Introduction
Breast cancer is the most frequent malignancy among
females worldwide, and results in ~0.5 million mortalities per year
(1). Chemotherapy is the standard
treatment method for breast cancer. The anthracycline antibiotic
Adriamycin® (doxorubicin), isolated from Streptomyces
peucetius, is a commonly used chemotherapeutic agent for the
treatment of breast carcinoma (2,3).
However, development of DOX resistance is one of the main reasons
for low chemotherapeutic efficiency in patients with breast cancer
(4). Therefore, improved treatment
strategies for doxorubicin-resistant breast cancer may improve its
clinical application.
Drug resistance may be mediated by different
mechanisms. One of the most important mechanisms underlying
chemoresistance involves the overexpression of ATP-binding cassette
(ABC) transporters. The ABC transporters area large group of
proteins involved in material transportation, cellular homeostasis
and chemoresistance (5). The
majority of eukaryotic ABC transporters are efflux transporters
that facilitate the removal of a variety of structurally distinct
chemotherapy drugs from the cytoplasm into the extracellular space
(5). Elevated expression of ABC
transporters has been reported to be associated with drug
resistance in vivo and in vitro (6,7). ABC
sub-family B member 4 (ABCB4) exhibits high sequence homology
(~80%) with the classic drug efflux transporter ABCB1 (8). ABCB4 functions as an efflux pump to
limit the intracellular accumulation of drugs, including taxanes,
anthracyclines and vinca alkaloids (9). Increasing evidence suggests that
ABCB4 is overexpressed in cancer cells following exposure to
anticancer drugs including doxorubicin, and may serve an important
role in drug resistance (10–12).
A previous study revealed that ABCB4 is overexpressed in
doxorubicin-resistant breast cancer cells, reduces chemotherapy
drug sensitivity and may cause drug resistance (13). Thus, inhibiting the activity of
ABCB4 may be an important target for the development of therapeutic
approaches to counteract doxorubicin resistance.
Previous studies explored the use of combining
traditional chemotherapeutic agents with natural compounds in
vitro, with encouraging results (14,15).
Curcumin, a yellow pigment derived from Curcuma longa, has
been used as a traditional medicine for centuries (16). It has been reported to exhibit
anti-inflammatory, antioxidant, antimicrobial and anticancer
properties, and has been investigated for the treatment of
postoperative inflammation, osteoarthritis, diabetes,
cardiovascular disease and cancer (17–24).
It suppressed the initiation and progression of breast cancer in
in vitro and in vivo and models (25,26).
Additionally, curcumin may increase the therapeutic efficacy of
chemotherapeutic agents and overcome multiple drug resistance in
cancer through inhibition of ABC transporters activity, including
ABCB1, ABCG2 and ABCCs (27–31).
However, studies investigating the effect of curcumin on drug
resistance in breast cancer remain limited and further research may
identify novel transporters involved in the chemotherapy resistance
reversal of curcumin.
The present study used doxorubicin-resistant
MCF-7/DOX and MDA-MB-231/DOX breast cancer cell lines to
investigate the doxorubicin resistance reversal effect of curcumin
and to explore its possible mechanism of action in vitro.
The results revealed that curcumin inhibited the doxorubicin efflux
function of ABCB4 without altering its protein expression, and may
be one of the molecular mechanisms underlying the reversal of
doxorubicin resistance. Curcumin appears to be a promising
chemosensitizer and chemotherapeutic drug for breast cancer
treatment.
Materials and methods
Cell culture
The human breast cancer cell lines MCF-7 and
MDA-MB-231, and Madin-Darby Canine Kidney (MDCKII) cells were
purchased from the Institute of Basic Medical Sciences, Chinese
Academy of Medical Sciences (Beijing, China). MDCKII cells are an
epithelial cell line of canine kidney origin that are frequently
used to investigate drug transporters in vitro, as they
exhibit low expression levels of transporter proteins and low
metabolic activity (32). MCF-7
and MDCKII cells were cultured in complete DMEM (Thermo Fisher
Scientific Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.). MDA-MB-231 cells were maintained in RPMI-1640
(Thermo Fisher Scientific Inc.) based culture medium supplemented
with 10% FBS. The LZRS-IRES-eGFP plasmid expressing human ABCB4 was
kindly donated by Professor Alfred Schinkel (Division of Molecular
Oncology, The Netherlands Cancer Institute). For knockdown studies,
the ABCB4 shRNA (5′-CUCAAUACGCGGCUAACAG-3′; Shanghai GenePharma
Co., Ltd.) was cloned into the LZRS-IRES-eGFP vector.
Doxorubicin-resistant breast cancer MCF-7/DOX and MDA-MB-231/DOX
cells, MDCKII-patent (transfected with LZRS-IRES-eGFP-empty
vector), MDCKII-ABCB4 (transfected with human LZRS-IRES-eGFP-ABCB4
expression vector), MCF-7/DOX and MDA-MB-231/DOX cells with ABCB4
knockdown (transfected with LZRS-IRES-eGFP-shABCB4) or
overexpression (transfected with LZRS-IRES-eGFP-ABCB4 expression
vector) were established in our laboratory as previously described
(13). MCF-7/DOX and
MDA-MB-231/DOX without transfection or treatment were used controls
for ABCB4 knockdown/overexpression in the respective cell types.
All the cell lines were maintained in an atmosphere containing 5%
CO2 at 37°C as previously reported (13).
Cell cytotoxicity assay
The cytotoxicity of curcumin and the combination of
curcumin (Sigma-Aldrich; Merck KGaA) and doxorubicin
(Sigma-Aldrich; Merck KGaA) was assessed using the Cell Counting
Kit-8 (CCK-8; Dojindo Molecular Technologies) assay. Cells were
seeded in a 96-well plate at a density of 5×103
cells/well. Cells were incubated with various concentrations of
curcumin (0, 2.5, 5, 10, 20, 50 and 100 µM) for 72 h. For the
doxorubicin chemosensitivity assay, cells were incubated with a
combination of 10 µM curcumin and varying concentrations of
doxorubicin (0, 2.5, 5, 10, 20, 50 and 100 µM) for 72 h. DMSO was
used as the control. Following incubation, 10 µl CCK-8 solution was
added to each well, and cells were incubated for an additional 4 h.
The absorbance was measured at a wave length of 570 nm using a
fluorescence spectrofluorometer (cat no. F-7000; Hitachi Ltd.).
IC50 values were calculated using the cell survival data
curve.
Measurement of intracellular
doxorubicin accumulation
The intracellular accumulation of doxorubicin in
cells was measured using a flow cytometer. The cells were cultured
in 6-well plates at a density of 1×106 per well, and
were cultured to 80% confluence with or without 10 µM curcumin
treatment for 72 h. The cells were subsequently treated with 30 µM
doxorubicin for 4 h, and washed three times with chilled PBS. The
cell-associated mean fluorescence intensity of doxorubicin was
detected using FACSCalibur (Beckman Coulter, Inc.) with
excitation/emission wavelengths of 485/580 nm. The results were
analyzed though CyExpert 2.0 (Beckman Coulter, Inc.) software.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from the cells with
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. The extracted RNA
was reverse transcribed into cDNA using the Revert Aid™ H Minus
First Strand cDNA Synthesis kit (Takara Bio, Inc.) according to the
manufacturer's protocols. qPCR was performed using the
SYBR® Green PCR Master mix (Takara Bio, Inc.). The
following primer pairs were used for the qPCR: ABCB4,
forward 5′-TGGCCCTGGTTGGAAGTAGTG-3′ and reverse
5′-AGAAGGATCTTGGGGTTGCGAA-3′; and β-actin, forward
5′-GGATGCAGGAGATCACTG-3′ and reverse 5′-CGATCCACACGGAGTACTT-3′.
qPCR was conducted as follows: 95°C for 30 sec, followed by 40
cycles of 95°C for 5 sec and 60°C for 30 sec. Relative
quantification of ABCB4 mRNA was analyzed using the
2−ΔΔCq method relative to the β-actin expression level
in each sample (33).
Protein extraction and western blot
analysis
Total protein was extracted using
radioimmunoprecipitation protein lysis buffer (Beyotime Institute
of Biotechnology) and quantified using the Pierce BCA Protein Assay
kit (Thermo Fisher Scientific, Inc.). Protein extracts (50 µg/lane)
were separated by via SDS-PAGE and transferred onto a
polyvinylidene difluoride membrane (Bio-Rad Laboratories, Inc.).
The membranes were blocked for 2 h at room temperature with 5%
non-fat milk. Subsequently, the membranes were incubated with
primary antibodies against ABCB4 (1:1,000; cat. no. ab24108, Abcam)
or β-actin (1:5,000; cat. no. A5441, Sigma-Aldrich; Merck KGaA) at
4°C overnight. The membranes were subsequently incubated with
horseradish peroxidase-conjugated secondary antibodies (1:2,000;
cat. no. ab97023, Abcam) for 2 h at room temperature. Protein bands
were visualized using the ECL™ Prime (GE Healthcare) and a LAS-3000
imager (Fujifilm).
MDCKII monolayer transport assay
Cellular transport assay was performed as described
previously (13). Briefly,
MDCKII-parent (transfected with LZRS-IRES-eGFP-empty vector) and
MDCKII-ABCB4 (transfected with LZRS-IRES-eGFP-ABCB4 expression
vector) cells (1×106/insert) were seeded onto
microporous polycarbonate membrane inserts (pore size, 3 µm;
diameter, 24 mm; Costar; Corning, Inc.). The plates were incubated
at 37°C with 5% CO2 for 3 days and subsequently used for
assays. Prior to experimentation, cells were cultured in Opti-MEM
(Invitrogen; Thermo Fisher Scientific, Inc.) with or without 100 µM
verapamil (Sigma-Aldrich; Merck KGaA) or 10 µM curcumin for 2 h.
Opti-MEM was subsequently replaced with fresh DMEM supplemented
with 10% fetal calf serum (Gibco; Thermo Fisher Scientific, Inc.)
and 100 nM (3H) doxorubicin with or without 100 µM
verapamil or 10 µM curcumin. The radioactivity of transported
(3H) doxorubicin was determined using a liquid
scintillation counter (cat no. LS6500; Beckman Coulter, Inc.). The
effect of curcumin on ABCB4-mediated doxorubicin transport was
calculated as the fraction of doxorubicin recovered in the acceptor
compartment vs. the fraction added in the donor compartment at the
beginning of the experiment.
ATPase activity assay
ATPase activity was assayed to determine the
functionality of the ABC transporter. MDCKII-ABCB4 cells
(1×106) were incubated at 37°C for 30 min in the
presence of sodium orthovanadate (0.3 mM) in ATPase assay buffer
(50 mM KCl, 5 mM sodium azide, 2 mM EDTA, 10 mM MgCl2
and 1 mM dithiothreitol; pH 6.8) to measure the amount of inorganic
phosphate that was released. Membrane proteins were isolated from
cells using a Membrane and Cytosol Protein Extraction kit (Beyotime
Institute of Biotechnology) according to the manufacturer's
protocol. A total of 30 µg membrane extracts were incubated with
increasing concentration of curcumin (0, 5, 10, 15 and 20 µM) in
the presence and absence of 5 µM verapamil. The reaction was
initiated by the addition of 5 mM ATP and incubated for 20 min at
37°C. The reaction was terminated by the addition of 0.1 ml 5% SDS
solution, and the inorganic phosphate released was quantified with
a colorimetric reaction at 340 nm using a microplate reader (Thermo
Fisher Scientific, Inc.).
Statistical analysis
Statistical analysis was performed using SPSS
software (version 20; IBM Corp.). All values are presented as the
mean ± SEM. One-way ANOVA and Tukey's post hoc test were performed
to analyze the differences between the different groups. P<0.05
was considered to indicate a statistically significant
difference.
Results
ABCB4 has a role in the effect of
curcumin on doxorubicin resistance
Prior to testing the reversal effects of curcumin,
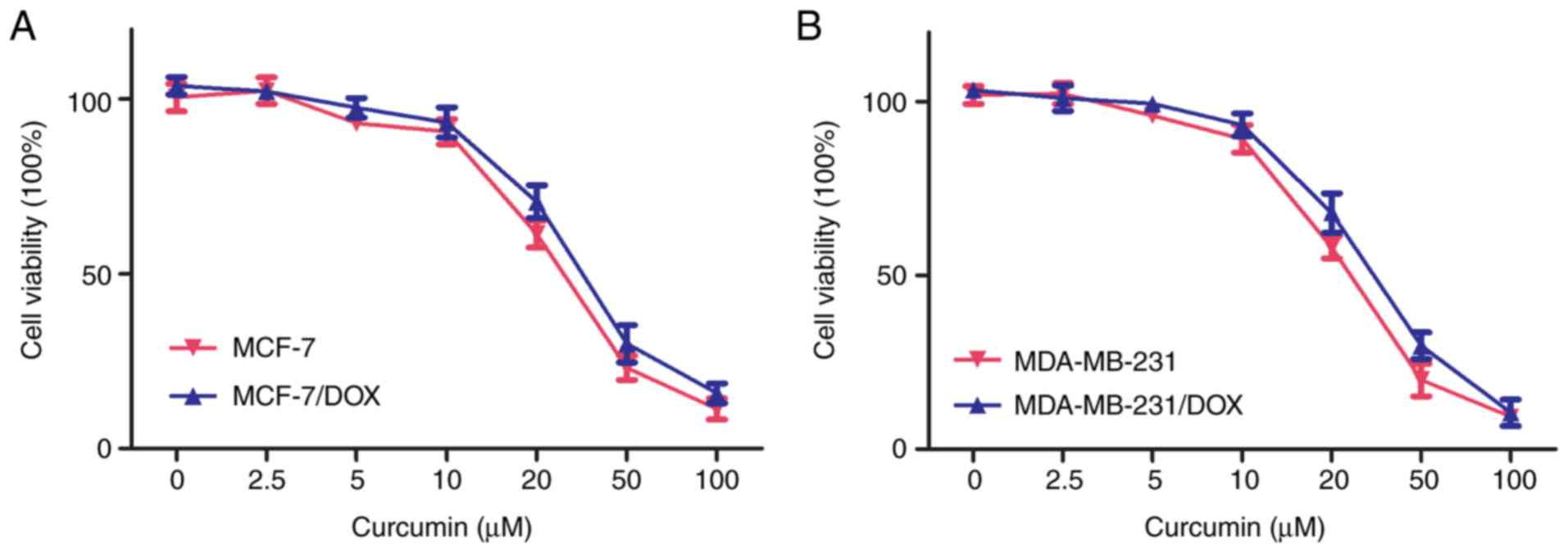
its cytotoxicity was evaluated using the CCK-8 assay. Curcumin (20
µM) exhibited moderate cytotoxic effects on parental and resistant
cells following 72 h of treatment (Fig. 1). The majority (>90%) of
parental and doxorubicin-resistant cells survived at a
concentration of 10 µM curcumin, suggesting that curcumin is safe
to be used up to a concentration of 10 µM. Thus, 10 µM curcumin was
used for the subsequent assays using MCF-7/DOX and MDA-MB-231/DOX
cells.
Preliminary results revealed that ABCB4 was
overexpressed in MCF-7/DOX and MDA-MB-231/DOX cells, and that it
results in drug resistance (12).
The present study investigated the role of ABCB4 in the effects of
curcumin on doxorubicin resistance. The effect of curcumin on
doxorubicin cytotoxicity in control the group, ABCB4 knockdown and
ABCB4 overexpression in MCF-7/DOX and MDA-MB-231/DOX cells was
investigated. As presented in Table
I, compared to the DMSO-treated cells, curcumin reduced the
IC50 value of doxorubicin in all the analyzed cells. The
most significant reduction was observed in ABCB4 overexpressing
cells, while the least significant reduction was found in ABCB4
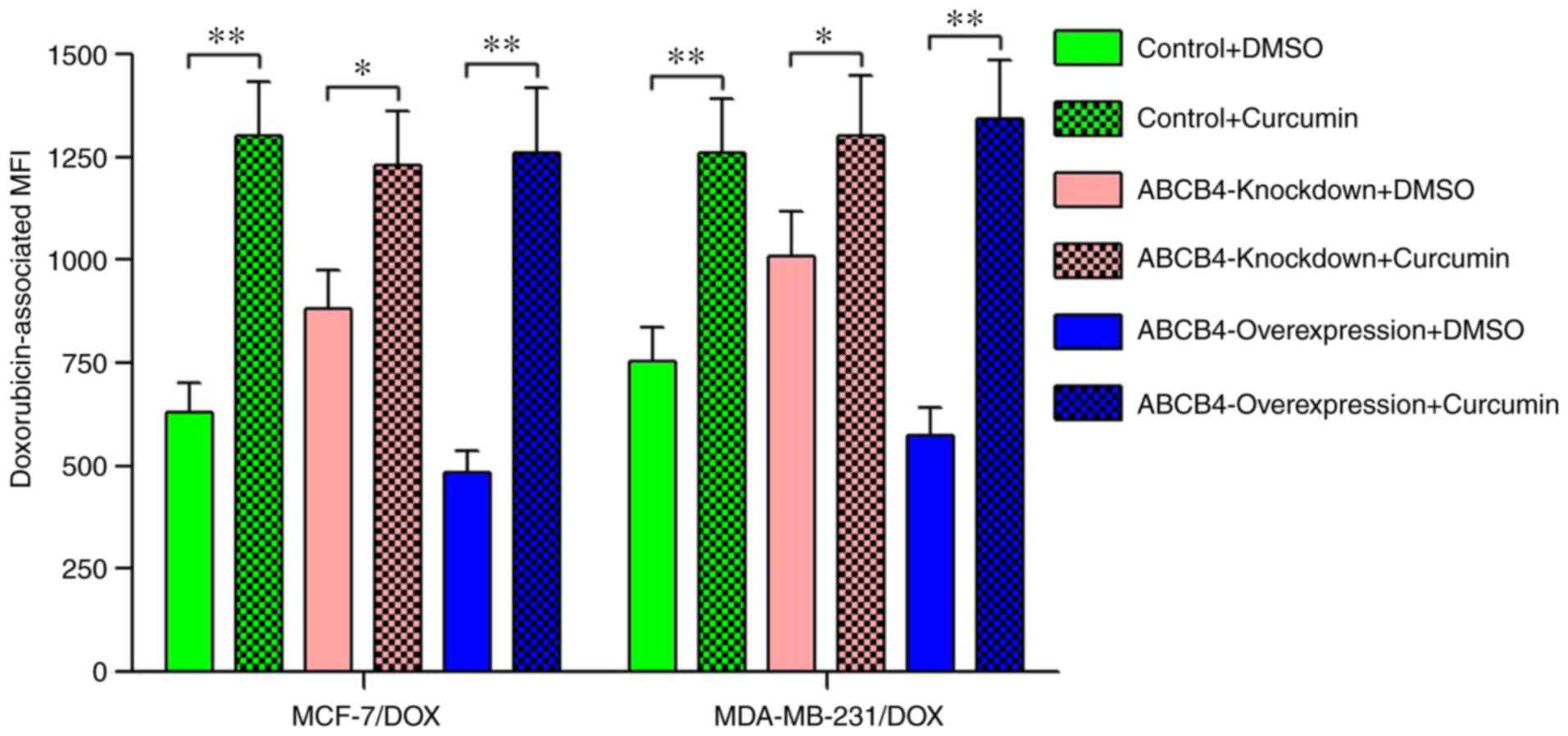
knockdown cells. The intracellular accumulation of doxorubicin in
these cells was subsequently detected in the presence or absence of
curcumin. As expected, compared with DMSO-treated group,
co-incubation of doxorubicin and curcumin resulted in a significant
increase in intracellular concentration of doxorubicin, and the
rate of increase was proportional to the level of ABCB4 expression
(Fig. 2).
 | Table I.Effect of curcumin on doxorubicin
sensitivity in MCF-7/DOX and MDA-MB-231/DOX cells with different
ABCB4 expression levels. |
Table I.
Effect of curcumin on doxorubicin
sensitivity in MCF-7/DOX and MDA-MB-231/DOX cells with different
ABCB4 expression levels.
|
| Half maximal
inhibitory concentration (µM) |
|---|
|
|
|
|---|
| Treatment
group | MCF-7/DOX | MDA-MB-231/DOX |
|---|
| Control+DMSO | 19.5±2.1 | 25.8±3.2 |
|
Control+curcumin |
12.8±1.4a |
15.3±1.3a |
|
ABCB4-knockdown+DMSO | 11.8±1.3 | 18.1±2.0 |
|
ABCB4-knockdown+curcumin |
8.6±0.9b |
12.9±1.1b |
|
ABCB4-overexpression+DMSO | 26.6±3.2 | 34.6±4.2 |
|
ABCB4-overexpression+curcumin |
10.9±1.4c |
14.1±1.6c |
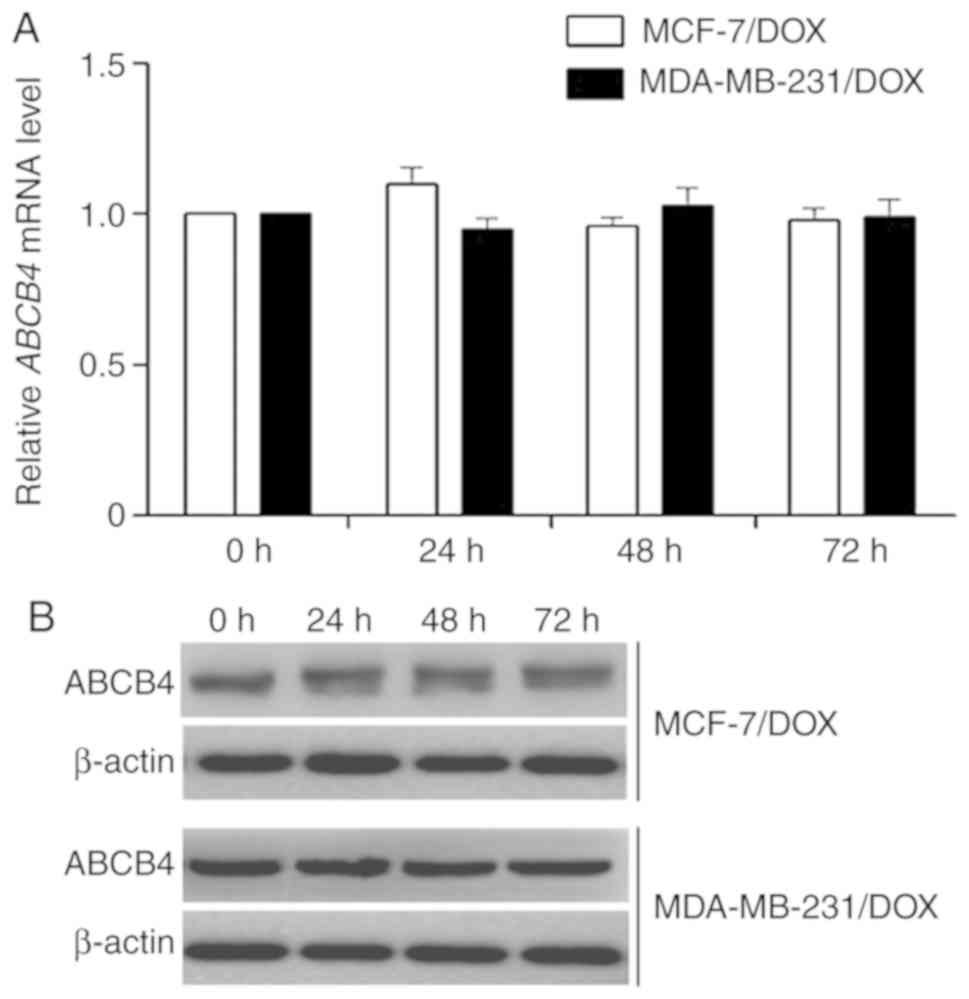
Curcumin did not alter the expression
level of ABCB4
The reversal of ABCB4-mediated doxorubicin
resistance may occur by ABCB4 down regulation or inhibition of the
function of ABCB4. Therefore, the effect of curcumin on the
expression level of ABCB4 following treatment of the
doxorubicin-resistant cell lines MCF-7/DOX and MDA-MB-231/DOX with
10 µM curcumin for 0, 24, 48 and 72 h was investigated. The results
revealed that following treatment with curcumin, the mRNA and
protein levels of ABCB4 in the two cell lines were not
significantly altered (Fig. 3).
Therefore, curcumin may inhibit the transport function of ABCB4 to
reduce doxorubicin resistance.
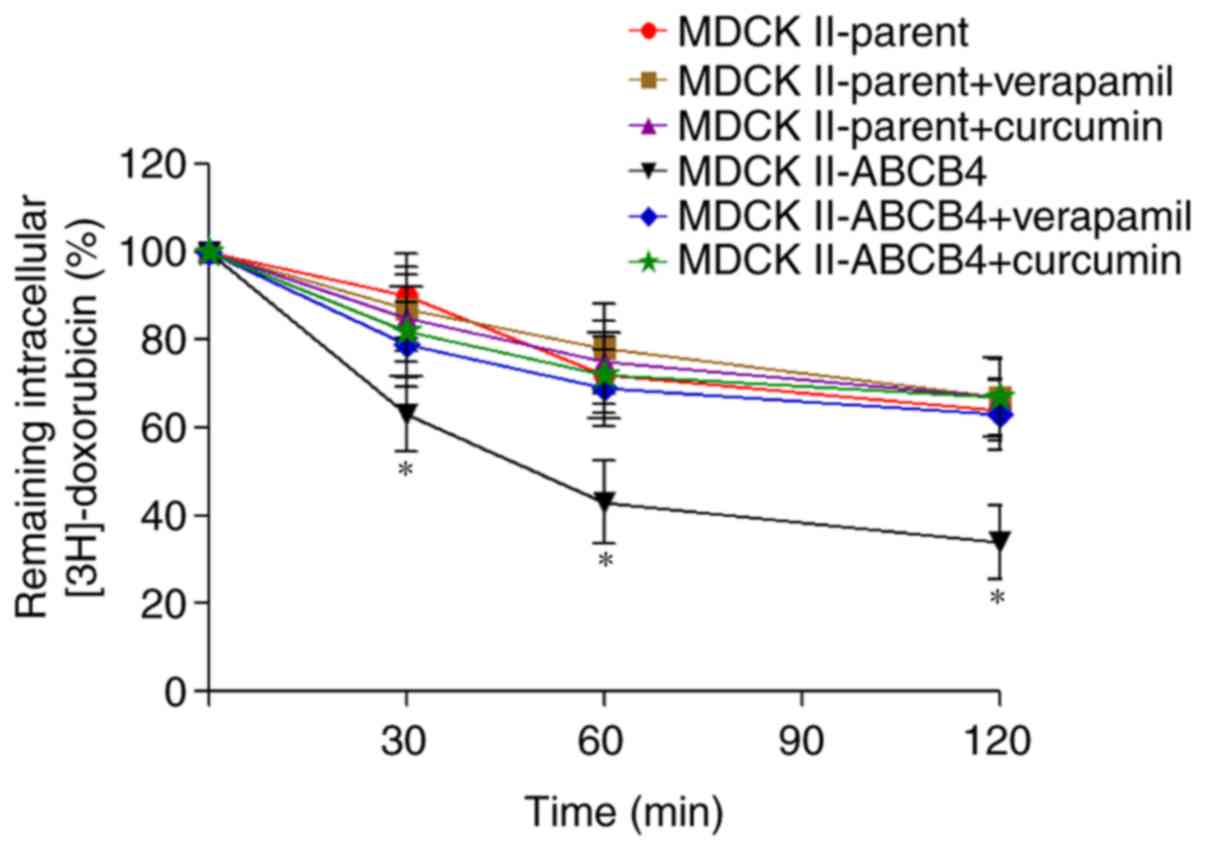
Curcumin inhibits ABCB4-mediated
doxorubicin transport
In order to clarify whether the reversal of
doxorubicin resistance by curcumin occurs due to inhibition of the
efflux function of ABCB4, an efflux assay was performed. The amount
of (3H)-doxorubicin that was pumped into the
extracellular medium was increased in MDCKII-ABCB4 cells compared
with MDCKII-parent cells (Fig. 4).
Incubation with curcumin significantly reduced the efflux of
(3H)-doxorubicin from MDCKII-ABCB4 cells. Verapamil is
an inhibitor of ABCB4 function and was used as control. Verapamil
decreased the efflux of (3H)-doxorubicin in MDCKII-ABCB4
cells. Curcumin and verapamil did not alter the rate of efflux of
(3H)-doxorubicin in the MDCKII-parent cells.
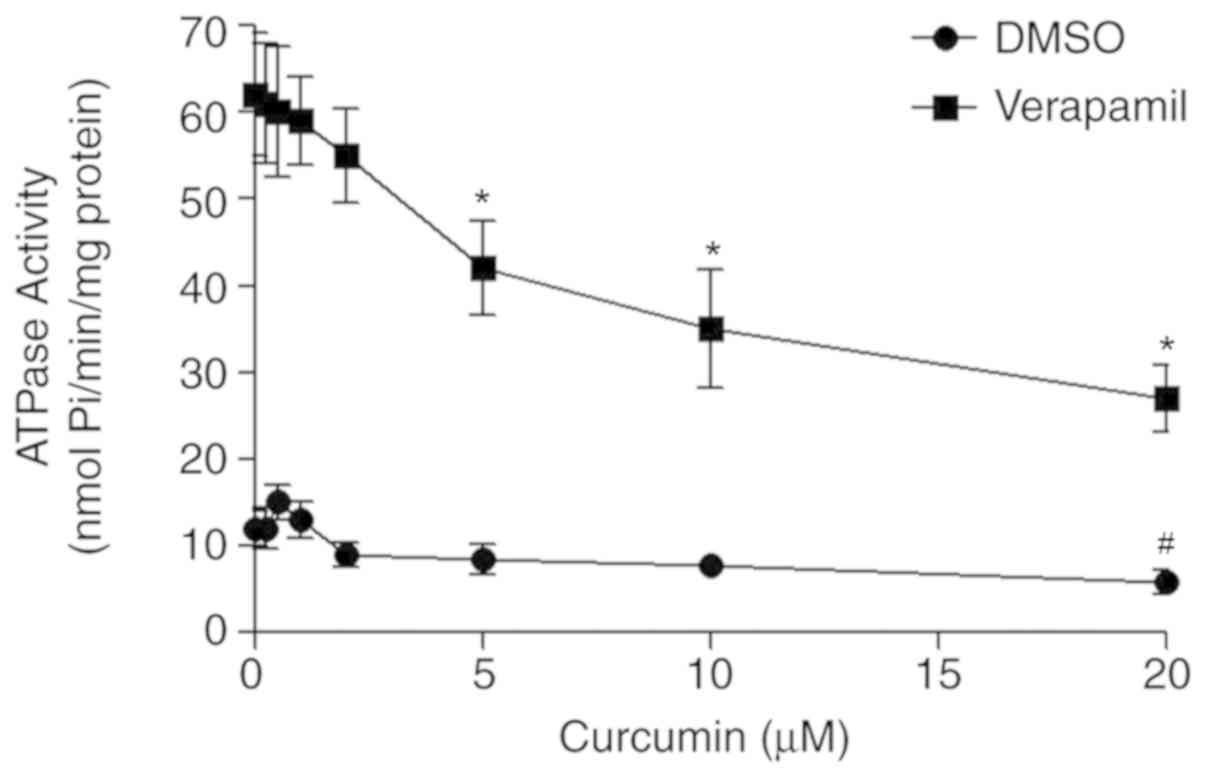
Curcumin inhibits ABCB4 ATPase
activity
To assess whether curcumin interacts directly with
ABCB4, the effect of curcumin on ABCB4 ATPase activity was
investigated in crude membranes expressing ABCB4. The results
revealed that curcumin slightly stimulated basal ATP hydrolysis by
ABCB4 at low concentrations (0.25–1 µM), but inhibited ATPase
activity at higher concentrations. In addition, curcumin inhibited
verapamil-stimulated ATPase activity in a concentration-dependent
manner (Fig. 5).
Discussion
Chemotherapy is the standard treatment for various
types of cancer including breast cancer. However, the development
of resistance to anticancer agents may complicate treatment.
Overexpression of the ABC transporters has been directly implicated
in resistance to a broad spectrum of chemotherapeutic drugs
(34). Thus, ABC proteins may
serve as a therapeutic target for overcoming drug resistance in
cancer. The use of ABC transporter modulators for overcoming drug
resistance has been previously investigated (35). There is an increasing interest in
the use of combination therapy consisting of natural compounds and
traditional chemotherapeutic agents, and this approach has produced
promising results in vitro (14,15).
There are an increasing number of studies
highlighting the anticancer and chemosensitizing abilities of
curcumin on various kinds of human cancer in vivo and in
vitro (36,37). A number of studies have
demonstrated the chemosensitizing ability of curcumin in
doxorubicin-based chemotherapy (38,39).
Curcumin reverses chemoresistance by inhibiting the expression and
function of ABC transporters, including ABCB1, ABCG2 and ABCCs
(27–31). One of the main mechanisms
underlying doxorubicin resistance is the increased expression of
the ABCB1 gene (40). High
homology exists between ABCB1 and ABCB4, suggesting that ABCB4 may
also serve a role in doxorubicin resistance (8). Doxorubicin-resistant variants of two
commonly used breast cancer cell lines, ERα-positive MCF-7 cells
and ERα-negative MDA-MB-231 cells, were previously established
(13). Preliminary results
revealed that ABCB4 was overexpressed in doxorubicin-resistant
breast cancer MCF-7/DOX and MDA-MB-231/DOX cells and was regulated
by promoter methylation. Furthermore, ABCB4 was upregulated in
response to chemotherapy, and may result in drug resistance
(13). Inhibition of the activity
of ABCB4 may be one of the mechanisms underlying the effect of
curcumin on doxorubicin resistance in breast cancer cells. In the
present study, a combination of curcumin and doxorubicin markedly
inhibited the proliferation of MCF-7/DOX and MDA-MB-231/DOX cells,
and reversed doxorubicin resistance, consistent with previous
observations (41). Furthermore,
the intracellular accumulation of doxorubicin was substantially
increased following curcumin treatment in doxorubicin-resistant
breast cells, in a manner that was inversely dependent on the
activity of ATP binding cassette subfamily B member 4 (ABCB4).
Based on the results obtained in the current study, curcumin may
reverse resistance by inhibiting ABCB4 activity. As the cells were
exposed to curcumin for 72 h in the present study, curcumin may
exert its effects by either down regulating ABCB4 expression or
inhibiting ABCB4 function. However, curcumin had no effect on the
expression level of ABCB4 in the present study. Although curcumin
down regulates a number of genes in cancer cells through epigenetic
modification, it mainly induces specific and modest effects, rather
than global and drastic changes (42). A previous study suggested that
curcumin-induced epigenetic changes were interpreted as secondary
to the effect of curcumin on gene expression, rather than direct
effects onthe epigenetic status (43). This may partly explain the negative
effects in this study. However, epigenetic modifications in the
control of ABCB4 expression were reported in a previous study
(13).
Curcumin may exert its effect by inhibiting the
ABCB4-mediated doxorubicin efflux, thus reversing doxorubicin
resistance. In order to investigate this potential mechanism,
anABCB4-mediated (3H)-doxorubicin efflux assay was
performed in the current study. The results revealed that curcumin
at 10 µM inhibited the efflux of doxorubicin in MDCKII-ABCB4 cells
following incubation fordifferent durations. Thus, curcumin
potentially reverses ABCB4-mediated doxorubicin resistance by
inhibiting the activity of ABCB4, rather than downregulating ABCB4
expression. An ATPase assay demonstrated that curcumin slightly
stimulated the basal ABCB4 ATPase activity at low concentrations
(0.25–1 µM), but inhibited this activity at higher concentrations.
Furthermore, curcumin had an inhibitory effect on the
verapamil-stimulated ABCB4 ATPase activity at all the
concentrations investigated. The results obtained suggested that
curcumin may inhibit ABCB4 activity, and that it may compete for
the binding site with substrates including verapamil and
doxorubicin. Further research is required to elucidate the
mechanism underlying any potential interaction between curcumin and
ABCB4.
To date, the majority of studies investigating the
effects of curcumin were performed in vitro (14,15,17,19,20).
Despite the promising in vitro results obtained with even
low concentrations of curcumin (10 µM) in the current study,
curcumin has extremely low solubility in aqueous solution and poor
bioavailability (44). Therefore,
the clinical potential of curcumin is currently limited. A number
of drug delivery systems have been developed to increase the
solubility, stability and bioavailability of curcumin, including
nanoparticles (45). Previous
studies revealed that novel drug delivery strategies may enhance
the delivery of curcumin to tumor tissues, and increase the
bioavailability in vivo with a good safety profile. Curcumin
did not induce hemolysis even at a high concentration of 120 µM
(46–48). Therefore, the development of novel
drug delivery technology may allow the clinical benefits of
curcumin to emerge.
In conclusion, the results obtained in the present
study may have important clinical implications for patients that
develop doxorubicin-resistant breast cancer. Curcumin inhibits
ATPase activity, reverses doxorubicin resistance and sensitizes
human doxorubicin-resistant MCF-7/DOX and MDA-MB-231/DOX breast
cancer cells, suggesting that curcumin may be a novel therapeutic
approach to overcome breast cancer drug resistance.
Acknowledgments
Not applicable.
Funding
This work was supported by the National Scientific
Foundation of China (grant nos. 81473284 and 81603201).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CW, LF and JH performed the experiments and analyzed
the data. YD, BW and GX interpreted the data and drafted the
manuscript. LW and HZ conducted the research design, supervised the
work and wrote the final version of the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
O'Shaughnessy JA: Pegylated liposomal
doxorubicin in the treatment of breast cancer. Clin Breast Cancer.
4:318–328. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bonadonna G, Monfardini S, De Lena M and
Fossati-Bellani F: Clinical evaluation of adriamycin, a new
antitumour antibiotic. Br Med J. 3:503–506. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Prados J, Melguizo C, Ortiz R, Vélez C,
Alvarez PJ, Arias JL, Ruíz MA, Gallardo V and Aranega A:
Doxorubicin-loaded nanoparticles: New advances in breast cancer
therapy. Anticancer Agents Med Chem. 12:1058–1070. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ween MP, Armstrong MA, Oehler MK and
Ricciardelli C: The role of ABC transporters in ovarian cancer
progression and chemoresistance. Crit Rev Oncol Hematol.
96:220–256. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gottesman MM, Fojo T and Bates SE:
Multidrug resistance in cancer: Role of ATP-dependent transporters.
Nat Rev Cancer. 2:48–58. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ozben T: Mechanisms and strategies to
overcome multiple drug resistance in cancer. FEBS Lett.
580:2903–2909. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Van der Bliek AM, Baas F, Ten Houte de
Lange T, Kooiman PM, Van der Velde-Koerts T and Borst P: The human
mdr3 gene encodes a novel P-glycoprotein homologue and gives rise
to alternatively spliced mRNAs in liver. EMBO J. 6:3325–3331. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Falguières T, Aït-Slimane T, Housset C and
Maurice M: ABCB4: Insights from pathobiology into therapy. Clin Res
Hepatol Gastroenterol. 38:557–563. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Němcová-Fürstová V, Kopperová D,
Balušíková K, Ehrlichová M, Brynychová V, Václavíková R, Daniel P,
Souček P and Kovář J: Characterization of acquired paclitaxel
resistance of breast cancer cells and involvement of ABC
transporters. Toxicol Appl Pharmacol. 310:215–228. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Januchowski R, Wojtowicz K, Andrzejewska M
and Zabel M: Expression of MDR1 and MDR3 gene products in
paclitaxel-, doxorubicin- and vincristine-resistant cell lines.
Biomed Pharmacother. 68:111–117. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hontecillas-Prieto L, Garcia-Dominguez DJ,
Vaca DP, Garcia-Mejias R, Marcilla D, Ramirez-Villar GL, Saez C and
de Álava E: Multidrug resistance transporter profile reveals MDR3
as a marker for stratification of blastemal Wilms tumour patients.
Oncotarget. 8:11173–11186. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang JF, Wen CJ, Zhao GZ, Dai Y, Li Y, Wu
LX and Zhou HH: Overexpression of ABCB4 contributes to acquired
doxorubicin resistance in breast cancer cells in vitro. Cancer
Chemother Pharmacol. 82:199–210. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Ye MX, Zhao YL, Li Y, Miao Q, Li ZK, Ren
XL, Song LQ, Yin H and Zhang J: Curcumin reverses cis-platin
resistance and promotes human lung adenocarcinoma A549/DDP cell
apoptosis through HIF-1α and caspase-3 mechanisms. Phytomedicine.
19:779–787. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yoshida K, Toden S, Ravindranathan P, Han
H and Goel A: Curcumin sensitizes pancreatic cancer cells to
gemcitabine by attenuating PRC2 subunit EZH2, and the lncRNA PVT1
expression. Carcinogenesis. 38:1036–1046. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gupta SC, Kismali G and Aggarwal BB:
Curcumin, a component of turmeric: From farm to pharmacy.
Biofactors. 39:2–13. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Banik U, Parasuraman S, Adhikary AK and
Othman NH: Curcumin: The spicy modulator of breast carcinogenesis.
J Exp Clin Cancer Res. 36:982017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Satoskar RR, Shah SJ and Shenoy SG:
Evaluation of anti-inflammatory property of curcumin (diferuloyl
methane) in patients with postoperative inflammation. Int J Clin
Pharmacol Ther Toxicol. 24:651–654. 1986.PubMed/NCBI
|
|
19
|
Mukhopadhyay A, Bueso-Ramos C, Chatterjee
D, Pantazis P and Aggarwal BB: Curcumin downregulates cell survival
mechanisms in human prostate cancer cell lines. Oncogene.
20:7597–7609. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shao ZM, Shen ZZ, Liu CH, Sartippour MR,
Go VL, Heber D and Nguyen M: Curcumin exerts multiple suppressive
effects on human breast carcinoma cells. Int J Cancer. 98:234–240.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shehzad A, Qureshi M, Anwar MN and Lee YS:
Multifunctional curcumin mediate multitherapeutic effects. J Food
Sci. 82:2006–2015. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li H, Sureda A, Devkota HP, Pittalà V,
Barreca D, Silva AS, Tewari D, Xu S and Nabavi SM: Curcumin, the
golden spice in treating cardiovascular diseases. Biotechnol Adv.
Feb 1–2019.(Epub ahead of print). View Article : Google Scholar
|
|
23
|
Perkins K, Sahy W and Beckett RD: Efficacy
of curcuma for treatment of osteoarthritis. J Evid Based
Complementary Altern Med. 22:156–165. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang DW, Fu M, Gao SH and Liu JL:
Curcumin and diabetes: A systematic review. Evid Based Complement
Alternat Med. 2013:6360532013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Sinha D, Biswas J, Sung B, Aggarwal BB and
Bishayee A: Chemopreventive and chemotherapeutic potential of
curcumin in breast cancer. Curr Drug Targets. 13:1799–1819. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lv ZD, Liu XP, Zhao WJ, Dong Q, Li FN,
Wang HB and Kong B: Curcumin induces apoptosis in breast cancer
cells and inhibits tumor growth in vitro and in vivo. Int J Clin
Exp Pathol. 7:2818–2824. 2014.PubMed/NCBI
|
|
27
|
Lopes-Rodrigues V, Sousa E and Vasconcelos
MH: Curcumin as a modulator of p-glycoprotein in cancer: Challenges
and perspectives. Pharmaceuticals (Basel). 9(pii): E712016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Panda AK, Chakraborty D, Sarkar I, Khan T
and Sa G: New insights into therapeutic activity and anticancer
properties of curcumin. J Exp Pharmacol. 9:31–45. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ge S, Yin T, Xu B, Gao S and Hu M:
Curcumin affects phase II disposition of resveratrol through
inhibiting efflux transporters MRP2 and BCRP. Pharm Res.
33:590–602. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wortelboer HM, Usta M, van der Velde AE,
Boersma MG, Spenkelink B, van Zanden JJ, Rietjens IM, van Bladeren
PJ and Cnubben NH: Interplay between MRP inhibition and metabolism
of MRP inhibitors: The case of curcumin. Chem Res Toxicol.
16:1642–1651. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Prehm P: Curcumin analogue identified as
hyaluronan export inhibitor by virtual docking to the ABC
transporter MRP5. Food Chem Toxicol. 62:76–81. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mukkavilli R, Jadhav G and Vangala S:
Evaluation of drug transport in MDCKII-Wild Type, MDCKII-MDR1,
MDCKII-BCRP and Caco-2 cell lines. Curr Pharm Biotechnol.
18:1151–1158. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Chen Z, Shi T, Zhang L, Zhu P, Deng M,
Huang C, Hu T, Jiang L and Li J: Mammalian drug efflux transporters
of the ATP binding cassette (ABC) family in multidrug resistance: A
review of the past decade. Cancer Lett. 370:153–164. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Li W, Zhang H, Assaraf YG, Zhao K, Xu X,
Xie J, Yang DH and Chen ZS: Overcoming ABC transporter-mediated
multidrug resistance: Molecular mechanisms and novel therapeutic
drug strategies. Drug Resist Updat. 27:14–29. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kuttan R, Bhanumathy P, Nirmala K and
George MC: Potential anticancer activity of turmeric (Curcuma
longa). Cancer Lett. 29:197–202. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Doello K, Ortiz R, Alvarez PJ, Melguizo C,
Cabeza L and Prados J: Latest in vitro and in vivo assay, clinical
trials and patents in cancer treatment using curcumin: A literature
review. Nutr Cancer. 70:569–578. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lv L, Qiu K, Yu X, Chen C, Qin F, Shi Y,
Ou J, Zhang T, Zhu H, Wu J, et al: Amphiphilic copolymeric micelles
for doxorubicin and curcumin Co-delivery to reverse multidrug
resistance in breast cancer. J Biomed Nanotechnol. 12:973–985.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Abouzeid AH, Patel NR, Rachman IM, Senn S
and Torchilin VP: Anti-cancer activity of anti-GLUT1
antibody-targeted polymeric micelles co-loaded with curcumin and
doxorubicin. J Drug Target. 21:994–1000. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kim HJ, Im SA, Keam B, Ham HS, Lee KH, Kim
TY, Kim YJ, Oh DY, Kim JH, Han W, et al: ABCB1 polymorphism as
prognostic factor in breast cancer patients treated with docetaxel
and doxorubicin neoadjuvant chemotherapy. Cancer Sci. 106:86–93.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Ma W, Guo Q, Li Y, Wang X, Wang J and Tu
P: Co-assembly of doxorubicin and curcumin targeted micelles for
synergistic delivery and improving anti-tumor efficacy. Eur J Pharm
Biopharm. 112:209–223. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Link A, Balaguer F, Shen Y, Lozano JJ,
Leung HC, Boland CR and Goel A: Curcumin modulates DNA methylation
in colorectal cancer cells. PLoS One. 8:e577092013. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Huminiecki L, Horbańczuk J and Atanasov
AG: The functional genomic studies of curcumin. Semin Cancer Biol.
46:107–118. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Anand P, Kunnumakkara AB, Newman RA and
Aggarwal BB: Bioavailability of curcumin: Problems and promises.
Mol Pharm. 4:807–818. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Shome S, Talukdar AD, Choudhury MD,
Bhattacharya MK and Upadhyaya H: Curcumin as potential therapeutic
natural product: A nanobiotechnological perspective. J Pharm
Pharmacol. 68:1481–1500. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Ghalandarlaki N, Alizadeh AM and
Ashkani-Esfahani S: Nanotechnology-applied curcumin for different
diseases therapy. Biomed Res Int. 2014:3942642014. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Subramani PA, Panati K and Narala VR:
Curcumin nanotechnologies and its anticancer activity. Nutr Cancer.
69:381–393. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Zanotto-Filho A, Coradini K, Braganhol E,
Schröder R, de Oliveira CM, Simões-Pires A, Battastini AM, Pohlmann
AR, Guterres SS, Forcelini CM, et al: Curcumin-loaded lipid-core
nanocapsules as a strategy to improve pharmacological efficacy of
curcumin in glioma treatment. Eur J Pharm Biopharm. 83:156–167.
2013. View Article : Google Scholar : PubMed/NCBI
|