Introduction
Hypercholesterolemia is one of the major risk
factors responsible for the occurrence and development of
atherosclerosis. This condition has become the primary therapeutic
focus of atherosclerosis treatment (1). The most common cholesterol-lowering
drugs are statins. These compounds decrease intracellular
cholesterol levels by selectively inhibiting the enzyme
3-hydroxy-3-methylglutaryl-coenzyme A reductase (2). This in turn inhibits cholesterol
biosynthesis and decreases hepatic cholesterol concentration
(2). Statins increase the
expression levels of the low density lipoprotein receptor (LDLR) in
liver cell membranes by the sterol regulatory element binding
protein (SREBP) pathway, leading to an increase in the clearance of
LDL particles from the blood circulation (2). However, the use of statins causes
several side effects. High doses of statins may increase the
incidence and severity of multiple adverse events, including
hepatotoxicity and myopathy, which are accompanied by muscle
cramps, stiffness and weakness (3). In addition, statins increase the
levels of the proprotein convertase subtilisin/kexin type 9
(PCSK9), which leads to LDLR degradation, thereby causing a
negative feedback response that attenuates their lipid lowering
effect (4). Therefore, the
development of PCSK9 inhibitors may, in theory, enhance the
lipid-lowering functions of statins.
PCSK9 is a newly identified serine protease that has
emerged as a critical regulator in the pathogenesis of
hypercholesterolemia and atherosclerosis (5). PCSK9 consists of 692 amino acids and
is synthesized in the cytoplasm of hepatocytes. This protein enters
the endoplasmic reticulum and the signal peptide of PCSK9 is
cleaved. Pro-PCSK9 undergoes autocatalytic cleavage at residue
Gln152 and binds to the catalytic domain of the protein to form a
mature protein by the secretory pathway (6,7). The
mature PCSK9 protein binds directly to the epidermal growth factor
repeat A of the LDLR, and the PCSK9: LDLR complex is transferred to
the lysosomes for degradation. Therefore, LDLR is no longer
recycled back to the cell membrane surface, which leads to elevated
LDL levels in the plasma resulting from decreasing binding of the
LDL to its receptor (8–10). Previous studies have indicated that
functional mutations of PCSK9 are associated with human
hypercholesterolemia (gain-of-function mutation) or
hypocholesterolemia (loss-of-function mutation), which are
associated with increased and decreased cardiovascular risk,
respectively (11–14). In human studies, PCSK9 has been
demonstrated to be an important target for the decrease of plasma
cholesterol concentration and the decrease in cardiovascular risk
(5).
The United States of America Food and Drug
Administration (FDA) has approved the marketing of 2 monoclonal
antibodies against PCSK9, namely alirocumab and evolocumab, which
are effective in decreasing levels of atherogenic lipoproteins and
are well tolerated (15,16). However, these 2 inhibitors are
expensive; their estimated cost ranges from $12,000-$15,000 for
each patient per year (17).
Therefore, the development of low-cost PCSK9 inhibitors will have
significant commercial interest. In contrast to these observations,
traditional Chinese medicine (TCM) has been used in clinical
practice for >2,000 years in China and has exhibited marked
beneficial effects on human health (18). Recently, several studies
demonstrated a favorable effect of TCM for the treatment of
dyslipidemia by the regulation of PCSK9, for example; berberine is
a compound isolated from a Chinese herb that has exhibited
cholesterol-lowering activity. Berberine inhibits PCSK9
transcription by downregulating hepatic hepatocyte nuclear factor 1
(HNF1) protein expression via the ubiquitin-proteasome degradation
pathway (19,20). Therefore, TCM may serve as a
promising candidate to screen functional PCSK9 inhibitors.
In the present study, a novel drug-screening assay
was developed based on the human PCSK9 promoter. The assay
used data from a dual-luciferase reporter assay and the compound
silibinin A (SIL), a TCM compound, was identified to repress
PCSK9 promoter activity. SIL has been demonstrated to have a
broad range of pharmacological activities, including
anti-inflammatory, anti-oxidant, anti-cancer, neuroprotective and
cardioprotective effects (21–26).
Therefore, SIL has been used as a popular dietary supplement due to
its optimal tolerability and low toxicity, and its diverse
biological functions. The present study suggested that SIL may be
developed as a novel PCSK9 inhibitor used in combination with
statins, in order to retain their lipid lowering activity.
Materials and methods
Cell culture
The 293 and human hepatoblastoma HepG2 cells were
obtained from the American Type Culture Collection and cultured in
Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal bovine serum (FBS;
Gibco; Thermo Fisher Scientific, Inc.) and 1%
penicillin/streptomycin solution. All cells were incubated in a
cell culture chamber at 37°C under a humidified atmosphere with 5%
CO2.
Promoter construction
The promoter of human PCSK9 (−1,833- +100 bp)
was retrieved from the National Centre for Biotechnology
Information. As demonstrated previously, PCSK9 transcription is
controlled through cis regulatory elements located in the
proximal promoter region of the PCSK9 gene where the transcription
factor Sp1, HNF1 and sterol regulatory element-binding protein 1
(SRE-1) sites are located (−430- −345) (27). Notably, the SRE-1 motif is
responsible for the statins-induced PCSK9 transcription (28). Therefore, the region containing all
these functional sites was selected for the drug screening
protocol. Genomic DNA was extracted from human liver HL7702 cells
using a Wizard® Genomic DNA Purification kit (Promega
Corporation). The primers were designed using Primer Premier 5
software (Premier Biosoft International), and MluI and
XhoI restriction sites were added upstream and downstream of
the promoter sequence. The specific sequences were as follows:
Forward (F), 5′-TGCAAAAAAGAGTATGCCCGT-3′; reverse (R),
5′-TCCCCAAACAGCGTCAGATTA-3′. The PCSK9 promoter fragment was
amplified by polymerase chain reaction (PCR). PCR was conducted by
activating the DNA polymerase at 94°C for 5 min, followed by 30
cycles of three-step PCR (94°C for 30 sec, 66.8°C for 20 sec and
72°C for 2 min), a final extension at 72°C for 10 min and holding
at 4°C (PrimeSTAR® HS DNA Polymerase; Takara Bio, Inc.),
and the band size was detected by 1% agarose gel electrophoresis.
The recovered product and the pGL3-basic vector (MiaoLingPlasmid)
were double-digested with MluI (Takara Bio, Inc.) and
XhoI (Takara Bio, Inc.) at 37°C for 4 h, and annealed
together using T4 DNA ligase (Takara Bio, Inc.) at 16°C overnight.
The purified product was ligated (the size of the PCSK9-luc
promoter plasmid was 6,751 bp), and finally the sequence of the
pGL3-basic PCSK9-luc promoter plasmid was verified by
GenScript Biotech Corp.
Drug screening and dual-luciferase
reporter assay
TCM compounds (detailed drug information were
presented in Table I) were used to
construct a library in Jiangsu Key Laboratory for Molecular Medical
Biotechnology. These compounds were purchased from Nanjing Cebai
Biotechnology Co., Ltd. and Shanghai Aladdin Biochemical Technology
Co., Ltd. The treatment doses for these compounds were selected
based on previous studies (Table
I) (29–66), which confirmed their functional
effects for the treatment of various diseases. To analyze the
activity of the PCSK9 promoter, pGL3-PCSK9-luc (60
ng) and internal control plasmid pRL-TK (10 ng; MiaoLingPlasmid)
was co-transfected into cells using Lipofectamine® 3000
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. Following 36 h of culture, 293 or HepG2
cells were incubated with serum-free DMEM containing 1% dimethyl
sulfoxide (DMSO) as a negative control, or with the indicated drugs
(Table I) for 12 h at 37°C. The
cell lysates were extracted and luciferase signal intensities
(ratios of firefly luciferase signal normalized to Renilla
luciferase) were measured using a Microplate Luminometer (Promega
Corporation).
 | Table I.Potential drugs that decrease
proprotein convertase subtilisin/kexin type 9 expression. |
Table I.
Potential drugs that decrease
proprotein convertase subtilisin/kexin type 9 expression.
| No. | Drugs | Concentration | (Refs.) |
|---|
| 1 | Isoorientin | 30 µM | (29) |
| 2 |
Dihydrokaempferol | 690 µM | (30) |
| 3 | Hyperin | 100 µM | (31) |
| 4 | Glycitein | 100 µM | (32) |
| 5 | Genistein | 60 µM | (33) |
| 6 | Calycosin | 100 µM | (34) |
| 7 | Formononetin | 200 µM | (35) |
| 8 | Irisflorentin | 40 µM | (36) |
| 9 | Dichotomitin | 3 µM | (37) |
| 10 | Iristectorigenin
A | 20 µM | (38) |
| 11 |
6″-O-xylosyl-glycitein | 350 µM | (32) |
| 12 | Corylin | 30 µM | (39) |
| 13 | Kaempferide | 50 µM | (40) |
| 14 | Isovitexin | 20 µM | (41) |
| 15 | Naringenin | 150 µM | (42) |
| 16 | Isofraxidin | 70 µM | (43) |
| 17 | Oleanolic acid | 80 µM | (44) |
| 18 | Berberine | 50 µM | (45) |
| 19 | Resveratrol | 20 µM | (46) |
| 20 | Rosiglitazone | 300 µM | (47) |
| 21 | Oridonin | 160 µM | (48) |
| 22 | Lithium
chloride | 100 mM | (49) |
| 23 | Chlorogenic
acid | 450 µM | (50) |
| 24 | Baicalin | 220 µM | (51) |
| 25 | Nicotinic acid | 10 mM | (52) |
| 26 |
Cycloastragenol | 20 µM | (53) |
| 27 |
Prim-O-glucosylcimifugin | 210 µM | (54) |
| 28 | Epimedin C | 500 µM | (55) |
| 29 | Linarin | 10 µM | (56) |
| 30 | Vitexin | 20 µM | (57) |
| 31 | Myricetin | 75 µM | (58) |
| 32 | Herbacetin | 100 µM | (59) |
| 33 | Typhaneoside | 10 µM | (60) |
| 34 | Afzelin | 460 µM | (61) |
| 35 | Taxifolin | 50 µM | (62) |
| 36 | Silibinin A | 100 µM | (63) |
| 37 | Daidzein | 10 µM | (64) |
| 38 |
6″-O-Xylosyltectoridin | 100 µM | (65) |
| 39 | Schaftoside | 2 µM | (66) |
Cytotoxicity assay
Cell viability was examined by the Cell Counting
Kit-8 assay (CCK-8; Nanjing Jiancheng Bioengineering Institute Co.,
Ltd.). To determine the non-toxic concentration for cells, SIL (1,
5, 10, 25, 50, 100 and 200 µM) was added to each well
(1×104 cells/well). The plates were subsequently
incubated for 12 h. CCK-8 solution (10 µl/well) was added to each
well and the cells were cultured for an additional 2 h. Finally, a
microplate reader was used to measure the absorbance at 450 nm.
In situ terminal deoxynucleotidyl
transferase-mediated dUTP nick end-labeling (TUNEL) assay
TUNEL assays were performed to detect DNA strand
breaks using a commercial kit provided by the Vazyme. In brief,
HepG2 cells were treated as aforementioned and fixed with 4%
paraformaldehyde for 15 min at room temperature. Following washing,
the cells were permeabilized with 0.1% Triton X-100, and finally
incubated with TUNEL labeling reagent for 60 min at 37°C. The cells
were double-stained with 10 µg/ml DAPI for 10 min at 37°C
(Sigma-Aldrich; Merck KGaA). The sections were captured in three
different fields per sample with a DP70 digital camera connected to
an ECLIPSE Ts2R-FL microscope (Nikon Corporation). Representative
images from at least three separate experiments were presented.
Reverse transcription quantitative PCR
(RT-qPCR) analysis
Total RNA was isolated using the TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and reverse
transcribed with the PrimeScript™ RT reagent kit (Takara Bio,
Inc.). The resulting cDNA was amplified by qPCR using SYBR Green
(Takara Bio, Inc.) and the LightCycler® 480 System
(Roche Diagnostics) under the following thermocycling conditions:
Denaturation at 95°C for 5 min, followed by 95°C for 10 sec and
60°C for 30 sec for 40 cycles. The relative expression of each
targeted gene was determined using the 2−∆∆Cq
comparative method (67). The
primers for human β-actin were included for normalization.
The primer sequences were as follows: PCSK9 F,
5′-AGGGGAGGACATCATTGGTG-3′; PCSK9 R,
5′-CAGGTTGGGGGTCAGTACC-3′; β-actin F,
5′-CACCCACACTGTGCCCATCTACGA-3′; β-actin R,
5′-CAGCGGAACCGCTCATTGCCAATGG-3′.
Western blot analysis
Total cellular proteins were isolated from HepG2
cells using radioimmunoprecipitation assay lysis buffer (Beyotime
Institute of Biotechnology). The protein concentration was
determined using the BCA protein assay reagent (Beyotime Institute
of Biotechnology). Equal amounts of protein (20 µg/lane) were
loaded and separated via 10% SDS-PAGE, and then transferred onto
PVDF membranes (EMD Millipore). Subsequently, the membranes were
blocked with 5% fat-free dry milk at room temperature for 1 h.
Membranes were incubated with rabbit anti-PCSK9 (1:1,000; cat. no.
BS71876; Bioworld Technology, Inc.), mouse anti-β-actin (1:1,000;
cat. no. AP0060; Bioworld Technology, Inc.), rabbit anti-p38 MAPK
(1:1,000; cat. no. 9212S; Cell Signaling Technology, Inc.) or
rabbit anti-phosphorylated (p)-p38 MAPK (p-Tyr182; (1:1,000; cat.
no. 11253; Signalway Antibody LLC) antibodies in 5% fat-free dry
milk overnight on a shaker at 4°C. Membranes were subsequently
washed three times in PBST and incubated with horseradish
peroxidase-conjugated goat anti-mouse IgG secondary antibodies
(1:2,000; cat. no. sc-2005; Santa Cruz Biotechnology, Inc.) or goat
anti-rabbit IgG secondary antibodies (1:2,000; cat. no. sc-2004;
Santa Cruz Biotechnology, Inc.) at room temperature for 1 h. The
immunoreactive bands were visualized using electrochemiluminescence
(Tanon-5200 Multi; Tanon Science and Technology Co., Ltd.) and
quantified with the AlphaEase FC version 3.1.2 software (Alpha
Innotech; Cell Biosciences).
Statistical analysis
Groups of data were presented as mean ± standard
deviation. The data were analyzed using one-way analysis of
variance followed by Fisher's Least Significant Difference post hoc
test. The calculations were performed using Origin 8 software
(v8.6, OriginLab Corporation). P<0.05 was considered to indicate
a statistically significant difference.
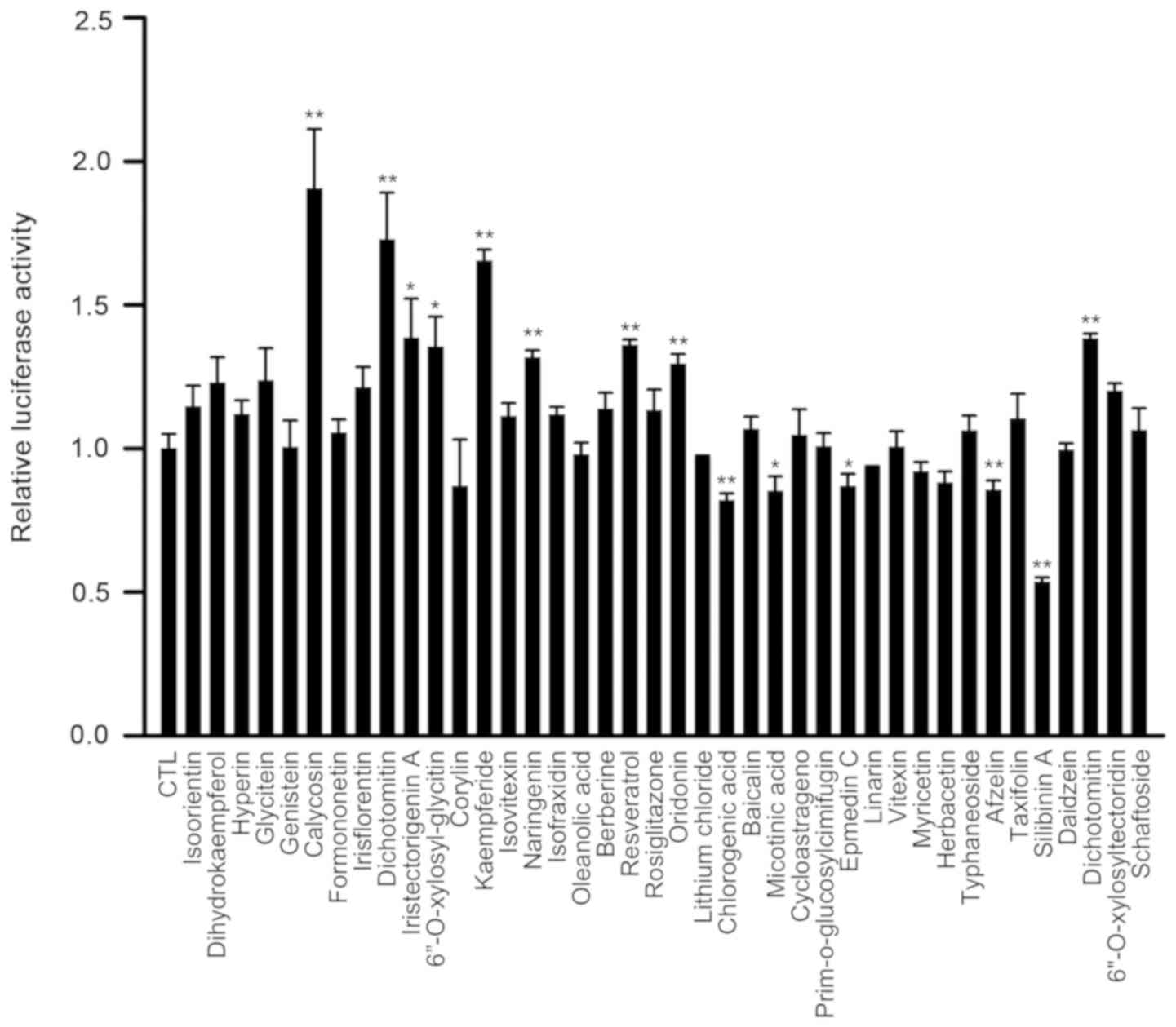
Results
SIL decreases the activity of
PCSK9-luc in 293 cells
The 293 cells transfected with PCSK9-luc were
used to screen the active ingredients present in the TCM compounds
(specific compounds were presented in Table I), which have been suggested to
inhibit the transcriptional activity of the PCSK9 promoter.
The transfected cells were incubated with various drug
concentrations for 12 h. Among all the tested drugs, chlorogenic
acid, nicotinic acid, epmedin C, linarin, herbacetin, afzelin and
SIL decreased the activity of PCSK9-luc (Fig. 1). In addition, SIL was the most
effective drug, which decreased PCSK9 promoter activity by
46.6% compared with that measured in the control samples.
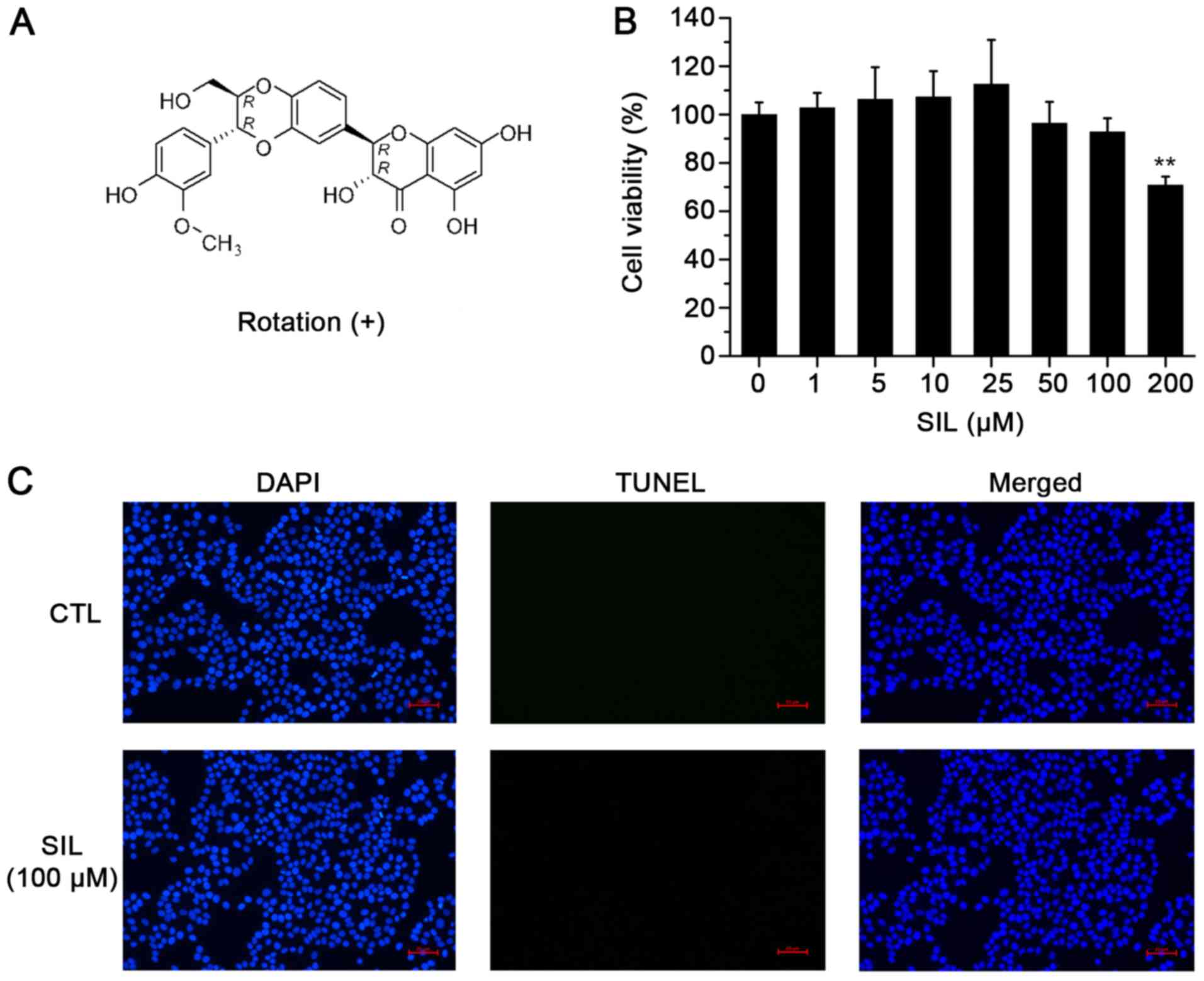
Detection of HepG2 cell cytotoxicity
and HepG2 apoptosis induction by SIL
The CCK-8 assay was used to assess the potential
cytotoxicity of SIL on human HepG2 cells. The data indicated that
treatment of SIL at the concentrations of 1, 5, 10 and 25 µM was
not cytotoxic to HepG2 cells. SIL at the doses of 50 and 100 µM
caused a slight decrease in cell viability, although the
differences were not significant (Fig.
2B). To exclude the possibility that the SIL-induced decrease
in PCSK9 would induce apoptosis in HepG2 cells, a TUNEL assay was
performed and it was identified that 100 µM of SIL did not induce
DNA fragmentation in these cells (Fig.
2C). This result indicated that the decrease of PCSK9
expression is not relevant to the apoptosis or cell growth.
Therefore, 100 µM SIL was selected as the maximum safe dose for
subsequent experiments.
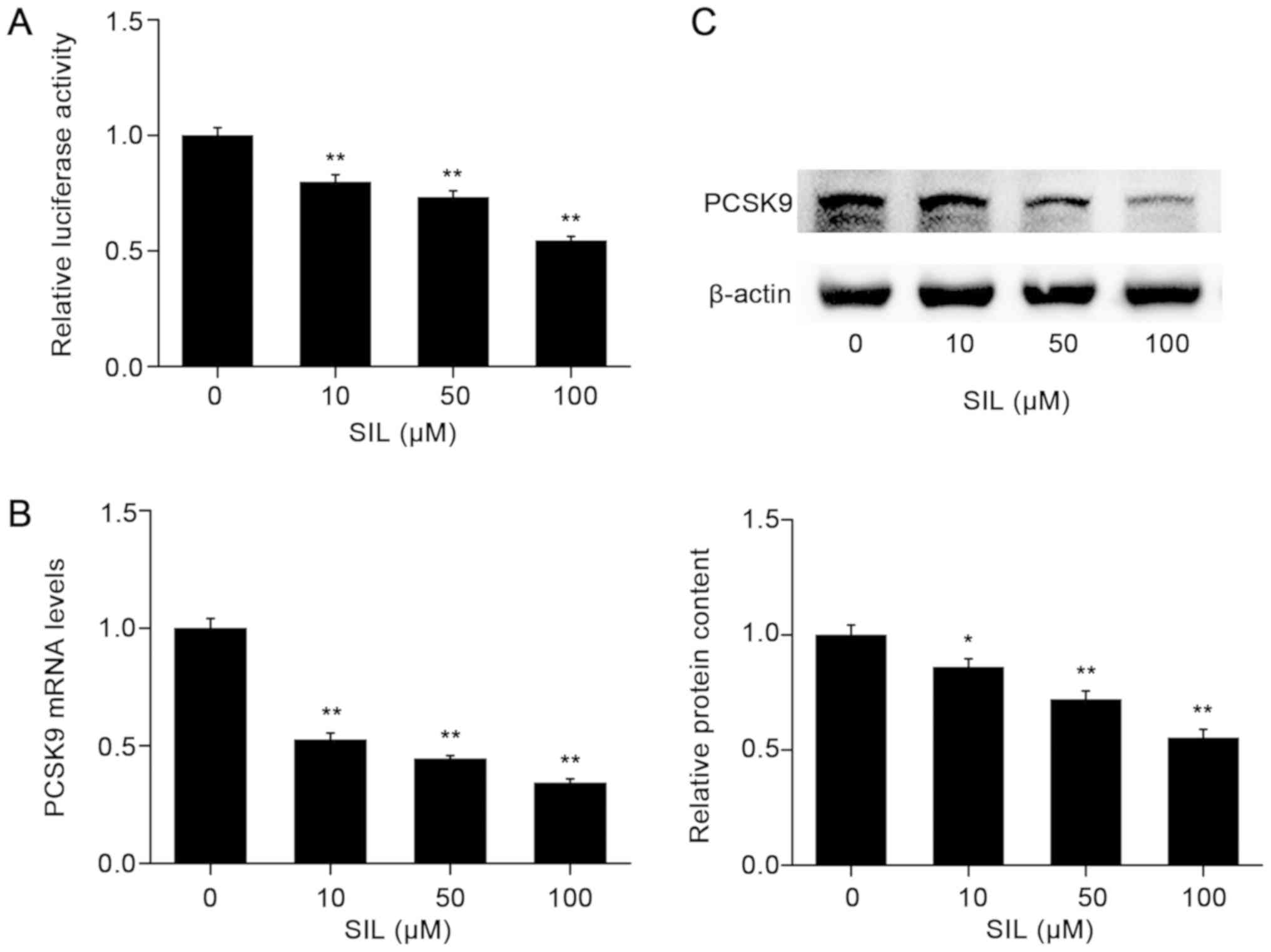
SIL decreases PCSK9 promoter activity
and the expression of PCSK9 in a dose-dependent manner
To confirm the results of the drug screening
experiments, HepG2 cells were transfected with a firefly luciferase
vector containing PCSK9-luc and subsequently treated with
SIL for 12 h. When compared with the cells treated with DMSO alone,
SIL decreases the activity of luciferase in a dose-dependent manner
(Fig. 3A). The maximum decrease in
the PCSK9 promoter activity was estimated to be 45.6% for
the cells treated with 100 µM SIL.
To examine whether SIL affected the expression
levels of PCSK9 mRNA, HepG2 cells were incubated with
increasing concentrations of SIL, and the mRNA levels of
PCSK9 were detected by RT-qPCR. The results indicated that
SIL decreased PCSK9 mRNA levels in a dose-dependent manner
(Fig. 3B). At the concentration of
100 µM, SIL inhibited PCSK9 mRNA levels by 65.8%
(P<0.01). The suppressive effects of SIL on the PCSK9 protein
expression levels were verified by western blot analysis (Fig. 3C).
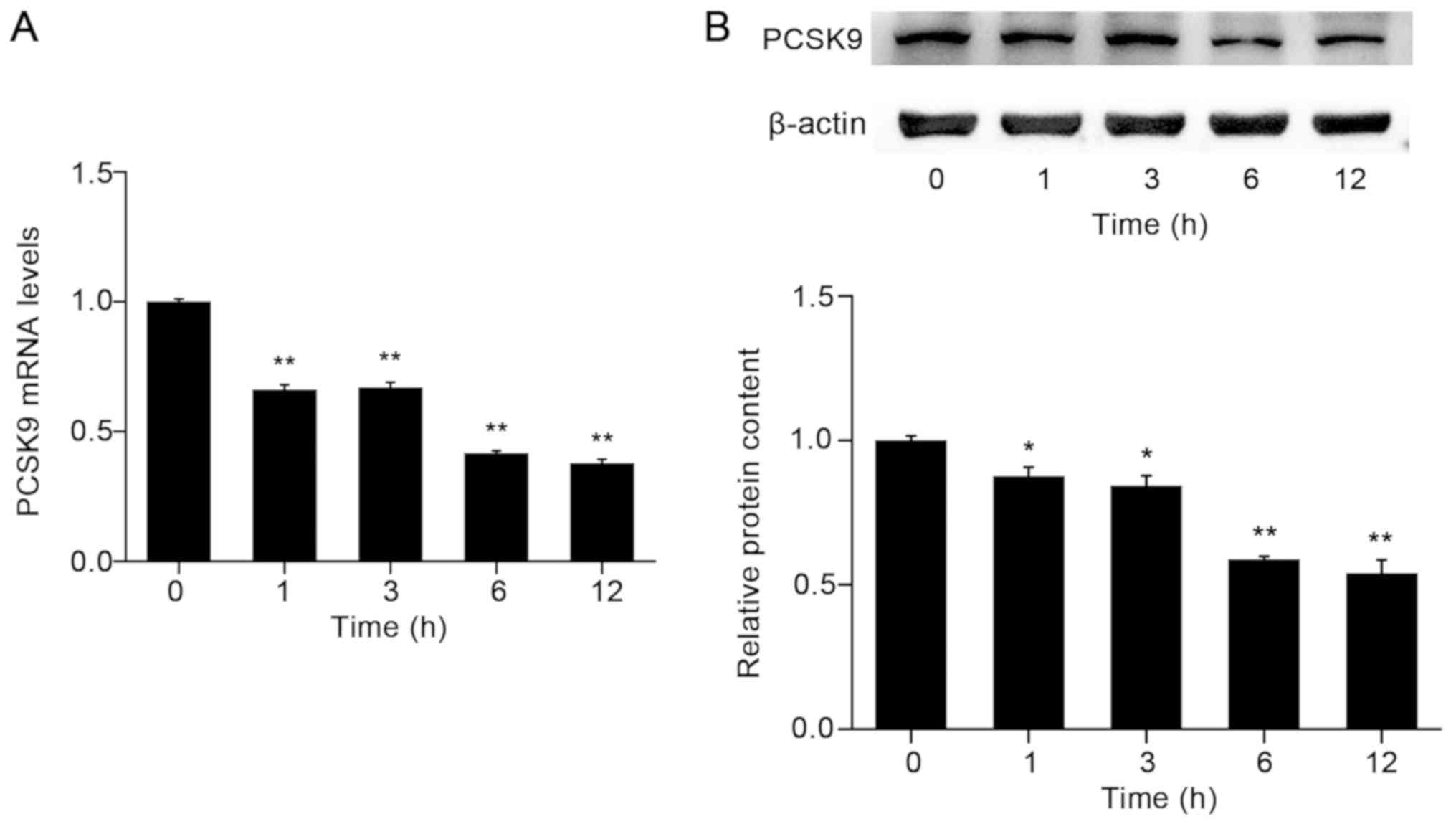
SIL decreases PCSK9 mRNA and protein
expression levels in a time-dependent manner
Subsequently, the effects of 100 µM SIL on PCSK9
expression in HepG2 cells at different time periods were assessed.
PCSK9 mRNA and protein expression levels were significantly
decreased following treatment of HepG2 cells with SIL in a
time-dependent manner (Fig. 4).
Notably, the maximum level of inhibition (62.3% decrease compared
with control; P<0.05) was observed at 12 h. A similar trend was
observed with regard to the expression of the PCSK9 protein.
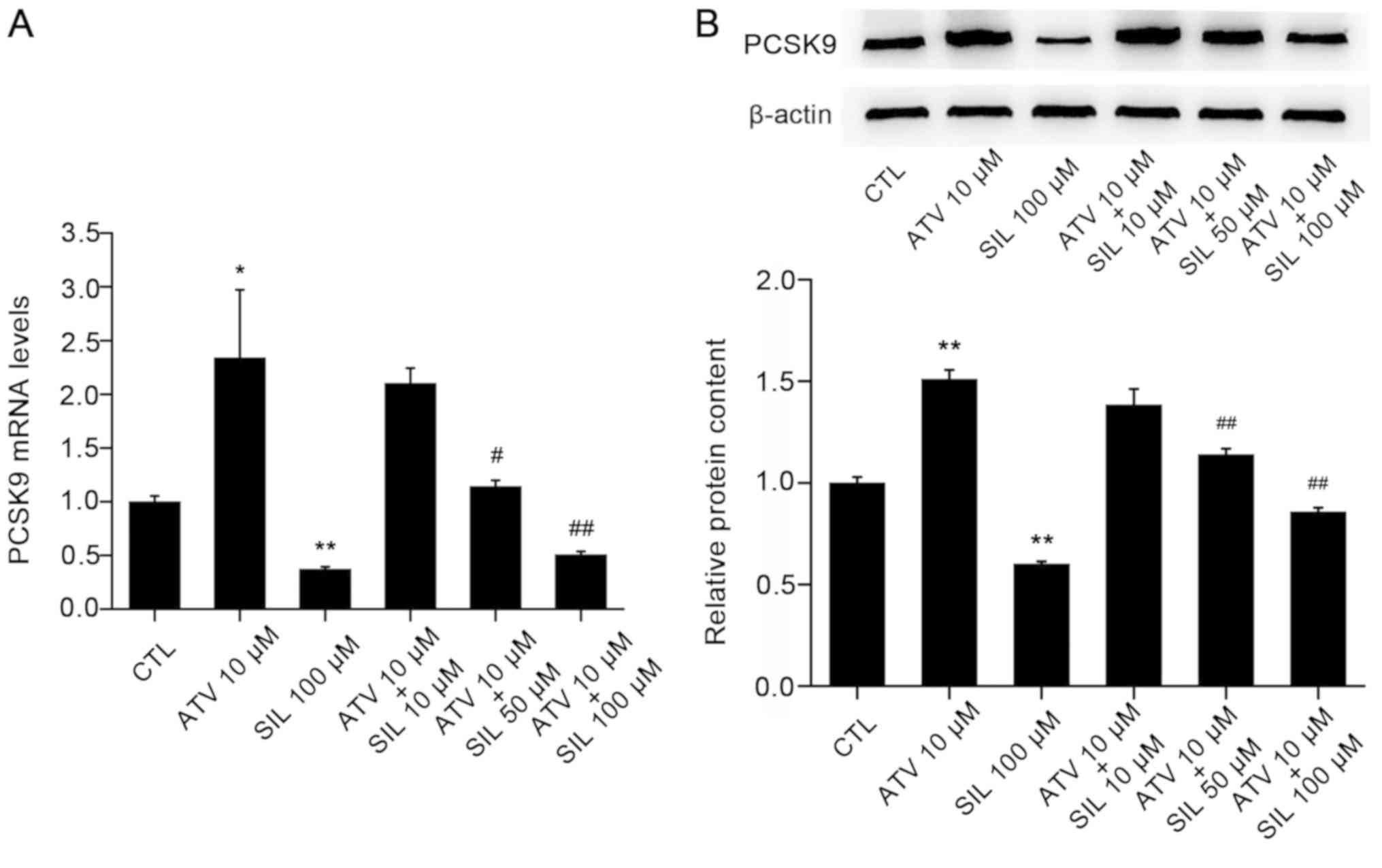
SIL antagonizes atorvastatin
(ATV)-induced upregulation of PCSK9 expression
It was previously reported that ATV may increase
PCSK9 expression in HepG2 cells (68). In the present study, PCSK9
mRNA expression levels were 2.3-fold increased following treatment
of HepG2 cells with 10 µM ATV, which was consistent with previous
studies (68,69). Notably, co-incubation of HepG2
cells with ATV and SIL for 12 h resulted in an inhibition of the
ATV-induced increase of PCSK9 mRNA levels by 78.1% (Fig. 5A). Similarly, western blot analysis
in HepG2 cell lysates revealed that ATV increased PCSK9 protein
expression levels by 51.0%, and SIL attenuated this effect by 43.2%
(Fig. 5B).
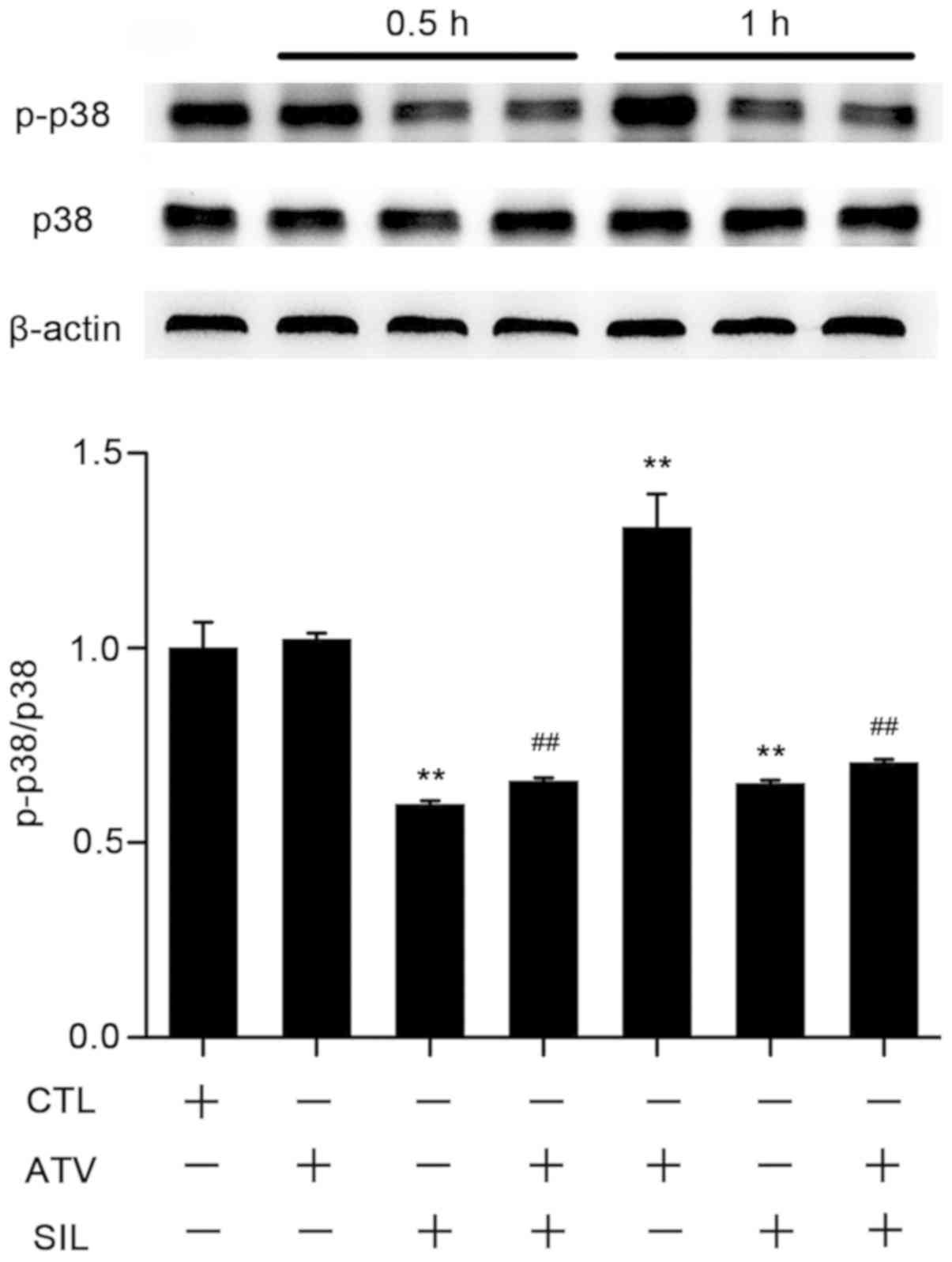
SIL decreases PCSK9 expression by
suppressing the p38 mitogen-activated protein kinases (MAPK)
pathway
In the liver, the p38 MAPK signaling pathway serves
a central role in lipid and cholesterol metabolism, and is induced
by statins to cause hepatic oxidative stress and apoptosis
(70). To investigate the cellular
and molecular mechanism underlying SIL-induced alleviation of
ATV-based PCSK9 accumulation, HepG2 cells were treated with ATV (10
µM) and SIL (100 µM) for 0.5 h and 1 h, respectively. As presented
in Fig. 6, SIL treatment decreased
the phosphorylation of p38 MAPK protein following ATV treatment for
0.5 h by ~34% in HepG2 cells. This effect occurred prior to the
induction of p38 phosphorylation triggered by 1 h of ATV treatment.
SIL consistently inhibited p38 phosphorylation induced by 1 h of
ATV treatment by ~46%. These data indicated that SIL may enhance
the lipid-lowering functions of statins.
Discussion
Hypercholesterolemia is a major risk factor for the
development of atherosclerosis. Decrease in plasma cholesterol
levels is beneficial for the treatment of
hypercholesterolemia-associated diseases. In a clinical setting,
statins are the most frequently used drugs for lowering blood lipid
levels (71). Statins increase
LDLR expression by the inhibition of cholesterol synthesis, leading
to the clearance of cholesterol in the blood. In addition, they
increase PCSK9 expression at the transcriptional level and
cause LDLR degradation within the cell membrane, therefore
attenuating their lipid-lowering effects (72). The present study aimed to examine
the ability of a novel compound to inhibit PCSK9 expression.
Therefore, 39 monomers isolated from TCM studies were screened, and
it was identified that SIL inhibited PCSK9 expression in a time-
and dose-dependent manner. In addition, SIL inhibited ATV-induced
upregulation of PCSK9. These results suggested that SIL may be a
promising compound for the attenuation of the negative feedback
response of statins with regard to PCSK9 expression.
The monomers isolated from TCM have several
advantages, including low cost, low toxicity and optimal isolation
procedures. Therefore, the present study focused on the screening
of those monomers that targeted PCSK9. Previous studies have
indicated that berberine decreases PCSK9 expression and improves
the LDL-C uptake from the hepatocytes (19,20,73).
However, berberine may also cause detrimental effects, which occur
primarily in the digestive system, including nausea, diarrhea,
constipation and abdominal pain (74,75).
Furthermore, the oral bioavailability of berberine is <1%, due
to its poor solubility. In contrast to berberine, the biological
half-life time and oral bioavailability levels of SIL are
considerably increased, which renders it a good candidate for the
development of novel PSCK9 inhibitors. Indeed, it has been
demonstrated that when administrated orally with 120 mg SIL, the
peak concentration in the plasma (Cmax) in patients was
2.7 µM (76). In addition, the
recommended oral dosage of SIL in phase I clinical trials
(ClinicalTrials.gov Identifier:
NCT00487721) for the treatment of prostate cancer is 13 g and
Cmax is 100 µM (77).
Taken together, the plasma levels of SIL administrated in the
clinical setting were comparable to the concentration used in the
present study.
SIL is a polyphenolic that belongs to the flavonoid
family of compounds (78). SIL has
been used clinically to treat acute and chronic hepatitis, early
cirrhosis and poisonous liver injury (22). SIL is a drug with multiple
biological targets, including PCSK9, and therefore has potential
use in the therapy of lipid metabolism disorders. Notably, the
plasma half-life of ATV and SIL is 7 and 6.21 h, respectively. The
corresponding time interval to reach Cmax levels is 1.5
h for ATV, and between 1–2 h for SIL, respectively (79,80).
These two important parameters have similar values, suggesting that
ATV and SIL may be orally administered simultaneously in order to
achieve an improved therapeutic effect of ATV.
The liver serves an important role in the
physiological process of lipid synthesis, gluconeogenesis and
cholesterol metabolism (81). It
has been suggested that ATV induces oxidative stress and apoptotic
injury in hepatocytes by increasing the phosphorylation of p38, JNK
and ERK MAPK enzymes (70). In
HepG2 cells, C-reactive protein increases PCSK9 expression by the
activation of the p38 MAPK-hepatocyte nuclear factor 1 homeobox A
signaling pathway (82).
Similarly, leptin additionally induced PCSK9 expression by the
activation of p38 MAPK (83).
Therefore, p38 MAPK is potentially involved in the regulation of
PCSK9 expression. In the present study, it was demonstrated that
ATV activated p38 MAPK, while SIL suppressed the ATV-induced
phosphorylation of p38 MAPK. The activation of the p38 MAPK
signaling pathway downregulated peroxisome proliferator-activated
receptor α and its transcriptional target genes carnitine
palmitoyltransferase 1A and Acyl-coenzyme A oxidase 1, leading to
the inhibition of fatty acid β-oxidation (84). Whether other MAPK enzymes are also
involved in the inhibition of PCSK9 by SIL remains unknown.
In conclusion, the present study demonstrated that
SIL inhibited the ATV-induced PCSK9 upregulation in HepG2 cells,
and that it may be used to decrease the adverse effects of statins
following simultaneous administration of these 2 drugs to patients
with hypercholesterolemia. Future studies are required to confirm
the beneficial effects of SIL in vivo by the use of
hyperlipidemic animal models.
Acknowledgements
Not applicable.
Funding
The present study was financially supported by
grants from the National Natural Science Foundation of China (grant
nos. 31800992, 31771298 and 81800512), the Natural Science
Foundation of the Jiangsu Higher Education Institutions of China
(grant nos. BK20180554 and BK20180577), the Project of State Key
Laboratory of Natural Medicines, China Pharmaceutical University
(grant no. SKLNMZZRC201803), the ‘Double First-Class’ University
Project (grant no. CPU2018GY17) and the Open Fund of State Key
Laboratory of Pharmaceutical Biotechnology, Nanjing University,
China (grant no. KF-GN-201901).
Availability of data and materials
The analyzed data sets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZD designed and performed the study, analyzed the
data and wrote the manuscript. WZ and SC performed the experiments
and analyzed the data. CL designed the experiments, analyzed the
data and wrote the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing
interests.
References
|
1
|
Benito-Vicente A, Uribe KB, Jebari S,
Galicia-Garcia U, Ostolaza H and Martin C: Familial
hypercholesterolemia: The most frequent cholesterol metabolism
disorder caused disease. Int J Mol Sci. 19(pii): E34262018.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Randomised trial of cholesterol lowering
in 4444 patients with coronary heart disease, . The Scandinavian
Simvastatin Survival Study (4S). Lancet. 344:1383–1389.
1994.PubMed/NCBI
|
|
3
|
Golomb BA and Evans MA: Statin adverse
effects: A review of the literature and evidence for a
mitochondrial mechanism. Am J Cardiovasc Drugs. 8:373–418. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dubuc G, Chamberland A, Wassef H, Davignon
J, Seidah NG, Bernier L and Prat A: Statins upregulate PCSK9, the
gene encoding the proprotein convertase neural apoptosis-regulated
convertase-1 implicated in familial hypercholesterolemia.
Arterioscler Thromb Vasc Biol. 24:1454–1459. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gouni-Berthold I: PCSK9 antibodies: A new
class of lipid-lowering drugs. Atheroscler Suppl. 18:21–27. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Horton JD, Cohen JC and Hobbs HH:
Molecular biology of PCSK9: Its role in LDL metabolism. Trends
Biochem Sci. 32:71–77. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Goldstein JL and Brown MS: A century of
cholesterol and coronaries: From plaques to genes to statins. Cell.
161:161–172. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Cunningham D, Danley DE, Geoghegan KF,
Griffor MC, Hawkins JL, Subashi TA, Varghese AH, Ammirati MJ, Culp
JS, Hoth LR, et al: Structural and biophysical studies of PCSK9 and
its mutants linked to familial hypercholesterolemia. Nat Struct Mol
Biol. 14:413–419. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Fisher TS, Lo Surdo P, Pandit S, Mattu M,
Santoro JC, Wisniewski D, Cummings RT, Calzetta A, Cubbon RM,
Fischer PA, et al: Effects of pH and low density lipoprotein (LDL)
on PCSK9-dependent LDL receptor regulation. J Biol Chem.
282:20502–20512. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang DW, Lagace TA, Garuti R, Zhao Z,
McDonald M, Horton JD, Cohen JC and Hobbs HH: Binding of proprotein
convertase subtilisin/kexin type 9 to epidermal growth factor-like
repeat A of low density lipoprotein receptor decreases receptor
recycling and increases degradation. J Biol Chem. 282:18602–18612.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Abifadel M, Varret M, Rabès JP, Allard D,
Ouguerram K, Devillers M, Cruaud C, Benjannet S, Wickham L, Erlich
D, et al: Mutations in PCSK9 cause autosomal dominant
hypercholesterolemia. Nat Genet. 34:154–156. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Maxwell KN and Breslow JL: Proprotein
convertase subtilisin kexin 9: The third locus implicated in
autosomal dominant hypercholesterolemia. Curr Opin Lipidol.
16:167–172. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Allard D, Amsellem S, Abifadel M, Trillard
M, Devillers M, Luc G, Krempf M, Reznik Y, Girardet JP, Fredenrich
A, et al: Novel mutations of the PCSK9 gene cause variable
phenotype of autosomal dominant hypercholesterolemia. Hum Mutat.
26:4972005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hallman DM, Srinivasan SR, Chen W,
Boerwinkle E and Berenson GS: Relation of PCSK9 mutations to serum
low-density lipoprotein cholesterol in childhood and adulthood
(from The Bogalusa Heart Study). Am J Cardiol. 100:69–72. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fala L: Repatha (Evolocumab): Second PCSK9
inhibitor approved by the FDA for patients with familial
hypercholesterolemia. Am Health Drug Benefits 9 (Spec Feature).
136–139. 2016.
|
|
16
|
Raedler LA: Praluent (Alirocumab): First
PCSK9 inhibitor approved by the FDA for hypercholesterolemia. Am
Health Drug Benefits 9 (Spec Feature). 123–126. 2016.
|
|
17
|
White CM: Therapeutic potential and
critical analysis of the PCSK9 monoclonal antibodies evolocumab and
alirocumab. Ann Pharmacother. 49:1327–1335. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Dai L, Lu A, Zhong LLD, Zheng G and Bian
Z: Chinese herbal medicine for hyperlipidaemia: A review based on
data mining from 1990 to 2016. Curr Vasc Pharmacol. 15:520–531.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kong W, Wei J, Abidi P, Lin M, Inaba S, Li
C, Wang Y, Wang Z, Si S, Pan H, et al: Berberine is a novel
cholesterol-lowering drug working through a unique mechanism
distinct from statins. Nat Med. 10:1344–1351. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dong B, Li H, Singh AB, Cao A and Liu J:
Inhibition of PCSK9 transcription by berberine involves
down-regulation of hepatic HNF1α protein expression through the
ubiquitin-proteasome degradation pathway. J Biol Chem.
290:4047–4058. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Flora K, Hahn M, Rosen H and Benner K:
Milk thistle (Silybum marianum) for the therapy of liver disease.
Am J Gastroenterol. 93:139–143. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wellington K and Jarvis B: Silymarin: A
review of its clinical properties in the management of hepatic
disorders. BioDrugs. 15:465–489. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Saller R, Meier R and Brignoli R: The use
of silymarin in the treatment of liver diseases. Drugs.
61:2035–2063. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zi X and Agarwal R: Silibinin decreases
prostate-specific antigen with cell growth inhibition via G1
arrest, leading to differentiation of prostate carcinoma cells:
Implications for prostate cancer intervention. Proc Natl Acad Sci
USA. 96:7490–7495. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gallo D, Giacomelli S, Ferlini C,
Raspaglio G, Apollonio P, Prislei S, Riva A, Morazzoni P,
Bombardelli E and Scambia G: Antitumour activity of the
silybin-phosphatidylcholine complex, IdB 1016, against human
ovarian cancer. Eur J Cancer. 39:2403–2410. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Saliou C, Rihn B, Cillard J, Okamoto T and
Packer L: Selective inhibition of NF-kappaB activation by the
flavonoid hepatoprotector silymarin in HepG2. Evidence for
different activating pathways. FEBS Lett. 440:8–12. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li H, Dong B, Park SW, Lee HS, Chen W and
Liu J: Hepatocyte nuclear factor 1alpha plays a critical role in
PCSK9 gene transcription and regulation by the natural
hypocholesterolemic compound berberine. J Biol Chem.
284:28885–28895. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Costet P, Cariou B, Lambert G, Lalanne F,
Lardeux B, Jarnoux AL, Grefhorst A, Staels B and Krempf M: Hepatic
PCSK9 expression is regulated by nutritional status via insulin and
sterol regulatory element-binding protein 1c. J Biol Chem.
281:6211–6218. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ko FN, Chu CC, Lin CN, Chang CC and Teng
CM: Isoorientin-6′-O-glucoside, a water-soluble antioxidant
isolated from Gentiana arisanensis. Biochim Biophys Acta.
1389:81–90. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Lu CL, Zhu W, Wang DM, Chen WL, Hu MM,
Wang M, Xu XJ and Lu CJ: Inhibitory effects of chemical compounds
isolated from the rhizome of Smilax glabra on nitric oxide and
tumor necrosis factor-α production in lipopolysaccharide-induced
RAW264.7 cell. Evid Based Complement Alternat Med. 2015:6024252015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Lee S, Jung SH, Lee YS, Yamada M, Kim BK,
Ohuchi K and Shin KH: Antiinflammatory activity of hyperin from
Acanthopanax chiisanensis roots. Arch Pharm Res. 27:628–632. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang B, Su JP, Bai Y, Li J and Liu YH:
Inhibitory effects of O-methylated isoflavone glycitein on human
breast cancer SKBR-3 cells. Int J Clin Exp Pathol. 8:7809–7817.
2015.PubMed/NCBI
|
|
33
|
Matsukawa Y, Marui N, Sakai T, Satomi Y,
Yoshida M, Matsumoto K, Nishino H and Aoike A: Genistein arrests
cell cycle progression at G2-M. Cancer Res. 53:1328–1331.
1993.PubMed/NCBI
|
|
34
|
Chen J, Zhao X, Ye Y, Wang Y and Tian J:
Estrogen receptor beta-mediated proliferative inhibition and
apoptosis in human breast cancer by Calycosin and Formononetin.
Cell Physiol Biochem. 32:1790–1797. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Auyeung KK, Law PC and Ko JK: Novel
anti-angiogenic effects of formononetin in human colon cancer cells
and tumor xenograft. Oncol Rep. 28:2188–2194. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gao Y, Fang L, Liu F, Zong C, Cai R, Chen
X and Qi Y: Suppressive effects of irisflorentin on LPS-induced
inflammatory responses in RAW 264.7 macrophages. Exp Biol Med
(Maywood). 239:1018–1024. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Effect of Dichotomitin on relieving cough
induced by cigarette and infection and serum cytokines of model
guinea pigs. Zhonghua Zhongyiyao Xuekan. 34:2902–2904. 2016.
|
|
38
|
Jun HJ, Hoang MH, Lee JW, Yaoyao J, Lee
JH, Lee DH, Lee HJ, Seo WD, Hwang BY and Lee SJ: Iristectorigenin B
isolated from Belamcanda chinensis is a liver X receptor modulator
that increases ABCA1 and ABCG1 expression in macrophage RAW 264.7
cells. Biotechnol Lett. 34:2213–2221. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chen CC, Chen CY, Ueng SH, Hsueh C, Yeh
CT, Ho JY, Chou LF and Wang TH: Corylin increases the sensitivity
of hepatocellular carcinoma cells to chemotherapy through long
noncoding RNA RAD51-AS1-mediated inhibition of DNA repair. Cell
Death Dis. 9:5432018. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Martineti V, Tognarini I, Azzari C,
Carbonell Sala S, Clematis F, Dolci M, Lanzotti V, Tonelli F,
Brandi ML and Curir P: Inhibition of in vitro growth and arrest in
the G0/G1 phase of HCT8 line human colon cancer cells by
kaempferide triglycoside from Dianthus caryophyllus. Phytother Res.
24:1302–1308. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Lin CM, Huang ST, Liang YC, Lin MS, Shih
CM, Chang YC, Chen TY and Chen CT: Isovitexin suppresses
lipopolysaccharide-mediated inducible nitric oxide synthase. Planta
Med. 71:748–753. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Zygmunt K, Faubert B, MacNeil J and Tsiani
E: Naringenin, a citrus flavonoid, increases muscle cell glucose
uptake via AMPK. Biochem Biophys Res Commun. 398:178–183. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Niu X, Xing W, Li W, Fan T, Hu H and Li Y:
Isofraxidin exhibited anti-inflammatory effects in vivo and
inhibited TNF-α production in LPS-induced mouse peritoneal
macrophages in vitro via the MAPK pathway. Int Immunopharmacol.
14:164–171. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shyu MH, Kao TC and Yen GC: Oleanolic acid
and ursolic acid induce apoptosis in HuH7 human hepatocellular
carcinoma cells through a mitochondrial-dependent pathway and
downregulation of XIAP. J Agric Food Chem. 58:6110–6118. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Ko BS, Choi SB, Park SK, Jang JS, Kim YE
and Park S: Insulin densitizing and insulinotropic sction of
Berberine from Cortidis Rhizoma. Biol Pharm Bull. 28:1431–1437.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Tang FY, Su YC, Chen NC, Hsieh HS and Chen
KS: Resveratrol inhibits migration and invasion of human
breast-cancer cells. Mol Nutr Food Res. 52:683–691. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Fryer LG, Parbu-Patel A and Carling D: The
Anti-diabetic drugs rosiglitazone and metformin stimulate
AMP-activated protein kinase through distinct signaling pathways. J
Biol Chem. 277:25226–25232. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Cui Q, Tashiro S, Onodera S, Minami M and
Ikejima T: Autophagy preceded apoptosis in oridonin-treated human
breast cancer MCF-7 cells. Biol Pharm Bull. 30:859–864. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Miyoshi K, Kasahara K, Miyazaki I and
Asanuma M: Factors that influence primary cilium length. Acta Med
Okayama. 65:279–185. 2011.PubMed/NCBI
|
|
50
|
Li X, Liu Y, Hou X, Peng H, Zhang L, Jiang
Q, Shi M, Ji Y, Wang Y and Shi W: Chlorogenic acid inhibits the
replication and viability of enterovirus 71 in vitro. PLoS One.
8:e760072013. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Zhang CJ and Yu HT: The signal pathways of
immune inflammation mediated By The Tlr3/Nf-Kappab and activator
protein-1 in cells infected with influenza A virus antagonized by
Baicalin. Adv Mat Res. 345:201–209. 2012.
|
|
52
|
Ida C, Ogata S, Okumura K and Taguchi H:
Changes in the gene expression of C-myc and CD38 in HL-60 cells
during differentiation induced by nicotinic acid-related compounds.
Biosci Biotechnol Biochem. 72:868–871. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Sun C, Jiang M, Zhang L, Jian Y, Zhang G,
Du B, Ren Y, Li X and Yao J: Cycloastragenol mediates activation
and proliferation suppression in concanavalin A-induced mouse
lymphocyte pan-activation model. Immunopharmacol Immunotoxicol.
39:131–139. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhou J, Sun YY, Sun MY, Mao WA, Wang L,
Zhang J and Zhang H: Prim-O-glucosylcimifugin attenuates
lipopolysaccharide induced inflammatory response in RAW 264.7
macrophages. Pharmacogn Mag. 13:378–384. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Liu TZ, Chen CY, Yiin SJ, Chen CH, Cheng
JT, Shih MK, Wang YS and Chern CL: Molecular mechanism of cell
cycle blockage of hepatoma SK-Hep-1 cells by Epimedin C through
suppression of mitogen-activated protein kinase activation and
increased expression of CDK inhibitors p21(Cip1) and p27(Kip1).
Food Chem Toxicol. 44:227–235. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Li J, Hao L, Wu J, Zhang J and Su J:
Linarin promotes osteogenic differentiation by activating the
BMP-2/RUNX2 pathway via protein kinase A signaling. Int J Mol Med.
37:901–910. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Zhou J, Hu H, Long J, Wan F, Li L, Zhang
S, Shi YE and Chen Y: Vitexin 6, a novel lignan, induces autophagy
and apoptosis by activating the Jun N-terminal kinase pathway.
Anticancer Drugs. 24:928–936. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Lu J, Papp LV, Fang J, Rodriguez-Nieto S,
Zhivotovsky B and Holmgren A: Inhibition of mammalian thioredoxin
reductase by some flavonoids: Implications for myricetin and
quercetin anticancer activity. Cancer Res. 66:4410–4418. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
59
|
Li L, Sapkota M, Kim SW and Soh Y:
Herbacetin inhibits inducible nitric oxide synthase via JNK and
nuclear factor-κB in LPS-stimulated RAW264.7 cells. Eur J
Pharmacol. 765:115–123. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
60
|
Cao S, Ni B, Feng L, Yin X, Dou H, Fu J,
Lin L and Ni J: Simultaneous determination of typhaneoside and
isorhamnetin-3-O-neohesperidoside in rats after oral administration
of pollen Typhae extract by UPLC-MS/MS. J Chromatogr Sci.
53:866–871. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
61
|
Shin SW, Jung E, Kim S, Kim JH, Kim EG,
Lee J and Park D: Antagonizing effects and mechanisms of Afzelin
against UVB-induced cell damage. PLoS One. 8:e619712013. View Article : Google Scholar : PubMed/NCBI
|
|
62
|
Guo H, Zhang X, Cui Y, Zhou H, Xu D, Shan
T, Zhang F, Guo Y, Chen Y and Wu D: Taxifolin protects against
cardiac hypertrophy and fibrosis during biomechanical stress of
pressure overload. Toxicol Appl Pharmacol. 287:168–177. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
63
|
Chu SC, Chiou HL, Chen PN, Yang SF and
Hsieh YS: Silibinin inhibits the invasion of human lung cancer
cells via decreased productions of urokinase-plasminogen activator
and matrix metalloproteinase-2. Mol Carcinog. 40:143–149. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
64
|
Sugimoto E and Yamaguchi M: Stimulatory
effect of Daidzein in osteoblastic MC3T3-E1 cells. Biochem
Pharmacol. 59:471–475. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
65
|
Lee KT, Sohn IC, Kim YK, Choi JH, Choi JW,
Park HJ, Itoh Y and Miyamoto K: Tectorigenin, an isoflavone of
Pueraria thunbergiana Benth., induces differentiation and apoptosis
in human promyelocytic leukemia HL-60 cells. Biol Pharm Bull.
24:1117–1121. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
66
|
Kim PS, Shin JH, Jo DS, Shin DW, Choi DH,
Kim WJ, Park K, Kim JK, Joo CG, Lee JS, et al: Anti-melanogenic
activity of schaftoside in Rhizoma Arisaematis by increasing
autophagy in B16F1 cells. Biochem Biophys Res Commun. 503:309–315.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
67
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
68
|
Davignon J and Dubuc G: Statins and
ezetimibe modulate plasma proprotein convertase subtilisin kexin-9
(PCSK9) levels. Trans Am Clin Climatol Assoc. 120:163–173.
2009.PubMed/NCBI
|
|
69
|
Welder G, Zineh I, Pacanowski MA, Troutt
JS, Cao GQ and Konrad RJ: High-dose atorvastatin causes a rapid
sustained increase in human serum PCSK9 and disrupts its
correlation with LDL cholesterol. J Lipid Res. 51:2714–2721. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
70
|
Pal S, Ghosh M, Ghosh S, Bhattacharyya S
and Sil PC: Atorvastatin induced hepatic oxidative stress and
apoptotic damage via MAPKs, mitochondria, calpain and caspase12
dependent pathways. Food Chem Toxicol. 83:36–47. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
71
|
Puccetti L, Acampa M and Auteri A:
Pharmacogenetics of statins therapy. Recent Pat Cardiovasc Drug
Discov. 2:228–236. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
72
|
Taylor BA and Thompson PD: Statins and
their effect on PCSK9-impact and clinical relevance. Curr
Atheroscler Rep. 18:462016. View Article : Google Scholar : PubMed/NCBI
|
|
73
|
Cameron J, Ranheim T, Kulseth MA, Leren TP
and Berge KE: Berberine decreases PCSK9 expression in HepG2 cells.
Atherosclerosis. 201:266–273. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
74
|
Kumar A, Ekavali, Chopra K, Mukherjee M,
Pottabathini R and Dhull DK: Current knowledge and pharmacological
profile of berberine: An update. Eur J Pharmacol. 761:288–297.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
75
|
Lan J, Zhao Y, Dong F, Yan Z, Zheng W, Fan
J and Sun G: Meta-analysis of the effect and safety of berberine in
the treatment of type 2 diabetes mellitus, hyperlipemia and
hypertension. J Ethnopharmacol. 161:69–81. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
76
|
Wu JW, Lin LC and Tsai TH: Drug-drug
interactions of silymarin on the perspective of pharmacokinetics. J
Ethnopharmacol. 121:185–193. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
77
|
Flaig TW, Gustafson DL, Su LJ, Zirrolli
JA, Crighton F, Harrison GS, Pierson AS, Agarwal R and Glodé LM: A
phase I and pharmacokinetic study of silybin-phytosome in prostate
cancer patients. Invest New Drugs. 25:139–146. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
78
|
Stolf AM, Cardoso CC and Acco A: Effects
of silymarin on diabetes mellitus complications: A review.
Phytother Res. 31:366–374. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
79
|
Lennernäs H: Clinical pharmacokinetics of
atorvastatin. Clin Pharmacokinet. 42:1141–1160. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
80
|
Wu JW, Lin LC and Tsai TH: Drug-drug
interactions of silymarin on the perspective of pharmacokinetics. J
Ethnopharmacol. 121:185–193. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
81
|
Pang Y, Zhu H, Xu J, Yang L, Liu L and Li
J: β-arrestin-2 is involved in irisin-induced glucose metabolism in
type 2 diabetes via p38 MAPK signaling. Exp Cell Res. 360:199–204.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
82
|
Cui CJ, Li S, Zhu CG, Sun J, Du Y, Zhang
Y, Wu NQ, Guo YL, Xu RX, Gao Y and Li JJ: Enhanced pro-protein
convertase subtilisin/kexin type 9 expression by C-reactive protein
through p38MAPK-HNF1α pathway in HepG2 cells. J Cell Mol Med.
20:2374–2383. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
83
|
Du Y, Li S, Cui CJ, Zhang Y, Yang SH and
Li JJ: Leptin decreases the expression of low-density lipoprotein
receptor via PCSK9 pathway: Linking dyslipidemia with obesity. J
Transl Med. 14:2762016. View Article : Google Scholar : PubMed/NCBI
|
|
84
|
Li J, Huang Q, Long X, Zhang J, Huang X,
Aa J, Yang H, Chen Z and Xing J: CD147 reprograms fatty acid
metabolism in hepatocellular carcinoma cells through
Akt/mTOR/SREBP1c and P38/PPARα pathways. J Hepatol. 63:1378–1389.
2015. View Article : Google Scholar : PubMed/NCBI
|