Introduction
Ischemia reperfusion injury (IRI) is one of the
primary causes of acute renal injury, and even acute renal failure
(1). It would be of great clinical
benefit to examine the physiological and pathological process of
IRI and to explore the molecular biological mechanisms governing
these processes in order to identify effective protective or
intervention measures for the improved treatment of acute renal
injury.
The present study demonstrated that when renal IRI
occurred, the increased expression of Toll-like receptor (TLR)-2
and TLR4 subsequently activated the downstream NF-κB signaling
pathway, which then produced a large number of inflammatory factors
such as tumor necrosis factor-α (TNF-α), interleukin (IL)-1 and
IL-6. Previous research has demonstrated that the apoptosis of
renal tubule cells is mediated by inflammatory injury (2,3). At
present, the expression of Bcl-2-like 1 (Bcl-x) and baculoviral IAP
repeat containing 8 is typically reported to be downregulated
during IRI, and the expression of Bcl-x, which serves an important
role in cell apoptosis, is usually upregulated (4,5).
Following IR, there is also an upregulation of adhesion factors in
the ischemic area, which leads to an inflammatory cascade reaction,
which is induced by the adhesion factors that mediate the adhesion
of endothelial cells to neutrophils (6). The aim of the present study was to
investigate how to inhibit the inflammatory reactions induced by
IRI, reduce the apoptosis of glomerular cells, promote the
regeneration and repair of renal tubular cells, and inhibit
adhesion factors, which are key issues in the intervention of
IRI.
At present, numerous miRNAs have been reported to
exert pivotal functions in renal IRI (7,8). An
experimental study has demonstrated that miR-21 is upregulated in
renal IRI (7). In a rat model of
renal IRI, researchers observed that miR-21 was involved in
regulating renal IRI by affecting cell adhesion and the structure
of the cytoskeleton (9,10). It has been reported that miR-494 is
highly expressed in a rat model of renal IRI, which can activate
transcription activator 3, thereby promoting apoptosis and further
aggravating renal impairment (11). However, despite the aforementioned
studies, the role of miR-27a in renal IRI has not been completely
elucidated.
In order to further identify the miRNAs regulated
during IRI, the present study investigated miRNA expression in
renal IRI, and demonstrated that the expression of miR-27 is
downregulated in renal IRI. miR-27a can inhibit renal IRI by
binding through the 3′UTR of TLR4, and miR-27 exhibits potential as
a novel therapeutic for the treatment of renal IRI.
Materials and methods
Ethics statement
All animal studies were conducted in accordance
with, and with the approval of the China Medical University Ethics
Committee for Animal Experimentation (Liaoning, China).
Renal IRI model
A total of 40 Sprague-Dawley female rats, 8 weeks of
age and weighing 200–250 g were acquired from Department of Animal
Department of China Medical University (Shenyang, China). The rats
were acclimated to standard experimental conditions: 12 h
light/dark cycle, 22±2°C room temperature, with humidity levels
ranging between 50–65%, and with food and water available ad
libitum. The animals were randomized into 4 groups comprised of
10 rats each, including a Sham group, IRI group, IRI+control group
and an IRI+miR-27a agomir group.
The sham group was anesthetized with 2% isoflurane
and received an abdominal incision. The IRI group, IRI+control
group and an IRI+miR-27a agomir group rats were also anesthetized
following the inhalation of 2% isoflurane. Subsequently, an
abdominal incision was created, and the renal pedicle was clamped
for 45 min with a microaneurysm clamp as previously described
(12). Following the removal of
the clamp, the kidney was inspected for the restoration of blood
flow. Animal wellbeing was monitored every 8 h. The rats were
sacrificed 7 days following the first procedure, through complete
exsanguination via cardiac puncture under anesthesia with 2%
isoflurane delivered via inhalation. The kidneys were harvested,
cut longitudinally and fixed in 10% buffered formalin (4°C,
overnight).
miR-27a control or agomir (Guangzhou RiboBio, Co.,
Ltd., Guangzhou, China) for in vivo use were delivered by
the protocol outlined in a previous study (13). Briefly, miR-27a agomir or control
in 0.1 ml of saline buffer were injected into the tail vein of
animals in the IRI+miR-27a agomir group or the IRI+control group
for 48 h prior to the aforementioned ischemic surgery via the tail
vein at 20–40 µl/sec.
Cell lines
Normal rat kidney epithelial cells (NRK52E cells)
were purchased from the Beijing Concorde Cell Resource Center
(Beijing, China), and were cultured in Dulbecco's modified Eagle's
medium (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) supplemented with 10% fetal bovine serum (Invitrogen; Thermo
Fisher Scientific, Inc.), at 37°C in 5% CO2.
Reverse transcription-quantitative PCR
(RT-qPCR) analysis
Total RNA was extracted from the NRK52E cells and
kidney tissues using the RNA Isolation kit (Tiangen Biotech Co.,
Ltd., Beijing, China) according to the manufacturer's protocol, and
samples were stored at −80°C until subsequent use. The RNA Reverse
Transcription kit (Beyotime Institute of Biotechnology, Shanghai,
China) was used for the RT of RNA into cDNA using the following
conditions: 37°C for 15 min, and followed by 85°C for 5 sec.
RT-qPCR was subsequently performed using a SYBR® Green
Real-time PCR Master Mix (Toyobo Co. Ltd., Osaka, Japan). RT-qPCR
was performed using MX3000P Real-time PCR instrument according to
the manufacturer's protocol (Beyotime Institute of Biotechnology),
using the following conditions: 94°C for 5 min, and followed by 30
cycles of 94°C for 30 sec, 58–61°C for 30 sec. GAPDH and U6 were
used as the internal controls. The expression of miRNAs was
detected using a miRcute miRNA qPCR Detection kit (SYBR Green,
Tiangen Biotech Co., Ltd.) (14,15).
The primer sequences used are listed in Table I. The relative gene expression
levels were calculated using the 2−ΔΔCq method (16). All experiments were performed in
triplicate.
 | Table I.Primer sequences. |
Table I.
Primer sequences.
| Name | Forward primer
(5′-3′) | Reverse primer
(5′-3′) |
|---|
| miR-448 |
ACACTCAUUGCAUAUGUAGG |
AUGGGACAUCCGAGAGTACA |
| miR-651-3p |
ACACTCAAAAGGAAAGUGU |
CUUUUAGGAUACGAGTACAT |
| miR-27a |
ACACTCAUUCACAGUGGC |
GCGGAACUUAGCAGTACAT |
| miR-21 |
ACACTCCAACACCAGUCG |
CAGCCCAUTTGAGAGTACAT |
| miR-24-3p |
ACACTCUGGCUCAGUUCAG |
CUGUUCCUGTTGAGAGTACA |
| miR-494-3p |
ACACTCUGAAACAUACACG |
GAGGUUUCCAGAGTACAT |
| U6 |
CTCGCTTCGGCAGCACA |
ACGCTTCACGAATTTGCGT |
| TLR4 |
TCCTGGCTAGGACTCTGATCAT |
CATGGCATGGCTTACACCACC |
| MyD88 |
GAAACTCCACAGGCGAGCG |
GTTAAGCGCGACCAAGGG |
| NF-κB |
GTGTGGAGGCTGCCTTGCG |
GGCTTTCAAGACTGGAACGGTC |
| TNF-α |
TCTCATTCCTGCTTGTGGC |
GCTGGCCCACTAGTTGGTT |
| IL-1β |
TGAGCACAGAAAGCATGATC |
CATCTGCTGGTACCACCAGTT |
| Bcl-2 |
TTCTTTGAGTTCGGTGGGGTC |
TGCATATTTGTTTGGGGCAGG |
| Bax |
TCCACCAAGAAGCTGAGCGAG |
GTCCAGCCCATGATGGTTCT |
| E-cad |
GGAGGCTCTCCCGTCTTTTG |
CTTTGTCGACCGGTGCAAT |
| Vimentin |
GGACCAGCTAACCAACGAC |
GGTCAAGACGTGCCAGAG |
| GAPDH |
CATCCCTTCTCCCCACACAC |
AGTCCCAGGGCTTTGATTTG |
miRNA microarray analysis
A total of 500 ng RNA, which was extracted as above,
was subjected to Agilent miRNA microarray analysis (Guangzhou
RiboBio, Co., Ltd.). The differences in miRNAs between the
experimental groups were examined for statistical significance
using an unpaired Student's t-test.
Western blot analyses
Tissues and 1×106 cells were lysed with
ice-cold lysis buffer containing NP40 buffer and a proteinase
inhibitor cocktail (Invitrogen; Thermo Fisher Scientific, Inc.).
The lysates were sonicated with an oscillation frequency of 15–25
kHz at 4°C for 10 sec, followed by centrifugation at 12,000 × g for
10 min at 4°C and the supernatants were retained. Total protein
concentration was quantified using a bicinchoninic acid protein
assay kit (Applygen Technologies, Inc., Beijing, China), and
western blot analysis was performed. A total of 30 µg protein/lane
were resolved by 10% SDS-PAGE and transferred onto polyvinylidene
fluoride membranes (Thermo Fisher Scientific, Inc.). Following
blocking with 3% BSA (Beijing Solarbio Science & Technology
Co., Ltd., Beijing, China) in TBS containing 0.1% Tween-20 for 3 h
at room temperature, the membranes were incubated overnight at 4°C
with primary antibody. After several washes, the membranes were
incubated with an appropriate horseradish peroxidase
(HRP)-conjugated secondary antibody for 1 h at room temperature.
The proteins bands were visualized using enhanced chemiluminescence
kits (GE Healthcare, Chicago, IL, USA), and the optical density of
the protein bands was quantified using the ImageJ v1.8.0 software
(National Institutes of Health, Bethesda, MD, USA), using GAPDH as
an internal control. The primary antibodies used included: TLR4
(1:1,000; cat. no. sc-13593), MyD88 (1:1,000; cat. no. sc-74532),
NF-κB (1:1,000; cat. no. sc-166588), TNF-α (1:1,000; cat. no.
sc-52746), IL-1β (1:1,000; cat. no. sc-52012), Bcl-2 (1:1,000; cat.
no. sc-56015), Bax (1:1,000; cat. no. sc-20067), E-cadherin (E-cad,
1:1,000; cat. no. sc-71009), Vimentin (vim, 1:1,000; cat. no.
sc-80975) and GAPDH (1:1,000; cat. no. sc-51907), all purchased
from Santa Cruz Biotechnology Inc. (Dallas, TX, USA). The secondary
antibodies used were HRP-conjugated anti-rabbit (1:1,000; cat. no.
sc-2370) and anti-mouse (1:1,000; cat. no. sc-516102, Santa Cruz
Biotechnology, Inc.) were used.
miRDB analysis
In order to analyze the target binding sites of
microRNA-27a and TLR4, miRDB software (http://mirdb.org/) was used.
Dual luciferase reporter assay
The TLR4 wild-type and TLR4 deletion (TLR DEL, where
the binding sites with miR-27a were removed) 3′-untranslated region
(3′-UTR) were PCR amplified and cloned into the pMIR-REPORT™ vector
(Ambion; Thermo Fisher Scientific, Inc.). A total of 100,000 NRK52E
cells were seeded in 24-well plates, and the experiment was
performed when cells reached 80% confluency. The plasmids were
transfected using 2.5 µl of HiGene (Beyotime Institute of
Biotechnology). Control, miR-27a and miR-27a antisense (AS;
Guangzhou RiboBio, Co., Ltd.) were simultaneously transfected.
After 24 h, passive lysis buffer (1×) was used to lyse the
transfected cells, and 20 µl supernatant was used to measure for
luciferase activity using a Dual-Luciferase Reporter Assay System
(BioTek Instruments, Inc., Winooski, VT, USA). Renilla
luciferase activity was used to normalize reporter activity.
Experiments were performed in triplicate.
MTT assay
MTT assay (Beijing Solarbio Science and Technology
Co., Ltd.) was used to measure cell viability. Cells were seeded at
a density of 1×103 cells per well in 96-well plates, and
transfected with 30 nM miR-27a mimic/control
(CGCCUUGAAUCGGUGACACUU/UUCUCCGAACGUGUCACGU) and miR-27a
inhibitor/control (GCGGAACUUAGCCACUGUGUGAA/UUCUCCGAACGUGUCACGU)
(Guangzhou RiboBio, Co., Ltd.) using HiGene transfection reagent
(Beyotime Institute of Biotechnology). Each transfection condition
was carried out in triplicate. At the end of the each time point,
50 µl of 5 mg/ml MTT was added to each well, and incubated at 37°C
for 4 h. A total of 500 µl of DMSO was added in to each well and
swirled gently to stop the reaction. The plate was left in the dark
for another 4 h at room temperature. Optical density at 490 nm was
measured by a microplate reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Apoptosis assay
After 24 h from transfection with miR-27a
mimic/control and miR-27a inhibitor/control, cells were
subsequently stained with Annexin V-fluorescein
isothiocyanate/propidium iodide (Shanghai Shenggong Biology
Engineering Technology Service, Ltd., Shanghai, China) for 20 min
at room temperature in the dark. Cell apoptosis was then detected
using a flow cytometry (BD FACSCalibur; BD Biosciences, San Jose,
CA, USA) at 488 nm. Data were analyzed using FlowJo version 10
(FlowJo LLC, Ashland, OR, USA). Experiments were carried out in
triplicate.
Immunohistochemical staining
Kidney samples were fixed in 10% neutral buffered
formalin (4°C, overnight), embedded in paraffin, sliced into
sections 5 µm thick. The paraffin sections were subsequently
de-waxed in water, and incubated with 3% H2O2
for 10 min at room temperature. The sections were incubated with 3%
goat serum (cat. no. 16210064; Thermo Fisher Scientific, Inc.) at
room temperature for 10 min, and subsequently incubated with a
primary antibody against TLR4 (1:100, cat. no. sc-13593, Santa Cruz
Biotechnology, Inc.) 4°C overnight. An avidin-biotin-HRP complex
immunodetection kit (Shanghai Shenggong Biology Engineering
Technology Service, Ltd.) was used to detect the reaction according
to the manufacturer's instructions, and the samples were examined
by light microscopy at ×400 magnification (Leica DM2500M; Leica
Microsystems GmbH, Wetzlar, Germany).
ELISA
Experiments were performed according to the protocol
outlined in the TNF-α (cat. no. ab236712; Abcam, Cambridge, UK) and
IL-1β ELISA kits (cat. no. ab100768; Abcam). Tissues and cells were
incubated at 37°C for 30 min. According to the manufacturer's
protocol: A total of 100 µl plasma or supernatant was added onto
the TNF-α/IL-1β antibody-coated plate, and incubated at 25°C for 2
h. After adding the biotin-conjugated detecting TNF-α/IL-1 antibody
and incubating for 2 h, streptavidin-HRP was added, and 3,3′-5,5′
tetramethylbenzidin was used for development. The optical density
value was measured at 450 nm by Multiskan spectrum (Thermo Fisher
Scientific, Inc.). Experiments were performed in triplicate.
Detection of blood urea nitrogen (BUN)
and serum creatinine (Scr)
The blood of rats in each group was extracted via
the abdominal aorta and centrifuged 37°C for 5 min at 3,000 × g.
The BUN and Scr levels of mice in each group were measured using an
automatic biochemical analyzer.
Statistical analysis
All data were analyzed with SPSS 17.0 software
(SPSS, Inc., Chicago, IL, USA). Data are presented as the mean ±
standard error of the mean. P<0.05 was considered to indicate a
statistically significant difference. Statistical analyses were
performed one-way analysis of variance when there were >2
groups, followed by Tukey's post hoc test for multiple comparisons.
A Student's t-test was used to compare differences between 2
groups. Actuarial survival rates were calculated using the
Kaplan-Meier method, and differences in survival in univariate
comparisons were compared using the log-rank test.
Results
Changes in miRNA levels in renal
IRI
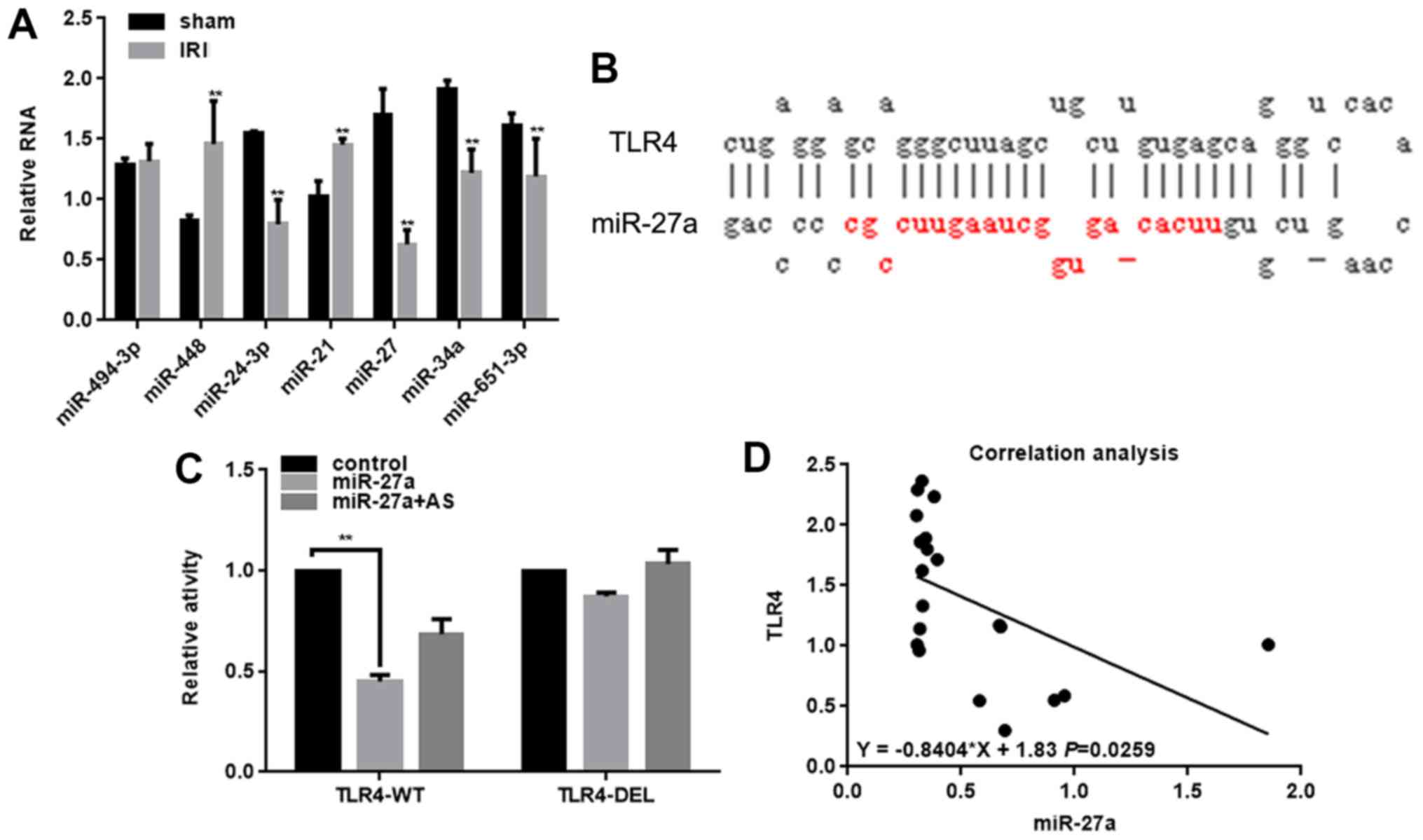
The miRNA microarray revealed that various miRNA
changes occurred following renal IRI when compared with the sham
group (Table II).
 | Table II.Differential miRNA expression in
IRI. |
Table II.
Differential miRNA expression in
IRI.
| miRNA | P-values | Fold change
(sham/IRI) | Regulation
trend |
|---|
| miR-494-3p | 0.8470 | – | – |
| miR-448 | 0.0002 | 19.875 | Up |
| miR-24-3p | 0.0012 | 20.221 | Down |
| miR-21 | 0.0010 |
9.283 | Up |
| miR-27a | 0.0001 | 35.298 | Down |
| miR-34a | 0.0010 |
7.192 | Down |
| miR-651-3p | 0.0001 |
18.28 | Down |
The expressions of these miRNAs in the IRI and sham
groups were detected by RT-qPCR (Fig.
1A). The results demonstrated that the expression of miR-27a
was significantly decreased in the IRI group. In addition, the
miRDB software indicated that miR-27a could bind to the 3′UTR of
TLR4 (Fig. 1B), which serves an
important role in renal IRI (17).
The luciferase reporter gene assay also confirmed that miR-27a
could inhibit the activity of TLR4 (Fig. 1C). However, when the miR-27a and
TLR4 binding sites were mutated, the inhibitory effect of miR-27a
on TLR4 was attenuated (Fig. 1C).
In addition, the co-transfection of miR-27a and miR-27a AS did not
affect the activity of TLR4 (Fig.
1C). miR-27a also inhibits the activity of TLR4, by binding to
the 3′UTR region of TLR4, as indicated by the negative correlation
between the expression of TLR4 and miR-27a in renal IRI (Fig. 1D). Taken together, the results of
the present study suggest that miR-27a serves an important role in
renal IRI by regulating TLR4.
Expression of TLR4 and its downstream
pathway in renal IRI
Immunohistochemical staining revealed that the
expression of TLR4 was higher in renal tissue following IRI
(Fig. 2A). The subsequent
detection of BUN and Scr levels demonstrated that their expressions
were upregulated following IRI (Fig.
2B). Western blot analysis and RT-qPCR also demonstrated that
TLR4 and its downstream signaling pathways were altered in renal
IRI (Fig. 2C and D). Among them,
the expressions of TLR4, MyD88, NF-κB, TNF-α, IL-1β, Bax and vim
were upregulated, and the expressions of Bcl-2 and E-cad were
downregulated. This phenomenon suggests that inflammation,
apoptosis and the enhancement of cell adhesion occurred in the
renal IRI tissues. An ELISA also demonstrated that there was an
upregulation of TNF-α and IL-1β in the IRI group, which
demonstrated that IRI can lead to inflammation (Fig. 2E).
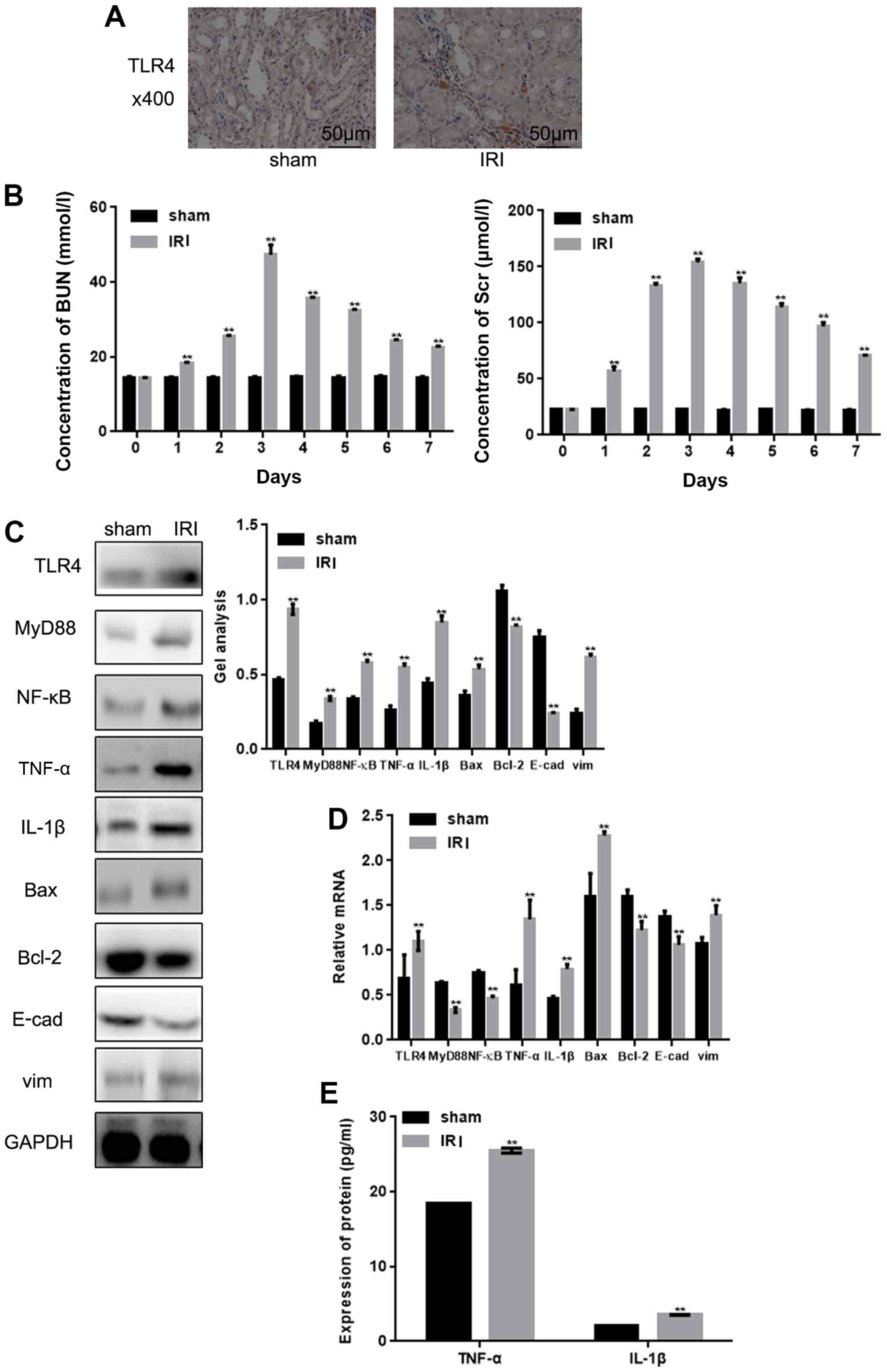 | Figure 2.Expression of TLR4 and its downstream
pathway in renal IRI. (A) TLR4 expression in renal tissues was
detected by immunohistochemical staining (magnification, ×400;
scale bars, 50 µm) (B) The expression of BUN and Scr were detected
by automatic biochemical analyzer. (C and D) Levels of TLR4, MyD88,
NF-κB, TNF-α, IL-1β, Bax, vim, Bcl-2 and E-cad in tissues were
detected with (C) western blotting and (D) reverse
transcription-quantitative PCR. (E) Levels of TNF-α and IL-1β in
tissues were detected by ELISA. Data are presented as the mean ±
standard error of the mean. **P<0.01 vs. sham group. IRI,
ischemia reperfusion injury; TLR4, Toll-like receptor 4; E-cad,
E-cadherin; TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β;
BUN, blood urea nitrogen; Scr, serum creatinine. |
Association between miR-27a and TLR4
in NRK52E cells
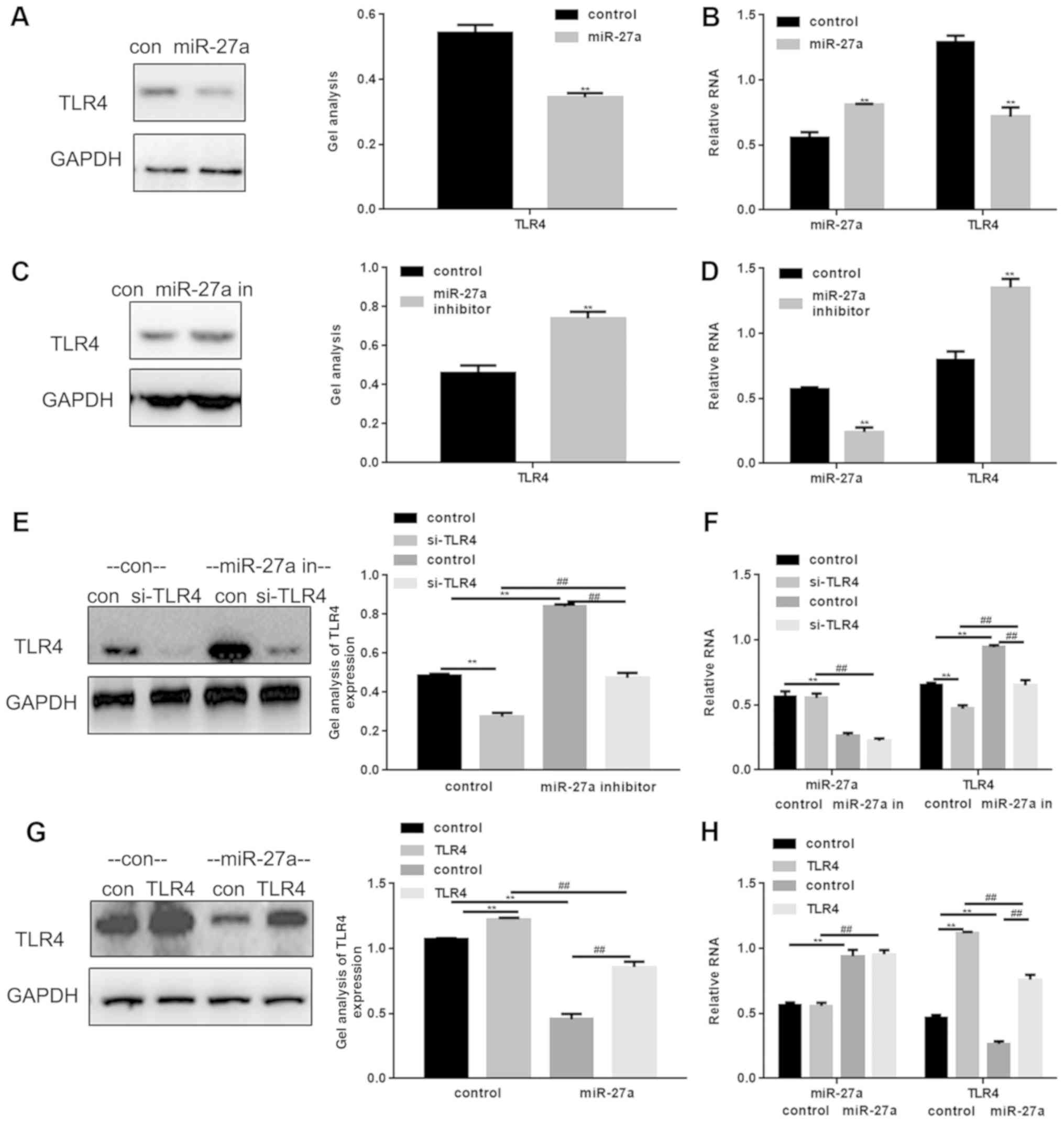
Following transfection with an miR-27a mimic or
miR-27a inhibitor into NRK52E cells, western blotting and RT-qPCR
were performed in order to detect the effect of miR-27a on TLR4
(Fig. 3). Transfection efficiency
was confirmed by RT-qPCR and western blotting (Fig. 3B and D-H). The results demonstrated
that the expression of TLR4 was markedly downregulated by miR-27a
(Fig. 3A and B) and upregulated
with miR-27a inhibitor (Fig. 3C and
D). The present study also observed that treatment with the
miR-27a inhibitor maintained the downregulated expression of TLR4
induced by si-TLR4 (Fig. 3E and
F). miR-27a was also observed to reduce the degree of the TLR4
expression upregulation induced by TLR4 transfection (Fig. 3G and H).
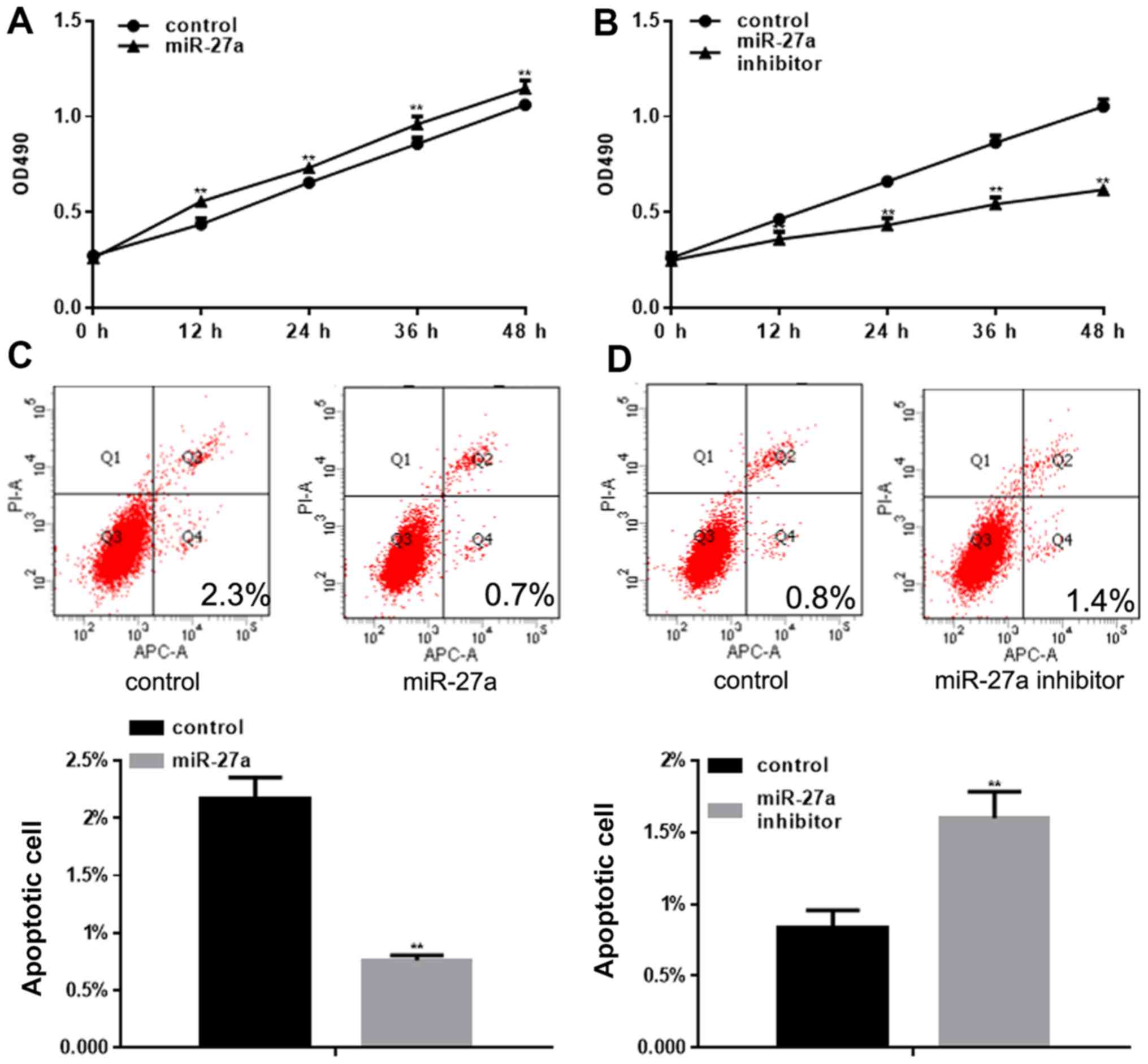
miR-27a promotes the growth of NRK52E
cells
An MTT assay was used to detect the proliferation of
NRK52E cells following transfection with miR-27a or the miR-27a
inhibitor. miR-27a was observed to promote the proliferation of
cells; however, the miR-27a inhibitor significantly inhibited the
proliferation of cells (Fig. 4A and
B). The present study also demonstrated that miR-27a could
inhibit cell apoptosis, and the miR-27a inhibitor significantly
promoted cell apoptosis (Fig. 4C and
D).
miR-27a inhibits renal IRI by
inhibiting TLR4
The present study increased the expression of
miR-27a in rats with renal IRI, and following this, the expression
of TLR4 was partially decreased (Fig.
5A). The subsequent detection of BUN and Scr demonstrated that
their expressions were downregulated following the overexpression
of miR-27a (Fig. 5B). Western
blotting and RT-qPCR also demonstrated that the overexpression of
miR-27a could downregulate the expressions of TLR4, MyD88, NF-κB,
TNF-α, IL-1β, Bax and vim, and upregulate the expressions of Bcl-2
and E-cad (Fig. 5C and D).
Furthermore, the ELISA also observed that the expressions of TNF-α
and IL-1β were significantly decreased by miR-27a (Fig. 5E).
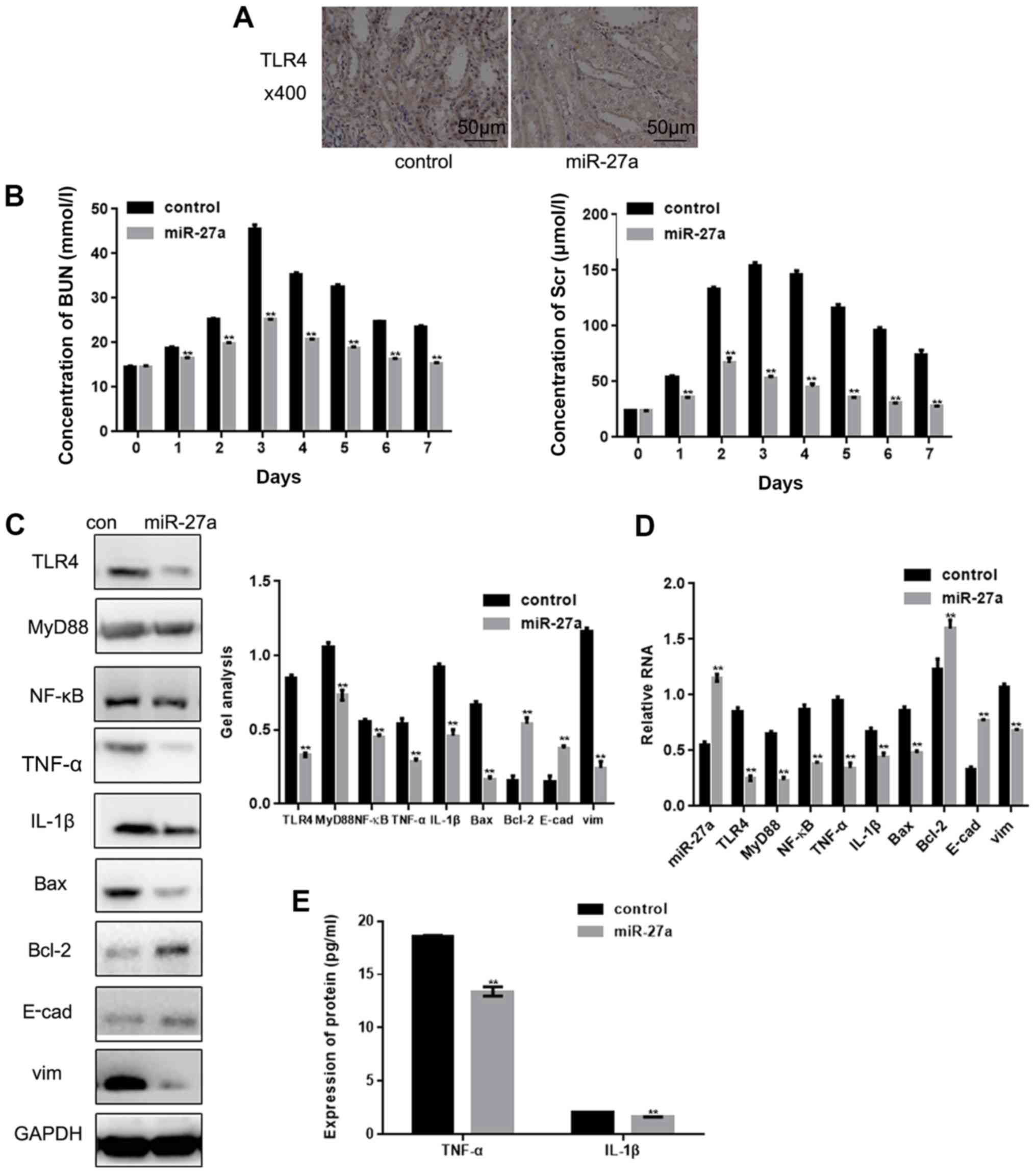 | Figure 5.miR-27a inhibits renal IRI by
inhibiting TLR4. (A) Expression of TLR4 in tissues was detected by
immunohistochemical staining (magnification, ×400; scale bars, 50
µm). (B) The expressions of BUN and Scr were detected by an
automatic biochemical analyzer. **P<0.01 vs. control group at
each time point. (C and D) The levels of miR-27a, TLR4, MyD88,
NF-κB, TNF-α, IL-1β, Bax, vim, Bcl-2 and E-cad in tissues were
detected by western blotting and reverse transcription-quantitative
PCR. (E) The levels of TNF-α and IL-1β in tissues were detected by
ELISA. Data are presented as the mean ± standard error of the mean.
**P<0.01 vs. control group. miR, microRNA; IRI, ischemia
reperfusion injury; TLR4, Toll-like receptor 4; E-cad, E-cadherin;
TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; BUN, blood
urea nitrogen; Scr, serum creatinine. |
Discussion
IRI is commonly observed in a clinical setting and
ailments such as shock, myocardial infarction and vascular
occlusion during surgery may lead to IRI (18).
A recent study has demonstrated that specific Dicer
enzymes in the epithelial cells of the proximal renal tubular
epithelial cells can mitigate, and to an extent cause, renal IRI by
regulating changes in the expression of various miRNAs (19). miR-126 and miR-24 are also
considered to be important miRNAs in regulating renal IRI (7,19–21).
The results of the present study indicated that miR-27a could
improve the pathological damage of the renal tubular epithelium by
reducing apoptosis, and also improved the repair of renal function
by alleviating inflammatory reactions. A previous study
demonstrated that the overexpression of miR-27 in a rat
subarachnoid IR model reduced the expression of Toll-like receptor
adaptor molecule-2 (22). It has
also been reported that the sustained intrathecal injection of an
miR-27a agomir prior to ischemia can significantly alleviate
ischemic symptoms (22). This
suggests that miR-27a may be particularly important for the
pathophysiology of IRI models. In the present study, a significant
downregulation of miR-27a was observed following renal IRI using
microarray technology.
The present study also observed a negative
correlation between miR-27a and the expression of TLR4 in a renal
IRI model, and that miR-27a and the 3′UTR of TLR4 have target
binding sites. Taken together, these results demonstrate that
miR-27a can inhibit the activity of TLR4.
In the early stage of IR, TLR4 recognizes the
activation of endogenous ligands released by injured kidney cells,
and subsequently activates downstream signaling pathways through
the binding protein MyD88 (21).
NF-κB is an important signal transduction pathway downstream of
TLR4, which controls the transcription of the majority of apoptotic
and immunoinflammatory genes (23). In addition, apoptosis can be
regulated by affecting the expression of Bax and Bcl-2 (24), and TLR4 also exerts specific
regulatory effects on E-cad and vim, which in turn affects cell
adhesion (25). The present study
constructed an IRI rat model, and observed that the expression of
TLR4 was significantly upregulated. The downstream signaling
molecules MyD88 and NF-κB of TLR4 were activated, the secretion of
pro-inflammatory cytokines and chemokines increased, and apoptosis
levels also increased, suggesting that the TLR4 signaling pathway
was overactivated in the IRI model.
A previous study confirmed that when IR occurs, cell
proliferation ability decreases and apoptosis increases (26). The present study demonstrated that
miR-27a can promote cell proliferation and inhibit cell apoptosis,
which may provide an alternative explanation for the protective
effect of miRNA-27a in the kidneys.
In the rat model of renal IRI, the present study
observed that the expressions of TLR4, MyD88, NF-κB TNF-α, IL-1β,
Bax and vim were upregulated, whereas the expressions of Bcl-2 and
E-cad were downregulated, and the changes in the expressions of
these proteins were partially restored following the overexpression
of miR-27a. This suggests that miR-27a can inhibit the inflammatory
response, cell adhesion enhancement and apoptosis by regulating the
expression of TLR4 in vivo.
In conclusion, the present study demonstrated that
miR-27a is downregulated in renal IRI, and that miR-27a may
partially influence the process of IRI by targeting TLR4.
Furthermore, miR-27a may serve as a potential therapeutic target
for the treatment of IRI in the future.
Acknowledgements
Not applicable.
Funding
The current study was funded by the National Natural
Fund (grant no. 81601289).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YW performed the majority of the experiments, and
was one of the contributors in writing the manuscript. DW conducted
the miRNA analysis, and ZJ designed the experiment and wrote the
manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to
participate
The current study followed internationally
recognized guidelines on animal welfare, and obtained approval of
China Medical University Ethics Committee for Animal
Experimentation (Liaoning, China; no. CMU201603). The care and use
of laboratory animals was performed in accordance to the National
Institutes of Health guide. All animal studies complied with the
ARRIVE guidelines and the AVMA euthanasia guidelines of 2013.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Li W, Ning JZ, Cheng F, Yu WM, Rao T, Ruan
Y, Yuan R, Zhang XB, Du Y and Xiao CC: MALAT1 promotes cell
apoptosis and suppresses cell proliferation in testicular
ischemia-reperfusion injury by sponging miR-214 to modulate TRPV4
expression. Cell Physiol Biochem. 46:802–814. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Xie T, Li K, Gong X, Jiang R, Huang W,
Chen X, Tie H, Zhou Q, Wu S, Wan J and Wang B: Paeoniflorin
protects against liver ischemia/reperfusion injury in mice via
inhibiting HMGB1-TLR4 signaling pathway. Phytother Res.
32:2247–2255. 2018. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Cheng J, Zhu P, Qin H, Li X, Yu H, Yu H
and Peng X: Dexmedetomidine attenuates cerebral
ischemia/reperfusion injury in neonatal rats by inhibiting TLR4
signaling. J Int Med Res. 46:2925–2932. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen H, Song Z, Ying S, Yang X, Wu W, Tan
Q, Ju X, Wu W, Zhang X, Qu J and Wang Y: Myeloid differentiation
protein 2 induced retinal ischemia reperfusion injury via
upregulation of ROS through a TLR4-NOX4 pathway. Toxicol Lett.
282:109–120. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Chen J, Yang C, Xu X, Yang Y and Xu B: The
effect of focal cerebral ischemia-reperfusion injury on TLR4 and
NF-κB signaling pathway. Exp Ther Med. 15:897–903. 2018.PubMed/NCBI
|
|
6
|
He Q, Zhao X, Bi S and Cao Y: Pretreatment
with erythropoietin attenuates lung ischemia/reperfusion injury via
toll-like receptor-4/nuclear factor-κB (TLR4/NF-κB) pathway. Med
Sci Monit. 24:1251–1257. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Di YF, Li DC, Shen YQ, Wang CL, Zhang DY,
Shang AQ and Hu T: miR-146b protects cardiomyocytes injury in
myocardial ischemia/reperfusion by targeting Smad4. Am J Transl
Res. 9:656–663. 2017.PubMed/NCBI
|
|
8
|
Fu BC, Lang JL, Zhang DY, Sun L, Chen W,
Liu W, Liu KY, Ma CY, Jiang SL, Li RK and Tian H: Suppression of
miR-34a expression in the myocardium protects against
ischemia-reperfusion injury through SIRT1 protective pathway. Stem
Cells Dev. 26:1270–1282. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jia P, Teng J, Zou J, Fang Y, Zhang X,
Bosnjak ZJ, Liang M and Ding X: miR-21 contributes to
xenon-conferred amelioration of renal ischemia-reperfusion injury
in mice. Anesthesiology. 119:621–630. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Song N, Zhang T, Xu X, Lu Z, Yu X, Fang Y,
Hu J, Jia P, Teng J and Ding X: miR-21 protects against
ischemia/reperfusion-induced acute kidney injury by preventing
epithelial cell apoptosis and inhibiting dendritic cell maturation.
Front Physiol. 9:7902018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Su S, Luo, Liu X, Liu J, Peng F, Fang C
and Li B: miR-494 up-regulates the PI3K/Akt pathway via targetting
PTEN and attenuates hepatic ischemia/reperfusion injury in a rat
model. Biosci Rep. 37(pii): BSR20170798. 2017.
|
|
12
|
Martin-Sole O, Rodo J, Garcia-Aparicio L,
Blanch J, Cusi V and Albert A: Effects of platelet-rich plasma
(PRP) on a model of renal ischemia-reperfusion in rats. PLoS One.
11:e01607032016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Huang X, Gao Y, Qin J and Lu S: The role
of miR-34a in the hepatoprotective effect of hydrogen sulfide on
ischemia/reperfusion injury in young and old rats. PLoS One.
9:e1133052014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Feng J, Wang K, Liu X, Chen S and Chen J:
The quantification of tomato microRNAs response to viral infection
by stem-loop real-time RT-PCR. Gene. 437:14–21. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen C, Ridzon DA, Broomer AJ, Zhou Z, Lee
DH, Nguyen JT, Barbisin M, Xu NL, Mahuvakar VR, Andersen MR, et al:
Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic
Acids Res. 33:e1792005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hua F, Ma J, Ha T, Kelley JL, Kao RL,
Schweitzer JB, Kalbfleisch JH, Williams DL and Li C: Differential
roles of TLR2 and TLR4 in acute focal cerebral ischemia/reperfusion
injury in mice. Brain Res. 1262:100–108. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li D, Wang J, Hou J, Fu J, Liu J and Lin
R: Salvianolic acid B induced upregulation of miR-30a protects
cardiac myocytes from ischemia/reperfusion injury. BMC Complement
Altern Med. 16:3362016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Diaz I, Calderon-Sanchez E, Toro RD,
Ávila-Médina J, de Rojas-de Pedro ES, Domínguez-Rodríguez A, Rosado
JA, Hmadcha A, Ordóñez A and Smani T: miR-125a, miR-139 and miR-324
contribute to Urocortin protection against myocardial
ischemia-reperfusion injury. Sci Rep. 7:88982017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dai Y, Jia P, Fang Y, Liu H, Jiao X, He JC
and Ding X: miR-146a is essential for lipopolysaccharide
(LPS)-induced cross-tolerance against kidney ischemia/reperfusion
injury in mice. Sci Rep. 6:270912016. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xie YL, Zhang B and Jing L: miR-125b
blocks bax/cytochrome C/caspase-3 apoptotic signaling pathway in
rat models of cerebral ischemia-reperfusion injury by targeting
p53. Neurol Res. 40:828–837. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Li XQ, Lv HW, Wang ZL, Tan WF, Fang B and
Ma H: miR-27a ameliorates inflammatory damage to the blood-spinal
cord barrier after spinal cord ischemia: Reperfusion injury in rats
by downregulating TICAM-2 of the TLR4 signaling pathway. J
Neuroinflammation. 12:252015. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee JW, Kim SC, Ko YS, Lee HY, Cho E, Kim
MG, Jo SK, Cho WY and Kim HK: Renoprotective effect of paricalcitol
via a modulation of the TLR4-NF-κB pathway in
ischemia/reperfusion-induced acute kidney injury. Biochem Biophys
Res Commun. 444:121–127. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Luo SY, Li R, Le ZY, Li QL and Chen ZW:
Anfibatide protects against rat cerebral ischemia/reperfusion
injury via TLR4/JNK/caspase-3 pathway. Eur J Pharmacol.
807:127–137. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Han Y, Liao X, Gao Z, Yang S, Chen C, Liu
Y, Wang WE, Wu G, Chen X, Jose PA, et al: Cardiac troponin I
exacerbates myocardial ischemia/reperfusion injury by inducing the
adhesion of monocytes to vascular endothelial cells via
TLR4/NF-κB-dependent pathway. Clin Sci (Lond). 130:2279–2293. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu X, Zhang L, Qin H, Han X, Zhang Z,
Zhang Z, Qin SY and Niu J: Inhibition of TRA F3 expression
alleviates cardiac ischemia reperfusion (IR) injury: A mechanism
involving in apoptosis, inflammation and oxid ative stress. Biochem
Biophys Res Commun. 506:298–305. 2018. View Article : Google Scholar : PubMed/NCBI
|