Introduction
Rheumatoid arthritis (RA), an immune-mediated
inflammatory disease of connective tissue, chronically accelerates
the erosion of cartilage and subchondral bone, characterized by
symmetrical inflammation in the joints of the hands and feet
(1,2). Hyperplasia of fibroblast-like
synoviocytes (FLSs) in pannus, the aggressive front of synovial
tissue, accompanied by the mass infiltration of macrophages and
lymphocytes, are the typical features of the pathogenesis in RA
(3). Inflammatory and
anti-inflammatory mediators exert significant effects in the
pathogenesis of RA (4–6). Previous studies have demonstrated
that the enhanced proliferation and attenuated apoptosis of FLSs
contribute to the invasion and destruction of connective tissue by
pannus in joints (7,8). Through the persistent efforts of
investigators, a number of natural and synthetic anti-inflammatory
and anticancer drugs have been found to suppress the abnormal
proliferation of FLSs in patients with RA, or in adjuvant arthritis
(AA) model rats (9–12).
Resveratrol, a bioactive compound predominantly
found in grapes and red wine, provides a wide range of properties
that are beneficial for health, including anticancer,
anti-inflammatory, antioxidant and cardiovascular protective
activities (13). Furthermore, it
has been suggested that resveratrol may not only suppress
proliferation, but may also induce apoptosis in various types of
cancer, including myeloid, breast, lung, liver, pancreatic,
prostate, colon and skin cancer (14–16).
The mechanism by which resveratrol exerts its pro-apoptotic
activities involves a wide range of signaling pathways, including
the mitochondrial, caspase 8- or 9-dependent, receptor-dependent
pathways and cell cycle arrest. Resveratrol-induced apoptosis has
also been demonstrated to be associated with the B-cell lymphoma 2
(Bcl-2)-associated X protein (Bax)/Bcl-2 ratio, driven by changes
in the transcriptional activity of P53 and nuclear factor-κB
(15,17). A previous study indicated that
reactive oxygen species (ROS) can function as upstream signaling
molecules that exert an influence on endoplasmic reticulum (ER)
stress-mediated apoptosis (18).
In addition, overexpressed ROS are able to induce the dysfunction
of ER foldase and chaperones, leading to an accumulation of
unfolded protein, reduced protein biosynthesis and alleviation of
the burden of the ER, which may afford a protective response
(19). However, continuous and
marked ER stress may lead to a prolonged period of marked C/EBP
homologous protein (Chop) expression, steering the cells towards
apoptosis (20,21). In our previous study, it was
demonstrated that levels of mitochondrial ROS and apoptosis were
elevated following resveratrol treatment in FLSs (22). Based on the close biological
association of mitochondria with the ER, the pro-apoptotic effects
of resveratrol may correlate with mitochondrial dysfunction and ER
stress. Furthermore, excessive ROS may trigger the oxidative
modification of ER proteins and suppress the
Ca2+-ATPase, leading to the depletion of calcium stores
and cell apoptosis, as revealed in hypoxic-ischemic model rats
(21). Cytokines are vital agents
in the inflammatory process. Markedly pro-inflammatory cytokines,
including interleukin (IL)-1, IL-6, IL-8 and tumor necrosis
factor-α (TNF-α), combined with markedly anti-inflammatory IL-10,
are ideal markers to assess the properties of resveratrol and its
influence on immune responses (4–6).
Considering the experimental evidence amassed to
date, resveratrol may be considered as a potential alleviator of
the symptoms of RA for patients, although its specific effects on
FLSs and the underlying mechanism remain to be elucidated. The
present study aimed to further investigate the pro-apoptotic
mechanism of resveratrol on FLSs with respect to mitochondrial and
ER dysfunction.
Materials and methods
Animal groupings and model
evaluation
A total of 60 2-month-old male Sprague-Dawley (SD)
rats (180±20 g) were obtained from the Laboratory Animal Center of
Anhui Medical University (Hefei, China). The present study was
approved by the Medical Ethics Committee of the Academic Committee
at Anhui Medical University. The animals were acclimatized in a
room at constant temperature (22–23°C) under a 12-h light-dark
cycle (light on at 7:00 AM), and the relative humidity was
controlled within the range 50–70%. The rats were provided with
access to standard mice chow and water ad libitum. Aliquots
of 150 µl Freund's complete adjuvant (FCA; Sigma-Aldrich; Merck
KGaA, Darmstadt, Germany) were injected into the left hind toe of
the male SD rats once, and evident arthritis was observed 20 days
later. Control rats were injected with 150 µl physiological saline
in the identical region of the limb. Subsequently, the AA model
rats were randomly divided into five groups that were respectively
treated with 0, 5, 15 or 45 mg/kg resveratrol and 200 mg/kg
N-acetyl-L-cysteine (NAC; Merck KGaA) for 12 days by continuous
intragastric administration. The selection of the doses used in the
present study were based on a study described previously (23). All animals were sacrificed on the
12th day upon completion of the above-mentioned treatments. Blood
samples were centrifuged at 1,500 × g for 20 min at −4°C to obtain
the supernatant fluid, which was stored at −80°C prior to further
analysis. The degree of paw swelling and the arthritis index scores
were determined to evaluate the severity of AA. Following the
injection of the FCA emulsion, the hind paw volume (ml) of all rats
was measured using a plethysmometer at 3-day intervals. The
arthritis index was classified using a four-points scale (0 points,
red spots or mild swelling; 2 points, moderate joint swelling; 3
points, severe joint swelling; 4 points, joint rigidity, deformity
or severe dysfunction), totaling 16 points for each rat. The degree
of paw swelling was also used to evaluate the severity of the
lesion in the affected limb. Prior to the induction of
inflammation, an animal volume detector (Jinan Yanyi Biotechnology
Corporation, Jinan, China) was applied to detect the affected hind
paw volume of each rat (F0). From the onset of inflammation, the
left hind paw volume was measured every 4 days (F1), and the degree
of paw swelling of the affected paw was calculated according to
F1/F0.
Cell culture and isolation
FLSs were isolated from the rats in the AA model
group as described previously (24). Sterile synovial tissue samples were
separated into 1-mm3-sized pieces, mixed with twice the
volume of 0.2% type II collagenase (Merck KGaA) containing 10%
Gibco® fetal bovine serum (FBS; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), and the tissues were digested
for 2–2.5 h at 25°C (percussing the samples once every 30 min).
Finally, 0.25% trypsin was added for a further digestion step for
30 min, and the isolated FLSs were cultured in Gibco®
Dulbecco's modified Eagle medium (Thermo Fisher Scientific, Inc.)
supplemented with 15% FBS in an incubator at 37°C with 5%
CO2 prior to performing the following experiments.
Evaluation of inflammatory injury,
apoptosis and intracellular ROS
Blood from the AA rats was obtained via removal of
the eyes, and serum was isolated via centrifugation (2,000 × g) at
4°C for 20 min. Serum levels of IL-1, IL-6, IL-8, IL10 and TNF-α
were detected using ELISA kits (all Abcam, Cambridge, MA, USA) for
mouse IL-1 (cat. no. ab100704), IL-6 (cat. no. ab100712), IL-8
(cat. no. ab46032), IL10 (cat. no. ab108870) and TNF-α (cat. no.
ab208348) according to the manufacturer's protocol. Cell apoptosis
was detected using a terminal deoxynucleotidyl-transferase-mediated
dUTP nick end labeling (TUNEL) assay (Roche Diagnostics,
Indianapolis, IN, USA) in situ cell death detection kit
(cat. no. 11684817910), according to the manufacturer's protocol.
Briefly, FLSs were washed 3 times with phosphate-buffered saline
(PBS) and incubated with reaction buffer at 37°C for 30 min in the
dark. FLS were then stained with DAPI at room temperature for 5 min
in the dark to visualize the nuclei, following which slices were
mounted (cat. no. ab64230; Abcam) at room temperature for ~5 min.
The number of apoptotic cells, and the total numbers of cells, were
counted from five random fields in each slide under a light
microscope (magnification, ×200). The results are presented as the
ratio of the apoptotic cell number to the total cell number (n=3
for each group) (25).
Apoptosis-associated proteins, including Bcl-2,
caspase-3, Bax, caspase-12 and Chop, were detected via western blot
analysis according to a protocol described previously (23). Briefly, cells were lysed using RIPA
buffer (Beyotime Institute of Technology, Shanghai, China) and
centrifuged at 12,000 × g for 10 min at 4°C. The protein
concentration was determined using a BCA Protein Assay kit (cat.
no. P0011; Beyotime Institute of Technology). The supernatants were
degenerated via heating at 100°C for 5 min with 1/5 volume of
loading buffer (Beyotime Institute of Technology), and 30 µg
samples were loaded in each well, separated via 10% SDS-PAGE and
transferred to PVDF membranes (EMD Millipore, Billicera, MA, USA).
Next, 5% nonfat milk in washing buffer was used to block the PVDF
membranes for 2 h at room temperature, which were then incubated at
4°C overnight with primary antibodies (all from Abcam) specific for
Bax (1:1,500; cat. no. ab32503), Bcl-2 (1:2,000; cat. no.
ab182858), caspase-3 (1:2,000; cat. no. ab13847), caspase-12
(1:2,500; cat. no. ab62484), Chop (1:3,000; cat. no. ab179823) and
β-actin (1:10,000; cat. no. ab115777). On the following day,
membranes were washed and incubated with horseradish
peroxidase-conjugated anti-rabbit Immunoglobulin G secondary
antibody (1:10,000; cat. no. A0208; Beyotime Institute of
Biotechnology) for 1 h at room temperature. Finally, protein bands
were visualized using enhanced chemiluminescence reagent (Boster
Biological Technology, Pleasanton, CA, USA), imaged using a Gel-Dox
XR+ imager (Bio-Rad Laboratories, Inc., Hercules, CA, USA) and
quantified using Image Lab v4.0 (Bio-Rad Laboratories, Inc.).
2′,7′-Dichlorodihydrofluorescein diacetate (DCFH-DA)
was utilized as the fluorescent probe to detect intracellular ROS
in the FLSs, also according to the manufacturer's protocol
(Bioluminor Biotechnology Co., Ltd., Xiamen, Fujian, China).
Briefly, 1×105/cm2 FLSs grown on glass
coverslips were cultured with 5 µM DCFH-DA at 37°C for 20 min.
Following staining, the slides were rinsed three times with PBS.
Subsequently, the fluorescence intensity was determined using a
confocal laser scanning microscope (Leica SP5-DMI6000-DIC; Leica
Microsystems GmbH, Wetzlar, Germany); its in-built evaluation
software (Leica LAS AF Lite 2.6.0 build 7288) was used to detect
the quantitative analysis of the green fluorescence signal with an
excitation wavelength of 488 nm and an emission wavelength of 522
nm. The ROS level was positively correlated with fluorescent
intensity.
Hematoxylin and eosin (H&E)
staining
Following corresponding animal experiments, the rats
were sacrificed via cervical dislocation. The knee joint was
extracted and fixed in 4% paraformaldehyde at 4°C. Subsequently,
the tissues were dehydrated in ethanol and finally embedded in
paraffin. Histologic cuts from the paraffin blocks (5-mm thickness)
were obtained and stained with hematoxylin and eosin as previously
described (22). Briefly, the
sections were attached to the slide and heated at 60°C. Next, the
sections were dewaxed with xylene twice for 5 min, rehydrated in a
graded ethanol series and then distilled water for 3 min, and then
stained with hematoxylin for 5 min and with eosin for 20 sec (both
steps at room temperature). Sections were then dehydrated in a
graded ethanol series and subsequently incubated with xylene for 5
min. Finally, sections were sealed with neutral paraffin. The
images of the stained tissue were captured via a light microscope
(magnification, ×100 and ×400). A total of six fields were randomly
selected from each group, and the fields of view were analyzed by
two different observers.
Mitochondrial membrane potential (Δψm)
determination
According to the manufacturer's protocol, a Δψm
assay kit with JC-1 (Thermo Fisher Scientific, Inc.) was used to
detect Δψm. In terms of the functioning of the assay, the JC-1
probe accumulates in the mitochondrial matrix to form a polymer,
which results in marked red fluorescence in normal mitochondria;
however, the JC-1 probe exists as a monomer in damaged
mitochondria, and appears as marked green fluorescence. Following
the specific experimental treatments with resveratrol and
H2O2, 1×105/cm2 FLSs,
grown on glass coverslips, were cultured with 5 µg/ml JC-1 at 37°C
for 20 min. Following staining, the slides were rinsed three times
with PBS. As described above, the fluorescence intensity was
subsequently determined using a Leica SP5-DMI6000-DIC confocal
laser scanning microscope (Leica Microsystems GmbH), and its
in-built evaluation software (Leica LAS AF Lite) was used to detect
the quantitative analysis of the green fluorescence signal, with an
excitation wavelength of 507 nm and an emission wavelength of 529
nm. Measurements for Δψm were determined as the ratios of
red-to-green fluorescence intensity.
Intracellular calcium [(Ca2+)i]
measurement
The measurement of [Ca2+]i was
performed as previously described (26). Briefly,
1×105/cm2 FLSs were seeded on circular
coverslips and incubated with 10 µmol/l Fluo-8 combined with 0.02%
pluronic acid F-127 at 37°C for 20 min. Ca2+ release was
triggered via treating FLSs with 4 µmol/l thapsigargin (TG) or 100
µmol/l ATP for 5 min in Ca2+-free PBS containing 140
mmol/l NaCl, 5 mmol/l KCl, 1 mmol/l MgCl2, 10 mmol/l
glucose, 0.2 mmol/l EGTA and 5 mmol/l
4-(2-hydroxyethyl)-1-piperazineethanesulphonic acid (pH 7.4).
Real-time fluctuations in fluorescence representing
[Ca2+]i (the internal concentration of
Ca2+) were recorded using a Leica SP5-DMI6000-DIC
confocal laser scanning microscope (Leica Microsystems GmbH) with
an excitation wavelength of 488 nm and a long-pass emission
wavelength of 515 nm. Variations in [Ca2+]i
are shown as the ratio of fluorescence relative to the intensity
prior to the administration of TG or ATP (F1/F0). The fluorescence
intensities were measured, based on an average of 20–30 cells for
each measurement.
Statistical analysis
The results were analyzed using SPSS 19.0
statistical software (IBM Corp., Armonk, NY, USA), and data are
shown as the mean ± standard deviation of experiments performed in
triplicate. All datasets were analyzed using one-way analysis of
variance followed by Tukey's post hoc test to compare two groups.
P≤0.05 was considered to indicate a statistically significant
difference.
Results
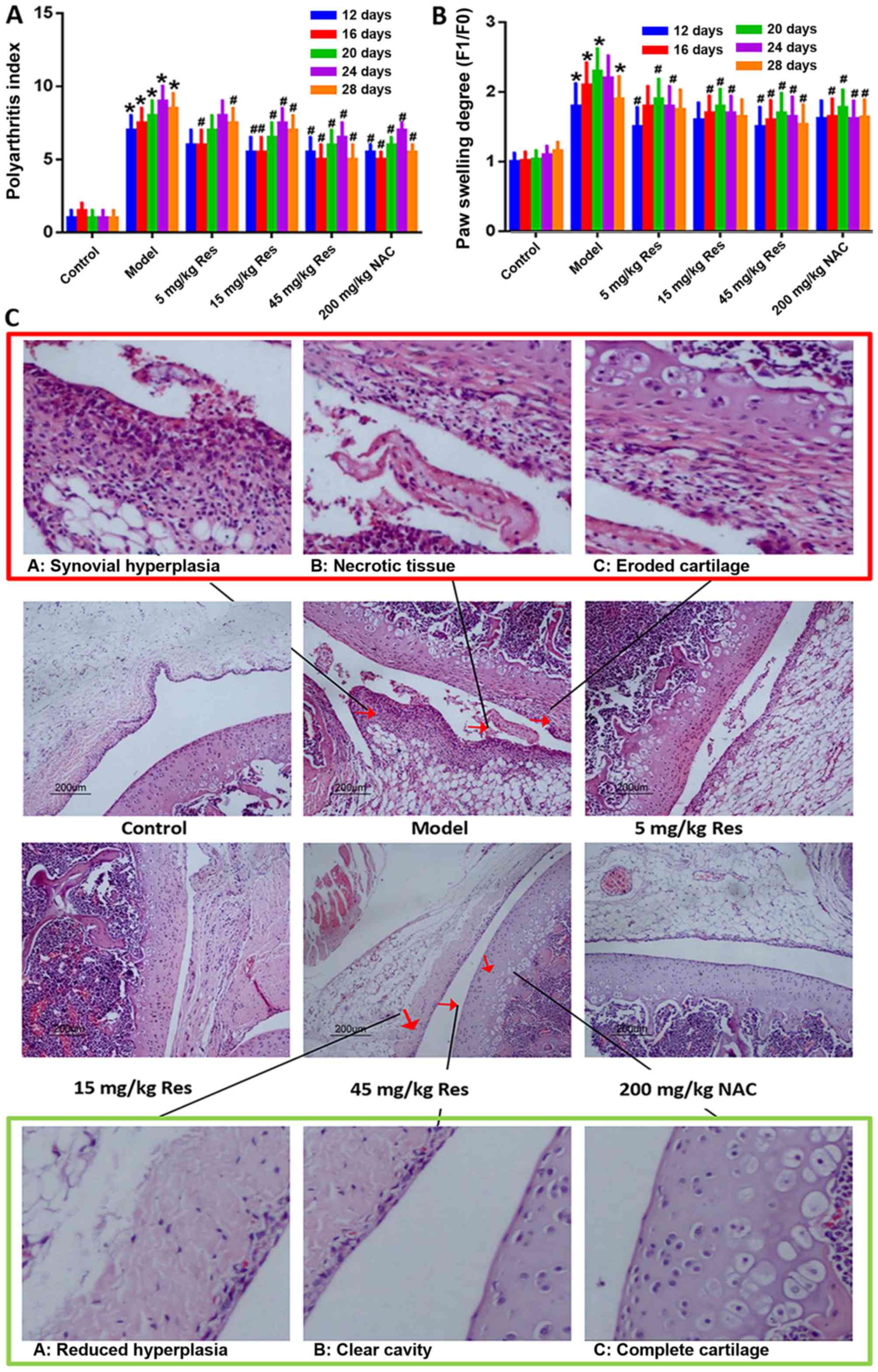
Resveratrol attenuates the severity of
arthritis induced by adjuvant in AA rats
Following the injection of FCA in SD rats, marked
paw swelling was observed. In order to identify the association
between the dose and pharmacological action of resveratrol, three
experimental groups, treated with low (5 mg/kg), middle (15 mg/kg)
and high (45 mg/kg) doses of resveratrol, were established in the
present study (23,27). NAC, the precursor of reduced
glutathione, is an important non-enzymatic antioxidant in the cell
that eliminates reactive oxygen groups, and has been demonstrated
to be effective in suppressing AA and RA. Therefore, NAC was
applied as a positive control in order to examine the functional
role of resveratrol (28–30). Compared with the AA model group,
treatment with resveratrol markedly reduced paw swelling and the
arthritis score in AA rats (Fig. 1A
and B). In addition, the effects of resveratrol on AA rats were
further demonstrated by H&E staining. FCA injection triggered
mass mononuclear cell infiltration, and hyperplasia of synovial
tissue. Upon microscopic examination, eroded cartilage and
thickened synovial tissue were clearly observed, with a large
number of fragments gathered in the synovial cavity of the AA model
group. Following treatment with resveratrol, the synovial samples
from rats revealed evidently attenuated inflammatory cell
infiltration and synovial hyperplasia; however, all the above
symptoms were relieved, or even eliminated, compared with the AA
model group (Fig. 1C).
Resveratrol alleviates inflammatory
injury in AA rats
The rats in the respective groups were fed for 27
days, and subsequently received intragastric administration of 5,
15 or 45 mg/kg resveratrol and 200 mg/kg NAC for 12 days.
Subsequently, the serum levels of IL-1, IL-6, IL-8, IL-10 and TNF-α
were detected using ELISA assay. Administration of resveratrol led
to a marginal decrease in the levels of IL-1, IL-6, IL-8 and TNF-α,
although the level of IL-10 in AA rats was increased compared with
that in the non-immunized controls in a dose-dependent manner. This
indicated that resveratrol was able to reduce the level of
inflammatory injury in the AA rats (Fig. 2A-E). Among the three experimental
groups treated with resveratrol, administration of 45 mg/kg
resveratrol exhibited the most marked suppression, which approached
the level of normal controls.
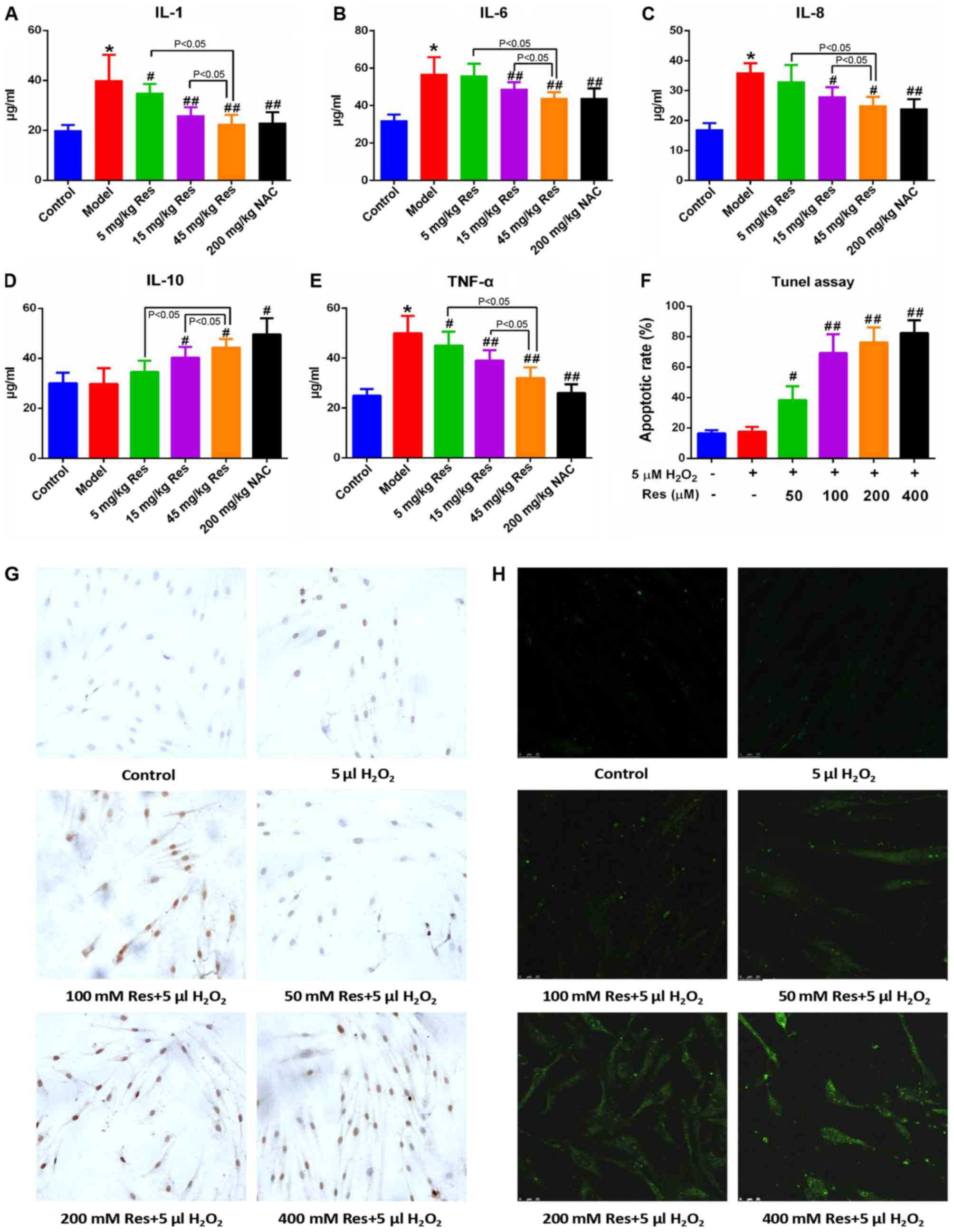 | Figure 2.Res alleviates inflammatory injury in
adjuvant arthritis rats and triggers apoptosis in FLSs.
Representative histograms show the serum levels of (A) IL-1, (B)
IL-6, (C) IL-8, (D) IL-10 and (E) TNF-α, from all grouped rats
detected via ELISA assay. (F) Quantified data and (G) images
(magnification, ×400) showing the apoptotic rates of FLSs following
Res and H2O2 treatment, detected via terminal
deoxynucleotidyl-transferase-mediated dUTP nick end labeling assay.
The apoptotic rate was determined by apoptotic cell number/total
cell number. (H) Representative images (magnification, ×400) show
the intracellular reactive oxygen species levels in FLSs, detected
via DCFH-DA following Res and H2O2 treatment.
Data are shown as the mean ± standard deviation. *P<0.05, vs.
control; #P<0.05, ##P<0.01, vs. model
(n=10/group). FLSs, fibroblast-like synoviocytes; Res, resveratrol;
NAC, N-acetyl-L-cysteine; IL, interleukin; TNF-α, tumor
necrosis factor-α; DCFH-DA, 2′,7′-dichlorodihydrofluorescein
diacetate. |
Resveratrol induces apoptosis of FLSs
within a 5µM H2O2 environment
Considering the anti-inflammatory and anticancer
effects of resveratrol reported previously in the literature, the
hypothesis of the present study was that resveratrol triggers the
apoptosis of FLSs. In order to test this hypothesis, a TUNEL assay
was used to examine the apoptotic rate of the FLSs. As shown in
Fig. 2F and G, no evident
differences in the apoptotic rates were observed when comparing
between the normal controls and FLSs; only when the cells were
incubated with 5 µM H2O2 did treatment with
resveratrol lead to an evident increase in the apoptotic rate, and
this increase occurred in a dose-dependent manner. Subsequently,
DCFH-DA was utilized to assess the levels of intracellular ROS, and
these results demonstrated that resveratrol led to an increase in
the level of intracellular ROS in FLSs in a dose-dependent manner,
and therefore it may be potential marker for apoptosis and ER
stress (Fig. 2H).
Mitochondrial dysfunction is involved
in the process of resveratrol-induced apoptosis in FLSs
As resveratrol induced the apoptosis of FLSs in an
environment containing 5 µM H2O2, further
elucidation of the intracellular mechanism became the subsequent
objective. It has been well established that the mitochondrial
signaling pathway has a vital role in cell apoptosis (31). In order to examine whether the
mitochondrial signaling pathway is involved in resveratrol-induced
apoptosis, the expression levels of mitochondria-associated
apoptotic proteins were initially examined. The immunoblotting
results suggested that, in the presence of 5 µM
H2O2 treatment, resveratrol led to a notable
increase in the expression levels of pro-apoptotic proteins,
including Bax and active caspase-3, whereas the anti-apoptotic
protein, Bcl-2, was evidently suppressed compared with that in
normal control cells and FLSs treated only with 5 µM
H2O2 (Fig.
3C-F).
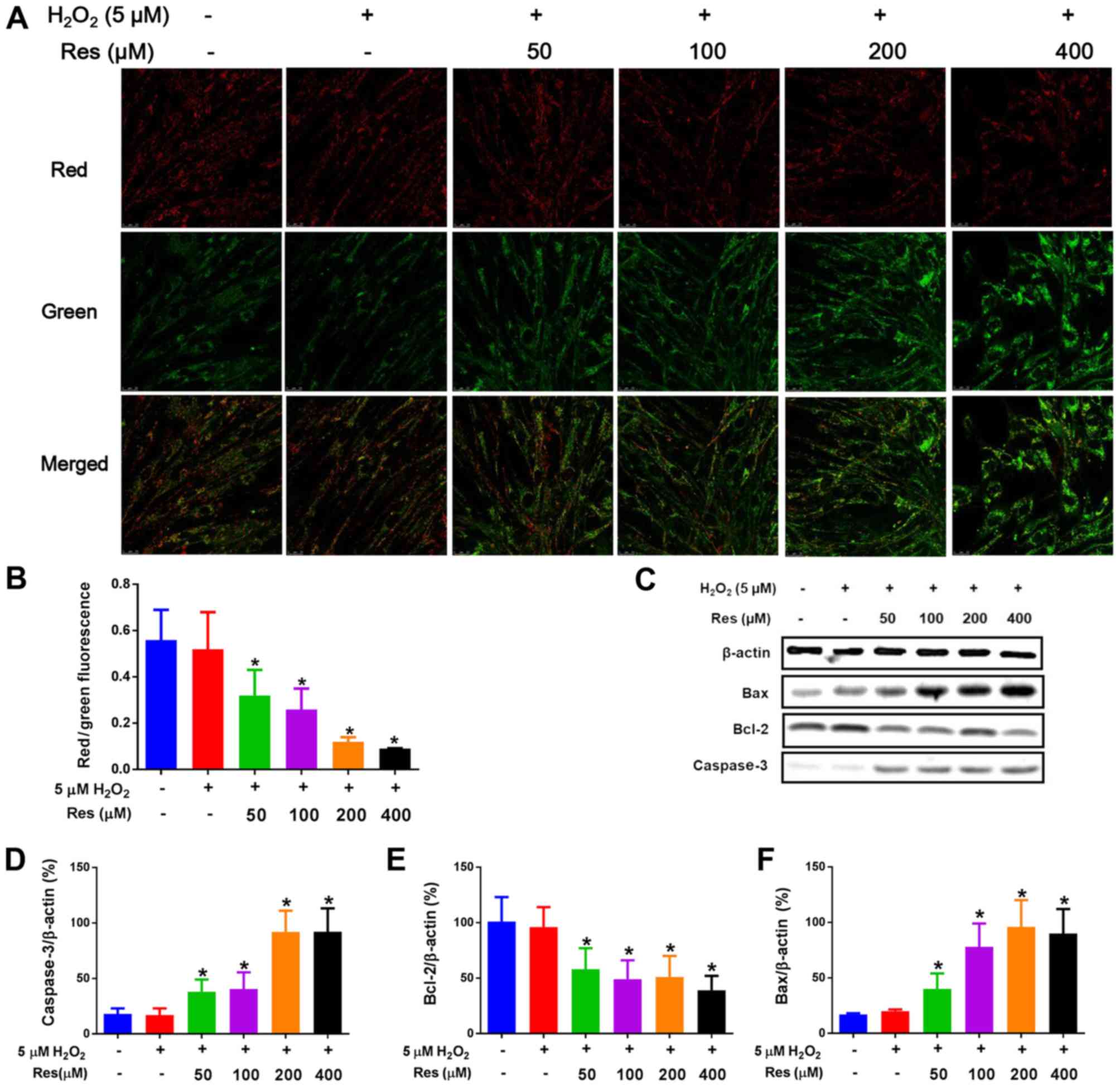 | Figure 3.Mitochondrial dysfunction is involved
in the process of Res-induced apoptosis in FLSs. (A) Δψm was
determined using confocal laser scanning microscopy and images were
captured (magnification, ×400). Red fluorescence represents JC-1
aggregates in matrix of mitochondria, whereas green fluorescence
represents JC-1 monomers, indicating a decreased Δψm. Merged images
show the overlap of JC-1 aggregates and monomers. (B) Δψm in each
group is presented as the red/green fluorescence. In addition, FLSs
were lysed and prepared for immunoblotting. (C) Representative
images and data showing the expression levels of (D) active
caspase-3, (E) Bcl-2 and (F) Bax. Data are shown as the mean ±
standard deviation. *P<0.05, vs. control. Δψm, mitochondrial
membrane potential; FLSs, fibroblast-like synoviocytes; Res,
resveratrol; Bcl-2, B-cell lymphoma 2; Bax, Bcl-2-associated X
protein. |
A reduction in Δψm or enhanced mitochondrial
membrane depolarization have been demonstrated to provide an early
sign of cell apoptosis, even in advance of DNA damage (32). JC-1, a widely used fluorescent
probe to detect Δψm, forms aggregates in the mitochondrial matrix
that yield red florescence when the value of Δψm is relatively
high, whereas it forms monomers, giving rise to green florescence,
when the value of Δψm is comparatively low (33). As shown in Fig. 3A and B, resveratrol was able to
suppress the ratio of the red-to-green fluorescent intensity in
FLSs in the presence of 5 µM H2O2 in a
dose-dependent manner compared with normal control cells and FLSs
treated only with 5 µM H2O2, indicating that
resveratrol was able to lead to a reduction in Δψm, which further
confirmed the pro-apoptotic effects of resveratrol.
Suppression of
[Ca2+]i release and ER stress in FLSs are
involved in the resveratrol-induced apoptosis of FLSs
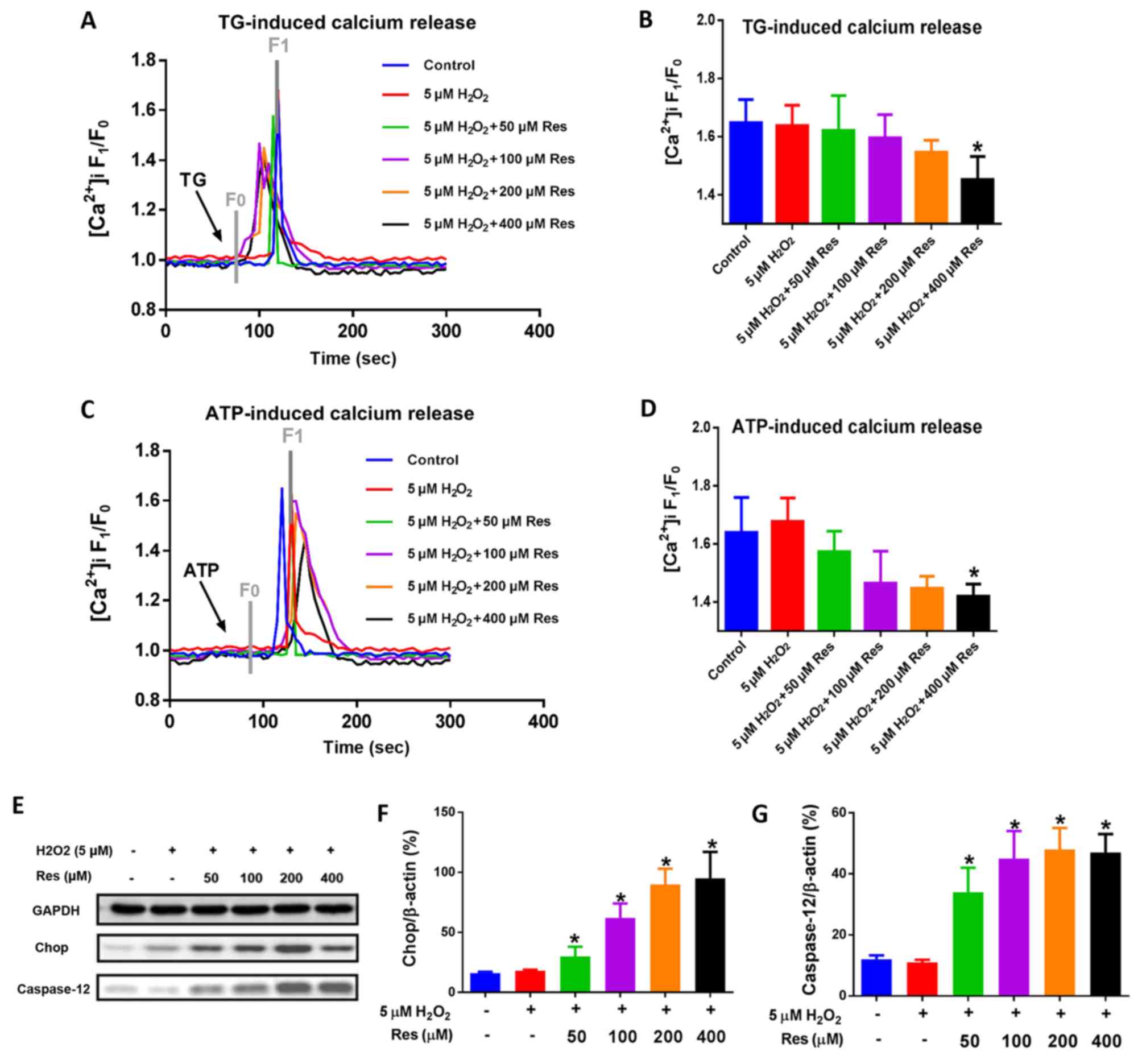
Apart from the mitochondria-associated apoptotic
pathway, the ER, which is known as an intracellular Ca2+
store, also serves a crucial role in cell apoptosis. Previous
reports have indicated that the depletion of ER Ca2+
stores may trigger cell apoptosis and growth arrest (34,35),
and the hypothesis of the present study was that resveratrol may
affect the depletion of the Ca2+ store. As
Ca2+ stores may be depleted by both ATP and TG, the
effect of resveratrol on TG- and ATP-induced Ca2+
release from the ER Ca2+ stores was subsequently
examined (36,37). Unexpectedly, the calcium imaging
experiments revealed that higher doses of resveratrol led to a
suppression of TG- and ATP-induced Ca2+ release in FLSs
in an environment containing 5 µM H2O2
(Fig. 4A-D). By contrast,
continuous and heavily applied ER stress has been suggested to be
another possible means of inducing cell apoptosis (38). Two essential terminal
apoptosis-associated proteins located downstream of the ER stress
pathway, Chop and caspase-12, have been revealed to be elevated
under aggravated ER stress (39).
In the present study, the immunoblotting data obtained revealed
that resveratrol led to an increase in the expression levels of
Chop and caspase-12 in FLSs in the presence of 5 µM
H2O2, in a dose-dependent manner, compared
with levels in normal control cells and FLSs treated only with 5 µM
H2O2 (Fig.
4E-G).
Discussion
In the present study, AA model rats were utilized to
examine the features of RA. Resveratrol injection led to an evident
reduction in the levels of IL-1, IL-6, IL-8 and TNF-α, and an
increase in the level of IL-10 in AA rats compared with levels in
non-immunized controls in a dose-dependent manner, indicating that
resveratrol was able to reduce the level of inflammatory injury,
and to enhance the anti-inflammatory capability, in AA rats. RA is
an autoimmune disease of the connective tissue characterized by
disrupted serum levels of inflammatory cytokines, including IL-1,
IL-6, IL-8 and TNF-α, which occur during its pathogenesis. The
results of the present study further corroborate the therapeutic
effects of resveratrol on RA identified in previous studies of
others (4–6). Cytokines are vital agents in the
inflammatory process. The markedly pro-inflammatory cytokines,
IL-1, IL-6, IL-8 and TNF-α, together with the markedly
anti-inflammatory cytokine, IL-10, were selected for assessment of
the properties of resveratrol and its influence on immune
responses. Resveratrol is a multifunctional compound, which reduced
the serum levels of IL-1, IL-6, IL-8 and TNF-α and simultaneously
increased the level of IL-10. It is an important marker in the
investigation to identify an effective pharmacological treatment of
RA based on the inflammatory etiology. Furthermore, the
intragastric administration of resveratrol potently reduced paw
swelling, the arthritis score, inflammatory cell infiltration and
synovial hyperplasia in AA rats compared with the AA model group
(Fig. 1D). Accumulating evidence
that resveratrol possesses certain tumor-like properties has
previously been reported, based on the fact that it not only
suppresses the proliferation of, but also induces apoptosis in
various cancer cell types and FLSs (22). Based on our former findings, the
proliferation of FLSs was identified as a typical abnormality in
RA, and 5 µM H2O2 was identified as the
optimal concentration for treatment; this concentration achieved
the highest proliferative rate of FLSs compared with higher or
lower doses of H2O2, and simulated the
physiological conditions of oxidative stress in AA model rats
(22).
The results of the in vitro TUNEL assay
revealed that the administration of resveratrol triggered the
apoptosis of FLSs in the presence of 5 µM
H2O2, and the apoptotic rate was increased
with increasing doses of resveratrol. As the majority of ROS are
produced in mitochondria, and resveratrol was able to reduce
excessive ROS production, it was hypothesized that the
mitochondrial apoptotic pathway may be involved in the
resveratrol-induced apoptosis of FLSs treated with 5 µM
H2O2. The levels of pro-apoptotic members of
the Bcl family, including Bax and caspase-3, were increased,
whereas that of the anti-apoptotic protein, Bcl-2, was suppressed
following administration of resveratrol in FLSs treated with 5 µM
H2O2. Furthermore, a reduced Δψm is known to
provide an early indicator for cellular apoptosis, and decreased
Δψm levels were detected upon treatment of resveratrol with 5 µM
H2O2.
Apart from the mitochondrial pathway, ER stress may
also be involved in the progression of apoptosis. An increasing
number of studies have revealed that ER stress, which refers to a
particular type of subcellular pathological state of ER that is
characterized by calcium dyshomeostasis, accumulation of unfolded
proteins, and ER dysfunction caused by intracellular and
extracellular stimuli, including viral infection and glucose
deficiency, serves vital roles in the process of cell apoptosis
(18). During the process of ER
stress, the unfolded protein reaction mediated by
chaperone-glucose-regulated protein 78/binding immunoglobulin
protein and three stress sensors, namely PKR-like ER kinase,
activating transcription factor 6 and inositol-requiring enzyme 1,
exert a protective role via reducing the accumulation of unfolded
protein, and gradually restoring the normal function of ER
(40). However, continual and
heavily applied ER stress can activate the downstream apoptotic
signaling molecules, Chop and caspase-12, leading to cell apoptosis
(20,41). In the present study, the levels of
these two essential mediators of ER stress-associated apoptosis,
Chop and caspase-12, were elevated following treatment with
resveratrol in the presence of 5 µM H2O2. As
the most important calcium store, ER undertakes vital tasks in
maintaining the normal biological activity of the cells, and
calcium overload is often linked to ER-associated apoptosis.
However, the processes of TG- and ATP-induced calcium release were
marginally suppressed following the administration of resveratrol
with 5 µM H2O2. This may due to a reduction
in the activity of ion channels that mediate the outflow of calcium
ions elicited by resveratrol; it is noteworthy that these results
were inconsistent with a former study, in which ATP-induced
apoptosis was accompanied by an elevated rate of calcium release in
HN4 cells (42). In the present
study, TG and ATP were used to induce calcium depletion of ER via
diverse mechanisms. TG prevents intracellular calcium from flowing
into the ER by blocking the sarco/endoplasmic reticulum
Ca2+-ATPase (SERCA), leading to a one-way efflux of
calcium from the ER to the cytosol. ATP binds to the purinergic
receptor (P2Y or P2X) on the cell membrane, and subsequently
couples with phospholipase C (PLC), leading to activation of the
inositol 1,4,5-trisphosphate receptor on the ER, which subsequently
leads to an emptying of the calcium pool (24). Therefore, the suppressive effects
of calcium release mediated by resveratrol in FLSs may be
associated with an activation of SERCA and inhibition of the
P2Y/PLC/IP3 pathway, although this hypothesis requires further
confirmation.
In conclusion, the present study demonstrated that
resveratrol was able to suppress the level of inflammatory injury
in AA and to trigger the apoptosis of FLSs through the
mitochondrial pathway and ER stress in the presence of 5 µM
H2O2, thereby alleviating the symptoms of AA
in SD rats (Fig. 5). However, the
effects of resveratrol on the bioactivity of ER-located calcium
channels require further investigation, and whether or not other
forms of programmed cell death may be involved in this progress
remains to be elucidated.
Acknowledgements
The authors would like to thank Professor Bing Shen
(Department of Physiology of Anhui Medical University), who
instructed on aspects of this study and who provided the
[Ca2+] measurements.
Funding
This study was supported by a grant from the
National Natural Science Foundation of China (grant nos.
NSFC:81373421 and 81270650).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JSL, SYF and XYC designed the experiments. JSL and
YSZ performed the experiments. JSL and JZY conducted the data
analysis. JQZ and WC contributed to data acquisition and analysis.
JSL drafted the manuscript.
Ethics approval and consent to
participate
The animal experiments was approved by the Medical
Ethics Committee of the Academic Committee at Anhui Medical
University. No human experiments were included in the present
study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Bellucci E, Terenzi R, La Paglia GM,
Gentileschi S, Tripoli A, Tani C and Alunno A: One year in review
2016: Pathogenesis of rheumatoid arthritis. Clin Exp Rheumatol.
34:793–801. 2016.PubMed/NCBI
|
|
2
|
Suzuki A and Yamamoto K: From genetics to
functional insights into rheumatoid arthritis. Clin Exp Rheuma 33
(4 Suppl 92). S40–S43. 2015.
|
|
3
|
Shin GC, Kim C, Lee JM, Cho WS, Lee SG,
Jeong M, Cho J and Lee K: Apigenin-induced apoptosis is mediated by
reactive oxygen species and activation of ERK1/2 in rheumatoid
fibroblast-like synoviocytes. Chem Biol Interact. 182:29–36. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Park JS, Kim NR, Lim MA, Kim SM, Hwang SH,
Jung KA, Choi J, Park SH and Cho ML: Deficiency of IL-1 receptor
antagonist suppresses IL-10-producing B cells in autoimmune
arthritis in an IL-17/Th17-dependent manner. Immunol Lett.
199:44–52. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jain S, Tran TH and Amiji M: Macrophage
repolarization with targeted alginate nanoparticles containing
IL-10 plasmid DNA for the treatment of experimental arthritis.
Biomaterials. 61:162–177. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Khandpur R, Carmona-Rivera C,
Vivekanandan-Giri A, Gizinski A, Yalavarthi S, Knight JS, Friday S,
Li S, Patel RM, Subramanian V, et al: NETs are a source of
citrullinated autoantigens and stimulate inflammatory responses in
rheumatoid arthritis. Sci Transl Med. 5:178ra402013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jackson JK, Higo T, Hunter WL and Burt HM:
Topoisomerase inhibitors as anti-arthritic agents. Inflamm Res.
57:126–134. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bottini N and Firestein GS: Duality of
fibroblast-like synoviocytes in RA: Passive responders and
imprinted aggressors. Nat Rev Rheumatol. 9:24–33. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dordunoo SK, Jackson JK, Arsenault LA,
Oktaba AM, Hunter WL and Burt HM: Taxol encapsulation in
poly(epsilon-caprolactone microspheres. Cancer Chemother Pharmacol.
36:279–282. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jackson JK, Tudan C, Sahl B, Pelech SL and
Burt HM: Calcium pyrophosphate dihydrate crystals activate MAP
kinase in human neutrophils: Inhibition of MAP kinase, oxidase
activation and degranulation responses of neutrophils by taxol.
Immunology. 90:502–510. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hui A, Kulkarni GV, Hunter WL, McCulloch
CA and Cruz TF: Paclitaxel selectively induces mitotic arrest and
apoptosis in proliferating bovine synoviocytes. Arthritis Rheum.
40:1073–1084. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tian J, Chen JW, Gao JS, Li L and Xie X:
Resveratrol inhibits TNF-α-induced IL-1β, MMP-3 production in human
rheumatoid arthritis fibroblast-like synoviocytes via modulation of
PI3kinase/Akt pathway. Rheumatol Int. 33:1829–1835. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Quiñonez-Flores CM, González-Chávez SA,
Del Río Nájera D and Pacheco-Tena C: Oxidative stress relevance in
the pathogenesis of the rheumatoid arthritis: A systematic review.
Biomed Res Int. 2016:60974172016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Han G, Xia J, Gao J, Inagaki Y, Tang W and
Kokudo N: Anti-tumor effects and cellular mechanisms of
resveratrol. Drug Discov Ther. 9:1–12. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sakamoto T, Horiguchi H, Oguma E and
Kayama F: Effects of diverse dietary phytoestrogens on cell growth,
cell cycle and apoptosis in estrogen-receptor-positive breast
cancer cells. J Nutr Biochem. 21:856–864. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Alkhalaf M: Resveratrol-induced apoptosis
is associated with activation of p53 and inhibition of protein
translation in T47D human breast cancer cells. Pharmacology.
80:134–143. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Luo H, Yang A, Schulte BA, Wargovich MJ
and Wang GY: Resveratrol induces premature senescence in lung
cancer cells via ROS-mediated DNA damage. PLoS One. 8:e600652013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Faitova J, Krekac D, Hrstka R and Vojtesek
B: Endoplasmic reticulum stress and apoptosis. Cell Mol Biol Lett.
11:488–505. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Szegezdi E, Logue SE, Gorman AM and Samali
A: Mediators of endoplasmic reticulum stress-induced apoptosis.
EMBO Rep. 7:880–885. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mihailidou C, Papazian I, Papavassiliou AG
and Kiaris H: CHOP-dependent regulation of p21/waf1 during ER
stress. Cell Physiol Biochem. 25:761–766. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bogeski I, Kilch T and Niemeyer BA: ROS
and SOCE: Recent advances and controversies in the regulation of
STIM and Orai. J Physiol. 590:4193–4200. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang J, Song X, Cao W, Lu J, Wang X, Wang
G, Wang Z and Chen X: Autophagy and mitochondrial dysfunction in
adjuvant-arthritis rats treatment with resveratrol. Sci Rep.
6:329282016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen X, Lu J, An M, Ma Z, Zong H and Yang
J: Anti-inflammatory effect of resveratrol on adjuvant arthritis
rats with abnormal immunological function via the reduction of
cyclooxygenase-2 and prostaglandin E2. Mol Med Rep. 9:2592–2598.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Omatsu-Kanbe M, Inoue K, Fujii Y, Yamamoto
T, Isono T, Fujita N and Matsuura H: Effect of ATP on preadipocyte
migration and adipocyte differentiation by activating P2Y receptors
in 3T3-L1 cells. Biochem J. 393:171–180. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shu S, Li CM, You YL, Qian XL, Zhou S and
Ling CQ: Electroacupuncture ameliorates cerebral
ischemia-reperfusion injury by regulation of autophagy and
apoptosis. Evidence-Based Complement Altern Med. 2016:72974252016.
View Article : Google Scholar
|
|
26
|
Shen B, Zhu J, Zhang J, Jiang F, Wang Z,
Zhang Y, Li J, Huang D, Ke D, Ma R and Du J: Attenuated mesangial
cell proliferation related to store-operated Ca2+ entry in aged
rat: The role of STIM 1 and Orai 1. Age (Dordr). 35:2193–2202.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Oliver SJ, Firestein GS, Arsenault L, Cruz
TF, Cheng TP, Banquerigo ML, Boyle DL and Brahn E: Vanadate, an
inhibitor of stromelysin and collagenase expression, suppresses
collagen induced arthritis. J Rheumatol. 34:1802–1809.
2007.PubMed/NCBI
|
|
28
|
Kim HR, Kim KW, Kim BM, Lee KA and Lee SH:
N-acetyl-l-cysteine controls osteoclastogenesis through regulating
Th17 differentiation and RANKL in rheumatoid arthritis. Korean J
Intern Med. 34:210–219. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Batooei M, Tahamoli-Roudsari A, Basiri Z,
Yasrebifar F, Shahdoust M, Eshraghi A, Mehrpooya M and Ataei S:
Evaluating the effect of oral N-acetylcysteine as an adjuvant
treatment on clinical outcomes of patients with rheumatoid
arthritis: A randomized, double blind clinical trial. Rev Recent
Clin Trials. 13:132–138. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Paul M, Hemshekhar M, Thushara RM,
Sundaram MS, NaveenKumar SK, Naveen S, Devaraja S, Somyajit K, West
R, Basappa, et al: Methotrexate promotes platelet apoptosis via
JNK-mediated mitochondrial damage: Alleviation by N-acetylcysteine
and N-acetylcysteine amide. PLoS One. 10:e01275582015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Zhang W, Wang X and Chen T: Resveratrol
induces mitochondria- mediated AIF and to a lesser extent
caspase-9-dependent apoptosis in human lung adenocarcinoma ASTC-a-1
cells. Mol Cell Biochem. 354:29–37. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Valdecantos MP, Pérez-Matute P, Quintero P
and Martínez JA: Vitamin C, resveratrol and lipoic acid actions on
isolated rat liver mitochondria: all antioxidants but different.
Redox Rep. 15:207–216. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
de la Lastra CA and Villegas I:
Resveratrol as an antioxidant and pro-oxidant agent: mechanisms and
clinical implications. Biochem Soc Trans. 35:1156–1160. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hamada R, Kaminaga K, Suzuki K and Yokoya
A: Mitochondrial membrane potential morphology atp production in
mammalian cells exposed to X-rays. Radiat Prot Dosimetry. Dec
13–2018.doi: 10.1093/rpd/ncy254. PubMed/NCBI
|
|
35
|
Sadi G, Bozan D and Yildiz HB: Redox
regulation of antioxidant enzymes: Post-translational modulation of
catalase and glutathione peroxidase activity by resveratrol in
diabetic rat liver. Mol Cell Biochem. 393:111–122. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Walia V, Kakar S and Elble R:
Micromanagement of the mitochondrial apoptotic pathway by p53.
Front Biosci (Landmark Ed). 16:749–758. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Bernardi P and Rasola A: Calcium and cell
death: The mitochondrial connection. Subcell Biochem. 45:481–506.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Xu Z, Lin S, Wu W, Tan H, Wang Z, Cheng C,
Lu L and Zhang X: Ghrelin prevents doxorubicin-induced
cardiotoxicity through TNF-alpha/NF-kappaB pathways and
mitochondrial protective mechanism. Toxicology. 247:133–138. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Contreras L, Drago I, Zampese E and Pozzan
T: Mitochondria: The calcium connection. Biochim Biophys Acta1.
797:607–618. 2010. View Article : Google Scholar
|
|
40
|
Fels DR and Koumenis C: The
PERK/eIF2alpha/ATF4 module of the UPR in hypoxia resistance and
tumor growth. Cancer Biol Ther. 5:723–728. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhong J, Kong X, Zhang H, Yu C, Xu Y, Kang
J, Yu H, Yi H, Yang X and Sun L: IInhibition of CLIC4 enhances
autophagy and triggers mitochondrial and ER stress-induced
apoptosis in human glioma U251 cells under starvation. PLoS One.
7:e393782012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Xue H, Lu J, Yuan R, Liu J, Liu Y, Wu J,
Wu K, Du J and Shen B: Knockdown of CLIC4 enhances ATP-induced HN4
cell apoptosis through mitochondrial and endoplasmic reticulum
pathways. Cell Bio. 6:52016.
|