Introduction
Considering the rapidly increasing aging population,
the number of elderly patients with fractures is likely to rise in
the near future (1). It has been
previously reported that the risk of non-union increases with age
and fracture in aged patients is associated with high morbidity and
mortality rates, in addition to increased healthcare costs
(2). Fracture healing in aged
patients is an emerging public health concern, and how to promote
fracture healing has been investigated extensively in recent
decades.
Remote ischemic preconditioning (RIPC) is the
process of inducing interspersed cycles of ischemia in a remote
organ to prevent ischemic damage of the target organ. In previous
years, RIPC has emerged as an innovative and successful therapeutic
procedure for ischemia and reperfusion (3). Recently, it has been revealed to be
effective in fracture healing in a rat model (4).
Compared with RIPC, intermittent hypoxia training
(IHT) has a long developmental history and there is a wealth of
information regarding its application in the biomedical field
(5). It has been demonstrated that
IHT has a marked effect on increasing resistance to severe
hypoxia/ischemia (6). Fractures
that disrupt the blood supply and isolate the damaged bone from
perfusion cause low oxygen tension and regional hypoxia (7). Therefore, it was hypothesized that
IHT may be critical in fracture healing. RIPC and IHT may become
widespread therapies due to their ease of access and effectiveness;
therefore, a comprehensive understanding of IHT and RIPC is
required to reduce potential harmful consequences and to maximize
potential utility in fracture healing.
The present study aimed to provide insight into the
role of IHT in fracture healing and the differing healing effects
of IHT and RIPC. To the best of our knowledge, this is the first
systematic study to compare the effects of IHT and RIPC in fracture
healing of the aged. It was hypothesized that IHT may have a
positive effect on fracture healing and that the application of IHT
may enhance bone healing. Furthermore, compared with RIPC, enhanced
healing results may be achieved through the use of IHT.
Materials and methods
Animal care and establishment of the
fracture model
All experimental procedures involving rats were
approved by the Ethics Committee on Animal Experimentation of
Capital Medical University (Beijing, China; no. AEEI-2017-098). A
total of 96 male Sprague-Dawley rats (485±60 g) were obtained from
the Laboratory Animal Center of Capital Medical University. In
preliminary experiments, 22–24-month-old rats were used. However,
the drop in weight usually resulted in a comminuted fracture of the
tibia and the number of rats with comminuted-fractures decreased
significantly when 18–20-month-old rats were used. In addition,
others have reported the use of 18–20-month-old rats as aged rats
(8,9), therefore, 18–20-month-old rats were
used in the present study. The animals were housed at a temperature
of 23–25°C, humidity of 50–60% and with unlimited access to food
and water. Upon arrival at the central animal facility, the rats
were allowed to acclimatize for 1 week prior to being randomly
assigned to three groups: IHT (n=32), RIPC (n=32) and control
(n=32). RIPC was initiated immediately following surgery by
occluding blood flow in the contralateral hind limb. Hind limb
occlusion was performed by three cycles of tightening and releasing
of a tourniquet (18-mm) around the upper thigh, with each occlusion
or the release phase lasting 10 min. This method has been
previously demonstrated to completely occlude the blood flow, as
assessed using the vascular assessments system (Periflux System
5000; Perimed AB, Järfälla, Sweden) (4). The procedure was performed once a day
for 28 consecutive days. The IHT training was performed in a
normobaric hypoxic cabin constructed by the Hypoxia Research Center
of Xuanwu Hospital (Beijing, China). The rats were placed in the
cabin immediately following surgery and the IHT regimen lasted 5
min with 12% O2 and 5-min breaks. A total of five cycles
were performed per day, and the control group underwent surgery
only.
Subsequent to peritoneal injection of sodium
pentobarbital (40 mg/kg body weight), fracture of the left tibia
was achieved with a blunt guillotine apparatus driven by a drop
weight, as previously described (4). To achieve intramedullary fixation, a
0.8-mm Kirschner-wire (K-wire) was inserted through the
intercondylar notch until it was seated in the distal cortex.
Radiographs were obtained immediately to confirm K-wire placement
and the extent of the fractures. Comminuted fractures or crack
fractures of the tibia were excluded from the study. The rats were
sacrificed by cervical dislocation following the same
administration of the anesthetic described above at 7, 14 and 28
days post-fracture (n=8 at each time point).
Following removal of the K-wires, the tibiae were
dissected and prepared for reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis, western blotting,
micro-computed tomography (micro-CT) and biomechanical testing.
Micro-CT analysis
A Siemens inveon micro-CT scanner (Siemens AG,
Munich, Germany) was used to scan the dissected tibia, and the
K-wires were removed carefully in order to protect the fracture
site. The beam protocol was set as follows: 15-µm isometric voxel
size, 800 mA and 80 kV. The proximal and distal bone tissues 5 mm
from the fracture line were selected as regions of interest (ROI).
The callus perimeter was determined using a semi-automated
contouring method. Contours were drawn to reveal the periosteal
surface of the ROI in the tibia. Mimics software version 20.0
(Materialise NV, Leuven, Belgium) was used for the
three-dimensional reconstruction of the tibia. The following bone
structural parameters were measured and statistically analyzed by
the internal software of the micro-CT system: Bone volume (BV,
mm3), bone volume/total volume (BV/TV, %), bone mineral
density (BMD, mg HA/cm3) and trabecular number (Tb.N,
1/mm).
Biomechanical testing
Three-point bending tests were applied to evaluate
the biomechanical properties of the fracture site. The
biomechanical tests were performed at room temperature using a
testing apparatus (ELF 3400; Enduratec Systems Group; Bose
Corporation, Framingham, MA, USA) with the distance between the
rollers set as 20-mm. Following careful removal of the K-wire, all
specimens were subjected to an axial compressive force (2 mm/min)
until fracture occurred. The applied forces and resulting
displacements were recorded. The stiffness (N/mm, the slope of the
linear portion of the load-deformation curve) and ultimate loading
(N; the maximum force that the specimen sustained) were
calculated.
RNA extraction and RT-qPCR
analysis
Total RNA was isolated from the callus tissue using
TRIzol according to the manufacturer's protocol (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA), and then reverse
transcribed into the cDNA) as following step: 42°C for 20 min, then
99°C for 5 min using the ReverTra Ace kit (Toyobo Co., Ltd., Osaka,
Japan). The reacted solution was stored at −20°C. RT-qPCR analysis
was performed to measure mRNA expression levels relative to the
expression of GAPDH using an icycle iq real-time PCR detection
system (Bio-Rad laboratories, Inc., Hercules, CA, USA) using
SYBR-Green Master mix. A total of 1 µl of cDNA (10 ng/µl) was used
for qPCR analysis in a 9 µl reaction volume with 5 µl of SYBR-Green
Master mix, 1 µl of forward primer (1 umol/l), 1 µl of reverse
primer (1 µmol/l), 2 µl of ddH2O. The sequences of the
primers used in the present study were as follows: Runt-related
transcription factor 2 (Runx2) forward, 5′-CCCACGAATGCACTATCCAG-3′
and reverse, 5′-GGCTTCCATCAGCGTCAACA-3′; alkaline phosphatase (ALP)
forward, 5′-GGACGGTGAACGGGAGAAC-3′ and reverse,
5′-CCCTCAGAACAGGGTGCGTAG-3′; hypoxia-inducible factor-1α (HIF-1α)
forward, 5′-CCCCTACTATGTCGCTTTCTTGG-3′ and reverse,
5′-GGTTTCTGCTGCCTTGTATGG-3′; vascular endothelial growth factor
(VEGF) forward, 5′-CGACAAGGCAGACTATTCAACG-3′ and reverse,
5′-GGCACGATTTAAGAGGGGAAT-3′; osteocalcin (OCN) forward,
5′-CGGACCACATTGGCTTCCAG-3′ and reverse,
5′-GCTGTGCCGTCCATACTTTCG-3′; GAPDH forward,
5′-TGACAACTTTGGCATCGTGG-3′ and reverse, 5′-GGGCCATCCACAGTCTTCTG-3′.
The thermocycling conditions were as follows: 95°C for 5 min
followed by 40 cycles of 95°C for 15 sec and 60°C for 1 min. The
expression values were normalized to GAPDH using the
2−∆∆Cq method (10). In
order to minimize confounding variance, two independent samples
were analyzed three times. Technical replicates were averaged prior
to all software analysis.
Western blotting
The dissected callus tissues from fractured tibia at
each stage (7, 14 and 28 days) were washed with PBS and ground into
a powder in liquid nitrogen, following which the tissue lysates
were prepared with RIPA buffer containing protease inhibitors
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). The protein
concentration was determined using a bicinchoninic acid protein
assay kit (Thermo Fisher Scientific, Inc.). The lysates (30 mg)
were separated on 10% SDS-polyacrylamide gels and transferred onto
polyvinylidene difluoride membranes (EMD Millipore, Bedford, MA,
USA). The membranes were blocked with 5% non-fat dry milk for 1 h
at room temperature and incubated overnight at 4°C with the
following primary antibodies: ALP (cat. no. ab84401; 1:2,000;
Abcam, Cambridge, UK), Runx2 (cat. no. H00000860-M04; 1:500;
Abnova, Taipei, Taiwan), HIF-1α (cat. no. ab463; 1:1,000), VEGF
(cat. no. ab46154; 1:1,000), OCN (cat. no. ab13420; 1:1,000), GAPDH
(cat. no. ab8245; 1:1,000; all Abcam). This was followed by
incubation for 1 h with a horseradish peroxidase-conjugated
secondary antibody (cat. no. 7076P2; 1:5,000; Cell Signaling
Technology, Inc, Danvers, MA, USA) at 37°C. The bands were
developed using chemiluminescence (Thermo Fisher Scientific, Inc.)
and the densitometric results were analyzed with Image Quant
LAS4000 software (GE Healthcare Life Sciences, Little Chalfont,
UK). GAPDH was used as a loading control.
Statistical analysis
Each experiment was repeated for three times. All
statistical analyses were performed with SPSS software, version
19.0 (IBM Corp., Armonk, NY, USA). Data are presented as the mean ±
standard deviation. The differences between groups were analyzed by
one-way analysis of variance followed by Dunnett's test. P<0.05
was considered to indicate a statistically significant
difference.
Results
IHT accelerates callus formation and
the subsequent remodeling process in fracture healing
Quantitative analysis of callus formation by
micro-CT is presented in Fig.
1A-D. The callus tissues formed in the experimental groups were
significantly different from those in the control group at all time
points. Although the callus in the IHT group exhibited a larger BV
than that in the RIPC group, no significant difference was observed
in any parameter at 7 days. At 14 days post-fracture, all
parameters were increased. At this time-point, the IHT group
exhibited significantly higher BV, BMD, BV/TV and Tb.N values than
in the RIPC group. At 28 days post-fracture, the BV of the IHT
group was markedly decreased compared with that of the RIPC group,
whereas the other parameters continued to increase. In addition,
higher BMD, BV/TV and Tb.N values were recorded in the IHT group
than the RIPC group. As low BV and high BMD are representative of
bone reconstruction in fracture healing, IHT may promote fracture
healing by accelerating callus formation and the subsequent
remodeling process.
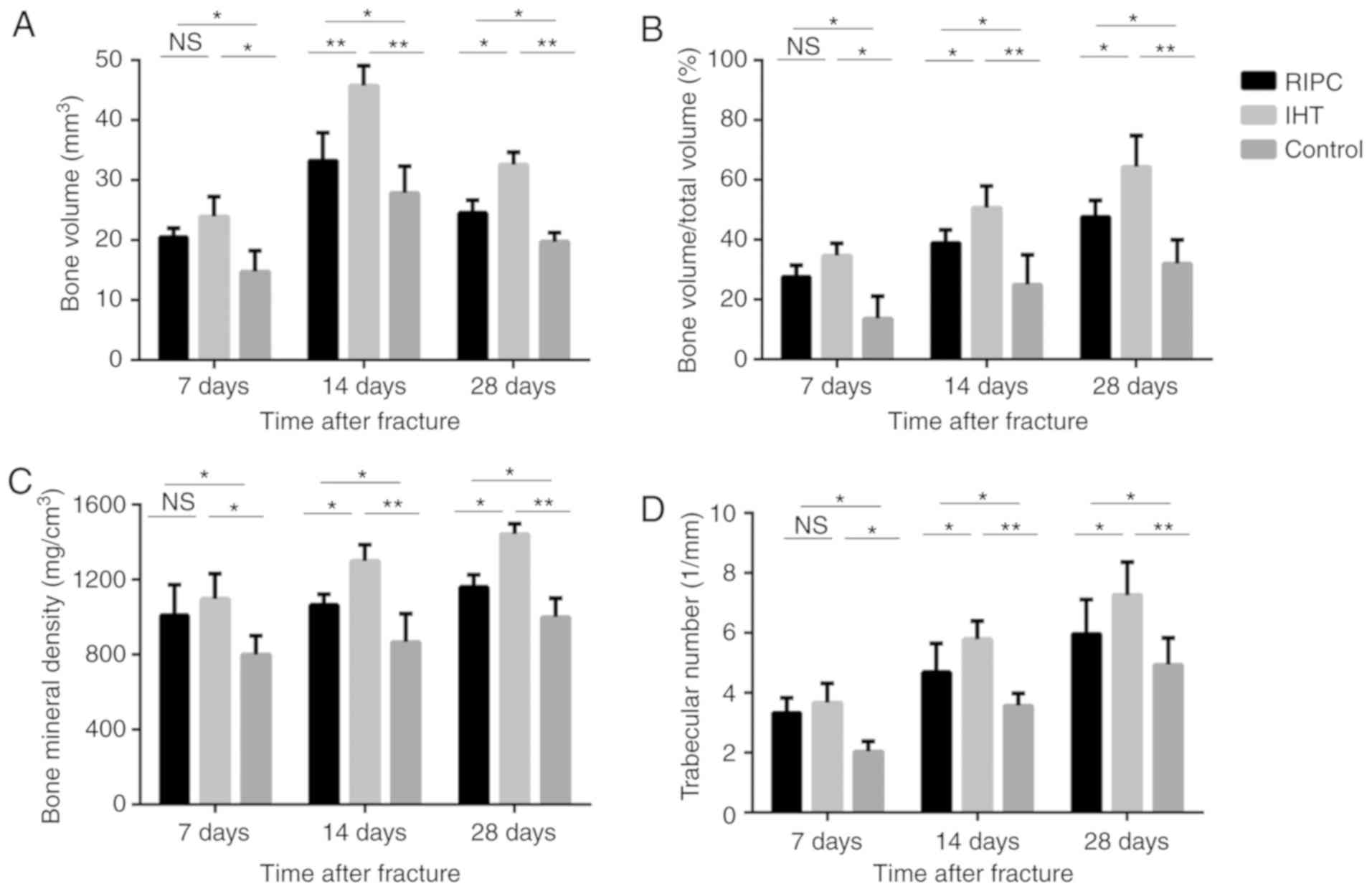 | Figure 1.Comparison of structural parameters
using micro-computed tomography among the IHT, RIPC and control
groups. Compared with the RIPC group, IHT accelerated the formation
of bone callus and the subsequent remodeling process. (A) Bone
volume, (B) total bone volume fraction, (C) bone mineral density
and (D) trabecular numbers. Values are presented as the mean ±
standard deviation, *P<0.05, **P<0.01, N.S., not significant;
IHT, intermittent hypoxia training; RIPC, remote ischemic
preconditioning. |
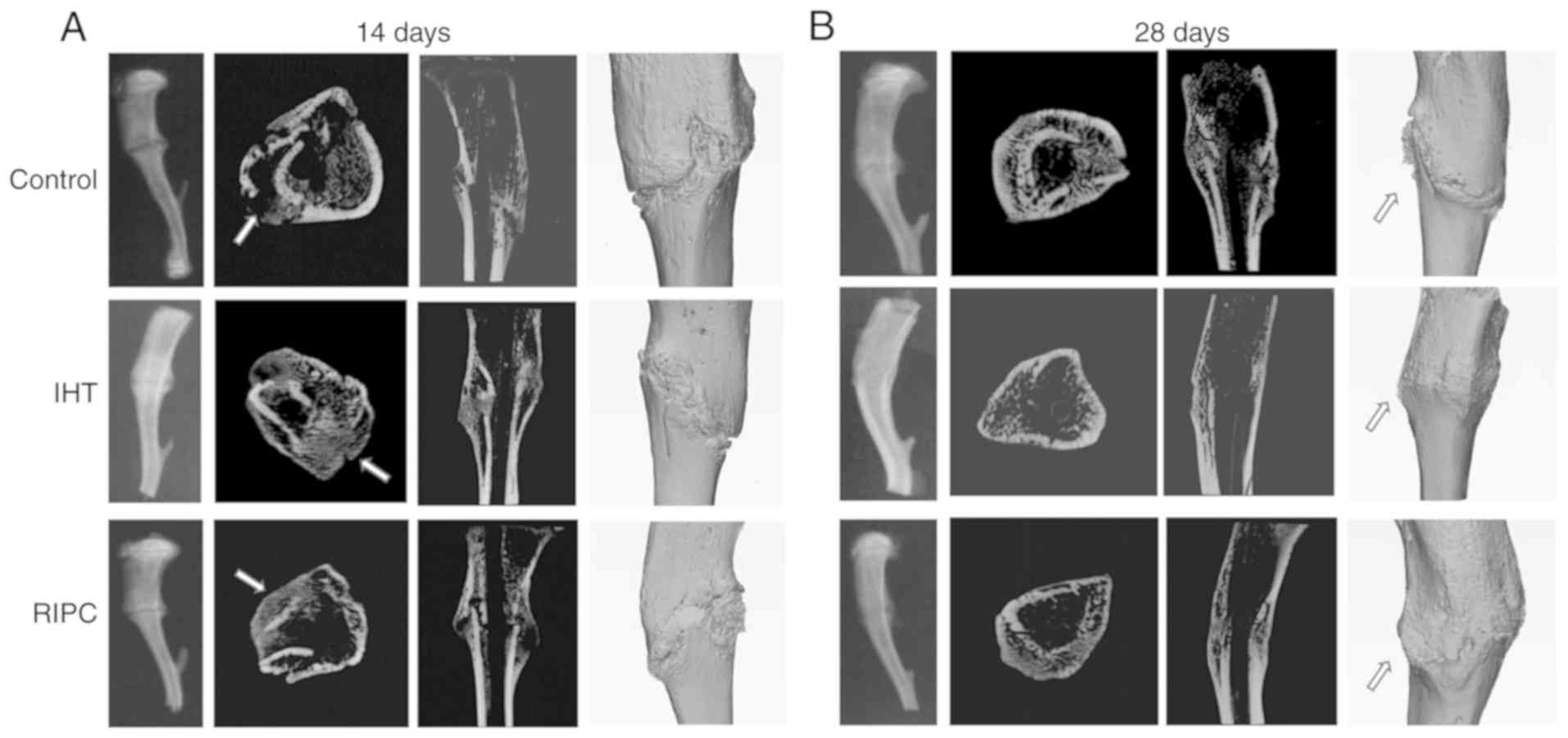
X-ray and three-dimensional reconstructions of the
tibiae are presented in Fig. 2A and
B, which provide a more intuitive delineation of the results.
The experimental groups exhibited more bridging callus at the
fracture site compared with the control group at 14 days. The IHT
group demonstrated loss of the fracture line at 28 days, whereas
the RIPC group exhibited dense bone callus around the fracture
line. These results indicated that the healing effect of IHT was
more efficient than that of RIPC at all time points.
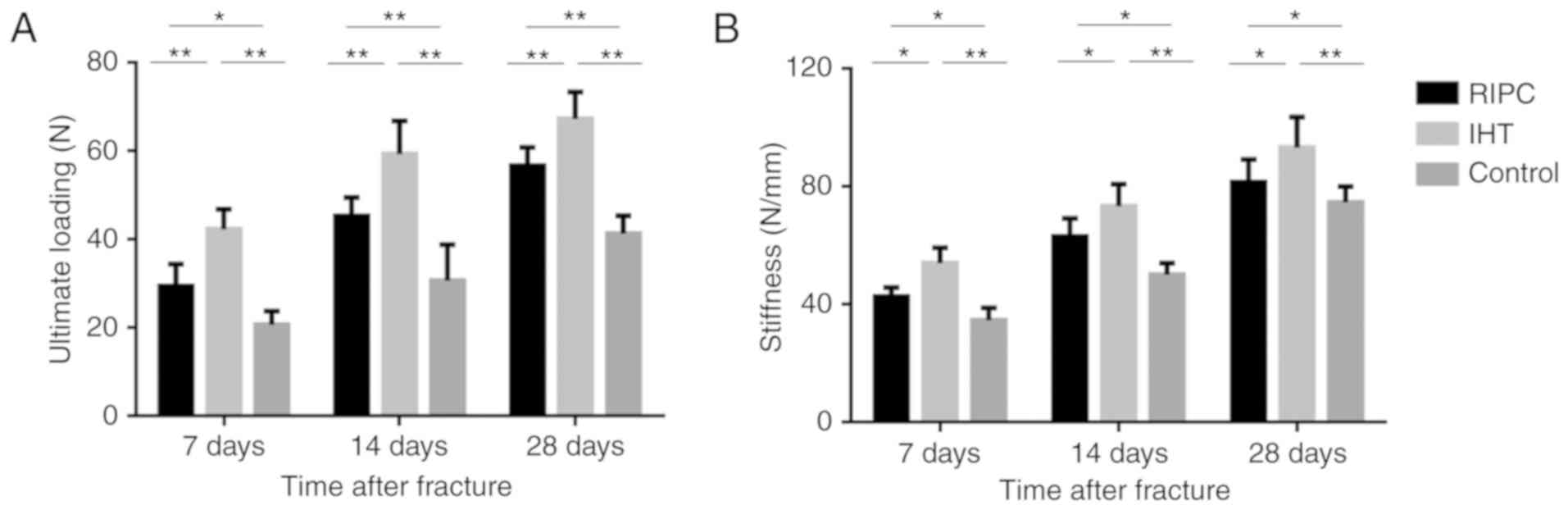
IHT improves the biomechanical
properties of the fractured tibia
The ultimate loading and stiffness values are
presented in Fig. 3A and B. From a
biomechanical perspective, the ultimate loading and stiffness
values are considered to be measures of resistance to failure and
deformation of the tibia. The experimental groups exhibited
enhanced biomechanical properties compared with the control group
in terms of ultimate loading and stiffness, at all time points
(P<0.05). In addition, the ultimate loading and stiffness values
were significantly higher in the IHT group than in the RIPC
group.
IHT promotes osteoblast
differentiation and mineralization
Osteoblasts are involved in the process of fracture
healing; therefore, the present study analyzed the expression of
osteoblast markers among the three groups, including VEGF, Runx2,
ALP and OCN. The results of the western blot (Figs. 4 and 5A-D) and RT-qPCR (Fig. 6A-D) analyses showed that the mRNA
and protein expression levels of VEGF, Runx2, ALP and OCN were
upregulated in the IHT and RIPC groups at all time points.
Furthermore, compared with the RIPC group, the IHT group exhibited
higher expression of all markers at all time points. These results
indicate that IHT may have a positive effect on osteoblast
differentiation, which promotes early fracture healing.
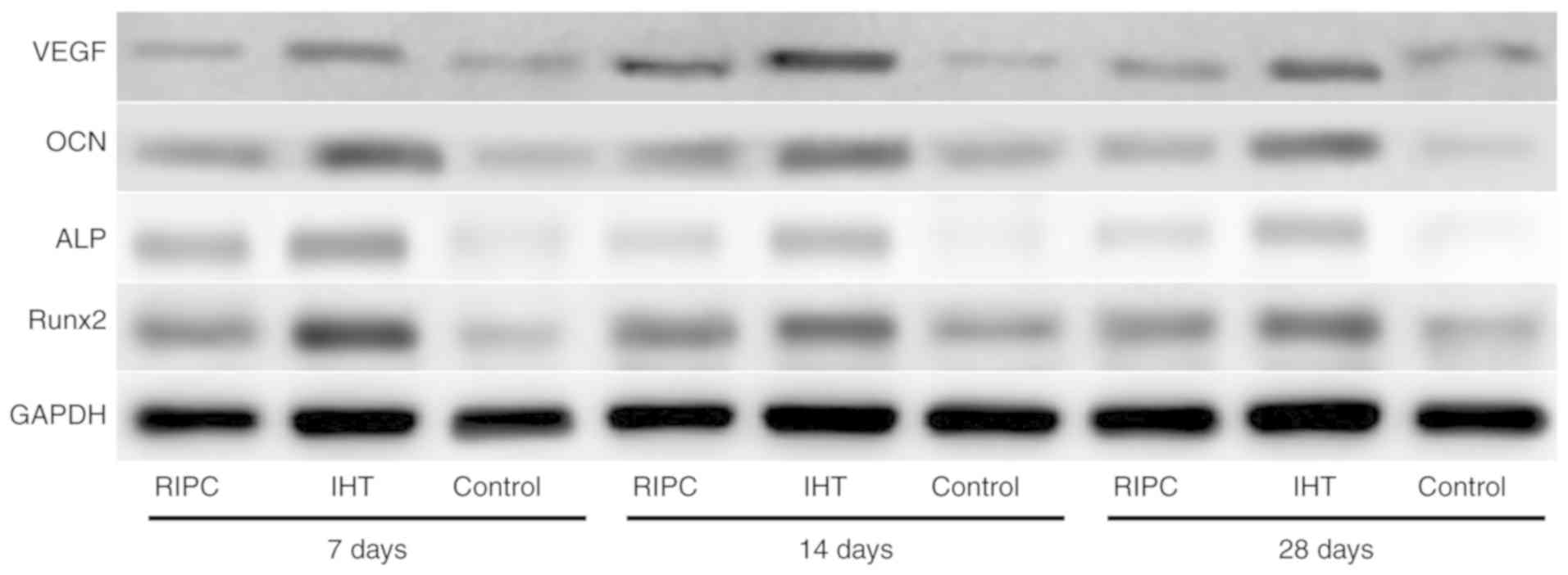 | Figure 4.Expression levels of VEGF, OCN, ALP
and Runx2 in bone callus tissues of the IHT, RIPC and control
groups, analyzed by western blotting. GAPDH was used as an internal
control. IHT, intermittent hypoxia training; RIPC, remote ischemic
preconditioning; VEGF, vascular endothelial growth factor; OCN,
osteocalcin; ALP, alkaline phosphatase; Runx2, runt-related
transcription factor 2. |
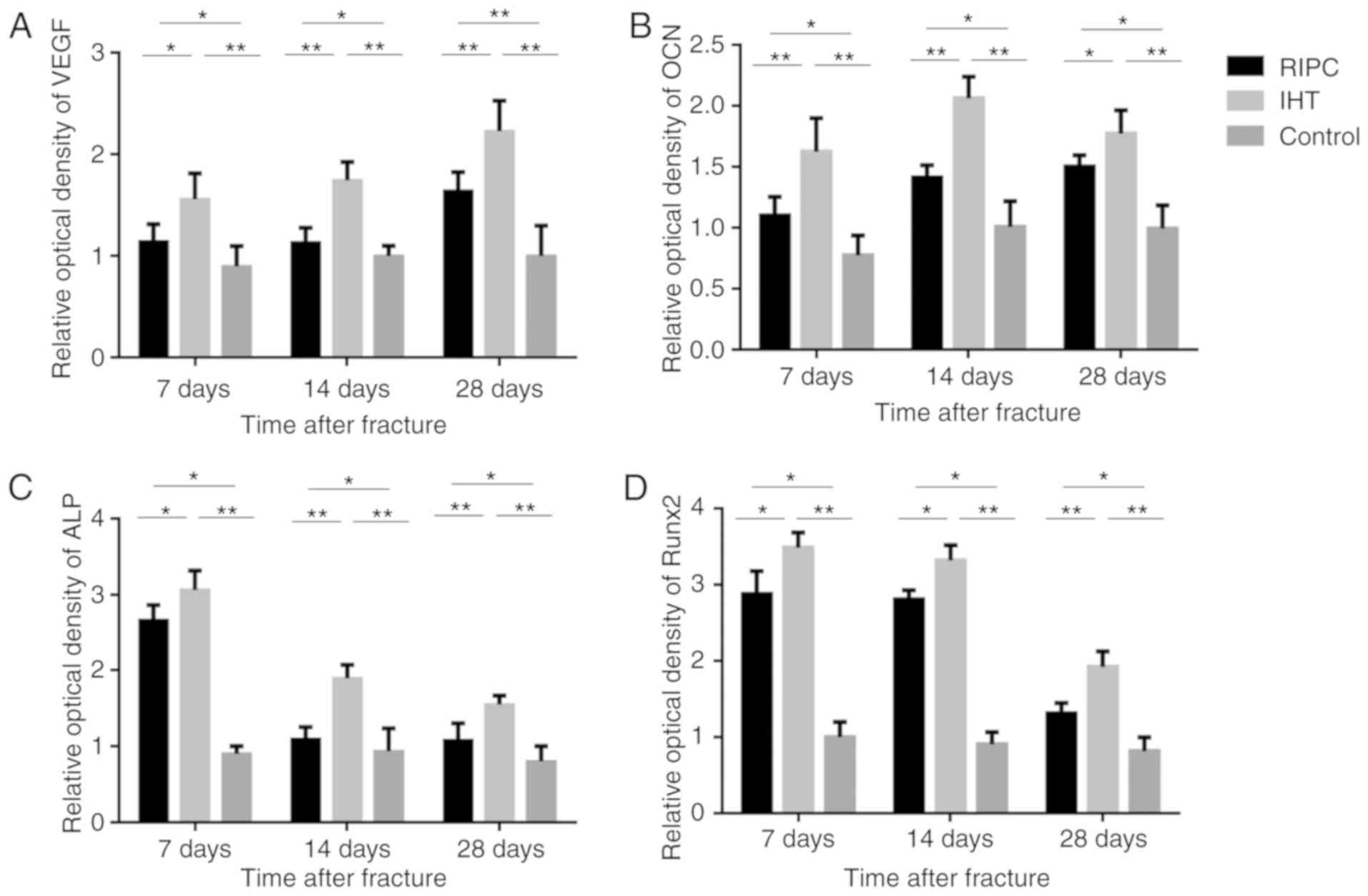 | Figure 5.Optical density of (A) VEGF, (B) OCN,
(C) ALP and (D) Runx2 in bone callus tissues of the IHT, RIPC and
control groups, analyzed by western blotting. GAPDH was used as an
internal control. *P<0.05, **P<0.01. IHT, intermittent
hypoxia training; RIPC, remote ischemic preconditioning; VEGF,
vascular endothelial growth factor; OCN, osteocalcin; ALP, alkaline
phosphatase; Runx2, runt-related transcription factor 2. |
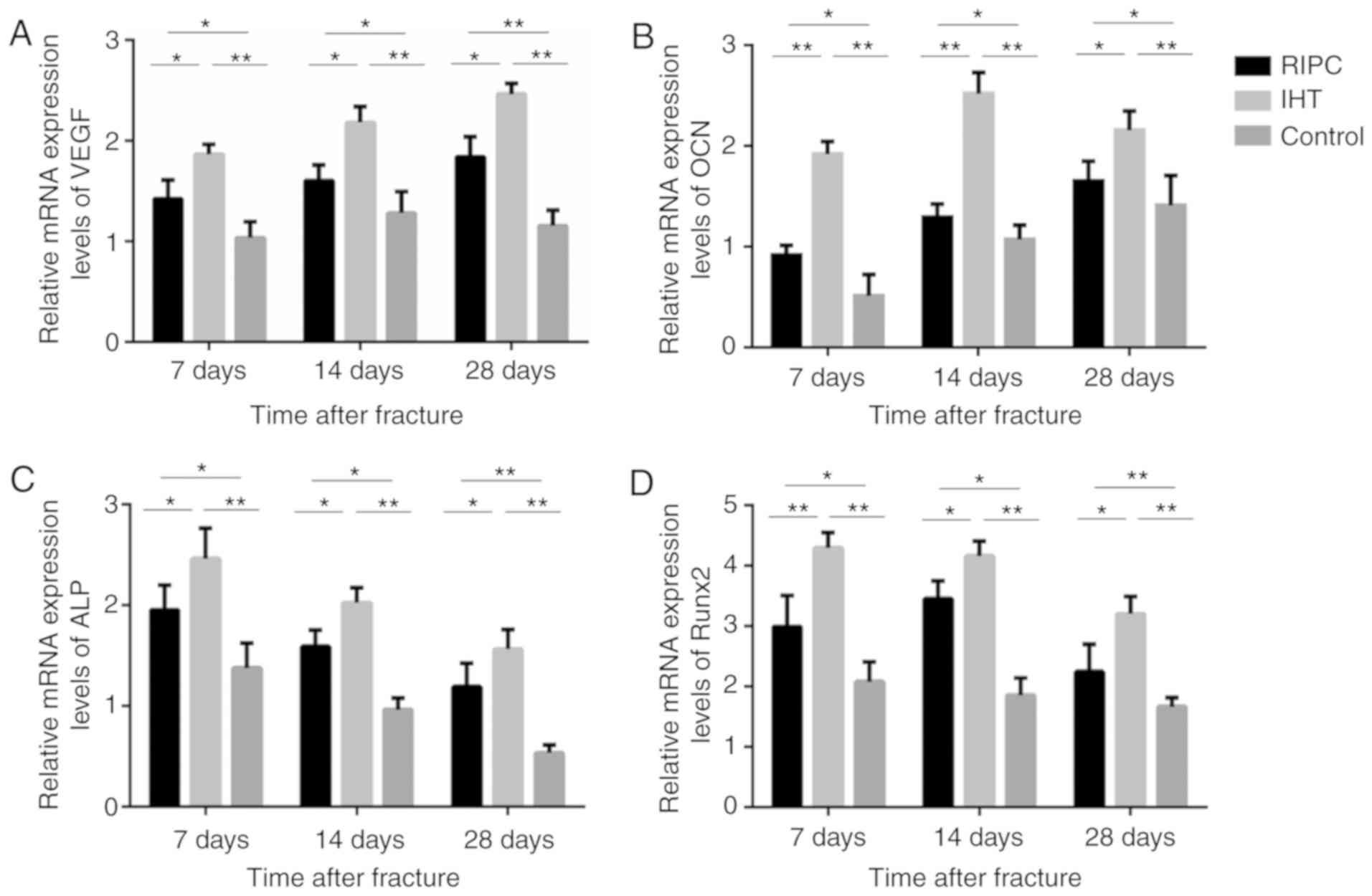 | Figure 6.mRNA analysis of VEGF, OCN, ALP and
Runx2 in bone callus tissues of the IHT, RIPC and control groups.
Expression levels of VEGF, Runx2, ALP and OCN were upregulated in
the IHT and RIPC groups, and the IHT group exhibited the highest
expression of the markers. Expression levels of (A) VEGF, (B) OCN,
(C) ALP, and (D) Runx2, with GAPDH as an internal control.
*P<0.05 and **P<0.01. IHT, intermittent hypoxia training;
RIPC, remote ischemic preconditioning; VEGF, vascular endothelial
growth factor; OCN, osteocalcin; ALP, alkaline phosphatase; Runx2,
runt-related transcription factor 2. |
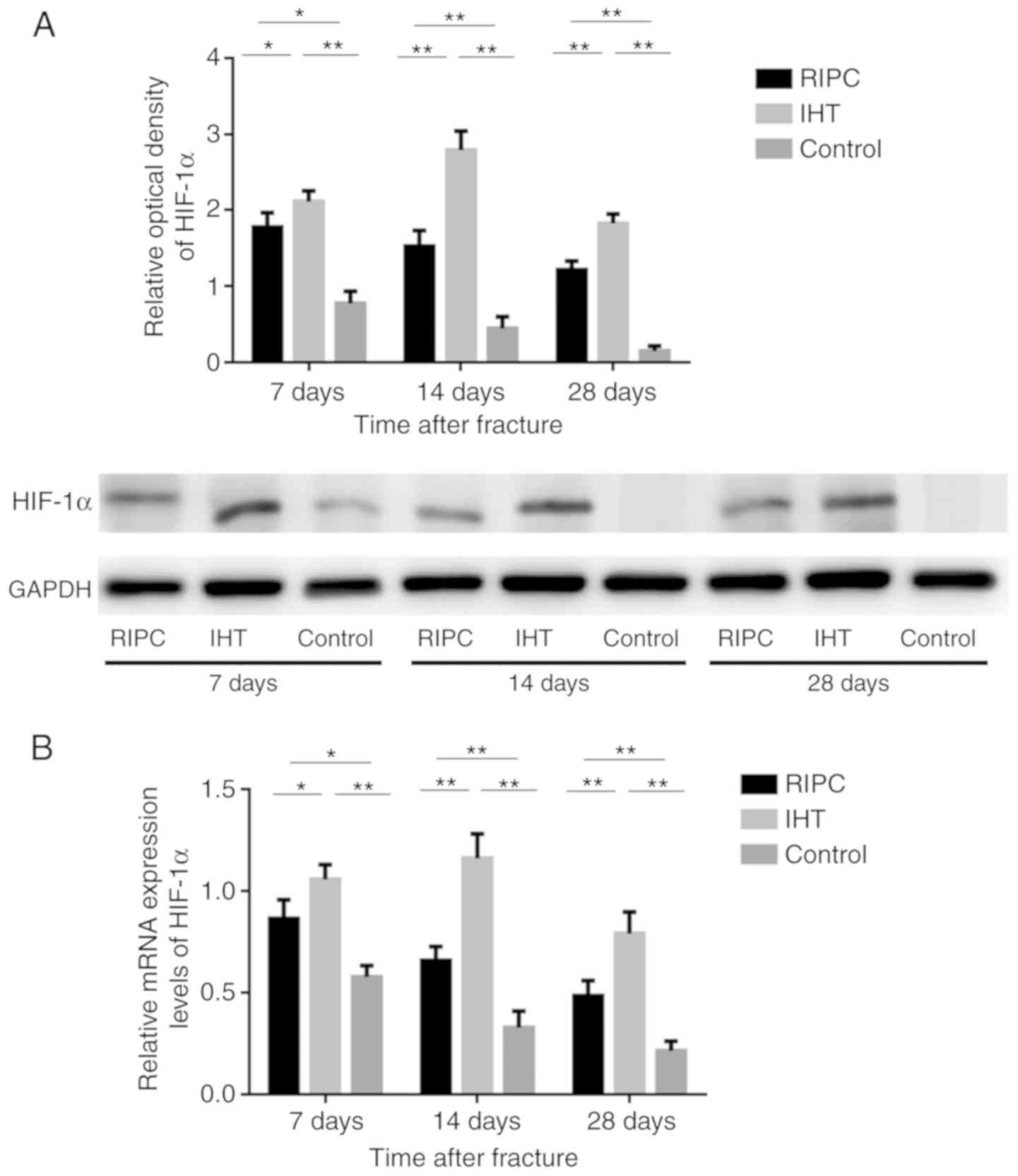
IHT induces the expression of
HIF-1α
It has been well established that VEGF, Runx2, ALP
and OCN are target genes of HIF-1α. Therefore, to elucidate the
mechanism underlying the induction of osteoblast marker expression,
the expression of HIF-1α was also detected by western blot and
RT-qPCR analyses. The results showed that the levels of HIF-1α were
significantly increased in all experimental groups compared with
levels in the control group at all time points (P<0.01).
Furthermore, the levels of HIF-1α in the IHT group were
significantly increased compared with levels in the in RIPC group
(Fig. 7A and B).
Discussion
The elderly population is increasing, and the repair
and regenerative capacity of bone tissue in elderly patients is
limited (11). It is also
important to consider the type and dosage of medicine administered
to elderly patients, due to their decreased drug metabolism
(12). Therefore, the
identification of a novel method to promote fracture healing in
elderly patients is required.
In the present study, the micro-CT findings support
the understanding that ITH and RIPC have a positive effects on
fracture healing. BV, which is sensitive to the detection of
changes in the early stage of fracture healing, was significantly
increased in the experimental groups compared with the control
group, and peaked at 14 days post-fracture (13). Among the other parameters, BMD,
which is the most sensitive in detecting changes in healing,
increased with time (14).
Compared with the RIPC group, BMD and Tb.N were increased in the
IHT group in the late stage of fracture healing. Combining these
results, it may be interpreted that the amount of mineralized
tissue and bone trabecula formed in the RIPC group are lower than
those in the IHT group, and that IHT accelerates the remodeling
process in fracture healing.
Biomechanical assessment is regarded as the gold
standard for evaluating the effects of various interventions on the
structural properties of a callus (15). Despite the fact that the RIPC
performed poorly compared with the IHT group at a relatively low
pressure, the IHT and RIPC groups had significantly higher ultimate
loading and stiffness values than the control group. The brittle
behavior of the callus in the IHT group is indicative of a high
mineralization rate at the fracture site. One possible explanation
is that the bony microarchitecture formed during the regenerative
process may influence the biomechanical properties of bone
directly, and that IHT may accelerate osteoblast proliferation and
subsequent mineralization in the early stage of fracture
healing.
Fracture healing is a regenerative process that
involves distinct temporal and spatial patterning of gene
expression among different cell types (16). To further illustrate the molecular
mechanisms of IHT and RIPC, the present study detected the levels
of ALP, Runx2, OCN and VEGF in the bone callus to investigate the
effect of IHT and RIPC on osteoblasts. Runx2 and OCN are considered
to be the primary controlling transcription factors in the early
and late stages of osteoblast differentiation, respectively
(17). VEGF is critical in the
extensive neovascularization of fracture sites, and has been
demonstrated to be important in stimulating bone healing (18). ALP is also a representative marker
of differentiation, secreted by osteoblasts in response to
osteogenic activity. This was confirmed by RT-qPCR and western blot
analyses. Furthermore, the IHT group exhibited significantly higher
expression of these markers at all time points.
It has been demonstrated that HIF-1α mainly responds
to changes in oxygen levels, and that it is important in adapting
to conditions including ischemia and hypoxia. Therefore, HIF-1α is
considered to be involved in IHT and RIPC (19). Consistent with previous studies,
the RIPC group exhibited a similar effect of upregulating the
expression of HIF-1α throughout the course of healing, which was
demonstrated at the mRNA and protein levels. In addition, the IHT
group exhibited higher differential expression levels of HIF-1α
compared with the RIPC group. HIF-1α is able to interact with the
core DNA sequence of the hypoxia response element, resulting in
upregulation of the expression of multiple hypoxia-sensitive target
genes (20). In the present study,
the results suggested that RIPC and IHT increased the expression of
HIF-1α and osteoblast markers. These hypoxia-sensitive genes were
further increased in the IHT group compared with the RIPC group,
which was in accordance with the micro-CT and biomechanical
results. Taken together, it was concluded that RIPC and IHT may
promote fracture healing by activating the HIF-1α pathway. The
expression level of HIF-1α was significantly higher in the IHT
group than in the RIPC group, which may explain the superior
fracture healing induced by IHT.
Various IHT protocols, considering the number of
hypoxic episodes, severity and total exposure duration, have been
implemented, and different combinations have resulted in various
responses (21). Accumulating
evidence suggests that ‘low dose’ IHT may be a simple, safe and
effective treatment, with considerable therapeutic potential for
multiple clinical disorders (22).
It has been reported that modest hypoxia (9–16% inspired
O2) and low cycle numbers (3–15 episodes per day) most
often lead to beneficial effects without pathological effects,
whereas severe hypoxia (2–8% inspired O2) and an
increased number of episodes per day (48–2,400 episodes/day) elicit
progressive pathological effects (23). The IHT regimen of 5 min, with 12%
O2, 5-min breaks and five cycles per day, used in the
present study, may be the most effective. Previous studies have
suggested that the clinical application of RIPC may be complex
(24,25). Although transient limb ischemia has
been applied as a remote conditioning stimulus in various clinical
settings (26), there is limited
data regarding the optimization of RIPC protocols. The majority of
investigations involving RIPC have been exploratory investigations
and based on clinical experiences.
In conclusion, the present study demonstrated that
RIPC and IHT at defined doses are efficient strategies to enhance
fracture healing, which may function by upregulation of the
expression of HIF-1α. Compared with RIPC, IHT more efficiently
promoted bone formation and guiding of a rapid fracture healing
course. Therefore, IHT may be an optimal choice for fracture
patients, and requires further investigation.
Acknowledgements
The authors give their thanks to the research
platform of Central Laboratory of Xuanwu Hospital Capital medical
university (Beijing, China).
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81541135) and
Capital's Funds for Health Improvement and Research (grant no.
2018-2-2012).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
JQ performed most of the experiments and manuscript
preparation. GG and JR performed the RT-qPCR analysis and western
blotting. GC and ZL analyzed the data. MZ, SL and HS designed the
experiments. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Xuanwu Hospital.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
IHT
|
intermittent hypoxia training
|
|
RIPC
|
remote ischemic preconditioning
|
|
HIF-1α
|
hypoxia-inducible factor-1α
|
|
BV
|
bone volume
|
|
BV/TV
|
total bone/total volume
|
|
BMD
|
bone mineral density
|
|
Tb.N
|
trabecular number
|
|
ALP
|
alkaline phosphatase
|
|
OCN
|
osteocalcin
|
|
Runx2
|
runt-related transcription factor
2
|
|
VEGF
|
vascular endothelial growth factor
|
|
Micro-CT
|
micro-computed tomography
|
|
RT-qPCR
|
reverse transcription-quantitative
polymerase chain reaction
|
References
|
1
|
Tarantino U, Saturnino L, Scialdoni A,
Feola M, Liuni FM, Tempesta V and Pistillo P: Fracture healing in
elderly patients: New challenges for antiosteoporotic drugs. Aging
Clin Exp Res. 25 Suppl 1:S105–S108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bliuc D, Nguyen ND, Alarkawi D, Nguyen TV,
Eisman JA and Center JR: Accelerated bone loss and increased
post-fracture mortality in elderly women and men. Osteoporos Int.
26:1331–1339. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Randhawa PK, Bali A and Jaggi AS: RIPC for
multiorgan salvage in clinical settings: Evolution of concept,
evidences and mechanisms. Eur J Pharmacol. 746:317–332. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhou M, Lu S, Lu G, Huang J, Liu L, An S,
Li Z and Shen H: Effects of remote ischemic postconditioning on
fracture healing in rats. Mol Med Rep. 15:3186–3192. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gabriele P, Ozello F, Tseroni V, Madon E
and Ragona R: Interstitial hyperthermia (IHT): Technical problems
and methodology. Adv Exp Med Biol. 267:121–127. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Korkushko OV, Shatilo VB and Ishchuk VA:
Effectiveness of intermittent normabaric hypoxic trainings in
elderly patients with coronary artery disease. Adv Gerontol.
23:476–482. 2010.(In Russian). PubMed/NCBI
|
|
7
|
Yuasa M, Mignemi NA, Barnett JV, Cates JM,
Nyman JS, Okawa A, Yoshii T, Schwartz HS, Stutz CM and Schoenecker
JG: The temporal and spatial development of vascularity in a
healing displaced fracture. Bone. 67:208–221. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hovens IB, Schoemaker RG, van der Zee EA,
Heineman E, Nyakas C and van Leeuwen BL: Surgery-induced behavioral
changes in aged rats. Exp Gerontol. 48:1204–1211. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zhang L, Yi L, Chopp M, Kramer BC, Romanko
M, Gosiewska A and Hong K: Intravenous administration of human
umbilical tissue-derived cells improves neurological function in
aged rats after embolic stroke. Cell Transplant. 22:1569–1576.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tinubu J and Scalea TM: Management of
fractures in a geriatric surgical patient. Surg Clin North Am.
95:115–128. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hefner G, Stieffenhofer V, Gabriel S,
Palmer G, Müller KM, Röschke J and Hiemke C: Side effects related
to potentially inappropriate medications in elderly psychiatric
patients under everyday pharmacotherapy. Eur J Clin Pharmacol.
71:165–172. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Peyrin F, Dong P, Pacureanu A and Langer
M: Micro- and nano-CT for the study of bone ultrastructure. Curr
Osteoporos Rep. 12:465–474. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
O'Neill KR, Stutz CM, Mignemi NA, Burns
MC, Murry MR, Nyman JS and Schoenecker JG: Micro-computed
tomography assessment of the progression of fracture healing in
mice. Bone. 50:1357–1367. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oftadeh R, Perez-Viloria M, Villa-Camacho
JC, Vaziri A and Nazarian A: Biomechanics and mechanobiology of
trabecular bone: A review. J Biomech Eng. 137:2015.doi:
10.1115/1.4029176. View Article : Google Scholar :
|
|
16
|
Einhorn TA and Gerstenfeld LC: Fracture
healing: Mechanisms and interventions. Nat Rev Rheumatol. 11:45–54.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Komori T: Regulation of osteoblast
differentiation by transcription factors. J Cell Biochem.
99:1233–1239. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Keramaris NC, Calori GM, Nikolaou VS,
Schemitsch EH and Giannoudis PV: Fracture vascularity and bone
healing: A systematic review of the role of VEGF. Injury. 39 Suppl
2:S45–S57. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kalakech H, Tamareille S, Pons S,
Godin-Ribuot D, Carmeliet P, Furber A, Martin V, Berdeaux A, Ghaleh
B and Prunier F: Role of hypoxia inducible factor-1alpha in remote
limb ischemic preconditioning. J Mol Cell Cardiol. 65:98–104. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Maes C, Carmeliet G and Schipani E:
Hypoxia-driven pathways in bone development, regeneration and
disease. Nat Rev Rheumatol. 8:358–366. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Serebrovskaya TV, Nosar VI, Bratus LV,
Gavenauskas BL and Mankovska IM: Tissue oxygenation and
mitochondrial respiration under different modes of intermittent
hypoxia. High Alt Med Biol. 14:280–288. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Serebrovska TV, Serebrovska ZO and Egorov
E: Fitness and therapeutic potential of intermittent hypoxia
training: A matter of dose. Fiziol Zh. 62:78–91. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Navarrete-Opazo A and Mitchell GS:
Therapeutic potential of intermittent hypoxia: A matter of dose. Am
J Physiol Regul Integr Comp Physiol. 307:R1181–R1197. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Anttila V, Haapanen H, Yannopoulos F,
Herajarvi J, Anttila T and Juvonen T: Review of remote ischemic
preconditioning: From laboratory studies to clinical trials. Scand
Cardiovasc J. 50:355–361. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Martin-Puig S, Tello D and Aragonés J:
Novel perspectives on the PHD-HIF oxygen sensing pathway in
cardioprotection mediated by IPC and RIPC. Front Physiol.
6:1372015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Candilio L, Malik A, Ariti C, Barnard M,
Di Salvo C, Lawrence D, Hayward M, Yap J, Roberts N, Sheikh A, et
al: Effect of remote ischaemic preconditioning on clinical outcomes
in patients undergoing cardiac bypass surgery: A randomised
controlled clinical trial. Heart. 101:185–192. 2015. View Article : Google Scholar : PubMed/NCBI
|