Introduction
Human gingival fibroblasts (HGFs) are the most
abundant resident cells in the periodontal tissue (1), and serve pivotal roles in the
maintenance of tissues and oral wound healing. When tissue damage
occurs, the wound healing process begins immediately to prevent
further damage or infection. This is a well-coordinated complex
process that involves four sequential overlapping phases, including
hemostasis, inflammation, proliferation and remodeling (2), which are orchestrated by
cross-talking of various cytokines, chemokines, growth factors and
cells that participate in the process (3). Among these, the proliferation phase
is characterized by migration and subsequent proliferation of
fibroblasts, and is important for proper healing and rebuilding of
damaged areas in the wound. Subsequently, fibroblasts secrete a new
collagen matrix and participate in wound closure by formation of
granulation tissue in preparation for the last remodeling phase.
Thus, fibroblasts have crucial roles in wound healing, and
elucidation of their associated characteristics is anticipated to
lead to the development of appropriate therapeutic agents.
Surface pre-reacted glass-ionomer (S-PRG) fillers
contain a stable glass ionomer that is generated by the reaction of
fluoroaluminosilicate glass with polyacrylic acid (4). These fillers have been developed as
GIOMER products for use as dental materials, such as fissure
sealants (5), direct filing
composite resins (6), and tooth
bonding and coating materials (7).
S-PRG fillers are characterized by the ability to release and
recharge fluoride (F), which makes them attractive materials for
the prevention of secondary dental caries (4). Furthermore, S-PRG fillers release
multiple other ions, including aluminum (Al), boron (B), sodium
(Na), silicon (Si), and strontium (Sr), which have been reported to
be effective in the prevention of oral bacteria adhesion (8), suppression of biofilm formation
(9), resistance to acid
demineralization (10) and
protection against dental caries (5,11,12).
Recently, an eluate solution from S-PRG fillers was demonstrated to
have a suppressive effect on periodontal disease in model mice
(13). Thus, the use of S-PRG
fillers containing dental materials may benefit oral health.
However, to the best of our knowledge, no known studies have
examined the influence of the multiple ions released from such
fillers on HGFs.
In the current study, the effects of multiple ions
released from S-PRG fillers on the proliferation, migration and
signaling of the HGF-1 cell line were investigated. This is the
first report to demonstrate the promotion of HGF-1 migration via
the extracellular signal-regulated kinase 1/2 (ERK1/2) signaling
pathway, induced by multiple ions present in the solution eluted
from S-PRG fillers. These results suggested that the combination of
multiple ions promotes cell migration, which assists in oral wound
healing.
Materials and methods
Reagents
The mitogen-activated protein kinase kinase (MEK)
inhibitor U0126 (cat. no. 9903), anti-p44/42 antibody (cat. no.
9102), anti-phosphorylated (p)–p44/42 antibody (cat. no. 9101S),
anti-p38 MAPK antibody (cat. no. 86905), anti-p-p38 MAPK antibody
(cat. no. 9215) and horseradish peroxidase (HRP)-conjugated
anti-rabbit immunoglobulin G secondary antibody (cat. no. 7074)
were purchased from Cell Signaling Technology, Inc. (Danvers, MA,
USA). The p38 mitogen-activated protein kinase (MAPK) inhibitor
SB203580 (cat. no. 152121-47-6) and β-actin antibody (cat. no.
GTX629630) were purchased from Wako Pure Chemical Industries, Ltd.
(Osaka, Japan) and GeneTex, Inc. (Irvine, CA, USA), respectively.
The multiple-ion solution (cat. no. 041402) eluted from S-PRG
fillers was provided by Shofu Dental Corporation (Kyoto, Japan).
For the preparation of the multiple-ion solution, S-PRG filler was
mixed with an equal volume of distilled water by a tumbler mixer at
23°C for 24 h, followed by centrifugation at 3,000 × g and 23°C for
6 h to separate the filler and the liquid. Next, the supernatant
was filtered to remove any residual insoluble material and used as
the S-PRG elute. The multiple-ion solution contained 32.0 ppm Al,
1,488.6 ppm B, 505.0 ppm Na, 12.9 ppm Si, 156.5 ppm Sr and 136.5
ppm F. The S-PRG elute was diluted with a 1:1 mixture of Dulbecco's
Modified Eagle's medium (DMEM) and F12 nutrient mixture (DMEM/F-12,
Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) at various
dilution ratios (0, 1:100, 1:1,000 and 1:10,000).
Cell culture
HGF-1 cells were purchased from ScienCell Research
Laboratories, Inc. (cat. no. 2620; San Diego, CA, USA). The cells
were cultured in DMEM/F-12, supplemented with 10% fetal bovine
serum (FBS; Biowest, Nuaille, France). Cultured fibroblasts were
maintained at 37°C in a humidified atmosphere of 5% CO2.
For the experiments, HGF-1 cells were cultured in DMEM/F-12
containing the multiple ion solution at various dilution
ratios.
Cell proliferation
The influence of the multiple-ion solution on HGF-1
cell proliferation was assessed using a Cell Counting Kit-8 (CCK-8)
purchased from Dojindo Molecular Technologies, Inc. (Kumamoto,
Japan). Briefly, cells were seeded into a 96-well plate at a
density of 1,000 cells/well, and then incubated with various
dilutions of the multiple-ion solution (0, 1:100, 1:1,000 and
1:10,000) for 24, 48 or 72 h. At each time point, 10 µl of solution
from the CCK-8 kit was added to each well and then incubation was
continued in an atmosphere that included 5% CO2 at 37°C
for 1 h. The absorbance at 450 nm was measured using a plate reader
(MultiSkan FC Basic; Thermo Fisher Scientific, Inc.).
Cell proliferation was also assessed using a cell
count method. For this, HGF-1 cells were seeded at a density of
1.0×103 cells/well in a 24-well plate and maintained
with various concentrations of the multiple-ion solution (0, 1:100,
1:1,000 and 1:10,000) for 24, 48 or 72 h. Subsequently, the total
cell number was counted in ten randomly selected fields of view in
a 24-well plate under an inverted microscope (magnification,
×20).
Cell migration
For in vitro cell migration assays, ibidi
Culture-Inserts (ibidi GmbH, Martinsried, Germany) were used in a
35 mm dish. The ibidi Culture-Insert has two cell culture wells,
which are separated by a 500-µm wall. HGF-1 cell suspension (70 µl;
5×105 cells/ml) was added to each well on the two sides
of the culture insert and cultured with DMEM/F-12 supplemented with
10% FBS for 24 h at 37°C. Then, the Culture Insert was gently
removed with sterile tweezers, and cells were cultured with various
concentrations of the multiple-ion solution (0, 1:100, 1:1,000 and
1:10,000) in medium containing 5 or 10% FBS for 12, 16 and 22 h at
37°C. Cell migration was observed and recorded using a Nikon
inverted microscope system (Nikon ECLIPSE TE2000-U; Nikon
Corporation, Tokyo, Japan). To quantify cell migration, the
uncovered area in which no cells were present was measured using
ImageJ 1.48v (National Institutes of Health, Bethesda, MD,
USA).
Furthermore, in order to assess the effect of
inhibitors on cell migration, HGF-1 cells were cultured in the
presence of the multiple-ion solution (diluted to 1:10,000) with or
without 10 µM U0126 (MEK inhibitor) or SB203580 (p38 MAPK
inhibitor). Subsequently, cell migration was examined as mentioned
earlier. An equivalent volume of dimethyl sulfoxide was used as
control treatment.
Western blotting
Following serum deprivation for 1 h, HGF-1 cells
were cultured in the presence of the multiple-ion solution (diluted
to 1:10,000) for various time periods and then washed three times
with ice-cold phosphate-buffer saline containing 1 mM sodium
vanadate (Na3VO4). Next, the cells were
solubilized with lysis buffer (containing 10 mM Tris-HCl, pH 7.4,
150 mM NaCl, 10 mM MgCl2, 0.5% Nonidet P-40, 1 mM
phenylmethylsulfonyl F and 20 units/ml aprotinin). The lysed cells
were centrifuged at 11,177 × g for 5 min at 4°C, and the protein
concentration in each sample was measured using a micro-BCA protein
assay reagent (Pierce; Thermo Fisher Scientific, Inc.). The samples
were then denatured in SDS sample buffer, and 20 µg/lane of lysate
protein was separated via 12% SDS-PAGE. Following separation, the
proteins were transferred onto a PVDF membrane and blocked with 5%
blocking solution (Cell Biolabs, Inc., San Diego, CA, USA) in
PBS-0.05% Tween 20 for 1 h at room temperature (RT). Then, the
blotted membrane was incubated for 1 h at RT using primary
antibodies (anti-p44/42, anti-p–p44/42, anti-p38 MAPK, anti-p-p38
MAPK and anti-β-actin; all 1:1,000 in the blocking solution).
Membranes were subsequently incubated with the HRP-conjugated
secondary antibody (1:2,000) for 1 h at RT. Subsequently, samples
were visualized using an enhanced chemiluminescence kit (GE
Healthcare Life Sciences, Little Chalfont, UK). The blot images
were acquired using an Amersham Imager 600 (GE Healthcare Life
Sciences).
Statistical analysis
All experiments were repeated at least three times.
All values are presented as the mean ± standard error of the mean.
Comparisons between more than two groups were assessed with
analysis of variance, followed by Bonferroni multiple comparisons
test. Comparisons between two groups were conducted with Student's
t-test. Differences with P<0.05 were considered as statistically
significant.
Results
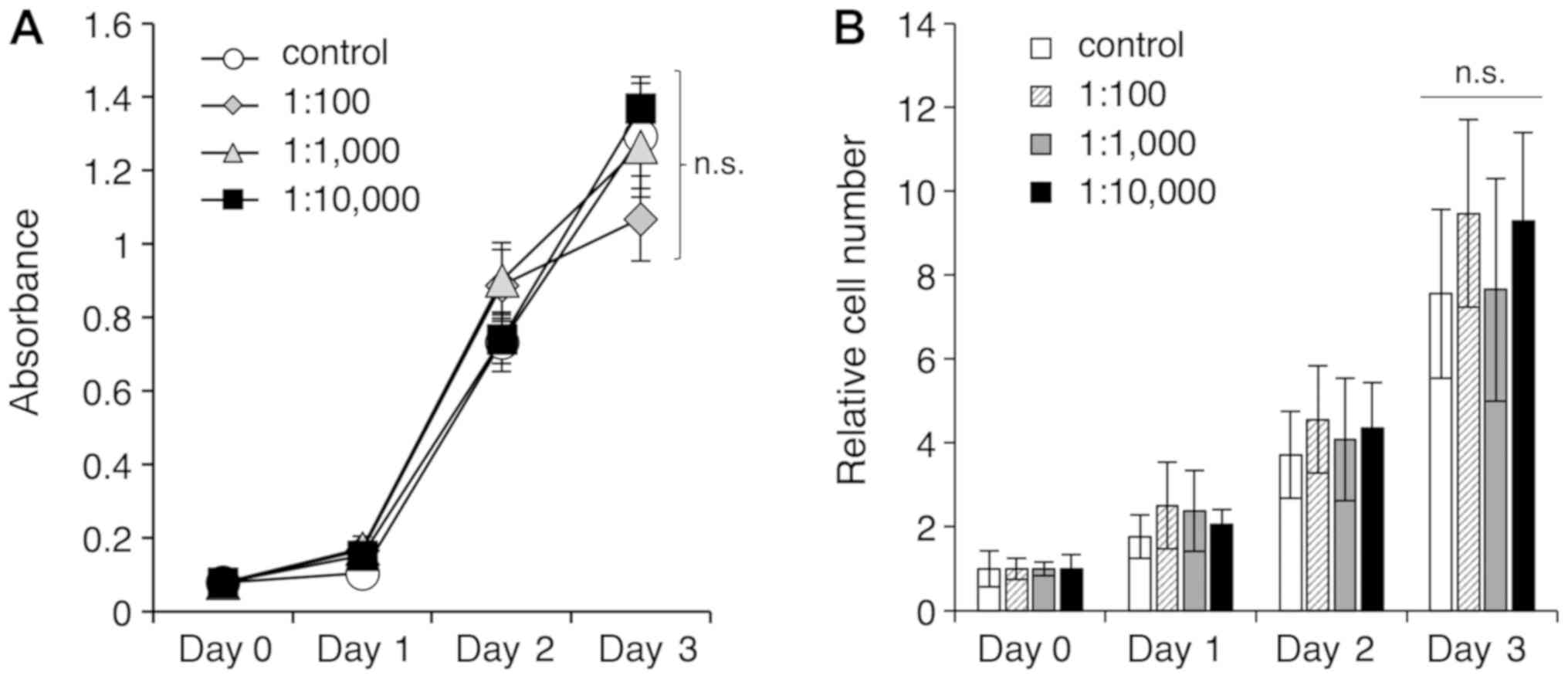
S-PRG filler solution has no effect on
cell proliferation
The solution eluted from the S-PRG filler contained
multiple ions, including F, Al, B, Na, Si, and Sr. Initially, the
study examined whether this multiple-ion solution at various
dilution ratios (1:100, 1:1,000 and 1:10,000) had an effect on the
proliferation of HGF-1 cells after 24, 48, and 72 h using a CCK-8
assay (Fig. 1A) and a cell count
method (Fig. 1B). At the final
time point of 72 h that was examined in the current study, no
significant differences were observed in regard to cell
proliferation between the control and experimental groups (Fig. 1). These results suggested that the
multiple-ion solution eluted from S-PRG fillers did not have an
effect on the proliferation of gingival fibroblasts.
S-PRG filler solution promotes cell
migration
Proper wound healing requires fibroblast migration;
thus, a cell migration assay was conducted in the present study
using culture inserts to examine whether the multiple-ion solution
eluted from the S-PRG filler had an effect on cell migration. HGF-1
cells were cultured with various dilutions of the multiple-ion
solution (1:100, 1:1,000 and 1:10,000) in medium containing 5% or
10% FBS for 12, 16 or 22 h. The results revealed that medium
containing multiple-ion solution at a dilution of 1:10,000 and 5%
FBS exhibited significant promotion of cell migration at 16 h of
incubation, as compared with the control cells (Fig. 2); however, other conditions did not
induce an increase in cell migration. These results suggested that
the multiple-ion solution eluted from the S-PRG filler promoted the
migration of gingival fibroblasts in a concentration and
time-dependent manner.
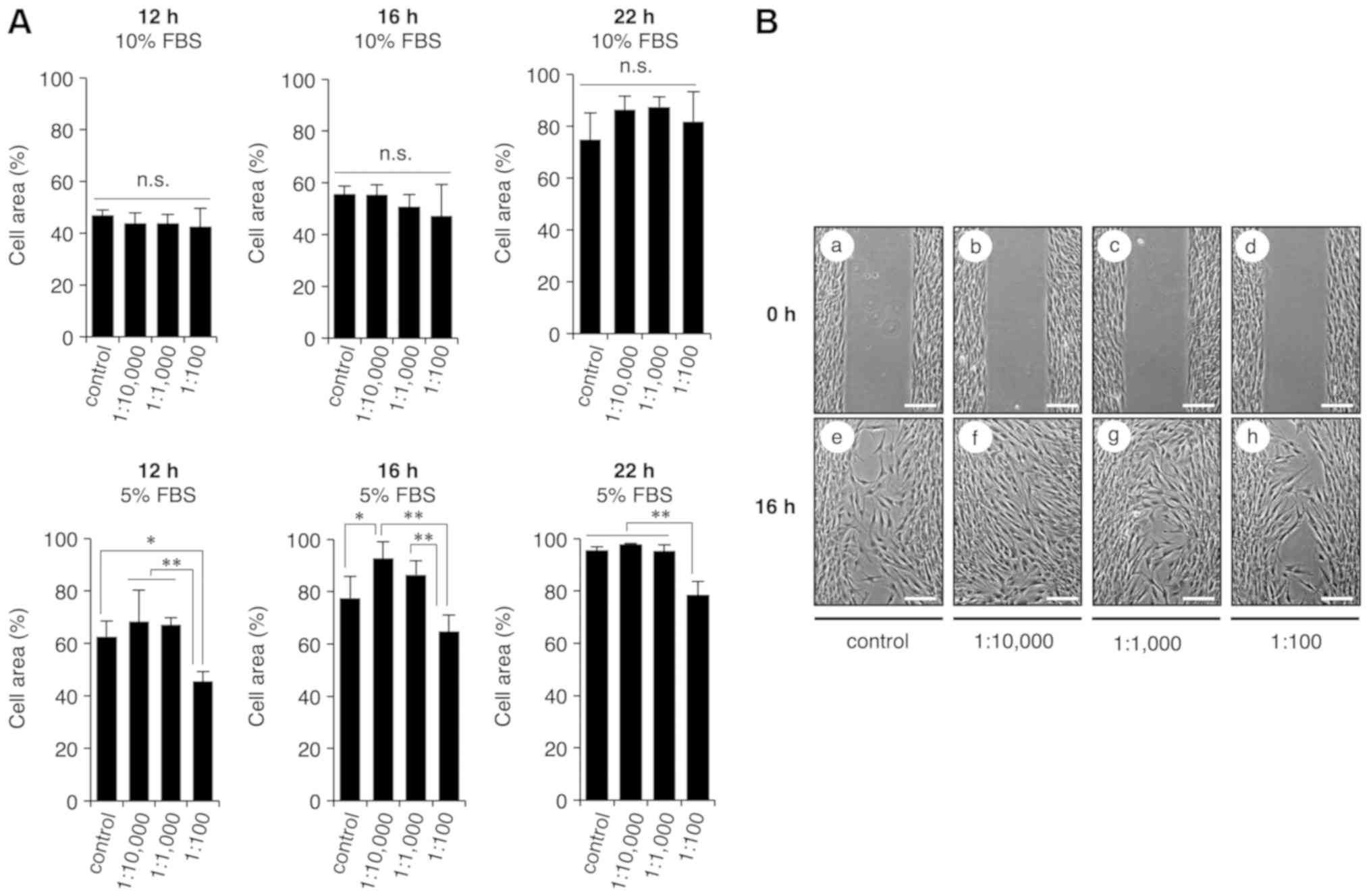 | Figure 2.Eluted solution from S-PRG filler
containing multiple ions promotes HGF-1 migration. HGF-1 cells were
seeded into wells on both sides of a cell culture insert, cultured
for 24 h and then exposed to the multiple-ion solution at various
dilutions (0, 1:100, 1:1,000 and 1:10,000) containing 5 or 10% FBS
at 12, 16 and 22 h for cell migration analysis. (A) Cell area
graphs and (B) images of cell culture (magnification, ×20; scale
bar, 200 µm) at different conditions are shown. Medium containing
the multiple-ion solution diluted at a ratio of 1:10,000 and
supplemented with 5% FBS was observed to significantly promote cell
migration as compared with the control cells at 16 h. To quantify
cell migration, the uncovered area was measured using ImageJ
software. Values are expressed as the mean ± standard error of the
mean (n=4). Repeatability of the results obtained experimentally
was confirmed by performing three independent trials *P<0.05 and
**P<0.01. S-PRG, surface pre-reacted glass-ionomer; HGF, human
gingival fibroblast; n.s., not significant. |
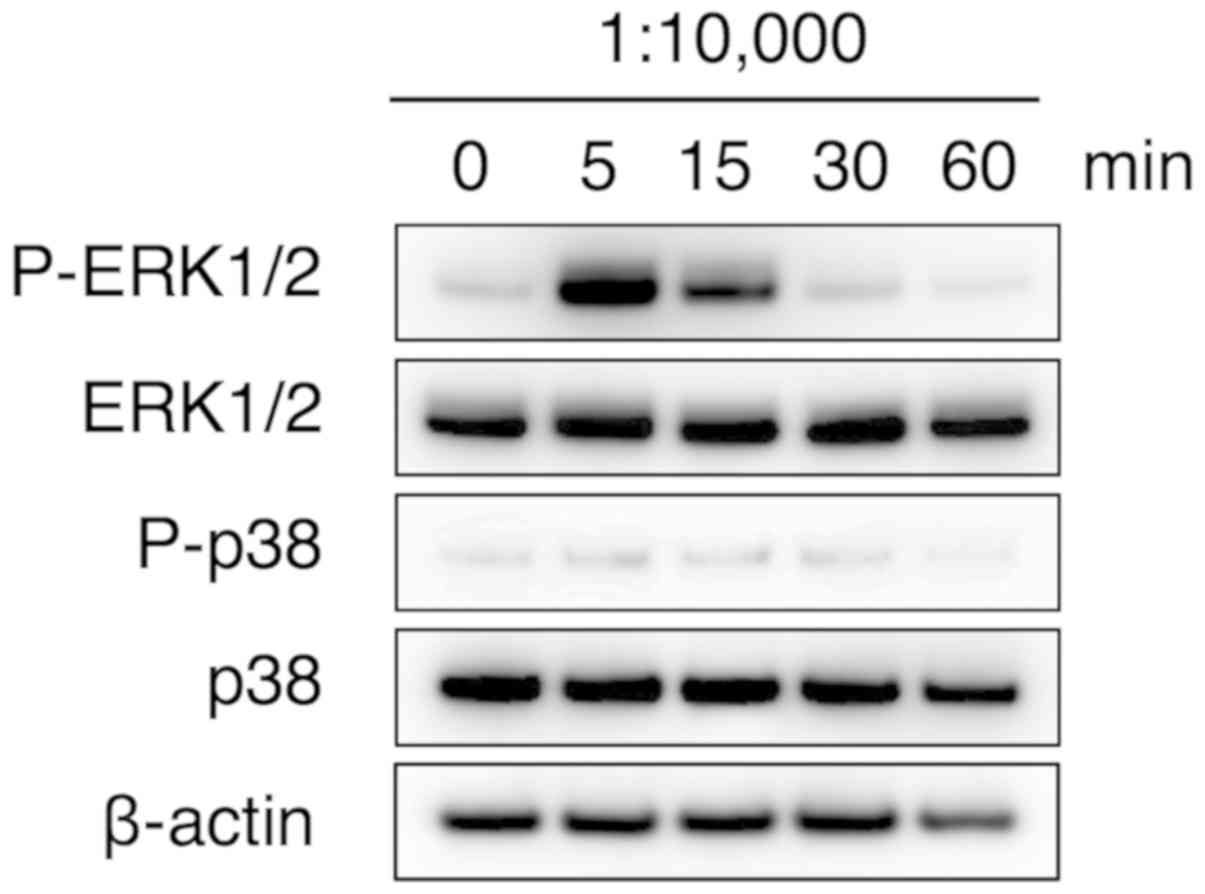
ERK signaling pathway is involved in
fibroblast migration induced by S-PRG filler solution
The effect of the multiple-ion solution eluted from
the S-PRG filler on intracellular signaling was also examined.
MAPKs, including ERK, p38 MAPK and Jun N-terminus kinase (JNK),
have a principal role in cell migration (14). In addition, a previous study has
suggested that sodium fluoride strongly induced the activation of
ERK1/2 and ERK5 signal transduction, rather than that of JNC and
p38 in MC3T3-E1 cells (15).
Therefore, the current study examined whether the multiple
ion-containing solution eluted from the S-PRG filler induced the
phosphorylation of ERK1/2 proteins. Marked ERK 1/2 phosphorylation
was observed at 5 min after stimulation with the diluted
multiple-ion solution, whereas p38 MAPK phosphorylation was not
induced at any of the investigated time points up to 60 min after
treatment (Fig. 3). These results
indicated that activation of ERK1/2, but not p38 MAPK, may be
involved in the promotion of HGF-1 cell migration by the
multiple-ion solution. Furthermore, the study examined whether the
MEK inhibitor U0126 had an effect on cell migration induced by the
S-PRG filler solution. Clear inhibition of cell migration was
observed following the addition of U0126, as compared with the
control (Fig. 4A and B). To
confirm the inhibition of ERK activity by U0126, western blotting
was performed in cells treated with U0126. It was observed that
U0126 evidently inhibited the phosphorylation of ERK1/2 in HGF-1
cells (Fig. 4C). By contrast, the
p38 MAPK inhibitor SB203580 had no effect on cell migration
(Fig. 4D and E) and the
phosphorylation of p38 MAPK (Fig.
4F) in HGF-1 cells. Since phosphorylation of p38 MAPK was not
observed in HGF-1 cells treated with a multiple ion-containing
solution (Fig. 3), p38 MAPK
signaling may not be involved in HGF-1 cell migration. Thus, these
results suggested that the multiple-ion solution may promote the
migration of gingival fibroblasts via an intracellular ERK1/2
signaling pathway.
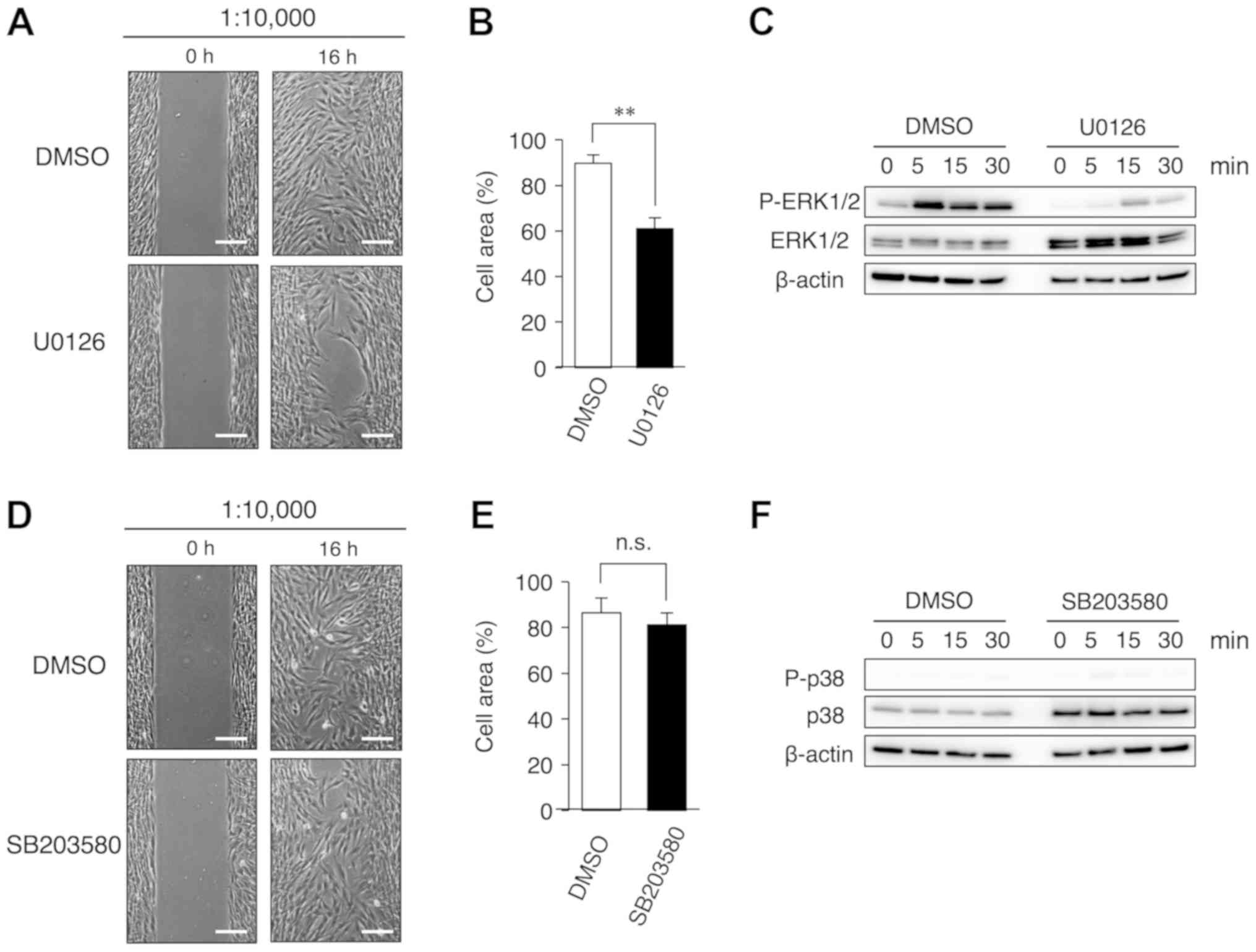 | Figure 4.Inhibition of cell migration is
induced by co-incubation with a MEK inhibitor and multiple-ion
solution eluted from the S-PRG filler. HGF-1 cells were cultured in
the presence of solution diluted to 1:10,000, and with or without
10 µM U0126 (a MEK inhibitor) or SB203580 (a p38 MAPK inhibitor)
for 16 h. An equal volume of DMSO was added to the control cells.
(A) Cell area percentage, (B) cell culture images (magnification,
×20; scale bar, 200 µm), and (C) western blot results following
incubation with U0126 are shown. (D) Cell area percentage, (E) cell
culture images (magnification, ×20; scale bar, 200 µm), and (F)
western blot results following incubation with SB203580 are
displayed. To quantify cell migration, the uncovered area was
measured using the ImageJ software. The time course of ERK1/2 and
p38 phosphorylation in the presence of U0126 and SB203580,
respectively, was analyzed by western blotting. Values are
presented as the mean ± standard error of the mean (n=4).
Repeatability of the results obtained experimentally was confirmed
by performing three independent trials. **P<0.01. S-PRG, surface
pre-reacted glass-ionomer; HGF, human gingival fibroblast; ERK1/2,
extracellular signal-regulated kinase 1/2; p-, phosphorylated;
DMSO, dimethyl sulfoxide; n.s., not significant. |
Discussion
The oral cavity functions as an entry way for the
digestive and respiratory systems, in addition to its important
roles in daily activities, such as eating, speaking and breathing;
thus, it is often exposed to various stimuli or injuries from
contact with external factors. Gingival fibroblasts serve crucial
roles in maintaining tissue homeostasis and recovery to a normal
condition following acute inflammation, migrating to the wound site
and proliferating in order to reconstitute connective tissue,
events that are regulated by various growth factors and cytokines,
including epidermal growth factor (16), basic fibroblast growth factor
(bFGF) (17), platelet-derived
growth factor (PDGF) (18) and
transforming growth factor-beta (TGFβ) (19). The molecular mechanisms of these
events are complex and have not been fully elucidated.
In the present study, it was observed that the
eluted S-PRG solution containing multiple ions promoted the
migration, but not the proliferation, of HGF-1 cells. S-PRG
fillers, composed of a powdery reaction product containing
polyalkenoic acid and fluorine-containing glass, are characterized
by sustained F release and have cariostatic properties (4). In fact, a previous study reported
that an S-PRG filler containing flowable resin had a high level of
F release as compared with other flowable resins tested (20). Furthermore, S-PRG filler is
produced by an acid-base reaction between acid-reactive glass and
polyacids in the presence of water (4), and has properties similar to glass
ionomer cement (21). Given these
properties, S-PRG filler can release Al, B, Na, Si and Sr ions, in
addition to F ion, and therefore the solution eluted from this
filler contains multiple ions. According to the results of the
present study, it is thus suggested that the combination of these
multiple ions promotes fibroblast migration.
Extracellular ions are extracellular environment
factors that have great effects on the physiological activity of
cells (22–30). The respective influence of
individual ions on cellular activity results in different effects
depending on the conditions present, including concentration and
combination with other ions. For instance, although Al is known to
be a toxic agent (22), a previous
study reported that micromolar concentrations of Al ions had a
direct effect on osteoblasts, stimulating their proliferation and
differentiation (23). Conversely,
another study observed that low concentrations of Al ions induced
no effects on osteoblast behaviors; however, in combination with
titanium ions (Ti), Al enhanced the deleterious effects of Ti on
osteoblast differentiation (24).
Furthermore, long-term exposure to F ions (>1 mM) inhibited the
proliferation of L-929 fibroblasts, whereas short-term exposure
stimulated the cell proliferation, with the stimulatory effect
further enhanced by 1 µM Al ions (29). A low-dose Sr was also found to
stimulate osteogenic differentiation, while higher doses induced
apoptosis of human adipose-derived stem cells (30). Thus, extracellular ions have
important functions in supporting fundamental cell activities and
cell death, and a change in extracellular ion composition can have
a great influence, either positive or negative, on affected
cells.
ERK1/2, a member of the MAPK family, is a dynamic
cell signaling pathway that functions with various cell responses,
including proliferation, migration, differentiation and death
(31). In the current study, the
examined solution eluted from S-PRG filler promoted the
phosphorylation of ERK1/2, but not of p38 MAPK. Furthermore, U0126
(a MEK inhibitor), but not SB203580 (a p38 MAPK inhibitor),
inhibited cell migration induced by the solution. Several studies
have indicated that the p38 inhibitors SB203580 and SB202190 also
inhibited cell migration that was induced by pigment
epithelium-derived factor PDGF, TGFβ and IL-1β (32–34).
These results suggest that p38 is involved in cell migration, while
it also has a principal role in growth factor or cytokine-induced
cell migration. Although further studies are required to identify
the molecular mechanism regulating cell migration in HGF-1, these
results indicated that the activation of ERK signaling may be
responsible for gingival fibroblast migration induced by the
multiple ions in the solution examined in the present study.
Dental restorative materials are designed to have
specific characteristics based on the biological, chemical and
mechanical properties of their components, and must not trigger
inflammation, toxic reactions or allergenic symptoms. However,
those materials in fact often cause allergic reactions, with
research in recent decades focusing on dental metals in particular.
The intraoral environment is susceptible to sudden changes in
temperature and pH caused by ingested food or drink, and is
subjected to mechanical or electronic forces caused by occlusion,
which have significant influence on the corrosion properties of
dental metals (35). Furthermore,
oxygen and chloride ions in saliva are involved in corrosion caused
by chemical processes (36). The
first reported dental metal allergy was in relation to mercury as
part of amalgam dental materials, which was demonstrated to cause
stomatitis and dermatitis (37).
Thereafter, nickel, chromium, palladium and cobalt, commonly used
as dental materials, were also reported to have associations with
dental metal allergies (38).
Resins are frequently used as an alternative dental material
instead of metals, including methyl methacrylate, 2-hydroxyethyl
methacrylate, ethylene glycol dimethacrylate and triethylene glycol
dimethacrylate; however, these can also be a cause of allergies
(39,40). Allergic reactions caused by resins
are mainly considered to be associated with an unreacted residual
monomer (41).
For effective use, biomaterials should be harmless
to the body; thus, the concept of the biocompatibility of
biomaterials used in regenerative medicine (42), associated with proper response by
surrounding tissues, has recently become an important issue. Second
generation biomaterials are characterized by their resorbable or
biological activity, while third generation materials possess both
of these characteristics (43).
For instance, hydroxyapatite, a widely-used bone graft biomaterial
with excellent biocompatibility and osteoconductive properties,
promotes self-healing processes (44). Thus, biomaterials are required to
have bioactive properties to induce and regulate specific cellular
responses at the molecular level, indicating that biocompatibility
is an essential concept for the design of dental materials.
A limitation of the current study is that only in
vitro experiments were conducted to evaluate the effect of the
multiple ion-containing solution eluted from the S-PRG filler.
Therefore, it is possible that a multiple-ion solution at a higher
dilution than 1:10,000 or a different combination of ions may have
a greater effect on cell migration. Furthermore, the current study
did not focus on which ion eluted from filler affected HGF-1 cell
migration. Mechanistic analysis to address whether the eluted
multiple ions had a direct or indirect effect on cell migration is
also lacking. The individual ion function and different combination
of ions should be assessed in further studies.
In conclusion, the results of the present study
demonstrated that the multiple ion-containing solution eluted from
S-PRG filler promoted the migration of HGF-1 cells via the ERK1/2
signaling pathway, potentially promoting oral mucosa wound healing.
These findings suggested that the application of such an S-PRG
filler may contribute not only to the prevention of dental caries,
but also to homeostasis of the oral mucosa. In addition, the
present study provides useful information for the development of
novel therapeutic drugs for oral diseases with materials composed
of multiple ions.
Acknowledgements
Not applicable.
Funding
This study was supported by grants-in-aid (grant
nos. 17H04414 and 15K11368) from the Ministry of Education,
Science, and Culture of Japan.
Availability of data and materials
The datasets generated and analyzed in this study
are available from the corresponding author upon reasonable
request.
Authors' contributions
TI conceived and designed the experiments, analyzed
the data, and drafted the manuscript. KYU, YA, KK, AS, HN, AM, RK,
KI, TK and TH performed the experiments, analyzed the data,
prepared the figures and reviewed cited literature. AY and SF
analyzed the data and reviewed drafts of the manuscript. All
authors have read and approved the final version of the
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ara T, Kurata K, Hirai K, Uchihashi T,
Uematsu T, Imamura Y, Furusawa K, Kurihara S and Wang PL: Human
gingival fibroblasts are critical in sustaining inflammation in
periodontal disease. J Periodontal Res. 44:21–27. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Soliman AM, Das S, Abd Ghafar N and Teoh
SL: Role of MicroRNA in proliferation phase of wound healing. Front
Genet. 9:382018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Heun Y, Pogoda K, Anton M, Pircher J,
Pfeifer A, Woernle M, Ribeiro A, Kameritsch P, Mykhaylyk O, Plank
C, et al: HIF-1α dependent wound healing angiogenesis in vivo can
be controlled by site-specific lentiviral magnetic targeting of
SHP-2. Mol Ther. 25:1616–1627. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ikemura K, Tay FR, Endo T and Pashley DH:
A review of chemical-approach and ultramorphological studies on the
development of fluoride-releasing dental adhesives comprising new
pre-reacted glass ionomer (PRG) fillers. Dent Mater J. 27:315–339.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Salmerón-Valdés EN, Scougall-Vilchis RJ,
Alanis-Tavira J and Morales-Luckie RA: Comparative study of
fluoride released and recharged from conventional pit and fissure
sealants versus surface prereacted glass ionomer technology. J
Conserv Dent. 19:41–45. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Itota T, Carrick TE, Yoshiyama M and
McCabe JF: Fluoride release and recharge in giomer, compomer and
resin composite. Dent Mater. 20:789–795. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Alsayed EZ, Hariri I, Nakashima S, Shimada
Y, Bakhsh TA, Tagami J and Sadr A: Effects of coating materials on
nanoindentation hardness of enamel and adjacent areas. Dent Mater.
32:807–816. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nomura R, Morita Y, Matayoshi S and Nakano
K: Inhibitory effect of surface pre-reacted glass-ionomer (S-PRG)
eluate against adhesion and colonization by Streptococcus
mutans. Sci Rep. 8:50562018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Tsutsumi C, Takakuda K and Wakabayashi N:
Reduction of Candida biofilm adhesion by incorporation of
prereacted glass ionomer filler in denture base resin. J Dent.
44:37–43. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kawasaki K and Kambara M: Effects of
ion-releasing tooth-coating material on demineralization of bovine
tooth enamel. Int J Dent. 2014:4631492014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hashimura T, Yamada A, Iwamoto T, Arakaki
M, Saito K and Fukumoto S: Application of a tooth-surface coating
material to teeth with discolored crowns. Pediat Dent J. 23:44–50.
2013. View Article : Google Scholar
|
|
12
|
Hirayama K, Hanada T, Hino R, Saito K,
Kobayashi M, Arakaki M, Chiba Y, Nakamura N, Sakurai T, Iwamoto T,
et al: Material properties on enamel and fissure of surface
pre-reacted glass-ionomer filler-containing dental sealant. Pediat
Dent J. 28:87–95. 2018. View Article : Google Scholar
|
|
13
|
Iwamatsu-Kobayashi Y, Abe S, Fujieda Y,
Orimoto A, Kanehira M, Handa K, Venkataiah VS, Zou W, Ishikawa M
and Saito M: Metal ions from S-PRG filler have the potential to
prevent periodontal disease. Clin Exp Dent Res. 3:126–133. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang C, Jacobson K and Schaller MD: MAP
kinases and cell migration. J Cell Sci. 117:4619–4628. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Iwatsuki M and Matsuoka M:
Fluoride-induced c-Fos expression in MC3T3-E1 osteoblastic cells.
Toxicol Mech Methods. 26:132–138. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ware MF, Wells A and Lauffenburger DA:
Epidermal growth factor alters fibroblast migration speed and
directional persistence reciprocally and in a matrix-dependent
manner. J Cell Sci. 111:2423–2432. 1998.PubMed/NCBI
|
|
17
|
Tan SS, Yeo XY, Liang ZC, Sethi SK and Tay
SSW: Stromal vascular fraction promotes fibroblast migration and
cellular viability in a hyperglycemic microenvironment through
up-regulation of wound healing cytokines. Exp Mol Pathol.
104:250–255. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Suetsugu S, Yamazaki D, Kurisu S and
Takenawa T: Differential roles of WAVE1 and WAVE2 in dorsal and
peripheral ruffle formation for fibroblast cell migration. Dev
Cell. 5:595–609. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Acharya PS, Majumdar S, Jacob M, Hayden J,
Mrass P, Weninger W, Assoian RK and Puré E: Fibroblast migration is
mediated by CD44-dependent TGF beta activation. J Cell Sci.
121:1393–1402. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Nakamura N, Yamada A, Iwamoto T, Arakaki
M, Tanaka K, Aizawa S, Nonaka K and Fukumoto S: Two-year clinical
evaluation of flowable composite resin containing pre-reacted
glass-ionomer. Pediat Dent J. 19:89–97. 2008. View Article : Google Scholar
|
|
21
|
Billington RW, Williams JA and Pearson GJ:
Ion processes in glass ionomer cements. J Dent. 34:544–555. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yokel RA: The toxicology of aluminum in
the brain: A review. Neurotoxicology. 21:813–828. 2000.PubMed/NCBI
|
|
23
|
Lau KH, Yoo A and Wang SP: Aluminum
stimulates the proliferation and differentiation of osteoblasts in
vitro by a mechanism that is different from fluoride. Mol Cell
Biochem. 105:93–105. 1991.PubMed/NCBI
|
|
24
|
Saldaña L, Barranco V, García-Alonso MC,
Vallés G, Escudero ML, Munuera L and Vilaboa N: Concentration-
dependent effects of titanium and aluminium ions released from
thermally oxidized Ti6Al4V alloy on human osteoblasts. J Biomed
Mater Res A. 77:220–229. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Benderdour M, Hess K, Dzondo-Gadet M,
Nabet P, Belleville F and Dousset B: Boron modulates extracellular
matrix and TNF alpha synthesis in human fibroblasts. Biochem
Biophys Res Commun. 246:746–751. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nzietchueng RM, Dousset B, Franck P,
Benderdour M, Nabet P and Hess K: Mechanisms implicated in the
effects of boron on wound healing. J Trace Elem Med Biol.
16:239–244. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Denker SP and Barber DL: Cell migration
requires both ion translocation and cytoskeletal anchoring by the
Na-H exchanger NHE1. J Cell Biol. 159:1087–1096. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Quignard S, Coradin T, Powell JJ and
Jugdaohsingh R: Silica nanoparticles as sources of silicic acid
favoring wound healing in vitro. Colloids Surf B Biointerfaces.
155:530–537. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kawase T and Suzuki A: Studies on the
transmembrane migration of fluoride and its effects on
proliferation of L-929 fibroblasts (L cells) in vitro. Arch Oral
Biol. 34:103–107. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Aimaiti A, Maimaitiyiming A, Boyong X, Aji
K, Li C and Cui L: Low-dose strontium stimulates osteogenesis but
high-dose doses cause apoptosis in human adipose-derived stem cells
via regulation of the ERK1/2 signaling pathway. Stem Cell Res Ther.
8:2822017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Murphy LO and Blenis J: MAPK signal
specificity: The right place at the right time. Trends Biochem Sci.
31:268–275. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Hedges JC, Dechert MA, Yamboliev IA,
Martin JL, Hickey E, Weber LA and Gerthoffer WT: A role for
p38(MAPK)/HSP27 pathway in smooth muscle cell migration. J Biol
Chem. 274:24211–24219. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kotlyarov A, Yannoni Y, Fritz S, Laass K,
Telliez JB, Pitman D, Lin LL and Gaestel M: Distinct cellular
functions of MK2. Mol Cell Biol. 22:4827–4835. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Konson A, Pradeep S, D'Acunto CW and Seger
R: Pigment epithelium-derived factor and its phosphomimetic mutant
induce JNK-dependent apoptosis and P38-mediated migration arrest.
Cell Physiol Biochem. 49:512–529. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kedici SP, Aksüt AA, Kílíçarslan MA,
Bayramoğlu G and Gökdemir K: Corrosion behaviour of dental metals
and alloys in different media. J Oral Rehabil. 25:800–808. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Porcayo-Calderon J, Casales-Diaz M,
Salinas-Bravo VM and Martinez-Gomez L: Corrosion performance of
Fe-Cr-Ni alloys in artificial saliva and mouthwash solution.
Bioinorg Chem Appl. 2015:9308022015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Syed M, Chopra R and Sachdev V: Allergic
reactions to dental materials-a systematic review. J Clin Diagn
Res. 9:ZE04–ZE09. 2015.PubMed/NCBI
|
|
38
|
Zhang X, Wei LC, Wu B, Yu LY, Wang XP and
Liu Y: A comparative analysis of metal allergens associated with
dental alloy prostheses and the expression of HLA-DR in gingival
tissue. Mol Med Rep. 13:91–98. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Leggat PA and Kedjarune U: Toxicity of
methyl methacrylate in dentistry. Int Dent J. 53:126–131. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Marquardt W, Seiss M, Hickel R and Reichl
FX: Volatile methacrylates in dental practices. J Adhes Dent.
11:101–107. 2009.PubMed/NCBI
|
|
41
|
Nik TH, Shahroudi AS, Eraghihzadeh Z and
Aghajani F: Comparison of residual monomer loss from cold-cure
orthodontic acrylic resins processed by different polymerization
techniques. J Orthod. 41:30–37. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Williams DF: There is no such thing as a
biocompatible material. Biomaterials. 35:10009–10014. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hench LL and Polak JM: Third-generation
biomedical materials. Science. 295:1014–1017. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Oliveira HL, Da Rosa WLO, Cuevas-Suárez
CE, Carreño NLV, da Silva AF, Guim TN, Dellagostin OA and Piva E:
Histological evaluation of bone repair with hydroxyapatite: A
systematic review. Calcif Tissue Int. 101:341–354. 2017. View Article : Google Scholar : PubMed/NCBI
|