Introduction
Lung cancer is the leading type of cancer in terms
of incidence and mortality worldwide (1,2).
Cytotoxic chemotherapy drugs represent the principal clinical
option for lung cancer therapy. However, the 1-year and 5-year
survival rates are <5% in certain Asian countries and regions
(1,3,4).
Additionally, chemotherapy drugs are associated with toxicity and
multiple side effects (5).
Targeted molecular therapy using small molecule drugs is a
promising novel approach that may be used in the future to treat
patients with lung cancer. Previous studies demonstrated the
efficacy of targeted therapy against lung cancer in preclinical and
clinical trials (5,6). Previous studies identified the
effects of novel anticancer compounds on lung cancer cells
(7–11), and inhibitors of the
phosphatidylinositol 3-kinase/AKT serine/threonine kinase 1 (AKT;
LY294002) and mitogen-activated protein kinase (MAPK; PD98059)
signaling pathways were used to examine the role of caspases, AKT
and/or MAPK in the apoptosis-inducing activity of the compounds
identified (8,9). Additionally, lung cancer cell lines
such as A549 cells and HepG2 liver cancer cells have been
previously used to perform pharmacodynamic screenings (7,8).
Various novel compounds have been identified as
promising agents in the treatment of lung cancer, and a number of
these drugs were able to effectively suppress cell proliferation
and metastasis in lung cancer (12). However, various drugs are
associated with multiple side effects, moderate response rates and
drug resistance (13,14). Therefore, the identification of
novel compounds exhibiting increased effectiveness and safety, and
the improvement of targeted lung cancer therapies, are urgently
required.
The Wnt signaling pathway and its downstream factors
serve an important role in carcinogenesis and may regulate cancer
progression by influencing various processes, including cancer
growth, metastasis and cell apoptosis (15). A previous study demonstrated that
the Wnt/β-catenin pathway may regulate oncogenesis in various types
of cancer, and hyperactivity of the Wnt/β-catenin pathway was
identified to promote carcinogenesis (16). The Wnt/β-catenin pathway regulates
the proteosomal degradation of β-catenin via the β-catenin
destruction complex, consisting of APC regulator of WNT signaling
pathway, axin 1/2 and glycogen synthase kinase 3β (GSK-3β)
(17). Therefore, the Wnt
signaling pathway is involved in regulating the cytoplasmic levels
of β-catenin. Extracellular Wnt ligands are able to bind to the
frizzled receptors, transducing the signal to the intracellular
protein dishevelled, thus inhibiting the β-catenin destruction
complex and promoting the nuclear translocation of β-catenin. In
the nucleus, β-catenin is able to induce the transcription of Wnt
ligands and multiple downstream effector genes (18,19).
The activation of the Wnt/β-catenin pathway, which
may lead to the upregulation of certain Wnt ligands, was identified
in ~50% of all cases of non-small cell lung cancer (NSCLC) and
primary lung cancer (20).
Additionally, a previous study demonstrated that the downregulation
of the Wnt signaling pathway was able to suppress cell
proliferation in NSCLC (20).
Therefore, Wnt may represent a promising therapeutic target for
NSCLC.
Apoptosis is a programmed cell death process
regulated by various cellular signals (21–25).
The induction of apoptosis is a promising approach in cancer
therapy, and various novel clinical chemotherapeutic strategies
have been developed, with the aim of increasing apoptosis
sensitivity by activating certain signaling pathways in cancer
cells (26). Furthermore, cell
proliferation serves an important role in the expansion of solid
tumors, and the inhibition of proliferation may prevent cancer
growth and metastasis (27). Our
previous studies identified novel small molecular compounds with
anticancer properties in preclinical models that may be used in
targeted therapies to treat cancer (7,28,29).
These compounds were identified to inhibit angiogenesis and cell
proliferation, and to promote apoptosis and autophagy, thus
exhibiting anticancer activity.
The aim of the present study was to investigate the
anticancer properties and the mechanisms of action of #2714, a
molecule identified in our previous study (7). The present study focused on the
therapeutic potential of #2714 in lung cancer. In particular, the
effects of #2714 on cancer cell proliferation and its ability to
influence the Wnt/β-catenin and mitochondria-mediated apoptotic
pathways were investigated.
Materials and methods
Preparation of #2714
In the present study, the pharmacological activity
of #2714 was investigated. The chemical structure of #2714 was
presented in our previous study (7). #2714 was one of the candidate
compounds identified in our previous study investigating the
targets of YL4073 (8). A
computer-aided drug design method was used to examine the molecular
docking of #2714 (7,8,26,29).
The results suggested that #2714 may be associated with cell
apoptosis and proliferation. Following drug design, #2714 was
synthesized in our laboratory (7).
To perform the in vitro experiments, #2714 was dissolved in
dimethyl sulfoxide (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany)
and stored at 4°C. #2714 was diluted in culture medium to a final
concentration of 0.1% (v/v) and cells were treated with various
concentrations of #2714 (0–20 µM). For the in vivo
experiments, #2714 was resuspended in 1.0% sodium carboxymethyl
cellulose (Sigma-Aldrich; Merck KGaA) for a final concentration of
10 mg/ml, and was administered daily to each animal by gavage at
various concentrations (25–100 mg/kg/day) at volumes of 10
ml/kg/day.
Materials
The Cell Counting Kit-8 (CCK-8) was purchased from
Dojindo Molecular Technologies, Inc. (Kumamoto, Japan). The
5-ethynyl-2-deoxyuridine (EdU) proliferation assay kit was
purchased from Guangzhou RiboBio Co., Ltd. (Guangzhou, China).
Anti-marker of proliferation Ki-67 (MKI67, 1:50 dilution, cat. no.
550609) and Matrigel were purchased from BD Biosciences (San Jose,
CA, USA). The primary antibodies Wnt3a (cat. no. ab28472) were
obtained from Abcam Company (Cambridge, MA, USA), the primary
antibodies Wnt5a/b (cat. no. 2530), t-β-catenin (cat. no. 9562),
p-β-catenin (cat. no. 2009), t-GSK-3β (cat. no. 12456), p-GSK-3β
(cat. no. 9336), t-p38 (MAPK14, cat. no. 9212); p-MAPK14 (cat. no.
4511), β-actin (cat. no. 4967) and horseradish peroxidase
(HRP)-conjugated secondary antibodies (cat. nos. 7074 and 7076)
were obtained from Cell Signaling Technology, Inc. (Danvers, MA,
USA). All the chemicals used in the present study were of
analytical grade (30).
Cell culture
The human NSCLC cell line SPC-A1 and human 293 cells
were purchased from the American Type Culture Collection (Manassas,
VA, USA). SPC-A1 cells were cultured in RPMI-1640 medium and 293
cells were cultured in Dulbecco's modified Eagle's medium (both
from Thermo Fisher Scientific, Inc., Waltham, MA, USA); media were
supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.), 2 mM glutamine and 1% antibiotic-antimycotic
solution at 37°C with 5% CO2.
Animals
A total of 108 female BALB/c nude mice (age, 6–8
weeks; weight, 18–22 g) were purchased from Beijing Animal Center
(Beijing, China), and maintained under controlled specific
pathogen-free experimental conditions at 21°C with 55% humidity
under a 12:12-h light/dark cycle, with free access to food and
water. For in vivo experiment, when tumor size reached
~1,500 mm3, animals were sacrificed, and tumor tissues
were harvested. All animal experiments were performed in compliance
with the ARRIVE guidelines and the Guide for the Care and Use of
Laboratory Animals (31). Animal
experiments were approved by The Experimental Animal Ethics
Committee of Anhui Medical University (Hefei, China).
Cell proliferation assay
Cell proliferation was examined by CCK-8 assay
following treatment with #2714. In total, 3–5×103 cells
were seeded in 96-well plates in triplicate at 37°C with 5%
CO2 for 24 h. The 293 cells were treated with #2714
(0–20 µM) at 37°C for 48 h, whereas SPC-A1 cells were treated at
37°C for 6–72 h. Subsequently, 100 µl culture medium containing 10
µl CCK-8 solution was added to the wells and incubated at 37°C for
2–4 h in the dark. The absorbance values were measured using a
Spectra MAX M5 microplate spectrophotometer (Molecular Devices,
LLC, Sunnyvale, CA, USA) at 450 nm. The inhibitory rate was
calculated as previously described (7). Subsequently, the half-maximal
inhibitory concentration (IC50) values of #2714 were
determined by using GraphPad Prism 7.0 Software (GraphPad Software,
Inc., La Jolla, CA, USA).
Flow cytometry analysis
Flow cytometry (FCM) was used to analyze cell
apoptosis and mitochondrial membrane potential (ΔΨm) by staining
the cells with propidium iodide (PI) and rhodamine-123 (Rho 123)
(both from Sigma-Aldrich; Merck KGaA), respectively. In total,
1×105 SPC-A1 cells were cultured in 6-well plates for 24
h. Following treatment with #2714 for 48 h, SPC-A1 cells were
incubated with 50 µl/ml PI solution, 0.1% Triton X-100 and 0.1%
sodium citrate, or 5 µg/ml Rho 123 at 37°C with 5% CO2
for 30 min in darkness. PI-stained and Rho 123-stained cells were
analyzed using a flow cytometer (FACS Verse; BD Biosciences). Data
were analyzed using FlowJo software Version 7.6 (FlowJo LLC,
Ashland, OR, USA).
EdU assay
In total, 1×105 SPC-A1 cells were
cultured on coverslips in triplicate in 24-well plates and treated
with 0, 2.5, 5 or 10 µM #2714 for 48 h as aforementioned. Cell
proliferation was determined using an EdU incorporation assay kit,
in which Hoechst 33342 was included (Guangzhou RiboBio Co., Ltd.)
according to the manufacturer's protocol. Briefly, following
treatment with #2714, cells were incubated with 500 µl EdU solution
(50 µM) diluted with completed medium at 37°C in darkness for 2 h,
then fixed with 4% paraformaldehyde at room temperature for 30 min
and stained with 500 µl Hoechst 33342 solution at room temperature
in darkness for a further 30 min. The percentage of proliferating
cells was determined using an inverted fluorescence microscope
(magnification, ×40; Zeiss AG, Oberkochen, Germany).
Matrigel invasion assay
Transwell filter inserts (EMD Millipore, Billerica,
MA, USA) were precoated with Matrigel (BD Biosciences) for 30 min
at 37°C. RPMI-1640 with 10% FBS was plated in the lower chamber,
and 4×104 cells were seeded in the upper chamber in
serum-free RPMI-1640 medium. Various concentrations of #2714 were
placed in the upper chambers (0–10 µM), and plates were incubated
for 24 h at 37°C with 5% CO2. Subsequently, 0.1% crystal
violet was used to stain the cells that had migrated from the upper
chamber to the lower chamber for 4 h at 37°C. Cells were visualized
using a fluorescence microscope equipped with a digital camera
(magnification, ×40; Olympus Corporation, Tokyo, Japan). Cells were
counted, and the results are presented as the percentage of
invasive cells in the treatment group compared with the control
(28).
Western blot analysis
Western blot analysis was performed to investigate
the mechanisms underlying #2714 function, according to previous
studies (7,8). Cells were lysed in
radioimmunoprecipitation buffer (1% NP-40, 0.5% deoxycholate, 0.2%
SDS, 150 mM sodium chloride, 50 mM Tris-HCl, pH 7.4) containing
0.1% phenylmethane sulfonyl fluoride and 1% proteinase inhibitor
cocktail (Sigma-Aldrich; Merck KGaA). Protein concentration was
measured using the Bio-Rad protein assay kit (Bio-Rad Laboratories,
Inc., Hercules, CA, USA). The lysates were loaded with 5X SDS
sample buffer and denatured, then proteins (20 µg/lane) were
subjected to 6–12% SDS-PAGE and subsequently transferred onto
polyvinylidene difluoride membranes. Then, the membranes were
blocked for 1 h in 5% dried milk in TBS-0.1% Tween-20 at room
temperature, and the protein expression levels of factors
associated with the Wnt/β-catenin pathway, apoptosis and
proliferation were detected in SPC-A1 cells with the primary
antibodies overnight at 4°C (1:500 for Wnt3a, and 1:1,000 for
Wnt5a/b, t-/p-β-catenin, t-/p-GSK-3β, t-/p-MAPK14 and β-actin),
prior to incubation with HRP-conjugated secondary antibodies for 2
h at 37°C (1:5,000-8,000). The protein bands were visualized using
Amersham enhanced chemiluminescence reagent (GE Healthcare Life
Sciences, Shanghai, China). Densitometric analysis was performed
using Quantity One 1-D analysis software Version 4.6.6 (Bio-Rad
Laboratories, Inc.). β-actin was used as the loading control.
Tumorigenesis in vivo
In total, 5×106 SPC-A1 cells were
subcutaneously injected into the right side of the back of female
BALB/c nude mice. The tumor volumes were measured every other day,
and mice carrying a tumor of 100–300 mm3 in volume were
randomly divided into four groups (n=5 in each group).
Subsequently, #2714 was administered by gavage at various
concentrations (0, 25, 50 and 100 mg/kg/day) every day. The
clinical symptoms of NSCLC were observed daily. After 18 days, mice
were sacrificed with CO2 (air displacement rate,
20%/min), and tumor tissues were removed, weighed and their volumes
were calculated. A bilateral Vernier caliper was used to measure
the tumor length (long diameter) and width (short diameter) every
three days. The tumor volume was calculated as: Tumor volume
(mm3)=0.5 × length (mm) × width2 (mm)
(32).
Immunohistochemical (IHC) analysis in
vivo
IHC analysis was performed on 4%
paraformaldehyde-fixed (4°C for 3–7 days) paraffin-embedded tumor
tissues from the mice injected with SPC-A1 cells and treated with
#2714 at a concentration of 100 mg/kg/day for 14 days (n=8 in each
group). MKI67 staining of 4-µm sections was performed to the detect
malignant features of the tumors, as previously described (33). Briefly, the sections were heated at
65°C for 2 h, deparaffinized and rehydrated with xylene and a
graded ethanol series (100, 95, 80 and 50%). Then, the sections
were heated for 10 min at ~100°C in citrate buffer (10 mM citric
acid, 0.05% Tween-20, pH 7.4) using a pressure cooker, followed by
cooling for antigen retrieval. To block endogenous peroxidase
activity, the sections were immersed in 0.3%
H2O2 for 10 min at room temperature, and
washed in distilled water. Then, the sections were incubated with
3% FBS (Gibco; Thermo Fisher Scientific, Inc.) at 37°C for 30 min
to block non-specific binding of immunoglobulin. Sections were
subsequently incubated with primary antibody MKI67 (1:50) overnight
at 4°C, washed with PBS three times for 5 min, and then incubated
with HRP-conjugated anti-mouse IgG (1:5,000; cat. no. 7076) for 20
min at 37°C. Sections were then reacted with 3,3′-diaminobenzidine
solution (1:200; ZSGB-BIO; OriGene Technologies, Inc., Beijing,
China) for 2 min at room temperature, following which the reaction
was stopped with dH2O, and sections were counterstained
with hematoxylin for 1 min at room temperature. Finally, sections
were washed with dH2O, dehydrated in a graded ethanol
series (50, 75 and 100%) and incubated in xylene prior to mounting.
Sections were observed under a light microscope (magnification,
×200; Zeiss AG); three equal-sized fields per slide were
analyzed.
H&E staining was performed to investigate the
morphology of heart, liver, spleen, lung and kidney tissue
following treatment with #2714. Briefly, following collection and
fixed with 4% paraformaldehyde (4°C for 3–7 days),
paraffin-embedded tissues were sectioned (4 um), deparaffinized,
rehydrated and stained at room temperature with hematoxylin for 3–5
sec and eosin (both from Poly Scientific R&D Corp., Bayshore,
NY, USA) for 20–30 sec. Sections were observed under a light
microscope (magnification, ×100; Zeiss AG); three equal-sized
fields per slide were analyzed.
Statistical analysis
Data are presented as the mean ± standard deviation
or standard error of the mean of three experiments. SPSS software
(version 13.0; SPSS, Inc., Chicago, IL, USA) was used to perform
statistical analysis. Student's t-test or one-way analysis of
variance followed by Dunnett's t-test was used to analyze the
differences between groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of #2714 on tumor cell
proliferation in vitro
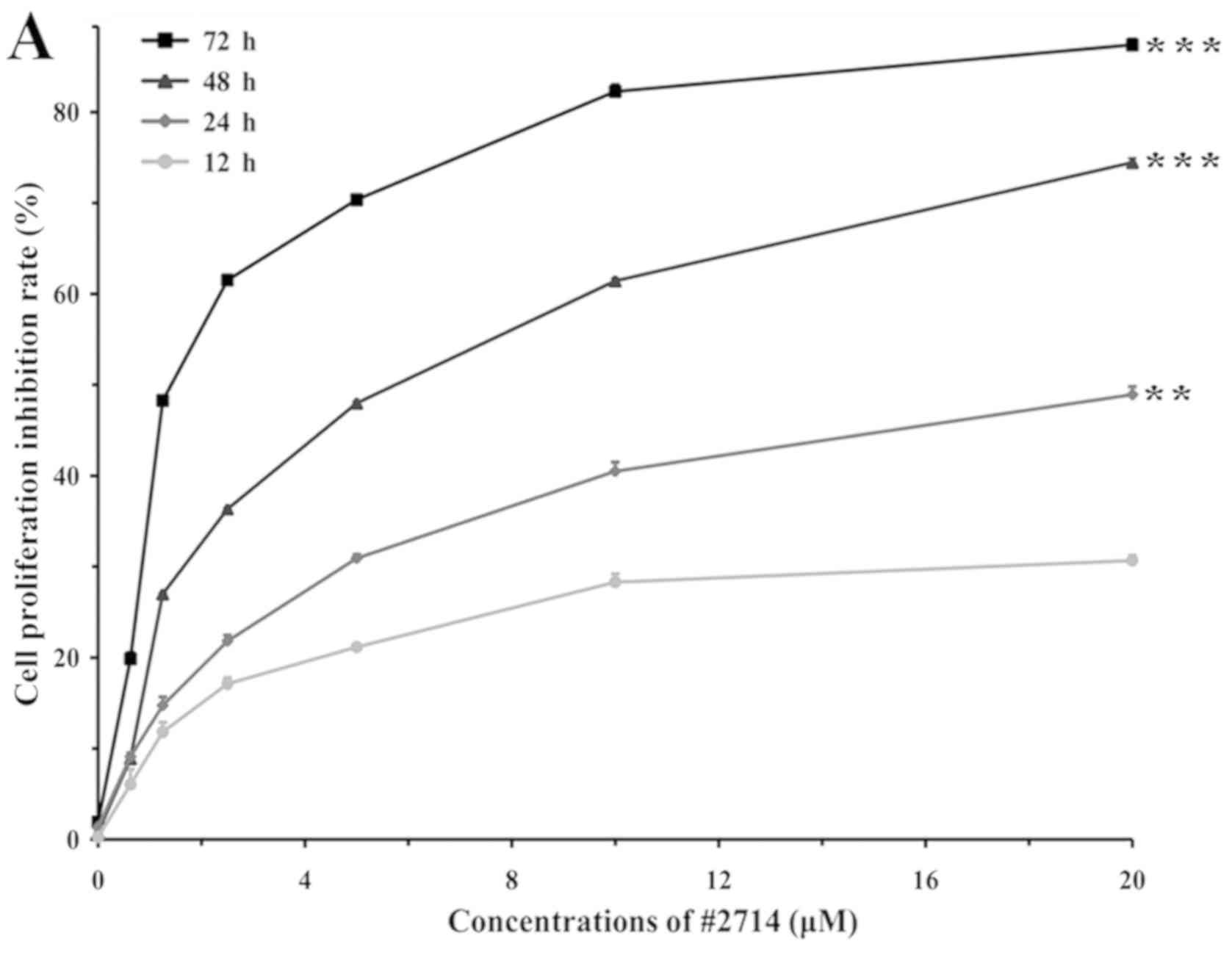
A CCK-8 assay was used to detect the proliferation
of cancerous and non-cancerous cells following treatment with #2714
or vehicle for 48 h. Subsequently, the IC50 values of
#2714 were determined. The present results suggested that #2714
decreased tumor cell proliferation. The IC50 in the
NSCLC cell line SPC-A1 was 5.54 µM after 48 h (Fig. 1A). Furthermore, #2714 exhibited a
favorable safety profile in the non-cancerous embryonic human cell
line 293 (data not shown). Furthermore, the present CCK-8 results
suggested that #2714 inhibited the proliferation of SPC-A1 cells in
a time- and concentration--dependent manner. The IC50
values of #2714 were 18.8 and 1.91 µM after 24 and 72 h of
treatment, respectively.
Treatment with #2714 induces apoptosis
and inhibits proliferation in vitro
Treatment with #2714 for 48 h markedly induced
apoptosis in SPC-A1 cells. The FCM results suggested that the
percentage of apoptotic cells following treatment with #2714
increased in a concentration-dependent manner. The percentage of
apoptotic cells following a 48-h treatment with #2714 at 2.5 µM was
17.83% and it increased to 47.70% at 10 µM (Fig. 1B). Furthermore, the effects of
#2714 on ΔΨm were assessed by measuring the intensity of Rho 123
(Fig. 1C). The percentage of Rho
123-positive cells was 92.2% in the vehicle group; however, it
decreased to 70.4% following a 48 h treatment with #2714 at 10 µM.
The present results suggested that the permeability of the
mitochondrial membrane and ΔΨm were severely impaired following
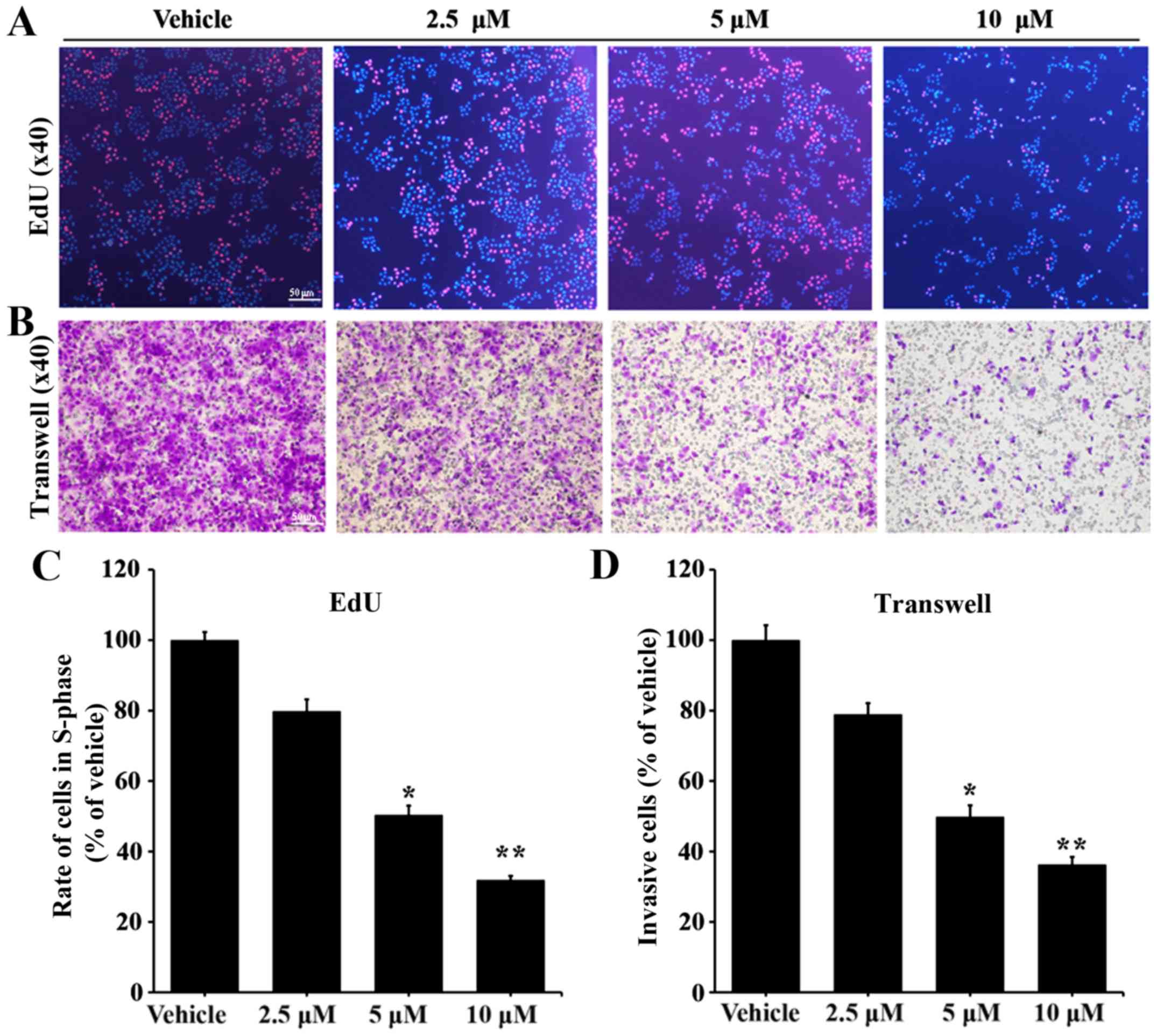
treatment with #2714. The EdU assay results suggested that
treatment with #2714 inhibited SPC-A1 cell proliferation in a
concentration-dependent manner (Fig.
2A). Additionally, the invasive ability of SPC-A1 cells was
decreased following treatment with #2714 and the percentage of
invasive cells was inversely proportional to the concentration of
#2714 (Fig. 2B). The EdU assay
results suggested that the percentage of cells in the S phase
decreased by 20.2–68.08% in a concentration-dependent manner
following treatment with 2.5–10 µM (Fig. 2C). Additionally, the invasion assay
results suggested that the percentage of invasive SPC-A1 cells
decreased by 21.08–63.70% depending on the concentration of #2714
(Fig. 2D).
Effects of #2714 on the Wnt/β-catenin
signaling pathway in vitro
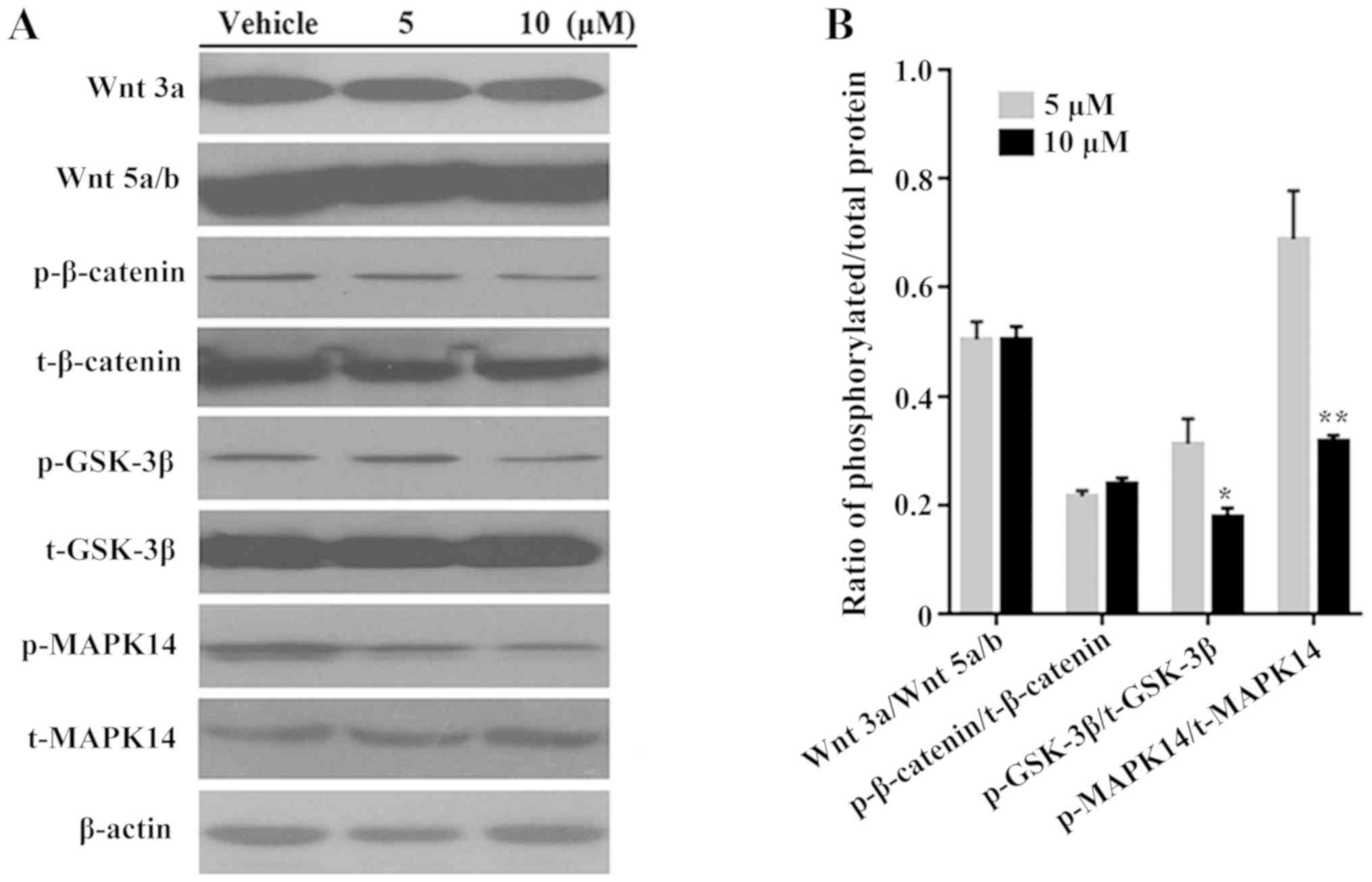
Western blotting was used to investigate the
mechanism underlying the function of #2714. The protein expression
levels of Wnt3a, Wnt5a/b and p-β-catenin were markedly
downregulated following treatment with #2714 compared with vehicle
group; however, there was no significant difference in expression
following treatment with 10 µM compared with 5 µM #2714 (Fig. 3A). Furthermore, treatment with
#2714 at 10 µM significantly downregulated the ratios of p-MAPK14
to total (t-)MAPK14 and of p-GSK-3β to t-GSK-3β in a
concentration-dependent manner in SPC-A1 cells, compared with
treatment at 5 µM (Fig. 3B).
Antitumor activity and safety
evaluation of #2714 in vivo
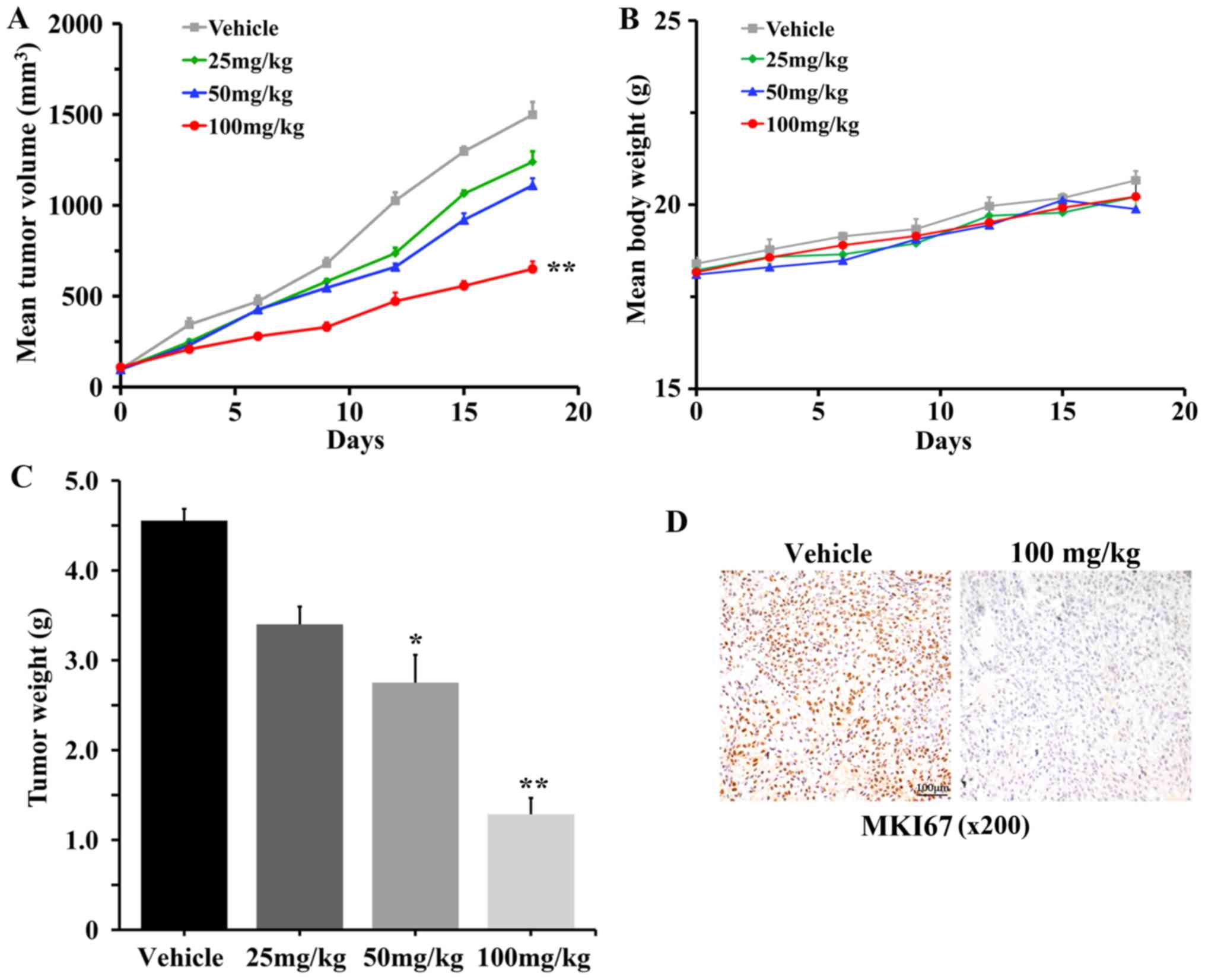
To determine the antitumor effects of #2714 in
vivo, SPC-A1 cells were subcutaneously injected into the right
side of the back of mice to establish a xenograft tumor model.
Treatment with #2714 at 100 mg/kg inhibited tumor growth by 68.56%
compared with the vehicle after 18 days of treatment (Fig. 4A). Notably, body weight was not
altered following treatment with #2714 (Fig. 4B). Furthermore, the tumor weights
were significantly decreased following treatment with #2714
(Fig. 4C). In addition, IHC
analysis suggested that the number of proliferating cells, as
assessed by MKI67 staining, was markedly decreased in tumor tissues
following treatment with #2714 at 100 mg/kg/day for 14 days
(Fig. 4D). Additionally, H&E
staining was conducted to investigate the morphology of the heart,
liver, spleen, lungs and kidneys following treatment with #2714; no
abnormal or pathological alterations were detected (data not
shown).
Discussion
NSCLC accounts for ~85% of all lung cancer cases
worldwide. Lung cancer is the most common type of cancer and the
leading cause of cancer-associated mortality worldwide (1). The 1-year and 5-year survival rates,
and the progression-free survival rate remain poor. The Wnt
signaling pathway was identified to be active in ~50% of human
NSCLC cell lines and primary tumors (17). Notably, suppression of the activity
of the Wnt signaling pathway may inhibit cell proliferation in
human NSCLC (17). Therefore, the
identification of novel compounds able to suppress the Wnt
signaling pathway may facilitate the development of effective NSCLC
targeted therapies. Previous studies have demonstrated that Wnt
ligands and downstream effectors of the Wnt/β-catenin pathway may
serve an important role in the regulation of tumor cell apoptosis
and tumor metastasis (15,19,25).
The Wnt/β-catenin pathway regulates the stability of β-catenin via
its proteasomal degradation (19).
Specifically, extracellular Wnt ligands are able to bind to the
frizzled and LDL receptor related protein 5/6 receptors,
transducing the signal to intracellular disheveled, leading to the
inhibition of the β-catenin destruction complex, thus inducing the
translocation of β-catenin into the nucleus and the transcription
of its target genes (34).
Decreased apoptotic and increased proliferation
rates serve important roles in the pathogenesis, metastasis and
chemoresistance of solid tumors (35). The use of chemically-synthesized
small molecules to increase apoptosis sensitivity and inhibit
proliferation of cancer cells is a promising and effective strategy
to treat various types of cancer (36).
In the present study, the pharmacological activity
of the novel compound #2714 was investigated. In previous studies
investigating the therapeutic targets of YL4073 (7,8,28),
#2714 was synthesized and identified as a promising candidate
compound with anticancer properties (8,30).
Additionally, in our previous studies, a computer-aided drug design
method was used to investigate the molecular docking of #2714, and
#2714 was predicted to be associated with cell apoptosis and
proliferation (7,8,28).
The present results suggested that #2714 significantly inhibited
the proliferation of lung cancer cells via the Wnt/β-catenin
signaling pathway, and induced cell apoptosis via the
mitochondria-dependent pathway without affecting the functions of
non-cancerous cells.
The in vitro CCK-8 experiments suggested that
#2714 exhibited anticancer activities in lung cancer cells with a
low IC50 value. Notably, #2714 inhibited the
proliferation of SPC-A1 cells in a concentration-dependent manner
as assessed by EdU assay, in line with the CCK-8 proliferation
assay results. Furthermore, MAPK14 was previously identified to be
associated with the activity of various small molecular anticancer
agents able to inhibit tumor cell proliferation and to promote
apoptosis (37). In the present
study, treatment with #2714 markedly downregulated the protein
expression levels of components of the Wnt/β-catenin pathway. The
present study aimed to investigate the effects of #2714 on tumor
cell proliferation and on the protein expression level of p-MAPK14
in SPC-A1 cells. The present results suggested that #2714 inhibited
cell proliferation by influencing the Wnt/β-catenin pathway in a
time- and concentration-dependent manner.
Apoptosis induction is one of the most promising and
effective strategies in cancer therapy (35). In the present study, #2714 was
identified to be able to decrease the viability of SPC-A1 cells by
inducing apoptosis, as assessed by FCM. Furthermore, the mechanism
underlying apoptosis induction was investigated in the present
study. Following treatment with #2714, SPC-A1 cells exhibited loss
of ΔΨm. The impairment and loss of ΔΨm are important factors
involved in the intrinsic apoptotic pathway (38). The present result suggested that
the loss of ΔΨm may be associated with the anticancer effects of
#2714.
In the present study, in order to investigate the
pharmacodynamic activity and the mechanism of action of #2714, the
antitumor activity of #2714 was examined using a mouse xenograft
model in vivo. SPC-A1 cells were injected into mice, and
tumor growth of mouse xenograft model were decreased by 68.56%
following treatment with 100 mg/kg/day #2714. Furthermore, IHC
analysis suggested that treatment with #2714 decreased the
proliferation of cancer cells in vivo. Following treatment
with #2714, mouse body weight was not altered, and abnormal
pathological alterations were not observed, suggesting that #2714
may serve as an anticancer agent with a favorable safety
profile.
In conclusion, the present study suggested that
#2714 may represent a novel anticancer compound, exhibiting
antiproliferative effects associated with the suppression of the
Wnt/β-catenin and the mitochondria-mediated apoptosis pathways.
Acknowledgements
Not applicable.
Funding
The present study was funded by The National Natural
Sciences Foundation of China (grant nos. 81402947, 81700763 and
81671327), Natural Sciences Foundation of Anhui Province (grant
nos. 1508085QH162, 1608085QH209 and 1808085MH273) and Postdoctoral
Science Foundation of Anhui Province (grant no. 2017B162).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YX and SW conceived and designed the study. WL, QS
and YX performed the experiments, collected and analyzed the data,
and wrote the original drafts of manuscript. BC and YL made
substantial contributions to the analysis and interpretation of
data. WL, QS, BC, YL and YX revised the manuscript. All authors
read and approved the final version of the manuscript.
Ethics approval and consent to
participate
The animal experiments were performed in compliance
with the ARRIVE guidelines and the Guide for the Care and Use of
Laboratory Animals. The present study was approved by The
Experimental Animal Ethics Committee of Anhui Medical University
(Hefei, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Blandin Knight S, Crosbie PA, Balata H,
Chudziak J, Hussell T and Dive C: Progress and prospects of early
detection in lung cancer. Open Biol. 7:1700702017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Akamatsu H, Mori K, Naito T, Imai H, Ono
A, Shukuya T, Taira T, Kenmotsu H, Murakami H, Endo M, et al:
Progression-free survival at 2 years is a reliable surrogate marker
for the 5-year survival rate in patients with locally advanced
non-small cell lung cancer treated with chemoradiotherapy. BMC
Cancer. 14:182014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Allemani C, Matsuda T, Di Carlo V,
Harewood R, Matz M, Nikšić M, Bonaventure A, Valkov M, Johnson CJ,
Estève J, et al: Global surveillance of trends in cancer survival
2000–14 (CONCORD-3): Analysis of individual records for 37 513 025
patients diagnosed with one of 18 cancers from 322 population-based
registries in 71 countries. Lancet. 391:1023–1075. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fennell DA, Summers Y, Cadranel J, Benepal
T, Christoph DC, Lal R, Das M, Maxwell F, Visseren-Grul C and Ferry
D: Cisplatin in the modern era: The backbone of first-line
chemotherapy for non-small cell lung cancer. Cancer Treat Rev.
44:42–50. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Brückl W, Tufman A and Huber RM: Advanced
non-small cell lung cancer (NSCLC) with activating EGFR mutations:
First-line treatment with afatinib and other EGFR TKIs. Expert Rev
Anticancer Ther. 17:143–155. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lu WJ, Peng W, Sun QQ, Li YH, Chen B, Yu
LT, Xu YZ, Wang SY and Zhao YL: #2714, a novel active inhibitor
with potent arrested G2/M phase and antitumor efficacy in
preclinical models. Cell Death Discov. 4:242018. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu YZ, Zheng RL, Zhou Y, Peng F, Lin HJ,
Bu Q, Mao YQ, Yu LT, Yang L and Yang SY: Small molecular anticancer
agent SKLB703 induces apoptosis in human hepatocellular carcinoma
cells via the mitochondrial apoptotic pathway in vitro and inhibits
tumor growth in vivo. Cancer Lett. 313:44–53. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xiao GH, Jeffers M, Bellacosa A, Mitsuuchi
Y, Vande Woude GF and Testa JR: Anti-apoptotic signaling by
hepatocyte growth factor/Met via the phosphatidylinositol
3-kinase/Akt and mitogen-activated protein kinase pathways. Proc
Natl Acad Sci USA. 98:247–252. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Downward J: Use of RNA interference
libraries to investigate oncogenic signalling in mammalian cells.
Oncogene. 23:8376–8383. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Li P, Nijhawan D, Budihardjo I,
Srinivasula SM, Ahmad M, Alnemri ES and Wang X: Cytochrome c and
dATP-dependent formation of Apaf-1/caspase-9 complex initiates an
apoptotic protease cascade. Cell. 91:479–489. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Printz C: Targeted therapy in lung cancer:
Survival, quality of life improved for some patients. Cancer.
120:2625–2626. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ramalingam SS, Blackhall F, Krzakowski M,
Barrios CH, Park K, Bover I, Seog Heo D, Rosell R, Talbot DC, Frank
R, et al: Randomized phase II study of dacomitinib (PF-00299804),
an irreversible pan-human epidermal growth factor receptor
inhibitor, versus erlotinib in patients with advanced
non-small-cell lung cancer. J Clin Oncol. 30:3337–3344. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hartmann JT, Haap M, Kopp HG and Lipp HP:
Tyrosine kinase inhibitors-a review on pharmacology, metabolism and
side effects. Curr Drug Metab. 10:470–481. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Anastas JN and Moon RT: WNT signalling
pathways as therapeutic targets in cancer. Nat Rev Cancer.
13:11–26. 2012. View
Article : Google Scholar
|
|
16
|
Brown K, Yang P, Salvador D, Kulikauskas
R, Ruoholabaker H, Robitaille AM, Chien AJ, Moon RT and Sherwood V:
WNT/β-catenin signaling regulates mitochondrial activity to alter
the oncogenic potential of melanoma in a PTEN-dependent manner.
Oncogene. 36:3119–3136. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Regmi SC, Park SY, Kim SJ, Banskota S,
Shah S, Kim DH and Kim JA: The anti-tumor activity of Succinyl
Macrolactin A is mediated through the β-catenin destruction complex
via the suppression of tankyrase and PI3K/Akt. PLoS One.
10:e01417532015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhan T, Rindtorff N and Boutros M: Wnt
signaling in cancer. Oncogene. 36:1461–1473. 2018. View Article : Google Scholar
|
|
19
|
Clevers H and Nusse R: Wnt/β-catenin
signaling and disease. Cell. 149:1192–1205. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Akiri G, Cherian MM, Vijayakumar S, Liu G,
Bafico A and Aaronson SA: Wnt pathway aberrations including
autocrine Wnt activation occur at high frequency in human
non-small-cell lung carcinoma. Oncogene. 28:2163–2172. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Kreuzaler P and Watson CJ: Killing a
cancer: What are the alternatives? Nat Rev Cancer. 12:411–424.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shalini S, Dorstyn L, Dawar S and Kumar S:
Old, new and emerging functions of caspases. Cell Death Differ.
22:526–539. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Pradelli LA, Bénéteau M and Ricci JE:
Mitochondrial control of caspase-dependent and -independent cell
death. Cell Mol Life Sci. 67:1589–1597. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Denisenko TV, Budkevich IN and Zhivotovsky
B: Cell death-based treatment of lung adenocarcinoma. Cell Death
Dis. 9:1172018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Brentnall M, Rodriguez-Menocal L, De
Guevara RL, Cepero E and Boise LH: Caspase-9, caspase- 3 and
caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell
Biol. 14:322013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Azmi AS, Wang Z, Philip PA, Mohammad RM
and Sarkar FH: Emerging Bcl-2 inhibitors for the treatment of
cancer. Expert Opin Emerg Drugs. 16:59–70. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wells A, Grahovac J, Wheeler S, Ma B and
Lauffenburger D: Targeting tumor cell motility as a strategy
against invasion and metastasis. Trends Pharmacol Sci. 34:283–289.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Xu YZ, Li YH, Lu WJ, Lu K, Wang CT, Li Y,
Lin HJ, Kan LX, Yang SY, Wang SY and Zhao YL: YL4073 is a potent
autophagy-stimulating antitumor agent in an in vivo model of
Lewis lung carcinoma. Oncol Rep. 35:2081–2088. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu Y, Lu W, Yang P, Peng W, Wang C, Li M,
Li Y, Li G, Meng N, Lin H, et al: A small molecular agent YL529
inhibits VEGF-D-induced lymphangiogenesis and metastasis in
preclinical tumor models in addition to its known antitumor
activities. BMC Cancer. 15:5252015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zheng RL, Zeng XX, He HY, He J, Yang SY,
Yu YT and Yang L: Facile synthesis of
6-Aryl-3-cyanopyridine-2-(1H)-thiones from Aryl Ketones. J
Synthetic Commun. 42:1521–1531. 2012. View Article : Google Scholar
|
|
31
|
Kilkenny C, Browne W, Cuthill IC, Emerson
M and Altman DG; NC3Rs Reporting Guidelines Working Group, : Animal
research: Reporting in vivo experiments: The ARRIVE guidelines. J
Physiol. 160:1577–1579. 2010.
|
|
32
|
Mitrofanova A, Aytes A, Zou M, Shen MM,
Abate-Shen C and Califano A: Predicting drug response in human
prostate cancer from preclinical analysis of in vivo mouse models.
Cell Rep. 12:2060–2071. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Awazu Y, Nakamura K, Mizutani A, Kakoi Y,
Iwata H, Yamasaki S, Miyamoto N, Imamura S, Miki H and Hori A: A
novel inhibitor of c-Met and VEGF receptor tyrosine kinases with a
broad spectrum of in vivo antitumor activities. Mol Cancer Ther.
12:913–924. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Niehrs C: The complex world of WNT
receptor signalling. Nat Rev Mol Cell Biol. 13:767–779. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Villanueva MT: Targeted therapies: Priming
apoptosis. Nat Rev Clin Oncol. 10:672013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Sarosiek KA, Fraser C, Muthalagu N, BholaP
D, Chang WT, McBrayer SK, Cantlon A, Fisch S, Golomb-Mello G, Ryan
JA, et al: Developmental regulation of mitochondrial apoptosis by
c-Myc governs age- and tissue-specific sensitivity to cancer
therapeutics. Cancer Cell. 31:142–156. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Fulda S: The PI3K/Akt/mTOR pathway as
therapeutic target in neuroblastoma. Curr Cancer Drug Targets.
9:729–737. 2014. View Article : Google Scholar
|
|
38
|
Davidson SM, Lopaschuk GD, Spedding M and
Beart PM: Mitochondrial pharmacology: Energy, injury and beyond. Br
J Pharmacol. 171:1795–1797. 2014. View Article : Google Scholar : PubMed/NCBI
|