Introduction
Colorectal cancer (CRC) is a major public health
problem since it is the third most commonly diagnosed cancer
resulting in mortality worldwide (1). Moreover, the incidence rates of
colorectal cancer in developing countries, including China, have
risen due to the growth of the aging population and adoption of
westernized behaviors and lifestyles. Surgery and adjuvant
chemotherapy are the main treatments for colorectal cancer.
However, 40–50% of patients succumb to this disease due to
recurrence, metastases, and drug resistance (2,3). In
addition, severe side effects caused by chemotherapeutic agents
lead to the deterioration of the quality of life of patients.
Therefore, it is necessary to develop tolerable treatment
strategies with increased sensitivity in order to improve the
clinical outcome and overall survival rates.
The most widely used chemotherapeutic drug,
5-fluorouracil (5-FU), is a first-line base treatment of colorectal
cancer (4–6). 5-FU inhibits cancer cell growth and
initiates apoptosis by inducing DNA damage during replication and
hindering its repair. It could disturb the synthesis of the
pyrimidine thymidine, a nucleoside required for DNA replication,
and block the activity of thymidylate synthase (TS) (7). Although 5-FU treatment has been
demonstrated to be effective for CRC, it is associated with severe
side effects and acquired drug resistance (8,9).
Therefore, further studies are required to identify agents that can
increase the efficacy of 5-FU and reduce its side effects.
Clinically, patients with low TS expression in tumor
tissues exhibit improved response to 5-FU-based therapy, indicating
that TS expression may be involved in the development of 5-FU
resistance. A previous study revealed that cancer cells acquire
5-FU resistance when stimulated with low doses of 5-FU for a
prolonged period, which is accompanied by high expression levels of
TS (10–12). The results of these studies
demonstrated that TS overexpression is closely related to the
occurrence of 5-FU resistance (13). Thus, TS is not only considered to
be a target of 5-FU but also an oncogene participating in 5-FU
resistance (14).
Recent studies indicated that prolonged exposure to
5-FU could activate several signaling pathways, including the
PI3K/Akt pathway, which is a major downstream effector pathway
leading to chemoresistance (15,16).
This pathway is involved in cell growth and drug resistance. Recent
evidence indicates that activation of the PI3K/AKT pathway
contributes to resistance to multiple cancer therapies and is
deemed a poor prognostic factor for cancers (17).
Combination studies are widely used in treating
dreadful diseases, including cancer (18), and aim to achieve synergistic
therapeutic effects, minimize toxicity, and delay the induction of
drug resistance. Several compounds from nature, such as medicinal
plants, are pharmacologically safe and have been demonstrated to be
potent chemosensitizers in combination with conventional
chemotherapeutic drugs. Therefore, phytochemicals have a good
application prospect in the treatment of cancer and adjuvant
chemotherapy (19–22).
Kaempferol is an ideal chemosensitizer owing to its
diverse pharmacological actions and nontoxic nature. Fig. 1 illustrates the structure of
kaempferol, which is known to exert antitumor effects in various
cancer models (23–26). However, no study has been conducted
on the effect of 5-FU and kaempferol in cancer. The purpose of this
study was to investigate the synergistic antitumor effects of 5-FU
and kaempferol in CRC and elucidate the possible mechanisms
underlying this effect.
Materials and methods
Chemicals and reagents
Roswell Park Memorial Institute (RPMI) 1640 medium,
fetal bovine serum (FBS), penicillin-streptomycin, trypsin-EDTA,
and BCA Protein Assay Kit were obtained from Thermo Fisher
Scientific, Inc. An Annexin V-FITC Apoptosis Detection Kit was
purchased from Nanjing KeyGen Biotech Co., Ltd. TRIzol Reagent and
PrimeScript RT Reagent Kit were provided by Takara Bio, Inc. 5-FU,
kaempferol, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium
bromide (MTT), and the remaining chemicals used in the present
study, unless otherwise stated, were obtained from Sigma-Aldrich;
Merck KGaA. Primary antibodies for Bax, Bcl-2, TS, PI3K, Akt, and
phosphorylated (p)-Akt and β-actin horseradish peroxidase
(HRP)-conjugated secondary antibodies were provided by Cell
Signaling Technology, Inc.
Cell line and cell culture
The CRC cell lines HCT-8, HCT-116 and the normal
human embryonic kidney cell line 293 were purchased from the Type
Culture Collection of the Chinese Academy of Sciences. The cells
were maintained in RPMI-1640 medium supplemented with 10% FBS, 100
unit/ml benzyl penicillin, and 100 µg/ml streptomycin in 5%
CO2 and 95% air at 37°C.
Cell viability assay
The HCT-8 cells and 293 cells (6×103
cells/well) were seeded in 96-well plates and exposed to serial
dilutions of 5-FU and/or kaempferol for 24 h. Then, MTT reagent
(0.5 mg/ml in PBS) was added, and the cells were cultured for a
further 4 h at 37°C prior to the addition of dimethyl sulfoxide.
Absorbance at 570 nm was measured using an ELISA reader (Model
ELX800; BioTek Instruments, Inc.). All assays were independently
performed in triplicates. The cell inhibition ratio was calculated
as follows:
(Acontrol-Atreated)/Acontrol
×100%, where Atreated and Acontrol were the
absorbance from treated and control groups, respectively. The
IC50 values (dose of 5-FU and kaempferol required to
inhibit cell growth by 50%) were assessed using nonlinear
regression analysis.
Calculation of combination index
(CI)
During the different dose combinations (ratios of
IC50 as 5-FU: kaempferol: 2:1, 1:1, 1:2, and 1:4), the
HCT-8 and 293 cells were treated with various concentrations of
kaempferol and 5-FU; a new concentration-dependent curve was
constructed using the MTT assay. In different combination ratios,
we used the chessboard concentration dilution method to design drug
combinations of different concentrations and then calculated the CI
according to the dose-effect curve. The Chou-Talalay method for
drug combination is based on the median-effect equation (27,28).
Based on these algorithms, CalcuSyn was used for determining
synergism and antagonism at all doses or effect levels. The CI was
analyzed by CalcuSyn where values <1, =1, and >1 indicated
synergism, an additive effect, and antagonism, respectively.
Identification of apoptosis by Annexin
V/propidium iodide (PI) staining
The percentages of cells undergoing apoptosis with
or without 5-FU and/or kaempferol were assessed by Annexin
V-FITC/PI kit-based FACS (BD Biosciences). Cells were plated
(3×105 cells/well) in a 6-well plate and then incubated
for 48 h with 100 µM kaempferol or 50 µM 5-FU alone or in
combination. Subsequently, the cells were washed twice with cold
PBS and stained with Annexin V/PI before being analyzed by FACS,
according to the manufacturer's instructions. All assays were
performed independently in triplicates.
Hoechst staining
HCT-8 cells were grown in a 6-well plate and treated
with 100 µM kaempferol and/or 50 µM 5-FU for 48 h. The cells were
fixed in ice-cold 4% paraformaldehyde for 10 min. Following washing
with PBS, the cells were incubated with 1 µg/ml of Hoechst 33258
solution for 5 min in the dark. The cells were washed with PBS
again and observed under a fluorescent microscope (DMI4000B; Leica
Microsystems); the apoptotic cells appeared condensed and displayed
fragmented nuclei.
Western blot analysis
Total protein extracts were obtained using lysis
buffer and concentrations were determined by the BCA assay (both
from Pierce Chemical Co.; Thermo Fisher Scientific, Inc.). Equal
amounts of protein (50 µg) from each sample were separated by 12%
SDS-PAGE gels and transferred to PVDF membranes (EMD Millipore).
The membranes were blocked with 5% skimmed milk for 1 h at room
temperature and probed with specific a primary antibody Bax
(1:1,000; cat. no. 5023, Cell Signaling Technology, Inc.), Bcl-2
(1:1,000; cat. no. 4223; Cell Signaling Technology, Inc.), TS
(1:1,000; cat. no. 5449; Cell Signaling Technology, Inc.), β-actin
(1:1,000; cat. no. 4967, Cell Signaling Technology, Inc.), PI3K
(1:1,000; cat. no. 4257; 1:1,000), AKT (1:1,000; cat. no. 2938;
Cell Signaling Technology, Inc.), p-Akt (1:1,000; ser473; cat. no.
4060, Cell Signaling Technology, Inc.), PTEN (1:1,000; cat. no.
4257; Cell Signaling Technology, Inc.) overnight at 4°C. After
being washed three times with TBST, the membranes were incubated
with horseradish peroxidase-conjugated goat anti-rabbit IgG
secondary antibody (1:25,000; cat. no. 31460; Thermo Fisher
Scientific, Inc.) or rabbit anti-mouse immunoglobulin G secondary
antibody (1:25,000; cat. no. 27025; Thermo Fisher Scientific Inc.)
at room temperature for 2 h. Following washing again in TBST,
protein signals were visualized by an enhanced chemiluminescence
reaction system (Bio-Rad Laboratories, Inc.) and quantified using
the ImageQuant software (Version 3.0; Bio-Rad Laboratories, Inc.).
β-actin served as the loading control. All protein quantifications
were normalized to their respective β-actin expression levels.
Statistical analysis
Three independent experiments were performed in
triplicate. Data are presented as the mean ± standard deviation
(SD). Differences between two groups were analyzed using unpaired
Student's t-test. Datasets that involved more than two groups were
assessed with one-way analysis variance (ANOVA), along with the
Tukey-Kramer test. A P-value <0.05 was regarded as statistically
significant. Regular analysis was carried out using the SPSS
package for Windows (version 17.0; SPSS, Inc.).
Results
5-FU and kaempferol cause greater
inhibition of cell viability in CRC cells
Firstly, the cell viability inhibition potential of
each drug was examined in the HCT-8 and HCT-116 cells. As
anticipated, the growth of the cells was significantly decreased by
treatment with kaempferol and 5-FU in a dose-dependent manner. The
IC50 values of 5-FU and kaempferol were 177.78 and 350
µM, respectively, in HCT-8 cells and 77.63 and 184.33 µM,
respectively, in the HCT-116 cells. The IC50
concentrations were then used to generate fixed ratios for
subsequent combination studies and to calculate the CI. Among them,
50 µM of 5-FU combined with 100 µM of kaempferol exhibited
synergistic anticancer effects on HCT-8 cells (CI value, 0.351;
Table I), when compared with the
effects of the two compounds used alone. Consistent results were
found in the HCT-116 cell line (CI, 0.621; Table II).
 | Table I.CI for different ratios of 5-FU and
kaempferol on HCT-8 cells. |
Table I.
CI for different ratios of 5-FU and
kaempferol on HCT-8 cells.
| Ratio (5-FU:
kaempferol) | CI | Effect |
|---|
| 1:2 | 0.351 | Synergistic |
| 1:1 | 0.378 | Synergistic |
| 2:1 | 0.808 | Synergistic |
| 4:1 | 0.800 | Synergistic |
 | Table II.CI for different ratios of 5-FU and
kaempferol on HCT-116 cells. |
Table II.
CI for different ratios of 5-FU and
kaempferol on HCT-116 cells.
| Ratio (5-FU:
kaempferol) | CI | Effect |
|---|
| 1:10 | 0.828 | Synergistic |
| 1:5 | 0.621 | Synergistic |
| 1:2.5 | 0.716 | Synergistic |
| 1:1.25 | 0.895 | Synergistic |
The combination of 5-FU and kaempferol
has no synergistic effect on 293 cells
The combination of 100 µM of kaempferol with 50 µM
of 5-FU did not exhibit greater cytotoxic effects on the 293 cells
(CI, >1) when compared with either of the two agents used alone
(Table III).
 | Table III.CI for different ratios of 5-FU and
kaempferol on 293 cells. |
Table III.
CI for different ratios of 5-FU and
kaempferol on 293 cells.
| Ratio (5-FU:
kaempferol) | CI | Effect |
|---|
| 12.5:25 | 2.852 | Antagonistic |
| 25:50 | 1.574 | Antagonistic |
| 50:100 | 4.039 | Antagonistic |
| 100:200 | 0.895 | Antagonistic |
The combination of 5-FU and kaempferol
exhibits greater inhibition on cell growth and cell viability
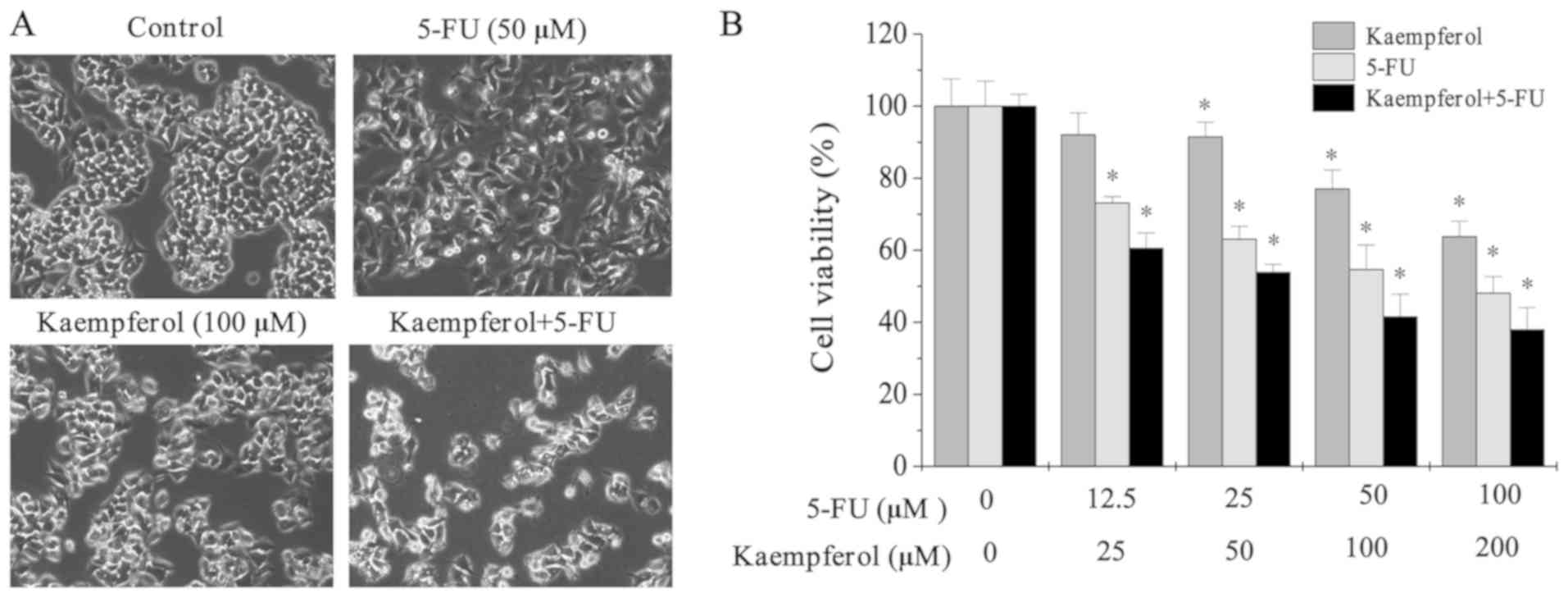
Microscopy was used to observe the cell morphology.
Cells treated with 5-FU and kaempferol were crenulated, and the
nuclei were dim (Fig. 2A). Cell
viability was significantly lower in cells subjected to treatment
with 5-FU and kaempferol alone when compared with that of the
untreated control cells (Fig. 2B).
These results indicated that combined treatment with kaempferol and
5-FU could inhibit the growth of HCT-8 cells.
Enhancement of 5-FU-induced apoptosis
by kaempferol
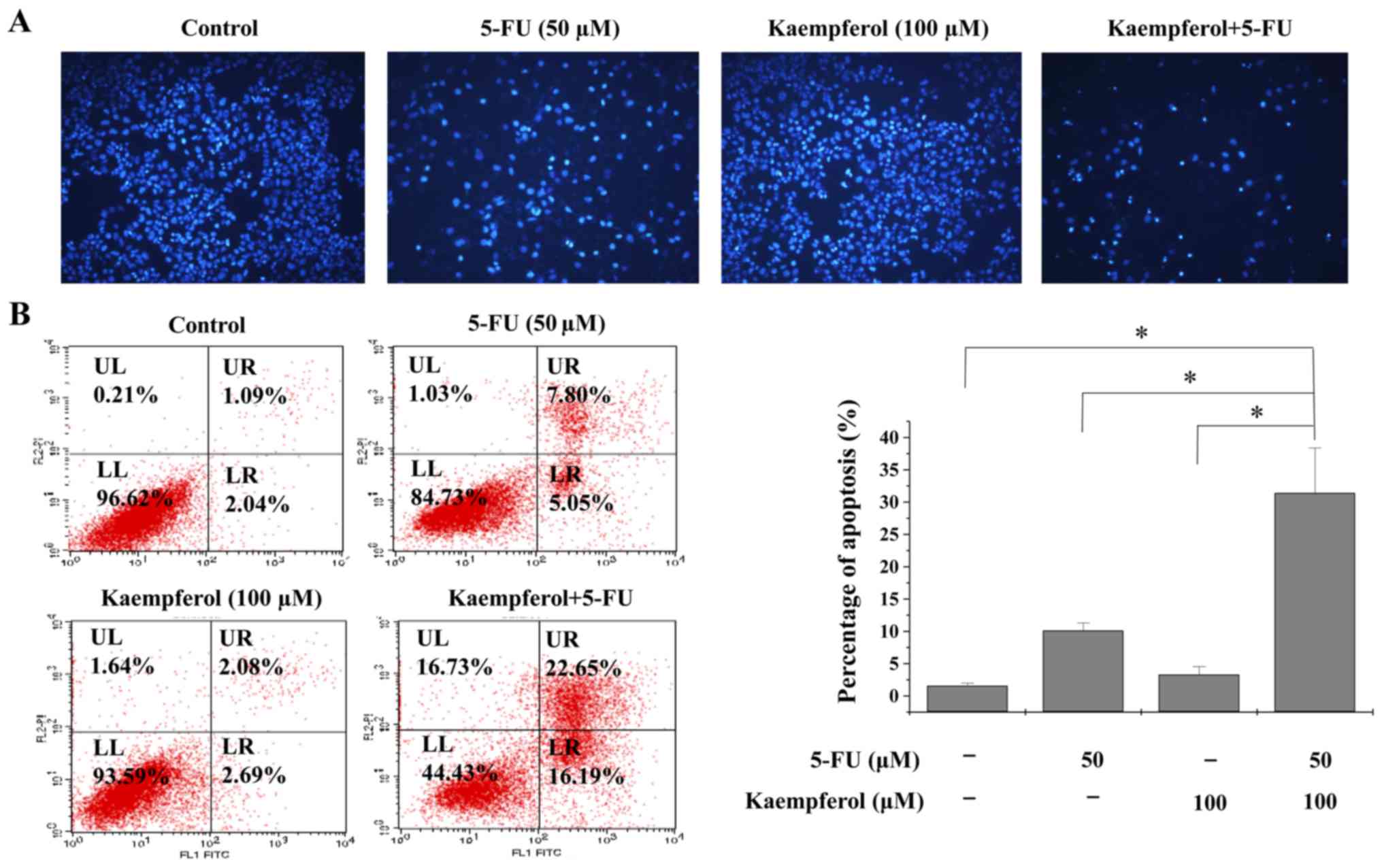
To assess if kaempferol plus 5-FU could induce
apoptosis, Hoechst staining and flow cytometric analysis were
performed to detect cell apoptosis (Fig. 3A). Kaempferol combined with 5-FU
treatment significantly induced cancer cell apoptosis in HCT-8
cells when compared with either of the two compounds alone.
Although 50 µM of 5-FU and 100 µM of kaempferol induced 10.13 and
3.31% apoptosis, respectively, in HCT-8 cells, a combination of
these two induced 31.41% apoptosis, which is almost three times
that induced by 50 µM 5-FU (Fig.
3B).
Kaempferol combined with 5-FU
upregulates the expression levels of apoptosis-associated proteins
Bax, Bcl-2, and 5-FU metabolic enzyme TS
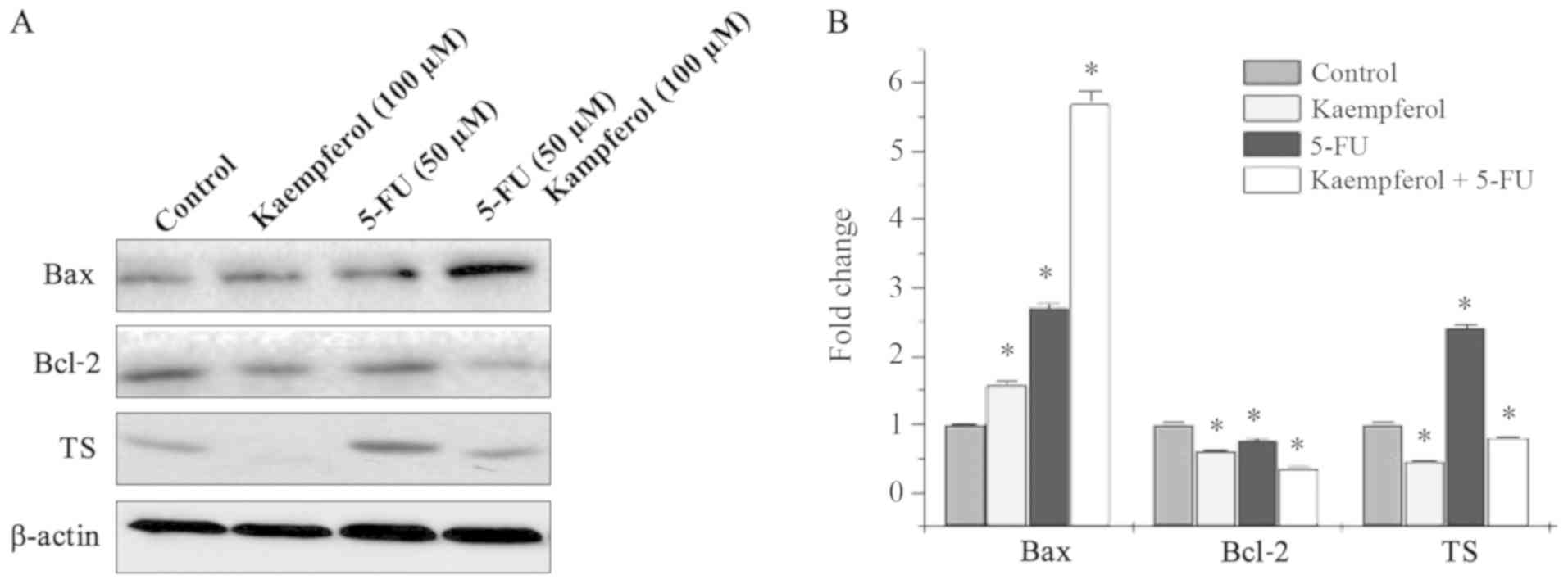
Apoptosis-related gene expression of Bax, Bcl-2, and
5-FU metabolic enzyme TS was detected by western blotting. The
expression levels of Bax were higher in cells subjected to
combination treatment when compared with those with either agent
alone (Fig. 4). Conversely, the
expression levels of Bcl-2 were decreased in the combination group
when compared with single-agent treatment; TS expression levels
were significantly decreased in HCT-8 cells when treated with
kaempferol and 5-FU (Fig. 4).
These results indicated that kaempferol combined with 5-FU exhibits
synergistic anticancer effects by inducing CRC cell apoptosis and
altering the expression levels of TS.
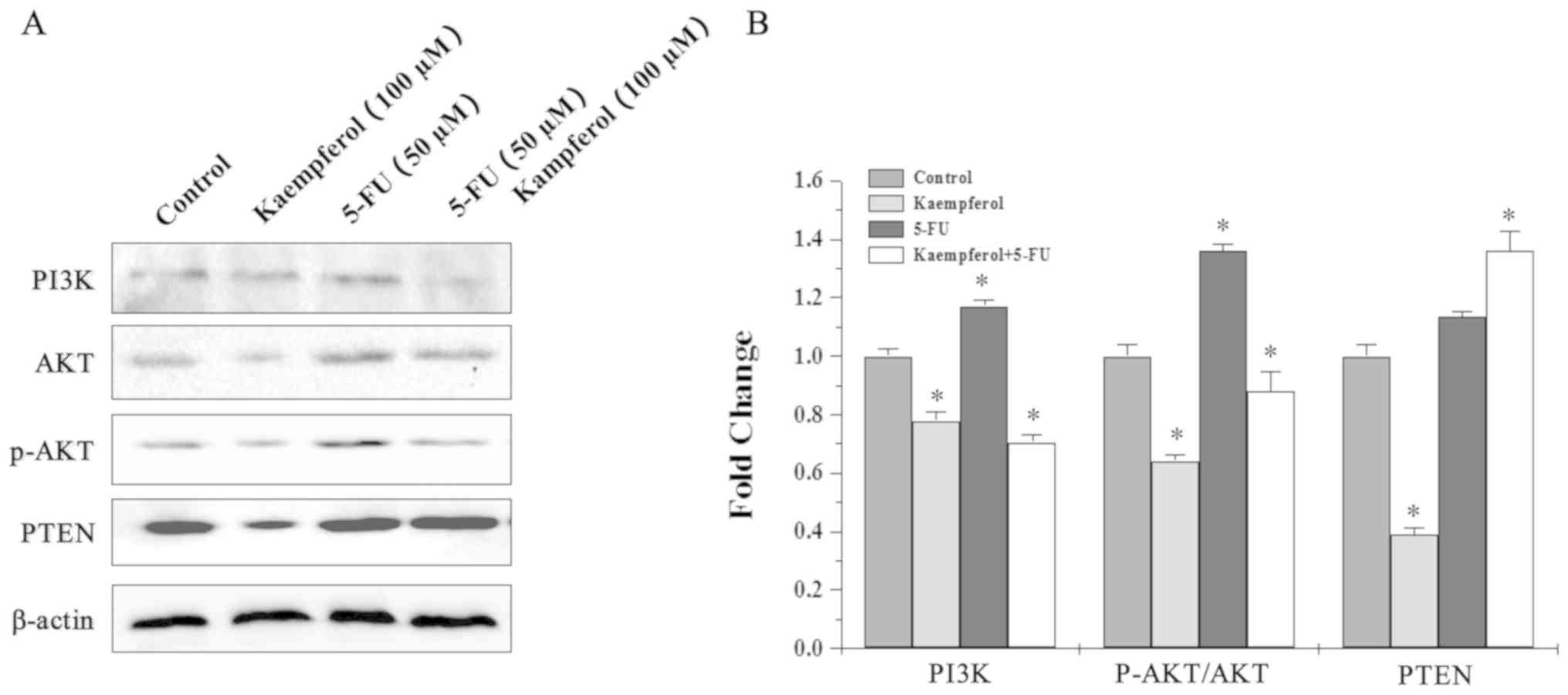
Role of the PI3K/AKT pathway in the
synergistic effects of kaempferol and 5-FU
To determine whether AKT activation was involved in
the synergistic effects of kaempferol and 5-FU, the levels of PI3K,
PTEN, AKT, and p-AKT in HCT-8 cells were examined by western
blotting. The p-AKT levels were attenuated in cells treated with
kaempferol and increased after 5-FU treatment. However, PI3K and
p-AKT levels were significantly lower in cells subjected to
combination treatment when compared with 5-FU alone (Fig. 5). Thus, kaempferol and 5-FU may
have synergistically suppressed CRC cell growth by inhibiting the
activation of the PI3K/Akt pathway.
Discussion
Chemotherapy is considered as the most potent
treatment option to improve poor survival rates in cancer. Although
the combination of 5-FU, oxaliplatin, and irinotecan are being used
in the clinical setting, their effects are not entirely
satisfactory (2). The two main
problems associated with chemotherapy are drug toxicity and the
development of resistance of the tumor cells toward apoptosis.
Thus, the combination of 5-FU with chemosensitizers could minimize
the occurrence of side effects and maximize efficacy. Several
synthetic chemosensitizers have been developed, but their cytotoxic
effects and adverse pharmacokinetics have prohibited their use in
clinical trials.
Hedyotis diffusa Willd is a major component
frequently used in traditional Chinese medicine for the clinical
treatment of CRC and is associated with drug resistance (29,30).
Kaempferol is one of the main active components of Hedyotis
diffusa and has been revealed to possess anticancer effects in
several cancer cell lines both in vitro and in vivo
(31–36). Notably, they exhibit almost no or
minor toxicity against normal epithelial, peripheral blood, and
myeloid cells. In the present study, the effects of different
combinations of kaempferol and 5-FU were examined; the inhibition
rates were analyzed by the method described by Chou and Talalay. As
revealed in Table I, the combined
inhibitory effect of kaempferol and 5-FU (CI, <1) on the growth
of the CRC cells was stronger than that of kaempferol or 5-FU
alone. The following combination was used for further evaluations
and comparisons in this study: kaempferol (100 µM) and 5-FU (50
µM).
The effect of kaempferol (100 µM) and 5-FU (50 µM)
on apoptosis induction in HCT-8 cells was higher than that of
either of the agents used alone. These results encouraged further
evaluations into the mechanism of this synergistic effect.
It is widely accepted that the PI3K/Akt pathway
plays an important role in drug resistance. Overexpression of this
PI3K/Akt pathway has been identified in 5-FU-resistant cell lines,
and the blocking of this pathway could sensitize cancer cells to
5-FU in vitro (37).
However, activation of the PI3K/Akt pathway was revealed to induce
5-FU resistance in cancer cells (38). Furthermore, this pathway has a
major function in cell proliferation and apoptosis.
Apoptosis-related proteins, such as Bax, Bcl-2, and the 5-FU
metabolic enzyme TS, are major downstream effectors of the PI3K/Akt
signaling pathway (39).
The intrinsic apoptotic pathway is largely
controlled by proapoptotic (Bax) and the antiapoptotic (Bcl-2)
proteins (40). In the present
study, kaempferol combined with 5-FU decreased the expression
levels of Bcl-2 and Bax when compared with kaempferol or 5-FU
alone. Thus, based on the results of Hoechst nuclear staining, and
flow cytometric and western blot analyses, the synergistic effect
of 5-FU and kaempferol on apoptosis induction was confirmed. The
formation of the apoptosome causes cleavage of procaspases (caspase
family) which are responsible for activating effector caspases,
such as caspase-3, which is a key protease of the apoptotic
machinery and ultimately resulting in apoptosis (41). Among them, it is unclear how the
synergy of kaempferol and 5-FU affects the caspase family and
ultimately promotes apoptosis. In addition, it was also revealed
that combination of 5-FU and kaempferol could arrest the cell cycle
in the S phase, while the specific regulatory mechanism of
kaempfetol is unknown. All of these issues rquire further
study.
TS, a critical 5-FU-targeted enzyme, participates in
5-FU resistance in cancer patients receiving chemotherapy. TS has
been well accepted as one of the most important targets of 5-FU
resistance (42). A previous study
indicated that TS was dramatically increased following prolonged
exposure to 5-FU (43). Consistent
with previous research, the expression level of TS was increased
along with the decrease in 5-FU sensitivity after 5-FU treatment in
the present study. Additionally, TS levels could be downregulated
by kaempferol; thus, kaempferol may increase 5-FU sensitivity by
upregulating the expression levels of TS, thereby contributing to
the synergistic effects of kaempferol and 5-FU.
In the present study, kaempferol combined with 5-FU
demonstrated synergistic anticancer effects by inducing apoptosis
and altering the expression levels of TS in CRC cells. These
effects may have occurred via attenuation of the activation of
p-AKT and suppression of the PI3K/AKT pathway.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JP designed the present study. QL, LW, SL performed
the experiments. QL, JL and YC analyzed the data. QL and JL and YC
wrote the paper. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
CRC
|
colorectal cancer
|
|
5-FU
|
5-fluorouracil
|
|
TS
|
thymidylate synthase
|
|
MTT
|
3-(4,5-dimethyl-thiazol-2-yl)-2,
5-diphenyltetrazolium bromide
|
|
FBS
|
fetal bovine serum
|
|
FACS
|
fluorescence-activated cell
sorting
|
|
SDS-PAGE
|
sodium dodecyl sulfate-polyacrylamide
minigel
|
|
DMSO
|
dimethyl sulfoxide
|
|
PI3K
|
phosphatidylinositol-3-kinase
|
|
AKT
|
protein kinase B
|
|
CI
|
combination index
|
References
|
1
|
Miller KD, Siegel RL, Lin CC, Mariotto AB,
Kramer JL, Rowland JH, Stein KD, Alteri R and Jemal A: Cancer
treatment and survivorship statistics, 2016. CA Cancer J Clin.
66:271–289. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
O'Dwyer PJ, Eckhardt SG, Haller DG, Tepper
J, Ahnen D, Hamilton S, Benson AB III, Rothenberg M, Petrelli N,
Lenz HJ, et al: Priorities in colorectal cancer research:
Recommendations from the gastrointestinal scientific leadership
council of the coalition of cancer cooperative groups. J Clin
Oncol. 25:2313–2321. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mody K and Bekaii-Saab T: Clinical trials
and progress in metastatic colon cancer. Surg Oncol Clin N Am.
27:349–365. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Montagnani F, Chiriatti A, Turrisi G,
Francini G and Fiorentini G: A systematic review of FOLFOXIRI
chemotherapy for the first-line treatment of metastatic colorectal
cancer: Improved efficacy at the cost of increased toxicity.
Colorectal Dis. 13:846–852. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kanazawa Y, Yamada T, Fujita I, Kakinuma
D, Matsuno K, Arai H, Shimoda T, Ko K, Kato S, Matsutani T, et al:
In vitro chemosensitivity test for gastric cancer specimens
predicts effectiveness of oxaliplatin and 5-fluorouracil.
Anticancer Res. 37:6401–6405. 2017.PubMed/NCBI
|
|
6
|
Pardini B, Kumar R, Naccarati A, Novotny
J, Prasad RB, Forsti A, Hemminki K, Vodicka P and Lorenzo Bermejo
J: 5-Fluorouracil-based chemotherapy for colorectal cancer and
MTHFR/MTRR genotypes. Br J Clin Pharmacol. 72:162–163. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ghoshal K and Jacob ST: An alternative
molecular mechanism of action of 5-fluorouracil, a potent
anticancer drug. Biochem Pharmacol. 53:1569–1575. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Latchman J, Guastella A and Tofthagen C:
5-fluorouracil toxicity and dihydropyrimidine dehydrogenase enzyme:
Implications for practice. Clin J Oncol Nurs. 18:581–585. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Papanastasopoulos P and Stebbing J:
Molecular basis of 5-fluorouracil-related toxicity: Lessons from
clinical practice. Anticancer Res. 34:1531–1535. 2014.PubMed/NCBI
|
|
10
|
Wang W, McLeod HL, Cassidy J and
Collie-Duguid ES: Mechanisms of acquired chemoresistance to
5-fluorouracil and tomudex: Thymidylate synthase dependent and
independent networks. Cancer Chemother Pharmacol. 59:839–845. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sigmond J, Backus HH, Wouters D, Temmink
OH, Jansen G and Peters GJ: Induction of resistance to the
multitargeted antifolate Pemetrexed (ALIMTA) in WiDr human colon
cancer cells is associated with thymidylate synthase
overexpression. Biochem Pharmacol. 66:431–438. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Peters GJ, Backus HH, Freemantle S, van
Triest B, Codacci-Pisanelli G, van der Wilt CL, Smid K, Lunec J,
Calvert AH, Marsh S, et al: Induction of thymidylate synthase as a
5-fluorouracil resistance mechanism. Biochim Biophys Acta.
1587:194–205. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Etienne MC, Chazal M, Laurent-Puig P,
Magné N, Rosty C, Formento JL, Francoual M, Formento P, Renée N,
Chamorey E, et al: Prognostic value of tumoral thymidylate synthase
and p53 in metastatic colorectal cancer patients receiving
fluorouracil-based chemotherapy: Phenotypic and genotypic analyses.
J Clin Oncol. 20:2832–2843. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rahman L, Voeller D, Rahman M, Lipkowitz
S, Allegra C, Barrett JC, Kaye FJ and Zajac-Kaye M: Thymidylate
synthase as an oncogene: A novel role for an essential DNA
synthesis enzyme. Cancer Cell. 5:341–351. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cassinelli G, Zuco V, Gatti L, Lanzi C,
Zaffaroni N, Colombo D and Perego P: Targeting the Akt kinase to
modulate survival, invasiveness and drug resistance of cancer
cells. Curr Med Chem. 20:1923–1945. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Das D, Satapathy SR, Siddharth S, Nayak A
and Kundu CN: NECTIN-4 increased the 5-FU resistance in colon
cancer cells by inducing the PI3K-AKT cascade. Cancer Chemother
Pharmacol. 76:471–479. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Xu J, Zhang S, Wang R, Wu X, Zeng L and Fu
Z: Knockdown of PRDX2 sensitizes colon cancer cells to 5-FU by
suppressing the PI3K/AKT signaling pathway. Biosci Rep. 37(pii):
BSR201604472017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Das R, Bhattacharya K, Samanta SK, Pal BC
and Mandal C: Improved chemosensitivity in cervical cancer to
cisplatin: Synergistic activity of mechanism through STAT3
inhibition. Cancer Lett. 351:81–90. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li CJ, Chu CY, Huang LH, Wang MH, Sheu LF,
Yeh JI and Hsu HY: Synergistic anticancer activity of triptolide
combined with cisplatin enhances apoptosis in gastric cancer in
vitro and in vivo. Cancer Lett. 319:203–213. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vinod BS, Antony J, Nair HH,
Puliyappadamba VT, Saikia M, Narayanan SS, Bevin A and Anto RJ:
Mechanistic evaluation of the signaling events regulating
curcumin-mediated chemosensitization of breast cancer cells to
5-fluorouracil. Cell Death Dis. 4:e5052013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee YJ, Lee S, Ho JN, Byun SS, Hong SK,
Lee SE and Lee E: Synergistic antitumor effect of ginsenoside Rg3
and cisplatin in cisplatin-resistant bladder tumor cell line. Oncol
Rep. 32:1803–1808. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Bi T, Zhu A, Yang X, Qiao H, Tang J, Liu Y
and Lv R: Metformin synergistically enhances antitumor activity of
cisplatin in gallbladder cancer via the PI3K/AKT/ERK pathway.
Cytotechnology. 70:439–448. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li C, Zhao Y, Yang D, Yu Y, Guo H, Zhao Z,
Zhang B and Yin X: Inhibitory effects of kaempferol on the invasion
of human breast carcinoma cells by downregulating the expression
and activity of matrix metalloproteinase-9. Biochem Cell Biol.
93:16–27. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Hung TW, Chen PN, Wu HC, Wu SW, Tsai PY,
Hsieh YS and Chang HR: Kaempferol inhibits the invasion and
migration of renal cancer cells through the downregulation of AKT
and FAK pathways. Int J Med Sci. 14:984–993. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Huang WW, Tsai SC, Peng SF, Lin MW, Chiang
JH, Chiu YJ, Fushiya S, Tseng MT and Yang JS: Kaempferol induces
autophagy through AMPK and AKT signaling molecules and causes G2/M
arrest via downregulation of CDK1/cyclin B in SK-HEP-1 human
hepatic cancer cells. Int J Oncol. 42:2069–2077. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Feng J, Jin Y, Peng J, Wei L, Cai Q, Yan
Z, Lai Z and Lin J: Hedyotis diffusa willd extract
suppresses colorectal cancer growth through multiple cellular
pathways. Oncol Lett. 14:8197–8205. 2017.PubMed/NCBI
|
|
27
|
Chou TC: Synergy determination issues. J
Virol. 76:10577–10578. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Chou TC: Drug combination studies and
their synergy quantification using the Chou-Talalay method. Cancer
Res. 70:440–446. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Q, Lai Z, Yan Z, Peng J, Jin Y, Wei L
and Lin J: Hedyotis diffusa Willd inhibits proliferation and
induces apoptosis of 5-FU resistant colorectal cancer cells by
regulating the PI3K/AKT signaling pathway. Mol Med Rep. 17:358–365.
2018.PubMed/NCBI
|
|
30
|
Li Q, Wang X, Shen A, Zhang Y, Chen Y,
Sferra TJ, Lin J and Peng J: Hedyotis diffusa Willd
overcomes 5-fluorouracil resistance in human colorectal cancer
HCT-8/5-FU cells by downregulating the expression of P-glycoprotein
and ATP-binding casette subfamily G member 2. Exp Ther Med.
10:1845–1850. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Diantini A, Subarnas A, Lestari K, Halimah
E, Susilawati Y, Supriyatna, Julaeha E, Achmad TH, Suradji EW,
Yamazaki C, et al: Kaempferol-3-O-rhamnoside isolated from the
leaves of Schima wallichii Korth. inhibits MCF-7 breast cancer cell
proliferation through activation of the caspase cascade pathway.
Oncol Lett. 3:1069–1072. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Luo H, Rankin GO, Li Z, Depriest L and
Chen YC: Kaempferol induces apoptosis in ovarian cancer cells
through activating p53 in the intrinsic pathway. Food Chem.
128:512–519. 2011. View Article : Google Scholar
|
|
33
|
Jo E, Park SJ, Choi YS, Jeon WK and Kim
BC: Kaempferol suppresses transforming growth factor-β1-induced
epithelial-to-mesenchymal transition and migration of A549 lung
cancer cells by inhibiting Akt1-mediated phosphorylation of Smad3
at threonine-179. Neoplasia. 17:525–537. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tu LY, Bai HH, Cai JY and Deng SP: The
mechanism of kaempferol induced apoptosis and inhibited
proliferation in human cervical cancer SiHa cell: From macro to
nano: From macro to nano. Scanning. 38:644–653. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu LY, Lu HF, Chou YC, Shih YL, Bau DT,
Chen JC, Hsu SC and Chung JG: Kaempferol induces DNA damage and
inhibits DNA repair associated protein expressions in human
promyelocytic leukemia HL-60 cells. Am J Chin Med. 43:365–382.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Song H, Bao J, Wei Y, Chen Y, Mao X, Li J,
Yang Z and Xue Y: Kaempferol inhibits gastric cancer tumor growth:
An in vitro and in vivo study. Oncol Rep. 33:868–874.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ishida K, Ito C, Ohmori Y, Kume K, Sato
KA, Koizumi Y, Konta A, Iwaya T, Nukatsuka M, Kobunai T, et al:
Inhibition of PI3K suppresses propagation of drug-tolerant cancer
cell subpopulations enriched by 5-fluorouracil. Sci Rep.
7:22622017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Kim EJ, Kang GJ, Kang JI, Boo HJ, Hyun JW,
Koh YS, Chang WY, Kim YR, Kwon JM, Maeng YH, et al: Over-activation
of AKT signaling leading to 5-Fluorouracil resistance in
SNU-C5/5-FU cells. Oncotarget. 9:19911–19928. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Nagaraju GP, Alese OB, Landry J, Diaz R
and El-Rayes BF: HSP90 inhibition downregulates thymidylate
synthase and sensitizes colorectal cancer cell lines to the effect
of 5FU-based chemotherapy. Oncotarget. 5:9980–9991. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kang MH and Reynolds CP: Bcl-2 inhibitors:
Targeting mitochondrial apoptotic pathways in cancer therapy. Clin
Cancer Res. 15:1126–1132. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Larsen BD and Sørensen CS: The
caspase-activated DNase: Apoptosis and beyond. FEBS J.
284:1160–1170. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Jiang B, Liu F, Liu Z, Zhang T and Hua D:
B7-H3 increases thymidylate synthase expression via the PI3k-Akt
pathway. Tumor Biol. 37:9465–9472. 2016. View Article : Google Scholar
|
|
43
|
Milczarek M, Rossowska J, Klopotowska D,
Stachowicz M, Kutner A and Wietrzyk J: Tacalcitol increases the
sensitivity of colorectal cancer cells to 5-fluorouracil by
downregulating the thymidylate synthase. J Steroid Biochem Mol
Biol. 190:139–151. 2019. View Article : Google Scholar : PubMed/NCBI
|