experimental animals and was approved by the Animal Use
and Protection Committee of Soochow University. Adequate measures
were taken to minimize the number of experimental animals used and
to ensure minimal pain or discomfort to the animals throughout the
study.
Animal experimental procedure
A total of 48 apoM−/− mice were
randomly divided into six groups (n=8/group), according to the time
of drug administration: 0, 1, 3, 6, 12 and 24 h groups; the 48
apoM+/+ mice were also randomly divided into the
same groups as the apoM−/− mice. Mice in each of
the six groups received an intraperitoneal (i.p.) injection of 10
mg/kg lipopolysaccharide (LPS; Escherichia coli serotype
O111:B4 L2630; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) in
100 µl pyrogen-free 0.9% saline, and the mice were sacrificed at 0,
1, 3, 6, 12 and 24 h, respectively; mice sacrificed at 0 h served
as the control. Liver tissues were collected for reverse
transcription-quantitative polymerase chain reaction (RT-qPCR).
Cell culture
The permanent human hybrid endothelial cell line,
EA.hy926, was purchased from the American Type Culture Collection
(Manassas, VA, USA). EA.hy926 cells were cultured in Dulbecco's
modified Eagle's medium (DMEM; HyClone; GE Healthcare Life
Sciences, Logan, UT, USA) supplemented with 10% fetal bovine serum
(FBS, Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) and
1% penicillin/streptomycin. Cells were incubated at 37°C in an
atmosphere containing 5% CO2. Cells were seeded in
6-well plates or 60-mm dishes and were grown to 60–80% confluence
prior to use.
Lentiviral infection
EA.hy926 cells were cultured in a 60-mm dish
containing DMEM supplemented with 10% FBS under standard culture
conditions (5% CO2, 37°C). Empty lentivirus (LV) vectors
with green fluorescent protein (GFP; apoMTgN) and
LV-mediated human apoM overexpression vectors
(apoMTg) with GFP were prepared by Shanghai
GeneChem Co., Ltd. (Shanghai, China). Cells grown to 50% and then
were infected with LV at a multiplicity of infection of 20
transducing units per cell, in the presence of 10 mg/ml polybrene.
After 24 h, cells were washed with fresh complete medium.
GFP-positive cells were counted 96 h post-transduction, and
underwent RT-qPCR and western blot analysis.
Cell experiments
EA.hy926 cells were incubated under standard
conditions, as aforementioned, grown to 60–80% confluence and
divided into four groups; the groups were plated in four 6-well
plates at 4×105 cells/well. The four groups were as
follows: Control group, rec-apoM group, TNF-α group and
TNF-α + rec-apoM group. Cells were incubated in serum-free
medium for 12 h prior to treatment. No treatment was administered
to the control group cells, whereas the rec-apoM group cells
were treated with rec-apoM [40 µg/ml; Abgent Biotech
(SuZhou) Co., Ltd., Suzhou, China] for 1.5 h in 37°C, and then
incubated with PBS for 3 h in 37°C. The TNF-α group cells were
pretreated with PBS for 1.5 h and were then treated with TNF-α (10
ng/ml; Cell Signaling Technology, Inc., Danvers, MA, USA) for 3 h.
The TNF-α + rec-apoM group cells were treated with
rec-apoM (40 µg/ml) for 1.5 h prior to treatment with TNF-α
(10 ng/ml) for 3 h. Finally, cells were collected for RT-qPCR
analysis.
EA.hy926 cells were infected as aforementioned and
were divided into two further groups: The apoMTgN
and apoMTg groups. These were then subdivided
into two additional subgroups in two 6-well plates, according to
the different treatments administered, resulting in a control
subgroup and a TNF-α subgroup for each of the original groups.
Cells were incubated in serum-free medium for 12 h prior to drug
treatment. The control group cells did not undergo any treatment,
whereas the TNF-α subgroup cells were treated with TNF-α (10 ng/ml)
for 3 h and were then collected for RT-qPCR analysis.
EA.hy926 cells were infected as aforementioned and
divided into two groups: The apoMTgN and
apoMTg groups, which were further subdivided into
two subgroups in two 6-well plates, according to the different drug
treatments administered, resulting in a control group and a W146
group. All cells in each of the four groups were treated with TNF-α
(10 ng/ml) for 3 h. The control group cells were treated with
ethanol, as a vehicle, for 30 min prior to TNF-α treatment. The
W146 group cells were treated with W146 (10 µM, Cayman Chemical
Company, Ann Arbor, MI, USA) for 30 min prior to TNF-α treatment.
After treatment, the cells were collected for RT-qPCR analysis.
In addition, EA.hy926 cells were infected as
aforementioned and divided into two groups: The
apoMTgN and apoMTg groups,
which were then subdivided into a control group and a block lipid
transport-1 (BLT-1) treatment group. All cells in each of the four
groups were treated with TNF-α (10 ng/ml) for 6 h. The control
group cells were treated with ethanol, as a vehicle, for 12 h prior
to TNF-α treatment. The BLT-1 group cells were treated with BLT-1
(10 µM, Merck KGaA) for 12 h prior to TNF-α treatment. Finally,
cells were collected for RT-qPCR analysis.
RNA isolation and RT-qPCR
analysis
Total RNA was isolated from mouse liver tissues and
cultured cells using the Total RNA Purification kit (Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol; sample
quality was determined by measuring absorbance at 260/280 nm.
Overall, 2 µg total RNA was reverse-transcribed into cDNA using a
RevertAid First Strand cDNA Synthesis kit (Qiagen, Inc., Valencia,
CA, USA), according to the manufacturer's protocol. The mRNA
expression levels of target and reference genes were performed
using Immolase™ DNA Polymerase (Bioline USA, Inc.,
Taunton, MA, USA). GAPDH was used as a reference control. The
quantification cycles (24) of the
target and reference genes were read through the FAM channel
(465–510 nm) and the CY5 channel (618–660 nm), respectively. All
primer/probe sets were designed using Primer Premier 5.0 software
(Premier Biosoft International, Palo Alto, CA, USA) using
information derived from GenBank (https://www.ncbi.nlm.nih.gov/genbank/; Table I). Quantification of interleukin-1β
(IL-1β), monocyte chemotactic protein-1 (MCP-1), S1PR1 and DHCR24
mRNA expression levels was determined relative to GAPDH mRNA
expression levels. qPCR was performed using the LightCycler
480® II (Roche Diagnostics) with a final volume of 25
µl. Each well of a 96-well plate contained 2.5 µl PCR buffer (10X),
2.5 µl MgCl2 (25 mM), 0.5 µl dNTPs (10 mM), 0.25 µl Taq
DNA polymerase, 0.04 µl 100 µM solutions of each primer and probe,
2 µl cDNA, and 17.01 µl of ddH2O. Thermal cycling
conditions included the following steps: Denaturation at 95°C for 3
min, followed by 40 cycles at 95°C for 5 sec and 60°C for 15
sec.
 | Table I.Sequences of primers and probes used
for polymerase chain reaction. |
Table I.
Sequences of primers and probes used
for polymerase chain reaction.
| Gene | Primer/probe | Sequence
(5′-3′) |
|---|
| Human |
|
ApoM | Forward primer |
CTGACAACTCTGGGCGTGGAT |
|
| Reverse primer |
TGTCCACAGGGTCAAAAGTTGC |
|
| Probe |
FAM-AGTTCCCAGAGGTCCACTTGGGCCA-BHQ1 |
|
S1PR1a | Forward primer
1 |
GGGCTCTCCGAACGCAAC |
|
| Forward primer
2 |
GGACCCCGACTCGAGCTG |
|
| Reverse primer |
GTTCGATGAGTGATCCAGGCTT |
|
| Probe |
FAM-TCCGAGGCCCTCTCCAGCCAA-BHQ1 |
|
IL-1β | Forward primer |
TCCTGCGTGTTGAAAGATGATAAG |
|
| Reverse primer |
ATCGCTTTTCCATCTTCTTCTTTG |
|
| Probe |
FAM-CCACTCTACAGCTGGAGAGTGTAGATCCCA-BHQ1 |
|
MCP-1 | Forward primer |
GCTCATAGCAGCCACCTTCAT |
|
| Reverse primer |
GCGAGCCTCTGCACTGAGAT |
|
| Probe |
FAM-CCAAGGGCTCGCTCAGCCAGAT-BHQ1 |
|
GAPDH | Forward primer |
GGAAGGTGAAGGTCGGAGTC |
|
| Reverse primer |
CGTTCTCAGCCTTGACGGT |
|
| Probe |
CY5-TTTGGTCGTATTGGGCGCCTG-BHQ2 |
| Mouse |
|
|
|
S1PR1 | Forward primer |
CAGCTTCGTCCGGCTTGAG |
|
| Reverse primer |
GTTACAGCAAAGCCAGGTCAGC |
|
| Probe |
FAM-AGGCTGCTGTTTCTCGGAGGCCTC-BHQ1 |
|
IL-1β | Forward primer |
GCAGGCAGTATCACTCATTGTGG |
|
| Reverse primer |
GAGTCACAGAGGATGGGCTCTTC |
|
| Probe |
FAM-TGGAGAAGCTGTGGCAGCTACCTGTGT-BHQ1 |
|
MCP-1 | Forward primer |
GCTGGAGAGCTACAAGAGGATCAC |
|
| Reverse primer |
CCTTCTTGGGGTCAGCACAG |
|
| Probe |
FAM-CAGCAGGTGTCCCAAAGAAGCTGTAGTT-BHQ1 |
| GAPDH | Forward primer |
TCTTGTGCAGTGCCAGCCT |
|
| Reverse primer |
TGAGGTCAATGAAGGGGTCG |
|
| Probe |
CY5-AGGTCGGTGTGAACGGATTTGGC-BHQ2 |
Western blot analysis
The proteins ApoM, S1PR1 and GAPDH were detected by
western blot analysis. Protein samples were extracted from cultured
cells using a total protein extraction kit (BestBio, Shanghai,
China), and were harvested and washed with ice-cold PBS. Proteins
were then quantified using a Bicinchoninic Acid Protein Assay kit
(BestBio). Equal amounts of protein (2.4 g/ml) were loaded into all
wells, separated by 10% SDS-PAGE and transferred to polyvinylidene
fluoride membranes (EMD Millipore, Billerica, MA, USA).
Subsequently, membranes were blocked in 3% bovine serum albumin
(Sigma-Aldrich; Merck KGaA) in PBS with 5% Tween-20 at room
temperature 1 h. The membranes were then incubated overnight at 4°C
with mouse polyclonal anti-ApoM (1:2,000; cat. no. H00055937,
Abnova, Taipei, Taiwan), rabbit polyclonal anti-S1PR1 (1:1,000;
catalog no. ab11424, Abcam, Cambridge, MA, USA) and mouse
polyclonal anti-GAPDH antibodies (1:1,000, cat. no. 10494-1-AP,
ProteinTech Group, Inc., Chicago, IL, USA). Subsequently, the
membranes were incubated with secondary antibodies [anti-rabbit
immunoglobulin G (IgG) (cat. no. SA00001-2, ProteinTech Group,
Inc., Chicago, IL, USA) or anti-mouse IgG (cat. no. SA00001-1,
ProteinTech Group, Inc.), 1:3,000] for 1 h at room temperature. The
proteins were visualized using enhanced chemiluminescence (ECL; ECL
Plus Western Blot Detection system; GE Healthcare, Chicago, IL,
USA).
H&E staining
Thoracic aorta samples were harvested for
histological examination and fixed in 4% paraformaldehyde in room
temperature for 12 h then the myocardial tissue was dehydrated with
ethanol and embedded in paraffin. Thereafter, the myocardial
tissues were cut into serial sections (5 µm) and stained with
H&E dye in room temperature for 30 sec. The staining was
observed under a light microscope (magnification, ×400, Olympus
Corporation, Tokyo, Japan).
Evaluation of S1PR1
immunohistochemical staining
Thoracic aorta samples were obtained from 8–10 week
old male apoM−/− and apoM+/+
mice, fixed in 3.7% paraformaldehyde 24 h at room temperature and
embedded in paraffin 40 min. A series of 2–3 µm sections were
collected. Briefly, the sections were pretreated with
dimethylbenzene and ethanol, blocked with 5% bovine serum albumin
for 20 min at room temperature, and incubated with primary
polyclonal S1PR1 antibody (1:50 dilution in PBS; cat. no. ab11424,
Abcam) at 4°C overnight. A negative control sample was prepared by
omitting the primary antibody. After washing with PBS, the sections
were incubated with horseradish peroxidase-labeled goat anti-rabbit
secondary antibody (cat. no. 200374. Dako; Agilent Technologies,
Inc., Santa Clara, CA, USA). The sections were stained using a
Diaminobenzidine Solution kit (OriGene Technologies, Inc.,
Rockville, MD, USA), counterstained with hematoxylin for 5 min,
dehydrated with increasing concentrations of ethanol (30, 50, 70,
80, 90, 95 and 100%) and mounted for 1–3 min at room temperature.
The staining was observed under a light microscope (magnification,
×400, Olympus Corporation). The sections were then dehydrated,
cleared and mounted.
Statistical analysis
All experiment repeated three times, data are
expressed as the means ± standard deviation. Statistical analyses
were performed using the GraphPad Prism 7.0 software (GraphPad
Software, Inc., La Jolla, CA, USA). Multiple comparisons were
performed with one-way analysis of variance (ANOVA), followed by
Dunnett's multiple comparison test. Two-way ANOVA was used to
analyze the interaction between two factors. Comparisons between
two groups were statistically evaluated using a two-tailed
Mann-Whitney U test. Two-tailed P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of apoM on the mRNA expression
levels of IL-1β and MCP-1 during inflammation
As shown in Fig.
1A, the mRNA expression levels of IL-1β were similar in
apoM−/− and apoM+/+ mice
without LPS administration. However, following LPS injection,
although the mRNA expression levels of IL-1β were increased at all
time intervals in both apoM−/− and
apoM+/+ mice; the levels were significantly
higher in apoM−/− mice compared with in
apoM+/+ mice. The same phenomenon was observed
with regards to MCP-1 mRNA expression (Fig. 1B). These data suggested that LPS
significantly amplified the inflammatory response in
apoM−/− mice compared with in
apoM+/+ mice.
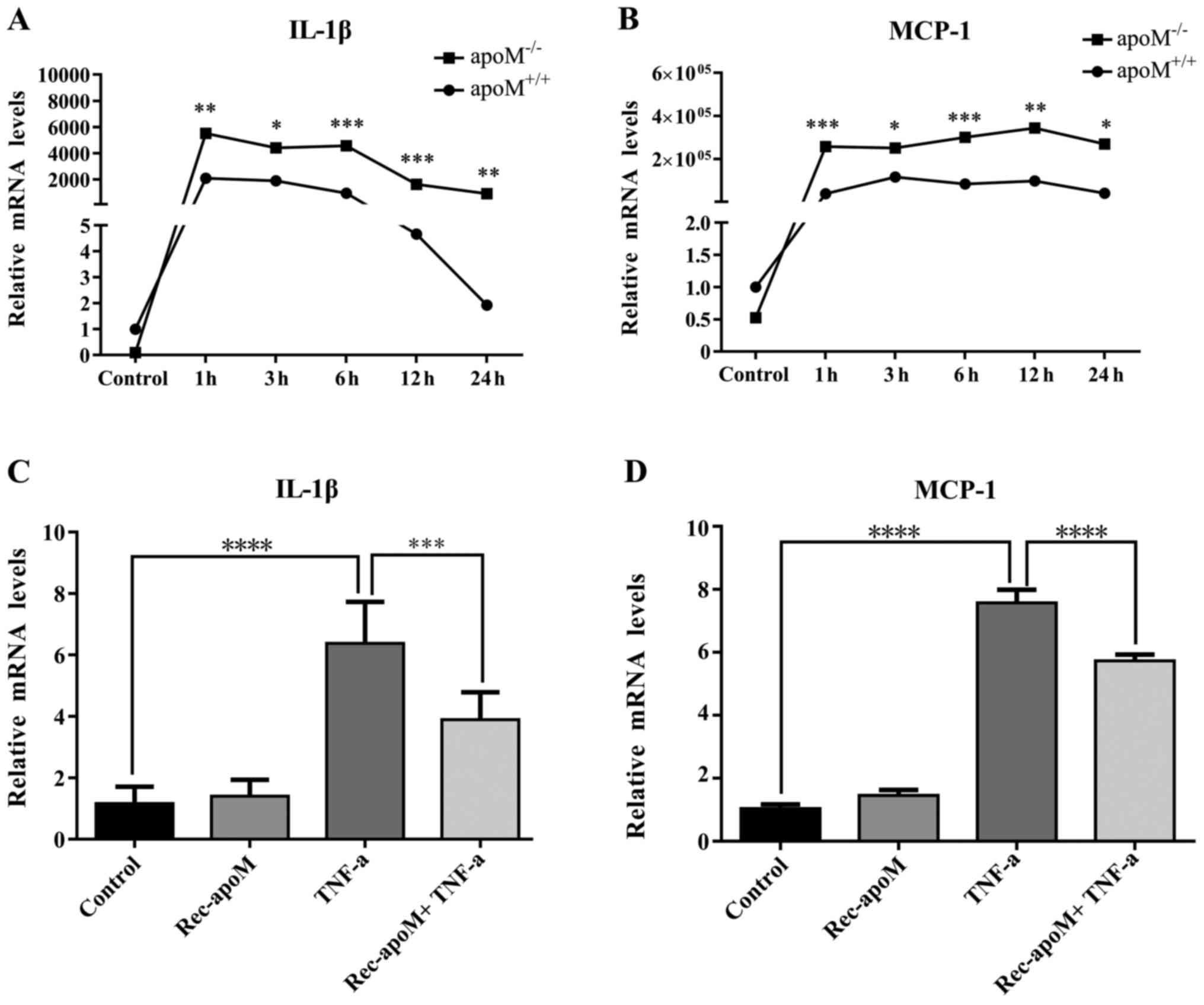 | Figure 1.mRNA expression levels of IL-1β and
MCP-1 in mice and EA.hy926 cells. The mRNA expression levels of (A)
IL-1β and (B) MCP-1 were detected in the livers of
apoM−/− and apoM+/+ mice
following administration of lipopolysaccharide. The mRNA expression
levels of IL-1β and MCP-1 in the control group
(apoM+/+ mice) were set as 1 (n=8, one-way ANOVA
followed by Dunnett's test). *P<0.05, **P<0.01, ***P<0.005
vs. control. The mRNA expression levels of (C) IL-1β and (D) MCP-1
were detected in EA.hy926 cells, which were treated with TNF-α (10
ng/ml) and/or rec-apoM (40 µg/ml). The mRNA expression
levels of IL-1β and MCP-1 in the control group were set as 1 (n=6,
one-way ANOVA followed by Dunnett's test). ***P<0.005,
****P<0.001. ANOVA, analysis of variance; apoM,
apolipoprotein M; IL-1β, interleukin-1β; MCP-1, monocyte
chemotactic protein-1; Rec-apoM, recombinant human
apoM; TNF-α, tumor necrosis factor-α. |
Rec-apoM inhibits the expression
levels of IL-1β and MCP-1 stimulated by TNF-α in EA.hy926
cells
As shown in Fig. 1C and
D, analysis by one-way ANOVA, the mRNA expression levels of
IL-1β and MCP-1 were significantly decreased in EA.hy926 cells that
had been stimulated by TNF-α (10 ng/ml) in the presence of
rec-apoM (40 µg/ml), particularly compared with in cells
that were not treated with rec-apoM (n=6, P<0.01 and
P<0.005). Furthermore, two-way ANOVA demonstrated that
apoM and TNF-α had additive effects on the mRNA expression
levels of IL-1β and MCP-1.
ApoM upregulates the expression of
S1PR1 and DHCR24 in EA.hy926 cells and mouse tissue
The effects of ApoM were detected on S1PR1 in cells
and mouse tissues (Fig. 2A-E). As
shown in Fig. 2B, C and E, the
mRNA and protein expression levels of S1PR1 were higher in
apoMTg cells compared with in the control group
(n=6; P<0.001, respectively). As shown in Fig. 2D, the mRNA expression levels of
S1PR1 were significantly decreased in the thoracic aorta of
apoM−/− mice (n=7-8; P<0.01);
immunohistochemical staining further confirmed that the abundance
of S1PR1 protein in aortic tissue from apoM−/−
mice was markedly lower than in apoM+/+ mice
(n=7-8; Fig. 2F and G). These data
suggested that ApoM significantly increased the expression of S1PR1
in vivo and in vitro. In addition, as shown in
Fig. 2H and I, compared with in
the control group, the mRNA expression levels of DHCR24 were
increased in apoMTg cells and
apoM+/+ mouse liver tissues without inflammatory
stimulation.
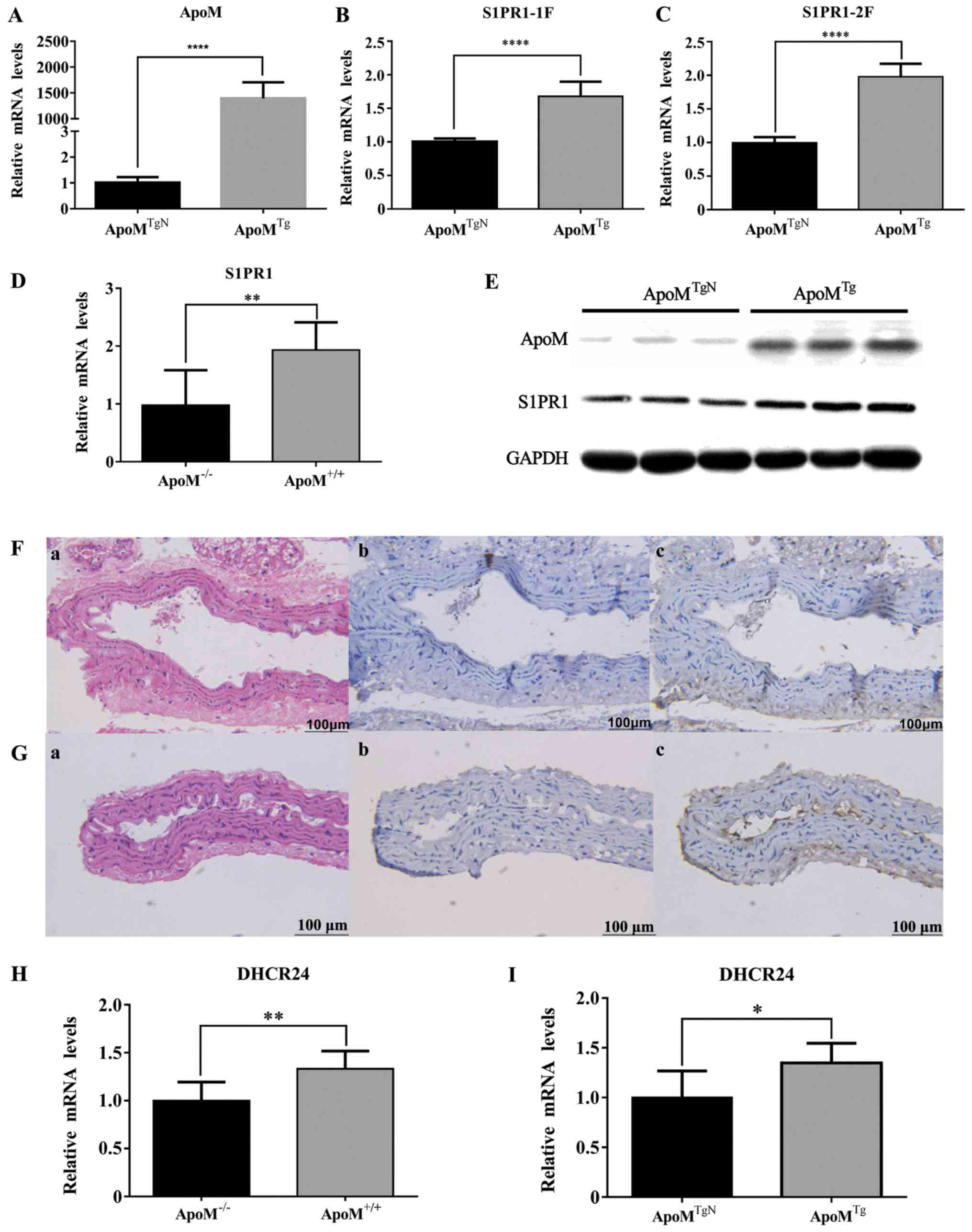 | Figure 2.Expression levels of S1PR1 and DHCR24
in mice and EA.hy926 cells. (A) mRNA expression levels of
apoM were detected in apoMTgN and
apoMTg transduced cells. (B and C) S1PR1 mRNA
levels were significantly increased in EA.hy926 cells when ApoM was
overexpressed. S1PR1-1F and S1PR1-2F represent the transcript
levels of each fragment of the S1PR1 gene. (D) mRNA levels of S1PR1
in mouse aortas were significantly lower in
apoM−/− mice than in apoM+/+
mice. (E) Total protein in apoMTgN cells was
analyzed by western blotting. Compared with in the control group,
S1PR1 protein levels were higher in apoMTg cells.
(Fa-c) Expression of S1PR1 in apoM−/− mouse
aorta. (Ga-c) H&E staining and immunohistochemical staining;
original magnification, ×400. Immunohistochemical staining of mouse
aorta samples indicated that S1PR1 levels were higher in
apoM+/+ mice compared with in
apoM−/−. Expression of S1PR1 in
apoM+/+ mouse aorta. H&E staining and
immunohistochemical staining; original magnification, ×400. (H) In
the absence of inflammatory stimulation, the mRNA expression levels
of DHCR24 in the liver of apoM−/− mice were lower
than in the liver of apoM+/+ mice. The mRNA
expression levels of DHCR24 in the apoM−/− mouse
group were set as 1. (I) mRNA expression levels of DHCR24 were
increased in apoMTg cells. The mRNA expression
levels of DHCR24 were set as 1 in the apoMTgN
group. (n=6–8 mice) *P<0.05, **P<0.01, ****P<0.001.
apoM, apolipoprotein M; DHCR24, 3β-hydroxysterol
Δ-24-reductase; H&E, hematoxylin and eosin; S1PR,
sphingosine-1-phosphate receptor-1. |
Effects of ApoM on the mRNA expression
levels of IL-1β, MCP-1, S1PR1 and DHCR24 in TNF-α-stimulated
EA.hy926 cells
As shown in Fig. 3A and
B, analysis by one-way ANOVA, the mRNA expression levels of
IL-1β and MCP-1 were significantly increased in
apoMTgN and apoMTg cells
following treatment with TNF-α. Compared with in
apoMTgN cells, the mRNA expression levels of
IL-1β and MCP-1 were markedly reduced in apoMTg
cells (n=6, P<0.01 and P<0.005). Two-way ANOVA revealed that
ApoM and TNF-α respectively affected the mRNA expression levels of
IL-1β and MCP-1. These results verified the anti-inflammatory role
of ApoM in EA.hy926 cells. As shown in Fig. 3C-E, compared with the increase in
IL-1β expression following TNF-α treatment, the mRNA expression
levels of S1PR1 and DHCR24 were decreased in
apoMTgN cells. However, this phenomenon was not
observed in apoMTg cells. These data suggested that apoM
may inhibit the expression of IL-1β and MCP-1 by increasing S1PR1
and DHCR24.
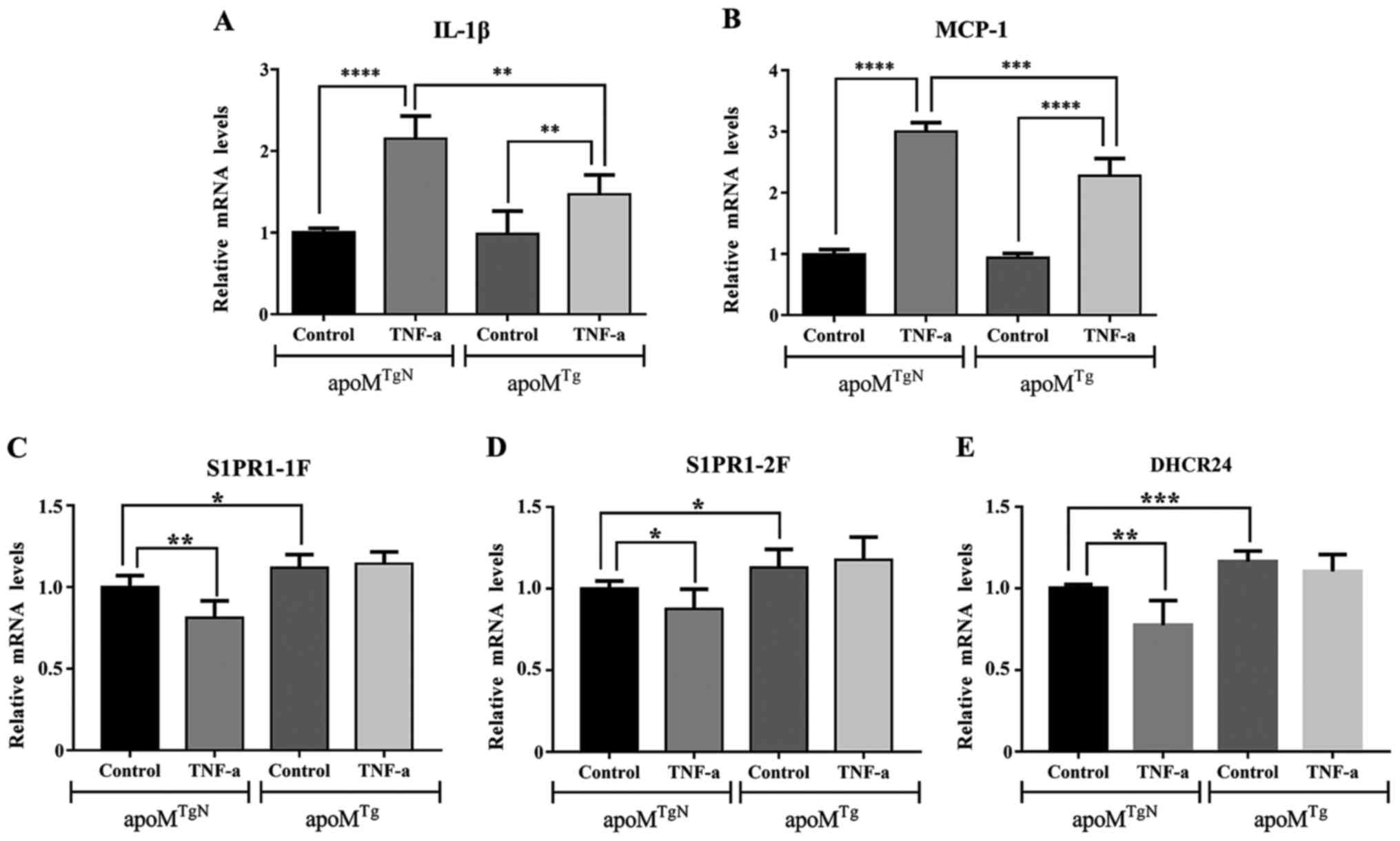 | Figure 3.mRNA expression levels of IL-1β,
MCP-1, S1PR1 and DHCR24 in apoMTgN and
apoMTg cells with or without TNF-α treatment. The
mRNA expression levels of (A) IL-1β and (B) MCP-1 were increased in
apoMTgN and apoMTg cells
following treatment with TNF-α (10 ng/ml) for 3 h. Compared with in
apoMTgN cells, the mRNA expression levels were
markedly reduced in apoMTg cells treated with
TNF-α for 3 h. Two-way ANOVA revealed that ApoM and TNF-α had
additive effects on the mRNA expression levels of IL-1β and MCP-1.
(C and D) mRNA expression levels of S1PR1 were decreased after
treatment with 10 ng/ml TNF-α for 3 h in apoMTgN
cells, but not in apoMTg cells. (E) mRNA
expression levels of DHCR24 were decreased after treatment with 10
ng/ml TNF-α for 3 h in apoMTgN cells, but not in
apoMTg cells. Two-way ANOVA revealed that
apoM and TNF-α had no interaction effects on the mRNA
expression levels of DHCR24. The mRNA expression levels of IL-1β,
MCP-1, S1PR1 and DHCR24 in the control group were set as 1 (n=6,
one-way ANOVA followed by Dunnett's test). *P<0.05, **P<0.01,
***P<0.005, ****P<0.001). ANOVA, analysis of variance;
apoM, apolipoprotein M; DHCR24, 3β-hydroxysterol
Δ-24-reductase; IL-1β, interleukin-1β; MCP-1, monocyte chemotactic
protein-1; S1PR, sphingosine-1-phosphate receptor-1; TNF-α, tumor
necrosis factor-α. |
Effects of W146 on the mRNA expression
levels of IL-1β, MCP-1 and DHCR24 during inflammation
As shown in Fig. 4A and
B, the mRNA expression levels of IL-1β and MCP-1 in the W146
apoMTg group were significantly elevated compared
with in the control apoMTg group. Conversely,
there were no detectable differences between the W146
apoMTgN group and the control
apoMTgN group. These data suggested that W146
amplified inflammation in EA.hy926 cells. As shown in Fig. 4C, the mRNA expression levels of
DHCR24 exhibited no obvious change between the W146
apoMTg group and the control
apoMTg group. These data suggested that the S1PR1
inhibitor W146 did not regulate DHCR24 expression during
inflammation.
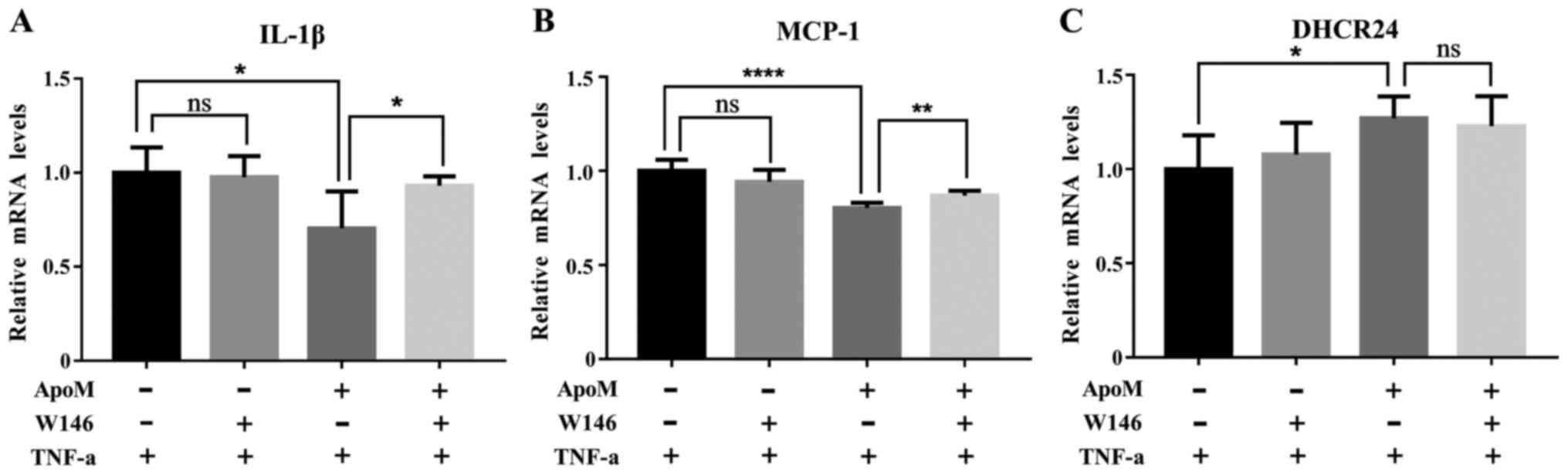 | Figure 4.mRNA expression levels of IL-1β,
MCP-1 and DHCR24 in apoMTgN and
apoMTg cells, which were treated with or without
W146 prior to TNF-α. The mRNA expression levels of (A) IL-1β and
(B) MCP-1 were significantly elevated when W146 was introduced for
30 min prior to TNF-α treatment in apoMTg cells.
However, there were no differences in the expression of these
inflammatory biomarkers in apoMTgN cells. (C) In
apoMTg cells, there was no significant difference
in the mRNA expression levels of DHCR24 between cells treated with
or without W146 before TNF-α. A similar phenomenon was observed in
apoMTgN cells. The mRNA expression levels of
IL-1β, MCP-1 and DHCR24 in the control groups were set as 1 (n=6,
one-way analysis of variance followed by Dunnett's test).
*P<0.05, **P<0.01, ****P<0.001. apoM,
apolipoprotein M; DHCR24, 3β-hydroxysterol Δ-24-reductase; IL-1β,
interleukin-1β; MCP-1, monocyte chemotactic protein-1; ns, not
significant. |
Effects of BLT-1 on the mRNA
expression levels of IL-1β, MCP-1 and DHCR24 during
inflammation
As shown in Fig. 5A and
B, the mRNA expression levels of IL-1β and MCP-1 were no
different between the BLT-1 apoMTg group and the
control apoMTg group. These data suggested that
the SR-B1 receptor inhibitor BLT-1 did not affect the ability of
ApoM to reduce IL-1β and MCP-1 during inflammation. As shown in
Fig. 5C, the mRNA expression
levels of DHCR24 in the BLT-1 apoMTg group were
significantly elevated compared with in the control
apoMTg group. These data suggested that the SR-B1
receptor inhibitor BLT-1 regulated the mRNA expression of DHCR24
during inflammation.
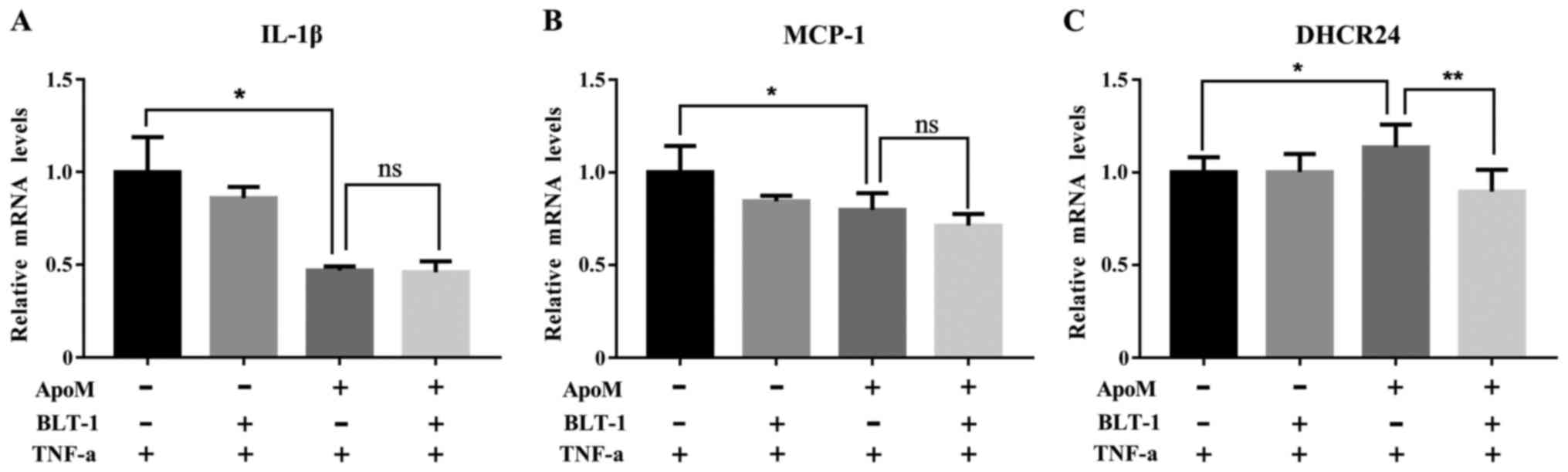 | Figure 5.mRNA expression levels of IL-1β,
MCP-1 and DHCR24 in apoMTgN and
apoMTg cells, which were treated with or without
BLT-1 prior to TNF-α. Compared with in the control
apoMTg cells, the mRNA expression levels of (A)
IL-1β and (B) MCP-1 were no different following treatment with
BLYT-1 prior to TNF-α exposure. A similar phenomenon was observed
in apoMTgN cells. (C) mRNA expression levels of
DHCR24 were significantly reduced in response to treatment with
BLT-1 for 12 h prior to TNF-α in apoMTg cells;
however, there were no differences in DHCR24 expression in
apoMTgN cells. The mRNA expression levels of
IL-1β, MCP-1 and DHCR24 in the control groups were set as 1 (n=6,
one-way analysis of variance followed by Dunnett's test).
*P<0.05, **P<0.01. apoM, apolipoprotein M; BLT-1,
block lipid transport-1; DHCR24, 3β-hydroxysterol Δ-24-reductase;
IL-1β, interleukin-1β; MCP-1, monocyte chemotactic protein-1; ns,
not significant. |
Discussion
It has been well documented that HDL exerts
anti-atherosclerotic effects, which may be partly mediated via its
anti-inflammatory properties (2).
As an essential component of HDL, ApoM is a novel apolipoprotein,
which was initially discovered by Xu and Dahlback in 1999 (3). The anti-atherosclerotic effects of
ApoM have been at the forefront of research for many years.
Christoffersen et al (25)
studied ApoM-overexpressing mice and revealed that mice with
low-density lipoprotein (LDL) receptor deficiencies exhibit a
two-fold increase in apoM compared with in control mice, and
display decreased occurrence of atherosclerosis. A previous study
by our group revealed that deletion of T-855C and C-724del
decreases ApoM expression. Furthermore, the occurrence of these two
single nucleotide polymorphisms is significantly increased in
patients with coronary artery disease (CAD) compared with in
non-CAD patients (26). Recent
research has revealed that HDL serves a role in minimizing
atherosclerosis and may inhibit activation of inflammatory pathways
normally activated by cholesterol crystals, through modulating the
expression of several key components of the inflammatory process
(27). Previous studies further
demonstrated that ApoM is required for pre-β-HDL formation, and
lack of apoM affects cholesterol reverse transport, leading
to atherosclerosis (28,29). ApoM may extend its anti-atherogenic
properties via anti-inflammatory effects (4) and the anti-inflammatory effects of
HDL may be partially mediated by ApoM. ApoM is mainly expressed in
liver cells and renal proximal tubule cells, and its expression is
affected by cytokines, leptin, insulin and other hormones (30). In addition, evidence has indicated
that serum ApoM concentrations are decreased in patients with
infection and inflammation (31–33).
Gao et al reported that ApoM suppresses TNF-α-induced
expression of intercellular adhesion molecule 1 and vascular cell
adhesion molecule 1 (VCAM-1), via inhibition of the activation
pathway for nuclear factor (NF)-κB (34). Our previous study demonstrated that
ApoM may regulate cluster of differentiation 4+ T
lymphocytes and may modify T-lymphocyte subgroups during host
immunomodulation in the murine spleen (23). IL-1β expression is secondary to the
activation of microglia cells, which is promoted by mediators of
inflammation (35) and serves an
important role in upregulating the expression of leukocyte adhesion
molecules in endothelial cells, vascular smooth muscle cell
proliferation, and production of other cytokines that have been
implicated in the development of atherosclerosis (36). Compared with normal coronary
arteries, arteries with atherosclerotic lesions exhibit higher
IL-1β concentrations (37). MCP-1
is a small cytokine that belongs to the CC chemokine family, which
can recruit monocytes, memory T cells and dendritic cells to the
site of inflammation (23). During
early atheroma formation, MCP-1 is considered the link between
oxidized LDL (oxLDL) and foam cell recruitment to vessel walls, and
oxLDL (but not native LDL) induces MCP-1 expression (38). Other than facilitating migration of
macrophages, MCP-1 is also involved in the differentiation of
macrophages to foam cells. During this process, the expression of
LDL receptors decreases, whereas the expression of scavenger
receptors responsible for phagocytosis of oxLDL increases (39). The results of the present study
demonstrated that the hepatic mRNA expression levels of IL-1β and
MCP-1 were higher in apoM−/− mice compared with
in apoM+/+ mice following LPS injection, whereas
two-way ANOVA suggested that there was an interaction effect
between LPS and ApoM with regards to the hepatic expression of
IL-1β and MCP-1 mRNA. Furthermore, the results of cell culture
experiments suggested that ApoM may significantly inhibit the mRNA
expression levels of IL-1β and MCP-1 following TNF-α treatment, and
a two-way ANOVA deduced the interaction effect between TNF-α and
ApoM with regards to the mRNA expression of these factors. In
vivo and in vitro, the mRNA expression levels of IL-1β
and MCP-1 were significantly increased following treatment with LPS
or TNF-α, whereas ApoM was a negative regulatory factor, thus
indicating that ApoM exerts an anti-inflammatory effect; however,
the mechanism underlying this phenomenon remains ambiguous.
A recent study (40) have demonstrated that S1P is a
bioactive lipid regulator that corresponds with S1PRs, which belong
to the G-protein coupled receptor family. There are five subtypes
of S1PRs, S1PR1-S1PR5, which are present on the cytoplasmic
membrane. A previous study (41)
demonstrated that EA.Hy926 cells mainly express S1PR1, S1PR2 and
S1PR3. Various S1PRs are usually present in distinct combinations
in various cell types to perform specific biological functions.
Recent research demonstrated that ApoM expression may be influenced
by numerous factors, including TNF-α, epidermal growth factor,
vascular endothelial growth factor (VEGF), leptin and insulin
levels (30). Christoffersen et
al reported that in a mouse model with 10-fold and 2-fold
increased ApoM concentrations, S1P expression is increased by 267
and 71%, respectively, whereas levels are decreased by 46% in
apoM−/− mice (9). Sutter et al revealed that HDL
may facilitate S1P efflux from erythrocytes through ApoM-dependent
and ApoM-independent processes in LDL receptor related protein
2−/−, chloride voltage-gated channel 5−/−,
cystinosin, lysosomal cystine transporter−/− and
apoM−/− mice. Furthermore, ApoM facilitates
tubular reabsorption of S1P from urine; however, no effect has been
detected on the concentration of plasma S1P in response to ApoM
(42). These results suggested
that ApoM is not only a physiological carrier of S1P, but may also
affect the secretion and clearance of S1P. The present study
demonstrated that the mRNA and protein expression levels of S1PR1
in apoMTg cells were increased compared with in
the control group. Similarly, aortic S1PR1 mRNA and protein
expression in apoM−/− mice was reduced compared
with in apoM+/+ mice. As part of the inflammatory
response, the mRNA expression levels of S1PR1 were decreased in
apoMTgN cells, but not in
apoMTg cells. These results further confirmed
that ApoM may be capable of upregulating S1PR1 and could eliminate
resistance to S1PR1. The present findings established that ApoM
promoted the expression of S1PR1 in endothelial cells and murine
aorta. It was hypothesized that the ApoM-induced increase in S1PR1
expression may lead to enhanced cellular responses to subsequent
S1P treatment; therefore, the increase in S1PR1 may be due to the
upregulation of S1P by ApoM.
Igarashi et al proposed that VEGF may
enhance cellular responses to S1P by increasing the expression of
S1PR1 and regulating the eNOS signaling pathway, which serves an
important role in vascular protection (43). S1P promotes eNOS expression via the
S1PR1 and S1PR3 pathways to produce more nitrogen oxide (NO) and
decreases the activity of the mononuclear phagocyte system to
resist atherosclerosis (8,44). In the present study, ApoM was
revealed to upregulate S1PR1 expression; therefore, it was
hypothesized that ApoM may have an impact on the eNOS signaling
pathway by means of elevation of S1PR1 expression and activation to
protect the vasculature. However, the mechanism underlying this
phenotype requires further study.
In the present study, mice of the same gender were
used to reduce the effects of differences between the genders. Due
to reduced hormonal influences, male mice may be considered better
models. Notably, our previous studies reported that estrogen
upregulates the expression of ApoM in vivo and in
vitro, and DHCR24 may also be regulated by estrogen receptors.
In addition, estrogen significantly increases the expression of
DHCR24 (12,13). Since estrogen may interfere with
the experiments, male mice were used in the present study. This
study demonstrated that the mRNA expression levels of DHCR24 were
higher in the liver samples of apoM+/+ mice
compared with in apoM−/− mice in the absence of
inflammatory stimulation. In addition, the mRNA expression levels
of DHCR24 were significantly increased in EA.hy926 cells
overexpressing apoM compared with in control cells. In
response to TNF-α treatment in apoMTgN and
apoMTg cells, the mRNA expression levels of
DHCR24 were markedly reduced in apoMTgN cells,
compared to apoMTg. This phenomenon was very
similar to changes observed in S1PR1 expression during
inflammation; however, two-way ANOVA revealed that ApoM and TNF-α
had no interaction effects on the mRNA expression levels of DHCR24.
These data suggested that ApoM may significantly increase DHCR24
gene expression in vivo and in vitro, although ApoM
alone does not induce notable alterations in DHCR24 expression to
directly affect the inflammatory response caused by TNF-α. The
detailed molecular mechanism underlying the effects of DHCR24 on
ApoM-inhibited inflammatory responses require further
investigation.
To further elucidate the role of S1PR1 and DHCR24
in the anti-inflammatory response of ApoM, the S1PR1 antagonist
W146 was administered prior to TNF-α treatment; the results
revealed that IL-1β and MCP-1 mRNA expression was significantly
elevated in apoMTg cells that received W146
treatment. However, there were no differences in the expression of
these inflammatory biomarkers in apoMTgN cells.
In addition, the mRNA expression levels of DHCR24 were not affected
in W146-treated apoMTg or
apoMTgN cells. These data suggested that ApoM
induced inhibition of the inflammatory response via the S1PR1
pathway, but did not regulate DHCR24 through S1PR1 during
inflammation. Furthermore, this result verified the hypothesis that
ApoM-mediated anti-inflammation may occur through both S1PR1 and
DHCR24, but that they operate in divergent signal pathways. The
vascular protective (45),
cardioprotective (46) and
anti-apoptotic (47,48) properties of HDL are associated with
S1PRs. ApoM is a component of HDL and a carrier of S1P; the
apoM/S1P axis was initially proposed by Arkensteijn et
al (5). This axis was
originally thought to include ApoM, S1P and S1PRs, and it was
suggested that an ApoM-S1P complex combined with S1PR1 could
regulate the occurrence and development of inflammatory-associated
diseases, such as atherosclerosis, diabetes mellitus, venous
thromboembolism, hepatic fibrosis and neuroinflammation (40,49–53).
Christensen et al suggested that vascular leakage of
albumin-sized particles in apoM−/− mice is
S1PR1-dependent and that this dependency exacerbates the response
to inflammatory stimuli (54).
Ruiz et al demonstrated that ApoM limits endothelial
inflammation by delivering S1P to S1PR1 (55). Our previous study revealed that
ApoM protects against LPS-induced acute lung injury via S1PR1
signaling (56). The present study
further confirmed that ApoM may be an anti-inflammatory molecule
that acts on the S1PR1 pathway. Notably, to the best of our
knowledge, the present study is the first to reveal ApoM may exert
anti-inflammatory properties by regulating the expression of
DHCR24, which is independent of S1PR1, and therefore, may be
another signaling pathway associated with the anti-inflammatory
effects of ApoM. The connections within this pathway require
further elucidation.
It has previously been reported that upregulation
of DHCR24 gene expression by (A-I) recombinant HDL is independent
of cholesterol biosynthesis and the efflux of cholesterol from
endothelial cells (11).
Furthermore, HCAECs may be activated by TNF-α, and HDL suppresses
VCAM-1 promoter activity by inhibiting the NF-κB pathway;
suppression of NF-κB and VCAM-1 expression by HDL are dependent on
alteration of DHCR24 protein levels. Elevated VCAM-1 and NF-κB
expression in small interfering RNA-DHCR24-treated cells is induced
by TNF-α, but can no longer be suppressed by HDL (11). Similarly, Patel et al
reported that ApoA-I inhibits vascular inflammation in New Zealand
white rabbits by increasing the expression of DHCR24 (57). HDL inhibits inflammation by
increasing DHCR24 expression, which activates PI3K/Akt and induces
heme oxygenase-1 (HO-1) expression through the SR-BI pathway
(58). The present results
demonstrated that ApoM may be involved in the regulation of HDL and
expression of DHCR24. Connelly et al reported that
SR-B1-mediated expression of NO and DHCR24 leads to reduced
inflammation, thus reducing monocyte recruitment into the intima
(59). SR-B1 interaction with HDL
prevents endothelial cell inflammation by controlling eNOS
activation and DHCR24 expression (60). S1PR1 and SR-B1 interactions are
thought to be involved in the ApoM-mediated anti-inflammatory
process. S1PR1 and SR-B1 are co-localized in the caveolar regions
of plasma membranes (20,21). Al-Jarallah et al
demonstrated that inactivation of the expression of SR-B1, PDZ
domain containing 1 or Akt1, or antagonism of S1PR1, impairs the
ability of macrophages to undergo chemotaxis towards HDL (61). HDL activates eNOS and S1P promotes
interaction of SR-B1 with S1PR1 to activate the latter (62). To investigate whether ApoM can
regulate DHCR24 through SR-B1 to exert an anti-inflammatory effect,
the SR-B1 antagonist BLT-1 was used prior to TNF-α treatment. Under
these conditions, DHCR24 mRNA expression was significantly reduced
in apoMTg cells, but no differences in DHCR24
mRNA expression were detected in apoMTgN cells.
These data suggested that ApoM may upregulate DHCR24 via SR-B1 but
ApoM may not induce inhibition inflammation via SR-B1. The present
study further demonstrated that ApoM not only promoted
anti-inflammatory action through S1PR1, but also possibly through
DHCR24. ApoM may regulate DHCR24 through SR-B1, but not enough to
induce an anti-inflammatory effect through this pathway. It has
recently been demonstrated that HDL inhibits inflammation by
increasing DHCR24 expression, which activates PI3K/Akt and induces
HO-1 through the SR-B1 pathway (55). Whether regulation of DHCR24 through
SR-B1 signaling may lead to oxidative stress or other pathways
inducing inflammatory changes remains to be determined.
In conclusion, the present study investigated the
anti-inflammatory potential of ApoM, and demonstrated that ApoM
regulated the expression levels of S1PR1 in vivo and in
vitro, with or without inflammation. Additional studies
revealed that ApoM regulated DHCR24 expression in vivo and
in vitro. These results demonstrated that ApoM-induced
inhibition of inflammatory responses may occur via the S1PR1
pathway, and suggested that the anti-inflammatory properties of
ApoM might be due to its regulation of DHCR24. However, the S1PR1
and DHCR24 pathways of ApoM anti-inflammatory action were not
overlapping; therefore, the detailed mechanism of action requires
further investigation.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (NSFC) (grant nos. 81370372,
81201352 and 81701584), the Natural Science Foundation of Jiangsu
Province (grant nos. BK2012154, BK20130244 and BK20151179) ‘333
Project’ of Jiangsu Province (grant nos. BRA2013062) and the
Changzhou High-Level Medical Talents Training Project (grant no.
2016ZCLJ002).
Availability of data and materials
All data generated or analyzed during this study
are included in this published article.
Authors' contributions
MW and YPZ performed the experiments and wrote the
manuscript. XYZ and NX made substantial contributions to conception
and experimental design. YY and SY performed the cell experiments,
HL, XHZ and BW performed the animal experiment and helped perform
the analysis with constructive discussions. GHL and DMD made
substantial contributions in acquiring, analyzing and interpreting
the data. All authors approved the final version of the manuscript
for publication.
Ethics approval and consent to
participate
Experimental protocols were approved by the Animal
Use and Protection Committee of Soochow University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Tall AR: An overview of reverse
cholesterol transport. Eur Heart J. 19 Suppl A:A31–A35.
1998.PubMed/NCBI
|
|
2
|
Feig JE, Shamir R and Fisher EA:
Atheroprotective effects of HDL: Beyond reverse cholesterol
transport. Curr Drug Targets. 9:196–203. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Xu N and Dahlbäck B: A novel human
apolipoprotein (apoM). J Biol Chem. 274:31286–31290. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Huang XS, Zhao SP, Hu M and Luo YP:
Apolipoprotein M likely extends its anti-atherogenesis via
anti-inflammation. Med Hypotheses. 69:136–140. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Arkensteijn BW, Berbée JF, Rensen PC,
Nielsen LB and Christoffersen C: The apolipoprotein
m-sphingosine-1-phosphate axis: Biological relevance in lipoprotein
metabolism, lipid disorders and atherosclerosis. Int J Mol Sci.
14:4419–4431. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ishii I, Fukushima N, Ye X and Chun J:
Lysophospholipid receptors: Signaling and biology. Annu Rev
Biochem. 73:321–354. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Potì F, Simoni M and Nofer JR:
Atheroprotective role of high-density lipoprotein (HDL)-associated
sphingosine-1-phosphate (S1P). Cardiovasc Res. 103:395–404. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
De Palma C, Meacci E, Perrotta C, Bruni P
and Clementi E: Endothelial nitric oxide synthase activation by
tumor necrosis factor alpha through neutral sphingomyelinase 2,
sphingosine kinase 1, and sphingosine 1 phosphate receptors: A
novel pathway relevant to the pathophysiology of endothelium.
Arterioscler Thromb Vasc Biol. 26:99–105. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Christoffersen C, Obinata H, Kumaraswamy
SB, Galvani S, Ahnström J, Sevvana M, Egerer-Sieber C, Muller YA,
Hla T, Nielsen LB and Dahlbäck B: Endothelium-protective
sphingosine-1-phosphate provided by HDL-associated apolipoprotein
M. Proc Natl Acad Sci USA. 108:9613–9618. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zerenturk EJ, Sharpe LJ, Ikonen E and
Brown AJ: Desmosterol and DHCR24: Unexpected new directions for a
terminal step in cholesterol synthesis. Prog Lipid Res. 52:666–680.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
McGrath KC, Li XH, Puranik R, Liong EC,
Tan JT, Dy VM, DiBartolo BA, Barter PJ, Rye KA and Heather AK: Role
of 3beta-hydroxysteroid-delta 24 reductase in mediating
antiinflammatory effects of high-density lipoproteins in
endothelial cells. Arterioscler Thromb Vasc Biol. 29:877–882. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wei J, Shi Y, Zhang X, Feng Y, Luo G,
Zhang J, Mu Q, Tang Y, Yu Y, Pan L, et al: Estrogen upregulates
hepatic apolipoprotein M expression via the estrogen receptor.
Biochim Biophys Acta. 1811:1146–1151. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Benvenuti S, Luciani P, Vannelli GB,
Gelmini S, Franceschi E, Serio M and Peri A: Estrogen and selective
estrogen receptor modulators exert neuroprotective effects and
stimulate the expression of selective Alzheimer's disease
indicator-1, a recently discovered antiapoptotic gene, in human
neuroblast long-term cell cultures. J Clin Endocrinol Metab.
90:1775–1782. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nofer JR and van Eck M: HDL scavenger
receptor class B type I and platelet function. Curr Opin Lipidol.
22:277–282. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pei Y, Chen X, Aboutouk D, Fuller MT,
Dadoo O, Yu P, White EJ, Igdoura SA and Trigatti BL: SR-BI in bone
marrow derived cells protects mice from diet induced coronary
artery atherosclerosis and myocardial infarction. PLoS One.
8:e724922013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tao H, Yancey PG, Babaev VR, Blakemore JL,
Zhang Y, Ding L, Fazio S and Linton MF: Macrophage SR-BI mediates
efferocytosis via Src/PI3K/Rac1 signaling and reduces
atherosclerotic lesion necrosis. J Lipid Res. 56:1449–1460. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Linton MF, Tao H, Linton EF and Yancey PG:
SR-BI: A multifunctional receptor in cholesterol homeostasis and
atherosclerosis. Trends Endocrinol Metab. 28:461–472. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pan B, Ma Y, Ren H, He Y, Wang Y, Lv X,
Liu D, Ji L, Yu B, Wang Y, et al: Diabetic HDL is dysfunctional in
stimulating endothelial cell migration and proliferation due to
down regulation of SR-BI expression. PLoS One. 7:e485302012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
van Wijk DF, Stroes ES and Dallinga-Thie
GM: Novel insights into anti-inflammatory actions of HDL.
Atherosclerosis. 212:388–389. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang QH, Zu XY, Cao RX, Liu JH, Mo ZC,
Zeng Y, Li YB, Xiong SL, Liu X, Liao DF and Yi GH: An involvement
of SR-B1 mediated PI3K-Akt-eNOS signaling in HDL-induced
cyclooxygenase 2 expression and prostacyclin production in
endothelial cells. Biochem Biophys Res Commun. 420:17–23. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yuhanna IS, Zhu Y, Cox BE, Hahner LD,
Osborne-Lawrence S, Lu P, Marcel YL, Anderson RG, Mendelsohn ME,
Hobbs HH and Shaul PW: High-density lipoprotein binding to
scavenger receptor-BI activates endothelial nitric oxide synthase.
Nat Med. 7:853–857. 2001. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee MH, Appleton KM, El-Shewy HM,
Sorci-Thomas MG, Thomas MJ, Lopes-Virella MF, Luttrell LM, Hammad
SM and Klein RL: S1P in HDL promotes interaction between SR-BI and
S1PR1 and activates S1PR1-mediated biological functions: Calcium
flux and S1PR1 internalization. J Lipid Res. 58:325–338. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang Z, Luo G, Feng Y, Zheng L, Liu H,
Liang Y, Liu Z, Shao P, Berggren-Söderlund M, Zhang X and Xu N:
Decreased splenic CD4(+) T-lymphocytes in apolipoprotein M gene
deficient mice. Biomed Res Int. 2015:2935122015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Christoffersen C, Jauhiainen M, Moser M,
Porse B, Ehnholm C, Boesl M, Dahlbäck B and Nielsen LB: Effect of
apolipoprotein M on high density lipoprotein metabolism and
atherosclerosis in low density lipoprotein receptor knock-out mice.
J Biol Chem. 283:1839–1847. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zheng L, Luo G, Zhang J, Mu Q, Shi Y,
Berggren-Söderlund M, Nilsson-Ehle P, Zhang X and Xu N: Decreased
activities of apolipoprotein m promoter are associated with the
susceptibility to coronary artery diseases. Int J Med Sci.
11:365–372. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Thacker SG, Zarzour A, Chen Y, Alcicek MS,
Freeman LA, Sviridov DO, Demosky SJ Jr and Remaley AT: High-density
lipoprotein reduces inflammation from cholesterol crystals by
inhibiting inflammasome activation. Immunology. 149:306–319. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wolfrum C, Poy MN and Stoffel M:
Apolipoprotein M is required for prebeta-HDL formation and
cholesterol efflux to HDL and protects against atherosclerosis. Nat
Med. 11:418–422. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mulya A, Seo J, Brown AL, Gebre AK,
Boudyguina E, Shelness GS and Parks JS: Apolipoprotein M expression
increases the size of nascent pre beta HDL formed by ATP binding
cassette transporter A1. J Lipid Res. 51:514–524. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ren K, Tang ZL, Jiang Y, Tan YM and Yi GH:
Apolipoprotein M. Clin Chim Acta. 446:21–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kumaraswamy SB, Linder A, Åkesson P and
Dahlbäck B: Decreased plasma concentrations of apolipoprotein M in
sepsis and systemic inflammatory response syndromes. Crit Care.
16:R602012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Christoffersen C and Nielsen LB:
Apolipoprotein M-a new biomarker in sepsis. Crit Care. 16:1262012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Feingold KR, Shigenaga JK, Chui LG, Moser
A, Khovidhunkit W and Grunfeld C: Infection and inflammation
decrease apolipoprotein M expression. Atherosclerosis. 199:19–26.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Gao JJ, Hu YW, Wang YC, Sha YH, Ma X, Li
SF, Zhao JY, Lu JB, Huang C, Zhao JJ, et al: ApoM Suppresses
TNF-α-Induced Expression of ICAM-1 and VCAM-1 Through Inhibiting
the Activity of NF-κB. DNA Cell Biol. 34:550–556. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Leyva-López N, Gutierrez-Grijalva EP,
Ambriz-Perez DL and Heredia JB: Flavonoids as cytokine modulators:
A possible therapy for inflammation-related diseases. Int J Mol
Sci. 17(pii): E9212016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Khan R, Spagnoli V, Tardif JC and L'Allier
PL: Novel anti-inflammatory therapies for the treatment of
atherosclerosis. Atherosclerosis. 240:497–509. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Galea J, Armstrong J, Gadsdon P, Holden H,
Francis SE and Holt CM: Interleukin-1 beta in coronary arteries of
patients with ischemic heart disease. Arterioscler Thromb Vasc
Biol. 16:1000–1006. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Freitas Lima LC, Braga VA, do Socorro de
França Silva M, Cruz JC, Sousa Santos SH, de Oliveira Monteiro MM
and Balarini CM: Adipokines, diabetes and atherosclerosis: An
inflammatory association. Front Physiol. 6:3042015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Stephen SL, Freestone K, Dunn S, Twigg MW,
Homer-Vanniasinkam S, Walker JH, Wheatcroft SB and Ponnambalam S:
Scavenger receptors and their potential as therapeutic targets in
the treatment of cardiovascular disease. Int J Hypertens.
2010:6469292010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Nádró B, Juhász L, Szentpéteri A, Páll D,
Paragh G and Harangi M: The role of apolipoprotein M and
sphingosine 1-phosphate axis in the prevention of atherosclerosis.
Orv Hetil. 159:168–175. 2018.(In Hungarian). View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Guo S, Yu Y, Zhang N, Cui Y, Zhai L, Li H,
Zhang Y, Li F, Kan Y and Qin S: Higher level of plasma bioactive
molecule sphingosine 1-phosphate in women is associated with
estrogen. Biochim Biophys Acta. 1841:836–846. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sutter I, Park R, Othman A, Rohrer L,
Hornemann T, Stoffel M, Devuyst O and von Eckardstein A:
Apolipoprotein M modulates erythrocyte efflux and tubular
reabsorption of sphingosine-1-phosphate. J Lipid Res. 55:1730–1737.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Igarashi J, Erwin PA, Dantas AP, Chen H
and Michel T: VEGF induces S1P1 receptors in endothelial cells:
Implications for cross-talk between sphingolipid and growth factor
receptors. Proc Natl Acad Sci USA. 100:10664–10669. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Nofer JR, van der Giet M, Tölle M,
Wolinska I, von Wnuck Lipinski K, Baba HA, Tietge UJ, Gödecke A,
Ishii I, Kleuser B, et al: HDL induces NO-dependent vasorelaxation
via the lysophospholipid receptor S1P3. J Clin Invest. 113:569–581.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Wang X and Wang F: Vascular protection by
high-density lipoprotein-associated sphingosine-1-phosphate. J
Geriatr Cardiol. 14:696–702. 2017.PubMed/NCBI
|
|
46
|
Brinck JW, Thomas A, Brulhart-Meynet MC,
Lauer E, Frej C, Dahlbäck B, Stenvinkel P, James RW and Frias MA:
High-density lipoprotein from end-stage renal disease patients
exhibits superior cardioprotection and increase in
sphingosine-1-phosphate. Eur J Clin Invest. 48:e128662018.
View Article : Google Scholar
|
|
47
|
Ruiz M, Okada H and Dahlbäck B:
HDL-associated ApoM is anti-apoptotic by delivering sphingosine
1-phosphate to S1P1 & S1P3 receptors on vascular endothelium.
Lipids Health Dis. 16:362017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Feuerborn R, Becker S, Potì F, Nagel P,
Brodde M, Schmidt H, Christoffersen C, Ceglarek U, Burkhardt R and
Nofer JR: High density lipoprotein (HDL)-associated sphingosine
1-phosphate (S1P) inhibits macrophage apoptosis by stimulating
STAT3 activity and survivin expression. Atherosclerosis. 257:29–37.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Bosteen MH, Madsen Svarrer EM, Bisgaard
LS, Martinussen T, Madsen M, Nielsen LB, Christoffersen C and
Pedersen TX: Effects of apolipoprotein M in uremic atherosclerosis.
Atherosclerosis. 265:93–101. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Frej C, Mendez AJ, Ruiz M, Castillo M,
Hughes TA, Dahlbäck B and Goldberg RB: A shift in ApoM/S1P between
HDL-particles in women with type 1 diabetes mellitus is associated
with impaired anti-inflammatory effects of the ApoM/S1P complex.
Arterioscler Thromb Vasc Biol. 37:1194–1205. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Memon AA, Sundquist J, Zöller B, Wang X,
Dahlbäck B, Svensson PJ and Sundquist K: Apolipoprotein M and the
risk of unprovoked recurrent venous thromboembolism. Thromb Res.
133:322–326. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Ahmad A, Sundquist K, Zöller B, Dahlbäck
B, Elf J, Svensson PJ, Strandberg K, Sundquist J and Memon AA:
Evaluation of expression level of apolipoprotein M as a diagnostic
marker for primary venous thromboembolism. Clin Appl Thromb Hemost.
24:416–422. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Hajny S and Christoffersen C: A novel
perspective on the ApoM-S1P axis, highlighting the metabolism of
ApoM and its role in liver fibrosis and neuroinflammation. Int J
Mol Sci. 18(pii): E16362017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Christensen PM, Liu CH, Swendeman SL,
Obinata H, Qvortrup K, Nielsen LB, Hla T, Di Lorenzo A and
Christoffersen C: Impaired endothelial barrier function in
apolipoprotein M-deficient mice is dependent on
sphingosine-1-phosphate receptor 1. FASEB J. 30:2351–2359. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Ruiz M, Frej C, Holmér A, Guo LJ, Tran S
and Dahlbäck B: High-density lipoprotein-associated apolipoprotein
M limits endothelial inflammation by delivering
sphingosine-1-phosphate to the sphingosine-1-phosphate receptor 1.
Arterioscler Thromb Vasc Biol. 37:118–129. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Zhu B, Luo GH, Feng YH, Yu MM, Zhang J,
Wei J, Yang C, Xu N and Zhang XY: Apolipoprotein M protects against
lipopolysaccharide-induced acute lung injury via
sphingosine-1-phosphate signaling. Inflammation. 41:643–653. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Patel S, Di Bartolo BA, Nakhla S, Heather
AK, Mitchell TW, Jessup W, Celermajer DS, Barter PJ and Rye KA:
Anti-inflammatory effects of apolipoprotein A-I in the rabbit.
Atherosclerosis. 212:392–397. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Wu BJ, Chen K, Shrestha S, Ong KL, Barter
PJ and Rye KA: High-density lipoproteins inhibit vascular
endothelial inflammation by increasing 3β-hydroxysteroid-Δ24
reductase expression and inducing heme oxygenase-1. Circ Res.
112:278–288. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Connelly MA, de la Llera-Moya M, Monzo P,
Yancey PG, Drazul D, Stoudt G, Fournier N, Klein SM, Rothblat GH
and Williams DL: Analysis of chimeric receptors shows that multiple
distinct functional activities of scavenger receptor, class B, type
I (SR-BI), are localized to the extracellular receptor domain.
Biochemistry. 40:5249–5259. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Banerjee S, de Freitas A, Friggeri A,
Zmijewski JW, Liu G and Abraham E: Intracellular HMGB1 negatively
regulates efferocytosis. J Immunol. 187:4686–4694. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Al-Jarallah A, Chen X, González L and
Trigatti BL: High density lipoprotein stimulated migration of
macrophages depends on the scavenger receptor class B type I, PDZK1
and Akt1 and is blocked by sphingosine 1 phosphate receptor
antagonists. PLoS One. 9:e1064872014. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Sahoo D, Peng Y, Smith JR, Darlington YF
and Connelly MA: Scavenger receptor class B, type I (SR-BI)
homo-dimerizes via its C-terminal region: Fluorescence resonance
energy transfer analysis. Biochim Biophys Acta. 1771:818–829. 2007.
View Article : Google Scholar : PubMed/NCBI
|



















