Introduction
Camptothecin, a pentacyclic quinoline alkaloid, was
first isolated by Wall et al (1) in 1966 from the bark of Camptotheca
acuminata Decne, a plant native to Southeast China.
Camptothecin has been reported to exhibit antitumor, antifungal,
antiviral and insecticidal activities (2,3). As
anticancer drugs, camptothecins have attracted increasing interest
and attention from both the academic community and the
pharmaceutical industry (4).
Additionally, camptothecins have been used as pesticides in
agriculture due to their insecticidal activity.
Hsiang et al (5) reported that camptothecin could
selectively block topoisomerase I in complex with DNA. Since the
first identification of camptothecin, multiple camptothecin
derivatives have been synthesized (6). In total, three water-soluble
derivatives of its analogues have gained approval for the treatment
of colon, breast, ovarian and small cell lung cancers. The three
analogues are irinotecan (NSC no. 616348), sold under the brand
name Camptosar® by Pharmacia & Upjohn (7), topotecan (NSC no. 609699), marketed
under the name Hycamtin® by SmithKline Beecham (8) and belotecan, sold under the brand
name Camtobell® by Chong Kun Dang Pharmaceutical
Corporation (9). Various other
analogues of camptothecin are under various stages of clinical
development (10). However,
topotecan, irinotecan and belotecan, drugs used to manage and treat
cancer that possess antineoplastic activity, are enzymatically
degraded to camptothecin and its metabolites, with toxic side
effects (11). Therefore, it is
important to detect the degradation products of camptothecins in
human plasma.
In agriculture, camptothecin and camptothecin
analogues have been reported to have a broad insecticidal activity
spectrum, and its action on Trichoplusia ni and
Spodoptera exigua induces alterations in the midgut, loss of
the single layer of epithelial cells and disruption of the
peritrophic membrane (12). Liu
et al (13,14) synthesized and tested the
insecticidal activity of numerous camptothecin derivatives with a
series of modifications at different sites. The use of
camptothecins as field pesticides may require the monitoring of
camptothecin residues, its metabolites and its degradation products
in plants, water and soil.
Immunoassay is a technique used to analyze a
particular substance with an antibody or a mixture of antibodies as
the main analytical reagent. Immunoassay techniques provide
qualitative and quantitative methods for analyzing a substance.
Since they are simple, rapid and cost-effective, immunoassays have
become widely used analysis systems, particularly in clinical
settings and in the detection of pesticides (15). Camptothecin and its derivatives
have emerged as a promising group of chemotherapeutic agents due to
their biological activities in clinical settings and in agriculture
(16); however, as the number of
drugs based on camptothecin analogues have increased,
camptothecins, along with their derivatives, metabolites and
degradation products, are increasingly found in humans, plants,
animals and the environment. Therefore, to develop an ELISA
suitable for the quantification of camptothecins in human plasma,
plants and other matrices is required. The aim of the present study
was to develop an ELISA selective for camptothecins using
monoclonal antibodies (MAbs).
Materials and methods
Reagents and instruments
All reagents and solvents used in the present study
were of analytical grade. Camptothecin was provided by Professor
Liu Yingqian (Lanzhuo University). N-hydroxysuccinimide (NHS),
N,N-dicyclohexylcarbodiimide (DCC), dimethyl formamide (DMF),
succinic anhydride, 1-(3-dimethylaminopropyl)-3-ethyl carbon
diimide hydrochloride (EDCI), toluene, potassium dichromate,
anhydrous pyridine, dimethylaminopyridine (DMAP),
isobutylchlorocarbonate, tri-n-butylamine, tetramethylbenzidine
(TMB) and DMSO were purchased from Sangon Biotech Co., Ltd. BSA,
keyhole limpet hemocyanin (KLH), ovalbumin (OVA), Freund's complete
and incomplete adjuvants, and Tween-20 were purchased from
Sigma-Aldrich (Merck KGaA). Goat anti-mouse immunoglobulin G
(IgG)-horseradish peroxidase (HRP) antibody (cat. no. 31432) was
obtained Thermo Fisher Scientific, Inc. Sp2/0 murine myeloma cells
was obtained from the American Type Culture Collection. RPMI 1640
medium (cat. no. 11875) was obtained from Thermo Fisher Scientific,
Inc. The SBA Clonotyping™ System/HRP kit (cat. no. 5300-05) was
obtained from SouthernBiotech.
The instruments used were the following: UV-visible
(vis) spectrometer (DU-640; Beckman Coulter, Inc.), mass
spectrometer (HP-5988; Agilent Technologies, Inc.), nuclear
magnetic resonance (NMR) spectrometer (Mercury 300 BB; Varian
Medical Systems), 96-well polystyrene microplates (MaxiSorp; Thermo
Fisher Scientific, Inc.) and Multiskan EX version 1.0 (Thermo
Fisher Scientific, Inc.). Electrospray ionization mass spectrometry
was conducted as previously described, and 1HNMR and
13CNMR spectra were recorded at 400 MHz and 100 MHz as
previously described using tetramethylsilane as the reference
(17,18).
In addition, PBS (10 mM, Ph 7.4; NaCl 8.0 g, KCl 0.2
g, Na2HPO4 2.9 g,
KH2PO4 0.2 g, H2O 1 l),
carbonate-buffered saline (CBS; 50 mM, pH 9.6;
Na2CO3 1.59 g, NaHCO3 2.93 g,
H2O 1 l) and citrate-PBS (CPBS; 50 mM, pH 5.5;
C6H7O8 21 g,
Na2HPO4 71.6 g, H2O 1 l) were
used.
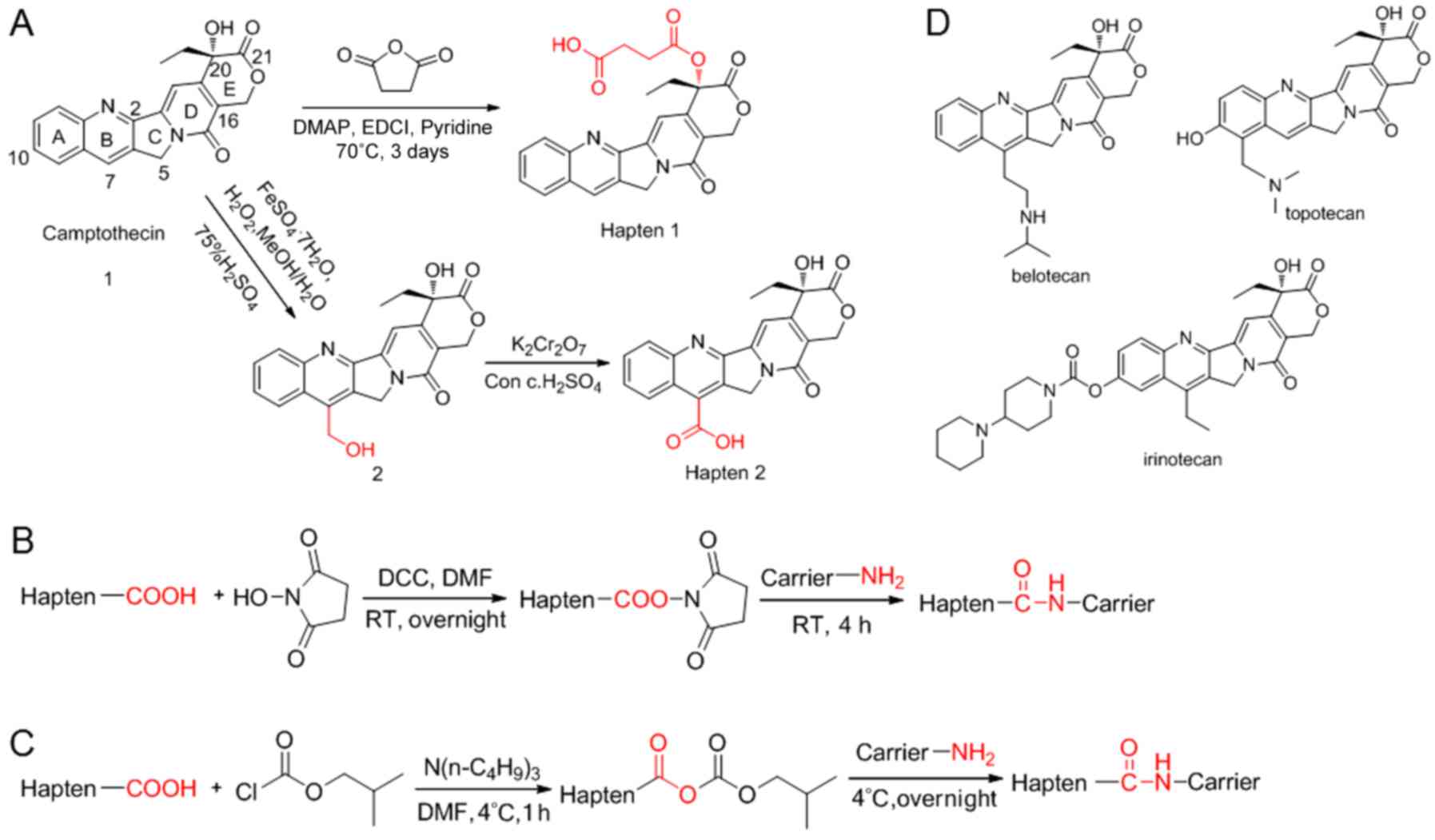
20(s)-O-succinyl-camptothecin (hapten
1)
The structure of camptothecin and the synthetic
route of ‘hapten 1’ (17) are
presented in Fig. 1A. Camptothecin
(0.352 g, 1.01 mmol) was dissolved in anhydrous pyridine (50 ml).
Then, DMAP (0.122 g, 1.00 mmol), EDCI (0.767 g, 4.03 mmol) and
succinic anhydride (0.201 g, 2.01 mmol) were added. The mixture was
stirred at 70°C for 3 days. The solution was then cooled to room
temperature, diluted with toluene and evaporated under vacuum.
Chloroform was added and the organic phase was washed with 0.1 M
HCl and saturated NaCl. The solution was then dried using anhydrous
MgSO4. The solvent was removed, and the crude product
was purified by silica gel chromatography (eluent,
CHCl3:CH3OH, 96:4) and recrystallised from
methanol to obtain the pale-yellow solid compound ‘hapten 1’
(Fig. 1A) with a melting point of
232.1°C (0.235 g, 51.9%).
The results of the 1H-NMR and
13C NMR [500 MHz; DMSO-d6; δ parts per million (ppm)]
were as follows: 0.91 (3H, t, J=7.0 Hz, C19-H), 2.16 (2H, t, J=7.4
Hz, C23-H), 2.50 (2H, t, J=7.4 Hz, C24-H,), 2.69–2.83 (2H, m,
C18-H), 5.29 (2H, s, C5-H), 5.48 (2H, s, C17-H), 7.12 (1H, s,
C14-H), 7.71 (1H, t, J=7.0 Hz, C10-H), 7.86 (1H, t, J=7.0 Hz,
C11-H), 8.12–8.19 (2H, dd, J=8.0 Hz, J=8.5 Hz, C9-H, C12-H), 8.68
(1H, s, C7-H), 12.14 (1H, s, -COOH); and 8.05 (C19), 29.01, 29.16
(C23, C24), 30.97 (C18), 50.73 (C5), 66.87 (C17), 76.45 (C20),
95.61 (C14), 119.45 (C16), 128.20, 128.53, 129.02, 129.58, 130.33,
130.96, 132.08, 145.83, 146.46, 148.47, 152.93, 154.21 (C2, C3,
C6-C15), 157.05 (C16a), 167.68 (C21), 171.83, 173.53 (C22, C25) [MS
m/z 449 (M+H)+].
7-Hydroxymethyl camptothecin
A solution of 75% H2SO4 (75
ml) was added dropwise to a suspension containing 3.00 g (8.6 mmol)
of camptothecin (Fig. 1A) mixed
with MeOH (90 ml) and H2O (75 ml). Subsequently,
FeSO4.7H2O (2.4 g, 8.6 mmol) was
added. To this ice-cold solution, 30% H2O2
(15 ml, 6.6 mmol) was added dropwise for 2 h with constant stirring
at room temperature. This solution was stirred at room temperature
for 14 h then diluted with H2O, and the precipitate was
collected on a celite pad by vacuum filtration. The pad was eluted
with hot DMF and the eluent was evaporated to dryness, and the
resulting pale-solid compound ‘2’ was obtained (2.70 g, 82%
yield).
Camptothecin 7-carboxylic acid (hapten 2). The
structure and synthetic route of 'hapten 2′ (18) are presented in Fig. 1A. Starting from a solution
containing the compound '2′ (1.00 g, 2.6 mmol) dissolved in 10 ml
H2SO4 (18 mmol), potassium dichromate (1.12
g, 3.82 mmol) was added while stirring in a cooling bath containing
ice and salt. The mixture was stirred at room temperature for 2 h
and then diluted with H2O. The precipitate was collected
by suction, and the solid substance was purified by
recrystallisation (815 mg, 79% yield) to give the 'hapten 2′
(Fig. 1A), exhibiting a melting
point of 280°C.
The results of the 1H-NMR and
13C NMR (500 MHz; DMSO-d6; δ ppm) were as follows: 0.85
(3H, t, J =15.0 Hz, C19-H), 1.84 (2H, q, J =15.0 Hz, C18-H), 5.23
(2H, s, C5-H), 5.44 (2H, s, C17-H), 6.58 (1H, brs), 7.19 (1H, s,
C14-H), 7.56–8.21 (4H, m, ArH); and 8.03 (C19), 30.91 (C18), 50.70
(C5), 66.82 (C17), 76.39 (C20), 95.56 (C14), 119.41 (C16), 128.16,
128.48, 129.01, 129.53, 130.29, 130.92, 132.04, 145.81, 146.43,
148.44, 152.95 (C2, C3, C6, C8-C15), 157.02 (C16a), 167.54 (C21),
172.83 (C22) [MS m/z 393 (M+H)+].
Immunogen production by conjugation via the active
ester method. Haptens were attached to BSA or KLH to use them as
immunogens. The haptens were conjugated to produce immunogens using
the active ester method, as previously described (19). The synthetic route for the active
ester method is presented in Fig.
1B. Hapten 1 (22.4 mg, 0.05 mmol) was dissolved in DMF (0.5
ml). Then, NHS (6.9 mg, 0.06 mmol) was added, followed by addition
of DCC (12.36 mg, 0.06 mmol) dissolved in 0.2 ml DMF. After
stirring overnight at room temperature in the dark, the mixture was
centrifuged at 10,000 × g at room temperature for 10 min. Then, the
supernatant was collected for the following step. BSA (33 mg, 0.5
µmol) was dissolved in 5 ml 10% CBS-PBS. The supernatant was added
to the protein solution dropwise and stirred for 4 h at room
temperature. The method used to conjugate KLH to hapten 1 was the
same that used for BSA, with the exception that KLH was 75 mg (0.25
µmol). Conjugates were dialyzed in PBS at 4°C for 2 days to allow
separation of the unreacted haptens. Purified conjugate solutions
were then freeze-dried. UV-vis spectral data were used to confirm
the structures of the final conjugates as previously described
(18). Same method were used for
the synthesis of the active ester of hapten 2 conjugated to BSA.
The hapten densities of the conjugates, defined as the number of
hapten molecules per molecule of protein, were directly estimated
by the molar absorbance ε (20),
with the following formula: Hapten
density=(εconjugates-εprotein)/εhapten.
Coating antigen production by conjugation via the
mixed anhydride method. Haptens were attached to OVA as coating
antigens. The method of conjugation used for coating antigens was
the mixed anhydride method, as previously described (21). The synthetic route for the mixed
anhydride method is presented in Fig.
1C. Hapten 1 (22.4 mg, 0.05 mmol) and 23 µl tri-n-butylamine
(9.26 mg, 0.05 mmol) were dissolved in DMF (0.5 ml) and cooled to
4°C. To the resulting solution, 7 µl isobutylchlorocarbonate (0.05
mmol, 6.829 mg) was added, and the mixed anhydride was stirred at
4°C for 1 h. Then, the mixed anhydride was collected for the
following steps. OVA (30 mg, 0.5 µmol) was dissolved in 5 ml PBS.
The OVA solution was added to the protein solution dropwise, and
stirred gently for 30 min at room temperature and then overnight at
4°C. Conjugates were dialyzed in PBS at 4°C for 2 days to allow the
separation of unreacted haptens. The purified conjugate solutions
were then freeze-dried. The same method was used for the synthesis
of the mixed anhydride of hapten 2 conjugated to OVA. Confirmation
of the structures of the final conjugates was performed by UV-vis
spectroscopy as aforementioned in the description of the active
ester method.
MAb preparation
Female BABL/c mice, (n=9; 6–8 weeks; 15–18 g) were
supplied by the Xi'an Jiaotong University Medical Laboratory Animal
Center. Mice were were housed at a temperature of 23°C with 50%
humidity under a standard 12:12-h light/dark cycle, with access to
specific pathogen-free-grade food and water ad libitum. Mice
were subcutaneously immunized with a mixture of an immunogen (100
µg/mouse) diluted in PBS and Freund's complete adjuvant (v/v 1:1).
At 2 weeks following the initial injection, booster injections of
an equal quantity of immunogen were given, with Freund's incomplete
adjuvant instead of Freund's complete adjuvant. Then, blood (0.2
ml/mouse) was collected from the tail vein, centrifuged at 1,000 ×
g at 4°C for 3 min, and the antisera were collected and tested to
determine the anti-hapten antibody titer by a non-competitive
indirect ELISA coated with the corresponding antigen. Immunized
mouse splenocytes were obtained by extruding the mouse spleen and
filtering with 200-mesh stainless steel mesh. Then, splenocytes
were mixed with Sp2/0 murine myeloma cells (ratio of mice
splenocytes to myeloma cells, 5–10:1) and centrifuged at 200 × g at
4°C for 10 min. Then, 50% polyethylene glycol 1500,
hypoxanthine-aminopterin-thymidine medium (RPMI 1640 supplemented
with 100 µM hypoxanthine, 16 µM thymidine and 0.4 µM aminopterine)
and hypoxanthine-thymidine medium (RPMI 1640 supplemented with 100
µM hypoxanthine and 16 µM thymidine) were used for the selection of
targeted hybridoma cells. The cells were cultivated at 37°C and 5%
CO2 for 7 days. The supernatants of targeted hybridoma
cell cultures were collected, and antibodies were detected using
non-competitive indirect ELISAs for hybridoma screening. Selected
hybridomas were cloned by limiting dilution (22), and stable antibody-producing clones
were expanded. Competitive indirect ELISA was then employed to
identify the antibodies that reacted with camptothecin. The
selected clones were cryopreserved in liquid nitrogen.
MAb purification
Saturated ammonium sulfate precipitation was
performed for the purification of MAb 5A3. Mice were
intraperitoneally injected with hybridoma cells 5A3, then ascites
were removed from mice 1 week later, and centrifuged at 2,000 × g
at 4°C for 5 min. The supernatant was mixed with PBS, then equal
volumes of saturated ammonium sulfate solution was added and
stirred evenly; the mixture was then refrigerated at 4°C overnight.
The mixture was centrifuged at 4°C at 10,000 × g for 20 min. The
precipitate was dissolved in PBS, and the procedure described above
was repeated twice. Finally, the precipitate was dissolved in 5 ml
PBS and dialyzed with a large volume of PBS at 4°C, and then frozen
at −20°C. The subtypes of purified antibodies were identified using
an SBA Clonotyping System/HRP kit according to the manufacturer's
protocols.
ELISA
For the non-competitive indirect ELISA, 96-well
plates were coated with 100 µl/well of coating antigens (2 µg/ml in
CBS) and incubated overnight at 4°C. The plates were washed three
times with PBST solution (10 mM PBS containing 0.05% Tween 20; pH
7.4) and then blocked with 200 µl/well 2% BSA (mg/ml) for 2 h at
37°C. After three washes with PBST, the 96-well plates were
incubated with 100 µl/well of serum, supernatant (containing 0.4
mg/ml antibody) or purified antibody solution (2.8 mg/ml) serially
diluted in PBS for 1 h at 37°C. The plates were washed three times
with PBST. Then, 100 µl/well of goat anti-mouse IgG-HRP diluted
1:4,000 with 1% BSA (mg/ml) was added. After incubation for 1 h at
37°C and three washes with PBST, 100 µl/well TMB solution (400 µl
0.6% TMB-DMSO and 100 µl 1% H2O2 diluted in
25 ml CPBS) was added, and plates were incubated for 10 min at
37°C. To stop the reaction, 50 µl H2SO4 (2 M)
was added per well, and the absorbance was measured at 450 nm.
For checkerboard assays (22), antibodies (2.8 mg/ml) and coating
antigens (2 mg/ml) were titrated using sequential concentrations.
All procedures were the same as the non-competitive indirect ELISA,
and were conducted under the same conditions. These assays were
used to obtain an approximate estimate of the appropriate coating
antigen and antibody concentrations for further competitive
assays.
In the competitive indirect ELISA, the coating
antigen (2 µg/ml) and antibodies (0.014 µg/ml) were used. All
procedures were the same as those used for the non-competitive
indirect ELISA, and were performed under similar conditions with
the exception of the addition of antigen homologues to
competitively bind the antibodies. To each well, 50 µl
camptothecins dissolved in 10% methanol-PBS and 50 µl antibody
(0.014 µg/ml) diluted in PBS were added. All samples were tested on
three different days, and the final titer value was calculated as
the average of three separate experiments. The cross-reactivity was
calculated with the following formula:
Cross-reactivity=(IC50 of camptothecin/IC50
of other compounds) ×100%.
Linear regression analysis
Competition curves were obtained by plotting the
inhibition rate against the logarithm of the analyte concentration.
The five values showing a linear trend in the sigmoidal curves were
selected as the numerical basis of the linear regression analysis
and the linear regression equation. Using the linear regression
equation, the concentration corresponding to the inhibition rate
within this range was calculated. The IC50 and
IC10 were determined with the following formula based on
the optical density (OD) of samples: Inhibition
rate=[(ODpositive-ODsample)/ODpositive]
×100%. Hapten 1 was used as a positive control.
Statistical analysis
Data were collected and plotted using Microsoft
Excel v16.0 (Microsoft Corporation) and SPSS 22.0 software (IBM
Corp.). Data are presented as the mean ± standard deviation. Data
were analyzed with ANOVA followed by Tukey's test for multiple
comparisons. P<0.05 was considered to indicate a statistically
significant difference.
Results
Hapten synthesis
Hapten 1, a 20-O-linked succinate-based camptothecin
ester derivative, and hapten 2, a water-soluble 7-substituted
camptothecin analogue, were synthesized (Fig. 1A). Camptothecin was reacted with
succinic anhydride to synthesize hapten 1 as previously described
(23). By contrast, hapten 2 was
synthesized from camptothecin by substitution and oxidation, as
previously described (24). The
structures of these products were confirmed by 1H-NMR,
13C-NMR and mass spectrometry. Haptens with substituents
at different sites were designed to investigate the effect of
various hapten analogues on the immunisation sensitivity. In
addition, the structures of the spacer arms of these two haptens
varied, potentially affecting the sensitivities of the two
haptens.
Identification of artificial antigens
and coupling ratio
The immunizing conjugates were prepared using
different activation methods. The mixed anhydride method was used
to prepare the coating antigens, whereas the active ester method
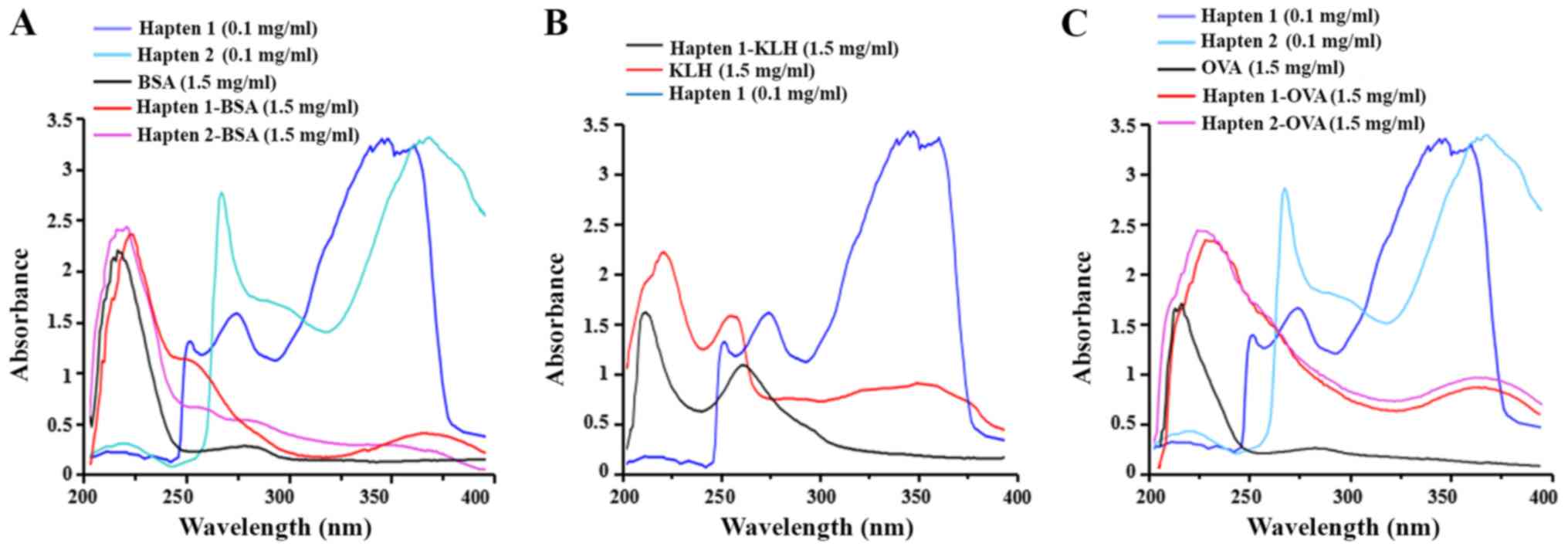
was conducted to obtain the immunogens. Analysis of the UV-vis
spectra is an effective method for the verification of the
conjugation and estimation of the hapten/protein ratio. UV-vis
spectra were obtained by scanning the samples using wavelengths of
200–400 nm (25). The haptens,
carrier proteins and conjugates were all analyzed by UV-vis
spectrometry (Fig. 2). The
profiles of the three curves were distinct, and the values differed
between haptens, carrier proteins and conjugates. The absorbance
patterns of the conjugates were different from the corresponding
carriers, suggesting that the hapten was successfully conjugated to
the carrier protein. Hapten/protein ratios were estimated by
spectroscopy at the same wavelength (18). A specific wavelength for each
hapten was selected to detect the absorbance values of haptens,
carrier protein and conjugate at selected concentrations, and the
values of the molar absorbance coefficients were subsequently
calculated. The concentration of all analytes tested was 100 µg/ml,
and the results are presented in Table
I. The present results suggested that the hapten/protein ratios
varied with different carrier proteins and coupling methods.
 | Table I.Ratios of hapten/protein
conjugates. |
Table I.
Ratios of hapten/protein
conjugates.
| Hapten | Conjugate | Binding ratio to
carrier protein | Wavelength, nm |
|---|
| Hapten 1 | BSA | 48 | 347 |
|
| KLH | 65 |
|
|
| OVA | 30 |
|
| Hapten 2 | BSA | 43 | 276 |
|
| OVA | 28 |
|
Selection of the immunogens
To determine the ideal hapten for eliciting the
production of antibodies against camptothecin, 6 mice were divided
into two groups, and injected with hapten 1-BSA or hapten 2-BSA.
Following the administration of booster injections (22), antisera from these mice were
collected and tested for the presence of antibodies that recognized
the corresponding immunizing hapten by non-competitive indirect
ELISAs using hapten 1-OVA and hapten 2-OVA. The results of the
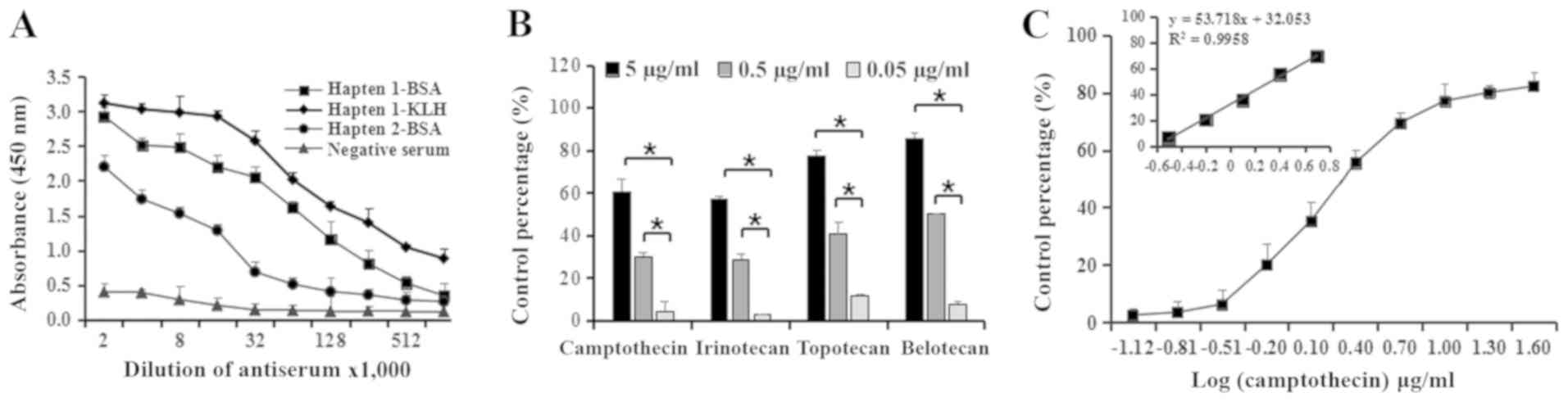
titration experiments are presented in Table II. The present results suggested
the presence of a marked difference in titer values between hapten
1-BSA and hapten 2-BSA, with a higher antibody titer generated from
the immunogen hapten 1-BSA compared with the hapten 2-BSA. The
antiserum titer curves of hapten 1-BSA and hapten 2-BSA determined
by indirect ELISA are presented in Fig. 3A. The present results suggested
that hapten 1 may be more suitable for the generation of antibodies
against camptothecin. Different carrier proteins were conjugated
with hapten 1 to examine the effects of the carrier protein on the
immunogenicity of hapten. Mice were immunized with hapten 1-KLH and
hapten 1-BSA. Mouse antisera samples were screened against hapten
1-OVA using a non-competitive indirect ELISA. The results of the
titration experiments are presented in Table II. The titration of hapten 1-KLH
was observed to be higher than hapten 1-BSA, and the antiserum
titer curves of hapten 1-KLH and hapten 1-BSA determined by
indirect ELISA exhibited distinct profiles (Fig. 3A). The titer of antibodies
generated from the immunogen hapten 1-KLH was higher compared with
hapten 1-BSA. Therefore, the present results suggested that KLH was
a superior carrier protein for MAb preparation. Therefore, hapten
1-KLH was selected for the generation of antibodies against
camptothecin.
 | Table II.Summary of titers of antisera. |
Table II.
Summary of titers of antisera.
|
|
| Boost |
|---|
|
|
|
|
|---|
| Antiserum | Sample | 1st | 2nd | 3rd | 4th | 5th |
|---|
| Hapten 1-BSA |
|
| Mouse 1A | 1:3,200 | 1:16,000 | 1:256,000 | 1:256,000 | 1:512,000 |
|
| Mouse 2A | 1:1,600 | 1:8,000 | 1:128,000 | 1:256,000 | 1:256,000 |
|
| Mouse 3A | 1:800 | 1:4,000 | 1:32,000 | 1:32,000 | 1:64,000 |
| Hapten 2-BSA | Mouse 1B | 1:400 | 1:800 | 1:3,200 | 1:3,200 | 1:6,400 |
|
| Mouse 2B | 1:200 | 1:600 | 1:1,600 | 1:3,200 | 1:3,200 |
|
| Mouse 3B | 1:800 | 1:1,600 | 1:3,200 | 1:6,400 | 1:12,000 |
| Hapten 1-KLH | Mouse 1C | 1:6,400 | 1:128,000 | 1:256,000 | 1:512,000 | 1:512,000 |
|
| Mouse 2C | 1:6,400 | 1:256,000 | 1:512,000 | 1:1,024,000 | 1:1,024,000 |
|
| Mouse 3C | 1:6,400 | 1:128,000 | 1:512,000 | 1:512,000 | 1:1,024,000 |
Establishment of ELISA using MAb
5A3
The MAb against camptothecin, MAb 5A3, was purified
from ascites in sensitized BALB/c mice. The identified subtype of
MAb 5A3 against camptothecin was IgG1 with κ-light chains, as
determined by using an SBA Clonotyping System/HRP kit. An ELISA was
used to analyze the titer and the limit of determination value of
MAb 5A3. The inhibition rate for camptothecin was determined using
checkerboard assays, and the concentration of hapten 1-OVA and MAb
5A3 was determined as aforementioned. Then, a standard curve for
camptothecin was obtained by plotting the inhibition rate against
the concentration of camptothecin, and the linear relation graph
for camptothecin was calculated (Fig.
3C). The present results suggested an I50 value of
2.1898 µg/ml with a detection limit of 0.3886 µg/ml
(I10) for MAb 5A3. According to the linear relation
diagram of the sigmoidal curve, the working range for MAb 5A3
ranged between 0.5965–7.8085 µg/ml, which were defined as
I20 and I80, respectively. The present
results suggested that the ELISA established using MAb 5A3 was
stable and sensitive for the detection of camptothecins. Therefore,
MAb 5A3 was selected for subsequent experiments.
Cross-reactivity
To evaluate the specificity of MAb 5A3, irinotecan,
topotecan and belotecan were used to test the cross-reactivity of
the developed assay. The inhibition rate for the four camptothecins
is presented in Fig. 3B. The
present results suggested that the inhibition rate of topotecan and
belotecan was increased compared with camptothecin and irinotecan.
The IC50, IC10, cross-reactivity and the
angular coefficient of the linear equations for each molecule are
presented in Table III. The
highest cross-reactivity was identified for topotecan (321.27%),
followed by belotecan (250.84%) and irinotecan (76.63%). The
present results suggested that the developed assay was more
sensitive for topotecan and belotecan than irinotecan. The
structures of irinotecan, topotecan and belotecan are presented in
Fig. 1D. The structures of
topotecan and belotecan are similar to the structure of
camptothecin, whereas irinotecan present marked differences
compared with camptothecin; however, these three compounds
exhibited high cross-reactivity with camptothecin (Fig. 1A).
 | Table III.Cross-reactivities and limit of
determination of camptothecins. |
Table III.
Cross-reactivities and limit of
determination of camptothecins.
| Compound | IC50,
µg/ml | IC10,
µg/ml | Cross-reactivity,
% | R2 of
the linear equation |
|---|
| Camptothecin | 2.19±0.08 | 0.39±0.02 | 100.00 | 0.9958 |
| Irinotecan | 2.85±0.10 | 0.47±0.01 |
76.63 | 0.9943 |
| Topotecan | 0.68±0.01 | 0.19±0.01 | 321.27 | 0.9884 |
| Belotecan | 0.87±0.03 | 0.22±0.01 | 250.84 | 0.9958 |
Discussion
For a specific immunoassay, a critical step is the
selection of the haptens (22).
Importantly, the selected hapten should preserve the structure of
the target compound (26). Hapten
design is important for the development of effective immunoassays
for small molecular compounds (27). In the present study, various
haptens for camptothecin were designed. As pentacyclic alkaloids
maintain a planar structure, numerous analogues of camptothecin
were synthesized by semi-synthetic approaches based on A-, B-, C-,
D- and E-ring modifications (28,29).
Due to high toxicity, relative instability and rapid inactivation
of camptothecins by lactone ring hydrolysis at a physiological pH,
the present study designed haptens based on B- and E-ring
modifications in order to investigate the effects of hapten
analogues on the sensitivity of immunization.
The prediction of the effects of heterogeneous
haptens on the immune response is challenging, due to differences
in the immune response among individual animals (30). Additionally, the ability to
discriminate the immune responses induced by haptens containing
distinct groups in different sites is limited (31). However, the present results
suggested conserved trends among the antibodies induced by the
injection of hapten 1. Hapten 1 was synthesized using succinic
anhydride. Notably, the biological life span of the lactone form of
20-O-alkyl camptothecin ester in human and mouse plasma is
significantly higher than succinate-based camptothecin ester
(32). Therefore, the
succinate-based camptothecin ester may be more suitable for
designing haptens, as succinate is not only relatively stable under
physiological conditions, but it has also been extensively used as
a spacer in the preparation of conjugates or pro-drugs (33). Hapten 1 contains a more stable
lactone form and demonstrated decreased cytotoxicity of the
20-hydroxyl group, which was masked by esterification with succinic
anhydride. In addition, the lactone E-ring, which includes an
α-hydroxy lactone ring with (S)-configuration, is characterized by
the presence of an (R)-enantiomer and (S)-enantiomer (34). Therefore, hapten 1 may possess more
exposed camptothecin-specific functional groups compared with
hapten 2.
The carrier protein is another important factor that
affects the immune effects of immunogens (35). The differences in antisera affinity
between hapten 1-KLH and hapten 1-BSA may be due to distinct
immunogenicities and hapten-carrying capabilities of the carrier
proteins. KLH, derived from molluscs, is less conserved in
mammalian species than BSA, and therefore produces antibodies that
are less likely to cross-react with typical target samples
(34). Due to its high molecular
weight and complexity, KLH elicited a stronger immune response than
other carrier proteins (32). KLH
is a large protein that possesses hundreds of primary amines and
carboxyl groups, whereas BSA contains a total of 59 lysine-amine
groups that are prone to be conjugated with other factors (35). Collectively, KLH is a more suitable
carrier protein for haptens.
The present study aimed to develop an ELISA for the
detection of camptothecins, and a MAb 5A3 against the designed
camptothecin hapten 1, 20(s)-O-succinyl-camptothecin, was
identified. Additionally, the characteristics of MAb 5A3 were
examined with a competitive indirect ELISA. MAb 5A3 showed high
specificity to camptothecin and its three derivatives. Although the
developed assay does not meet the criteria for the detection of all
camptothecins, the present results suggested that it was able to
efficiently detect various common camptothecins. Therefore, the
present study suggested the feasibility of the detection of certain
camptothecins using immunochemical methods. Collectively, the
developed assay could be used for a variety of applications such as
compound analysis, clinical applications, and analyses of food and
environmental samples.
Acknowledgements
Not applicable.
Funding
The present study was supported by The National
Natural Science Foundation of China (grant nos. 30800720 and
31371975) and The National Key Research and Development Program of
China (grant no. 2016YFD0500700).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
CZ, LY and YL conceived and designed the
experiments. LY and XN performed the experiments. LY drafted the
manuscript. HW and XH analyzed the data. JH interpreted the data
and critically revised the manuscript for important intellectual
content. YL supervised all research and revised the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The protocols for animal studies were approved by
The Committee on the Ethics of Animal Experiments of the Shaanxi
Provincial People's Hospital (approval no. 01-0420).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wall ME, Wani MC, Cook CE, Palmer KH,
McPhail AT and Sim GA: Plant antitumor agents I. The isolation and
structure of camptothecin, a novel alkaloidal leukemia and tumor
inhibitor from Camptotheca acuminata. J Am Chem Soc. 88:3888–3890.
1966. View Article : Google Scholar
|
|
2
|
Liu YQ, Liu ZL, Tian X and Yang L:
Anti-HSV activity of camptothecin analogues. Nat Prod Res.
24:509–514. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bodley AL, Cumming JN and Shapiro TA:
Effect of camptothecin, a topoisomerase I inhibitor on Plasmodium
falciparum. Biochem Pharmacol. 55:709–711. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Jensen NF, Agama K, Roy A, Smith DH,
Pfster TD, Romer MU, Zhang HL, Doroshow JH, Knudsen BR, Stenvang J,
et al: Characterization of DNA topoisomerase I in three SN-38
resistant human colon cancer cell lines reveals a new pair of
resistance-associated mutations. J Exp Clin Cancer Res. 35:562016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hsiang YH, Hertzberg R, Hecht S and Liu
LF: Camptothecin induces protein-linked DNA breaks via mammalian
DNA topoisomerase I. J Biol Chem. 260:14873–14878. 1985.PubMed/NCBI
|
|
6
|
Liu YQ, Li WQ, Morris-Natschke SL, Qian K,
Yang L, Zhu GX, Wu XB, Chen AL, Zhang SY, Nan X and Lee KH:
Perspectives on biologically active camptothecin derivatives. Med
Res Rev. 35:753–89. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kawato Y, Aonuma M, Hirota Y, Kuga H and
Sato K: Intracellular roles of SN-38, a metabolite of the
camptothecin derivative CPT-11, in the antitumor effect of CPT-11.
Cancer Res. 51:4187–4191. 1991.PubMed/NCBI
|
|
8
|
Kingsbury WD, Boehm JC, Jakas DR, Holden
KG, Hecht SM, Gallagher G, Caranfa MJ, McCabe FL, Faucette LF,
Johnson RK, et al: Synthesis of water-soluble (aminoalkyl.
camptothecin analogues: inhibition of topoisomerase I and antitumor
activity. J Med Chem. 34:98–107. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
LEE JH, Lee JM, Kim JK, Ahn SK, Lee SJ,
Kim MY, Jew SS, Park JG and Hong CI: Antitumor activity of
7-(2-(N-Isopropylamino)ethyl)-(20S)-camptothecin, CKD602, as a
potent DNA topoisomerase I inhibitor. Arch Pharm Res. 21:581–590.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Pommier Y: Topoisomerase I inhibitors:
Camptothecins and beyond. Nat Rev Cancer. 6:789–802. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Süsskind D, Hagemann U, Schrader M,
Januschowski K, Schnichels S and Aisenbrey S: Toxic effects of
melphalan, topotecan and carboplatin on retinal pigment epithelial
cells. Acta Ophthalmol. 94:471–478. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma JY, Tong SM, Wang PW, Liao WL, Liu HB
and Zhang LQ: Insecticidal activity of camptothecin against
Nilaparvata lugens, Brevicoryne brassicae, and Chilo suppressalis.
J Econ Entomol. 10:492–496. 2010. View
Article : Google Scholar
|
|
13
|
Liu YQ, Yang L, Zhao YL and Li HY:
Synthesis of novel derivatives of camptothecin as potential
insecticides. Pest Biochem Physio. 198:219–223. 2010. View Article : Google Scholar
|
|
14
|
Liu YQ, Dai W and Tian J: Synthesis and
insecticidal activities of novel spin-labeled derivatives of
camptothecin. Heteroatom Chem. 22:687–691. 2011. View Article : Google Scholar
|
|
15
|
Tang D, Cui Y and Chen G:
Nanoparticle-based immunoassays in the biomedical field. Analyst.
138:981–90. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Samori C, Guerrini A, Varchi G, Fontana G,
Bombardelli E and Tinelli S: Semisynthesis, biological activity,
and molecular modeling studies of C-ring-modified camptothecins. J
Med Chem. 52:1029–1039. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu H, Lin H, Jiang Y, Zhou X, Wu B and
Chen J: Synthesis and antitumor activity of 20-O-linked
succinate-based camptothecin ester derivatives. Lett Drug Design
Discovery. 3:83–86. 2006. View Article : Google Scholar
|
|
18
|
Seigo S, Ken-ichiro N, Tomio F, Teruo Y
and Tadashi M: Chemical modification of an antitumor alkaloid
camptothecin: synthesis and antitumor activity of 7-C-substitude
camptothecins. Chem Pharm Bull. 39:2574–2580. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Peeters JM, Hazendonk TG, Beuvery EC and
Tesser GI: Comparison of four bifunctional reagents for coupling
peptides to proteins and the effect of the three moieties on the
immunogenicity of the conjugates. J Immunol Methods. 120:133–143.
1989. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hua Lu, Haixia Lin, Yi Jiang, Xinguang
Zhou, Beili Wu and Jianmin Chen: Synthesis and Antitumor Activity
of 20-O-Linked Succinate-Based Camptothecin Ester Derivatives.
Letters in Drug Design & Discovery. 3:83–86. 2006. View Article : Google Scholar
|
|
21
|
Gendloff EH, Casale WL, Ram BP, Tai JH,
Pestka JJ and Hart LP: Hapten-protein conjugates prepared by the
mixed anhydride method. Cross-reactive antibodies in heterologous
antisera. J Immunol Methods. 92:15–20. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jang MS, Lee SJ, Xue X, Kwon HM, Ra CS,
Lee YT and Chung TW: Production and characterization of monoclonal
antibodies to a generic hapten for-class-specific determination of
organophosphorus pesticides. Bulletin of the Korean Chemical Soc.
23:1116–1119. 2002. View Article : Google Scholar
|
|
23
|
Vladu B, Woynaowski JM, Manikumar G, Wani
MC, Wall ME, Von Hoff DD and Wadkins RM: 7- and 10-Substituted
Camptothecins: Dependence of Topoisomerase I-DNA Cleavable Complex
Formation and Stability on the 7- and 10-Substituents. Mol
Pharmacol. 57:243–51. 2000.PubMed/NCBI
|
|
24
|
Trier NH, Hansen PR and Houen G:
Production and characterization of peptide antibodies. Methods.
56:136–144. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liang Y, Liu XJ, Liu Y, Yu XY and Fan MT:
Synthesis of three haptens for the class-specific immunoassay of
O,O-dimethyl organophosphorus pesticides and effect of hapten
heterology on immunoassay sensitivity. Anal Chim Acta. 615:174–83.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Burkin MA and Galvidis IA: Hapten
modification approach for switching immunoassay specificity from
selective to generic. J Immunol Methods. 388:60–67. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Esteve-Turrillas FA, Agulló C, Mercader
JV, Abad-Somovilla A and Abad-Fuentes A: Rationally designed
haptens for highly sensitive monoclonal antibody-based
immunoanalysis of fenhexamid. Analyst. 143:4057–4066. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wang MJ, Liu YQ, Chang LC, Wang CY, Zhao
YL, Zhao XB, Qian KD, Nan X, Yang L, Yang XM, et al: Design,
synthesis, mechanisms of action, and toxicity of novel
20(S)-sulfonylamidine derivatives of camptothecin as potent
antitumor agents. J Med Chem. 57:6008–6018. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sriram D, Yogeeswari P, Thirumurugan R and
Bal TR: Camptothecin and its analogues: A review on their
chemotherapeutic potential. Mat Prod Res. 19:393–412. 2005.
|
|
30
|
Sawada H and Matsuoka YG: Effect of a
nitrofuran derivative (AF2) on the immune response of mice. Gan.
67:693–701. 1976.PubMed/NCBI
|
|
31
|
Cao Z, Harris N, Kozielski A, Vardeman D,
Stehlin JS and Giovanella B: Alkyl esters of camptothecin and
9-nitrocamptothecin: Synthesis, in vitro pharmacokinetics,
toxicity, and antitumor activity. J Med Chem. 41:31–37. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Safavy A, Georg G, Vander Velde D, Raisch
KP, Safavy K, Carpenter M, Wang W, Bonner JA, Khazaeli MB and
Buchsbaum DJ: Site-specifically traced drug release and
biodistribution of a paclitaxel-antibody conjugate toward
improvement of the linker structure. Bioconjug Chem. 15:1264–1274.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Huang Q, Wang L and Lu W: Evolution in
medicinal chemistry of E-ring-modified Camptothecin analogs as
anticancer agents. Eur J Med Chem. 63:746–57. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Giesecke C, Meyer T, Durek P, Maul J,
Preiß J, Jacobs JFM, Thiel A, Radbruch A, Ullrich R and Dörner T:
Simultaneous presence of non- and highly mutated keyhole limpet
hemocyanin (KLH)-Specific Plasmablasts Early after Primary KLH
immunization suggests cross-reactive memory B Cell Activation. J
Immunol. 200:3981–3992. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cruz LJ, Cabrales A, Iglesias E, Aguilar
JC, González LJ and Reyes O: Enhanced immunogenicity and
cross-reactivity of HIV-1 V3-peptide and multiple antigen peptides
conjugated to distinct carrier proteins. Int Immunopharmacol.
9:1452–1459. 2009. View Article : Google Scholar : PubMed/NCBI
|