Introduction
The function of the ‘blood-biliary barrier’ is to
ensure bile secretion without leakage from the centrizonal region
to the periportal zone and then to bile ducts, in a highly ordered
manner (1). Because gap junctions
(GJs) regulate direct intercellular communication and tight
junctions (TJs) completely seal the bile canaliculi, these are
essential to maintain the function of the blood-biliary barrier
(1). The most common GJ proteins
in the liver, the connexins (Cx, also termed gap junction proteins)
Cx26 (gap junction protein β2) and Cx32 (gap junction protein β1)
have been found to be downregulated during obstructive cholestasis
and lipopolysaccharide (LPS)-induced hepatocellular cholestasis
(2). In addition, expression of TJ
proteins, such as tight junction protein 1 (ZO1/TJP1), has also
been reported to be influenced by LPS or liver injury (3,4).
Alterations in GJ and TJ composition are associated with hepatic
disease and lead to cholangiovenous reflux, liver injury, and even
systemic disease (1). Acute
obstructive cholangitis (AOC) is a bacterial infection caused by
biliary obstruction, and it leads to systemic signs of infection
(5). Due to the high biliary
pressure and the presence of LPS, blood-biliary barrier disruption
may occur in cases of AOC (5). In
severe cases of AOC, LPS and cholochrome may be continuously
released into the blood until functional restoration of the barrier
occurs (5). Therefore,
accelerating the restoration of hepatocyte GJs and TJs may improve
patient prognosis and shorten the course of AOC.
Molecular hydrogen (H2) is regarded as an
important physiological regulatory factor with antioxidant effects,
which protect cells and organs from injury caused by reactive
oxygen species (ROS) and oxidative stress (6–8).
Anti-inflammatory effects are another important physiological
regulatory function of H2 (6). H2 may therefore be a
promising therapeutic strategy to combat certain pathologies and
sepsis (9–11). Given that H2 serves
important physiological functions, it is possible that
H2 may have a role in the process of AOC.
Therefore, the present study hypothesized that
H2 administration may help reverse the AOC-induced
disruption of GJs and TJs in hepatocytes and accelerate the tissue
recovery process.
Materials and methods
Experimental animals
Male Wistar rats, weighing 300–350 g (13–16 weeks
old) were purchased from Shanghai SLAC Laboratory Animal Co., Ltd.
All procedures were approved by the Ethics Committee of Zhejiang
University, and conformed to the Care and Use of Laboratory Animals
Guide published by the US National Institutes of Health (NIH
Publication no. 85e23, revised 1996). The rats had ad
libitum access to food and water, and were maintained at 20°C,
with 50% humidity under 12:12-h light-dark cycles.
Establishment of rat AOC models
A total of 19 rats were randomly assigned to the
following three groups (n≥6 rats/group): Sham; bile duct ligation
(BDL); and acute obstructive cholangitis (AOC) groups.
Intraperitoneal injections of pentobarbital (50 mg/kg) were used to
anaesthetize the animals. In the BDL and AOC groups, the distal
common bile ducts were dissociated and ligated with 6–0 silk
sutures. PE-10 polyethylene catheters (~3 cm), which were long
enough to reach the skin surface of the animals, were inserted into
the proximal bile ducts as previously described (12). Intra-bile duct infusions were
performed immediately following surgery. For intra-bile duct
infusions, 0.2 ml of saline or LPS (2 mg/ml, purified from
Escherichia coli O111:B4; Sigma-Aldrich, Merck KGaA) was
injected into the proximal bile ducts through the catheters. After
injection of 0.1 ml air, the catheter was sealed with a sealing cap
and the abdominal cavity was closed using silk sutures. Rats in the
sham group underwent a sham operation.
According to the specific symptoms associated with
this model and the guidelines suggested by previous studies
(13,14), humane endpoints were defined as
lethargy (lack of response to stimulus), hypopnea, cyanosis or
hypothermia at 34°C. Animals that exhibited any of these symptoms
were sacrificed via cervical dislocation under isoflurane
anesthesia. After a 12-h observation period, during which 1 rat was
sacrificed after reaching the aforementioned humane endpoints, the
remaining 18 rats were sacrificed, and blood and tissue samples
were harvested. Blood samples were centrifuged (1,000 × g for 5 min
at 4°C) and serum was stored at −80°C prior to subsequent analysis.
Liver fractions were snap-frozen in liquid nitrogen and then stored
at −80°C.
Preparation of hydrogen-rich saline
(HRS)
HRS was prepared as previously described (15,16).
In brief, HRS was prepared by dissolving hydrogen in physiological
saline for 12 h under high pressure (0.4 MPa). HRS was stored under
atmospheric pressure at 4°C in a sealed bag with no dead volume.
Using gas chromatography according to a previously described
protocol (17), the hydrogen
concentration on the first day after preparation was 0.76±0.05 and
0.62±0.04 mmol/l at 7 days post-preparation. HRS was prepared
weekly to ensure that the concentration of hydrogen was sufficient
for experiments.
HRS administration experiment
A total of 30 AOC rats were divided into three
groups in this part of experiment (n=10). The catheter sealing caps
were removed and bile was allowed to flow out from the catheters at
12 h after LPS infusion. Normal saline (NS, 5 ml/kg) or HRS (5 and
10 ml/kg) was administered intraperitoneally from the day of
surgery (once daily) until the end of the experiment (72 h
following LPS infusion). All rats remained under strict observation
(every 6 h) and time of death was recorded over a 72 h observation
period after LPS infusion. During this observation period, animals
that reached the aforementioned humane endpoints were sacrificed
humanely under anesthesia. All other animals were sacrificed after
the 72 h observation period, and blood and tissue samples were
harvested from these animals only.
Evaluation of liver function
The serum levels of aspartate aminotransferase
(AST), alanine aminotransferase (ALT) and total bilirubin (TBIL)
were measured using an Automated Chemical Analyzer (Dimension
RxLMax HM; Siemens AG) to evaluate the degree of liver injury and
function.
Cell experiments
The rat hepatic cell line BRL was obtained from the
Chinese Academy of Sciences Shanghai Branch Cell Bank. BRL cells
were cultured in DMEM (Gibco, Thermo Fisher Scientific. Inc.)
supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.),
100 IU/ml penicillin and 100 µg/ml streptomycin, at 37°C in an
atmosphere with 5% CO2.
The BRL cells (1×106) were seeded into 60
mm dishes. After 24 h of culture to allow cells to attach, cells
were treated with or without LPS, at 400 ng/ml for 6 h. Cells were
harvested and total proteins were extracted for western blot
analysis.
Similar to the aforementioned method, BRL cells were
treated with or without H2O2, at 200 µmol/l
for 6 h. Cells were harvested and total proteins were extracted for
western blot analysis.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was isolated from liver tissue samples
using TRIzol® (Invitrogen; Thermo Fisher Scientific,
Inc.) and reverse transcribed into cDNA using the High-Capacity
cDNA Reverse Transcription kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.), according to the manufacturer's instructions.
Briefly, RT was conducted at 25°C for 10 min, 37°C for 120 min and
85°C for 5 min; cDNA was stored at 4°C until further use. The
RT-qPCR was performed using SYBR®-Green PCR Master Mix
and the ABI 7500 Real-time PCR system (both Applied Biosystems;
Thermo Fisher Scientific, Inc.). Primers sequences used are
provided in Table I. β-actin was
used as an endogenous control. The RT-qPCR was performed according
to the manufacturer's instructions. Briefly, the PCR conditions
included 95°C for 5 min, and a total of 40 cycles of 95°C, 60°C and
72°C for 30 sec, followed by a final extension at 72°C for 5 min.
All assays were performed three times. Relative expression levels
were then determined using the 2−ΔΔCq method (18).
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Name | Symbol | Forward
(5′-3′) | Reverse
(5′-3′) |
|---|
| β-actin | ACTB |
ACACCCGCCACCAGTTCG |
CCCACGATGGAGGGGAAGA |
| ZO1 | TJP1 |
TCGGAGCTCGGGCATTATTC |
CAGGGCACCATACCAACCAT |
| Occludin | OCLN |
CCCTTCTTTCCTTAGGCGACC |
TGGGTTTGAATTCATCCGGC |
| Cx26 | GJB2 |
CCACTTCTGACCAACCCAGG |
CTCTGGATGGTTGGCACTGT |
| Cx32 | GJB1 |
GACACGCCTGCATACATTCC |
TCCTGCCTCATTCACACCTCC |
| Cx43 | GJA1 |
TGAAAGAGAGGTGCCCAGACA |
CACCCCAAGCTGACTCAACA |
| IL-6 | IL6 |
TCTGCCCTTCAGGAACAGCTAT |
TGTCAACAACATCAGTCCCAAGA |
| TNF-α | TNF |
ACAGCAACTCCAGAACACCC |
GGAGGGAGATGTGTTGCCTC |
| IL-1β | IL1β |
GCTTCCTTGTGCAAGTGTCTG |
AGTCAAGGGCTTGGAAGCAA |
Western blot analysis
Total proteins were extracted from tissue samples or
cells using lysis buffer containing phenylmethyl sulfonylfluoride
(both from Beyotime Institute of Biotechnology) at 25°C, and the
protein concentration was determined using a BCA Protein Assay kit
(Beyotime Institute of Biotechnology). On a 10% SDS-PAGE gel, 20 µg
total protein was electrophoresed, transferred onto to
polyvinylidene fluoride membranes, blocked with 5% non-fat milk for
1 h at room temperature, and incubated with the following primary
antibodies: Anti-ZO1 (1:250; cat. no. ab59720; Abcam);
anti-occludin, (1:500; cat. no. 13409-1-AP; ProteinTech Group,
Inc.); anti-Cx26 (1:500; cat. no. 16960-1-AP; ProteinTech Group,
Inc.); anti-Cx43 (gap junction protein α1; 1:1,000; cat. no. 3512;
Cell Signaling Technology, Inc.); anti-Cx32 (1:250; cat. no.
sc-59948, Santa Cruz Biotechnology, Inc.); and anti-β-actin
(1:1,000; cat. no. sc-4778; Santa Cruz Biotechnology, Inc.). All
primary antibody incubations were performed overnight at 4°C. The
membranes were subsequently incubated with horseradish
peroxidase-conjugated secondary antibodies (1:8,000; cat. nos.
A0216 and A0208; Beyotime Institute of Biotechnology) for 2 h at
room temperature. Immunoreactive bands were visualized using
enhanced chemiluminescence reagent (Beyotime Institute of
Biotechnology). β-actin was employed as an endogenous control.
Quantity One 4.6.8 (Bio-Rad Laboratories, Inc.) was used for the
quantification of expression.
Immunohistochemical staining
Liver tissue samples were fixed with 10% neutral
formalin at room temperature overnight. ZO1 expression was detected
immunohistochemically by sectioning (3-µm thickness)
paraffin-embedded specimens from the different groups. After
deparaffinization and rehydration of the sections, endogenous
peroxidase activity was blocked using 0.3% hydrogen peroxide. The
sections were blocked with 1% bovine serum albumin (Beyotime
Institute of Biotechnology) for 2 h at room temperature. The
sections were incubated with primary anti-ZO1 antibody (1:100; cat.
no. ab59720; Abcam) overnight at 4°C, followed by incubation with
appropriate horseradish peroxidase-conjugated secondary antibodies
(1:4,000; cat. no. A0208; Beyotime Institute of Biotechnology) for
1.5 h at room temperature. After a thorough washing, the sections
were developed using a 3,3′-diaminobenzidine kit (cat. no. P0202;
Beyotime Institute of Biotechnology) and counterstained with
hematoxylin staining solution for 10 min at room temperature (cat.
no. C0107; Beyotime Institute of Biotechnology). Each stained
sample was observed using a light microscope (×200; Leica
Microsystems, Inc.).
Measurement of superoxide dismutase
(SOD) and malondialdehyde (MDA)
Samples were homogenized and sonicated (25°C, ~20–25
kHz, 2 sec) in cold saline to generate 5% homogenates. Aliquots of
supernatants were prepared by centrifugation (4°C, 12,000 × g, 5
min) and used for subsequent experiments. The supernatant was
assayed for protein concentration using an enhanced bicinchoninic
acid protein assay kit (Beyotime Institute of Biotechnology). Both
measurements of SOD activity and MDA content in tissue homogenates
were performed in accordance with the manufacturer's instructions
(cat. nos. A001-3–1 and A003-1-1, respectively; Nanjing Jiancheng
Bio-Engineering Institute Co., Ltd.). All assays were performed
three times.
Gelatin gel zymography
Gelatin gel zymography was performed using a gelatin
zymography assay kit (Shanghai Genmed Pharmaceutical Technology
Co., Ltd.). Briefly, gels with gelatin were prepared in accordance
with the manufacturer's instructions. After electrophoresis, gels
were treated with a renaturation buffer, a digestive buffer, a
staining buffer, an eluent buffer and a stop buffer, all supplied
with the assay kit. The clear bands on the blue background
reflected the proteolytic activity of matrix metalloproteinase
(MMP)-2 and MMP-9.
Statistical analysis
Data are presented as the mean ± standard deviation.
All assays were performed at least three times. Statistical
significance between two groups was determined using the Student's
t-test. One-way analysis of variance followed by Tukey-Kramer
post-hoc tests were performed to examine differences among multiple
groups. P<0.05 was considered to indicate a statistically
significant difference. All statistical analyses were conducted
using SPSS 13.0 (SPSS, Inc., Chicago, IL, USA).
Results
AOC induces disruption of hepatocyte
GJs and TJs
Firstly, hepatic tests were used to verify liver
function in the AOC animal model. ALT, AST and TBIL levels
increased rapidly after BDL compared with those in the sham
operation group, and these were further significantly increased in
the AOC group (data not shown), which is similar to a previous
study (19) and the classical
changes observed in patients with AOC (Table II).
 | Table II.Liver function test results of the
different rat models. |
Table II.
Liver function test results of the
different rat models.
|
| Sham | BDL | AOC |
|---|
| ALT, IU/l | 57.50±7.46 |
518.83±71.86a |
848.67±67.74a,b |
| AST, IU/l | 77.67±8.30 |
798.83±72.78a |
1321.67±121.28a,b |
| TBIL, µmol/l | 10.02±1.65 |
46.72±12.88a |
68.93±10.21a,b |
Secondly, GJ and TJ mRNA and protein expression in
liver tissues were examined. RT-qPCR revealed that TJ protein mRNA
expression in the AOC group, including ZO1 and occludin, decreased
significantly compared with the BDL and sham groups (Fig. 1A). GJ protein expression, including
Cx26 and Cx32, was also downregulated in the AOC group. Cx43
expression, another important GJ protein, was upregulated
significantly in the AOC group compared with the other groups
(Fig. 1A). Western blot analysis
also confirmed that ZO1, occludin, Cx26 and Cx32 expression was
downregulated in the AOC group (Fig.
1B and C). However, Cx43 protein levels did not change, which
was unexpected (Fig. 1B and C).
These results indicated that AOC induces aberrant TJ and GJ mRNA
and protein expression.
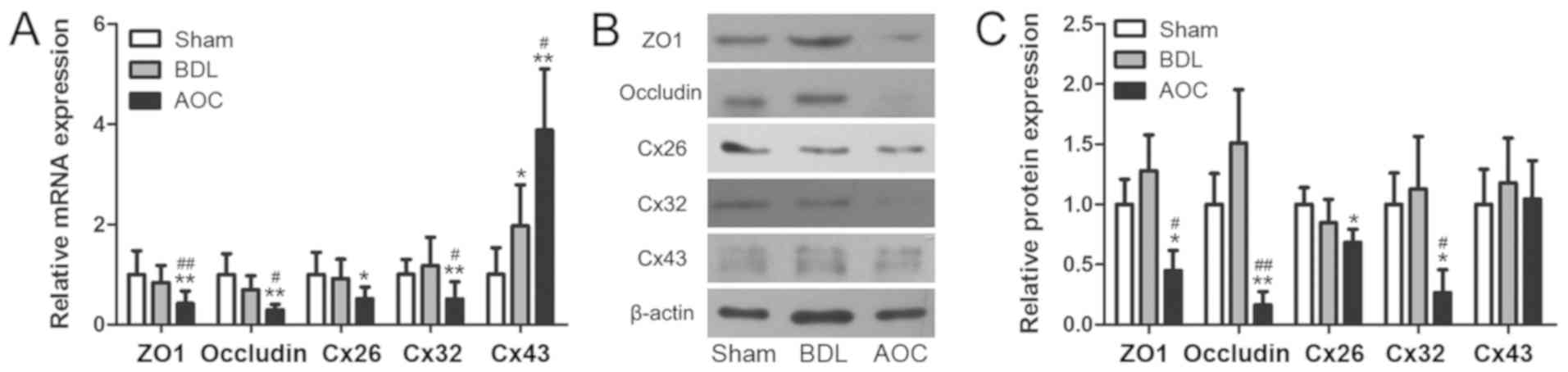 | Figure 1.AOC induces disruptions in hepatocyte
GJs and TJs. (A) Reverse transcription-quantitative PCR was
performed to examine the expression of GJ and TJ genes in liver
tissues from Sham, BDL and AOC rat models. The results demonstrated
that AOC affected the expression level of junction genes. (B)
Western blot and (C) densitometry analyses were performed to
examine the protein levels of GJ and TJ proteins in the same liver
tissues from the rat models, further corroborating the results for
mRNA expression. *P<0.05 and **P<0.01 vs. sham group;
#P<0.05 and ##P<0.01 vs. BDL group.
AOC, acute obstructive cholangitis; BDL, bile duct ligation; ZO1,
tight junction protein 1; Cx26, gap junction protein β2; Cx32, gap
junction protein β1; Cx43, gap junction protein α1; GJ, gap
junction; TJ, tight junction. |
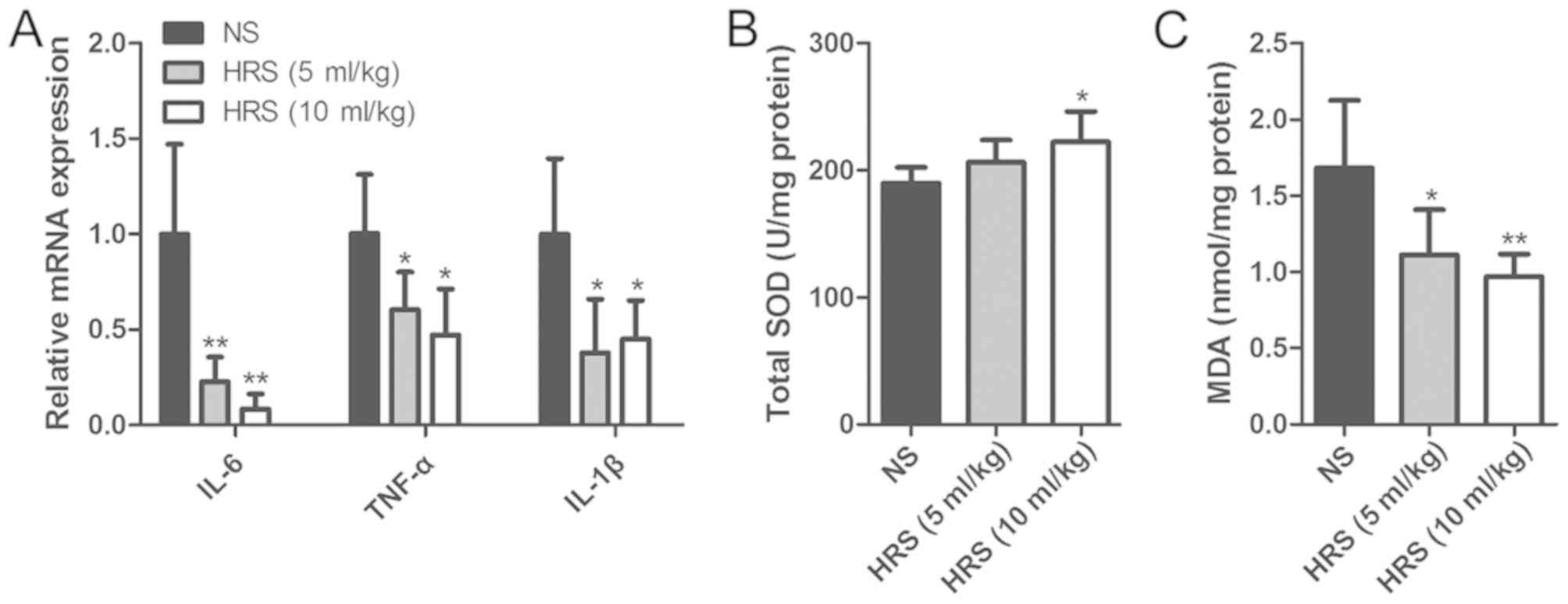
Inflammatory and oxidative damage
induces disruption of junction proteins in hepatocytes
Inflammatory and oxidative damage occurs across a
variety of liver injuries (20,21).
Therefore, the role of inflammatory and oxidative damage in
AOC-induced liver injury was also evaluated in the present study.
The results showed that the mRNA levels of inflammatory cytokines,
including interleukin (IL)-6, tumor necrosis factor-α (TNF-α) and
IL-1β, were upregulated significantly in the AOC group compared
with the other groups (Fig. 2A).
Hepatic SOD and MDA were also measured to evaluate oxidative damage
during AOC. Compared with the BDL group, the AOC group had higher
levels of MDA and lower levels of SOD activity (Fig. 2B and C).
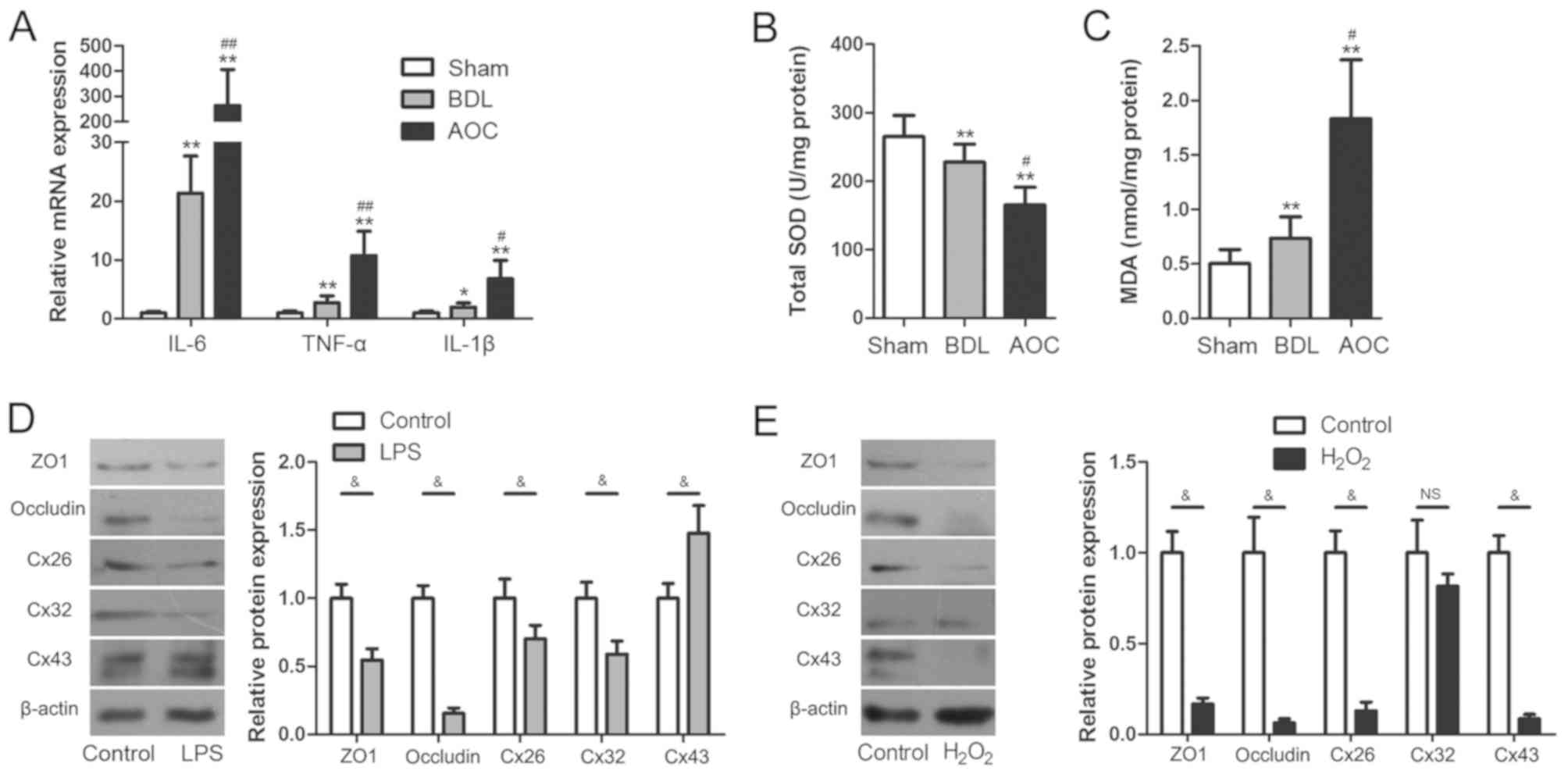 | Figure 2.Inflammatory and oxidative damage
arises during AOC. (A) Reverse transcription-quantitative PCR was
performed to examine the expression of pro-inflammatory cytokines
in liver tissues from different rat models, demonstrating that
these were increased in the BDL and AOC groups compared with the
Sham-operated group. (B) SOD levels decreased, while (C) MDA levels
increased in liver tissues from animals in the BDL and AOC groups
compared with the sham-operated group. (D) Western blotting was
performed to examine the expression level of gap and tight junction
proteins in BRL cells with or without LPS treatment. The protein
levels following LPS treatment were similar to those observed in
vivo. (E) Western blotting was performed to examine the
expression level of gap and tight junction proteins in BRL cells
with or without H2O2 treatment. The levels of
all examined proteins, with the exception of Cx32, were reduced
following H2O2 treatment. *P<0.05 and
**P<0.01 vs. sham group; #P<0.05 and
##P<0.01 vs. BDL group; &P<0.05.
AOC, acute obstructive cholangitis; BDL, bile duct ligation; IL,
interleukin; TNF-α, tumor necrosis factor-α; SOD, superoxide
dismutase; MAD, malondialdehyde; ZO1, tight junction protein 1;
Cx26, gap junction protein β2; Cx32, gap junction protein β1; Cx43,
gap junction protein α1; LPS, lipopolysaccharide; NS,
non-significant. |
In vitro cell experiments were performed to
further elucidate the association between the disruption of
junction proteins and inflammatory or oxidative damage. This was
performed by examining TJ and GJ protein expression levels in
cultured BRL cells after LPS or H2O2
treatment, respectively. The results showed that the expression of
ZO1, occludin, Cx26 and Cx32 was significantly downregulated
following treatment with LPS, while Cx43 expression was upregulated
(Fig. 2D). Following exposure to
H2O2, Cx32 expression did not change
significantly, while ZO1, occludin, Cx26 and Cx43 expression levels
were significantly decreased (Fig.
2E). The cell experiments showed that both inflammatory damage
and oxidative damage induced aberrant TJ and GJ protein expression.
Notably, these changes were similar to those observed in the livers
of AOC rats. These results indicated that aberrant TJ and GJ
protein expression during AOC may be attributed to inflammatory and
oxidative damage.
Molecular hydrogen attenuates
AOC-induced inflammatory and oxidative damage
H2 has been reported to exhibit
antioxidant and anti-inflammatory properties (6). Thus, the potential protective role of
H2 against inflammatory and oxidative liver damage
induced by AOC was further assessed. IL-6, TNF-α and IL-1β were
analyzed to evaluate the levels of inflammation with or without HRS
administration. The results showed that HRS administration
significantly mitigated the increase in inflammatory cytokine
expression (Fig. 3A). Compared
with the NS group, SOD activity was increased in the liver tissues
after administration of HRS (Fig.
3B). Conversely, treatment with HRS significantly lowered the
levels of MDA (Fig. 3C). These
results indicated that H2 may have attenuated the
AOC-induced inflammatory and oxidative damage.
H2 reverses AOC-induced
disruption of junction proteins and decreases the activity of
MMPs
To clarify the relationship between the function of
H2 and the blood-biliary barrier, GJ and TJ mRNA
expression were evaluated after biliary drainage and HRS
administration. The results showed that occludin, Cx26 and Cx32
mRNA expression were upregulated significantly after treatment with
HRS compared with rats that received NS (Fig. 4A). The other junction proteins, ZO1
and Cx43, were not affected by either treatment (Fig. 4A). However, the western blotting
results indicated that all GJ and TJ proteins, including ZO1 and
Cx43, were upregulated significantly following HRS administration
(Fig. 4B and C).
Immunohistochemistry also confirmed that ZO1 protein levels were
seemingly higher in the HRS group (Fig. 4D). The results suggested that the
disruptions to ZO1 and Cx43 were alleviated by HRS treatment.
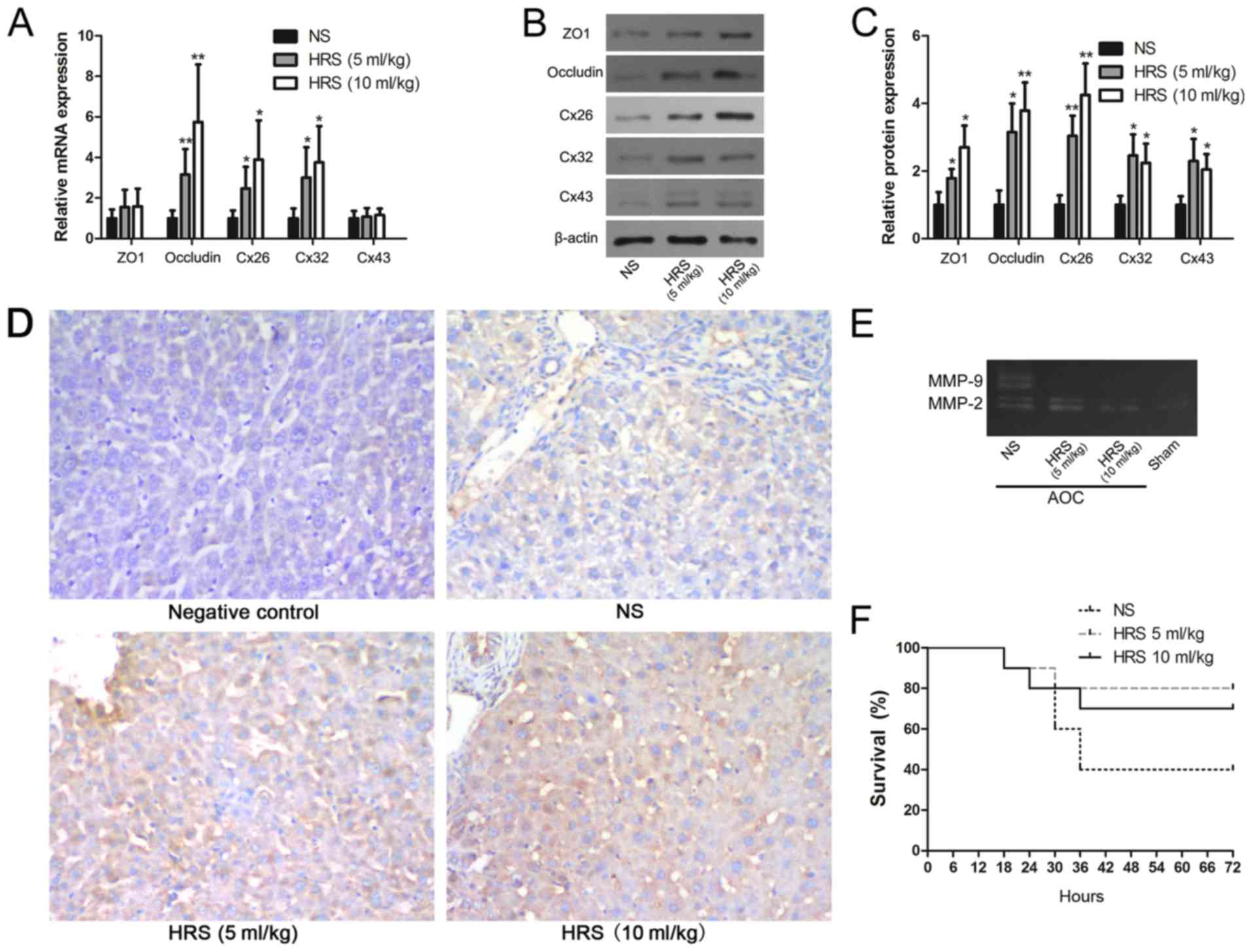 | Figure 4.Molecular hydrogen reverses the
AOC-induced disruption of GJs and TJs. (A) Reverse
transcription-quantitative PCR, (B) western blotting and (C)
respective densitometry analysis were performed to evaluate the
expression of GJ and TJ proteins with or without HRS treatment in
AOC model rats. (D) Immunohistochemical staining of ZO1
(counterstained with hematoxylin) in liver tissues from AOC model
animals treated with NS or different doses of HRS, and from a
control animal (magnification, ×200). (E) Gelatin zymography
demonstrating the downregulation of MMP-2 and MMP-9 activity
following the administration of HRS in AOC rats compared with
NS-treated ones. (F) Survival rates of AOC animals that received
biliary drainage and were subsequently treated with HRS or NS,
showing that HRS treatment increased the survival rates of AOC
animals. *P<0.05 and **P<0.01 vs. respective NS group. AOC,
acute obstructive cholangitis; HRS, hydrogen-rich saline; NS,
normal saline; ZO1, tight junction protein 1; Cx26, gap junction
protein β2; Cx32, gap junction protein β1; Cx43, gap junction
protein α1; MMP, matrix metalloproteinase; GJ, gap junction; TJ,
tight junction. |
MMPs are classically known as matrix-degrading and
barrier-regulating enzymes that are involved in many physiological
and pathological processes (22,23).
Previous studies have shown that MMP-2 and MMP-9 are important
proteins that can degrade ZO1 and Cx43 (24,25).
MMP activity has also been reported to be regulated by oxidative
stress and inflammation (20,26).
The results of the gelatin gel zymography showed that MMP-2 and
MMP-9 activity in the liver decreased markedly in response to
treatment with HRS (Fig. 4E).
Given that MMPs can be influenced by oxidative stress (23), this result suggested another
possible mechanism for AOC-induced GJ and TJ disruption, in
addition to the observed changes in transcription and
translation.
HRS accelerates the reversal of
AOC-induced liver dysfunction in rats
The role of H2 as a potential treatment
for AOC-induced liver dysfunction was further evaluated. Timely
drainage and relief of obstruction are the key treatments for
patients with AOC (5). Thus, the
catheter sealing caps were removed and bile was allowed to flow out
12 h after LPS infusion into the bile duct. Moreover, the animals
in the various groups were subsequently treated with HRS or NS
intraperitoneally, and their hepatic function was evaluated. The
results showed that HRS, especially at a higher dose, potentially
accelerated the reversal of AOC-induced liver dysfunction (Table III). Moreover, survival
statistics also indicated that more AOC model rats survived
following treatment with HRS (Fig.
4F).
 | Table III.Liver function test results used to
evaluate the degree of liver injury with or without HRS
administration in AOC model rats. |
Table III.
Liver function test results used to
evaluate the degree of liver injury with or without HRS
administration in AOC model rats.
|
| NS | HRS (5 ml/kg) | HRS (10 ml/kg) |
|---|
| ALT, IU/l | 229.75±44.79 |
161.38±26.56a |
151.14±37.39a |
| AST, IU/l | 341.75±43.97 |
251.63±52.53a |
261.86±45.95a |
| TBIL, µmol/l | 33.83±5.33 | 24.43±8.18 | 21.19±5.46 |
Discussion
The blood-biliary barrier is essential to maintain
liver function (1). As functional
components of the blood-biliary barrier, both TJs and GJs have been
reported to be disrupted by cholestasis (1,27).
AOC occurs as a result of biliary obstruction, which causes a rapid
decrease in liver function and increases liver damage (5). Thus, it was speculated that
AOC-induced liver injury may be associated with disrupted TJs and
GJs. Unexpectedly, the present results indicated that TJs and GJs
did not change significantly after BDL, as shown in previous
studies (1,27). An acute biliary obstruction animal
model was used in the present experiments, while animal models with
chronic biliary obstruction were used in previous studies. However,
AOC induced in rats via LPS infusion following BDL caused a notable
change in TJ and GJ mRNA and protein expression levels. Compared
with simple biliary obstruction models, compound injury is
simultaneously induced by cholestasis and LPS, which is the most
noteworthy characteristic of AOC (5). The above results revealed that
abnormally expressed TJ and GJ proteins may have promoted the
disruption of the blood-biliary barrier and worsened AOC-induced
liver injury.
Reducing excessive inflammatory and oxidative damage
may be an effective approach to accelerate the reversal of
AOC-induced liver dysfunction. Due to its anti-oxidative and
anti-inflammatory properties, H2 has been reported to
protect various organs, such as the heart, lung, intestine and
brain, from injury (11,28–30).
For hepatic diseases, H2 has also been found to have a
protective role in a variety of injuries, such as
ischemia-reperfusion damage, drug-induced liver injury and
hepatitis (7,31). Although studies have revealed that
H2 may also protect liver tissues from obstructive
jaundice and sepsis (32,33), its role in AOC, a more rapidly
progressing liver injury, remains elusive. The present results
showed that hydrogen effectively accelerated the reversal of
AOC-induced liver dysfunction in rats. Previous studies revealed
that H2 attenuated postoperative liver failure and
accelerated liver regeneration after hepatectomy (34,35).
Therefore, it is possible that molecular hydrogen may have not only
a protective but also a restorative role in liver injury. However,
the chemical instability of H2 is still the main
limitation of its clinical application (6). Hydrogen poses no risk of explosion in
air when present at concentrations <4.6% by volume, and hydrogen
inhalation at 2% has been used in previous studies (36–38).
Due to the advantages in controlling the dose and route of
administration, administration of hydrogen using an injectable
hydrogen-rich vehicle may be a more suitable choice for specific
target organs, including the liver, brain and pancreas (6). Future studies may provide a more
convenient and safer delivery method of hydrogen application.
Both inflammatory and oxidative damage are common
during various liver injuries (39,40).
GJ protein expression is closely associated with inflammatory and
oxidative damage (2,41,42).
However, the relationship between TJ proteins and inflammatory or
oxidative damage during liver injury remained unclear. The present
results confirmed that both inflammatory and oxidative damage
induced aberrant TJ and GJ protein expression in hepatocytes in
vitro. It also provided a possible mechanism underlying the
H2-mediated rescue of GJ and TJ protein expression, due
to its reported anti-oxidant and anti-inflammatory properties.
Moreover, as MMPs are important regulatory enzymes that may affect
both GJ and TJ proteins (24,25),
their activity levels were evaluated. A number of studies have
verified that junction proteins, such as occludin, ZO1, Cx32 and
Cx43, are substrates of MMP-2/9 (24,43–46).
Additionally, MMPs have been reported to be activated by oxidative
damage, mediating junction protein cleavage (25,45).
The present results showed that HRS treatment may have attenuated
AOC-induced inflammatory and oxidative damage, and significantly
reduced MMP-2/9 activity. This suggests that the antioxidant and
anti-inflammatory properties of H2 may reverse the
disruption of junction proteins by decreasing MMP activity.
In conclusion, the present results indicated that
H2 may have accelerated the reversal of AOC-induced
liver dysfunction, and this phenomenon may depend on the reversal
of inhibition of GJ and TJ protein expression.
Acknowledgements
Not applicable.
Funding
This research was supported by the Zhejiang
Provincial Natural Science Foundation of China (grants nos.
LY17H030001 and LQ14H160001), the National Natural Science
Foundation of China (grant no. 81602044), Zheng Shu Medical Elite
Scholarship Fund, the Zhejiang Provincial Public Welfare Technology
Application Research Projects (grants nos. 2013C33214, 2015C33293
and LGF18H030008) and the Research Foundation of Health Bureau of
Zhejiang Province (grants nos. 2018238887 and 2018RC077).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BL designed the present study. ZZ, JY and HT
performed the experiments. WL and WZ analyzed the data. JY, WZ and
BL drafted and revised the paper. BL, JY, WZ and ZZ provided
funding for this study. All authors reviewed the manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
Zhejiang University (Hangzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kojima T, Yamamoto T, Murata M, Chiba H,
Kokai Y and Sawada N: Regulation of the blood-biliary barrier:
Interaction between gap and tight junctions in hepatocytes. Med
Electron Microsc. 36:157–164. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
González HE, Eugenín EA, Garcés G, Solís
N, Pizarro M, Accatino L and Sáez JC: Regulation of hepatic
connexins in cholestasis: Possible involvement of Kupffer cells and
inflammatory mediators. Am J Physiol Gastrointest Liver Physiol.
282:G991–G1001. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zhang Y, Sun K, Liu YY, Zhang YP, Hu BH,
Chang X, Yan L, Pan CS, Li Q, Fan JY, et al: Ginsenoside Rb1
ameliorates lipopolysaccharide-induced albumin leakage from rat
mesenteric venules by intervening in both trans- and paracellular
pathway. Am J Physiol Gastrointest Liver Physiol. 306:G289–G300.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang X, Wang T, Gui P, Yao C, Sun W, Wang
L, Wang H, Xie W, Yao S, Lin Y and Wu Q: Resolvin D1 reverts
lipopolysaccharide-induced TJ proteins disruption and the increase
of cellular permeability by regulating IκBα signaling in human
vascular endothelial cells. Oxid Med Cell Longev. 2013:1857152013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mosler P: Diagnosis and management of
acute cholangitis. Curr Gastroenterol Rep. 13:166–172. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Huang CS, Kawamura T, Toyoda Y and Nakao
A: Recent advances in hydrogen research as a therapeutic medical
gas. Free Radic Res. 44:971–982. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fukuda K, Asoh S, Ishikawa M, Yamamoto Y,
Ohsawa I and Ohta S: Inhalation of hydrogen gas suppresses hepatic
injury caused by ischemia/reperfusion through reducing oxidative
stress. Biochem Biophys Res Commun. 361:670–674. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sun H, Chen L, Zhou W, Hu L, Li L, Tu Q,
Chang Y, Liu Q, Sun X, Wu M and Wang H: The protective role of
hydrogen-rich saline in experimental liver injury in mice. J
Hepatol. 54:471–480. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xie K, Liu L, Yu Y and Wang G: Hydrogen
gas presents a promising therapeutic strategy for sepsis. Biomed
Res Int. 2014:8076352014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu W, Shan LP, Dong XS, Liu XW, Ma T and
Liu Z: Combined early fluid resuscitation and hydrogen inhalation
attenuates lung and intestine injury. World J Gastroenterol.
19:492–502. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Xie K, Yu Y, Huang Y, Zheng L, Li J, Chen
H, Han H, Hou L, Gong G and Wang G: Molecular hydrogen ameliorates
lipopolysaccharide-induced acute lung injury in mice through
reducing inflammation and apoptosis. Shock. 37:548–555.
2012.PubMed/NCBI
|
|
12
|
Yang J and Lu B: Establishment of a novel
rat model of severe acute cholangitis. Iran J Basic Med Sci.
18:1124–1129. 2015.PubMed/NCBI
|
|
13
|
Guidelines for the welfare of animals in
rodent protection tests, . A report from the rodent protection test
working party. Lab Anim. 28:13–18. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Soothill JS, Morton DB and Ahmad A: The
HID50 (hypothermia-inducing dose 50): An alternative to the LD50
for measurement of bacterial virulence. Int J Exp Pathol. 73:95–98.
1992.PubMed/NCBI
|
|
15
|
Ohsawa I, Nishimaki K, Yamagata K,
Ishikawa M and Ohta S: Consumption of hydrogen water prevents
atherosclerosis in apolipoprotein E knockout mice. Biochem Biophys
Res Commun. 377:1195–1198. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu P, Wang Z, Sun X, Chen X, Zeng S, Chen
L and Li S: Hydrogen-rich medium protects human skin fibroblasts
from high glucose or mannitol induced oxidative damage. Biochem
Biophys Res Commun. 409:350–355. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ohsawa I, Ishikawa M, Takahashi K,
Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S
and Ohta S: Hydrogen acts as a therapeutic antioxidant by
selectively reducing cytotoxic oxygen radicals. Nat Med.
13:688–694. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Method. 25:402–408. 2001.
View Article : Google Scholar
|
|
19
|
Yu J, Zhang W, Qian H, Tang H, Lin W and
Lu B: SOCS1 regulates hepatic regenerative response and provides
prognostic makers for acute obstructive cholangitis. Sci Rep.
7:94822017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cannistrà M, Ruggiero M, Zullo A, Gallelli
G, Serafini S, Maria M, Naso A, Grande R, Serra R and Nardo B:
Hepatic ischemia reperfusion injury: A systematic review of
literature and the role of current drugs and biomarkers. Int J
Surg. 33 (Suppl 1):S57–S70. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
de Andrade KQ, Moura FA, dos Santos JM, de
Araújo OR, de Farias Santos JC and Goulart MO: Oxidative stress and
inflammation in hepatic diseases: Therapeutic possibilities of
N-acetylcysteine. Int J Mol Sci. 16:30269–30308. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Rodrigues SF and Granger DN: Blood cells
and endothelial barrier function. Tissue Barriers. 3:e9787202015.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rosenberg GA and Yang Y: Vasogenic edema
due to tight junction disruption by matrix metalloproteinases in
cerebral ischemia. Neurosurg Focus. 22:E42007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu J, Han W, Chen X, Guo W, Liu K, Wang R,
Zhang J and Sai N: Matrix metalloproteinase-2 and −9 contribute to
functional integrity and noise-induced damage to the
blood-labyrinth-barrier. Mol Med Rep. 16:1731–1738. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Barteková M, Šimončíková P, Fogarassyová
M, Ivanová M, Okruhlicová Ľ, Tribulová N, Dovinová I and Barančík
M: Quercetin improves postischemic recovery of heart function in
doxorubicin-treated rats and prevents doxorubicin-induced matrix
metalloproteinase-2 activation and apoptosis induction. Int J Mol
Sci. 16:8168–8185. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Amin M, Pushpakumar S, Muradashvili N,
Kundu S, Tyagi SC and Sen U: Regulation and involvement of matrix
metalloproteinases in vascular diseases. Front Biosci (Landmark
Ed). 21:89–118. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Balasubramaniyan V, Dhar DK, Warner AE,
Vivien Li WY, Amiri AF, Bright B, Mookerjee RP, Davies NA, Becker
DL and Jalan R: Importance of connexin-43 based gap junction in
cirrhosis and acute-on-chronic liver failure. J Hepatol.
58:1194–1200. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tao B, Liu L, Wang N, Tong D, Wang W and
Zhang J: Hydrogen-rich saline attenuates lipopolysaccharide-induced
heart dysfunction by restoring fatty acid oxidation in rats by
mitigating C-Jun N-terminal kinase activation. Shock. 44:593–600.
2015.PubMed/NCBI
|
|
29
|
Zheng X, Mao Y, Cai J, Li Y, Liu W, Sun P,
Zhang JH, Sun X and Yuan H: Hydrogen-rich saline protects against
intestinal ischemia/reperfusion injury in rats. Free Radic Res.
43:478–484. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bai X, Liu S, Yuan L, Xie Y, Li T, Wang L,
Wang X, Zhang T, Qin S, Song G, et al: Hydrogen-rich saline
mediates neuroprotection through the regulation of endoplasmic
reticulum stress and autophagy under hypoxia-ischemia neonatal
brain injury in mice. Brain Res. 1646:410–417. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kajiya M, Sato K, Silva MJ, Ouhara K, Do
PM, Shanmugam KT and Kawai T: Hydrogen from intestinal bacteria is
protective for Concanavalin A-induced hepatitis. Biochem Biophys
Res Commun. 386:316–321. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Liu Q, Shen WF, Sun HY, Fan DF, Nakao A,
Cai JM, Yan G, Zhou WP, Shen RX, Yang JM and Sun XJ: Hydrogen-rich
saline protects against liver injury in rats with obstructive
jaundice. Liver Int. 30:958–968. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Iketani M, Ohshiro J, Urushibara T,
Takahashi M, Arai T, Kawaguchi H and Ohsawa I: Preadministration of
hydrogen-rich water protects against lipopolysaccharide-induced
sepsis and attenuates liver injury. Shock. 48:85–93. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tan YC, Xie F, Zhang HL, Zhu YL, Chen K,
Tan HM, Hu BS, Yang JM and Tan JW: Hydrogen-rich saline attenuates
postoperative liver failure after major hepatectomy in rats. Clin
Res Hepatol Gastroenterol. 38:337–345. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yu J, Zhang W, Zhang R, Ruan X, Ren P and
Lu B: Lactulose accelerates liver regeneration in rats by inducing
hydrogen. J Surg Res. 195:128–135. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Cai J, Kang Z, Liu WW, Luo X, Qiang S,
Zhang JH, Ohta S, Sun X, Xu W, Tao H and Li R: Hydrogen therapy
reduces apoptosis in neonatal hypoxia-ischemia rat model. Neurosci
Lett. 441:167–172. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Matchett GA, Fathali N, Hasegawa Y, Jadhav
V, Ostrowski RP, Martin RD, Dorotta IR, Sun X and Zhang JH:
Hydrogen gas is ineffective in moderate and severe neonatal
hypoxia-ischemia rat models. Brain Res. 1259:90–97. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hayashida K, Sano M, Ohsawa I, Shinmura K,
Tamaki K, Kimura K, Endo J, Katayama T, Kawamura A, Kohsaka S, et
al: Inhalation of hydrogen gas reduces infarct size in the rat
model of myocardial ischemia-reperfusion injury. Biochem Biophys
Res Commun. 373:30–35. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Li S, Tan HY, Wang N, Zhang ZJ, Lao L,
Wong CW and Feng Y: The role of oxidative stress and antioxidants
in liver diseases. Int J Mol Sci. 16:26087–26124. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gao B: Hepatoprotective and
anti-inflammatory cytokines in alcoholic liver disease. J
Gastroenterol Hepatol. 27 (Suppl 2):89–93. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Santolim LV, Amaral MECD, Fachi JL, Mendes
MF and Oliveira CA: Vitamin E and caloric restriction promote
hepatic homeostasis through expression of connexin 26, N-cad, E-cad
and cholesterol metabolism genes. J Nutr Biochem. 39:S86–S92. 2017.
View Article : Google Scholar
|
|
42
|
Gagliano N, Donne ID, Torri C, Migliori M,
Grizzi F, Milzani A, Filippi C, Annoni G, Colombo P, Costa F, et
al: Early cytotoxic effects of ochratoxin A in rat liver: A
morphological, biochemical and molecular study. Toxicology.
225:214–224. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Chaturvedi M and Kaczmarek L: Mmp-9
inhibition: A therapeutic strategy in ischemic stroke. Mol
Neurobiol. 49:563–573. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Ren C, Li N, Wang B, Yang Y, Gao J, Li S,
Ding Y, Jin K and Ji X: Limb ischemic perconditioning attenuates
blood-brain barrier disruption by inhibiting activity of MMP-9 and
occludin degradation after focal cerebral ischemia. Aging Dis.
6:406–417. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Bauer AT, Bürgers HF, Rabie T and Marti
HH: Matrix metalloproteinase-9 mediates hypoxia-induced vascular
leakage in the brain via tight junction rearrangement. J Cereb
Blood Flow Metab. 30:837–848. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lischper M, Beuck S, Thanabalasundaram G,
Pieper C and Galla HJ: Metalloproteinase mediated occludin cleavage
in the cerebral microcapillary endothelium under pathological
conditions. Brain Res. 1326:114–127. 2010. View Article : Google Scholar : PubMed/NCBI
|