Introduction
Advances in perinatal medicine and neonatal
intensive care have increased the survival rate of premature
infants, and the incidence of respiratory distress syndrome (RDS)
and bronchopulmonary dysplasia (BPD). A total of 80,000 cases of
RDS have been reported in the United States per year, of which
8,500 cases are cases of neonatal mortality (1). The incidence of BPD is estimated to
be 12–32% in preterm infants born at <32 weeks of gestation, and
even up to 50% in infants with very low birth weight (<1,000 g)
and preterm infants with gestational age <28 weeks (2,3). At
lower gestational ages, the lungs are less developed, and incidence
of RDS and BPD is higher (4).
Although RDS, BPD and other neonatal pulmonary diseases have been
studied extensively, these studies have primarily focused on the
pathogenesis, progression and prognoses of the diseases examined. A
limited number of studies have been associated with the common
physiological basis of immature pulmonary development. Thus,
investigating of the potential mechanism underlying lung
development is imperative to provide important insights for the
prevention and control of lung developmental diseases.
MicroRNAs (miRNAs/miRs) are a group of noncoding
RNAs (typically 21–24 nucleotides) that have an important role in
regulating the expression of target genes at the
post-transcriptional level (5,6).
miRNA functions have been recently identified to be associated with
cell development, cell proliferation, signal transduction, stem
cell differentiation and tumor progression (7). To date, a limited number of studies
have reported whether miRNAs are involved in lung development.
Bhaskaran et al (8)
reported that overexpression of miR-127 in fetal lung organ culture
significantly decreased the terminal bud count, while increasing
terminal and internal bud sizes, and causing unevenness in bud
sizes, which suggested improper development. Carraro et al
(9) reported that miR-142-3p
balances proliferation and differentiation of mesenchymal cells
during lung development. miR-124 levels were also reported to be
downregulated during the later stages of fetal lung development and
these alterations could inhibit fetal lung epithelial maturation
(10). Despite these promising
findings, knowledge is limited regarding the regulation of miRNA
expression and their potential role in lung pathophysiology. In our
previous study, an miRNA microarray was used to compare the
expression levels of miRNAs in venous blood between infants with
RDS and infants without RDS. miR-431 was differentially expressed
between the two groups, which indicated that it may be associated
with occurrence of RDS (11). In
addition, we revealed that the expression levels of miR-431 were
downregulated gradually following rat lung tissue development as
demonstrated by miRNA microarrays (12). Taken collectively, it was
hypothesized that miR-431 has an important role in lung tissue
development.
In the present study, bioinformatic analysis was
performed and demonstrated that SMAD4 (also termed drosophila
mothers against decapentaplegic protein 4) is one of the target
genes of miR-431. The transforming growth factor-β (TGF-β)/SMAD
pathway has been characterized as an important signaling axis that
regulates lung development (13).
In addition, SMAD4 is an important transcription factor required
for the regulation of the TGF-β pathway throughout lung tissue
development (14).
Lung tissue development is a continuous and gradual
process. The development of the lung tissues in Sprague-Dawley rats
can be divided into the following five stages: embryonic stage
(E0-E13), the glandular stage (E13-E18), the canalicular stage
(E18-E20), the saccular stage (E20 to full term) and the alveolar
stage (after birth) (15,16). It is important to note that the
onset of surfactant synthesis and microvascular development in the
early saccular stage is necessary for lung function at birth. The
lack of surfactant proteins (SPs) leads to RDS, and it is also
considered a risk factor of BPD (16,17).
Among these stages, E19, E21 and P3 time points representing the
canalicular, the saccular and the alveolar phases of lung
development, respectively, were selected, and the expression and
potential regulation of miR-431 in rat lung development by
targeting SMAD4 was explored at each stage.
Materials and methods
Rats
The animal procedures in this study were approved by
the Nanjing Medical University Animal Care and Use Committee
(Nanjing, China). Healthy adult Sprague-Dawley rats (9 female and 9
male; age, 2 months; weight, 350–450 g) were purchased from The
Experimental Animal Center of Nanjing Medical University. Animals
were housed in a specific pathogen-free animal environment with the
temperature between 18–23°C and humidity between 60–65%. Rats had
access to food and water ad libitum. Whole lungs were isolated from
nine rat fetuses on gestational days 19 and 21 (E19 and E21) and
from nine 3-day-old rats (weight, 9.361±0.742 g; postnatal day 3;
P3). The three time points represent the key three stages of
mammalian lung development [the canalicular stage (E18-E20), the
saccular stage (E20 to full term), and the alveolar stage], and
were designated as groups S1 (E19), S2 (E21) and S3 (P3). For timed
pregnancy, the day on which vaginal plugs were discovered was
denoted as gestational day 1. For to obtain fetal lungs, pregnant
Sprague-Dawley rats were sacrificed using CO2. Fetuses
were removed from the uterus, and the lungs were isolated from
these fetuses without the surrounding tissues. For pup lungs, male
Sprague-Dawley rats were sacrificed by cervical dislocation before
isolation of the lungs. The left lungs were used for light
microscopy observation, miRNA and mRNA detection, while the right
lungs were used for electron microscopy observation, fluorescence
in situ hybridization (FISH) and western blot analysis.
Light microscopy
After isolation, a randomly selected fetal lung was
retained for histological observation. Left lung tissue specimens
were fixed in formalin at 37°C for 24 h, dehydrated and embedded in
paraffin. Continuous sections (thickness, 3 µm) were stained with
hematoxylin and eosin for 10 min at room temperature for subsequent
morphological observation. Morphological changes were evaluated
using an optical microscope (×20 or ×40 magnification; DM2500;
Leica Microsystems GmbH, Wetzlar, Germany). Three sections from
each mouse were analyzed. In total, ten fields from each section
were randomly selected for analysis.
Electron microscopy
The lobe of right lung was cut and divided into
several sections, with the size of 0.1×0.1×0.1 cm, then fixed in
2.5% glutaraldehyde at 4°C for 4 h, dehydrated, embedded in epoxy
resin, and cut into ultrathin sections (thickness of sections,
50–80 nm) for analysis of the changes of organelles by transmission
electron microscopy (JEM-1010; Jeol, Ltd., Tokyo, Japan).
FISH
FISH was performed using 5′ fluorescein amidite
(FAM)-labeled probes (TsingKe Biological Technology, Beijing,
China) for miRNA-431. The lung tissue specimens were fixed in 4%
paraformaldehyde (prepared from diethyl pyrocarbonate) for 1–2 h at
37°C. Fixed specimens were dehydrated in 15% sucrose solution for 8
h and in 30% sucrose solution overnight at room temperature.
Samples were frozen at −30°C and cut into 4-µm-thick slides, then
dried at room temperature. The slides were again fixed in 4%
paraformaldehyde at 37°C for 10 min, and rinsed in PBS (pH 7.4)
three times in rocking device for decoloring, for 5 min each time.
According to the characteristics of rat lung tissue and index, the
slides were digested in proteinase K (20 µg/ml; cat. no. G1205;
Servicebio Inc., Wuhan, China) for 8 min at 37°C, and then washed
in PBS three times for 15 min. The slides were pre-hybridized in
hybridization buffer (Abnova, Taipei, Taiwan) for 1 h at 37°C in a
humidified chamber. The pre-hybridization buffer was removed and
the FAM-labeled probes for rno-mir-431 was added at a concentration
of 8 ng/µl and incubated overnight at the hybridization temperature
(37°C). The slides were rinsed in 2X saline-sodium citrate (SSC)
for 10 min, 1X SSC twice for 10 min, and washed 0.5X SSC for 10
min, 2X SSC and 1X SSC solution at the same hybridization
temperature. The slides were stained with DAPI (cat. no. G1012;
Servicebio Inc.) and incubated for 8 min at 4°C, avoiding the
light. The anti-fluorescence quenching reagent (cat. no. G1401;
Servicebio Inc.) was dripped on after washing. The slides were then
mounted, coverslipped, and observed using a Nikon DS-U3
fluorescence microscope (magnification, ×40; Nikon Corporation,
Tokyo, Japan). The sequence of the rno-miR-431 probe for FISH was
5′-GCATGACGGCCTGCAAGACA-3′. The probe was labeled with fluorescein
amidite (Biosearch Technologies, Petaluma, CA, USA).
Target genes prediction and
bioinformatics analysis
MiRBase sequence database (http://www.mirbase.org/) was used to identify the
nucleotide sequence for different species. The target genes of
miR-431 were predicted using TargetScan database (www.targetscan.org). The results indicated SMAD4 was a
potential target of miR-431.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA from lung tissue was extracted with TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) according to manufacturer instructions. For miR-431
quantification in rat lungs, cDNA was prepared in an RT reaction
using the PrimeScript™ RT Reagent kit (Takara Bio, Inc., Otsu,
Japan). The reactions were incubated at 16°C for 30 min, 42°C for
40 min and at 85°C for 5 min. RT-qPCR was performed using an
Applied Biosystems 7500 Fast real-time PCR cycler (Applied
Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA). U6
small nuclear 6 was used as an internal control. The primers
(Table I) for miR-431-5p and U6
were designed and purchased from Kangchen Biotech Co., Ltd (Wuhan,
China). Amplification was performed with the SYBR®
Premix Ex Taq™ (Takara Bio, Inc.) according to the manufacturer's
protocol. Thermocycling conditions included an initial step at 95°C
for 5 min, and 40 cycles at 90°C for 15 sec, 60°C for 15 sec and
72°C for 1 min, and final extension at 72°C for 10 min.
 | Table I.Primer sequences for reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences for reverse
transcription-quantitative polymerase chain reaction.
| Gene | Primer
sequence |
|---|
| MicroRNA-431 | F:
5′-AGGTGTCTTGCAGGCCGT-3′ |
|
| R:
5′-GTGCGTGTCGTGGAGTCG-3′ |
| U6 | F:
5′-GCTTCGGCAGCACATATACTAAAAT-3′ |
|
| R:
5′-CGCTTCACGAATTTGCGTGTCAT-3′ |
| SMAD4 | F:
5′-ATCACTATGAGCGGGTTGT-3′ |
|
| R:
5′-TTGGTGGATGTTGGATGG-3′ |
| SP-A | F:
5′-AGAACGTGGAGACAAGGG-3′ |
|
| R:
5′-TGATCTCATAGAGTTCAGTCTGG-3′ |
| SP-B | F:
5′-AGCCTGGAGCAAGCGATAC-3′ |
|
| R:
5′-AAGCGTCTTCCTTGGTCATC-3′ |
| SP-C | F:
5′-CCTTGTCGTCGTGGTGAT-3′ |
|
| R:
5′-AGGTAGCGATGGTGTCTGT-3′ |
| SP-D | F:
5′-GGAATCAAAGGCGAAAGTGG-3′ |
|
| R:
5′-TGCTGTGGGCTGTGACGAG-3′ |
| β-actin | F:
5′-CGAGTACAACCTTCTTGCAGC-3′ |
|
| R:
5′-ACCCATACCCACCATCACAC-3′ |
For mRNA quantification, cDNA was synthesized using
PrimeScript™ RT Master Mix (Takara Bio, Inc.). The reactions were
incubated at 37°C for 15 min and at 85°C for 5 sec. Expression of
SMAD4 and SPs was determined by RT-qPCR using the SYBR-Green PCR
Master Mix (cat. no. RR036A; Takara Bio, Inc.) with the mouse
primers. GAPDH primers were used for normalization. All primers
(Table I) were designed and
purchased from Kangchen Biotech Co., Ltd. Thermocycling conditions
included an initial step at 95°C for 30 sec, and 40 cycles at 95°C
for 5 sec and 60°C for 34 sec, and final extension at 72°C for 10
min. The relative gene expression was calculated using the
2−∆∆Cq method (18).
Dual luciferase-reporter assay
The wild-type (WT) and mutated (MUT) 3′ untranslated
region (3′-UTR) sequences of SMAD4 (Guangzhou RiboBio Co., Ltd.,
Guangzhou, China), which were predicted to have an interaction with
miR-431, were inserted into firefly luciferase expressing
pmiR-RB-REPORT™ vector (Guangzhou RiboBio Co., Ltd.) in accordance
with the manufacturer's protocol. The primer sequences of
hsa-SMAD4-3′UTR were the following: forward
GCGGCTCGAGACAAGGTTGGTTGCTAAGA and reverse
AATGCGGCCGCGGCTGTTGCCTGTCATTTA. Genomic DNA was extracted from 293T
cells (Guangzhou RiboBio Co., Ltd.) using the TIANamp Genomic DNA
kit (Tiangen Biotech Co., Ltd., Beijing, China). The thermocycling
conditions of the PCR were as follows: initial denaturation at 98°C
for 2 min, followed by 10 cycles of 98°C for 10 sec, 65°C for 15
sec and 72°C for 45 sec, followed by 15 cycles of 98°C for 10 sec,
60°C for 15 sec and 72°C for 45 sec, with a final extension at 72°C
for 3 min. For the luciferase reporter assay, 293T cells were
seeded into 96-well plates (1.5×104 cells/well).
Following culturing for 24 h, cells were co-transfected with the
indicated vectors (250 ng/well), 50 nM miR-431 mimics (cat. no.
miR10001625; Guangzhou RiboBio Co., Ltd.) or miR-431 mimic negative
control (NC) (cat. no. miR1N0000001-1-5; Guangzhou RiboBio Co.,
Ltd.) using 250 ng/well Lipofectamine® 3000 (Invitrogen;
Thermo Fisher Scientific, Inc.). At 48 h post-transfection, the
cells were lysed and luciferase activity was assayed using the
Dual-Luciferase Reporter Assay system (Promega Corporation,
Madison, WI, USA). The Firefly luciferase activity was evaluated
after normalization to Renilla activity.
Western blot analysis
The following antibodies were used as primary
antibodies for western blot analysis: monoclonal rabbit anti-mouse
SMAD4 antibody (1:2,000; cat. no. ab40759; Abcam), polyclonal
rabbit anti-mouse SP-A antibody (1:500; cat. no. SAB4300719;
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany), polyclonal rabbit
anti-mouse SP-B antibody (1:1,000; cat. no. NBP1-57977; Novus
Biologicals, LLC, Littleton, CO, USA), monoclonal mouse anti-mouse
SP-C antibody (1:1,000; cat. no. MA5-17172; Invitrogen; Thermo
Fisher Scientific, Inc.), monoclonal rabbit anti-mouse SP-D
antibody (1:1,000; cat. no. ab168366; Abcam), or monoclonal rabbit
anti-mouse β-actin antibody (1:2,000; cat. no. 4970; Cell Signaling
Technology, Inc., Danvers, MA, USA).
The isolated lung specimens were washed three times
with PBS, and then lysed with phenylmethylsulfonyl fluoride (1 mM;
cat. no. G2008; Servicebio Inc.). Total protein was extracted from
these specimens and then quantified using a bicinchoninic acid
protein assay kit (cat. no. G2026; Servicebio Inc.). A total of 50
µg protein was loaded in each well. Proteins were separated by 10%
SDS-PAGE. Electrotransfer onto PVDF membranes was performed for 1.5
h on ice. The membranes were blocked for 1 h at room temperature in
5% non-fat dry milk in TBS+0.05% Tween-20 (TBST), followed by
incubation at 4°C overnight with primary antibodies. After washing
with TBST three times, the membranes were incubated with
horseradish peroxidase (HRP)-conjugated goat anti-mouse secondary
antibody (1:3,000; cat. no. GB23303; Servicebio Inc.) or
HRP-conjugated goat anti-rabbit secondary antibody (1:3,000; cat.
no. GB23302; Servicebio Inc.) at 37°C for 1 h, followed by a
further three washes with TBST. The protein expression levels were
normalized to that of β-actin on the same membrane to confirm equal
loading. The protein signals were detected using an enhanced
chemiluminescence kit (cat. no. G2014; Servicebio Inc.) and
analyzed with ImageJ software (version 2.1; National Institutes
Health).
Statistical analysis
Data were analyzed using the SPSS 19.0 software (IBM
Corp., Armonk, NY, USA). Values are presented the mean ± standard
deviation. One-way analysis of variance followed by Tukey's test
was applied to analyze statistical significance among three groups,
while Student's t-test was used to compare two groups. All
experiments were repeated independently at least three times.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Light microscopy
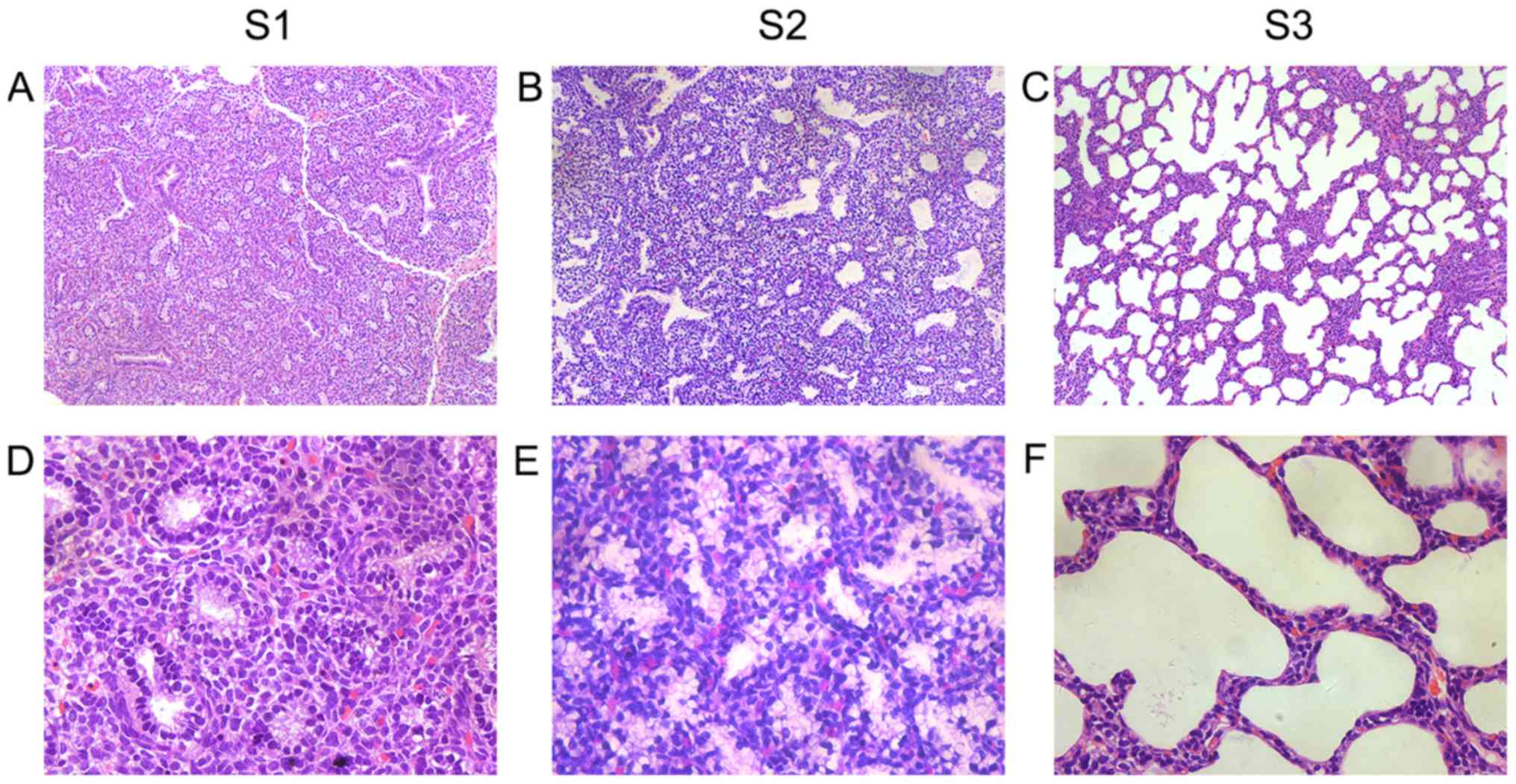
In group S1 (E19), bronchioles were evident. The
interstitium was thick and primal alveolar cells surrounded by
monolayer cubic epithelial cells were visible (Fig. 1A and D). In group S2 (E21), the
number of alveoli increased and the alveolar structures expanded.
The stromal layer developed and became thinner than that noted
earlier. The cuboidal epithelial cells were gradually flattened
(Fig. 1B and E). In group S3 (P3),
the shaping of the secondary septum was initiated. The stromal
layer was thinner than that noted in the earlier groups and
alveolar cavities were considerably expanded (Fig. 1C and F).
Electron microscopy
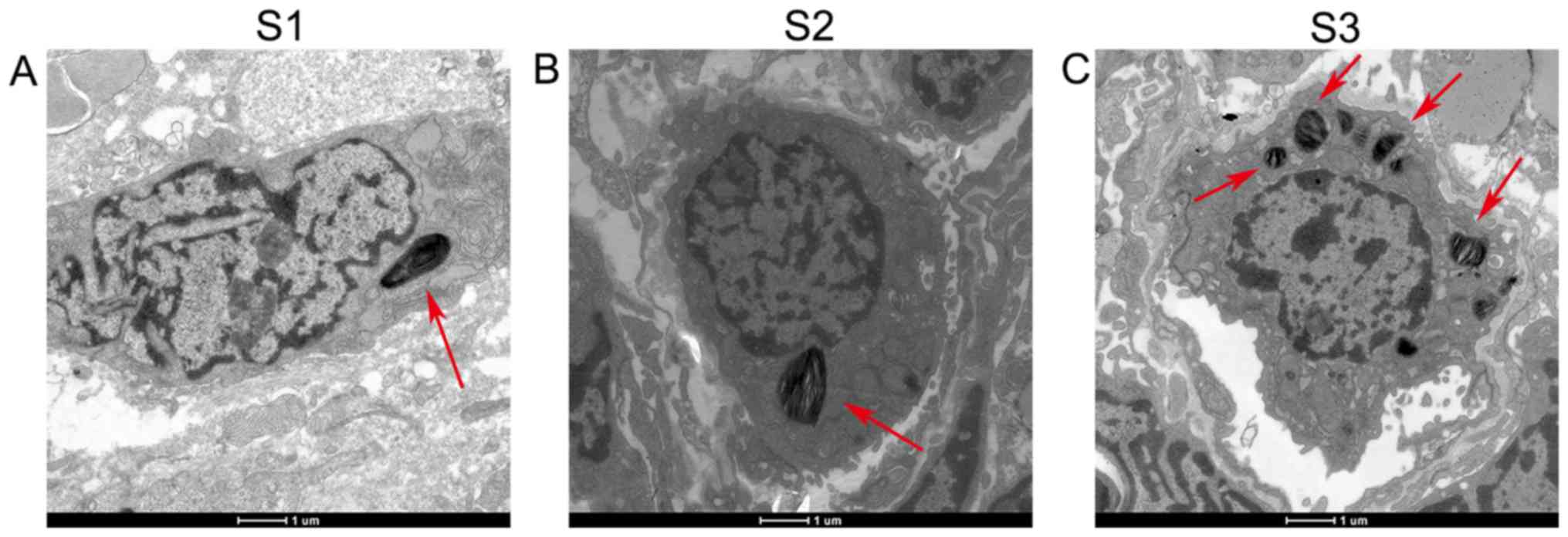
Type II alveolar epithelial differentiation was
observed in group S1 (E19). A limited number of microvilli were
present on the free surface of the cells. Osmiophilic multilamellar
bodies appeared for the first time in the cytoplasm, which were
polycentric or parallel lamellar structures with deep staining
(Fig. 2A). In group S2 (E21), type
II alveolar epithelial cells were enlarged. The number of short
microvilli on the cell surface and lamellar bodies increased. A
higher number of mitochondria and multivesicular bodies were
simultaneously observed in the cytoplasm (Fig. 2B). In group S3 (P3), the lamellar
structure became more dense. The number of lamellar bodies was
increased and tended to aggregate in the cytoplasm (Fig. 2C).
Expression levels of miR-431 during
rat lung development
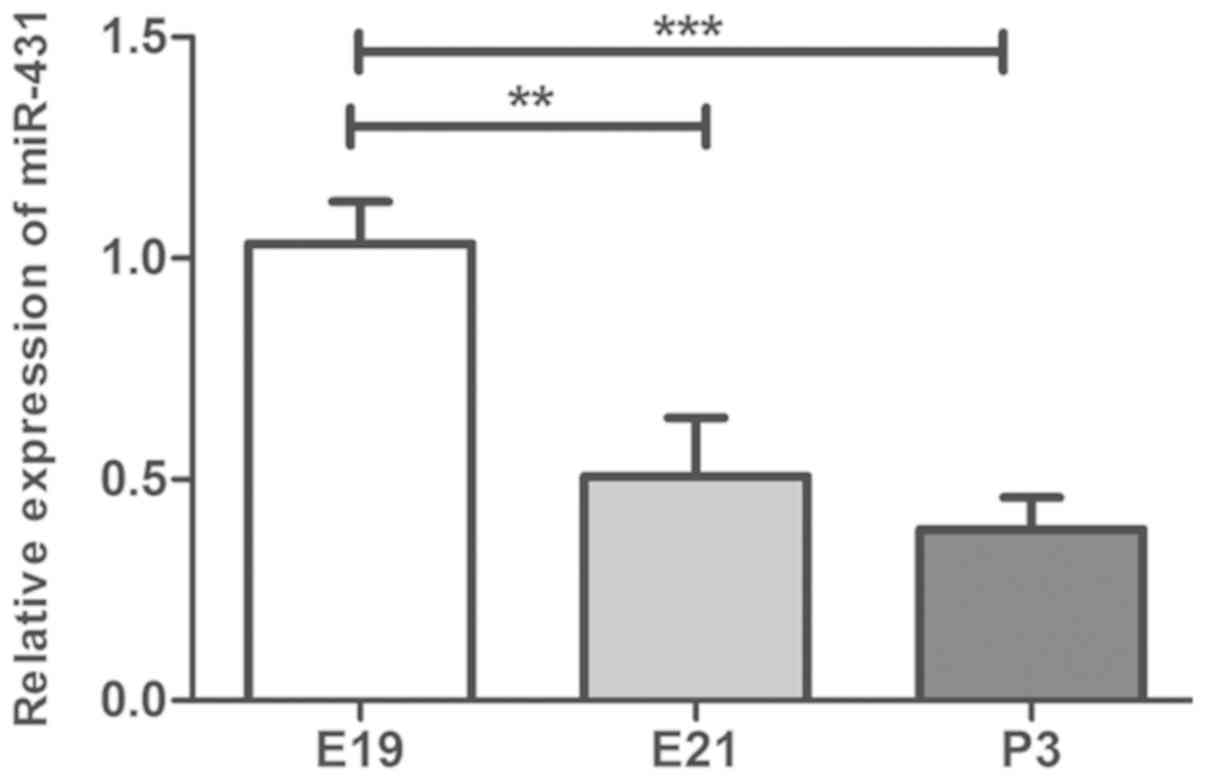
The expression levels of miR-431 were measured by
RT-qPCR during the three different stages of the rat lung tissue
development, namely gestational days 19 and 21 (E19, E21), and
postnatal day 3 (P3; Fig. 3). The
three time points represent the key three stages of mammal lung
development [the canalicular stage (E18-E20), the saccular stage
(E20 to full term), and the alveolar stage]. The expression levels
of miR-431 were significantly decreased at E21 compared with E19
(P<0.05) and were continuously decreased at P3 compared with
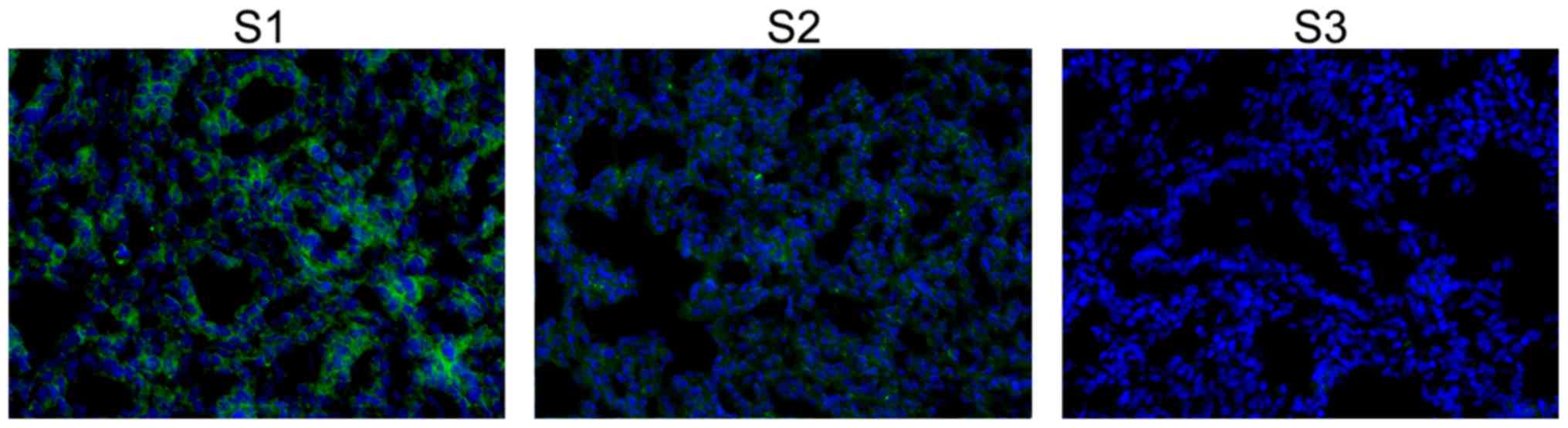
E21. FISH analysis of miR-431 expression is shown in Fig. 4. miR-431 was localized in the
cytoplasm and exhibited higher signal intensity at E19. The
fluorescence intensity was gradually decreased from E19 to P3
(Fig. 4).
SMAD4 was identified as a target of
miR-431
Target prediction was performed using TargetScan to
identify the potential target of miR-431. As shown in Fig. 5A, SMAD4 contained the putative
binding sites for miR-431 and was predicted as one of the target
genes. The sequence ‘GCAAGAC’ of the SMAD4 3′-UTR that matches
‘CGUUCUG’ in miR-431 is highly conserved among species (Fig. 5C). In order to validate the
accuracy of the prediction, a dual luciferase reporter assay was
performed. Upregulation of miR-431 significantly downregulated the
luciferase activity of the SMAD4-WT 3′-UTR (P<0.01; Fig. 5B). These results indicated that
miR-431 directly targeted SMAD4-WT. In addition, there was a
significant increase in the luciferase activity of 293T cells
transfected with a mutant SMAD4 3′UTR (SMAD4-mut) in the miR-431
mimics compared with the NC groups (P<0.001).
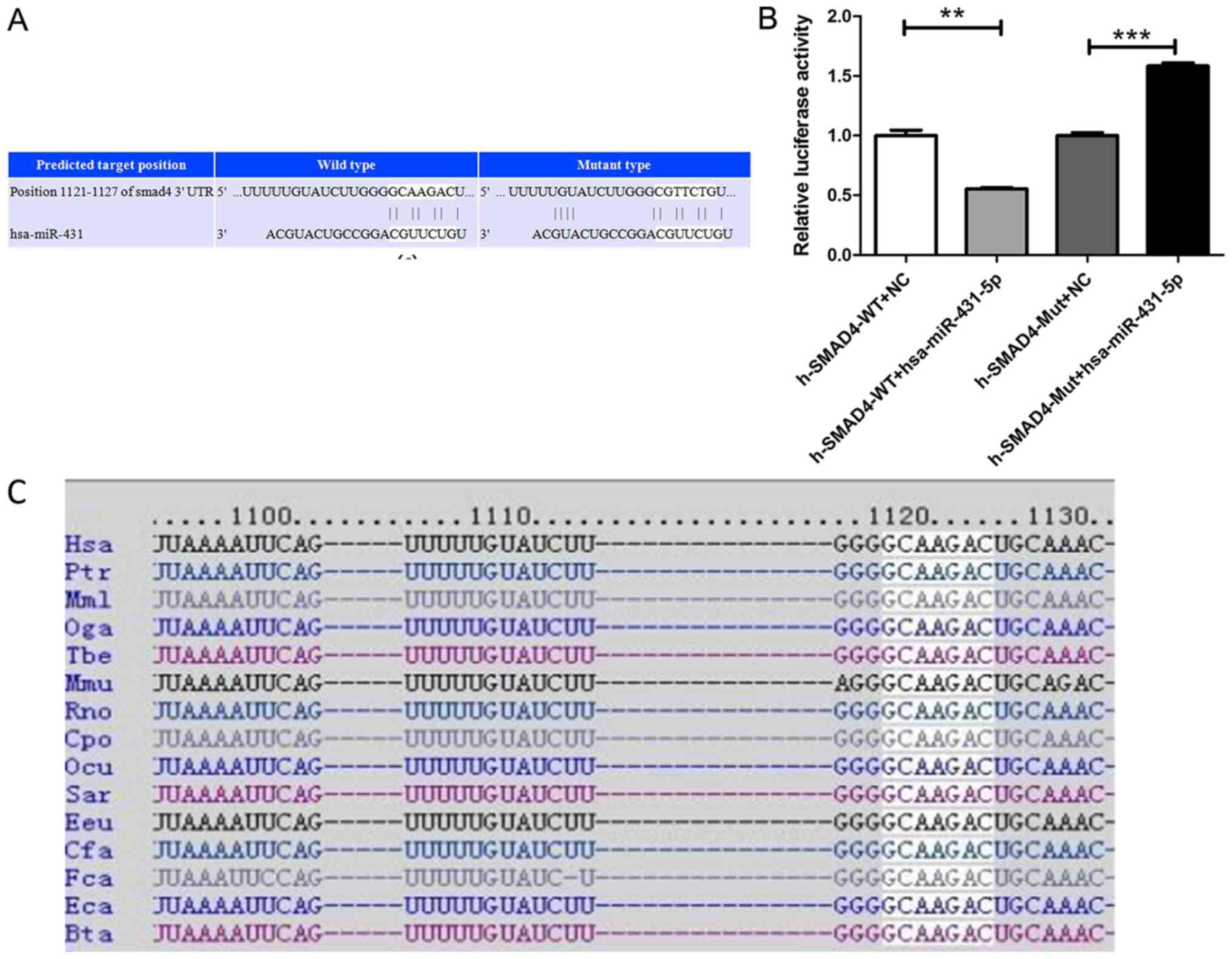 | Figure 5.SMAD4 is a target of miR-431-5p. (A)
TargetScan predicted position 1,121-1,127 of the SMAD4 3′-UTR as a
binding site for miR-431. (B) Luciferase activity of SMAD4
3′-UTR-WT and SMAD4 3′-UTR-MUT in the presence of miR-431-5p mimic
or NC. (C) GCAAGAC is a highly conserved sequence of Smad4, and it
is present in different species, as predicted by TargetScan
database. **P<0.01, ***P<0.001. 3′-UTR, 3′ untranslated
region; miR-431-5p, microRNA-431-5p; WT, wild-type; NC, negative
control; MUT, mutant. |
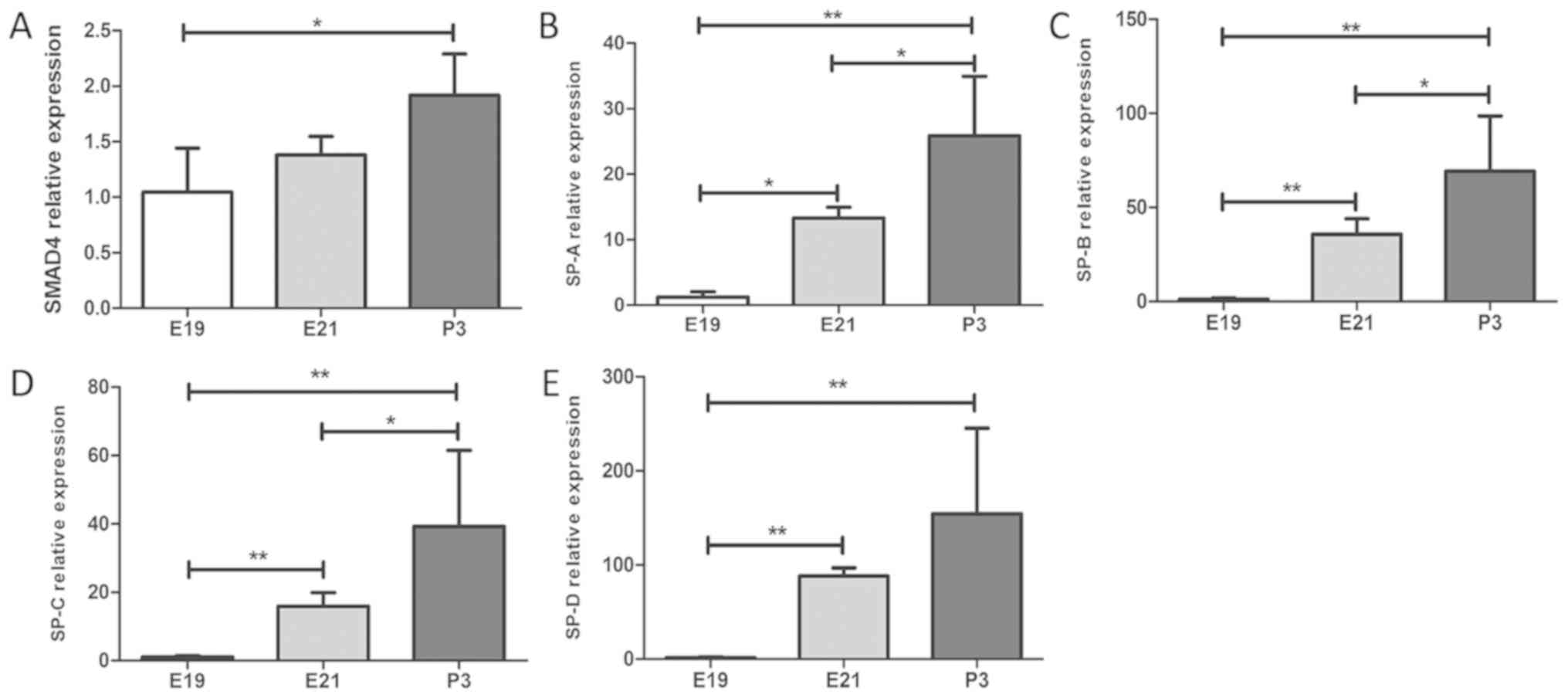
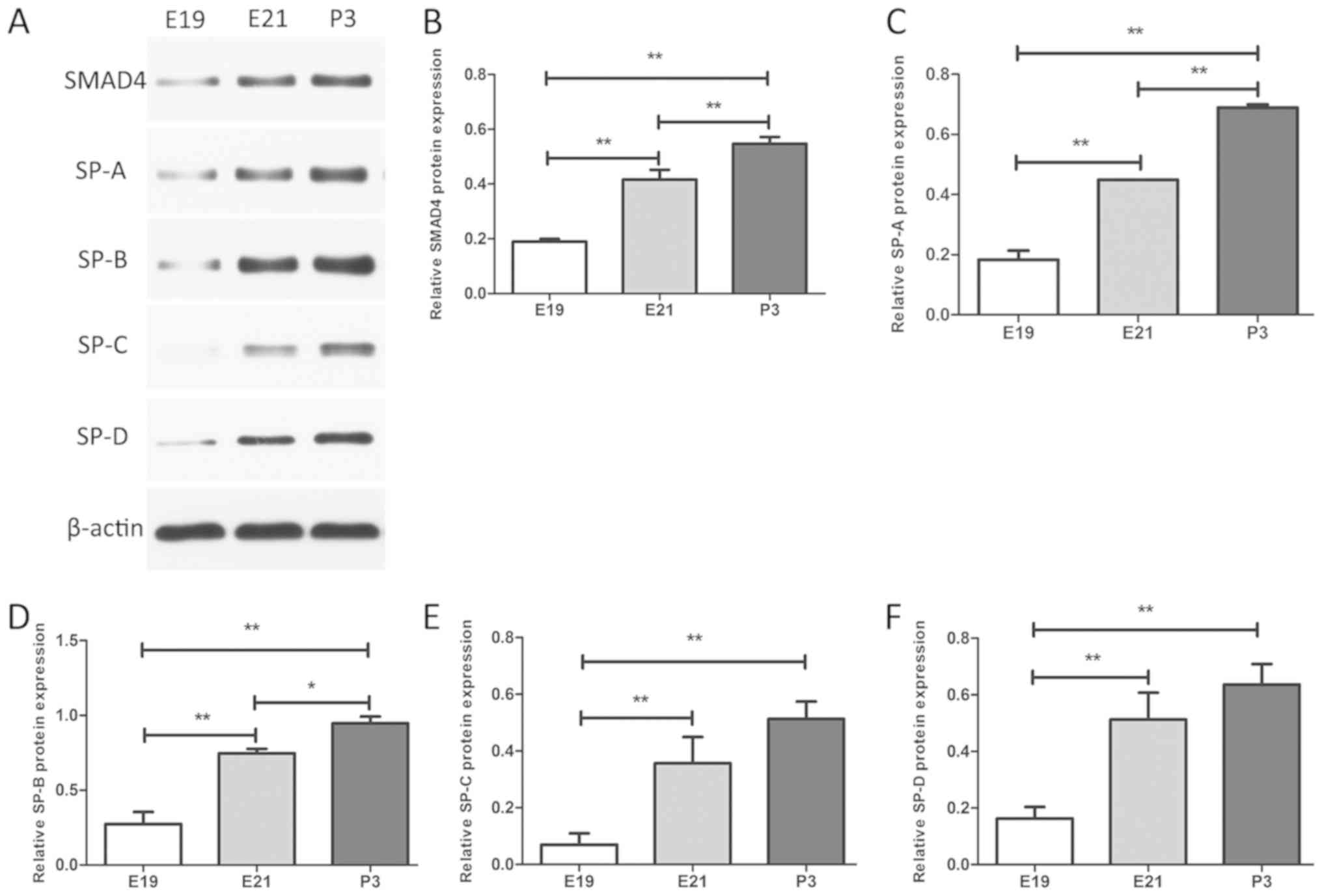
Expression levels of SMAD4 and SPs
during rat lung development
In order to explore the effects of miRNA-431 on
SMAD4 and the synthesis and secretion of the surfactants during rat
lung development, rat lung tissues were isolated during three
development stages and measured the expression levels of SMAD4,
SP-A, SP-B, SP-C and SP-D. The expression levels of miR-431 were
downregulated from E19 to P3, whereas the mRNA levels of SMAD4,
SP-A, SP-B, SP-C and SP-D were increased gradually, as determined
by RT-qPCR (Fig. 6). These changes
were statistically significant (Fig.
6). In addition western blot analysis was performed to
determine the expression of SMAD4 and SPs at the protein level.
Similar statistically significant trends were noted for SMAD4 and
SP markers with regard to the expression of their mRNA and protein
levels (Fig. 7).
Discussion
miR-431 is an oncogenic miRNA associated with the
initiation and development of human cancer. Currently, certain
studies have provided new insight regarding the biological
functions of miR-431. Han et al (19) reported that miR-431 had protective
effects against cerebral ischemia-reperfusion injury in rats by
targeting the Rho/Rho-kinase signaling pathway. Wu and Murashov
(20) demonstrated that miR-431
regulated axon regeneration in mature sensory neurons. An
additional study has shown that miR-431 regulates differentiation
and regeneration of old skeletal muscle (21). The common findings of these studies
indicate that miR-431 is associated with organ maturation. In the
present study, we hypothesized miR-431 is associated with the
regulation of lung tissue development. RT-qPCR and FISH analyses
demonstrated that the expression levels of miR-431 were decreased
from E19 to P3. In a previous study, our group used miRNA
microarray analysis to compare the expression levels of miR-431,
and found higher expression levels of this miRNA in patients with
RDS than in infants without RDS (11). Therefore, miR-431 may have a
negative impact on the regulation on lung development.
Lung development extends from the embryonic period
to the fetal period, up to birth and afterwards (22). During the pseudoglandular stage,
the epithelial tube undergoes branching morphogenesis so that the
conduction part of the respiratory system reaches to the level of
distal bronchioles. At this stage the bronchial tree and parts of
the parenchyma are formed. During the canalicular stage of lung
development, distal airway bronchioles begin to shape and this
process is accompanied by proximal to distal epithelial
differentiation. One of the main features of this stage is the
onset of the air-blood barrier. In the saccular stage of lung
development, the formation of terminal saccules was gradually
formed, and the epithelial cell differentiation into secretory
rounded type II and squamous type I pneumonocytes was initiated.
Airspaces expand and the surfactant forms. During the alveolar
stage (last stage of lung development), alveolarization aims to
increase the gas exchange surface area. In humans, the alveolar
stage is initiated during the late fetal period and continues into
childhood. In rodents, alveolarization is predominantly postnatal
(15). In the present study, it
was observed that lamellar bodies, which are intracellular storage
units of pulmonary surfactant, were present in alveolar cells on
E19. Following lung development, the number of lamellar bodies was
gradually increased, and the staining was enhanced, which suggested
enhanced function. From E19 to P3, the stromal layer became thinner
and capillary networks were gradually generated.
Several signaling pathways are associated with lung
tissue development, such as the TGF-β, bone morphogenetic proteins
and Wnt pathways (23–25). Notably, TGF-β exerts a key role in
normal lung morphogenesis and function (26,27).
Previous studies have demonstrated that TGF-β is expressed at high
levels during normal lung development and that the expression
levels of TGF-β determine branching morphogenesis and epithelial
cell differentiation with maturation of surfactant synthesis
(13). The SMAD family of proteins
is an important intracellular mediator of TGF-β signaling and can
be divided into three functional classes as follows:
Receptor-regulated SMAD (R-SMAD), the co-mediator SMAD (Co-SMAD),
and the inhibitory SMAD (I-SMAD). R-SMADs (SMAD1, 2, 3, 5 and 8)
are directly phosphorylated and activated by the type I receptor
kinases and can undergo homotrimerization in order to form
heteromeric complexes with Co-SMAD and SMAD4 (28,29).
As a key nuclear transcription factor, SMAD4 transfers the signal
from the activated receptor to the nucleus to regulate gene
expression. It has been demonstrated that miR-27a regulates the
TGF-β signaling pathway by targeting SMAD4 in lung cancer (30). Cui et al (31) indicated that miR-27a-3p acted as a
negative regulator of lung fibrosis by directly targeting SMAD2 and
SMAD4. In the present study, bioinformatic analysis suggested that
SMAD4 is a potential target gene of miR-431. The sequence of SMAD4
3′-UTR binding with miR-431 is highly conserved among species. A
dual luciferase reporter assay was performed to demonstrate that
miR-431 directly targets SMAD4-WT. Notably, there was a statistical
difference in luciferase activity when comparing the
Smad4-WT+miR-431-5p group and the Smad4-WT+NC group. Interestingly,
the luciferase activity in the Smad4-MUT+miR-431-5p group increased
significantly compared with the Smad4-MUT+NC group. This may be due
to the fact that miRNAs have pleiotropic roles, and the expression
of target genes is influenced by various unknown factors. The dual
luciferase-reporter assay actually reflected the interaction of
miRNA-431 with various potential molecules in cells. Therefore, the
increase in luciferase activity in the Smad4-MUT+miR-431-5p group
may be influenced by multiple factors, which require further
investigation. The expression levels of SMAD4 were subsequently
compared among the three development stages. The expression levels
of SMAD4 were increased with the increasing age of the rats (E19,
E21, P3), which was the opposite of the trend noted for miR-431.
Taken collectively, the data suggested that miR-431 negatively
regulates SMAD4 expression.
Pulmonary surfactant is a complex with a unique
phospholipid and protein composition. Its specific function is to
reduce surface tension at the pulmonary air-liquid interface
(32). Lack of pulmonary
surfactant due to lung immaturity can lead to RDS. To date, four
types of SPs (SP-A, SP-B, SP-C and SP-D) have been identified. It
was previously reported that overexpression of miR-124 inhibited
the mRNA expression levels of SP-A, SP-B and SP-C (10). Another previous study demonstrated
that overexpression of miR-26a in alveolar epithelial type II cells
inhibited the synthesis of SP-B and SP-C (33). In the present study, the expression
levels of SP mRNA and protein were increased with lung development
(from E19 to P3), which was contradictory to the trend noted for
miR-431 expression. However, the precise association between
miR-431 and SP protein expression requires further
investigation.
In conclusion, the current study demonstrated that
the expression levels of miR-431 were decreased from E19 to P3
during rat lung development. SMAD4 was identified as a target gene
of miR-431 and was negatively regulated by miR-431, with SMAD4
expression levels increased in the rat lung tissue from E19 to P3.
Surfactant synthesis was additionally increased from E19 to P3.
Whether miR-431 can downregulate pulmonary surfactant synthesis by
targeting SMAD4 expression requires further investigation. In
summary, these results add provide more insight on the mechanism of
lung development and provide a potential direction for the
prevention or treatment of RDS, and for the treatment of chronic
lung diseases.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Scientific Grand (grant nos. 81601321, 81741052 and
81871195) from the Government of China, and by the Jiangsu Province
Young Medical Talents' Project of ‘Science Education Facilitating
Health’ (grant no. QNRC2016092).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZS, YS and YY contributed to the conception and
design of the experiments, the analysis and interpretation of data,
and the manuscript preparation and critical evaluation. ZB and XC
contributed to the experimental study, the data interpretation and
the statistical analysis and manuscript preparation. XZ and RC
contributed to the experimental study conduct, the data
interpretation and the statistical analysis. All authors reviewed
the manuscript.
Ethics approval and consent to
participate
All experimental protocols were conducted with the
approval of the Nanjing Medical University Animal Care and Use
Committee (Nanjing, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Edwards MO, Kotecha SJ and Kotecha S:
Respiratory distress of the term newborn infant. Paediatr Respir
Rev. 14:29–36; quiz 36–37. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jensen EA and Schmidt B: Epidemiology of
bronchopulmonary dysplasia. Birth Defects Res A Clin Mol Teratol.
100:145–157. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Strueby L and Thébaud B: Advances in
bronchopulmonary dysplasia. Expert Rev Respir Med. 8:327–338. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Stoll BJ, Hansen NI, Bell EF, Shankaran S,
Laptook AR, Walsh MC, Hale EC, Newman NS, Schibler K, Carlo WA, et
al Eunice Kennedy Shriver National Institute of Child Health and
Human Development Neonatal Research Network, : Neonatal outcomes of
extremely preterm infants from the NICHD Neonatal Research Network.
Pediatrics. 126:443–456. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yates LA, Norbury CJ and Gilbert RJ: The
long and short of microRNA. Cell. 153:516–519. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bhaskaran M, Wang Y, Zhang H, Weng T,
Baviskar P, Guo Y, Gou D and Liu L: MicroRNA-127 modulates fetal
lung development. Physiol Genomics. 37:268–278. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Carraro G, Shrestha A, Rostkovius J,
Contreras A, Chao CM, El Agha E, Mackenzie B, Dilai S, Guidolin D,
Taketo MM, et al: miR-142-3p balances proliferation and
differentiation of mesenchymal cells during lung development.
Development. 141:1272–1281. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang Y, Huang C, Chintagari NR, Xi D, Weng
T and Liu L: miR-124 regulates fetal pulmonary epithelial cell
maturation. Am J Physiol Lung Cell Mol Physiol. 309:L400–L413.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kan Q, Ding S, Yang Y and Zhou X:
Expression profile of plasma microRNAs in premature infants with
respiratory distress syndrome. Mol Med Rep. 12:2858–2864. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang Y, Kai G, Pu XD, Qing K, Guo XR and
Zhou XY: Expression profile of microRNAs in fetal lung development
of Sprague-Dawley rats. Int J Mol Med. 29:393–402. 2012.PubMed/NCBI
|
|
13
|
Bartram U and Speer CP: The role of
transforming growth factor β in lung development and disease.
Chest. 125:754–765. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
ten Dijke P and Hill CS: New insights into
TGF-β-Smad signalling. Trends Biochem Sci. 29:265–273. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zoetis T and Hurtt ME: Species comparison
of lung development. Birth Defects Res B Dev Reprod Toxicol.
68:121–124. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Burri PH: Fetal and postnatal development
of the lung. Annu Rev Physiol. 46:617–628. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bernhard W: Lung surfactant: function and
composition in the context of development and respiratory
physiology. Ann Anat. 208:146–150. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Han XR, Wen X, Wang YJ, Wang S, Shen M,
Zhang ZF, Fan SH, Shan Q, Wang L, Li MQ, et al: Protective effects
of microRNA-431 against cerebral ischemia-reperfusion injury in
rats by targeting the Rho/Rho-kinase signaling pathway. J Cell
Physiol. 233:5895–5907. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu D and Murashov AK: MicroRNA-431
regulates axon regeneration in mature sensory neurons by targeting
the Wnt antagonist Kremen1. Front Mol Neurosci. 6:35–48. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee KP, Shin YJ, Panda AC, Abdelmohsen K,
Kim JY, Lee SM, Bahn YJ, Choi JY, Kwon ES, Baek SJ, et al: miR-431
promotes differentiation and regeneration of old skeletal muscle by
targeting Smad4. Genes Dev. 29:1605–1617. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mullassery D and Smith NP: Lung
development. Semin Pediatr Surg. 24:152–155. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Hata A and Chen YG: TGF-β Signaling from
receptors to smads. Cold Spring Harb Perspect Biol. 8:a0220612016.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Shi Y and Massagué J: Mechanisms of TGF-β
signaling from cell membrane to the nucleus. Cell. 113:685–700.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Weng T and Liu L: The role of pleiotrophin
and β-catenin in fetal lung development. Respir Res. 11:80. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Roth-Kleiner M and Post M: Similarities
and dissimilarities of branching and septation during lung
development. Pediatr Pulmonol. 40:113–134. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Chen XQ, Wu SH, Luo YY, Li BJ, Li SJ, Lu
HY, Jin R and Sun ZY: Lipoxin A4 attenuates bronchopulmonary
dysplasia via upregulation of Let-7c and downregulation of TGF-β1
signaling pathway. Inflammation. 40:2094–2108. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Verhamme FM, Bracke KR, Joos GF and
Brusselle GG: Transforming growth factor-β superfamily in
obstructive lung diseases. more suspects than TGF-β alone. Am J
Respir Cell Mol Biol. 52:653–662. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mehra A and Wrana JL: TGF-beta and the
Smad signal transduction pathway. Biochem Cell Biol. 80:605–622.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chae DK, Ban E, Yoo YS, Kim EE, Baik JH
and Song EJ: MIR-27a regulates the TGF-β signaling pathway by
targeting SMAD2 and SMAD4 in lung cancer. Mol Carcinog.
56:1992–1998. 2017. View
Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cui H, Banerjee S, Xie N, Ge J, Liu R-M,
Matalon S, Thannickal VJ and Liu G: MicroRNA-27a-3p is a negative
regulator of lung fibrosis by targeting myofibroblast
differentiation. Am J Respir Cell Mol Biol. 54:843–852. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Whitsett JA and Weaver TE: Hydrophobic
surfactant proteins in lung function and disease. N Engl J Med.
347:2141–2148. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang XQ, Zhang P, Yang Y, Qiu J, Kan Q,
Liang HL, Zhou XY and Zhou XG: Regulation of pulmonary surfactant
synthesis in fetal rat type II alveolar epithelial cells by
microRNA-26a. Pediatr Pulmonol. 49:863–872. 2014. View Article : Google Scholar : PubMed/NCBI
|