Introduction
Esophageal carcinoma (EC) is one of the most common
types of malignant tumor of the digestive tract, with high
morbidity and mortality (1,2). In
developed countries, the morbidity and mortality of EC ranks 20 and
11th, respectively, whereas in developing countries, the morbidity
and mortality rank eighth and fifth, respectively (3). China accounts for >50% of the
world's annual incidence (4).
Esophageal squamous cell carcinoma (ESCC) and esophageal
adenocarcinoma are the main subtypes, with ESCC most commonly
reported, particularly in China (5). As of the lack of clear symptoms
during the early stages of EC, the majority of patients are first
diagnosed with advanced EC (6,7).
Tumor metastasis and recurrence are the leading causes of poor
prognosis of EC; the 5-year survival rate of patients with EC is
9.4–35% (8,9). Radiotherapy is an important
therapeutic strategy for the treatment of EC to decrease the risk
of recurrence, and improve quality of life and survival; however,
numerous factors affect the efficacy of radiotherapy, with
resistance to radiotherapy the main reason for the failure of
treatment of patients with EC (10–12).
Therefore, improved understanding of the mechanisms underlying the
metastasis and radiotherapy resistance of EC is required.
MicroRNAs (miRNAs/miRs) are a class of small (17–24
nucleotides) noncoding RNAs that regulate gene expression at the
post-transcriptional level via interactions with the
3′-untranslated region (3′-UTR) of target mRNAs (13). Increasing evidence suggests that
miRNAs are involved in the differentiation, proliferation,
invasion, metastasis and apoptosis of cells, and may represent
novel diagnostic biomarkers and therapeutic targets for various
cancers, such as EC (14–18). For example, miRNA-377 inhibits the
initiation and progression of esophageal cancer via suppression of
cluster of differentiation 133 and vascular endothelial growth
factor (19). miRNA-200c enhanced
the radiosensitivity of esophageal cancer by arresting the cell
cycle and targeting p21 (20).
Analysis of miRNA expression profiles using
Affymetrix GeneChip® technology revealed the miR-30
family as downregulated in numerous tumors, including lung,
prostate, thyroid and liver cancers (21,22).
A member of this family, miR-30a-3p, exhibited reduced expression
in various tumors (23,24). Insulin-like growth factor type 1
receptor (IGF-1R) is a heterotetrameric tyrosine kinase belonging
to the receptor tyrosine kinase superfamily. In the present study,
putative miR-30a-3p targets were identified using TargetScan,
suggesting that IGF-1R may be a potential target gene of
miR-30a-3p. It was demonstrated that miR-30a-3p was downregulated
in EC tissues and cell lines. Additionally, the role of miR-30a-3p
in the migration, invasion and radiosensitivity of EC cells was
investigated in vitro. Furthermore, it was revealed that
IGF-1R was a direct functional target of miR-30a-3p. The findings
of the present study suggested the regulation of IGF-1R by
miR-30a-3p as a potential mechanism underlying cellular migration
and invasion in EC, and also demonstrated that miR-30a-3p may be a
novel biomarker to determine the radiosensitivity of tumors in
patients with EC.
Materials and methods
Patients and tissue specimens
A total of 30 patients (17 female and 13 male
patients), who were histopathologically and clinically diagnosed at
Jiangsu Cancer Hospital (Nanjing, China) were included in the
present study. Patients with EC were diagnosed via a combination of
esophageal X-ray barium meal examination, three-dimensional
computerized tomography imaging and histopathological examination.
Clinicopathological data were collected, including gender, age,
tumor location, differentiation grade, tumor size and tumor,
necrosis and metastasis stage (25) (Table
I). EC tissues and paired normal tissues were obtained from
patients (age, 51±12 years) that underwent esophagectomy or
endoscopic submucosal dissection without radiotherapy or
chemotherapy in Jiangsu Cancer Hospital (Nanjing, China) from
August 2016 to September 2017. The present study was approved by
the Ethics Committee of Nanjing Medical University (Nanjing,
China), and each patient provided written informed consent. All
specimens were snap-frozen in liquid nitrogen within 2 h and stored
at −80°C until use.
 | Table I.Associations between levels
of'miR-30a-3p expression and the clinicopathological data of
patients with esophageal carcinoma. |
Table I.
Associations between levels
of'miR-30a-3p expression and the clinicopathological data of
patients with esophageal carcinoma.
| Clinical
characteristics | Nο. of
patients | Relative
expression | P-value |
|---|
| Age (years) |
|
| 0.572 |
|
≤60 | 18 | 0.425 |
|
|
>60 | 12 | 0.581 |
|
| Sex |
|
| 0.653 |
|
Male | 20 | 0.366 |
|
|
Female | 10 | 0.418 |
|
| Drinking |
|
| 0.544 |
|
Yes | 11 | 0.545 |
|
| No | 19 | 0.411 |
|
| Smoking |
|
| 0.661 |
|
Yes | 14 | 0.622 |
|
| No | 16 | 0.541 |
|
| pT stage |
|
| 0.526 |
| T1 +
T2 | 13 | 0.366 |
|
| T3 +
T4 | 17 | 0.521 |
|
| TNM stage |
|
| 0.492 |
|
≤II | 10 | 0.512 |
|
|
>II | 20 | 0.478 |
|
| Lymphatic
metastasis |
|
| 0.517 |
|
Positive | 11 | 0.362 |
|
|
Negative | 19 | 0.428 |
|
Cell culture and transfection
Human EC cell lines (EC9706 and EC109) and a human
esophageal epithelial cell line (HET-1A) were purchased from the
Cell Type Culture Collection of the Chinese Academy of Sciences
(Shanghai, China). Human cell lines were cultured in RPMI-1640
medium (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
containing 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.), 100 µg/ml streptomycin and 100 U/ml penicillin
(Gibco; Thermo Fisher Scientific, Inc.), and maintained at 37°C in
a humidified incubator with 5% CO2 for 48 h prior to
further experiments.
miR-30a-3p mimics, miRNA mimics negative control
(mimics NC), miR-30a-3p inhibitors, miRNA inhibitors negative
control (inhibitors NC), small interfering RNA (siRNA) targeted
against IGF-1R (siRNA IGF-1R) and negative control siRNA (siRNA NC)
were chemically synthesized by Shanghai GenePharma Co., Ltd.
(Shanghai, China). The sequences were as follows: miR-30 mimics
forward, 5′-ACUGGUAGUAAGUUGUAAUGCU-3′ and reverse,
5′-UCAUAUAACUUCAUACCUGCCU-3′; and miR-30 inhibitors,
5′-CGTGGCACCAATAGAATTGAGA-3′; NC forward,
5′-GGCTGCATTGGCTGGCGAAACCCGUC-3′ and reverse,
5′-ATGCGUGCCCTGCTGTTGCTCCATGTCG-3′. Cells were seeded into 6-well
plates at 60–70% confluence 1 day prior to transfection. Cell
transfection was conducted using Lipofectamine® 2000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocols. The RNA was transfected at a
concentration of 50 nM.
RNA extraction and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from EC tissues, paired
normal tissues, EC cells and normal cells using TRIzol®
solution (Invitrogen; Thermo Fisher Scientific, Inc.). For miRNA
expression, RT reactions were performed with a One Step PrimeScript
miRNA cDNA Synthesis kit (Takara Biotechnology Co., Ltd., Dalian,
China) at 37°C for 30 min according to the manufacturer's
instructions, followed by qPCR with SYBR® Premix Ex Taq
(Takara Biotechnology Co., Ltd.). For mRNA expression, cDNA was
synthesized from total RNA using a PrimeScript RT Reagent kit
(Takara Biotechnology Co., Ltd.). For miRNA and mRNA
amplifications, analysis was performed with an ABI 7500 Fast
Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc). qPCR was performed using the SYBR Premix ExTaq (Takara Bio,
Inc., Otsu, Japan) according to the manufacturer's protocols. The
thermocycling conditions comprised one cycle at 95°C for 30 sec,
followed by 40 cycles of amplification (95°C for 3 sec and 60°C for
30 sec). All alterations in the expression of miR and mRNA were
calculated via the 2−ΔΔcq method using U6 or GAPDH for
normalization, respectively (26).
The primer sequences used were as follows: miR-30a-3p, forward
5′-CCCTGCTCTGGCTGGTCAAACGGAAC-3′, reverse,
5′-TTGCCAGCCCTGCTGTAGCTGGTTGAAG-3′; U6, forward
5′-CTCGCTTCGGCAGCACA-3′, reverse, 5′-AACGCTTCACGAATTTGCGT-3′;
IGF-1R, forward 5′-CAACGGCAACCTGAGTTAC-3′, reverse,
5′-GCACGAAGATGGAGTTGTG-3′; and GAPDH, forward
5′-ACAACTTTGGTATCGTGGAAGG-3′ and reverse,
5′-GCCATCACGCCACAGTTTC-3′. Each experiment was performed in
triplicate.
Scratch-wound assay
EC9706 and EC109 cells were seeded into a 6-well
plate following transfection at a density of 5×105
cells/well. When 90% confluence was attained, a sterilized plastic
scraper was used to scratch the well axis, and the floating cells
were removed with PBS. The medium was subsequently replaced with
fresh culture medium and incubated at 37°C for 48 h. The cells were
then washed twice with PBS and were photographed at 0 and 48 h
under a light microscope (Nikon Corporation, Tokyo, Japan) in five
random fields of view (magnification, ×200). Then, the distance of
cell migration was determined.
Transwell assay
The migratory and invasive abilities of transfected
EC cells were evaluated using Transwell inserts (Costar; Corning,
Inc., Corning, NY, USA) with polycarbonate membrane filters (8-µm
pores). For the Transwell migration assay, EC9706 and EC109 cells
at density of 5×104 cells/well were plated in the upper
chambers of Transwell plates in fresh culture media. For the
Transwell invasion assay, Matrigel (BD Biosciences, San Jose, CA,
USA) was dissolved at 4°C overnight, diluted with fresh culture
media (1:3), added to the upper chambers (30 µl/well) and incubated
for 30 min at 37°C prior to the addition of cells. Culture medium
containing 20% FBS was added to the lower chambers. Following
incubation with RPMI-1640 medium supplemented with 10% FBS (Gibco;
Thermo Fisher Scientific, Inc.) for 48 h at 37°C, filter inserts
were removed from the wells and the cells on the upper surface were
removed with a cotton swab. The filters were fixed in 4%
paraformaldehyde for 30 min at room temperature and then stained
with 0.1% crystal violet for 30 min at room temperature. The
migratory and invasive cells were observed and counted in six
fields with a light microscope (magnification, ×200).
Cell Counting Kit-8 (CCK-8) assay
Cell proliferation was determined using a CCK-8
assay (Dojindo Molecular Technologies, Inc., Kumamoto, Japan).
EC9706 and EC109 cells were cultured in a 96-well plate at a
density of 3×103 cells/well, and transfected with
miR-30a-3p mimics, miR-30a-3p mimics NC, miR-30a-3p inhibitors or
miR-30a-3p inhibitors NC. Following incubation for 24, 48 or 72 h
at 37°C with 5% CO2, CCK-8 solution (10 µl) was added to
each well, followed by incubation for a further 4 h at room
temperature. The optical density values were measured at 450 nm
using a microplate reader (Shanghai, China).
Western blotting
Tissues and transfected cells were lysed using
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology, Haimen, China). Total protein was quantified using a
bicinchoninic acid protein assay kit (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany). Supernatant samples (20 µg) were heated at
99°C for 5 min prior to loading, separated via 10% SDS-PAGE
(Bio-Rad Laboratories, Inc., Hercules, CA, USA), and transferred to
polyvinylidene difluoride membranes (Merck KGaA) according to the
manufacturer's protocols. The membranes were then blocked with 5%
non-fat milk for 1 h at room temperature prior to incubation
overnight with primary antibodies at 4°C. The specific primary
antibodies from Cell Signaling Technology, Inc. (Danvers, MA, USA)
used during the study were as follows: IGF-1R (1:1,000; cat. no.
3027); E-cadherin (1:1,000, cat. no. 4A2); N-cadherin (1:1,000;
cat. no. 13A9); and vimentin (1:1,000; cat. no. 49636). Following
three washes with 0.1% TBS-Tween 20 (TBS-T). Subsequently, the
membranes were incubated with the horseradish peroxidase-conjugated
anti-rabbit secondary antibody (cat. no. ab6721; 1:2,000; Abcam)
for 1 h at room temperature. The samples were washed three times
using TBS-T and agitated for 5 min at room temperature, and the
proteins were then visualized using an enhanced chemiluminescence
system (Pierce; Thermo Fisher Scientific, Inc.). Protein expression
was quantified using ImageJ software (version 1.48; National
Institutes of Health, Bethesda, MD, USA). GAPDH were used as a
loading control (ab9485; 1:1,000 dilution; Abcam, Cambridge, MA,
USA). The following antibodies were purchased from Abcam: IGF-1R
(ab39675; 1:1,000 dilution); matrix metalloproteinase-2 (MMP-2;
ab37150; 1:1,000 dilution); MMP-9 (ab73734; 1:1,000 dilution);
E-cadherin (ab1416; 1:1,000 dilution); vimentin (ab8978; 1:1,000
dilution); N-cadherin (ab18203; 1:1,000 dilution) and GAPDH
(ab8245; 1:1,000 dilution).
Flow cytometry analysis
A total of 5×104 cells were seeded in
6-well plates, cultured for 48 h at 37°C and subsequently cells
were collected. Cells were fixed with precooled 70% ethanol for 30
min at room temperature, and collected following centrifugation at
12,000 × g for 5 min at room temperature. The cells were
resuspended in PBS containing 50 mg/ml propidium iodide and 50
mg/ml RNaseA (cat. no. 40711ES10; Shanghai Yeasen Biotechnology,
Co., Ltd., Shanghai, China) for 30 min at room temperature. Cells
were incubated for 1 h at 37°C in the dark, and analyzed using flow
cytometry (FACSCalibur; BD Biosciences) and analyzed using FlowJo
10.06 software (FlowJo LLC, Ashland, OR, USA). The flow cytometry
analyses were repeated three times.
Immunohistochemical analysis
The surgical EC tissues and paired adjacent tissues
were fixed in 10% neutral buffered formalin at room temperature for
20 min and embedded in paraffin wax. Tissue sections with a
thickness of 4 µm were mounted onto slides, then the slides were
deparaffinized with xylene at room temperature, rehydrated with a
graded alcohol series, and incubated with
H2O2 at 37°C for 10 min. Following blocking
using 1.5% normal goat serum (Shanghai Yeasen Biotechnology Co.,
Ltd.) at 37°C for 20 min, the primary IGF-1R antibody (CST;
Danvers, MA, USA; 1:1,000; cat. no. 3027) was incubated on the
slides at 4°C overnight. The slides were washed with PBS three
times, then incubated with a secondary antibody (horseradish
peroxidase-conjugated; cat. no. ab6721; 1:2,000; Abcam) and stained
with 3,3′-diaminobenzidine. Images were obtained using a
fluorescence microscope in five randomly-selected fields of view
(magnification, ×200) (FSX100; Olympus Corporation, Tokyo,
Japan).
Radiosensitivity assay
Transfected EC9706 and EC109 cells were seeded at a
density of 3×103 cells/well in a 96-well plate, and
irradiated with various doses of radiation (0, 2, 4, 6 and 8 Gy)
using an X-RAD 320 X-ray radiator (Softex Co., Ltd., Tokyo, Japan)
at a dose rate of 2 Gy/min. A CCK-8 assay was subsequently
performed as previously described to determine cell
proliferation.
Luciferase assay
A search for putative targets of miR-30a-3p was
performed with TargetScan Human 7.2 (www.targetscan.org/vert_72/) and miRanda software
(www.microrna.org/). The 3′-untranslated region
(3′-UTR) of IGF-1R was cloned into an miRNA Expression Reporter
Vector psiCheck-2 (Promega Corporation, Madison, WI, USA). Cells
were plated in 48-well plates for 24 h, psiCheck-2 with the
wild-type (WT) or mutant (MUT) 3′-UTR of IGF-1R was cotransfected
with miR-30a-3p mimics or miR-NC using Lipofectamine®
3000 (Invitrogen; Thermo Fisher Scientific, Inc.). Then, 48 h
following transfection, cells were collected and subjected to a
luciferase assay using a Dual Luciferase Reporter Assay kit
(Promega Corporation). Luciferase activity was normalized using
Renilla luciferase activity (Promega Corporation).
Statistical analysis
Data are expressed as the mean ± standard deviation
and analyzed with SPSS 19.0 (IBM Corp., Armonk, NY, USA).
Comparisons between two groups were performed using Student's
t-tests. Comparisons across three or more groups were performed
using one-way analyses of variance and a Tukey's post-hoc test.
P<0.05 was considered to indicate a statistically significant
difference. All experiments were repeated a minimum of three
times.
Results
miR-30a-3p is downregulated in EC
tissues and cell lines
miR-30a-3p, a member of the miR-30 family, has been
reported as downregulated in numerous tissues (24,27).
In the present study, to investigate the role of miR-30a-3p in the
development of EC, the expression of miR-30a-3p in EC tissues and
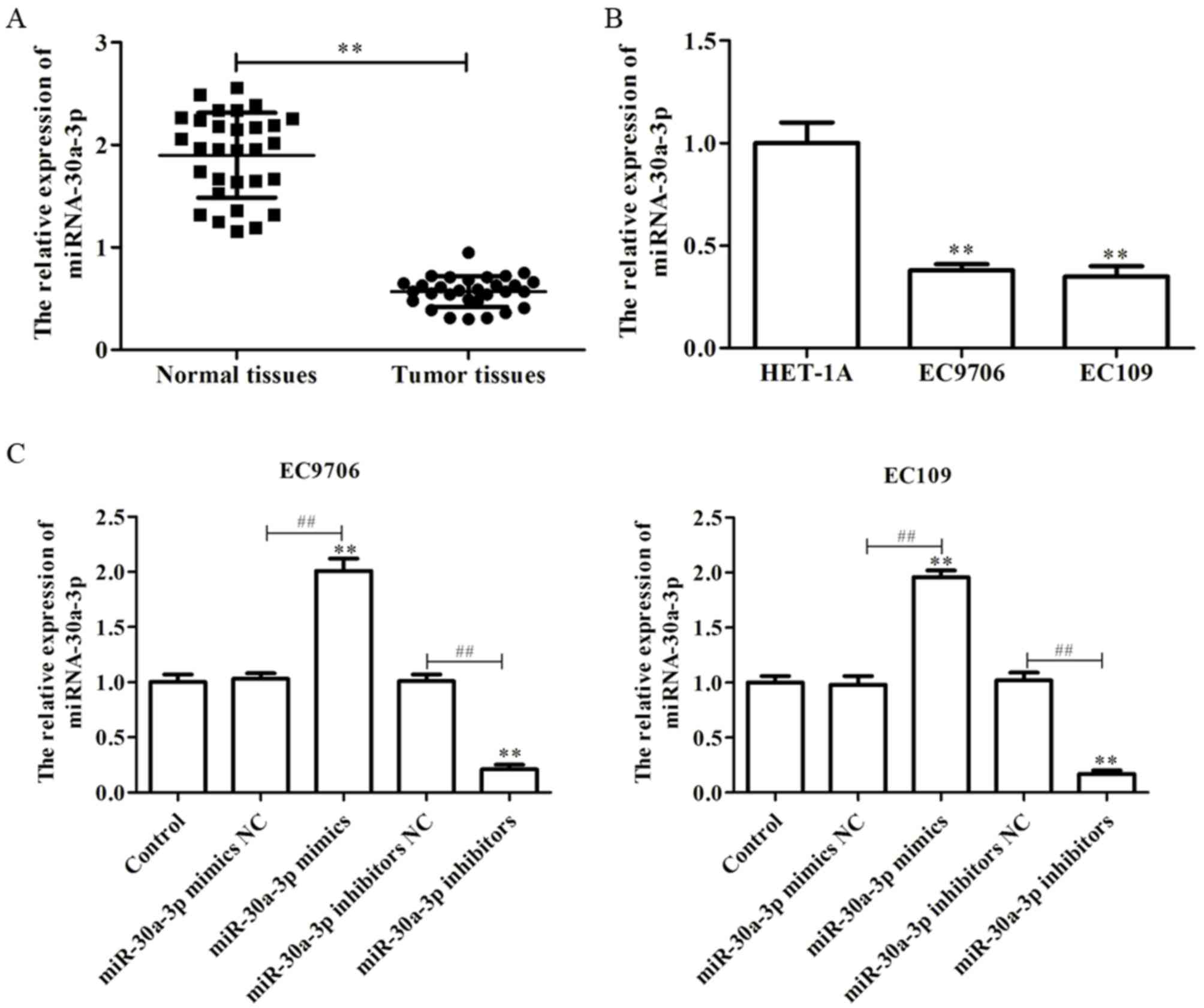
cell lines was determined via RT-qPCR. As presented in Fig. 1A, it was observed that the levels
of miR-30a-3p expression were significantly downregulated in EC
tissues compared with in paired normal tissues. Furthermore, it was
demonstrated that the levels of miR-30a-3p expression in the EC
cell lines, EC9706 and EC109, were significantly decreased compared
with in a human esophageal epithelial cell line, HET-1A (Fig. 1B). The findings suggested that
miR-30a-3p is downregulated in EC tissues and cell lines.
In order to investigate the effects of miR-30a-3p on
EC, miR-30a-3p mimics, mimics NC, miR-30a-3p inhibitors or
inhibitors NC were transfected into EC9706 and EC109 cells. RT-qPCR
was performed to determine the efficiency of transfection, and the
results revealed that miR-30a-3p mimics could significantly promote
the expression of miR-30a-3p compared with the control, while
miR-30a-3p inhibitors significantly reduced the expression of
miR-30a-3p in EC9706 and EC109 cells (Fig. 1C).
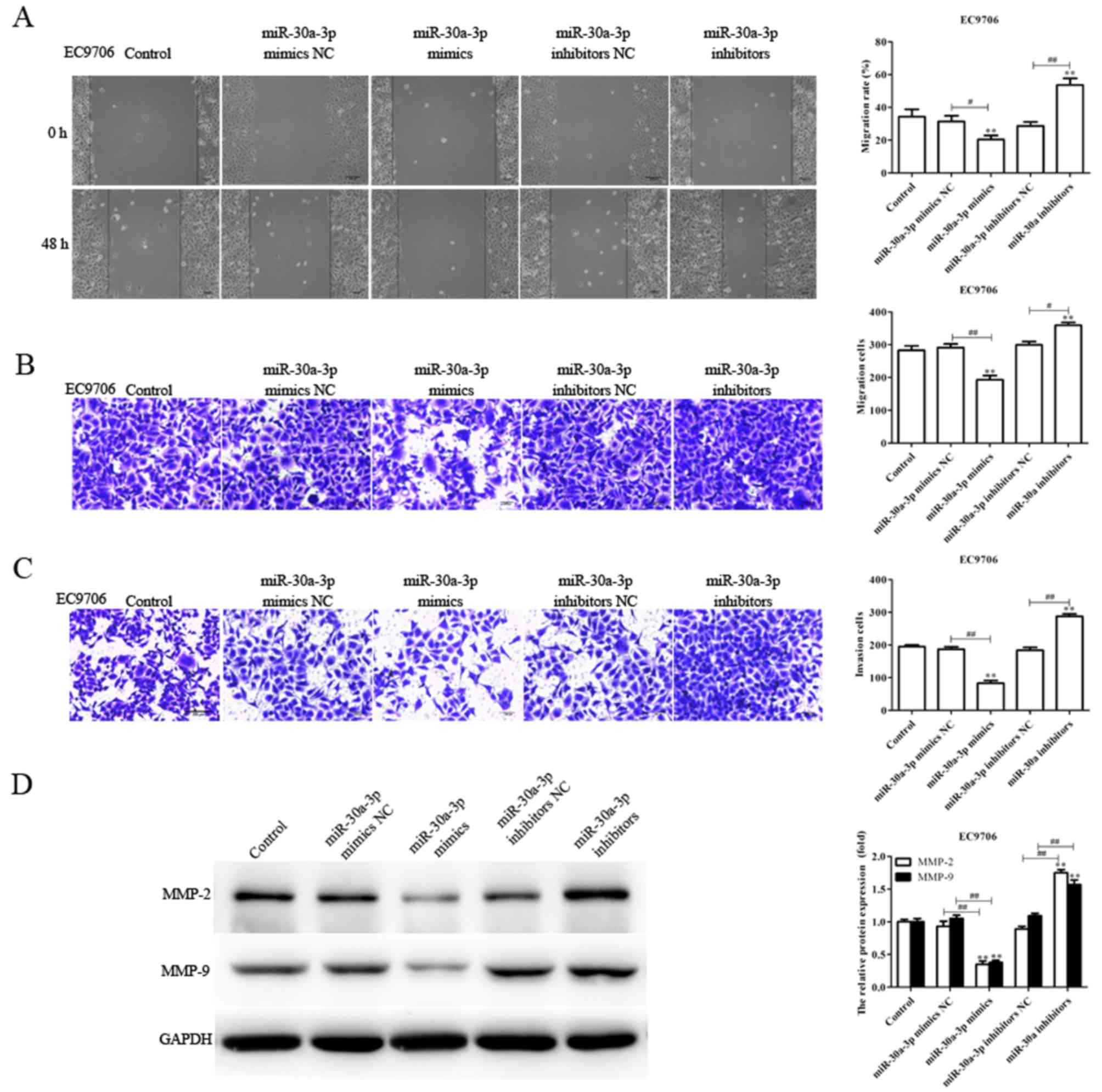
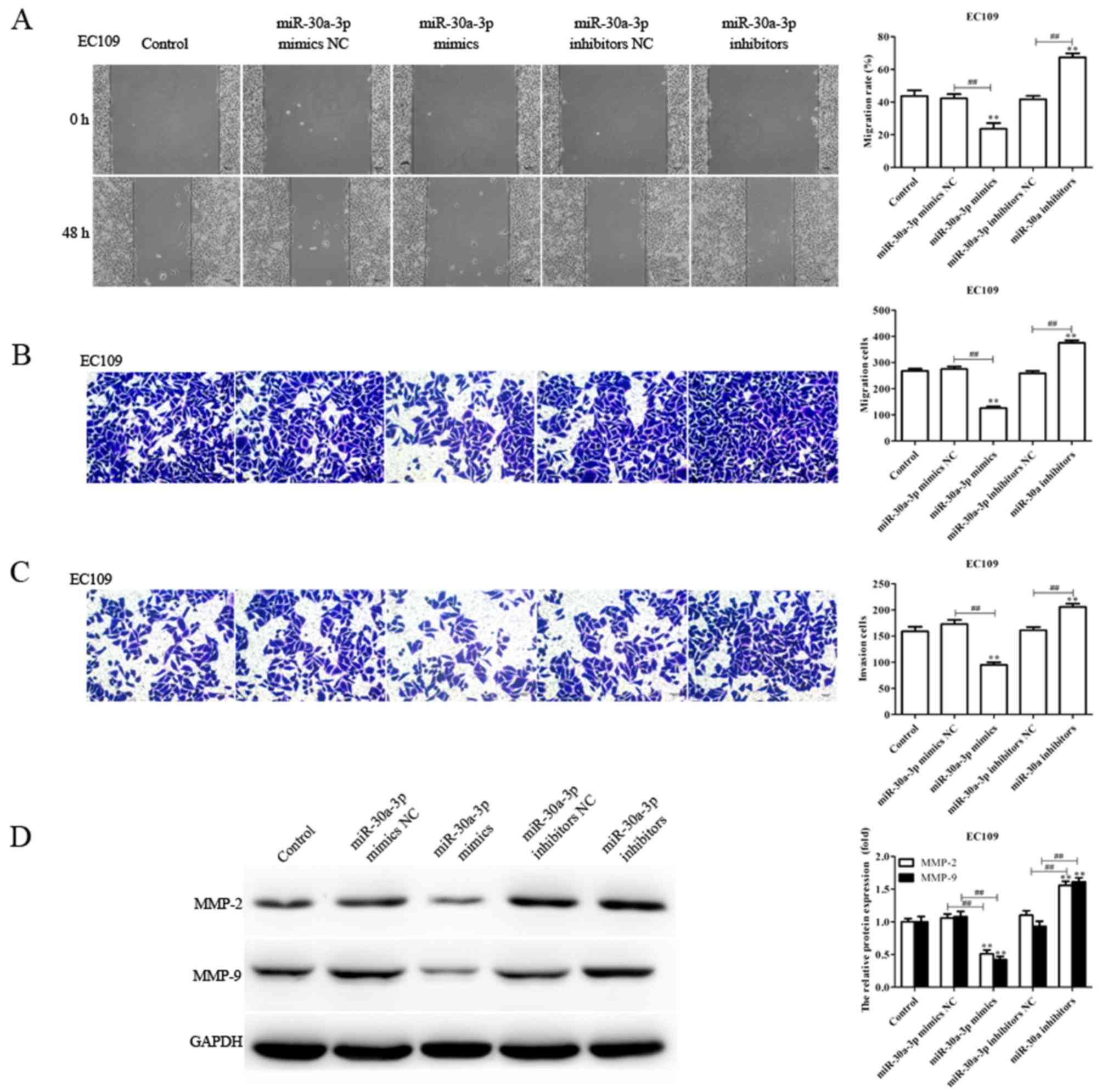
miR-30a-3p suppresses the migration
and invasion of EC cells
As the levels of miR-30a-3p expression are
associated with lymph node metastasis (28), the migratory and invasive abilities
of EC9706 and EC109 cells transfected with miR-30a-3p mimics,
mimics NC, miR-30a-3p inhibitors and inhibitors NC were determined
via a scratch-wound, and Transwell migration and invasion assays,
respectively. Compared with the control, the migration and invasion
of EC9706 and EC109 cells transfected with miR-30a-3p mimics were
significantly reduced, whereas miR-30a-3p inhibitors significantly
promoted the migratory and invasive abilities of EC9706 and EC109
cells (Figs. 2 and 3). The results indicated that miR-30a-3p
inhibits EC cell migration and invasion in vitro.
Tumor metastasis is a sequential process involving
interactions between tumor cells, host cells and the tissue
microenvironment, in which MMPs serve an essential role; MMP-2 and
MMP-9 have been reported to be associated with the migration and
invasion of various tumors (29,30).
In the present study, the levels of MMP-2 and MMP-9 protein
expression in transfected EC9706 and EC109 cells were determined.
The expression levels of MMP-2 and MMP-9 protein were significantly
decreased in EC9706 and EC109 cells transfected with miR-30a-3p
mimics compared with the control; however, the expression levels
were significantly increased in EC9706 and EC109 cells transfected
with miR-30a-3p inhibitors (Figs.
2D and 3D). The results
suggested that miR-30a-3p serves an inhibitory role in EC cell
metastasis.
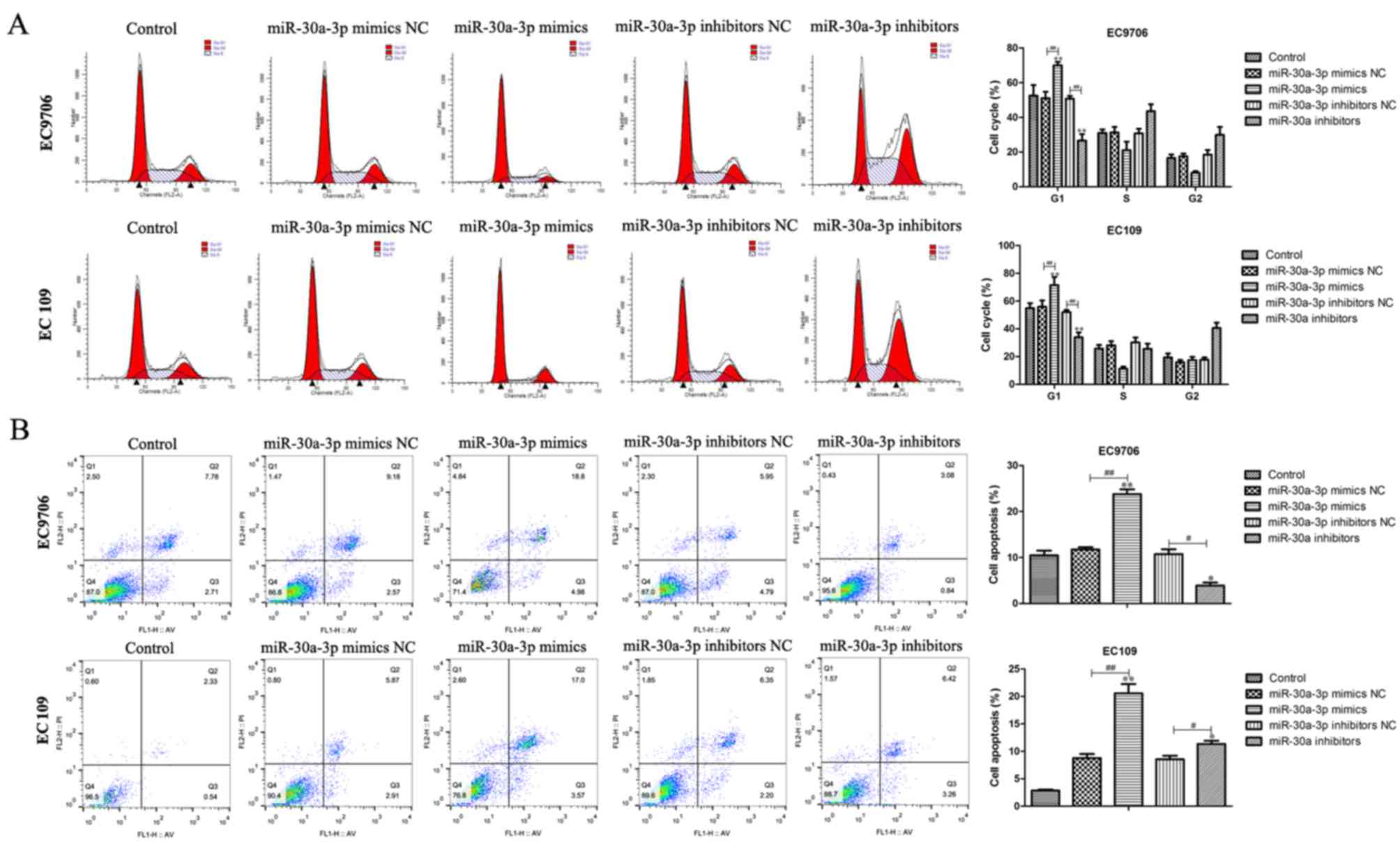
Effects of miR-30a-30p on the
apoptosis and cell cycle of EC cells
To determine whether miR-30a-3p is able to affect
the cell cycle and apoptosis, flow cytometry was performed. The
results demonstrated that overexpression of miR-30a-3p markedly
increased the number of cells arrested in the G1 phase compared
with the control group (Fig. 4A).
Furthermore, the number of cells in the G1 phase was notably
reduced following transfection with miR-30a-3p inhibitors compared
with the control group. Additionally, it was revealed that the
number of apoptotic cells was markedly decreased following
transfection with miR-30a-3p inhibitors, whereas, overexpression of
miR-30a-3p notably increased the number of apoptotic cells compared
with the control group (Fig. 4B).
The results indicated that miR-30a-3p may suppress cell cycle
progression and induce the apoptosis of EC cells.
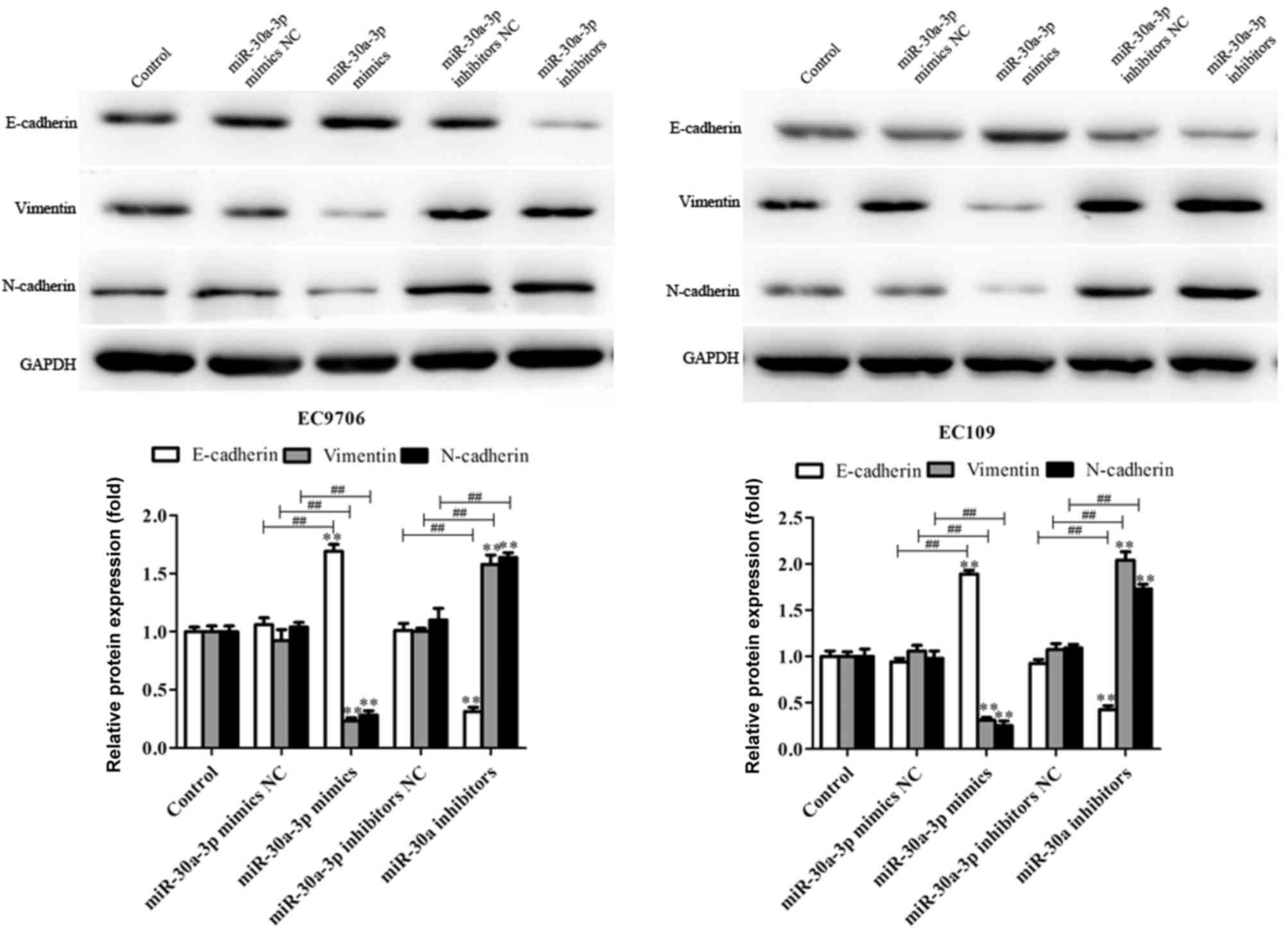
miR-30a-3p inhibits
epithelial-mesenchymal transition (EMT) in EC cells
EMT is characterized by the loss of differentiation
of epithelial cells. Important features of EMT include loss of the
epithelial cell phenotype and the acquisition of interstitial
properties, represented by the downregulation of E-cadherin, and
upregulated expression of N-cadherin and vimentin. This results in
reduced adhesion between cells, and enhanced migration and invasion
(31–33). In the present study, the effects of
miR-30a-3p on the EMT of EC9706 and EC109 cells transfected with
miR-30a-3p mimics, mimics NC, miR-30a-3p inhibitors and inhibitors
NC were investigated. As presented in Fig. 5, miR-30a-3p mimics significantly
upregulated the levels of E-cadherin protein expression, and
decreased those of N-cadherin and vimentin compared with the
control; opposing effects were observed in EC9706 and EC109 cells
transfected with miR-30a-3p inhibitors. The results suggested that
miR-30a-3p inhibits EC cell metastasis via the regulation of
EMT.
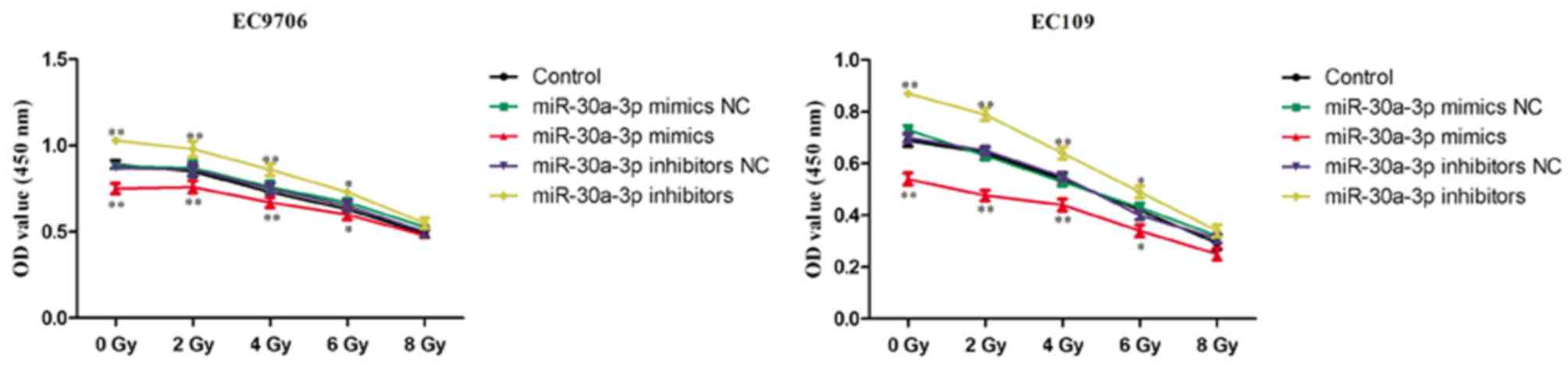
miR-30a-3p enhances the
radiosensitivity of EC cells
Radiotherapy is commonly used in the treatment of
EC; however, patients may exhibit recurrence of EC following
radiotherapy due to low radiosensitivity. It is important to
identify strategies that decrease the resistance of EC to
radiotherapy to improve the therapeutic effects (11). Therefore, the role of miR-30a-3p in
the radiosensitivity of EC cells was investigated. EC9706 and EC109
cells were transfected with miR-30a-3p mimics, mimics NC,
miR-30a-3p inhibitors and inhibitors NC, and irradiated with
various radiation doses (0, 2, 4, 6 and 8 Gy) for 48 h. The CCK-8
assay was subsequently performed to evaluate the proliferation of
cells. As presented in Fig. 6,
miR-30a-3p mimics significantly decreased the proliferation of
EC9706 and EC109 cells compared with the control group indicating
an increased radiosensitivity, whereas miR-30a-3p inhibitors
significantly promoted the proliferation of EC9706 and EC109 cells.
The results suggested that miR-30a-3p may act as a
radiosensitizer.
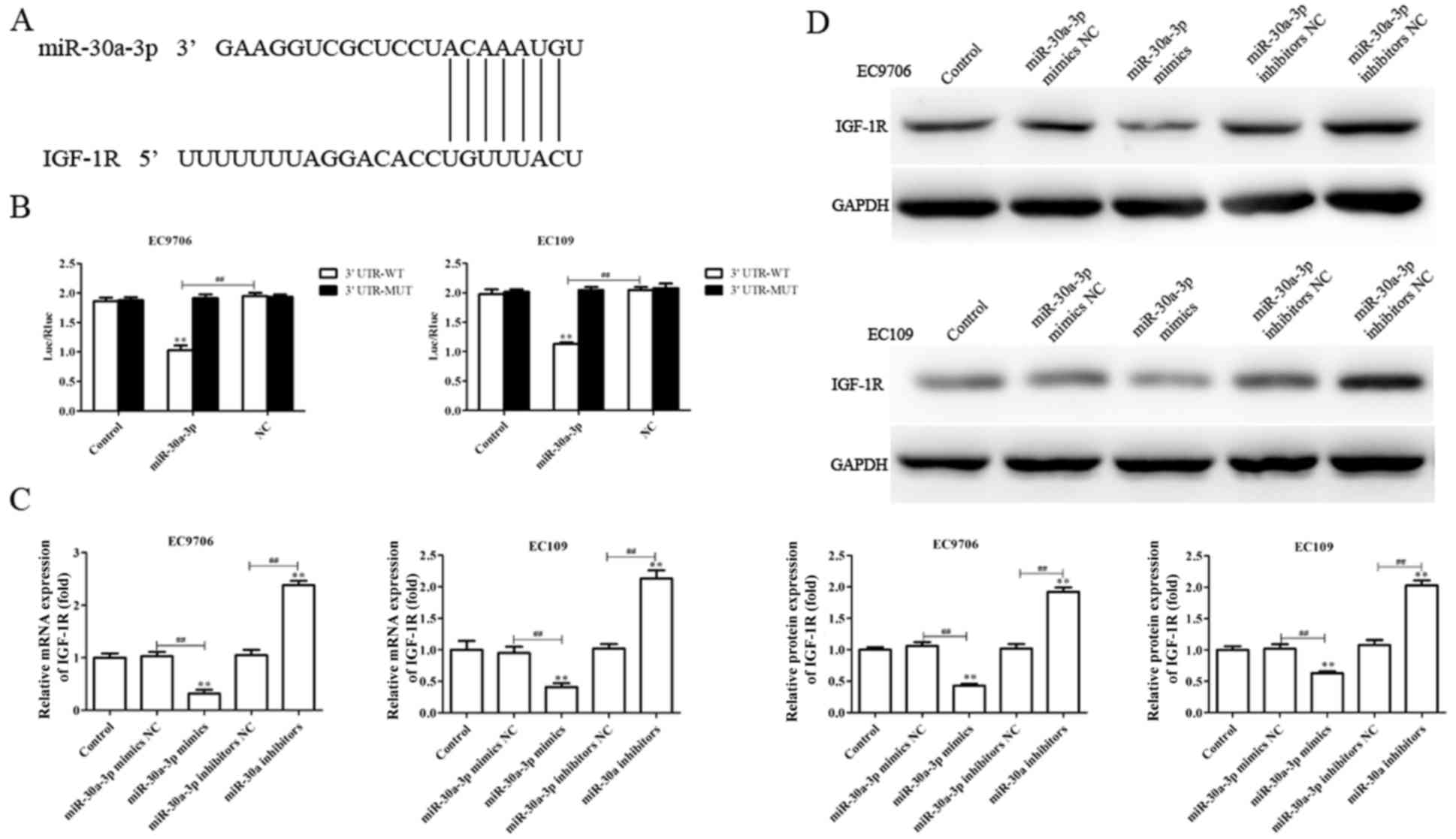
IGF-1R is a direct target of
miR-30a-3p
To investigate the molecular mechanisms by which
miR-30a-3p may suppress metastasis and EMT, and enhance
radiosensitivity in EC cells, putative miR-30a-3p targets were
determined using TargetScan. The software analysis suggested that
IGF-1R may present a potential candidate for regulation by
miR-30a-3p (Fig. 7A). Therefore,
dual luciferase reporter gene analysis was performed to investigate
whether miR-30a-3p directly targets the 3′-UTR of IGF-1R. As
presented in Fig. 7B, miR-30a-3p
mimics significantly reduced the luciferase activity of cells
containing the IGF-1R 3′-UTR-WT compared with the control, but not
3′-UTR-MUT, indicating that miR-30a-3p directly binds to the 3′UTR
of IGF-1R.
RT-qPCR and western blotting were utilized to
investigate the effects of miR-30a-3p on endogenous IGF-1R
expression in EC cells. As presented in Fig. 7C and D, the levels of IGF-1R mRNA
and protein expression were significantly downregulated in EC9706
and EC109 cells transfected with miR-30a-3p mimics compared with
the control, whereas miR-30a-3p inhibitors promoted IGF-1R
expression (Fig. 7C and D). The
results suggested that IGF-1R is a direct target gene of miR-30a-3p
in EC.
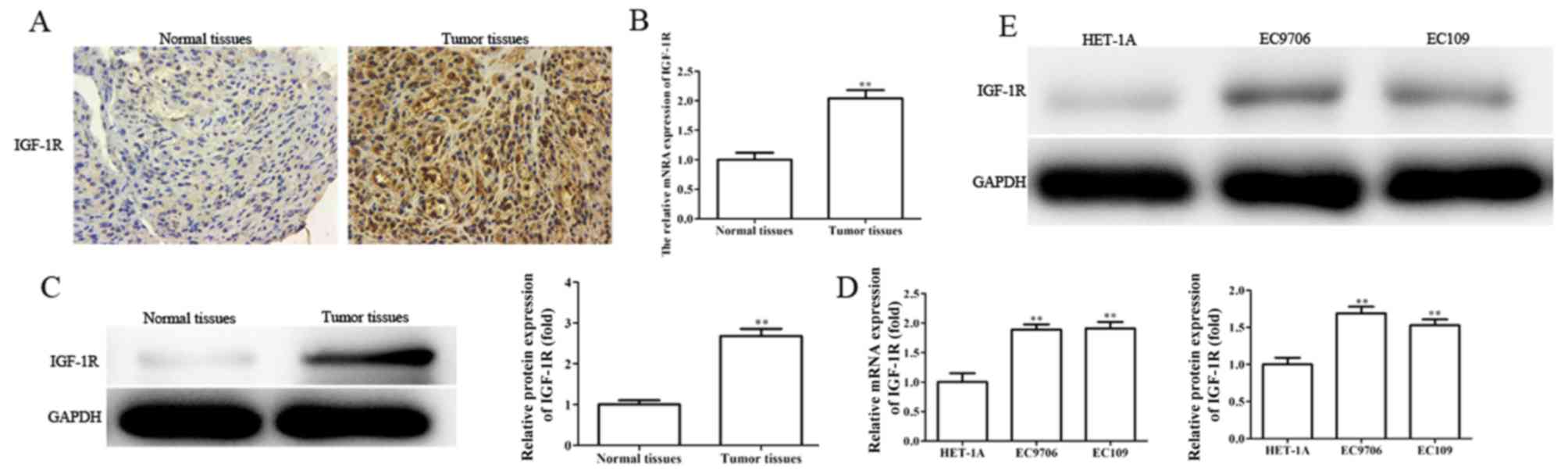
IGF-1R is upregulated in EC tissues
and cell lines, and silencing IGF-1R suppresses the migration,
invasion and EMT, and enhances the radiosensitivity of EC
cells
Providing IGF-1R is a direct target gene of
miR-30a-3p, the role of IGF-1R in EC was investigated. The levels
of IGF-1R mRNA and protein expression were determined in EC tissues
and cells by RT-qPCR, western blotting and immunohistochemical
analysis. It was revealed that the levels of IGF-1R mRNA and
protein expression were significantly upregulated in EC tissues and
cell lines (Fig. 8A-E).
To investigate the effects of IGF-1R on the
migration, invasion, EMT and radiosensitivity of EC cells, EC9706
and EC109 cells were transfected with si-IGF-1R, and RT-qPCR
analysis was performed to determine the transfection efficiency. As
presented in Fig. 9A, the levels
of IGF-1R mRNA expression were significantly reduced in EC9706 and
EC109 cells following transfection with si-IGF-1R. EC9706 and EC109
cells transfected with si-IGF-1R were irradiated with various
radiation doses (0, 2, 4, 6 and 8 Gy) for 48 h, and a CCK-8 assay
was performed to evaluate the proliferation of cells. As presented
in Fig. 9B, transfection with
si-IGF-1R significantly decreased the proliferation indicating
increased radiosensitivity of EC9706 and EC109 cells. Additionally,
western blotting was performed to evaluate the levels of expression
of EMT-associated proteins. It was demonstrated that, compared with
the control, E-cadherin protein was significantly upregulated, and
vimentin and N-cadherin proteins were significantly downregulated
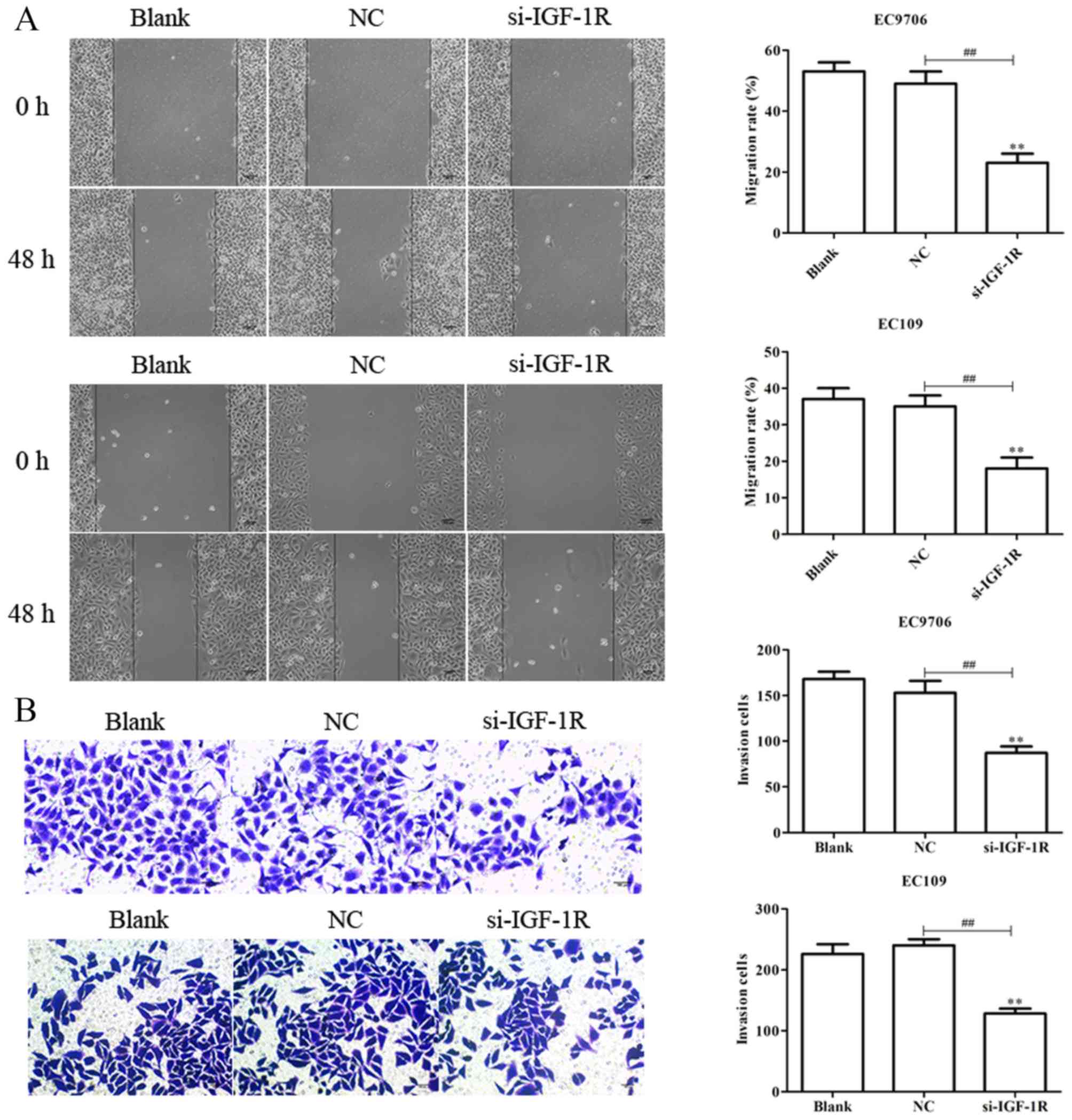
following transfection with si-IGF-1R (Fig. 9C). Scratch-wound, and Transwell
migration and invasion assays were subsequently performed to
determine the effects of IGF-1R on the migration and invasion of
EC9706 and EC109 cells. It was demonstrated that si-IGF-1R
significantly suppressed the migration and invasion of EC9706 and
EC109 cells compared with the control (Fig. 10A and B). The results indicated
that IGF-1R, a candidate target of miR-30a-3p, serves important
roles in the migration, invasion, EMT and radiosensitivity of EC
cells.
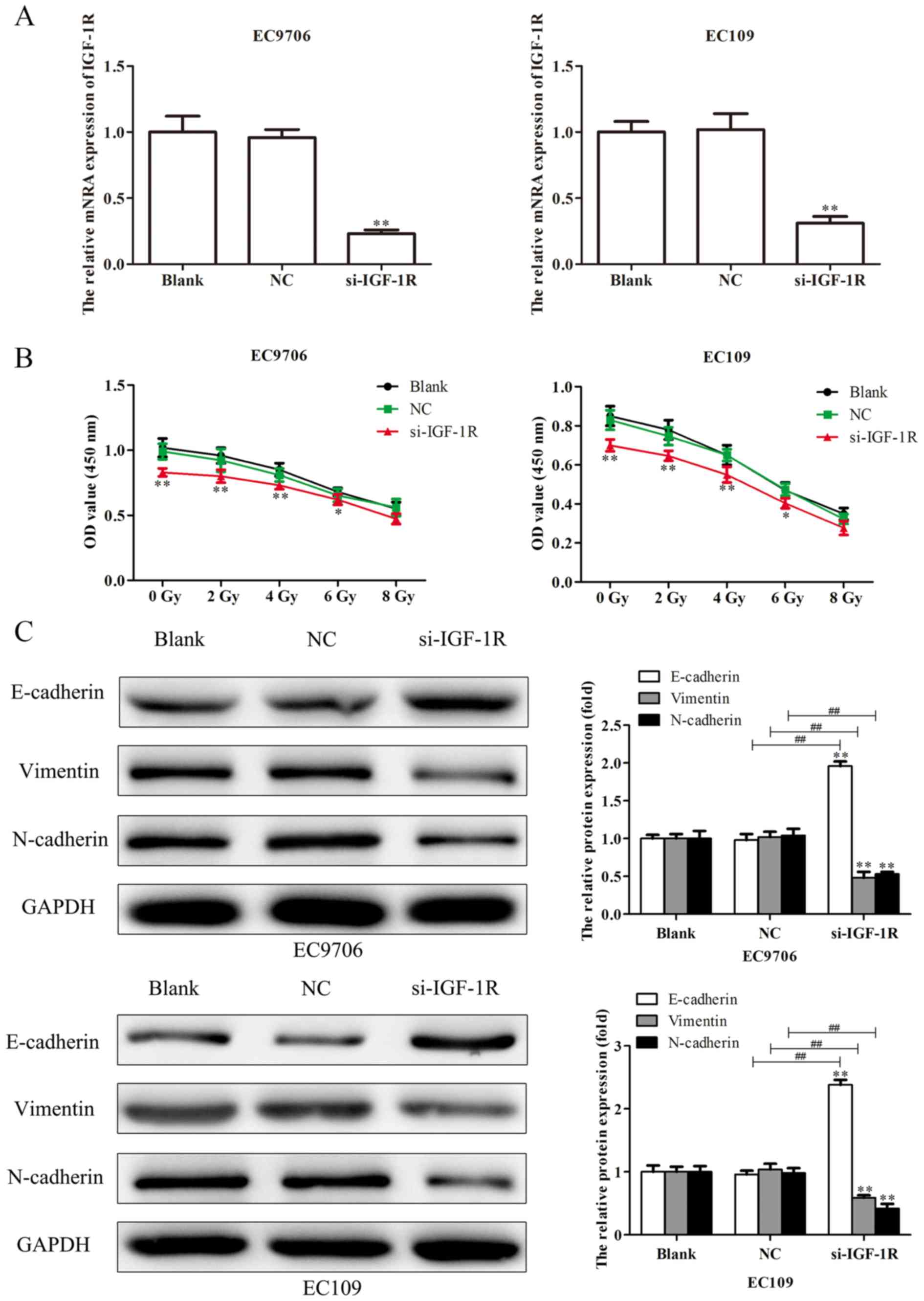 | Figure 9.Silencing IGF-1R suppresses the
proliferation and EMT of esophageal carcinoma cells. (A) Following
transfection of EC9706 and EC109 cells with si-IGF-1R, the
efficiency of transfection was determined via reverse
transcription-quantitative polymerase chain reaction analysis. (B)
EC9706 and EC109 cells transfected with si-IGF-1R were irradiated
with various radiation doses (0, 2, 4, 6 and 8 Gy) for 48 h, then a
Cell Counting Kit-8 assay was performed to evaluate the
proliferation of cells. (C) Expression levels of EMT-associated
proteins (E-cadherin, vimentin and N-cadherin) were determined in
EC9706 and EC109 cells transfected with si-IGF-1R by western blot
analysis. Band intensity was quantified using ImageJ software. Data
are presented as the mean ± standard deviation of three independent
experiments; each was performed in triplicate. *P<0.05 and
**P<0.01 vs. the blank group. ##P<0.01 vs. the NC
group. EMT, epithelial-mesenchymal transition; IGF-1R, insulin-like
growth factor 1 receptor; NC, negative control; si, small
interfering RNA. |
Discussion
Studies investigating the association between miRNAs
and tumor metastasis have focused on tumors located in various
regions and systems, including the head and neck, as well as the
respiratory, digestive and urinary systems (34–37).
For example, numerous miRNAs have been reported to contribute to
the regulation of breast cancer metastasis. miR-21 promoted the
invasion of breast cancer cells via negative regulation of its
target genes tropomyosin 1, programmed cell death 4 and maspin
(38); a mutual feedback loop
between zinc-finger E-box binding homeobox 1, miR-141 and miR-200c
in regulating the invasion and metastasis of breast, pancreatic and
colorectal cancer cells via EMT was reported (39).
miRNAs are not only involved in physiological
processes, including growth and development of the body, but also
serve important roles in various pathological processes, including
the proliferation, apoptosis, invasion, angiogenesis and metastasis
of various malignancies via downstream target genes (40–42).
miR-30a-3p has been reported as downregulated, and involved in the
progression and development of a number of tumors (28–30).
For example, miRNA-30a-3p is downregulated in hepatocellular
carcinoma, and inhibits the proliferation, invasiveness and
metastasis of tumors (43). In the
present study, it was demonstrated that the levels of miRNA-30a-3p
expression were decreased in EC tissues and cell lines, and
contributed to the migration and invasion of EC cells.
The role of EMT in tumor metastasis has received
increasing focus (44,45). EMT is a complex process in which
epithelial cells develop a mesenchymal-like phenotype. E-cadherin
downregulation is an important feature of EMT and has been
considered to be the most reliable indicator of EMT occurrence.
E-cadherin serves an important role in the homeostasis of
epithelial cells; its reduced expression during EMT leads to the
downregulation of epithelial cell-associated proteins or
reconstruction of complexes (including desmosome-associated
proteins, tight junction proteins and cell polarity complex
components), and upregulated expression of proteins associated with
mesenchymal cells (including vimentin and N-cadherin), which is
accompanied with reconstruction of the actin cytoskeleton (46–50).
Therefore, the expression levels of EMT-associated proteins were
evaluated in EC cells following transfection via western blotting,
and it was revealed that miR-30a-3p significantly altered the
expression of EMT-associated proteins.
Radiotherapy is an important therapy in the
treatment of EC, reducing the recurrence risk of EC, and improving
quality of life and survival; however, numerous factors affect the
efficacy of radiotherapy, including the degree of hypoxia,
glutathione content and individual differences in the sensitivity
to radiation, which may result in an unsuccessful response to
radiotherapy, and even induce severe radiation resistance (11,12,51,52).
Radiation resistance of tumors is a complex phenomenon, involving
complex molecular mechanisms and genetic alterations. DNA damage is
the main mechanism by which radiotherapy-induced cell death occurs,
including single and double strand breaks, and base damage
(50,53–55).
Ionizing radiation can provide sufficient time to repair damaged
DNA and enable cells to survive via cell cycle arrest; however,
when DNA repair fails, apoptosis is induced, an important defense
and protection mechanism for the maintenance of genomic stability
in normal cells. In tumor cells, cell cycle arrest and the
suppression of apoptosis are important factors associated with the
development of radiation resistance (51). Increasing evidence suggests that
miRNAs are involved in these processes (56,57).
Thus, CCK-8 assays were performed in the present study to evaluate
the role of miR-30a-3p in transfected EC9706 and EC109 cells
irradiated with various radiation doses. The results suggested that
miR-30a-3p may act to enhance the radiosensitivity of EC cells.
IGF-1R belongs to the insulin receptor (IR) family,
which also includes IR, IGF-1R and IGF-2R. Previous studies
reported that the IGF-1R signaling pathway serves a pivotal role in
the growth and progression of cancer, and resistance to anticancer
therapies (58,59). It was revealed that IGF-1R promoted
the growth of whole-body tissues and organs via the induction of
protein synthesis. Autocrine or paracrine IGF-2 from tumor cells
activated IGF-1R, and regulated the proliferation and
differentiation of tumor cells (60–63).
Nussbaum et al (63)
observed that autocrine IGF-2 release from hepatocytes signaled via
IGF-1R, interacting with hepatocyte growth factors to inhibit cell
apoptosis, and promote cell growth and metastasis. Kim et al
(64) reported that bronchial
epithelial cells lacking p53 or expressing mutations in v-Ki-Ras2
Kirsten rat sarcoma viral oncogene homolog exhibited upregulation
of IGF-1 and IGF-2; the transformed characteristics of these cells
could be suppressed by IGF-1R inactivation, or enhanced by
overexpression of IGF-1R. Furthermore, activated IGF-1R induced
cisplatin resistance in numerous ovarian cancer cell lines via its
downstream target gene phosphatidylinositol-3-kinase (65). IGF-1R has also been reported to
contribute to radiosensitivity in oral squamous cell carcinoma
(66). In the present study, the
luciferase assay indicated that miR-30a-3p binds to the 3′-UTR of
IGF-1R mRNA. Furthermore, silencing of IGF-1R affected the
migration, invasion and radiosensitivity of EC cells; however,
whether miR-30a-3p exerts its effects on EC via targeting IGF-1R
requires further investigation.
The present study is a preliminary study into the
role of miR-30a-3p in EC. Based on the findings, future work will
aim to further investigate the role and mechanisms of miR-30a-3p in
the progression and development of cancer in vitro and in
vivo. Collectively, it was demonstrated that miR-30a-3p
expression was upregulated in EC tissues and cell lines, and that
miR-30a-3p may function as a potential tumor suppressor in EC,
inhibiting metastasis and enhancing radiosensitivity via
downregulation of IGF-1R. Therefore, miR-30a-3p may represent a
potential therapeutic target in the treatment of EC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YF and QZ conceived and designed the study. PQ, PY
and JWe performed the experiments. YL, JWu and XB wrote the
manuscript and contibuted to the analysis or interpretation of the
data. All authors have read and approved the final manuscript and
agreed to be accountable for all aspects of the research in
ensuring that the accuracy and integrity of any part of the work
are appropriately investigated and resolved.
Ethics approval and consent to
participate
The present study was approved by The Ethics
Committee of Nanjing Medical University (Nanjing, China), and each
patient provided written informed consent.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Enzinger PC and Mayer RJ: Esophageal
cancer. N Engl J Med. 349:2241–2252. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Demeester SR: Epidemiology and biology of
esophageal cancer. Gastrointest Cancer Res. 32 (Suppl):S2–S5.
2009.
|
|
4
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang F, Yang Z, Cao M, Xu Y, Li J, Chen
X, Gao Z, Xin J, Zhou S, Zhou Z, et al: MiR-203 suppresses tumor
growth and invasion and down-regulates MiR-21 expression through
repressing Ran in esophageal cancer. Cancer Lett. 342:121–129.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Iyer RB, Silverman PM, Tamm EP, Dunnington
JS and DuBrow RA: Diagnosis, staging, and follow-up of esophageal
cancer. AJR Am J Roentgenol. 181:785–793. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Subasinghe D and Samarasekera DN: Delay in
the diagnosis of esophageal carcinoma: Experience of a single unit
from a developing country. Indian J Cancer. 47:151–155. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lv XB, Lian GY, Wang HR, Song E, Yao H and
Wang MH: Long noncoding RNA HOTAIR is a prognostic marker for
esophageal squamous cell carcinoma progression and survival. PLoS
One. 8:e635162013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Thompson SK, Ruszkiewicz AR, Jamieson GG,
Esterman A, Watson DI, Wijnhoven BP, Lamb PJ and Devitt PG:
Improving the accuracy of TNM staging in esophageal cancer: A
pathological review of resected specimens. Ann Surg Oncol.
15:3447–3458. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li CC, Chen CY and Chien CR: Comparative
effectiveness of image-guided radiotherapy for non-operated
localized esophageal squamous cell carcinoma patients receiving
concurrent chemoradiotherapy: A population-based propensity score
matched analysis. Oncotarget. 7:71548–71555. 2016.PubMed/NCBI
|
|
11
|
Kondo S, Tajika M, Tanaka T, Kodaira T,
Mizuno N, Hara K, Hijioka S, Imaoka H, Goto H, Yamao K and Niwa Y:
Prognostic factors for salvage endoscopic resection for esophageal
squamous cell carcinoma after chemoradiotherapy or radiotherapy
alone. Endosc Int Open. 4:E841–E848. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Roeder F, Nicolay NH, Nguyen T,
Saleh-Ebrahimi L, Askoxylakis V, Bostel T, Zwicker F, Debus J,
Timke C and Huber PE: Intensity modulated radiotherapy (IMRT) with
concurrent chemotherapy as definitive treatment of locally advanced
esophageal cancer. Radiat Oncol. 9:1912014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Li S, Li Z, Guo F, Qin X, Liu B, Lei Z,
Song Z, Sun L, Zhang HT, You J and Zhou Q: miR-223 regulates
migration and invasion by targeting Artemin in human esophageal
carcinoma. J Biomed Sci. 18:242011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hagman Z, Larne O, Edsjö A, Bjartell A,
Ehrnström RA, Ulmert D, Lilja H and Ceder Y: miR-34c is
downregulated in prostate cancer and exerts tumor suppressive
functions. Int J Cancer. 127:2768–2776. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Lin RJ, Xiao DW, Liao LD, Chen T, Xie ZF,
Huang WZ, Wang WS, Jiang TF, Wu BL, Li EM and Xu LY: MiR-142-3p as
a potential prognostic biomarker for esophageal squamous cell
carcinoma. J Surg Oncol. 105:175–182. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yokobori T, Suzuki S, Tanaka N, Inose T,
Sohda M, Sano A, Sakai M, Nakajima M, Miyazaki T, Kato H and Kuwano
H: MiR-150 is associated with poor prognosis in esophageal squamous
cell carcinoma via targeting the EMT inducer ZEB1. Cancer Sci.
104:48–54. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hong L, Han Y, Zhang H, Zhao Q and Qiao Y:
miR-210: A therapeutic target in cancer. Expert Opin Ther Targets.
17:21–28. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li B, Xu WW, Han L, Chan KT, Tsao SW, Lee
NPY, Law S, Xu LY, Li EM, Chan KW, et al: MicroRNA-377 suppresses
initiation and progression of esophageal cancer by inhibiting CD133
and VEGF. Oncogene. 36:3986–4000. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zheng R, Liu Y, Zhang X, Zhao P and Deng
Q: miRNA-200c enhances radiosensitivity of esophageal cancer by
cell cycle arrest and targeting P21. Biomed Pharmacother.
90:517–523. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Shao C, Yu Y, Yu L, Pei Y, Feng Q, Chu F,
Fang Z and Zhou Y: Amplification and up-regulation of microRNA-30b
in oral squamous cell cancers. Arch Oral Biol. 57:1012–1017. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jiang BY, Zhang XC, Su J, Meng W, Yang XN,
Yang JJ, Zhou Q, Chen ZY, Chen ZH, Xie Z, et al: BCL11A
overexpression predicts survival and relapse in non-small cell lung
cancer and is modulated by microRNA-30a and gene amplification. Mol
Cancer. 12:612013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Qi B, Wang Y, Chen ZJ, Li XN, Qi Y, Yang
Y, Cui GH, Guo HZ, Li WH and Zhao S: Down-regulation of
miR-30a-3p/5p promotes esophageal squamous cell carcinoma cell
proliferation by activating the Wnt signaling pathway. World J
Gastroenterol. 23:7965–7977. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Liu X, Ji Q, Zhang C, Liu X, Liu Y, Liu N,
Sui H, Zhou L, Wang S and Li Q: miR-30a acts as a tumor suppressor
by double-targeting COX-2 and BCL9 in H. pylori gastric cancer
models. Sci Rep. 7:71132017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Thomas W: Staging of esophageal cancer:
TNM and beyond. Esophagus. 7:189–195. 2010. View Article : Google Scholar
|
|
26
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wen XP, Ma HL, Zhao LY, Zhang W and Dang
CX: MiR-30a suppresses non-small cell lung cancer progression
through AKT signaling pathway by targeting IGF1R. Cell Mol Biol
(Noisy-le-Grand). 61:78–85. 2015.PubMed/NCBI
|
|
28
|
Wang X, Qiu H, Tang R, Song H, Pan H, Feng
Z and Chen L: miR-30a inhibits epithelial-mesenchymal transition
and metastasis in triple-negative breast cancer by targeting ROR1.
Oncol Rep. 39:2635–2643. 2018.PubMed/NCBI
|
|
29
|
Chiang AC and Massague J: Molecular basis
of metastasis. N Engl J Med. 359:2814–2823. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Nguyen DX, Bos PD and Massague J:
Metastasis: From dissemination to organ-specific colonization. Nat
Rev Cancer. 9:274–284. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Acloque H, Adams MS, Fishwick K,
Bronner-Fraser M and Nieto MA: Epithelial-mesenchymal transitions
the importance of changing cell state in development and disease. J
Clin Invest. 19:1438–1449. 2009. View Article : Google Scholar
|
|
32
|
Yang J and Weinberg RA: Epithelial
mesenchymal transition: At the crossroads of development and tumor
metastasis. Dev Cell. 14:818–829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Thiery JP and Sleeman JP: Complex networks
orchestrate epithelial-mesenchymal transitions. Nat Rev Mol Cell
Biol. 7:131–142. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tie J, Pan Y, Zhao L, Wu K, Liu J, Sun S,
Guo X, Wang B, Gang Y, Zhang Y, et al: MiR-218 inhibits invasion
and metastasis of gastric cancer by targeting the robo1 receptor.
PLoS Genet. 6:e10008792010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Liu ZL, Wang H, Liu J and Wang ZX:
MicroRNA-21 (miR-21) expression promotes growth, metastasis, and
chemo- or radioresistance in non-small cell lung cancer cells by
targeting PTEN. Mol Cell Biochem. 372:35–45. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Zhang Z, Liu S, Shi R and Zhao G: miR-27
promotes human gastric cancer cell metastasis by inducing
epithelial-to-mesenchymal transition. Cancer Genet. 204:486–491.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Sachdeva M and Mo YY: miR-145-mediated
suppression of cell growth, invasion and metastasis. Am J Transl
Res. 2:170–180. 2010.PubMed/NCBI
|
|
38
|
Zhu S, Wu H, Wu F, Nie D, Sheng S and Mo
YY: MicroRNA-21 targets tumor suppressor genes in invasion and
metastasis. Cell Res. 18:350–359. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Burk U, Schubert J, Wellner U, Schmalhofer
O, Vincan E, Spaderna S and Brabletz T: A reciprocal repression
between ZEBl and membem of the miR-200 family promotes EMT and
invasion in cancer cells. EMBO Rep. 9:582–589. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Lu J, Getz G, Miska EA, Alvarez-Saavedra
E, Lamb J, Peck D, Sweet-Cordero A, Ebert BL, Mak RH, Ferrando AA,
et al: MicroRNA expression profiles classify human cancers. Nature.
435:834–838. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Miranda KC, Huynh T, Tay Y, Ang YS, Tam
WL, Thomson AM, Lim B and Rigoutsos I: A pattern-based method for
the identification of MicroRNA binding sites and their
corresponding heteroduplexes. Cell. 126:1203–1217. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Liu H, Zhao J and Lv J: Inhibitory effects
of miR-101 overexpression on cervical cancer Siha cells. Eur J
Gynaecol Oncol. 38:236–240. 2017.PubMed/NCBI
|
|
43
|
Wang W, Lin H, Zhou L, Zhu Q, Gao S, Xie
H, Liu Z, Xu Z, Wei J, Huang X and Zheng S: MicroRNA-30a-3p
inhibits tumor proliferation, invasiveness and metastasis and is
downregulated in hepatocellular carcinoma. Eur J Surg Oncol.
40:1586–1594. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
De Craene B and Berx G: Regulatory
networks defining EMT during cancer initiation and progression. Nat
Rev Cancer. 13:97–110. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Vandewalle C, Comijn J, De Craene B,
Vermassen P, Bruyneel E, Andersen H, Tulchinsky E, Van Roy F and
Berx G: SIP1/ZEB2 induces EMT by repressing genes of different
epithelial cell-cell junctions. Nucleic Acids Res. 33:6566–6578.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Eades G, Yuan Y, Yang M, Zhang Y, Chumsri
S and Zhou Q: MiR-200a regulates SIRT1 and EMT-like transformation
in mammary epithelial cells. J Biol Chem. 286:25992–6002. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Xiang X, Zhuang X, Jiang H, Zhang S, Jiang
H, Mu J, Zhang L, Miller D, Grizzle W and Zhang HG: miR-155
promotes macroscopic tumor formation yet inhibits tumor
dissemination from mammary fat pads to the lung by preventing EMT.
Oncogene. 30:3440–3453. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Ke Y, Zhao W, Xiong J and Cao R: miR-149
inhibits non-small-cell lung cancer cells EMT by targeting FOXM1.
Biochem Res Int. 2013:5067312013. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Yin Z, Zhou B, He Q, Li M, Guan P, Li X,
Cui Z, Xue X, Su M, Ma R, et al: Association between polymorphisms
in DNA repair genes and survival of non-smoking female patients
with lung adenocarcinoma. BMC Cancer. 9:4392009. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Connell PP, Kron SJ and Weichselbaum RR:
Relevance and irrelevance of DNA damage response to radiotherapy.
DNA Repair (Amst). 3:1245–1251. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Woodward WA, Chen MS, Behbod F, Alfaro MP,
Buchholz TA and Rosen JM: WNT/beta-catenin mediates radiation
resistance of mouse mammary progenitor cells. Proc Natl Acad Sci
USA. 104:618–623. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Anastasov N, Höfig I, Vasconcellos IG,
Rappl K, Braselmann H, Ludyga N, Auer G, Aubele M and Atkinson MJ:
Radiation resistance due to high expression of miR-21 and G2/M
checkpoint arrest in breast cancer cells. Radiat Oncol. 7:2062012.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Naidu MD, Mason JM, Pica RV, Fung H and
Peña LA: Radiation resistance in glioma cells determined by DNA
damage repair activity of Ape1/Ref-1. J Radiat Res. 51:393–404.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Cho S, Cinghu S, Yu JR and Park WY:
Helicase-like transcription factor confers radiation resistance in
cervical cancer through enhancing the DNA damage repair capacity. J
Cancer Res Clin Oncol. 137:629–637. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Hazawa M, Hosokawa Y, Monzen S, Yoshino H
and Kashiwakura I: Regulation of DNA damage response and cell cycle
in radiation-resistant HL60 myeloid leukemia cells. Oncol Rep.
28:55–61. 2012.PubMed/NCBI
|
|
56
|
Liamina D, Sibirnyj W, Khokhlova A, Saenko
V, Rastorgueva E, Fomin A and Saenko Y: Radiation-induced changes
of microRNA expression profiles in radiosensitive and
radioresistant leukemia cell lines with different levels of
chromosome abnormalities. Cancers. 9(pii): E1362017. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Liu J, Li M, Wang Y and Luo J: Curcumin
sensitizes prostate cancer cells to radiation partly via epigenetic
activation of miR-143 and miR-143 mediated autophagy inhibition. J
Drug Target. 25:645–652. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Hartog H, Wesseling J, Boezen HM and van
der Graaf WT: The insulin-like growth factor 1 receptor in cancer:
Old focus, new future. Eur J Cancer. 43:1895–1904. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Bähr C and Groner B: The insulin like
growth factor-1 receptor (IGF-1R) as a drug target: Novel
approaches to cancer therapy. Growth Horm IGF Res. 14:287–295.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Lovly CM, McDonald NT, Chen H,
Ortiz-Cuaran S, Heukamp LC, Yan Y, Florin A, Ozretić L, Lim D, Wang
L, et al: Rationale for co-targeting IGF-1R and ALK in ALK fusion
positive lung cancer. Nat Med. 20:1027–1034. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Buck E and Mulvihill M: Small molecule
inhibitors of the IGF-1R/IR axis for the treatment of cancer.
Expert Opin Investig Drugs. 20:605–621. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Shiratsuchi I, Akagi Y, Kawahara A,
Kinugasa T, Romeo K, Yoshida T, Ryu Y, Gotanda Y, Kage M and
Shirouzu K: Expression of IGF-1 and IGF-1R and their relation to
clinicopathological factors in colorectal cancer. Anticancer Res.
31:2541–2545. 2011.PubMed/NCBI
|
|
63
|
Nussbaum T, Samarin J, Ehemann V,
Bissinger M, Ryschich E, Khamidjanov A, Yu X, Gretz N, Schirmacher
P and Breuhahn K: Autocrine insulin-like growth factor-II
stimulation of tumor cell migration is a progression step in human
hepatocarcinogenesis. Hepatology. 48:146–156. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Kim WY, Jin Q, Oh SH, Kim ES, Yang YJ, Lee
DH, Feng L, Behrens C, Prudkin L, Miller YE, et al: Elevated
epithelial insulin-like growth factor expression is a risk factor
for lung cancer development. Cancer Res. 69:7439–7448. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Eckstein N, Servan K, Hildebrandt B,
Pölitz A, von Jonquières G, Wolf-Kümmeth S, Napierski I, Hamacher
A, Kassack MU, Budczies J, et al: Hyperactivation of the
insulin-like growth factor receptor I signaling pathway is an
essential event for cisplatin resistance of ovarian cancer cells.
Cancer Res. 69:2996–3003. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Zhang B, Li Y, Hou D, Shi Q, Yang S and Li
Q: MicroRNA-375 inhibits growth and enhances radiosensitivity in
oral squamous cell carcinoma by targeting insulin like growth
factor 1 receptor. Cell Physiol Biochem. 42:2105–2117. 2017.
View Article : Google Scholar : PubMed/NCBI
|