Introduction
Keloids are benign dermal proliferative tumours that
develop as a result of abnormal wound-healing processes following
skin injury. Keloids are characterized by fibroblast proliferation,
excessive deposition of extracellular matrix (ECM), particularly
collagen and fibronectin, and increased infiltration of
inflammatory cells (1–3). Although it has been established that
excess deposition of ECM components, including collagen (4) by fibroblasts, is responsible for
keloids (5), the aetiology and
mechanisms underlying these effects remain poorly understood.
Keloids do not regress over time, and there is a high recurrence
rate following surgical excision.
MicroRNAs (miRNAs/miRs) are short non-coding RNAs
that serve critical roles in a number of important biological
processes, including cell proliferation, differentiation and
apoptosis (6–8). To date, hundreds of miRNAs have been
identified to be dysregulated in various diseased tissues; however,
only a fraction has been functionally characterized. miRNAs are
known to regulate skin development, and alterations in gene
expression and have been associated with skin pathologies,
including inflammatory disorders (8) and malignant lesions (9,10).
The primary function of miRNAs in skin fibrosis is to regulate the
expression of genes involved in its pathogenesis and maintenance
(11). However, the functional
role of miRNAs in the pathogenesis of keloids remains largely
unknown.
Trichostatin A (TSA) is a classical and widely used
histone deacetylase (HDAC) inhibitor (12). Inhibition of HDAC activity using
HDAC inhibitors results in cell growth inhibition. Thus, inhibition
of HDAC activity through the use of agents such as TSA has been
developed as a form of targeted therapy, which has demonstrated
promising antitumor effects in multiple malignancies, including
skin and colon cancer (13,14).
It has also been reported that TSA activity is associated with the
development and progression of certain chronic diseases
characterized by skin fibrosis (14). Therefore, it was hypothesized that
selective alterations in the miRNA expression profile caused by TSA
treatment may result in keloid fibroblast proliferation and the
accumulation of ECM.
In the current study, miR-30a-5p was observed to
induce apoptosis in keloid fibroblasts. In addition, the results
demonstrated that miR-30a-5p directly targeted and negatively
regulated B-cell lymphoma 2 (BCL2) by binding to its
3′-untranslated region (UTR), which resulted in the induction of
apoptosis. Notably, these effects were similar to those observed in
response to TSA exposure. These data provide novel and important
information that may help to elucidate the factors and mechanisms
underlying TSA exposure and miR-30a-5p in keloid fibroblasts.
Materials and methods
Samples
Keloid tissue samples were obtained from 15
patients, and healthy skin samples were obtained from 5 patients,
all admitted to the Department of Dermatology at No. 1 Hospital of
China Medical University (Shenyang, China) between June 2016 and
June 2017. The diagnosis of keloid samples was confirmed by
pathological examination. All protocols were approved by the Ethics
Committee of the No. 1 Hospital of China Medical University.
Cell culture
Human keloid tissues (n=8) were collected from
patients who underwent surgery at the Center for Plastic Surgery of
No. 1 Hospital of China Medical University between June 2016 and
June 2017. The skin specimens were washed in PBS containing 100
U/ml penicillin-streptomycin (Beyotime Institute of Biotechnology,
Haimen, China) and incubated with 2.5 mg/ml dispase II (Roche
Applied Science, Penzberg, Germany) overnight at 4°C. The following
day, the specimens were washed with PBS, and the dermis was
manually separated from the epidermis, cut into small 1-mm sections
and seeded in a culture flask. Fibroblasts were cultured in
Dulbecco's modified Eagle's medium (DMEM; Corning Inc., Corning,
NY, USA) supplemented with 10% fetal bovine serum (FBS; Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA). The cultures
were maintained at 37°C in an atmosphere with 5% CO2.
The medium was refreshed three times/week. Cells at passages 3–5
were used for the purposes of this study.
Cell proliferation assay
Cell proliferation was determined using a standard
MTT assay. In brief, keloid fibroblasts were first seeded at a
density of 5×103 cells/well into 96-well culture plates,
and cultured in DMEM containing 10% FBS for 24 h. The medium was
removed and replaced with DMEM containing 10% FBS and different
concentrations (0, 250, 500, 1,000 or 1,500 nM) of TSA (Enzo Life
Sciences, Inc., Farmingdale, NY, USA). Following incubation for a
further 24, 48 or 72 h, 20 µl MTT reagent (Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) was added, and the cells were incubated
at 37°C for 4 h. A total of 150 µl dimethyl sulfoxide was added to
stop the reaction. The effect of TSA on cell viability was
determined by measuring the absorbance at 490 nm.
Cell cycle analysis
Cells were washed three times with cold PBS and then
fixed in ice-cold 70% ethanol at 4°C overnight. The cells were
washed with cold PBS and each sample was incubated with 500 µl of
propidium iodide (PI; 50 µg/ml; Sigma-Aldrich; Merck KGaA) and 50
µl RNase A (50 µg/ml) at 37°C for 20 min in the dark. Analyses were
performed using a BD LSRFortessa Cell Analyzer (BD Biosciences, San
Jose, CA, USA) and the percentage of cells in the G0/G1, S or G2/M
cell cycle phases was analyzed using ModFit software version 3.0
(BD Biosciences). Experiments were repeated three times.
Apoptosis analysis
The number of apoptotic cells was quantified using
an Annexin-V-allophycocyanin (APC) Apoptosis Detection kit (cat.
no. 88-8007-74; Thermo Fisher Scientific, Inc.) according to the
manufacturer's instructions. Early apoptotic cells were defined as
Annexin-V-positive and PI-negative cells. Analyses were performed
using a BD LSRFortessa Cell Analyzer (BD Biosciences) and FlowJo
version 10.0.7 software (FlowJo LLC, Ashland, OR, USA). All
experiments were repeated three times.
Total RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Dermal tissue or dermal fibroblasts were first
crushed on ice and total RNA extracted using the miRNeasy mini kit
(Qiagen GmbH, Hilden, Germany), according to the manufacturer's
protocol. Fibroblasts at passages 3–5 were treated with TSA or
transfected with mimics and total RNA extracted using the miRNeasy
mini kit (Qiagen GmbH, Hilden, Germany), according to the
manufacturer's protocol. cDNA was synthesized from RNA using the
High Capacity cDNA Reverse Transcription kit or TaqMan MicroRNA
Reverse transcription kit (Thermo Fisher Scientific, Inc.). Each RT
reaction using the High Capacity cDNA Reverse Transcription kit was
performed in a 96 well Thermal Cycler (Applied Biosystems, USA) for
10 min at 25°C, 37°C for 120 min, 85°C for 5 min. Each RT reaction
using the TaqMan MicroRNA Reverse transcription kit was performed
in a 96 well Thermal Cycler (Applied Biosystems, USA) for 30 min at
16°C, 42°C for 30 min, 85°C for 5 min. Primer sequences are listed
in Table I. Realtime PCR of mRNA
was performed using GoTaq qPCR Master Mix reagent (Promega, WI,
USA) and a 7900HT Fast Real-Time PCR system (Thermo Fisher
Scientific, Inc.) with following program: Stage 1, 95°C for 2 min;
Stage 2, 40 cycles of 95°C for 15 sec, 60°C for 1 min. Analysis of
miRNA expression by qPCR was performed using TaqMan miRNA assays
(Thermo Fisher Scientific, Inc.) and a 7900HT Fast Real-Time PCR
system (Thermo Fisher Scientific, Inc.) with following program:
Hold 1, 50°C for 2 min; Hold 2, 95°C for 10 min; Hold 3, 40 cycles
of 95°C for 15 sec, then 60°C for 1 min. Relative expression was
calculated using the 2−ΔΔCq method or ΔCq method
(15). Confirmation of TSA-induced
miRNA clusters in keloid fibroblasts were normalized to the control
gene U6 small nuclear RNA and analysis of relative gene Expression
data performed with the 2−ΔΔCq method. Expression of
miR-30a-5p in keloid tissue as determined by use of RT-qPCR.
miR-30a-5p PCR data were compared with small nucleolar RNA, C/D box
48 and analysis of relative gene expression data performed using
the 2−ΔΔCq method.
 | Table I.Primers for reverse
transcription-quantitative polymerase chain reaction analysis with
the SYBR® Green system. |
Table I.
Primers for reverse
transcription-quantitative polymerase chain reaction analysis with
the SYBR® Green system.
| mRNA | Forward
(5′-3′) | Reverse
(5′-3′) |
|---|
| GAPDH |
AAGAGCACAAGAGGAAGAGAGAGAC |
GTCTACATGGCAACTGTGAGGAG |
| BCL2 |
GGATTGTGGCCTTCTTTGAG |
CCAAACTGAGCAGAGTCTTC |
| COL1A1 |
AGCCAGCAGATCGAGAACAT |
TCCTTGGGGTTCTTGCTGAT |
Western blotting
Cells were first washed with ice-cold PBS, and lysed
with phenylmethylsulfonyl fluoride (Beyotime Institute of
Biotechnology). Following centrifugation at 5,000 × g for 15 min at
4°C, the protein concentration was determined using a bicinchoninic
acid protein assay kit (cat. no. 23225; Thermo Fisher Scientific,
Inc.). Protein lysates (50 µg) were loaded onto 10% SDS-PAGE gels
before being transferred to polyvinylidene difluoride membranes.
The membranes were blocked with TBS with Tween 20 and 5% non-fat
milk for 1 h at room temperature, and subsequently incubated with
collagen I antibody (cat. no. BA0325; dilution, 1:200; Wuhan Boster
Biological Technology, Ltd., Wuhan, China), BCL2 antibody (cat. no.
ab196495; dilution, 1:1,000; Abcam, Cambridge, MA, USA), GAPDH
(cat. no. 10494-1-AP; dilution, 1:2,000; ProteinTech Group, Inc.,
Chicago, IL, USA) or β-actin (cat. no. ab90641; dilution, 1:5,000;
Abcam) overnight at 4°C. The membranes were subsequently incubated
with a horseradish peroxidase-conjugated secondary antibody (cat.
no. A0181; dilution, 1:1,000; Beyotime Institute of Biotechnology)
at room temperature for 1 h, and detected using enhanced
chemiluminescence (Amersham™ ECL™ Prime
Western Blotting Detection Reagent; GE Healthcare Life Sciences,
Little Chalfont, UK) and a MF-Chemi BIS 2.0 Imaging Systems(NDR Bio
Imaging Systems, Israel) the following day. Grayscale value
analysis of protein was performed by Image-Pro Plus 6.0 software
(Media Cybernetics, Inc., Rockville, MD, USA). The ratio of the
target protein to the internal reference protein was calculated for
each group.
Transient transfection
All oligonucleotides were synthesized by Ambion
(Thermo Fisher Scientific, Inc.). Transfections were performed
using Lipofectamine® RNAiMAX (cat. no. 13778-075; Thermo
Fisher Scientific, Inc.). Cells in the growth phase were seeded at
2×105 cells/well in six-well plates. To assess the
transfection efficiency of miR-30a-5p mimics, cells were
transfected with 40, 60 or 80 nM mimics for 24, 48 or 72 h,
respectively. Transfections of 60 nM miR-30a-5p mimics or negative
control mimics for 72 h was selected for all experiments.
TaqMan low-density miRNA RT-qPCR array
and miRNA analyses
The miRNA array was performed using three different
paired samples. RNA was first reverse transcribed using the TaqMan
miRNA Reverse Transcription kit and TaqMan miRNA Multiplex RT
assays, respectively; Thermo Fisher Scientific, Inc.) with
following program: Stage 1, 40 cycles of 16°C for 2 min, 42°C for 1
min, 50°C for 1 sec; Stage 2, 85°C for 5 min. Expression was
profiled using a TaqMan Human MicroRNA array card A, B (V2.1 and
V3.0, respectively) and a 7900HT Fast Real-Time PCR System (Thermo
Fisher Scientific, Inc.), according to the manufacturer's
recommended protocol. Briefly, the array was loaded and run using
the 384-well Taqman Low Density Array default thermal-cycling
conditions with following program: Stage 1, 94.5°C for 10 min;
Stage 2, 40 cycles of 97°C for 30 sec, 59.7°C for 1 min on a 7900HT
Fast Real-Time PCR System. The quantification cycle (Cq) values
were obtained with 900HT System Fast Real-Time PCR SDS software
version 2.3 (Applied Biosystems; Thermo Fisher Scientific, Inc.),
and the data were analyzed using RQ manager 1.2 software (Applied
Biosystems; Thermo Fisher Scientific, Inc.). U6 small nuclear RNA
was selected as a control. The -ΔCq[-(Cq-CqU6)] values
were calculated, and heatmap analyses were performed with
hierarchical clustering (16).
Verification of BCL2 as a direct
target gene of miR-30a-5p
The predicted (TargetScan Human 7.1 and miRanda) or
confirmed gene targets of these miRNAs were provided. A
dual-luciferase reporter assay was performed using 293T cells.
Briefly, the putative miR-30a-5-p binding sites within the 3′-UTR
of the BCL2 gene were amplified and cloned into the GV272 vector
(GeneChem, Inc., Shanghai, China), and the miR-30a-5p gene was
amplified and cloned into the GV251 vector (Shanghai GeneChem,
Inc.). Cells (1×105) were seeded in 24-well plates and
co-transfected with 100 ng wild-type or mutated BCL2 3′-UTR
constructs and 400 ng negative control or miR-30a-5p plasmids using
X-tremegene HP (Roche Diagnostics, Basel, Switzerland for ≤48 h.
Luciferase activity was determined using the Dual-luciferase
Reporter Assay System (cat. no. E1910; Thermo Fisher Scientific,
Inc.) following transfection for 48 h. Briefly,
Dual-Glo® Luciferase Assay Reagent was added to the
plate, the firefly luminescence measured, Dual-Glo® Stop
& Glo® Reagent added to the plate and Renilla
luminescence measured. The ratio of firefly:Renilla luminescence
for each well was calculated. The sample well ratio to the ratio
from control wells was normalized. Since the miRNA functions
primarily by targeting the 3′-UTR of the target gene, this region
may be cloned into a luciferase vector and positioned before the
luciferase reporter gene. Luciferase activity in mimic or negative
control-transfected cells was subsequently measured. Modifications
in gene expression are reflected in the change in luciferase
activity, and may quantitatively reflect the inhibitory effect of
miRNA on the target gene. With TRAF6-3′UTR as a positive control,
the expression of luciferase in the group was significantly
decreased (P<0.05), indicating that there was no problem in the
whole transfection detection system.
Statistical analysis
The results are expressed as the mean ± standard
deviation of at least three separate experiments, each performed in
triplicate. Differences between groups were analyzed using a
two-tailed Student's t-test, Mann-Whitney U test or one-way
analysis of variance with Tukeys post hoc test using the software
GraphPad Prism 7.0 (GraphPad Software, Inc., La Jolla, CA, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
TSA inhibits the growth of keloid
fibroblasts in a time- and dose-dependent manner
An initial dose titration was performed to determine
the appropriate concentration of TSA to be used in subsequent
experiments. Keloid fibroblasts were treated with media containing
increasing doses (0, 250, 500, 1,000 and 1,500 nM) of TSA. The
effects of TSA on cell viability were monitored using an MTT
proliferation assay. As presented in Fig. 1, keloid fibroblasts treated with
TSA demonstrated a statistically significant reduction in cell
growth following incubation with TSA for 24, 48 or 72 h.
Accordingly, TSA inhibited keloid fibroblast growth in a time- and
dose-dependent manner. The proliferation of keloid fibroblasts
treated with either 1,000 or 1,500 nM TSA was significantly
inhibited and morphological alterations were observed when compared
with the controls. Overall, cells treated with 1,000 nM TSA
tolerated the treatment well, and preserved their viability
compared with the control. Therefore, 1,000 nM TSA was used as the
working dose for all subsequent experiments.
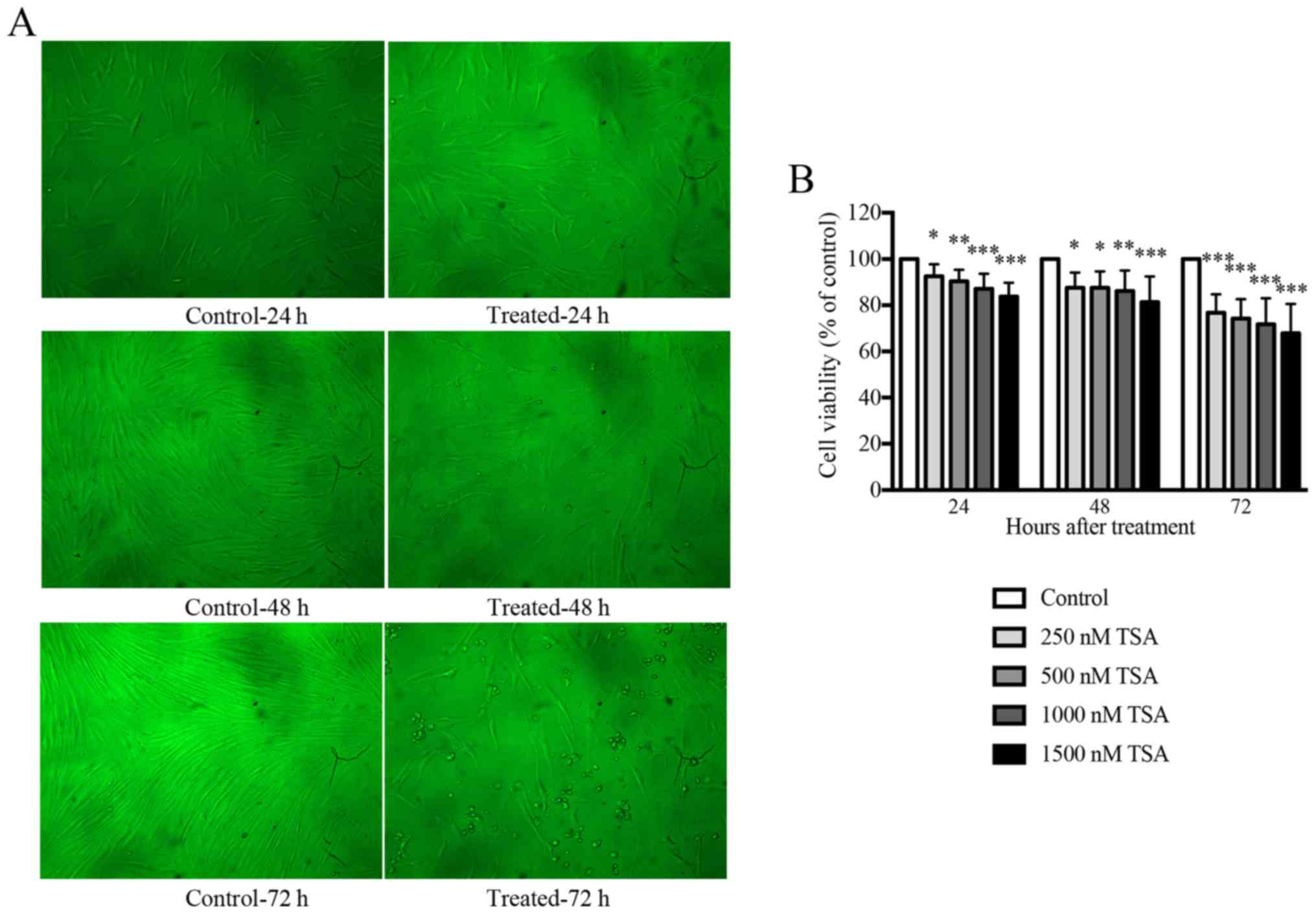 | Figure 1.TSA inhibits the growth of keloid
fibroblasts in a time- and dose-dependent manner. (A) Treatment
with 1,000 nM TSA altered the morphology of keloid fibroblasts at
24, 48 or 72 h in culture (×100 magnification). Keloid fibroblast
phenotypes were examined by phase-contrast microscopy for changes
in morphology. (B) The MTT assay indicated that TSA inhibited the
cell viability of keloid fibroblasts at concentrations of 250, 500,
1,000, 1,500 nM as observed after 24, 48 or 72 h in culture
compared with the control. Results are presented as the mean ±
standard deviation of three independent experiments (n=8). One-way
analysis of variance with Tukey's post-hoc test was used to compare
the groups. *P<0.05, **P<0.01 and ***P<0.001 vs.
respective control. TSA, trichostatin A. |
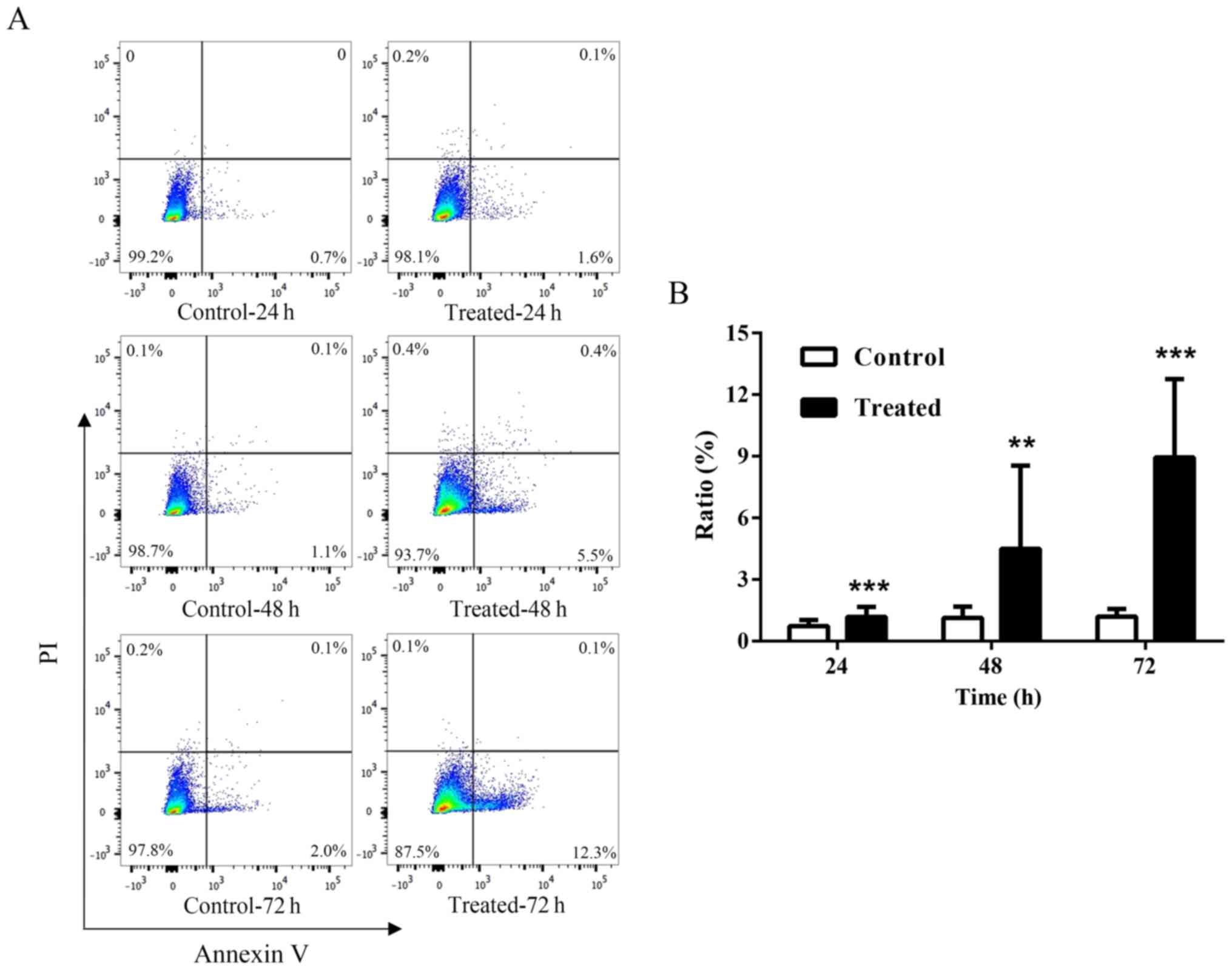
Apoptosis of keloid fibroblasts is
upregulated following TSA treatment
To investigate the effects of TSA on apoptosis,
cultured keloid fibroblasts were incubated with 1,000 nM TSA for
24, 48 or 72 h, and Annexin V-APC/PI staining for apoptosis
detection was performed, followed by flow cytometry. The results
demonstrated that keloid fibroblast apoptosis was upregulated
following TSA treatment at all three time points tested (P<0.01;
Fig. 2). In addition, the
apoptosis rate in cells pretreated with TSA for 72 h was increased
by 12.5% relative to the controls.
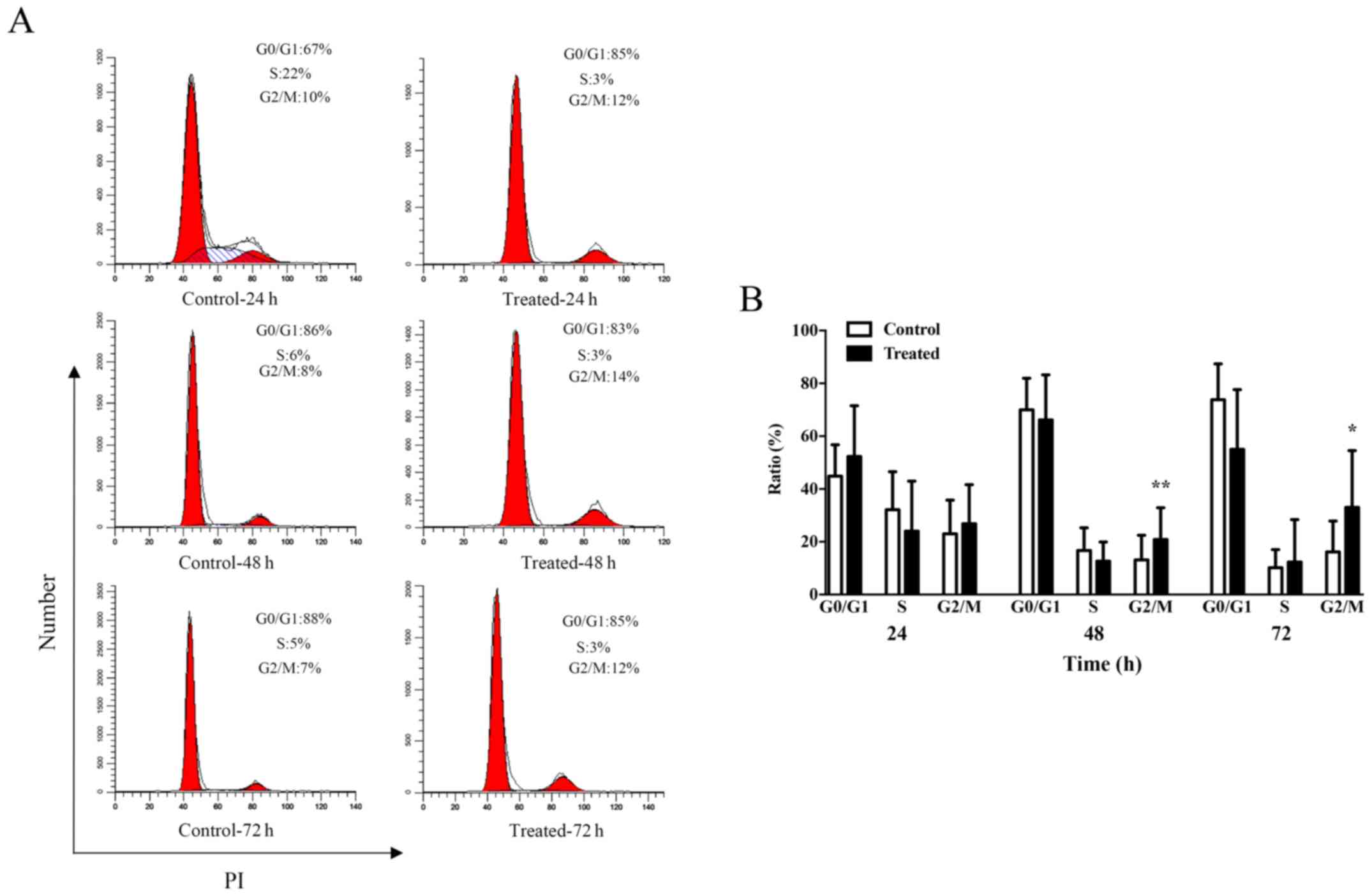
TSA blocks the G2/M cell cycle phase
of keloid fibroblasts
The results demonstrating the impact of TSA
treatment on keloid fibroblast proliferation prompted the
investigation of the role of cell cycle progression in this
phenotype. Following a 24 h incubation period, the average
percentage of cells in the G2/M phase increased from 23.0% in the
control group to 26.8% in the 1,000 nM TSA group (P=0.5287;
Fig. 3). Notably, G2/M arrest was
observed following longer incubation periods. After a 48 h
incubation period, the average percentage of cells in the G2/M
phase increased from 13.2% in the control group to 20.8% in the
1,000 nM TSA group (P=0.0064), while 72 h of TSA incubation was
associated with an average increase in the percentage of cells in
the G2/M phase from 16.2% in the control group to 33.0% in the
1,000 nM TSA group (P=0.0381; Fig.
3B). These results suggested that TSA may influence the cell
cycle of keloid fibroblasts.
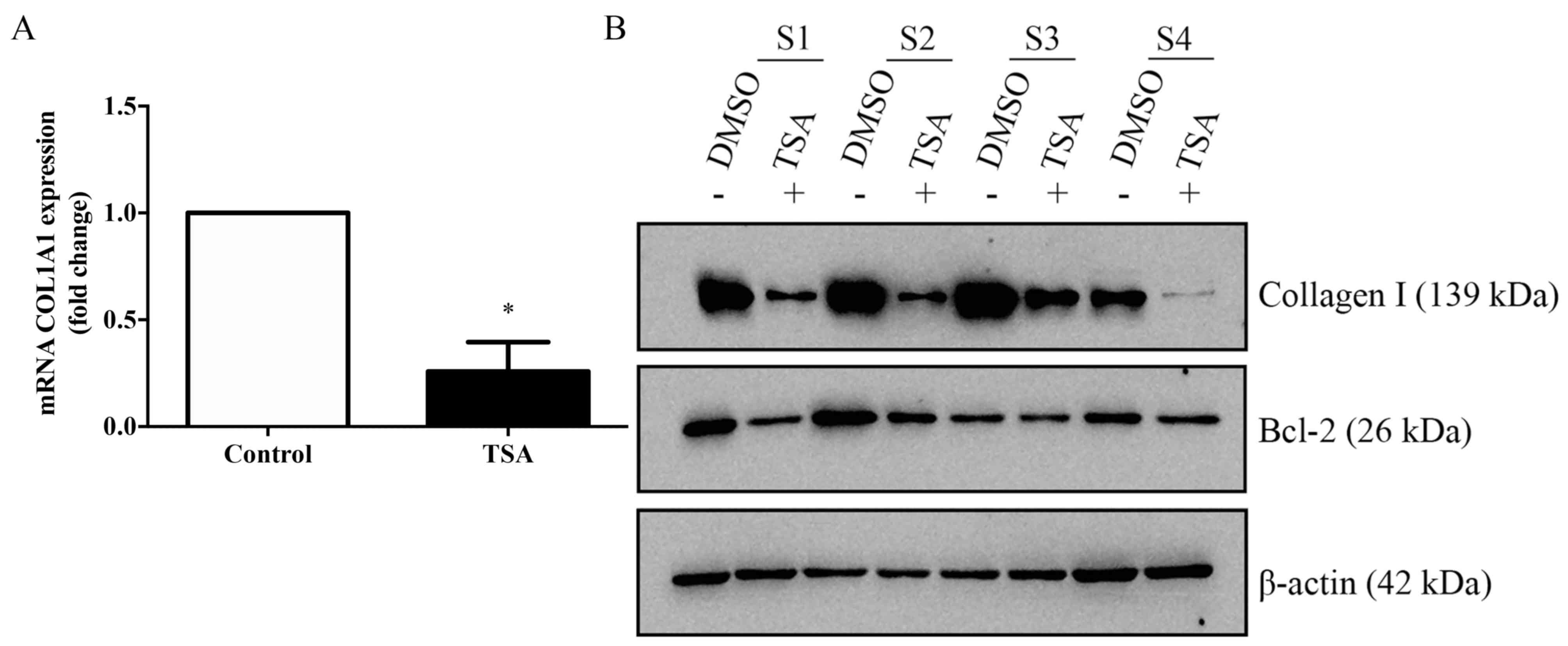
TSA inhibits the expression of
collagen and BCL2 in keloid fibroblasts
The ability of TSA to modulate the expression of
collagen type Iα 1 chain (COL1A1) in keloid fibroblasts was
investigated by RT-qPCR (Fig. 4A).
COL1A1 expression, as detected using western blotting, exhibited an
apparent decrease (Fig. 4B). In
addition, the protein expression levels of COL1A1 and BCL2 in
TSA-treated keloid fibroblasts were seemingly reduced compared with
the controls.
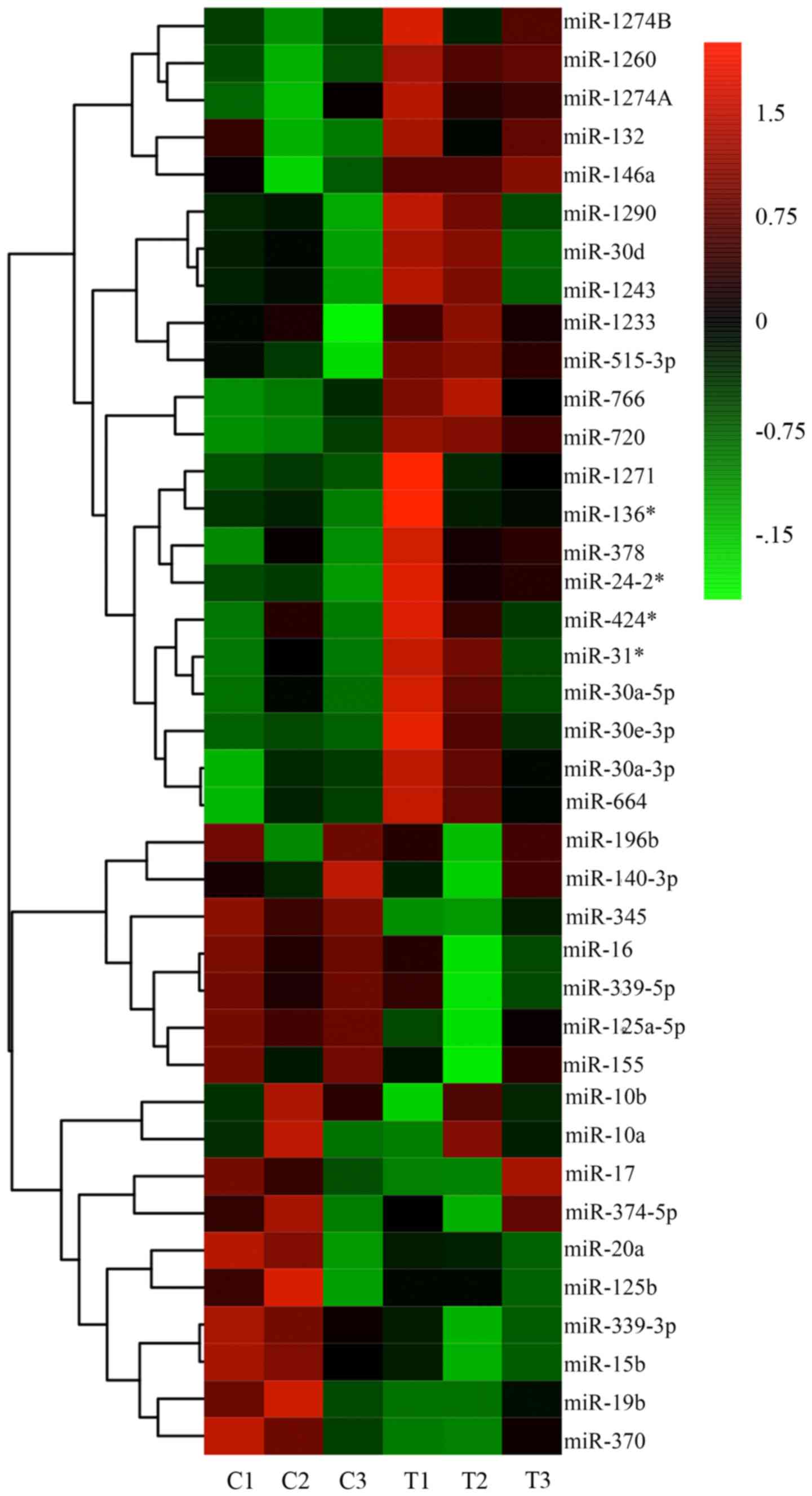
TSA interferes with the miRNA
expression profile in keloid fibroblasts
It has been previously reported that the expression
of specific miRNAs is significantly altered following TSA treatment
(17). The altered expression of
specific miRNAs has been associated with ECM synthesis, fibroblast
differentiation, epithelial-mesenchymal transition (EMT), in
addition to cancer initiation, invasion and metastasis (17,18).
Therefore, miRNA microarray analysis of keloid fibroblasts from
three biological samples treated with 1,000 nM TSA for 72 h was
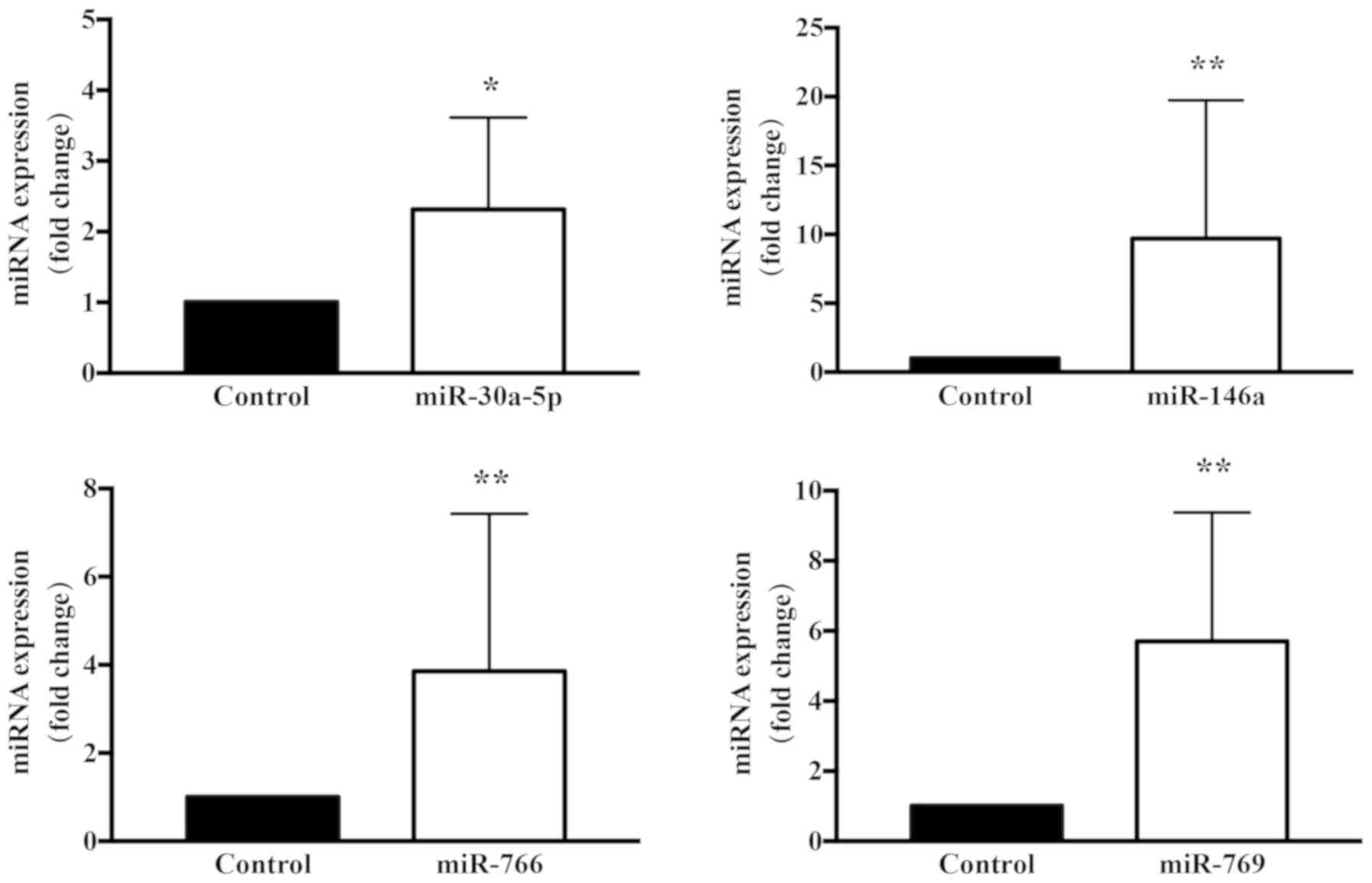
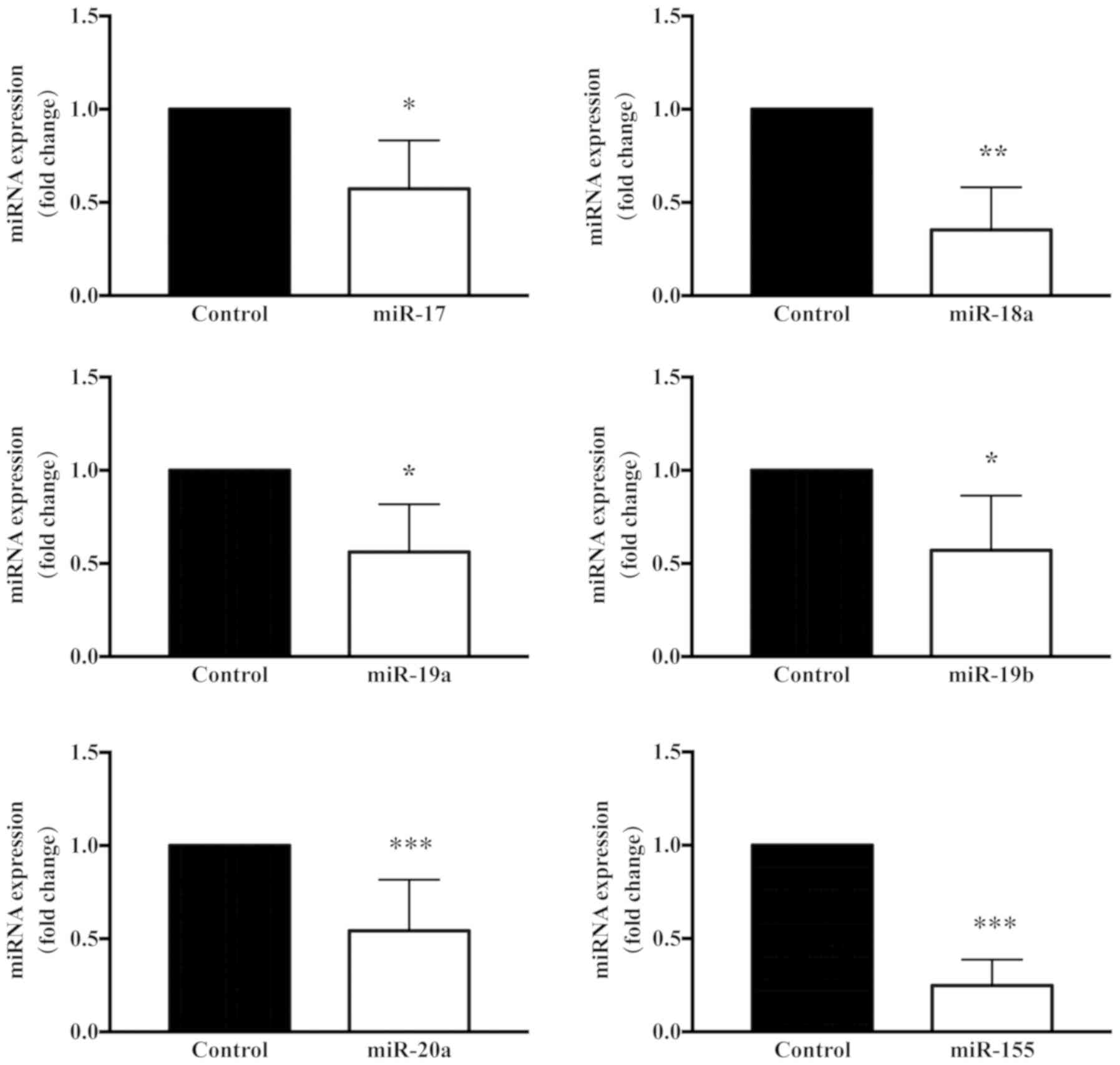
performed in the present study. As illustrated in the heatmap
presented in Fig. 5, a number of
alterations in miRNA expression were observed. Specifically, of the
miRNAs significantly altered by TSA treatment, 22 were upregulated
(Table II) and 10 were
downregulated (Table III). The
predicted (TargetScan Human 7.1 and miRanda) or confirmed gene
targets of these miRNAs are provided. Of particular relevance to
the present study, the miR-125a and miR-17 sequences, which have
been associated with fibroblast proliferation, were observed to be
downregulated, while miR-30a-5p and miR-146a, which have been
associated with transforming growth factor (TGF)-β signaling, were
upregulated. Considering previous studies, a number of miRNAs were
selected and alterations in their expression between treatment and
control were evaluated by RT-qPCR. The miRNA microarray and RT-qPCR
results verified the aforementioned observations, demonstrating
that that miR-30a-5p exhibited a marked alteration during
TSA-mediated inhibition of keloid fibroblast cell growth (Figs. 6 and 7). It has been reported that miR-30a-5p
targets BCL2 mRNA and induces apoptosis in non-small cell lung
cancer cells (19,20). Although the change observed was not
the most evident, it was found that potential targets of this
miR-30a-5p were more pertinent to the aims of the present study.
Therefore, the role of miR-30a-5p in keloid fibroblast regulation
was further investigated.
 | Table II.Upregulated microRNAs in keloid
fibroblasts treated with Trichostatin A. |
Table II.
Upregulated microRNAs in keloid
fibroblasts treated with Trichostatin A.
| miRs | Mean fold change ±
SD | Notable
targets | Key function |
|---|
| miR-1233 | 2.89±1.975 | HOXB3 | Invasion |
| miR-515-3p | 2.02±0.4761 |
|
|
| miR-132 | 2.05±0.4389 | RASA1 | Migration |
| miR-146a-5p | 4.89±3.199 | SMAD4, IRAK-1 | TGF-β
signaling |
| miR-30d | 2.12±0.7582 | CDH1 | EMT |
| miR-1243 | 2.27±0.9789 |
|
|
| miR-1271 | 1.95±1.263 | PDK1, CDK1,
FN1 | Proliferation,
apoptosis |
| miR-1260 | 3.64±0.8275 |
|
|
| miR-30e-3p | 2.79±1.92 | SNAIL1 | Invasion,
migration |
| miR-1274A | 3.84±2.318 | BMPR1B, FOXO4 | Proliferation,
migration |
| miR-424 | 2.85±2.822 | MEK1, CCNE1 | Proliferation |
| miR-1274B | 3.83±2.457 |
|
|
| miR-766 | 5.56±3.752 | MDM4, SOX6, | p53 signaling |
| miR-378 | 3.60±3.019 | FOXG1, GLI3 | MAPK/TGF-β
signaling |
| miR-30a-3p | 3.77±3.53 | IGF-1, PTEN,
BAFF | Invasion,
apoptosis |
| miR-31 | 3.83±3.626 | STK40, FIH-1 | Proliferation,
differentiation |
| miR-664 | 3.93±3.863 | IRS1, SOX7,
FOXO4 | Proliferation |
| miR-1290 | 5.86±4.125 | IRF2, LHX6,
BCL2 | EMT |
| miR-720 | 7.48±4.239 | CDH1 | EMT |
| miR-30a-5p | 5.18±5.965 | BCL2, NEUROD1,
Akt | Apoptosis |
| miR-24-2 | 7.44±6.375 | PKC-alpha,
BCL2 | Apoptosis |
| miR-136 | 6.11±7.416 | E2F1, MIEN1,
PMEL | Proliferation |
 | Table III.Downregulated microRNAs in keloid
fibroblasts treated with Trichostatin A. |
Table III.
Downregulated microRNAs in keloid
fibroblasts treated with Trichostatin A.
| miRs | Mean fold change ±
SD | Notable
targets | Key function |
|---|
| miR-10b | 0.22±0.1125 | CADM2, PIK3CA | Proliferation,
migration |
| miR-339-3p | 0.42±0.1862 | PRL-1 | Proliferation |
| miR-125a-5p | 0.42±0.1962 | BAX, BMF | Apoptosis |
| miR-155 | 0.43±0.1884 | SKI, TGFβ2R | TGF-β
signaling |
| miR-196b | 0.51±0.1001 | COL1A1, COL3A1 | ECM synthesis |
| miR-345 | 0.51±0.0151 | FOXQ1, YAP1,
IRF1 | Metastasis,
EMT |
| miR-15b | 0.44±0.1977 | RECK, ERK1 | Proliferation |
| miR-16 | 0.65±0.1977 | HGF, SESN1 | P53 signaling |
| miR-140-3p | 0.64±0.1564 | ATP6AP2,
ATP8A1 | Proliferation,
invasion |
| miR-339-5p | 0.66±0.166 | NACC1, MDM2 | P53 signaling |
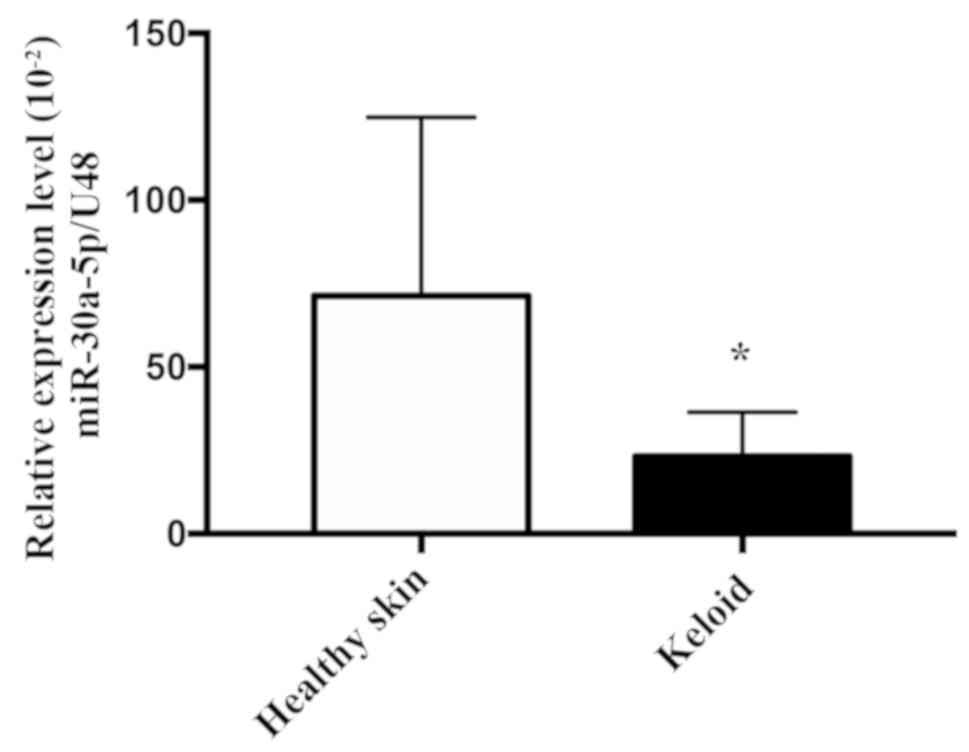
miR-30a-5p expression is downregulated
in keloid tissues
Based on the observation that TSA altered the
expression of miR-30a-5p in keloid fibroblasts, the expression of
miR-30a-5p in keloid tissues was investigated. To achieve this,
RT-qPCR was used to examine the expression levels of miR-30a-5p in
five healthy skin and seven keloid tissue samples. As exhibited in
Fig. 8, the mean expression levels
of miR-30a-5p in keloid tissue samples were significantly decreased
when compared with the healthy skin samples (P=0.0480). These
results suggested that miR-30a-5p may serve an important role in
inhibiting the pathological process of keloid formation.
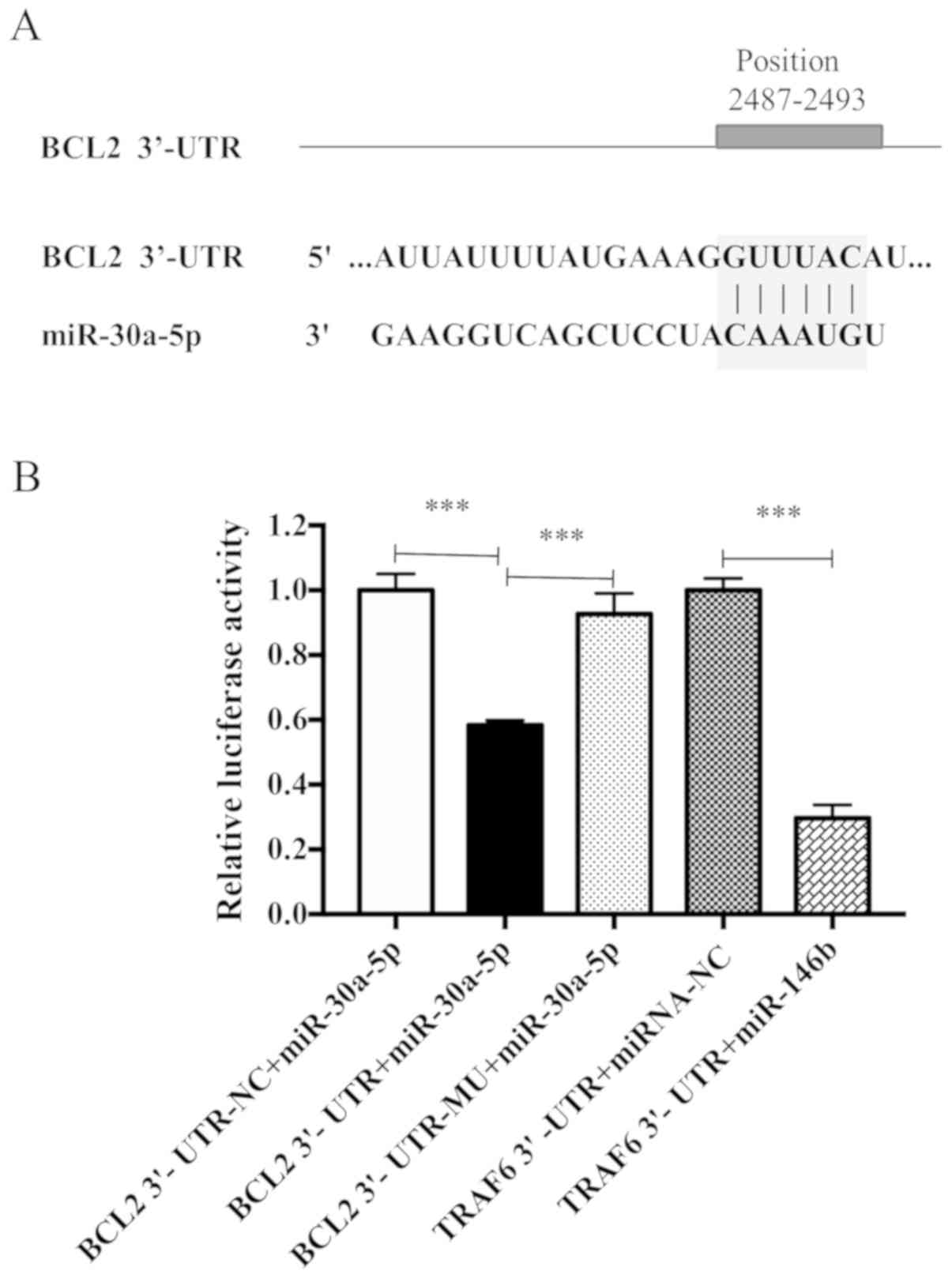
Predicted and confirmed mRNA
targets
The results presented thus far suggested that
miR-30a-5p may be associated with fibroblast proliferation and ECM
deposition. The 3′-UTR of BCL2 was predicted to contain a
miR-30a-5p binding site (Fig. 9A).
To experimentally validate this miRNA-mRNA interaction, a
luciferase assay was performed. As presented in Fig. 9B, miR-30a-5p significantly
decreased BCL2 3′-UTR reporter activity when compared with the BCL2
3′-UTR negative control group. The inhibitory effect of miR-30a-5p
was eliminated when the predicted miR-30a-5p binding site was
mutated. TRAF6-3′ UTR was used as a positive control; the
expression of luciferase in the group was significantly decreased
(P<0.05), indicating that there was no problem in the
transfection detection system.
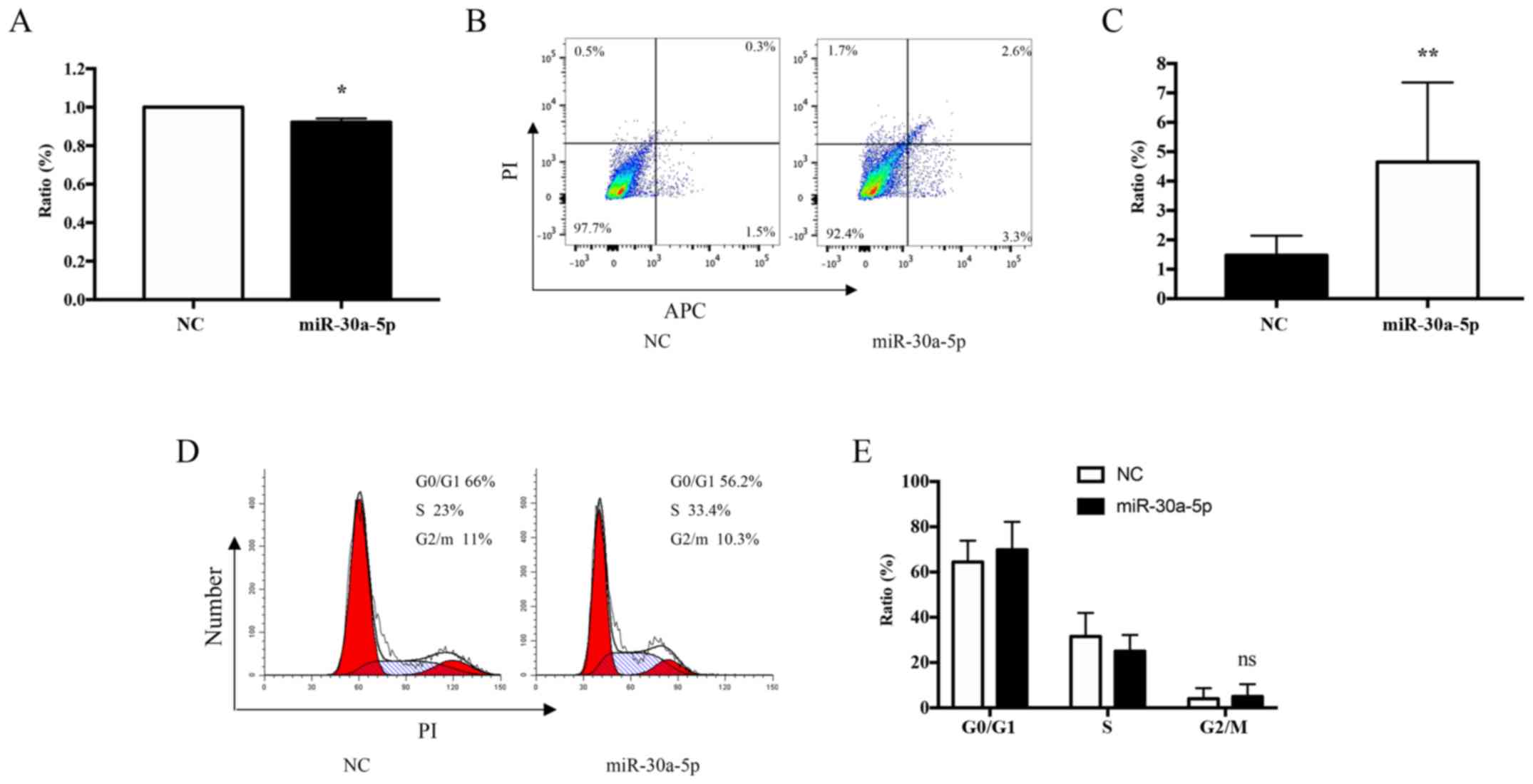
Overexpression of miR-30a-5p inhibits
apoptosis and the proliferation of keloid fibroblasts in vitro
To further investigate the role of miR-30a-5p in
keloid fibroblasts treated with TSA, the transfection efficiency of
miR-30a-5p mimics within keloid fibroblasts cultured for 72 h was
determined. The effect of miR-30a-5p mimics on cell viability was
monitored using MTT proliferation assays. The results demonstrated
that the proliferation of cultured keloid fibroblasts was reduced
by 8% when compared with the controls (P=0.0187; Fig. 10A). To study the inhibitory effect
of miR-30a-5p on apoptosis, keloid fibroblast cultures were
incubated with 60 nM miR-30a-5p for 72 h, and the level of
apoptosis was ascertained using Annexin V-APC/PI staining and flow
cytometry analysis. The results demonstrated that keloid fibroblast
apoptosis was increased by 5.9% relative to the control (P=0.0031;
Fig. 10B and C). However, no
effects on cell cycle progression were observed (P=0.6303; Fig. 10D and E).
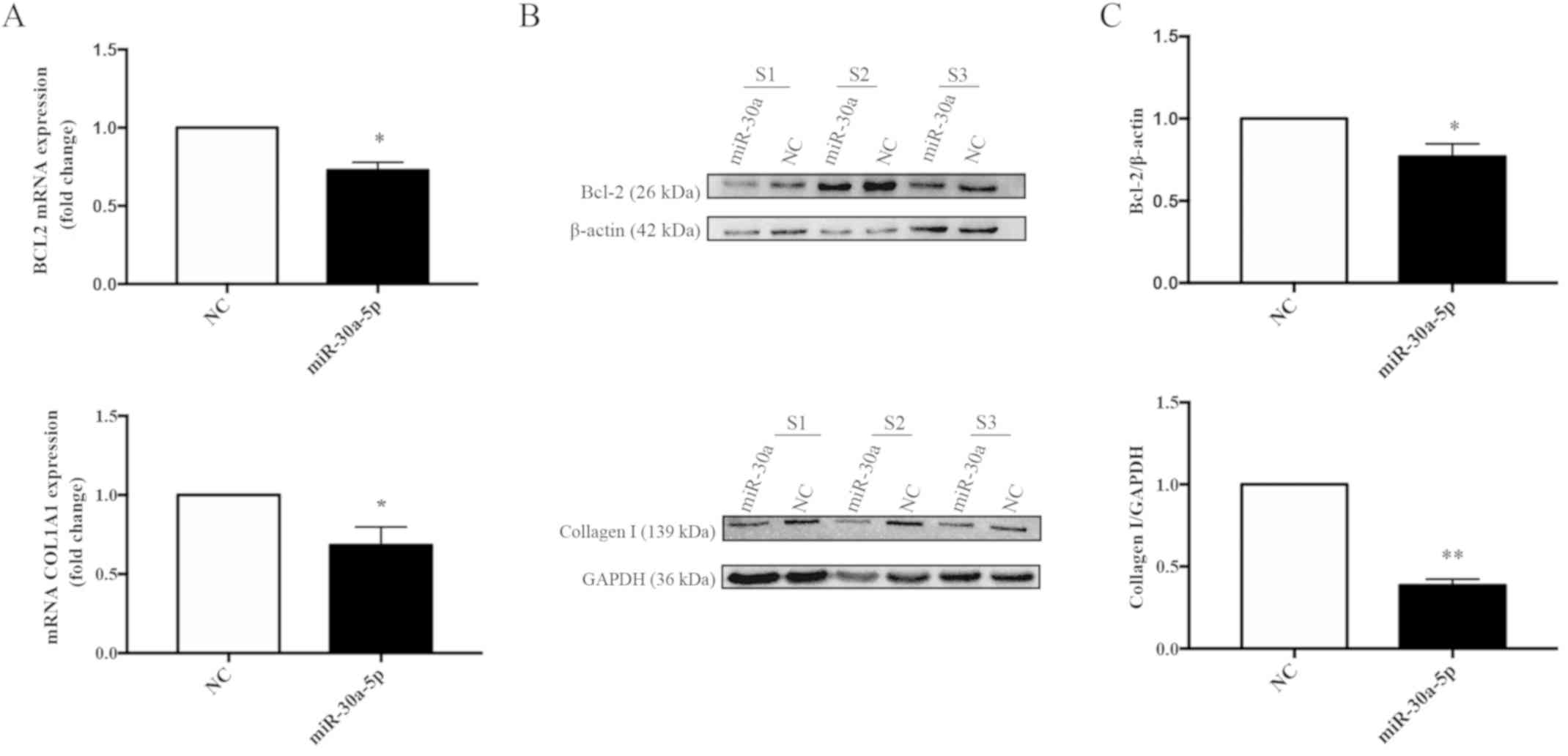
Overexpression of miR-30a-5p inhibits
the mRNA and protein expression levels of BCL2 and COL1A1 in keloid
fibroblasts in vitro
To investigate potential interactions between the
predicted target gene, BCL2, and miR-30a-5p, total RNA and protein
from keloid fibroblasts transfected with negative control or
miR-30a-5p mimics for 72 h was determined by RT-qPCR. Furthermore,
western blot analyses were performed to determine the mRNA and
protein expression levels of BCL2 and COL1A1. The mRNA and protein
levels of BCL2 (Fig. 11A-C) and
COL1A1 (Fig. 11D-E) were
significantly decreased in keloid fibroblasts. When compared with
the negative control mimic-transfected cells, BCL2 mRNA levels were
decreased by 0.73-fold (P=0.0116) and COL1A1 mRNA by 0.68-fold
(P=0.0118), and statistically significant reductions in BCL2
(P=0.0361) and COL1A1 (P=0.0012) protein expression levels were
also observed.
Discussion
In the current study, an important inhibitory role
of TSA and miR-30a-5p in the process of fibrosis in keloid
fibroblasts was demonstrated. TSA is a broad-spectrum HDAC
inhibitor that serves a key role in the epigenetic regulation of
multiple genes involved in fibrosis and adverse remodeling
(14,18–21).
TSA effectively inhibits the EMT of hepatic stellate cells
(22) and abrogates
TGF-β1-induced, fibrosis-associated gene expression in skin
fibroblasts (23). To date, a
considerable body of evidence has provided an insight into the
crosstalk among different signaling pathways in fibrosis, and has
elucidated the molecular actions of TSA in attenuating fibrogenesis
(22–25). Of particular note, one study
demonstrated that TSA induces apoptosis in keloid fibroblasts
(26). The results of the current
study demonstrated that 1,000 nM TSA produced a time-dependent
inhibition of keloid fibroblast proliferation and inhibited cell
growth in the G2/M cell cycle phase. Treatment with 1,000 nM TSA
also altered the miRNA expression profile of genes involved in cell
proliferation, apoptosis and migration. Previous research has
suggested that miRNAs serve important roles in fibrosis, and may be
useful targets for the treatment of this disease (27). The present study tested and
verified the differential expression of miRNAs, identified from an
array, through RT-qPCR analysis, and the results were consistent
with the TaqMan low-density miRNA array, RT-qPCR and miRNA
analyses. Notably, the miR-17-92 cluster was downregulated,
suggesting that it may contribute to cell proliferation and
fibrosis (28,29). By contrast, specific miRNAs,
reportedly associated with fibroblast differentiation, such as
miR-146a, were observed to be upregulated (30).
miR-30a-5p is a multifunctional miRNA that has been
implicated in numerous cell processes, including cell growth,
proliferation and migration (20,31,32).
To investigate the significance of miR-30a-5p in human keloid
fibroblasts in the current study, the expression of miR-30a-5p in
human keloid tissues and normal healthy tissue samples was first
compared. The results demonstrated that keloid tissues exhibited
decreased expression of miR-30a-5p when compared with healthy skin
tissues. miR-30a-5p overexpression was also observed to induce
apoptosis in keloid fibroblasts in vitro. These results
provide evidence of a functional, mechanistic and clinically
relevant role for this molecule. However, further research is
required to elucidate the targets of miR-30a-5p, in addition to the
molecular signaling pathways mediating these different biological
effects in human keloid fibroblasts.
A previous report demonstrated that miR-30a-5p
sensitizes non-small cell lung cancer cells to paclitaxel by
inducing apoptosis via BCL2 inhibition (20). BCL2 regulates cell death by
inhibiting apoptosis (20). BCL2
also serves as a phosphatase enzyme that is essential for the
specific and effective termination of fibrosis (33). With the use of bioinformatics
analysis software in the present study, BCL2 was identified as a
predicted target of miR-30a-5p, a result that was verified using a
dual-luciferase reporter assay. In addition, BCL2 was observed to
be downregulated in keloid fibroblasts transfected with a
miR-30a-5p mimics. As the miR-3a-5p mimic induced apoptosis and
decreased the expression of COL1A1, it is likely that miR-30a-5p
may induce apoptosis and inhibit the proliferation of keloid
fibroblasts by inhibiting BCL2.
Similar to the effects observed in fibroblasts
treated with 1,000 nM TSA, overexpression of miR-30a-5p resulted in
the inhibition of keloid fibroblast cell proliferation in
vitro, as well as apoptosis induction. However, the miR-30a-5p
mimic exerted no observable effect on the cell cycle progression of
active keloid fibroblasts. Thus, TSA may induce G2/M cycle arrest
in keloid fibroblast through other gene networks that require
further research. Moreover, miR-30a-5p mimics induced apoptosis and
inhibited proliferation, which was analogous but not identical to
the effects of TSA treatment in keloid fibroblasts. TSA may have
downregulated the mRNA and protein expression levels of BCL2 and
COL1A1 by upregulating the expression of miR-30a-5p.
In conclusion, possibly due to its capacity to
upregulate the expression of miR-30a-5p, TSA was observed to induce
apoptosis and inhibit the proliferation of keloid fibroblasts.
Using this process, TSA may inhibit the synthesis of ECM in keloid
fibroblasts. The results of the present study provide novel and
noteworthy information regarding the mechanisms underlying the
capacity for TSA to induce miR-30a-5p and thus regulate apoptosis
in keloid fibroblasts in vitro. The results present a
foundation for the potential use of TSA and miR-30a-5p in the
diagnosis and treatment of patients with keloids. However,
additional in vivo experiments are necessary to confirm
these results.
Acknowledgements
The authors would like to acknowledge the Key
Laboratory of Immunodermatology, Ministry of Health (China Medical
University, Shenyang, China) for providing the space and equipment
for conducting the experiments.
Funding
This study was supported by: The Science &
Technology Fund of Liaoning Province (grant no. 201501013); the
National Natural Science Fund of China (grant no. 81602741); the
Distinguished Professor Foundation of Liaoning Province [grant no.
Liao (2012) 145]; and the Major Science and Technology Platform of
Liaoning Province [grant no. Liao (2010) 191].
Availability of data and materials
The data used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CH and XJ conceived and designed the experiments; XJ
performed the experiments; XJ, LQ, QZo, QZh, SC and YW analyzed the
data; QZo, QZh, SC, YW, XG and HC contributed
reagents/materials/analysis tools; XJ wrote the manuscript. HC and
XG assessed the data and revised the manuscript. All authors read
and approved the final manuscript.
Ethics approval and consent to
participate
Informed consent was obtained from all individual
participants included in the present study. The present study was
approved by the Ethics Committee of the No.1 Hospital of China
Medical University [approval no. (2016)71; Shenyang, China].
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li Z and Jin Z: Comparative effect and
safety of verapamil in keloid and hypertrophic scar treatment: A
meta-analysis. Ther Clin Risk Manag. 12:1634–1641. 2016. View Article : Google Scholar
|
|
2
|
Xue M and Jackson CJ: Extracellular matrix
reorganization during wound healing and its impact on abnormal
scarring. Adv Wound Care (New Rochelle). 4:119–136. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wilgus TA and Wulff BC: The importance of
mast cells in dermal scarring. Adv Wound Care (New Rochelle).
3:356–365. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Younai S, Nichter LS, Wellisz T, Reinisch
J, Nimni ME and Tuan TL: Modulation of collagen synthesis by
transforming growth factor-beta in keloid and hypertrophic scar
fibroblasts. Ann Plast Surg. 33:148–151. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Suarez E, Syed F, Rasgado TA, Walmsley A,
Mandal P and Bayat A: Skin equivalent tensional force alters keloid
fibroblast behavior and phenotype. Wound Repair Regen. 22:557–68.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sonkoly E, Stahle M and Pivarcsi A:
MicroRNAs: Novel regulators in skin inflammation. Clin Exp
Dermatol. 33:312–315. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Igoucheva O and Alexeev V:
MicroRNA-dependent regulation of cKit in cutaneous melanoma.
Biochem Biophys Res Commun. 379:790–794. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Molnar V, Tamasi V, Bakos B, Wiener Z and
Falus A: Changes in miRNA expression in solid tumors: An miRNA
profiling in melanomas. Semin Cancer Biol. 18:111–122. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mottamal M, Zheng S, Huang TL and Wang G:
Histone deacetylase inhibitors in clinical studies as templates for
new anticancer agents. Molecules. 20:3898–3941. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Schneider MR: MicroRNAs as novel players
in skin development, homeostasis and disease. Br J Dermatol.
166:22–28. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhao LM and Zhang JH: Histone deacetylase
inhibitors in tumor immunotherapy. Curr Med Chem. 2017.(Epub ahead
of prin). View Article : Google Scholar
|
|
13
|
Singh A, Patel P, Jageshwar, Patel VK,
Jain DK, Kamal M and Rajak H: The safety, efficacy and therapeutic
potential of histone deacetylase inhibitors with special reference
to panobinostat in gastrointestinal tumors: A Review of Preclinical
and Clinical Studies. Curr Cancer Drug Targets. 18:720–736. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang L, Qu M, Wang Y, Duan H, Chen P, Wang
Y, Shi W, Danielson P and Zhou Q: Trichostatin a inhibits
transforming growth Factor-β induced reactive oxygen species
accumulation and myofibroblast differentiation via enhanced
NF-E2-related factor 2-antioxidant response element signaling. Mol
Pharmacol. 83:671–680. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rao X, Huang X, Zhou Z and Lin X: An
improvement of the 2ˆ(-delta delta CT) method for quantitative
real-time polymerase chain reaction data analysis. Biostat
Bioinforma Biomath. 3:71–85. 2013.PubMed/NCBI
|
|
17
|
Rhodes LV, Nitschke AM, Segar HC, Martin
EC, Driver JL, Elliott S, Nam SY, Li M, Nephew KP, Burow ME and
Collins-Burow BM: The histone deacetylase inhibitor trichostatin A
alters microRNA expression profiles in apoptosis-resistant breast
cancer cells. Oncol Rep. 27:10–16. 2012.PubMed/NCBI
|
|
18
|
Williams SM, Golden-Mason L, Ferguson BS,
Schuetze KB, Cavasin MA, Demos-Davies K, Yeager ME, Stenmark KR and
McKinsey TA: Class I HDACs regulate angiotensin II-dependent
cardiac fibrosis via fibroblasts and circulating fibrocytes. J Mol
Cell Cardiol. 67:112–125. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bodas M, Mazur S, Min T and Vij N:
Inhibition of histone-deacetylase activity rescues inflammatory
cystic fibrosis lung disease by modulating innate and adaptive
immune responses. Respir Res. 19:22018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xu X, Jin S, Ma Y, Fan Z, Yan Z, Li W,
Song Q, You W, Lyu Z and Song Y: miR-30a-5p enhances paclitaxel
sensitivity in non-small cell lung cancer through targeting BCL-2
expression. J Mol Med (Berl). 95:861–871. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Tung CW, Hsu YC, Cai CJ, Shih YH, Wang CJ,
Chang PJ and Lin CL: Trichostatin A ameliorates renal
tubulointerstitial fibrosis through modulation of the JNK-dependent
Notch-2 signaling pathway. Sci Rep. 7:144952017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kaimori A, Potter JJ, Choti M, Ding Z,
Mezey E and Koteish AA: Histone deacetylase inhibition suppresses
the transforming growth factor beta1-induced
epithelial-to-mesenchymal transition in hepatocytes. Hepatology.
52:1033–45. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rombouts K, Niki T, Greenwel P,
Vandermonde A, Wielant A, Hellemans K, De Bleser P, Yoshida M,
Schuppan D, Rojkind M and Geerts A: Trichostatin A, a histone
deacetylase inhibitor, suppresses collagen synthesis and prevents
TGF-beta(1)-induced fibrogenesis in skin fibroblasts. Exp Cell Res.
278:184–197. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Sun J, Wang Y, Cui W, Lou Y, Sun G, Zhang
D and Miao L: Role of epigenetic histone modifications in diabetic
kidney disease involving renal fibrosis. J Diabetes Res. 7:242–384.
2017.
|
|
25
|
Ghosh AK, Mori Y, Dowling E and Varga J:
Trichostatin A blocks TGF-beta-induced collagen gene expression in
skin fibroblasts: involvement of Sp1. Biochem Biophys Res Commun.
354:420–426. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Diao JS, Xia WS, Yi CG, Wang YM, Li B, Xia
W, Liu B, Guo SZ and Sun XD: Trichostatin A inhibits collagen
synthesis and induces apoptosis in keloid fibroblasts. Arch
Dermatol Res. 303:573–580. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Babalola O, Mamalis A, Lev-Tov H and
Jagdeo J: The role of microRNAs in skin fibrosis. Arch Dermatol
Res. 305:763–76. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Zhu H, Han C and Wu T: MiR-17-92 cluster
promotes hepatocarcinogenesis. Carcinogenesis. 36:1213–22. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Dakhlallah D, Batte K, Wang Y,
Cantemir-Stone CZ, Yan P, Nuovo G, Mikhail A, Hitchcock CL, Wright
VP, Piper MG and Marsh CB: Epigenetic regulation of miR-17~92
contributes to the pathogenesis of pulmonary fibrosis. Am J Respir
Crit Care Med. 187:397–405. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li YL, Wang J, Zhang CY, Shen YQ, Wang HM,
Ding L, Gu YC, Lou JT, Zhao XT, Ma ZL and Jin YX: MiR-146a-5p
inhibits cell proliferation and cell cycle progression in NSCLC
cell lines by targeting CCND1 and CCND2. Eur Respir J.
7:59287–59298. 2016.
|
|
31
|
He R, Yang L, Lin X, Chen X, Lin X, Wei F,
Liang X, Luo Y, Wu Y, Gan T, et al: MiR-30a-5p suppresses cell
growth and enhances apoptosis of hepatocellular carcinoma cells via
targeting AEG-1. Int J Clin Exp Pathol. 8:15632–15641.
2015.PubMed/NCBI
|
|
32
|
Wang Z, Dai X, Chen Y, Sun C, Zhu Q, Zhao
H, Liu G, Huang Q and Lan Q: MiR-30a-5p is induced by Wnt/β-catenin
pathway and promotes glioma cell invasion by repressing NCAM.
Biochem Biophys Res Commun. 465:374–380. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Safaeian L, Abed A and Vaseghi G: The role
of Bcl-2 family proteins in pulmonary fibrosis. Eur J Pharmacol.
741:281–9. 2014. View Article : Google Scholar : PubMed/NCBI
|