Introduction
The oestrogen steroid hormone 17β-oestradiol (E2) is
essential for normal spermatogenesis, while a surplus of oestrogen
along with a lack of testosterone usually causes infertility
(1,2). The general human population is
exposed to many chemicals. Among them, oestrogenic and other
endocrine disruptors (EDs) have been found to lead to a decline in
male reproductive ability (3).
Accumulated evidence has revealed that exposure to endocrine
disruptors is associated with reduced semen quality and impaired
fertility in men, as illustrated by slow development of gonad
testis and epididymis as well as testicular atrophy (4,5).
Bisphenol A (BPA) is the most widely used and well-studied
endocrine disruptor and has been implicated in the pathogenesis of
male reproductive disability (6).
High plasma BPA levels have been found in men with infertility, and
plasma BPA level is negatively correlated with sperm concentration
and total sperm count (7). Certain
mechanisms by which exogenous oestrogen destroys male fertility
have been revealed, such as the dysfunction of the
hypothalamic-pituitary-testicular axis, inhibition of germ cell
proliferation, germ cell apoptosis, and testicular oxidative stress
(8). Oestradiol benzoate (EB) has
been widely used to investigate the oestrogen-like effects of
various EDs, which can eliminate the interference of other types of
ED toxicity (9,10). Our previous study revealed that EB
disrupted spermatogenesis and induced infertility in male mice
through its effects on apoptosis and oestrogen receptor signaling
pathways (11). During the
previous study, we inadvertently determined that EB interferes with
testicular metabolic cooperation, but the underlying mechanism is
largely unknown.
Cellular energy metabolism affects various
physiological and pathological processes (12,13).
The spermatogenesis process is sensitive to changes in energy
metabolism. Sertoli cells (SCs) are pivotal to spermatogenesis by
providing nutritional support to germ cells throughout their
development. SCs preferentially export lactate to germ cells, which
utilise lactate as their main energy substrate (14). In vitro studies have
revealed that E2 controls glucose uptake and lactate production in
SCs by regulating glycolysis-related transporters [e.g., glucose
transporter 3 (GLUT3)] and enzymes [e.g., lactate dehydrogenase
(LDH)] at the transcriptional level (15,16).
Therefore, it was hypothesized that EB likely modulates the
glycolytic process in the testis, thereby inducing male
infertility. The present study investigated the expression levels
of glycolysis-related factors [including GLUT3, monocarboxylate
transporter 2 (MCT2), MCT4, and LDH] and the activity of
rate-limiting enzymes in mice.
Materials and methods
Animals and treatments
In total, 60 male Kunming mice (age, 4 weeks;
weight, 25 g) were purchased from the Laboratory Animal Center of
the Third Military Medical University (Chongqing, China). The mice
were raised in specific pathogen-free animal rooms with free access
to a rodent diet and water. Housing conditions comprised a
temperature-controlled polysulfone cage (23–25°C) under a 12-h
light/dark cycle.
All the mice were randomly divided into a control
group and 2 treatment groups (20 animals/group). The treatment
groups were intramuscularly injected with EB (Hangzhou
Pharmaceutical Factory, Hangzhou, Zhejiang, China) at a
concentration of 5 or 10 mg/kg body weight. This method was
described in a previous study (17). The control group was injected with
an equal quantity (150 µl) of corn oil (COFCO Grain Factory,
Chongqing, China). The injection was performed every other day for
4 weeks. The testes were immediately excised from euthanised mice
at the end of the experiments and then trimmed of fat and
connective tissue. One of the testes from each group was fixed in
Bouin's or 2.5% glutaraldehyde for histological analysis. The other
testis was frozen in liquid nitrogen for biochemical and RNA
detection. Cauda epididymis samples were collected for sperm
counts. The present study was approved and monitored by the Animal
Experiments Ethical Review Committee of Zunyi Medical University
(Zunyi, China) (no. ZMU210500326). All procedures strictly adhered
to the National Institutes of Health guidelines for the care and
use of animals (18).
Sperm and testicular cell count
Sperm counts were obtained as previously described
(19). The caudal epididymis was
dissected and placed in 500 µl of saline media heated to 37°C, and
then splayed openly using a back-cutting method. To give sperm time
to escape into liquid, the caudal epididymis was left undisturbed
for 30 sec. Subsequently, the number of sperm was assessed using a
haemocytometer under a light microscope (Olympus, Tokyo, Japan) and
expressed as 106/100 mg of epididymal weight.
Testes were stained with haematoxylin and eosin
(H&E) and haematoxylin-periodic acid-Schiff (PAS). Germ cells
at stage VII of the seminiferous cycle, including spermatogonia,
spermatocytes, and step 7 spermatids in 30 round seminiferous
tubules per testicle (20), were
counted using a conventional light microscope (Leica Microsystems
GmbH, Wetzlar, Germany).
Transmission electron microscopy
(TEM)
For TEM, the testis samples were cut into pieces
(2×2 mm) and fixed in 2.5% glutaraldehyde (pH=7.4) for 6–8 h at
4°C. Next, they were washed and fixed in 2% OSO4 for 1 h
at 4°C. The tissue was finally dehydrated with alcohol solutions
ascending in concentration gradient and embedded in araldite CY212.
Semithin sections (1 µm) were cut and stained with toluidine blue.
Ultrathin sections (60–70 nm) were cut and stained with uranyl
acetate and alkaline lead citrate.
Assessment of oxidative stress in the
testis
Oxidative stress in the testis was detected as
previously described (21). In
brief, the testis was homogenized in 0.8% sodium chloride solution
(pH 7.4) containing 0.01 mol/l Tris-HCl, 1 mmol/l EDTA-2Na, and
0.01 mol/l sucrose, with a glass homogenizer according to the
weight-to-volume ratio of 1 g:9 ml. After centrifugation of the
homogenate at 839 × g for 15 min at 4°C, the supernatant was
preserved. The activities of catalase (CAT), superoxide dismutase
(SOD), glutathione peroxidase (GPx), nitric oxide synthase (NOS),
malondialdehyde (MDA) and nitric oxide (NO) content were
specifically detected by colorimetric assay at different absorbable
wavelengths as the purchased kit (Nanjing Jiancheng Bioengineering
Institute, Nanjing, China) indicated.
High performance liquid chromatography
(HPLC)
Testis tissue was rapidly frozen in liquid nitrogen
and homogenized into powder. Adenosine phosphates were extracted
from the powder with 10 µl/mg of 0.1 M perchloric acid in an ice
bath for 1 min. The extraction mixture was centrifuged for 20 min
at 5,031 × g, and the supernatant was removed by paper filtration.
The filtrate solution was filtered again through a 0.45-mm filter.
HPLC separation was achieved using continuous gradient elution as
follows: Solution A, methanol; solution B, 0.1 M
NaH2PO4 and 0.1 M
Na2HPO4 mixed at a volume ratio of 9:1 and 10
mM tetrabutylammonium sulphate; solution A/B=12/88. The flow rate
of the mobile phase was 0.6 ml/min, while the injection volume was
5 µl. Total retention time was ~5 min, and the gradient was run for
6 min to ensure full separation. The concentration of ATP was
determined using the external standard method. Data were expressed
as means of three repeat determinations.
Measurement of rate-limiting enzyme
activities and metabolites
In the testis homogenate, hexokinase (HK), pyruvate
kinase (PK), and lactate dehydrogenase (LDH) activities and lactate
and pyruvate content were specifically detected by colorimetric
assay at different absorbable wavelengths as the purchased kit
(Nanjing Jiancheng Bioengineering Institute) indicated.
Quantitative real-time PCR (qPCR)
As reported previously (22), total RNA was isolated from the
testis and its quality was assessed by spectroscopy. Synthesis of
cDNA was performed with the TransScript II One-Step gDNA Removal
and cDNA Synthesis SuperMix Kit (Beijing Transgen Biotech Co.,
Ltd., Beijing, China), according to the manufacturer's protocols.
Quantitative PCR analysis for gene expression level was performed
by the SYBR-Green Assay System with the Applied Biosystems 7500
Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific,
Inc., Waltham, MA, USA). The primers used in the present study are
presented in Table I. PCR cycle
parameters were 95°C for 15 sec and 55°C for 1 min, lasting for 40
cycles. The threshold line was set in the linear region of the
plots above the baseline noise, and the value of the threshold
cycle (CT) was determined as the cycle number at which
the threshold line crossed the amplification curve. PCR without
template or with template substituted with total RNA was used as a
negative control to verify experimental results. After
amplification, the specificity of the PCR was determined by both
melting curve analysis and gel electrophoresis to verify that only
a single product of the correct size was present. Data were
normalized against GAPDH, and all samples were amplified for 3
replications. Data are presented as the average fold increase ±
standard error of mean (SEM).
 | Table I.Primers for reverse
transcription-quantitative PCR. |
Table I.
Primers for reverse
transcription-quantitative PCR.
| Accession no. | Gene | Sequence of forward
and reverse primers 5′-3′ | Amplicon length
(bp) |
|---|
| AF121906.1 | SOD | F
CTACCTTCCGGATAGAGGAT | 123 |
|
|
| R
TCCCTGTGATCTTGGATAAG |
|
| NM_009804.2 | CAT | F
TACACAAAGGTGTTGAACGA | 120 |
|
|
| R
GTGGACGTCAGTGAAATTCT |
|
| AF045768.1 | GPx4 | F
CAAGAAATCAAGGAGTTTGC | 107 |
|
|
| R
ACTTTCATCCATTTCCACAG |
|
| X61093.1 | GLUT3 | F
AGATCCAGGAGATGAAGGAT | 117 |
|
|
| R
GAGGACAATGGAGATGAGAA |
|
| AF058054.1 | MCT2 | F
GGATTGGGATTTGGAAGTAT | 119 |
|
|
| R
AGAACTGGACAACACTCCAC |
|
| AF178954.1 | MCT4 | F
GTGGTGAGCTATGCTAAGGA | 120 |
|
|
| R
CTTGAGGCCTGTTATGAAAC |
|
| GU214026.1 | GAPDH | F
ATTGTCAGCAATGCATCCTG | 102 |
|
|
| R
ATGGACTGTGGTCATGAGCC |
|
Western blot analysis
Tissues were lysed in RIPA buffer (Promega
Corporation, Madison WI, USA) containing protease inhibitor
cocktail (Roche Diagnostics, Basel, Switzerland). The
concentrations of total protein in the whole-cell lysate were
determined by the Bradford assay. An equal amount (20 µg) of
protein in each sample was separated by 12% sodium dodecyl
sulphate-polyacrylamide gel electrophoresis and then transferred to
a polyvinylidene difluoride membrane (EMD Millipore, Bedford, MA,
USA). The membrane was incubated with 5% (w/v) skim milk in
phosphate-buffered saline (PBS; pH 7.4) containing 0.05% Tween-20
(PBS-T) at 20°C for 1 h and then probed with anti-5′ adenosine
monophosphate-activated protein kinase (AMPK) antibody (1:800
dilution; cat. no. ab32047, Abcam, Cambridge, MA, USA), anti-p-AMPK
antibody (1:1,500 dilution; cat. no. ab23875, Abcam), and
anti-β-actin antibody (1:1,000 dilution; cat. no. ab8227, Abcam)
for 2 h at room temperature. The membrane was washed in PBS-T and
then incubated with horseradish peroxidase-conjugated goat
anti-rabbit IgG secondary antibody (dilution 1:1,500; cat. no.
sc-2004; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) for 1
h at room temperature. Reactive proteins were detected using
enhanced chemiluminscent and SuperSignal Chemiluminescent
Substrates (Pierce; Thermo Fisher Scientific, Inc.). The protein
intensity was semi-quantified using the ChemiDoc MP Imaging System
(version 5.1.2; Bio-Rad Laboratories, Inc.).
Statistical analysis
Data were analysed using SPSS 17.0 software (SPSS,
Inc., Chicago, IL, USA). All experimental data are presented as the
mean ± SEM. One-way analysis of variance (ANOVA) and Dunnett's post
hoc test were employed when comparisons were performed between a
control group and >1 experimental group to identify their
differences. A P-value <0.05 was considered to indicate a
statistically significant result.
Results
EB causes male mice azoospermia
The weights of the cauda epididymides and sperm
counts were recorded (Table II),
which revealed that there was a significant decrease in caudal
epididymis weight in EB-treated mice (both P<0.05). As indicated
by sperm suspension analysis, the control mice exhibited normal
sperm counts; however, no sperm were observed in EB-treated mice
(both P<0.01 vs. control).
 | Table II.Effect of EB on sperm quantity of
mice (mean ± SEM). |
Table II.
Effect of EB on sperm quantity of
mice (mean ± SEM).
| Dosages
(mg/kg) | Weight of cauda
epididymidis (mg) | Sperm count
(106/100 mg) |
|---|
| 0 | 12±16 | 14.56±1.48 |
| 5 | 7±12a | 0±0b |
| 10 | 6±11a | 0±0b |
H&E and PAS staining were performed to reveal
the morphology of spermatogenic cells at different developmental
stages in the seminiferous tubules. Representative spermatogonium,
primary spermatocyte, and step 7 spermatids were indicated by the
black arrows (Fig. 1A, right).
Through statistical analysis, it was determined that 5 and 10 mg/kg
EB decreased all the number of spermatogonium (P<0.05), primary
spermatocyte (P<0.05), and step 7 spermatids (P<0.05) in the
seminiferous tubules (Fig. 1A,
left).
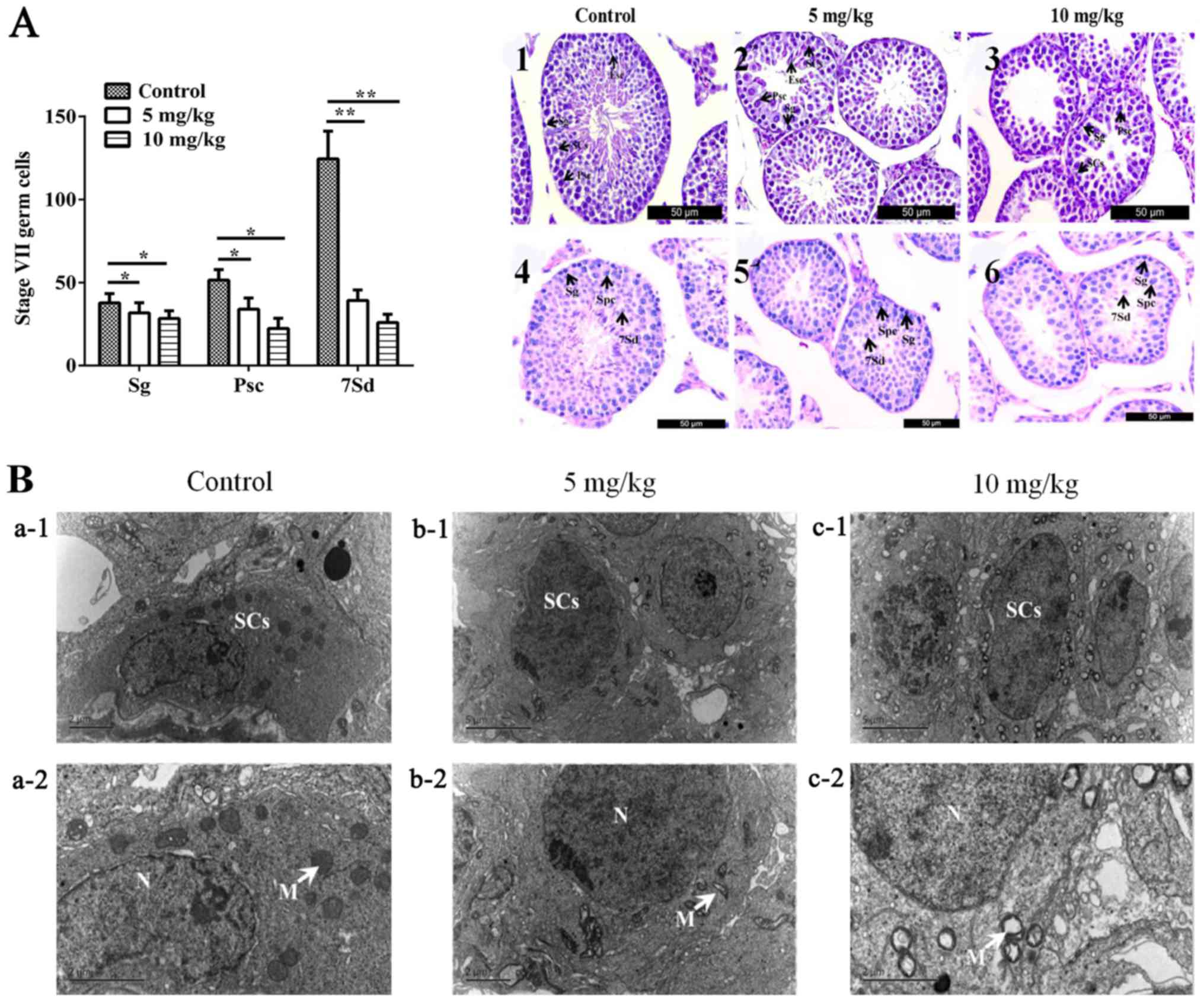 | Figure 1.Effects of EB on the number of
testicular cells and the ultrastructure of Sertoli cells. (A, left
panel) The numbers of spermatogonia and spermatocytes at stage VII
of the spermatogenic cycle in 100 seminiferous tubules. *P<0.05,
**P<0.01. (A, right panel) (1–3)
testes stained with H&E; (4–6)
testes stained with PAS. Sg, Psc, 7Sd, and SCs are indicated by
black arrows. (B) Control groups (a-1 and −2); mice treated with 5
mg/kg/day oestradiol benzoate (b-1 and −2); mice treated with 10
mg/kg/day oestradiol benzoate (c-1 and −2). N, means cell nucleus;
mitochondria are indicated by black arrows. EB, oestradiol
benzoate; H&E, haematoxylin and eosin; PAS, periodic
acid-Schiff; Sg, spermatogonia; Psc, primer spermatocytes; 7Sd,
step 7 spermatids; SCs, Sertoli cells. |
Ultrastructural changes of Sertoli
cells
The nuclei of SCs exhibited an irregular oval shape
at the ultrastructural level, and the nuclei were prominent in most
sections of the SCs (Fig. 1Ba-1).
The electron-dense perinuclear heterochromatin clumps could also be
observed in the SCs. The cytoplasm of SCs contained numerous
mitochondria, which had a circular outline with an electron-dense
matrix and complete cristae (Fig.
1Ba-2). The ultrastructure of SCs in EB-treated groups
displayed irregular oval-shaped nuclei and electron-dense
perinuclear heterochromatin clumps (Fig. 1Bb-1 and c-1). However, almost all
SCs had marked alterations in mitochondria. In the 5 mg/kg
EB-treated group, the mitochondria were transformed to vacuoles
with irregular cristae (Fig.
1Bb-2); in the 10 mg/kg EB-treated group, the mitochondria
became giant organelles with irregular cristae and vacuoles were
clearly observable (Fig.
1Bc-2).
Oxidative stress in the testis
Compared with the control group, the activities of
SOD, CAT, and GSH-Px were significantly decreased (P<0.05;
Fig. 2B) by EB treatments, while
the contents of MDA and NO as well as NOS activity (P<0.05 or
P<0.01, Fig. 2B) were
increased. In addition, the mRNA expression levels of SOD
(P<0.05), CAT (P<0.05), and GSH-Px (P<0.01) in the
EB-treated group were significantly reduced (Fig. 2A).
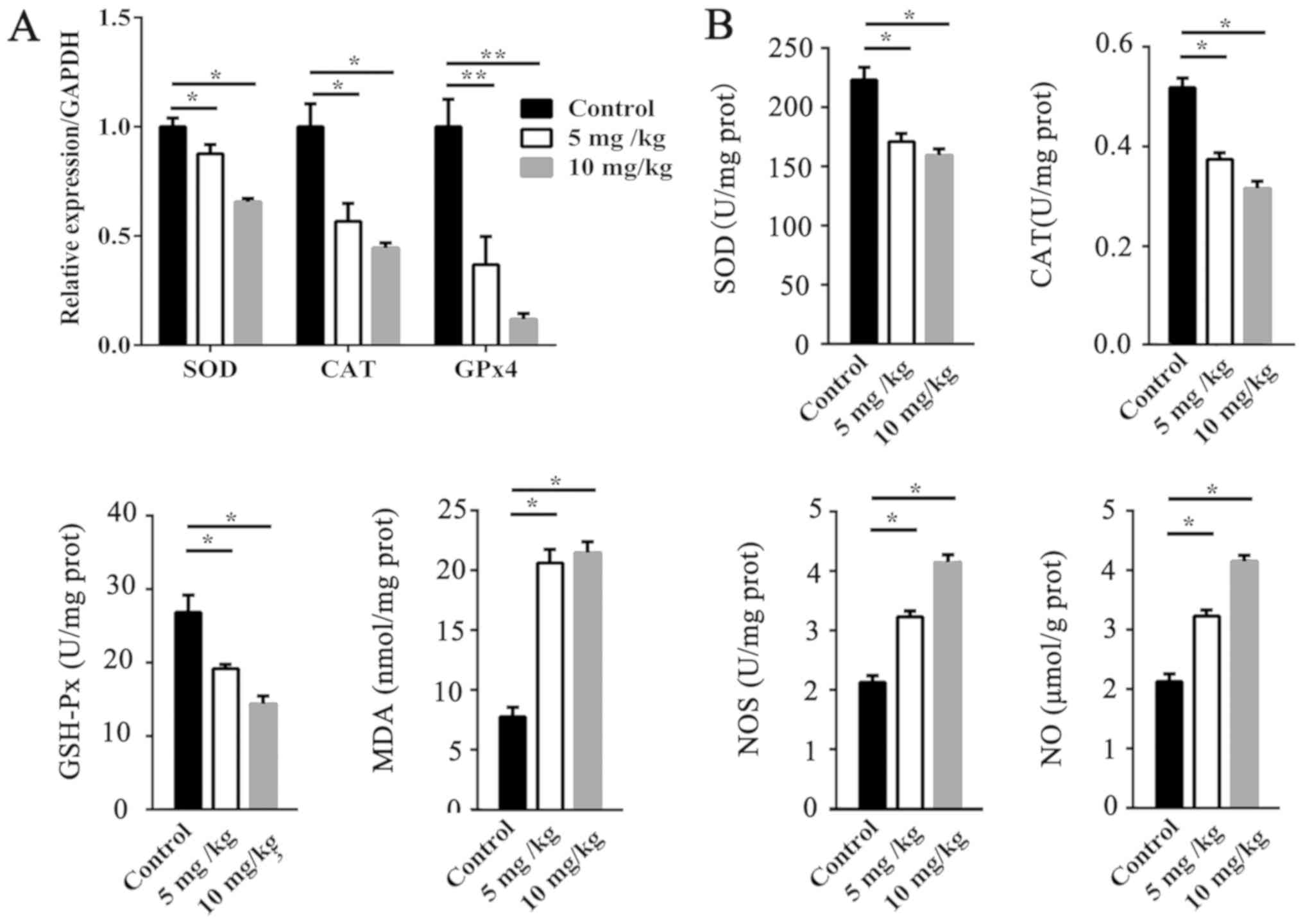 | Figure 2.Effect of EB on antioxidative balance
in testes. (A) SOD, CAT, and GPx4 mRNA levels in the testes. (B)
Activities of SOD, CAT, and GPxs and NOS as well as the levels of
MDA and NO in testes. Results are expressed as the means ± SEM (n=5
for each condition). *P<0.05, **P<0.01. EB, oestradiol
benzoate; SOD, superoxide dismutase; CAT, catalase; GPx,
glutathione peroxidise; NOS, nitric oxide synthase; MDA,
malondialdehyde; NO, nitric oxide; SEM, standard error of mean. |
Metabolic profile of the testis after
EB treatment
HPLC analysis confirmed that ATP content in
EB-treated groups was significantly decreased compared with the
control groups (P<0.05; Fig.
3A), while the testicular glucose and pyruvate were increased
(P<0.01; Fig. 3B). The activity
of AMPK is primarily assessed by the protein phosphorylation level.
GLUT3, MCT2 and MCT4 are transporters. Their functions are mainly
affected by their gene expression. Protein levels of AMPK and
p-AMPK were decreased by EB in a dose-dependent manner (Fig. 3C). The p-AMPK/AMPK ratio was
decreased by 5 mg/kg EB (P<0.05), but increased by 10 mg/kg EB
(P<0.05). The relative mRNA expression of GLUT3, MCT2, and MCT4
is displayed in Fig. 3D. The mRNA
expression level of GLUT3 was significantly upregulated by EB
(P<0.05), whereas MCT2 and MCT4 expression in the testis was
downregulated (P<0.05). There was no difference in the
concentration of lactate between EB-treated and control groups
(data not shown). The activities of HK and PK were significantly
decreased by EB (P<0.05; Fig.
3E), however the activity of LDH was almost unchanged between
the 3 groups (data not shown).
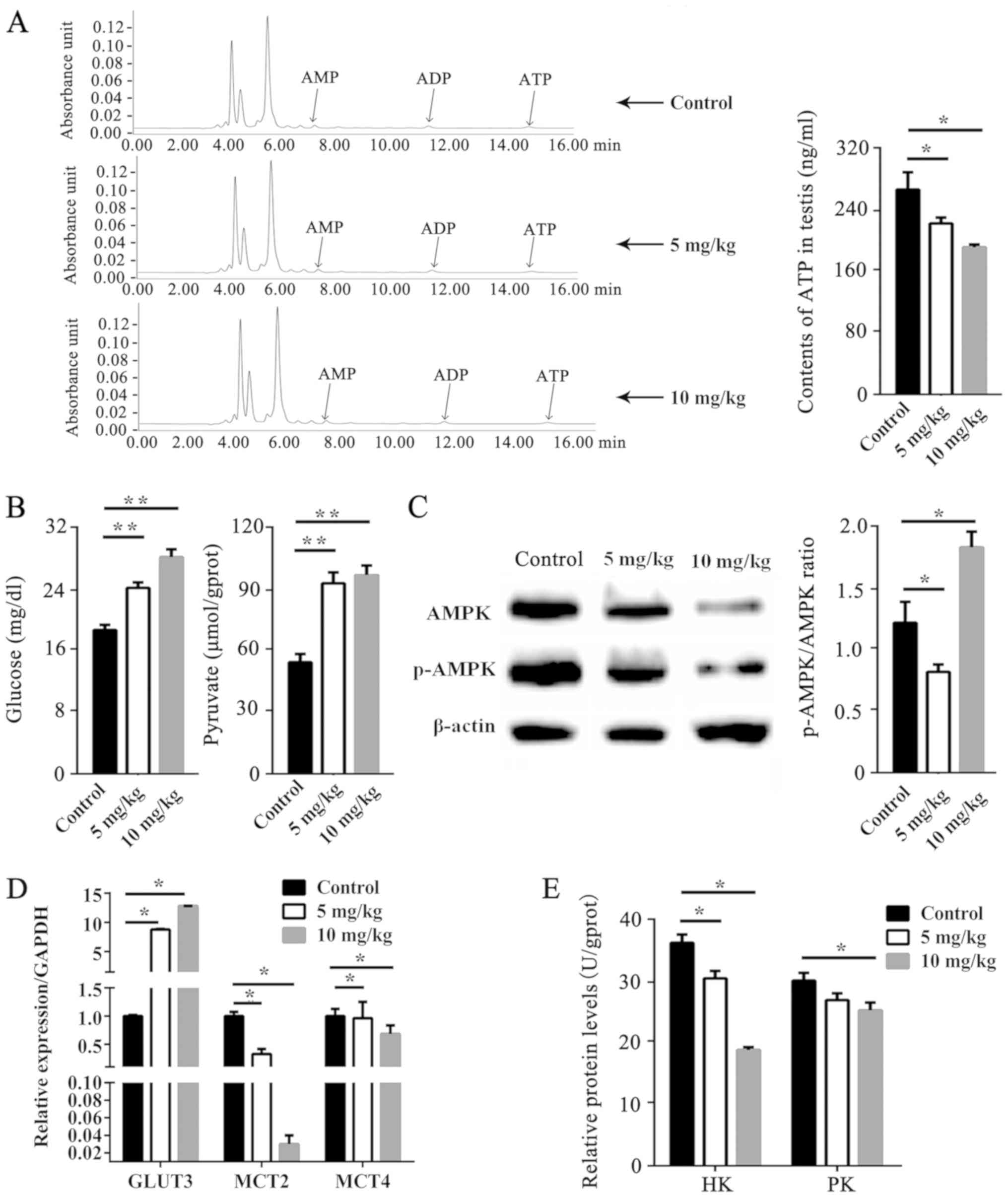 | Figure 3.Effect of EB on testicular energy
metabolism. (A) ATP content in mice testis. (B) Content of glucose
and pyruvate in mice testis. (C) AMPK and p-AMPK protein levels.
(D) mRNA levels of GLUT3, MCT2, and MCT4 in the testis. (E)
Activities of HK and PK in mice testis. Results are expressed as
the means ± SEM (n=5 for each condition). Relative to the control,
*P<0.05, **P<0.01. EB, oestradiol benzoate; ATP, adenosine
triphosphate; AMPK, 5′ adenosine monophosphate-activated protein
kinase; GLUT3, glucose transporter 3; MCT2, monocarboxylate
transporter 2; MCT4, monocarboxylate transporter 4; HK, hexokinase;
PK, pyruvate kinase; SEM, standard error of mean. |
Discussion
Environmental pollution by natural or chemically
synthetic oestrogen has been confirmed to disrupt the normal
function of male reproduction (23–25).
Epidemiological studies have suggested a strong correlation between
exposure to oestrogen and alterations to the reproductive system,
infertility, and increased risk of seminomas and malformations
(24). Our preliminary
experimental study indicated that EB impaired spermatogenesis
through apoptosis and ER signalling pathways (11). In agreement with previous studies,
decreased sperm counts in male mice were observed following EB
treatment. Exposure to EB decreased the number of spermatogonia,
spermatocytes, and step 7 spermatids, which was similar to the
effect caused by BPA (26). At the
ultrastructural level, the transformation of SC mitochondria to
vacuoles with irregular cristae in EB-treated groups indicated that
EB damaged the structure of SC mitochondria and induced
mitochondrial dysfunction. SCs are often referred to as ‘nurse
cells’, responsible for providing energy and nutrition to support
the growth and development of spermatogenic cells; therefore, the
structural destruction of mitochondria in SCs is very likely to
influence the energy supply to spermatogenic cells.
Oxidative damage induced by EB in the testis was
also observed in the present study. Previous studies have revealed
that oral gavage quinestrol causes testicular damage via oxidative
stress, as indicated by changes in MDA concentration and the
activities of SOD and GSH-Px (21). The present results revealed that
the activities of antioxidant enzymes including CAT, SOD, and
GSH-Px in the testis of the EB-treated animals were significantly
decreased, but the level of lipid peroxidation product (MDA) was
increased. Decreased CAT and SOD activities impair the elimination
of reactive oxygen species (ROS), which can lead to germ cell
dysfunction or even death (27).
GSH-Px can scavenge alkyl (R·), RO·, and ROO· radicals that may
form from oxidized membrane components and protect the structure
and function of testicular cells from oxidative damage (28). Thus, the depletion of testicular
GSH-Px levels by EB may ultimately render testicular cells even
more sensitive to oxidative stress. Polyunsaturated fatty acids are
important components of the cell membrane, and they play an
important role in the maintenance of membrane structure and
functions. Endogenous MDA mainly results from lipid peroxidation of
polyunsaturated fatty acids. Therefore, MDA is often used to assess
the impairment of the cell membrane in response to oxidative
species (29–31). The increase in MDA content of the
EB-treated group indicated the impairment of the cellular membrane
structure. Previous studies revealed that E2 induced NOS activation
along with increased NO levels in cerebral and hepatic cells
(32,33). The present study revealed that NO
and NOS were also significantly increased in the testis after EB
treatment, which may aggravate damage to sperm cells.
Mitochondria are the main organelles that produce
ROS; they can also be attacked by ROS in turn (34). It was thus suggested that the
ultrastructural changes to the mitochondria in SCs may result from
oxidative damage or direct impairment by toxic metabolites that are
accumulated during metabolic disorder (35). Since ATP production can be impaired
by oxidative stress (36), the
decreased ATP content in the testis following EB treatment may be
the result of EB disrupting ATP synthesis in SCs, ultimately
leading to energy supply disorder in germ cells. Theoretically, ATP
reduction could induce the activation of 5′ adenosine
monophosphate-activated protein kinase (AMPK); however, it was
revealed that the expression of AMPK and p-AMPK protein was
significantly decreased in mice testes. AMPK is a conserved sensor
of cellular energy change and maintains energy balance by
regulating ATP synthesis and consumption (37). Prior to the present study, there
was no evidence that the expression of AMPK in the testes is
affected by oestrogen. Furthermore, the causes of AMPK
downregulation are still unclear and require further investigation.
It was speculated that ROS is an important cause of the
downregulation of AMPK. On the one hand, ROS has an impact on
cell-signaling proteins (NF-κB, MAPKs, Keap1-Nrf2-ARE, and
PI3K-Akt), ion channels and transporters [Ca(2+) and mPTP], and
modifying protein kinase and ubiquitination/proteasome system, as
indicated by a literature review (38), therefore it is possible that ROS
downregulates AMPK by impacting other signaling pathways or
ubiquitination; on the other hand, overproduced ROS can directly
interact with proteins, resulting in the impairment of protein
functions. Theoretically, ROS-mediated damage of molecules
implicated in the transcriptional regulation of AMPK may cause its
downregulation.
Spermatogenesis is highly dependent on metabolic
cooperation between SCs and germ cells in growth. SCs produce
lactate primarily from glucose, and the rate-limiting step is the
membrane passage of glucose from the extracellular space via
specific GLUTs (14,39). However, 17β-oestradiol (E2)
downregulated the transcript levels of GLUT1 and GLUT3 in SCs in
vitro (16). In contrast to
previous research that revealed that BPA and oestradiol decrease
testicular glucose levels (40),
the present study found that EB increased glucose levels and GLUT3
mRNA expression in testis. Exogenous oestrogen may exert a
different effect on glucose metabolism. With the assistance of HK
and PK, glucose can be transformed to pyruvate, which is used as a
substrate in the tricarboxylic acid cycle in mitochondria, or
reduced to lactate in the cytoplasm (41,42).
The present data revealed that the amount of lactate in the testis
was not changed by EB, suggesting pyruvate was not used for the
production of lactate in any considerable quantity. In addition,
mitochondria in SCs were damaged after EB treatment, as indicated
by their anomalous structure and decreased ATP production.
Therefore, pyruvate could not be oxidized in the tricarboxylic acid
cycle, and thus it accumulated in the testis. High levels of
pyruvate adversely affect spermatogenesis (43,44).
Although EB increased glucose levels in the testis, it was not
effectively used for producing ATP; the question of why pyruvate
was not transformed to lactate in considerable quantities is an
interesting one. It was revealed that the transcriptional levels of
both MCT2 and MCT4 were significantly downregulated in the
EB-treated group. The lactate produced by SCs can be exported by
MCT4, and the lactate in the intratubular fluid is taken up by germ
cells through MCT2 (45).
Therefore, decreased MCT2 and MCT4 levels inhibit the export of
lactate from SCs and its import to germ cells. The accumulation of
lactate in SCs possibly prevents the transformation of pyruvate to
lactate by a negative feedback mechanism.
Based on our data, the detrimental effect of EB on
male reproductive function is associated with both the
overproduction of oxidative species and the alteration of
testicular metabolic cooperation. Thus, additional supplement of
conventional antioxidants may not be sufficient to eliminate the
detrimental effect of EB, although the alteration of testicular
metabolic cooperation was partly due to the detrimental function of
ROS. The improvement of the energy metabolism of testis in addition
to the supplement of an antioxidant after exposure to EB is
suggested.
Collectively, the present results revealed that the
exposure of male mice to EB induced azoospermia through oxidative
damage and the disruption of testicular metabolic cooperation
between SCs and germ cells. It is likely that EB downregulation of
MCT2 and MCT4 is the molecular basis underlying the metabolic
dysfunction of SCs and germ cells. Further study is required to
ascertain whether the resumption of MCT2 and MCT4 expression can
antagonise the destructive effect of EB on spermatogenesis.
Acknowledgements
Not applicable.
Funding
The present study was supported by the China
National Natural Science Fund (grant nos. 31460282 and 81860733),
The Science and Technology Fund of Guizhou province (grant. no.
Qian Basic[2019]1344), the Talent Growth Project of Youth Science
and Technology of Guizhou Province Education Department [no.
(2016)210], and the PhD Start-up Fund of Zunyi Medical University
(no. F-861).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
XL, ZL, and YR were responsible for the experimental
design. ZL, YR and JL performed the experiments of the study and
wrote the manuscript. HT, FD, XY and YZ analyzed the data. SZ and
QL interpreted and collected the data, and revised the manuscript.
All authors read and approved the manuscript and agree to be
accountable for all aspects of the research in ensuring that the
accuracy or integrity of any part of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
The study was approved by The Ethics Committee of
Zunyi Medical University (Guizhou, China). The present study was
approved and monitored by the Animal Experiments Ethical Review
Committee of the Zunyi Medical University (no. ZMU210500326). All
procedures strictly adhered to the National Institutes of Health
guidelines for the care and use of animals (18).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
O'Donnell L, Robertson KM, Jones ME and
Simpson ER: Estrogen and spermatogenesis. Endocr Rev. 22:289–318.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pavlovich CP, King P, Goldstein M and
Schlegel PN: Evidence of a treatable endocrinopathy in infertile
men. J Urol. 165:837–841. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jensen TK, Toppari J, Keiding N and
Skakkebaek NE: Do environmental estrogens contribute to the decline
in male reproductive health? Clin Chem. 41:1896–1901.
1995.PubMed/NCBI
|
|
4
|
Li X, Li H, Jia L, Li X and Rahman N:
Oestrogen action and male fertility: Experimental and clinical
findings. Cell Mol Life Sci. 72:3915–3930. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Giwercman A: Estrogens and phytoestrogens
in male infertility. Curr Opin Urol. 21:519–526. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
LaRocca J, Boyajian A, Brown C, Smith SD
and Hixon M: Effects of in utero exposure to Bisphenol A or
diethylstilbestrol on the adult male reproductive system. Birth
Defects Res B Dev Reprod Toxicol. 92:526–533. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vitku J, Sosvorova L, Chlupacova T, Hampl
R, Hill M, Sobotka V, Heracek J, Bicikova M and Starka L:
Differences in bisphenol A and estrogen levels in the plasma and
seminal plasma of men with different degrees of infertility.
Physiol Res. 64 (Suppl 2):S303–S311. 2015.PubMed/NCBI
|
|
8
|
Chaki SP, Misro MM, Gautam DK, Kaushik M,
Ghosh D and Chainy GB: Estradiol treatment induces testicular
oxidative stress and germ cell apoptosis in rats. Apoptosis.
11:1427–1437. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Walker DM, Kermath BA, Woller MJ and Gore
AC: Disruption of reproductive aging in female and male rats by
gestational exposure to estrogenic endocrine disruptors.
Endocrinology. 154:2129–2143. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Reilly MP, Weeks CD, Topper VY, Thompson
LM, Crews D and Gore AC: The effects of prenatal PCBs on adult
social behavior in rats. Horm Behav. 73:47–55. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lei X, Cui K, Liu Q, Zhang H, Li Z, Huang
B and Shi D: Exogenous estradiol benzoate induces spermatogenesis
disorder through influencing apoptosis and oestrogen receptor
signalling pathway. Reprod Domest Anim. 51:75–84. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cairns RA, Harris IS and Mak TW:
Regulation of cancer cell metabolism. Nat Rev Cancer. 11:85–95.
2011. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Patnaik A, Locasale JW and Cantley LC:
Cancer cell metabolism. Springer; US: 76. pp. 299–311. 2012
|
|
14
|
Rato L, Alves MG, Socorro S, Duarte AI,
Cavaco JE and Oliveira PF: Metabolic regulation is important for
spermatogenesis. Nat Rev Urol. 9:330–338. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Oliveira PF and Alves MG: Sertoli Cell
Metabolism and Spermatogenesis. Springerbriefs in Cell Biology.
1st. Springer International Publishing; pp. 982015
|
|
16
|
Martins AD, Alves MG, Simões VL, Dias TR,
Rato L, Moreira PI, Socorro S, Cavaco JE and Oliveira PF: Control
of Sertoli cell metabolism by sex steroid hormones is mediated
through modulation in glycolysis-related transporters and enzymes.
Cell Tissue Res. 354:861–868. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Putz O, Schwartz CB, Kim S, LeBlanc GA,
Cooper RL and Prins GS: Neonatal low- and high-dose exposure to
estradiol benzoate in the male rat: I. Effects on the prostate
gland. Biol Reprod. 65:1496–505. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
National Research Council (US) Institute
for Laboratory Animal Research, . Guide for the Care and Use of
Laboratory Animals. Astronomy & Astrophysics. 327:963–965.
2004.
|
|
19
|
Gouyandeh J, Modaresi M, Mansouri S and
Najafabadi FY: Long-term effects of betamethasone on epididymal
tissue, epididymal sperm counts and fertility in male mice. J Chem
Health Risks. 5:295–300. 2015.
|
|
20
|
Clermont Y and Perey B: The stages of the
cycle of the seminiferous epithelium of the rat: Practical
definitions in PA-Schiff-hematoxylin and hematoxylin-eosin stained
sections. Rev Can Biol. 16:451–462. 1957.PubMed/NCBI
|
|
21
|
Nikolaidou B, Nouris C, Lazaridis A,
Sampanis C and Doumas M: Diabetes mellitus and erectile
dysfunction. Springer International Publishing; pp. 119–128.
2015
|
|
22
|
Welborn JP, Davis MG, Ebers SD, Stodden
GR, Hayashi K, Cheatwood JL, Rao MK and MacLean JA III: Rhox8
ablation in the sertoli cells using a tissue-specific RNAi approach
results in impaired male fertility in mice. Biol Reprod. 93:82015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sharpe RM: Environmental estrogens and
male infertility. Pure App Chem. 70:1685–1701. 1998. View Article : Google Scholar
|
|
24
|
Rochester JR: Bisphenol A and human
health: A review of the literature. Reprod Toxicol. 42:132–155.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Rao MV and Chinoy NJ: Effect of oestradiol
benzoate on reproductive organs and fertility in the male rat. Eur
J Obstet Gynecol Reprod Biol. 15:189–198. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jin P, Wang X, Chang F, Bai Y, Li Y, Zhou
R and Chen L: Low dose bisphenol A impairs spermatogenesis by
suppressing reproductive hormone production and promoting germ cell
apoptosis in adult rats. J Biomed Res. 27:135–144. 2013.PubMed/NCBI
|
|
27
|
Samhan-Arias AK, Tyurina YY and Kagan VE:
Lipid antioxidants: Free radical scavenging versus regulation of
enzymatic lipid peroxidation. J Clin Biochem Nutr. 48:91–95. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Goc Z, Szaroma W, Kapusta E and Dziubek K:
Protective effects of melatonin on the activity of SOD, CAT, GSH-Px
and GSH content in organs of mice after administration of SNP. Chin
J Physiol. 60:1–10. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Omar SS, Aly RG and Badae NM: Vitamin E
improves testicular damage in streptozocin-induced diabetic rats,
via increasing vascular endothelial growth factor and
poly(ADP-ribose) polymerase-1. Andrologia. 50:2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wiktorowska-Owczarek A, Berezińska M and
Nowak JZ: PUFAs: Structures, metabolism and functions. Adv Clin Exp
Med. 24:931–941. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Schroeder F, Kier AB and Sweet WD: Role of
polyunsaturated fatty acids and lipid peroxidation in LM fibroblast
plasma membrane transbilayer structure. Arch Biochem Biophys.
276:55–64. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sakamoto M, Ueno T, Nakamura T, Sakata R,
Hasimoto O, Torimura T and Sata M: Improvement of portal
hypertension and hepatic blood flow in cirrhotic rats by oestrogen.
Eur J Clin Invest. 35:220–225. 2015. View Article : Google Scholar
|
|
33
|
Nevzati E, Shafighi M, Bakhtian KD,
Treiber H, Fandino J and Fathi AR: Estrogen induces nitric oxide
production via nitric oxide synthase activation in endothelial
cells. Acta Neurochir Suppl. 120:141–145. 2015.PubMed/NCBI
|
|
34
|
Al-Gubory KH: Mitochondria: Omega-3 in the
route of mitochondrial reactive oxygen species. Int J Biochem Cell
Biol. 44:1569–1573. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Stepien KM, Heaton R, Rankin S, Murphy A,
Bentley J, Sexton D and Hargreaves IP: Evidence of oxidative stress
and secondary mitochondrial dysfunction in metabolic and
non-metabolic disorders. J Clin Med. 6(pii): E712017. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Liang H, Remmen HV, Frohlich V, Lechleiter
J, Richardson A and Ran Q: GSH-Px4 protects mitochondrial ATP
generation against oxidative damage. Biochem Biophys Res Commun.
356:893–898. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ke R, Xu Q, Li C, Luo L and Huang D:
Mechanisms of AMPK in the maintenance of ATP balance during energy
metabolism. Cell Biol Int. 42:384–392. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang J, Wang X, Vikash V, Ye Q, Wu D, Liu
Y and Dong W: Ros and ros-mediated cellular signaling. Oxid Med
Cell Longev. 2016:43509652016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kishimoto A, Ishiguro-Oonuma T, Takahashi
R, Maekawa M, Toshimori K, Watanabe M and Iwanaga T:
Immunohistochemical localization of GLUT3, MCT1, and MCT2 in the
testes of mice and rats: The use of different energy sources in
spermatogenesis. Biomed Res. 36:225–234. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
D'Cruz SC, Jubendradass R and Mathur PP:
Bisphenol A induces oxidative stress and decreases levels of
insulin receptor substrate 2 and glucose transporter 8 in rat
testis. Reprod Sci. 19:163–172. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rato L, Alves MG, Dias TR, Cavaco JE and
Oliveira PF: Testicular metabolic reprogramming in neonatal
streptozotocin-induced type 2 diabetic rats impairs glycolytic flux
and promotes glycogen synthesis. J Diabetes Res. 2015:9731422015.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tavares RS, Portela JMD, Sousa MI, Mota
PC, Ramalho-Santos J and Amaral S: High glucose levels affect
spermatogenesis: An in vitro approach. Reprod Fertil Dev.
29:1369–1378. 2016. View Article : Google Scholar
|
|
43
|
Oliveira PF and Alves MG: Modulation of
sertoli cell metabolism. Springer International Publishing; pp.
57–71. 2015
|
|
44
|
Oishi S: Effects of phthalic acid esters
on testicular mitochondrial functions in the rat. Arch Toxicol.
64:143–147. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Oliveira PF, Martins AD, Moreira AC, Cheng
CY and Alves MG: The warburg effect revisited-lesson from the
sertoli cell. Med Res Rev. 35:126–151. 2015. View Article : Google Scholar : PubMed/NCBI
|

















