Introduction
Sepsis is a systemic inflammatory response syndrome
that can be caused by invading pathogens (1–3).
Severe sepsis leads to multiple organ dysfunction and circulatory
failure (1–3). Despite significant advances in the
understanding of the mechanisms of sepsis, it remains one of the
major causes of patient mortality in intensive care units. Sepsis
is a common critical condition that is difficult to treat and has a
high mortality rate (4,5). In the United States, the incidence of
severe sepsis is estimated to be 300 cases per 100,000 of the
population, and the age-adjusted mortality caused by sepsis is
>60 per 100,000 (6). Sepsis is
associated with gastrointestinal tract dysfunction, manifesting as
dysfunction in gastrointestinal motility, nutritional absorption
and the intestinal immunological barrier, which ultimately results
in intestinal mucosa atrophy, dysbacteriosis and enterogenic
infection, thereby causing or aggravating dysfunction in other
organs (7,8). Hiltebrand et al (9) reported that stomach blood flow was
reduced by 55% in a sepsis model in pigs. Lang et al
(5) also reported decreased
cardiac output during sepsis, and a similar observation has been
made in humans (10–12).
Ghrelin, which is predominantly secreted by the
gastric mucosa (~67%) and intestinal mucosa (~33%) (13–17),
mediates its effect by binding to growth hormone secretagogue
receptor-1a (15). Ghrelin has a
vital role in regulating appetite, promotes ingestion, increases
body weight, improves gastric contraction function, increases small
intestine transportation, promotes the secretion of growth hormones
and improves gastric mucosal blood flow (18–20).
Ghrelin may have some therapeutic potential for treating
gastrointestinal dysfunction in patients with diabetic
gastroparesis and in the ileus post-surgery (21). The protective effects of ghrelin in
the stomach are reported to be due to improved blood flow mediated
by prostaglandins and nitric oxide in a healthy rodent model
(22,23). Additionally, ghrelin has been shown
to suppress intestinal mucosal apoptosis in a non-inflammatory
animal model (24). However, it is
not clear whether the administration of ghrelin has a beneficial
effect on sepsis-induced gastric system complications. Therefore,
the present study aimed to explore the effects of ghrelin on
gastric blood flow and disease severity in septic rats, and
investigated the potential mechanisms by which ghrelin regulates
the expression of apoptosis-associated factors in gastric
tissues.
Materials and methods
Animals
Healthy male Wistar rats (n=36) aged 6–8 weeks and
weighing 180–250 g were purchased from the Gansu University of
Traditional Chinese Medicine (Lanzhou, China). Animals were
maintained under pathogen-free conditions at the animal facility of
Lanzhou University (Lanzhou, China). Rats had free access to food
and water and they were maintained under a 12-h light/dark cycle at
room temperature with 50–65% relative humidity. All procedures
involving mice were approved by the Institutional Animal Care and
Use Committee of Lanzhou University.
Development of sepsis
The rats were randomly divided into 3 groups
(n=12/group): Sham group, sepsis group and ghrelin group. The rats
in the sham group received sham surgery. Rats in the sepsis and
ghrelin groups underwent cecal ligation and puncture (CLP) surgery
to induce the sepsis model (25,26).
Briefly, rats were fasted for 12 h and intraperitoneally injected
with 1% pentobarbital sodium (50 mg/kg; production batch number
wS2016040l; West of Shanghai Tang Biotechnology Co., Ltd.,
Shanghai, China) for anesthesia prior to surgery. Following
conventional disinfection, a 2-cm incision was made along the
abdominal medial line. The cecum was circumferentially ligated 0.5
cm away from the ileocecal valve using 3/0 suture. An 18-gauge
syringe needle was used to cut through the cecum twice. The
intestinal contents were slightly pushed out by gently squeezing
the intestinal canal. The cecum was returned to its original site,
and the incision was sutured layer-by-layer. Sterile saline (3
ml/100 g) was immediately subcutaneously injected to compensate for
the loss of body fluid. For the rats in the sham group, after
locating the cecum, the intestine was placed outside of the abdomen
for 2 min and then returned to the abdominal cavity.
Ghrelin (Enzo Life Sciences, Inc., Farmingdale, NY,
USA) was dissolved in saline (1 ml) and injected intraperitoneally
at 13.3 nmol/kg/injection in the ghrelin group rats at 2, 4 and 8 h
post-surgery, following a previously established protocol (27,28).
The rats in the sham and sepsis groups were injected with 1 ml
saline at the same time points as the ghrelin group rats following
surgery.
Blood pressure monitoring
Blood pressure in the 3 groups of rats was measured
at the tail base 2 h post-surgery using a non-invasive blood
pressure system (IITC Life Science, Inc., Woodland Hill, CA, USA)
according to the manufacturer's instructions. Rats were fixed in
place using rat fixing apparatus (DCX II; JiXi RuiJi BioTechnology
Co., Ltd.).
Gastric perfusion imaging
A laser Doppler perfusion imaging instrument
(PeriScan PIM 1I; Perimed AB, Järfälla, Sweden) was used to assess
rat blood flow in the greater curvature of the stomach. The laser
wavelength used was 670 nm. The NR scanning mode and Min scanning
accuracy were used in this experiment. The imaging range was set at
25×30 mm, and pixel size was 0.5×0.5 px. LDPI imaging software
(version 2.5; Perimed AB, Järfälla, Sweden) was used to record,
analyze and process gastric blood flow images. The unit of blood
flow was expressed as perfusion units.
Levels of systemic and local ghrelin
and inflammatory factors assay
At 24 h following the operation, sera collected from
each experimental group were diluted and used in ELISAs to measure
protein levels. The rat gastrointestinal tract from the stomach to
the duodenum was isolated, and the food debris was carefully
removed under a dissection microscope. One part of the tissue was
mechanically homogenized in radioimmunoprecipitation assay (RIPA)
lysis buffer (Santa Cruz Biotechnology, Inc., Dallas, TX, USA)
containing protease inhibitors. The homogenized tissue was
incubated on ice for 45 min with brief vortexing every 5 min.
Tissue lysates were centrifuged at 4,000 × g for 40 min at 4°C, the
pellets were discarded and protein concentration of the supernatant
was measured using the Pierce Bicinchoninic Acid Protein Assay kit
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). The serum
(systemic) and protein lysate (local) ghrelin levels were then
measured using a Colorimetric Rat Ghrelin ELISA kit (cat. KA1863;
Bio-Techne, Minneapolis, MN, USA). The levels of inflammatory
factors [tumor necrosis factor (TNF)-α, interleukin (IL)-1β and
IL-6] were measured using the Rat Inflammation ELISA Strip (cat.
no. EA-1201; Signosis, Inc., Santa Clara, CA, USA) and Rat IL-10
ELISA Kit (cat. no. RAB0246; Merck KGaA, Darmstadt, Germany)
according to the manufacturer's protocol. The final local
inflammatory factor concentrations were normalized to the starting
initial protein concentration.
Isolation of rat primary gastric
epithelial cells (GECs)
Rat GECs were isolated as previously described
(29,30). Briefly, bacterial contaminants were
removed from the remaining part of the collected tissue (incubated
in 50 ml 0.04% sodium hypochlorite on ice for 15 min). The tissue
was minced and incubated in 1% pronase (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) dissolved in 1XPBS for 2 h at 37°C. The cells
were then treated with DNase I for 40 min at 37°C, washed with
ice-cold buffer and centrifuged at 1,000 × g for 5 min at 4°C. The
cells were then filtered with 40-mm nylon cell mesh and washed with
PBS three times at room temperature, and then cultured in
epithelial cell culture medium. The isolated GECs were validated
using specific markers anti-CD45 (1:200; cat. no. 202201;
Biolegend, Inc., San Diego, CA, USA) and anti-CD103 (1:200; cat.
no. 205505; Biolegend, Inc.) that were incubated for 30 min on ice.
Flow cytometry was performed using a flow cytometer (FACSCanto II;
BD Bioscience, San Jose, CA), and analyzed using the BD FACSDiva
software (version 7.0; BD Bioscience).
Immunohistochemistry and
immunofluorescence staining
Tissues were embedded in Optimal Cutting Temperature
compound solution and immediately frozen in dry ice/ethanol bath
and stored at −80°C. Sections (4 µm in thickness) were prepared
using a freezing microtome (wi78174; Leica Microsystems GmbH,
Wetzlar, Germany). Tissue sections (thickness, 4 µm) were
deparaffinized at room temperature with 100% xylene for 5 min and
rehydrated at room temperature using descending ethanol series
(100, 95, 80 and 70% ethanol). Each incubation in ethanol was
performed at room temperature for 3 min. The sections were then
subjected to antigen retrieval (citrate buffer; 10 mmol/l; pH 6.0)
for 15 min at room temperature. The samples were blocked with 5%
goat serum (1:50; cat. no. GTX30973; GeneTex, Inc.) for 10–15 min
at room temperature. Primary antibodies anti Bcl-2 (1:100; cat. no.
EKC1055; Wuhan Boster Biological Technology, Ltd.) and Bax (1:100;
cat. no. M00183-2; Wuhan Boster Biological Technology, Ltd.) were
incubated overnight at 4°C. After washing with PBS (pH 7.2–7.6),
biotin-labeled goat anti-rabbit immunoglobulin G was added and
incubated at room temperature for 10–15 min. Horseradish
peroxidase-labeled streptavidin working solution was incubated with
the samples at room temperature for 10 min. After washing with PBS,
staining was visualized with 0.01% 3,3′-diaminobenzidine
tetrahydrochloride for 5 min and counterstained with 0.05%
hematoxylin for 6–10 sec at room temperature. Positive was detected
using a SABC-POD kit according to the manufacturer's protocol
(Wuhan Boster Biological Technology, Ltd., Wuhan, China). Primary
antibodies against Bcl-2 (cat. no. EKC1055) or Bax (cat. no.
M00183-2) were purchased from Wuhan Boster Biological Technology,
Ltd. Primary antibodies (1:100 dilution) were incubated with
sections at 4°C overnight, then the SuperPicture™ 3rd
Gen IHC Detection kit (Thermo Fisher Scientific, Inc.) was used to
detect the protein signal. For cleaved caspase-3 detection,
immunofluorescent staining was performed. Primary rabbit
anti-cleaved caspase-3 (cat. no. 9664; Cell Signaling Technology,
Inc.) was incubated with the sections for 2 h at room temperature,
subsequently, the SuperPicture™ 3rd Gen IHC Detection
kit (Thermo Fisher Scientific, Inc.) was used to detect the protein
signal according to the manufacturer's protocol. Nuclei were marked
using DAPI (10 µg/ml; cat. no. ab228549; Abcam) at room temperature
for 5 min in the dark.
RNA extraction and semi-quantitative
polymerase chain reaction (sqPCR) detection of ghrelin
At 2 h following sepsis induction, GECs were
isolated from the sham and CLP group rats. Total RNA was extracted
from 1×106 GECs using the RNeasy RNA extraction kit
(Qiagen, Inc., Valencia, CA, USA) following the kit's instructions.
Extracted total RNA (1 µg) from each group of rats was reverse
transcribed in a 20 µl reaction using the Superscript III reverse
transcriptase (Thermo Fisher Scientific, Inc.), according to the
manufacturer's instructions, with hexamer primers supplied in the
kit. RT products were diluted (2-fold) in RNase-free, and 1:20 of
the cDNA was used to amplify ghrelin. GAPDH was used as an internal
control. The primers used in the PCR were as follows: Rat ghrelin,
forward 5′TGAGCCCAGAGCACCAGAAA3′ and reverse
5′GTTGCAGAGGAGGCAGAAGCT3′; rat GAPDH, forward
5′TGAAGGTCGGTGTGAACGGATTTGGC3′ and reverse
5′CATGTAGGCCATGAGGTCCACCAC3′. PCR was performed in a 25 µl PCR
system containing 50 mM KCl, 10 mM Tris-HCl, 2 mM MgCl2,
0.2 mM dNTP and 0.5 units Platinum Taq DNA polymerase (Thermo
Fisher Scientific, Inc.) using the following PCR program: Initial
denaturation at 95°C for 3 min, followed by 25 cycles of 94°C for
30 sec, 45°C for 30 sec and 72°C for 1 min/kb. PCR was carried out
in a thermal cycler (Bio-Rad Laboratories, Inc., Hercules, CA,
USA). Following reveres transcription-PCR, 10 µl of the reaction
mixture was electrophoresed on a 1% agarose gel containing ethidium
bromide (0.22 g/ml). The gel was then imaged and band intensities
were normalized to GAPDH using the Bio-Rad Image System (Gel Doc
XR+ System; Bio-Rad Laboratories, Inc.) and the Image Lab Software
(version number 1708195; Bio-Rad Laboratories, Inc.).
Western blotting
Isolated GECs were lysed in RIPA buffer for 15 min
on ice and cleared by centrifugation at 4,000 × g for 15 min at
4°C. Protein concentration was determined using a bicinchoninic
acid assay kit. Protein (50 µg) was separated by 12.5% SDS-PAGE and
transferred to 0.2-m PVDF membranes. The membranes were blocked by
incubation in TBS (10 mM Tris-HCl, pH 7.5 and 150 mM NaCl)
containing 5% milk for 1 h at room temperature. Blots were
incubated with rabbit anti-Bcl-2 (cat. no. D17C4), Bax (cat. no.
D3R2M) and cleaved caspase-3 (cat. no. Asp175) antibodies (1:5,000;
Alpha Diagnostic International, San Antonio, TX, USA) overnight at
4°C. The blots were then washed 3 times in TBS supplemented with
0.05% Tween for 10 min. Blots were incubated with horseradish
peroxidase-labeled secondary antibodies (cat. no. A0208; 1:3,000;
Beyotime Institute of Biotechnology, Haimen, China) for 1 h at room
temperature and then washed 3 times in TBST for 10 min. An enhanced
chemiluminescent peroxidase substrate (GE Healthcare, Chicago, IL,
USA) was applied according to the manufacturer's instructions and
the membranes were exposed to X-ray film. The band densities were
normalized to β-actin (cat. no. 20536-1-AP; 1:5,000; Proteintech
Group, Inc.) with the use of the Bio-Rad Image System (Bio-Rad
Laboratories, Inc.).
Assay for GEC viability following
lipopolysaccharide (LPS) stimulation
Cell viability was determined using the Annexin V
Apoptosis Detection kit (BD Biosciences; Becton, Dickinson and
Company, Franklin, Lakes, NJ, USA) according to the manufacturer's
instructions. Fresh isolated GECs (2×105) from naïve
rats were cultured in 24-well plates and stimulated using LPS (100
ng/ml; Sigma-Aldrich; Merck KGaA) with or without 0.1 nmol ghrelin
(Enzo Life Sciences, Inc.) for 2, 4 and 8 h. Following the
incubation period, the cells were stained with 5 µl Annexin V and
propidium iodide (PI) followed by flow cytometric analysis, using a
flow cytometer (LSR I; BD Bioscience). Data were analyzed using
FlowJo (version X.0.7; FlowJo LLC; Ashland, OR, USA) and expressed
as the percentage of Annexin V and PI-positive cells.
Statistical analysis
The results are expressed as the mean ± standard
error of the mean. SPSS 19.0 software (IBM Corp., Armonk, NY, USA)
was used to analyze the data. Comparisons among groups were
performed using two-way analysis of variance with the
Student-Newman-Keuls-q post-hoc test. P<0.05 was considered to
indicate a statistically significant difference.
Results
Ghrelin expression is downregulated
during CLP induced sepsis
To investigate the potential benefits of ghrelin
administration to septic patients, a CLP-induced rat sepsis model
was established in the present study, following a previously
published protocol (27,28). Ghrelin mRNA expression levels in
the sham and CLP group rats were determined at 2 h following CLP or
sham surgery. At 20 h post-CLP and ghrelin administration, the
protein levels of systemic and local ghrelin were determined by
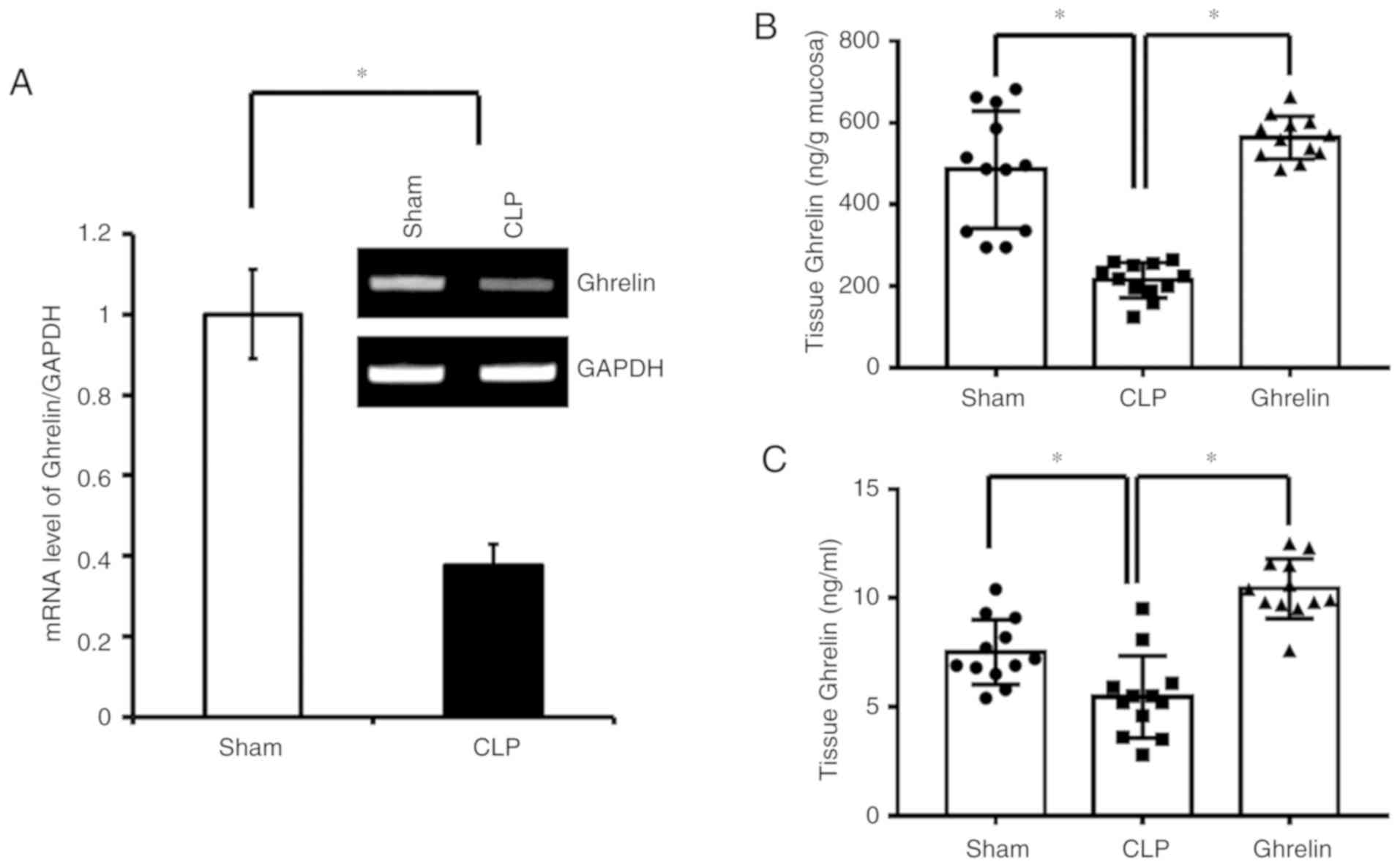
ELISA in the 3 experimental groups. As shown in Fig. 1A, at 2 h post-surgery, the ghrelin
mRNA level was significantly lower in the CLP sepsis group than in
the sham group. Consistently, at 20 h post-surgery, the protein
level of ghrelin in the stomach tissue (Fig. 1B), where ghrelin is secreted, and
the circulation (Fig. 1C) was also
downregulated in the CLP sepsis rats when compared with the sham
group. Notably, administration of exogenous ghrelin significantly
increased the ghrelin level in the stomach mucosa and in the serum
(Fig. 1B and C).
Ghrelin administration attenuates
sepsis symptoms induced by CLP
The altered ghrelin expression level indicated that
it may be involved in the regulation of sepsis development. It has
been previously reported that ghrelin attenuates sepsis-induced
acute lung injury and mortality in rats (31). Therefore, it was investigated
whether ghrelin has a similar role in the gastrointestinal tract
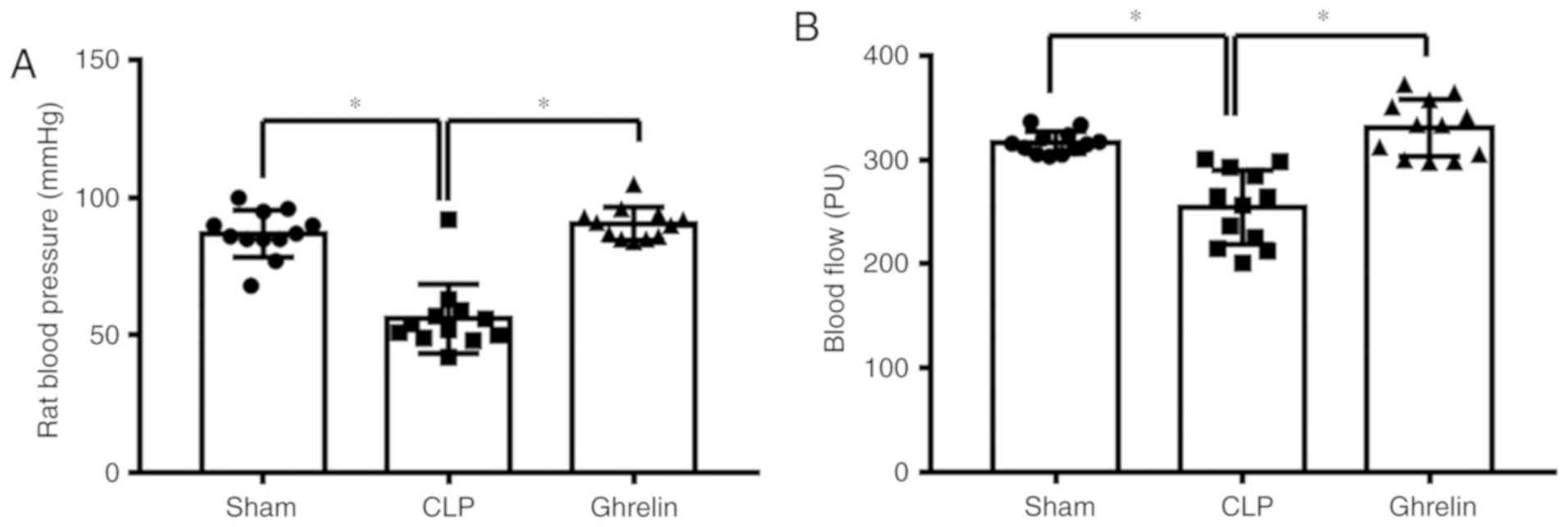
during sepsis. At 24 h post-CLP, blood pressure and blood flow were
measured to determine the sepsis status. As shown in Fig. 2, compared with the sham group, CLP
induced a significant decrease in blood pressure (Fig. 2A) and blood flow to the greater
curvature of the stomach (Fig.
2B). By contrast, the blood pressure and the blood flow were
increased in the ghrelin-treated group when compared with the CLP
group (Fig. 2A and B). Ghrelin
treatment restored the blood pressure and the blood flow to the
stomach almost back to the level of the sham group, indicating that
ghrelin treatment may be able to attenuate sepsis symptoms.
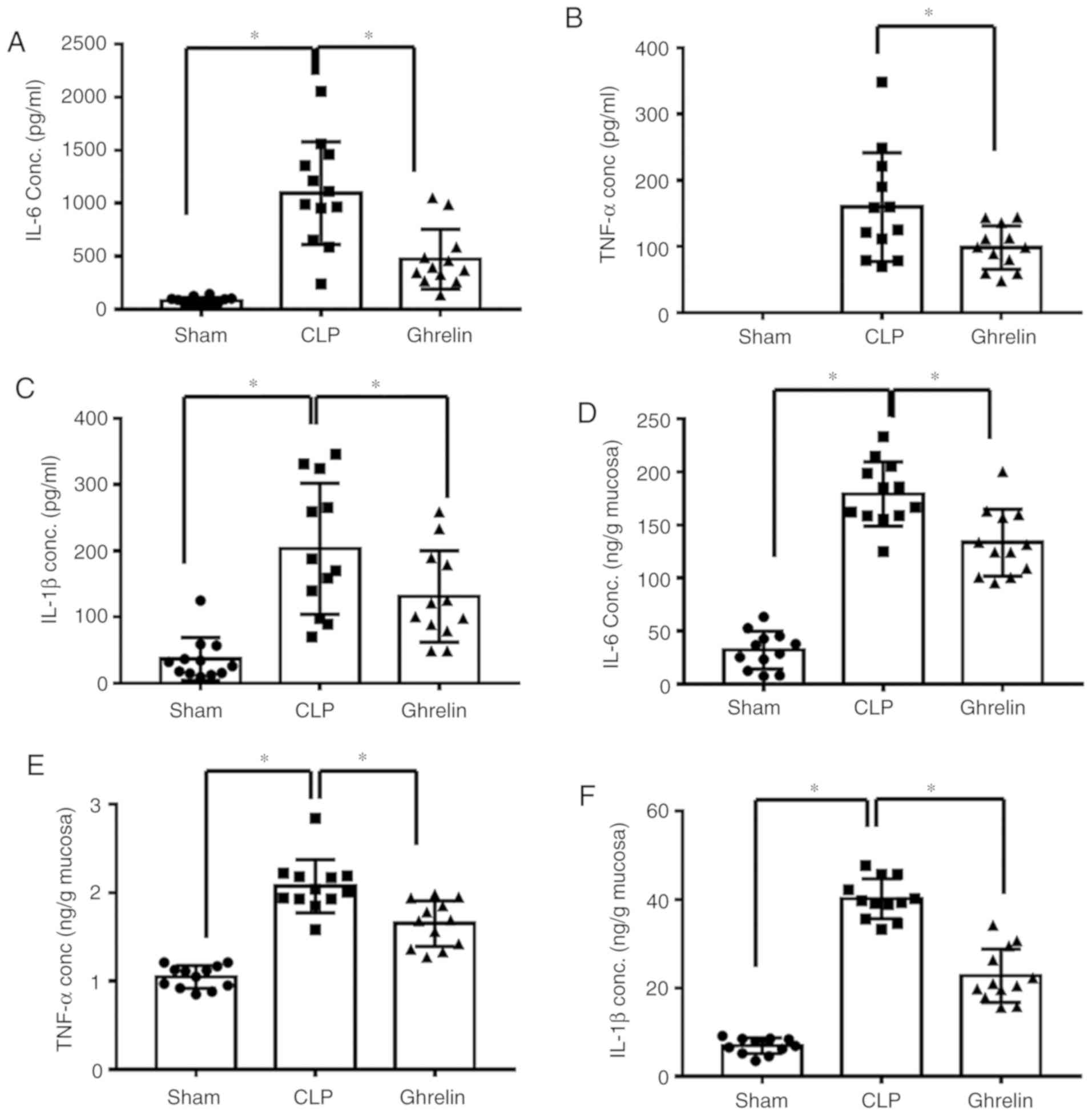
Increased pro-inflammatory cytokine levels are considered to be
another indicator of sepsis. The level of cytokines, including
TNF-α, IL-6 and IL-1β, were examined in the circulation (Fig. 3A-C) and in the stomach tissue
(Fig. 3D-F) of sepsis model rats.
As shown in Fig. 3, the levels of
the 3 proinflammatory cytokines were significantly increased in the
CLP group when compared with that of the sham group, while
administration of ghrelin reversed this CLP-induced increase,
indicating that ghrelin may have a protective role during
sepsis-induced systemic inflammatory response syndrome.
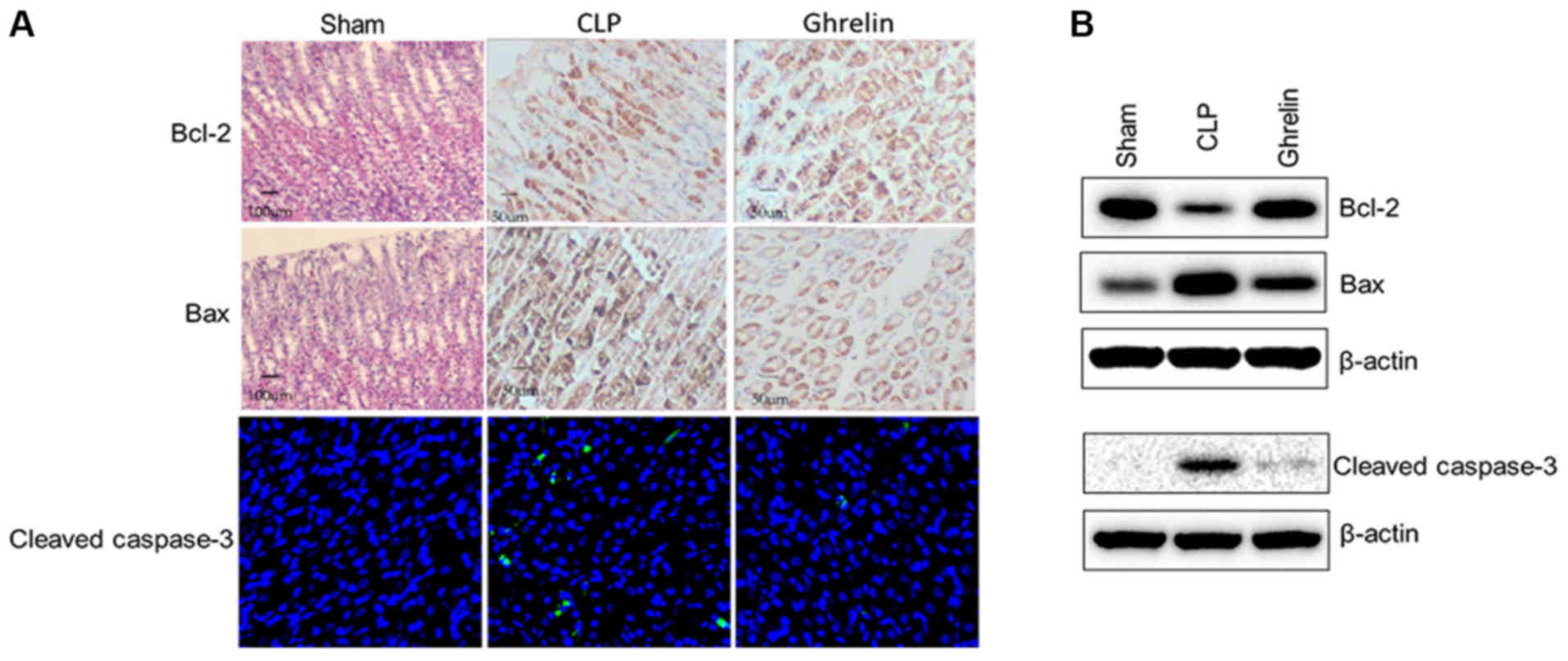
Apoptosis-associated protein
expression profile in stomach mucosa
Uncontrolled and abnormal inflammatory reactions in
tissues can lead to cell apoptosis. As sepsis induced systemic
inflammation in the present model, it was hypothesized that CLP may
also affect cell apoptosis in the stomach mucosa. To validate this
hypothesis, the expression of 3 representative apoptosis-associated
proteins was assessed in the 3 experimental groups.
Immunohistochemistry or immunofluorescent staining was used to
detect the protein expression of Bcl-2 and Bax, and cleaved
caspase-3, respectively, in the stomach mucosa. The most striking
result to emerge from the data, as shown in Fig. 4A, was that Bcl-2 and Bax expression
was barely detectable in the rat gastric mucosal tissues in the
sham group. CLP-induced sepsis decreased the level of Bcl-2 and
increased the level of Bax in the stomach mucosa compared with that
of the sham group, in terms of staining density and the number of
stained cells. When ghrelin was administered to the CLP-induced
sepsis rats, the staining of these 2 proteins was attenuated when
compared with CLP alone. Consistently, cleaved caspase-3, a marker
of cell apoptosis, exhibited the same trend (Fig. 4A). While CLP increased the cleaved
caspase-3 level, application of ghrelin significantly downregulated
its expression in the stomach mucosa. These observations were
further validated and semi-quantified by western blotting. Stomach
mucosa from the same rats was harvested as aforementioned, and
lysates were blotted to determine the protein level of Bcl-2, Bax
and cleaved caspase-3 (Fig. 4B).
CLP significantly reduced the expression of Bcl-2, and enhanced the
pro-apoptotic proteins Bax and cleaved caspase-3 compared with sham
rats; whereas, ghrelin application reversed the effects of CLP on
the expression of these apoptosis-associated proteins. Based on
these results, it was hypothesized that ghrelin protected the
stomach from CLP-induced sepsis injury by preventing the apoptosis
of mucosal cells.
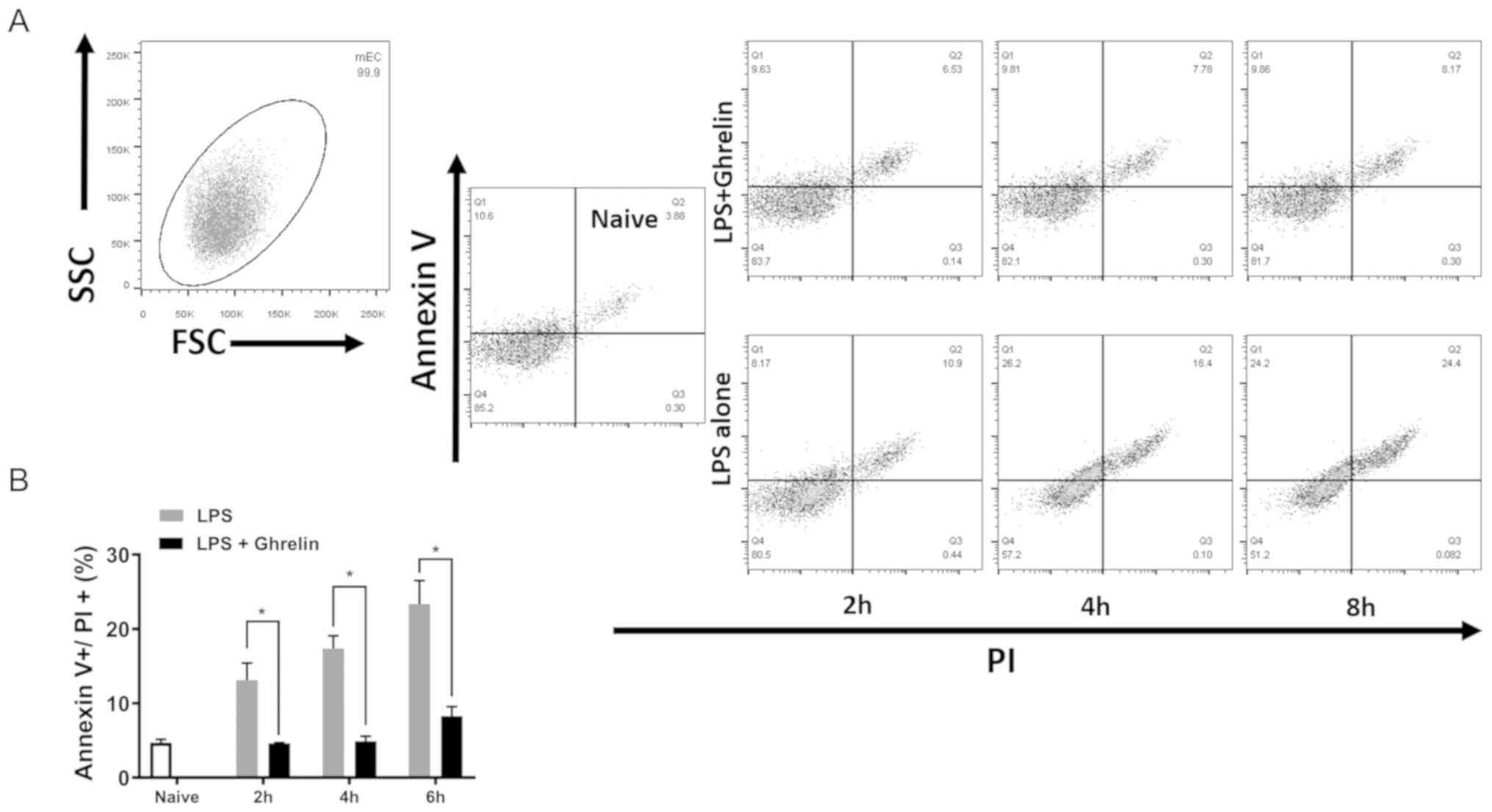
Ghrelin inhibits cell apoptosis in
stomach mucosa in vitro
To further elucidate the role of ghrelin in stomach
mucosa cell apoptosis, LPS, the trigger of septic shock, was used
to induce cell apoptosis in vitro. Following LPS
stimulation, GECs without ghrelin treatment exhibited a higher
proportion of apoptotic cells (Annexin V and PI double positive)
than the ghrelin treatment group, suggesting that ghrelin
attenuates sepsis-induced injury to stomach epithelial cells
(Fig. 5A and B). At each time
point tested, LPS increased cell apoptosis when compared with the
control group (naïve cells), which was significantly reduced by the
addition of ghrelin.
Discussion
The aim of the present study was to explore the
potential beneficial effect of ghrelin on sepsis symptoms in the
gastrointestinal tract, including the gastric blood flow and the
expression of Bcl-2 and Bax in gastric tissues. The results showed
that ghrelin could promote blood flow in the stomach of rats with
sepsis, and could upregulate the protein expression of Bcl-2 and
downregulate the expression of Bax in gastric tissues. Ghrelin was
first discovered by Kojima et al (32) in 1999. It is widely distributed in
the body, including in the stomach, intestine and pituitary gland.
Ghrelin regulates the secretion of many hormones (such as growth
hormone, prolactin and adrenal gland hormones), maintains hormonal
balance, promotes ingestion, decreases fat use, influences energy
metabolism, regulates hemodynamic force, decreases average
hemodynamic force, increases cardiac index, increases stroke
volume, and regulates gastric motility and gastric acid secretion,
all of which are associated with stomach functions (18,25,33).
In the present study, the CLP-induced sepsis group rats exhibited
significantly decreased blood flow in the stomach greater curvature
compared with the normal control group. In the ghrelin treatment
group, the gastric blood flow was significantly higher than in the
sepsis group, which suggested ghrelin may, at least in part,
reverse the pathological changes caused by CLP-induced sepsis.
These results support the findings of Wan et al (34). Their previous results indicated
that ghrelin could increase autophagy in sepsis patients and
alleviate the damage to the intestinal tract, suggesting that
ghrelin had protective effects on the gastrointestinal tract
(34). These results are
consistent with those obtained by other previous studies, which
reported that plasma levels of ghrelin were significantly reduced
after CLP, that ghrelin administration improves organ blood flow by
inhibiting nuclear factor-κB, it increases tissue perfusion in
sepsis by increasing cardiac output and cardiac output, and it
decreased vascular resistance by decrease norepinephrine level
(31,35,36).
As far as we known, ghrelin can regulate energy balance in addition
to appetite. The sepsis rat in a negative energy balance state
requires more energy, which may lead to the upregulation of ghrelin
(37). The sepsis rat may have a
poor appetite, which is the result of the downregulation of
ghrelin. So there may be some degree of balance between the up- and
downregulation of ghrelin in septic rats, and the end results
downregulate ghrelin; however, appetite cannot regulate ghrelin
levels (38), and Ariyasu et
al (39) demonstrated that
ghrelin secretion even increased in anorexia, which proved the
administration of ghrelin did not necessarily lead to the
amelioration of appetite. In addition, ghrelin treatment
significantly increased adenosine triphosphate values and improved
tissue histology in septic rats (40). Therefore, we believe that the
sepsis itself lead to the ischemia of stomach greater curvature by
downregulating ghrelin as opposed to other reasons. Furthermore,
the present results are similar to those of Lyra Junior et
al (41). Their previous
results indicated that ghrelin can increase local nitric oxide
release and reduce tissue ischemia of gut.
Bcl-2 is known to be an important apoptosis
regulator (42,43). The Bcl-2 family members function as
dimers; whereby, Bcl-2/Bcl-2, Bcl-2/Bax and Bcl-2/Bcl-xl dimers
inhibit apoptosis signaling, and Bax/Bax, Bax/Bad and Bcl-2/Bcl-xs
dimers promote apoptosis (42,43).
The interaction between Bcl-2 family proteins regulates the
survival and apoptosis of cells (44,45).
In the present study, in the normal control group, Bcl-2 and Bax
were not expressed in rat gastric mucosa and were barely detectable
in the gastric mucosa tissues of sham group rats. Bax protein
expression was higher than the Bcl-2 levels in the CLP-induced
sepsis group; however, following ghrelin treatment, Bcl-2 exhibited
strong positive expression in the gastric mucosa, and the number of
Bcl-2-positive cells was increased when compared with that of Bax.
The results suggested that ghrelin treatment might inhibit
apoptosis in gastric mucosa tissue.
Taken together, the results of the present study
revealed that ghrelin has the ability to increase blood flow in the
gastrointestinal tract in a sepsis model and regulate the
expressions of apoptosis-associated factors in gastric tissues,
inhibiting the expression of pro-apoptosis proteins and promoting
the expression of anti-apoptosis proteins. Nevertheless, it is
unclear whether the apoptotic factors can be directly regulated by
ghrelin. It has been reported that the effect of ghrelin on the
gastrointestinal tract is predominantly mediated through the
cholinergic pathway (34). Another
study reported that the protective effect of ghrelin on the
gastrointestinal tract was due to the increased expression of LC3,
Autophagy-related 7 and Beclin1 (46). Additional studies are still
necessary to determine the exact mechanisms of ghrelin in the
gastrointestinal tract in the context of sepsis. In conclusion, the
beneficial effects of ghrelin include the promotion of blood flow
in the stomach of rats with sepsis and the prevention of stomach
mucosa cell apoptosis, potentially through the regulation of Bcl-2,
Bax and caspase-3 protein expression.
Acknowledgements
Not applicable.
Funding
The present study was supported by Natural Science
Foundation of Gansu Province (grant nos. 1308RJZA240-01 and
1506RJZA255).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
BL and QL performed the experiments and analyzed the
data. LL and HG collected the samples, analyzed the data, and
reviewed and edited the manuscript. YL provided the reagents and
analyzed the data. BL, HG and YL also contributed to the
discussion, and reviewed and edited the manuscript. BL and YL
designed the experiments, summarized the data and wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
All procedures involving mice were approved by the
Institutional Animal Care and Use Committee of Lanzhou University
(Lanzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lever A and Mackenzie I: Sepsis:
Definition, epidemiology, and diagnosis. BMJ. 335:879–83. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Martin GS: Sepsis, severe sepsis and
septic shock: Changes in incidence, pathogens and outcomes. Expert
Rev Anti Infect Ther. 10:701–706. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Singer M, Deutschman CS, Seymour CW,
Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche
JD, Coopersmith CM, et al: The third international consensus
definitions for sepsis and septic shock (Sepsis-3). JAMA.
315:801–810. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kennelly PJ and Martin-Loeches I: Long
term mortality following sepsis. Ann Transl Med. 4:3872016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lang CH, Bagby GJ, Ferguson JL and Spitzer
J: Cardiac output and redistribution of organ blood flow in
hypermetabolic sepsis. Am J Physiol. 246:R331–R337. 1984.PubMed/NCBI
|
|
6
|
Moore JX, Donnelly JP, Griffin R, Howard
G, Safford MM and Wang HE: Defining sepsis mortality clusters in
the united states. Crit Care Med. 44:1380–1387. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dashwood AM, Mason R, Jennings C and
Dhillon P: Hepatic portal venous gas with associated bowel
ischaemia and intra-abdominal sepsis after recent chemotherapy. BMJ
Case Rep. 2016(pii): bcr2015213562016.
|
|
8
|
Zhang T, Yang J, Ding C, Li Y, Gu L, Wei
Y, Cao L, Gong J, Zhu W, Li N and Li J: Preoperative
intra-abdominal sepsis, not penetrating behavior itself, is
associated with worse postoperative outcome after bowel resection
for crohn disease: A Retrospective Cohort Study. Medicine
(Baltimore). 94:e19872015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hiltebrand LB, Krejci V, tenHoevel ME,
Banic A and Sigurdsson GH: Redistribution of microcirculatory blood
flow within the intestinal wall during sepsis and general
anesthesia. Anesthesiology. 98:658–669. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Albanèse J, Leone M, Delmas A and Martin
C: Terlipressin or norepinephrine in hyperdynamic septic shock: A
prospective, randomized study. Crit Care Med. 33:1897–1902. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Patel BM, Chittock DR, Russell JA and
Walley KR: Beneficial effects of short-term vasopressin infusion
during severe septic shock. Anesthesiology. 96:576–582. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
van Haren FM, Rozendaal FW and van der
Hoeven JG: The effect of vasopressin on gastric perfusion in
catecholamine-dependent patients in septic shock. Chest.
124:2256–2260. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Janiuk I, Kaleczyc J and Kasacka I:
Ghrelin-immunoreactive cells in the gastrointestinal tract of
hypertensive rats. Folia Histochem Cytobiol. 54:181–185. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sakata I, Nakamura K, Yamazaki M,
Matsubara M, Hayashi Y, Kangawa K and Sakai T: Ghrelin-producing
cells exist as two types of cells, closed- and opened-type cells,
in the rat gastrointestinal tract. Peptides. 23:531–536. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sakata I and Sakai T: Ghrelin cells in the
gastrointestinal tract. Int J Pept. 2010(pii):
9450562010.PubMed/NCBI
|
|
16
|
Shao Y, Liu S, Tang X, Gao J, Wu G and Li
Z: Ontogeny of ghrelin mRNA expression and identification of
ghrelin-immunopositive cells in the gastrointestinal tract of the
Peking duck, Anas platyrhynchos. Gen Comp Endocrinol. 166:12–18.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang JX, Peng KM, Liu H, Song H, Chen X
and Liu M: Distribution and developmental changes in
ghrelin-immunopositive cells in the gastrointestinal tract of
African ostrich chicks. Regul Pept. 154:97–101. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Kaplan RC, Strizich G, Aneke-Nash C,
Dominguez-Islas C, Bůžková P, Strickler H, Rohan T, Pollak M,
Kuller L, Kizer JR, et al: Insulinlike growth factor binding
protein-1 and ghrelin predict health outcomes among older adults:
Cardiovascular Health Study Cohort. J Clin Endocrinol Metab.
102:267–278. 2017.PubMed/NCBI
|
|
19
|
Mansson JV, Alves FD, Biolo A and Souza
GC: Use of ghrelin in cachexia syndrome: A systematic review of
clinical trials. Nutr Rev. 74:659–669. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sominsky L, Ziko I, Nguyen TX, Andrews ZB
and Spencer SJ: Early life disruption to the ghrelin system with
over-eating is resolved in adulthood in male rats.
Neuropharmacology. 113:21–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou D, Jiang X, Jian W, Zheng L, Lu L and
Zheng C: Comparing the effectiveness of total gastrectomy and
gastric bypass on glucose metabolism in diabetic rats. Obes Surg.
26:119–125. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Konturek PC, Brzozowski T, Pajdo R,
Nikiforuk A, Kwiecien S, Harsch I, Drozdowicz D, Hahn EG and
Konturek SJ: Ghrelin-a new gastroprotective factor in gastric
mucosa. J Physiol Pharmacol. 55:325–336. 2004.PubMed/NCBI
|
|
23
|
Bilgin HM, Tumer C, Diken H, Kelle M and
Sermet A: Role of ghrelin in the regulation of gastric acid
secretion involving nitrergic mechanisms in rats. Physiol Res.
57:563–568. 2008.PubMed/NCBI
|
|
24
|
Park JM, Kakimoto T, Kuroki T, Shiraishi
R, Fujise T, Iwakiri R and Fujimoto K: Suppression of intestinal
mucosal apoptosis by ghrelin in fasting rats. Exp Biol Med
(Maywood). 233:48–56. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wei C, Louis H, Schmitt M, Albuisson E,
Orlowski S, Levy B and Kimmoun A: Effects of low doses of esmolol
on cardiac and vascular function in experimental septic shock.
Criti Care. 20:4072016. View Article : Google Scholar
|
|
26
|
Chen YK, Xu YK, Zhang H, Yin JT, Fan X,
Liu DD, Fu HY and Wan B: Emodin alleviates jejunum injury in rats
with sepsis by inhibiting inflammation response. Biomed
Pharmacother. 84:1001–1007. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kouno T, Akiyama N, Fujieda K, Nanchi I,
Okuda T, Iwasaki T, Oka S and Yukioka H: Reduced intake of
carbohydrate prevents the development of obesity and impaired
glucose metabolism in ghrelin O-acyltransferase knockout mice.
Peptides. 86:145–152. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Miyatake Y, Shiuchi T, Mawatari K, Toda S,
Taniguchi Y, Futami A, Sato F, Kuroda M, Sebe M, Tsutsumi R, et al:
Intracerebroventricular injection of ghrelin decreases wheel
running activity in rats. Peptides. 87:12–19. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zavros Y, Van Antwerp M and Merchant JL:
Use of flow cytometry to quantify mouse gastric epithelial cell
populations. Dig Dis Sci. 45:1192–1199. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Zeineldin M and Neufeld K: Isolation of
epithelial cells from mouse gastrointestinal tract for western blot
or RNA analysis. Bio Protoc. 2:e2922012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu R, Dong W, Zhou M, Zhang F, Marini CP,
Ravikumar TS and Wang P: Ghrelin attenuates sepsis-induced acute
lung injury and mortality in rats. Am J Respir Crit Care Med.
176:805–813. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Kojima M, Hosoda H, Date Y, Nakazato M,
Matsuo H and Kangawa K: Ghrelin is a growth-hormone-releasing
acylated peptide from stomach. Nature. 402:656–660. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Shirshev SV, Nekrasova IV, Orlova EG and
Gorbunova OL: Roles of leptin and ghrelin in the regulation of the
phenotype and cytokine production by NK cells from peripheral
blood. Dokl Biol Sci. 470:249–252. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wan SX, Shi B, Lou XL, Liu JQ, MA GG,
Liang DY and Ma S: Ghrelin protects small intestinal epithelium
against sepsis-induced injury by enhancing the autophagy of
intestinal epithelial cells. Biomed Pharmacother. 83:1315–1320.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu R, Dong W, Zhou M, Cui X, Hank Simms H
and Wang P: Ghrelin improves tissue perfusion in severe sepsis via
downregulation of endothelin-1. Cardiovasc Res. 68:318–326. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Jacob A, Wu R, Zhou M, Coppa GF and Wang
P: Mechanism of the inhibitory effect of ghrelin in sepsis. Hepat
Med. 2:33–38. 2010.PubMed/NCBI
|
|
37
|
Pradhan G, Samson SL and Sun YX: Ghrelin:
Much more than a hunger hormone. Curr Opin Clin Nutr Metab Care.
16:619–624. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Diz-Chaves Y: Ghrelin, appetite
regulation, and food reward: Interaction with chronic stress. Int J
Pept. 2011:8984502011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Ariyasu H, Takaya K, Tagami T, Ogawa Y,
Hosoda K, Akamizu T, Suda M, Koh T, Natsui K, Toyooka S, et al:
Stomach is a major source of circulating ghrelin, and feeding state
determines plasma ghrelin-like immunoreactivity levels in humans. J
Clin Endocrinol Metab. 86:4753–4758. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Yorulmaz H, Ozkok E, Ates G, Aksu A,
Balkıs N and Tamer S: Ghrelin: Impact on muscle energy metabolism
in sepsis. Int J Pept Res Ther. 24:259–264. 2018. View Article : Google Scholar
|
|
41
|
Lyra Junior HF, Rodrigues IK, Schiavon LL
and D Acâmpora AJ: Ghrelin and gastrointestinal wound healing. A
new perspective for colorectal surgery. Acta Cir Bras. 33:282–294.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Czabotar PE, Lessene G, Strasser A and
Adams JM: Control of apoptosis by the BCL-2 protein family:
Implications for physiology and therapy. Nat Rev Mol Cell Biol.
15:49–63. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Tsujimoto Y: Role of Bcl-2 family proteins
in apoptosis: Apoptosomes or mitochondria? Genes Cells. 3:697–707.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
An HM, Tan YL, Shi J, Wang Z, Lv MH,
Soares JC, Zhou D, Yang F and Zhang XY: Ginkgo biloba leaf extract
and alpha-tocopherol attenuate haloperidol-induced orofacial
dyskinesia in rats: Possible implication of antiapoptotic
mechanisms by preventing Bcl-2 decrease and Bax elevation.
Phytomedicine. 23:1653–1660. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Jiang Z, Liu X, Chang K, Liu X and Xiong
J: Allyl Isothiocyanate inhibits the proliferation of renal
carcinoma cell line GRC-1 by inducing an imbalance between Bcl2 and
Bax. Med Sci Monit. 22:4283–4288. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Yorulmaz H, Ozkok E, Erguven M, Ates G,
Aydin I and Tamer S: Effect of simvastatin on mitochondrial enzyme
activities, ghrelin, hypoxia-inducible factor 1α in hepatic tissue
during early phase of sepsis. Int J Clin Exp Med. 8:3640–3650.
2015.PubMed/NCBI
|