Introduction
Preeclampsia (PE) is a complication of pregnancy,
which is characterized by proteinuria and hypertension after 20
weeks of gestation. It remains a leading cause of maternal
mortality and morbidity worldwide (1). Although great advances have been made
in this field, the precise mechanisms underlying PE remain unclear.
It is generally believed that the placenta serves an essential role
in PE pathogenesis and progression (2). During early development of the
placenta, the uterine spiral arteries of the decidua and the
myometrium are initially invaded by extravillous trophoblasts
(EVTs) of fetal origin; the invaded EVTs subsequently replace the
endothelial layers of the maternal spiral arteries (3,4).
This process causes an increase in utero-placental perfusion and a
decrease in maternal blood flow resistance. In PE, this process is
impaired, which may be due to increased apoptosis, decreased
proliferation of EVTs, and abnormal migration and invasion of EVTs
(5). A previous study reported
that apoptosis of placental villous trophoblasts in PE pregnancies
is increased due to hypoxia-reperfusion injury and oxidative stress
(6). Although it has been
suggested that increased apoptosis may promote syncytial
degeneration and release inflammatory mediators into the maternal
circulation, the role of increased apoptosis in the pathogenesis of
PE remains unclear (7). Therefore,
understanding the molecular mechanisms underlying the abnormal
functions of trophoblast cells may assist in improving the
management of PE.
Long non-coding RNAs (lncRNAs) are defined as a type
of non-coding RNA >200 nucleotides long. Previous studies have
demonstrated roles for lncRNAs in the pathogenesis and progression
of diseases, including cancer, neurodegenerative diseases and
cardiovascular diseases (8–10).
Several lncRNAs have been identified to serve biological roles in
PE. The lncRNA maternally expressed gene 3 (MEG3) is downregulated
in placental tissues from patients with PE, and is associated with
trophoblast cell apoptosis and migration (11). The lncRNA metastasis-associated
lung adenocarcinoma transcript 1 (MALAT1) has also been identified
to be downregulated in PE and to regulate JEG-3 trophoblast
cellular processes, including cell proliferation, cell apoptosis,
cell migration and invasion (12).
The lncRNA SPRY4 intronic transcript 1 (SPRY4-IT1) regulates the
epithelial-mesenchymal transition, and modulates trophoblast cell
invasion and migration (13).
Recently, data from microarray analysis revealed that the lncRNA
family with sequence similarity 99 member A (FAM99A) is
downregulated in PE (14),
suggesting a potential role for FAM99A in PE. However, the
biological role of FAM99A in PE remains unknown.
The present study examined the expression levels of
FAM99A in placental tissues from women with PE, and determined the
in vitro effects of FAM99A on trophoblast cell invasion,
migration and apoptosis. The present results demonstrated that
FAM99A may provide a potential novel approach for the diagnosis and
treatment of PE.
Materials and methods
Clinical samples
A total of 45 healthy pregnant women and 45 women
with severe PE (early onset, n=24; late onset, n=21) were included
in the present study. Placental tissues were collected from
primipara women who underwent a caesarean section between January
2015 and June 2017 at The Northwest Women and Children's Hospital.
All of the collected placental tissues were washed with sterile PBS
and immediately placed in liquid N2; the sections were
stored at −80°C for further experimentation. All experiments were
approved by the Ethics Committee of The Northwest Women and
Children's Hospital, and written informed consent was obtained from
all of the recruited subjects. All clinical studies were performed
according to the principles of the Declaration of Helsinki. None of
the patients had autoimmune diseases, chronic nephritis, diabetes,
heart diseases, chronic hypertension, thrombophilic conditions or
HELLP syndrome (hemolysis, elevated liver enzymes and low platelet
count), and none delivered vaginally or gave birth to infants with
fetal malformation. Severe PE was diagnosed based on the definition
provided in Williams Obstetrics (23rd edition) (15). The patients had no history of
preexisting or chronic hypertension, but had exhibited systolic
blood pressure >160 mmHg or diastolic blood pressure >110
mmHg on at least two occasions, accompanied by significant
proteinuria (>300 mg/24 h), or persistent and severe central
nervous system symptoms, or symptoms in multiple organs associated
with persistent epigastric or right upper-quadrant pain, after the
20th week of gestation. Early onset of PE is defined as PE that
develops prior to 34 weeks of gestation, whereas late onset of PE
develops at ≥34 weeks of gestation (16). The key clinical characteristics of
the recruited subjects are summarized in Table I.
 | Table I.Clinical characteristics of normal
and PE pregnancies. |
Table I.
Clinical characteristics of normal
and PE pregnancies.
|
| PE (n=45) |
|---|
|
|
|
|---|
|
Characteristics | Control (n=45) | Early onset
(n=22) | Late onset
(n=23) |
|---|
| Maternal weight
(kg) | 68.9±5.6 | 70.8±6.7 | 71.4±6.0 |
| Maternal age
(years) | 30.6±4.3 | 30.8±3.6 | 30.0±3.8 |
| Gestational age
(weeks) | 38.7±2.3 | 37.2±1.8 | 37.9±2.1 |
| Systolic blood
pressure (mmHg) | 113.2±9.8 |
166.2±5.9a |
170.6±6.8a |
| Diastolic blood
pressure (mmHg) | 75.3±3.4 |
108.2±7.2a |
108.6±6.5a |
| Proteinuria
(g/day) | Not detected |
4.4±1.4a |
4.6±1.1a |
| Body weight of
infant (g) | 3,556.5±346.1 |
2,524.8±146.5a |
2,560.8±184.7a |
Cell culture
HTR-8/SVneo cells (CRL-3271™) were purchased from
American Type Culture Collection and were authenticated by short
tandem repeat profiling (17).
HTR-8/SVneo cells were maintained in RPMI 1640 medium supplemented
with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific,
Inc.), 100 µg/ml streptomycin and 100 U/ml penicillin at 37°C in a
humidified incubator containing 5% CO2.
Transfection with plasmids and small
interfering RNAs (siRNAs)
The control plasmid (pcDNA3.1) and the FAM99A
overexpressing plasmid (pcDNA3.1-FAM99A) were commercially designed
and synthesized by Shanghai GenePharma Co., Ltd. Scrambled siRNA
(siRNA-negative control, si-NC) and FAM99A-specific siRNA
(si-FAM99A) were commercially synthesized by Guangzhou RiboBio Co.,
Ltd. Cell transfection (1×106 cells/ml) with plasmids (2
µg) or siRNAs (50 nM) was performed at room temperature using
Lipofectamine® 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. A total
of 48 h post-transfection, cells were used for further
experimentation.
RNA extraction and reverse
transcription-quantitative PCR (RT-qPCR)
Total RNA was isolated from placental tissues or
cells using TRIzol® reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. RNA was
reversed transcribed into cDNA using a Reverse Transcription kit
(Takara Biotechnology Co., Ltd.) according to the manufacturer's
protocol. SYBR Premix Ex Taq (Takara Biotechnology Co., Ltd.) was
used to perform qPCR in order to determine the expression levels of
FAM99A, according to the manufacturer's protocol. The primers were
as follow: FAM99A; forward, 5′-GTCCCTTGCCCTCTCTTGTC-3′ and reverse,
5′-ACACGCATCACAAAACAGCC-3′; and GAPDH; forward,
5′-GTCAACGGATTTGGTCTGTATT-3′ and reverse,
5′-AGTCTTCTGGGTGGCAGTGAT-3′. The thermocycling conditions were as
follows: 95°C for 30 sec, followed by 40 cycles at 95°C for 5 sec
and 60°C for 30 sec. Gene expression levels were normalized to
GAPDH. RT-qPCR assays were performed on an ABI 7500 system (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The gene expression
levels were calculated using the comparative Ct method (18).
Transwell invasion assay
The invasive ability of HTR-8/SVneo cells was
assessed using a Transwell invasion assay with Matrigel. Briefly,
transfected HTR-8/SVneo cells (1×106 cells/ml) were
re-suspended in 200 µl serum-free medium and cultured in the upper
chambers of Matrigel-coated (Corning, Inc.) Transwell inserts (8 µm
pore size; Corning Inc.). The lower chamber was filled with 800 µl
culture medium supplemented with 10% FBS. After culturing for 24 h,
the cells on the top surface were removed and the cells on the
bottom surface were fixed with 70% ethanol for 10 min at room
temperature and stained with 0.1% crystal violet for 10 min at room
temperature. The invaded cells were counted under a light
microscope.
Wound healing assay
The migratory ability of HTR-8/SVneo cells was
assessed using a wound healing assay. Briefly, the transfected
HTR-8/SVneo cells (1×106 cells/ml) were seeded in a
6-well plate and were grown until they reached 89–90% confluence.
Wounds were generated by scratching the cell layers with a 1-ml
pipette tip and cell proliferation was blocked with 40 µM mitomycin
C (Sigma-Aldrich; Merck KGaA). Cells were incubated for another 24
h at 37°C. Wound width was measured using a light microscope at 0
and 24 h after the wound was created, and the percentage of wound
closure was calculated.
Flow cytometry
Cell apoptosis was detected using a fluorescein
isothiocyanate (FITC) Annexin V Apoptosis Detection kit
(Sigma-Aldrich; Merck KGaA). Transfected HTR-8/SVneo cells
(1×107 cells/ml) were collected using trypsin and
double-stained with propidium iodide and FITC-Annexin V, according
to the manufacturer's protocol. Cell apoptosis was analyzed by flow
cytometry (FACScan; BD Biosciences) and apoptotic rate was
calculated using CellQuest analysis software (version 5.1; BD
Biosciences).
Caspase-3 activity
The caspase-3 activity of HTR-8/SVneo cells was
measured using a Caspase-3 assay kit (cat. no. CASP3C-1KT;
Sigma-Aldrich; Merck KGaA) according to the manufacturer's
protocol. Briefly, the transfected cells were collected and lysed
with the lysis buffer supplied with the kit. The supernatant of the
lysed cells was collected by centrifugation at 2,000 × g for 15 min
and the supernatant was incubated with Ac-DEVE-pNA and the reaction
buffer. Caspase-3 activity was determined by measuring optical
density at 405 nm.
TOP-FLASH luciferase assay
Cells (1×106 cells/ml) were transfected
with the TOP-FLASH reporter construct (0.5 µg) together with the
Renilla luciferase vector (0.05 µg) at room temperature for
48 h using Lipofectamine 2000 reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). A total of 48 h post-transfection, luciferase
activity was measured using the Dual Luciferase Reporter Assay
system (Promega Corporation) according to the manufacturer's
protocol. Renilla luciferase activity was used as an
internal control to normalize the TOP values.
Western blot analysis
Cells were lysed with RIPA lysis buffer (Beyotime
Institute of Biotechnology) for total protein extraction. Protein
concentrations were measured by BCA protein assay (Bio-Rad
Laboratories, Inc., Hercules, USA) and equal amounts of proteins
(50 µg) were separated by 10% sodium dodecyl sulfate-polyacrylamide
gel electrophoresis and transferred onto a polyvinylidene
difluoride (PVDF) membrane. The PVDF membrane was incubated with 5%
non-fat milk for 1 h at room temperature, followed by incubation
with corresponding primary antibodies against cleaved caspase-3
(1:1,000; cat. no. ab2302; Abcam, Cambridge, UK), cleaved caspase-9
(1:1,000; cat. no. ab2324; Abcam), Bcl-2 (1:1,000; cat. no.
ab32124; Abcam), BAX (1:1,500; cat. no. ab32503; Abcam), β-catenin
(1:2,000; cat. no. ab16051; Abcam), GSK-3β (1:500; cat. no.
ab131356; Abcam), DKK-1 (1:2,000; cat. no. ab109416; Abcam), c-myc
(1:1,000; cat. no. ab39688; Abcam) and β-actin (1:2,000; cat. no.
ab8229; Abcam) overnight at 4°C. The membrane was then washed three
times with PBS, followed by incubation with horseradish
peroxidase-conjugated secondary antibody (1:5,000; cat. no.
ab205718; Abcam) at room temperature for 1 h. The western blot
bands were detected using an enhanced chemiluminescence kit (Abcam)
and protein levels were semi-quantified using Image J software
(version 1.8.0; National Institutes of Health, Bethesda, MD,
USA).
Statistical analysis
All data are presented as the mean ± standard
deviation of three experimental repeats. Data analysis was
performed using GraphPad Prism (version 6.0; GraphPad Software,
Inc.). Student's t-test or one-way ANOVA followed by Bonferroni's
multiple comparison test were used to evaluate significant
differences between groups. P<0.05 was considered to indicate a
statistically significant difference.
Results
Clinical characteristics and FAM99A
expression in placental tissues
Clinical data were collected from all participants.
The patients were divided into two groups: Normal pregnancy (n=45)
and PE pregnancy (n=45). As shown in Table I, the gestational age in the early
and late onset PE groups was lower than that in the control group,
and the body weight of infants was lower in the early and late
onset PE group compared with the control group. Furthermore, the
patients with early or late onset PE had higher systolic and
diastolic blood pressure compared with the control group, and
exhibited proteinuria.
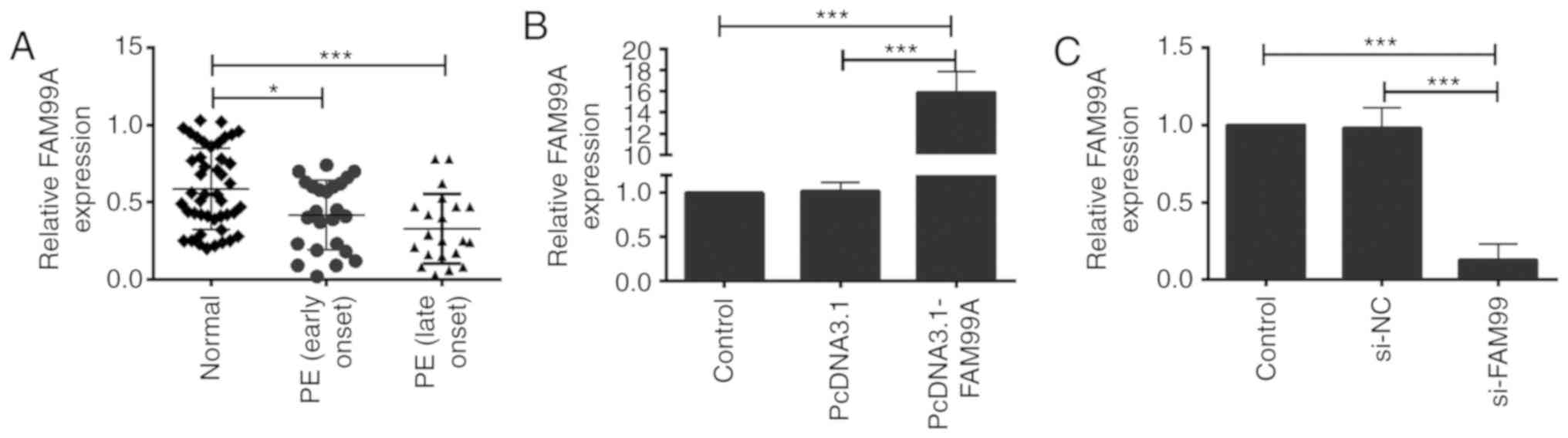
RT-qPCR was performed to determine the expression
levels of FAM99A in placental tissues from healthy women and women
with early onset (n=22) and late onset (n=23) PE. The expression
levels of FAM99A were significantly decreased in placental tissues
from women with early or late onset PE compared with in the control
group (Fig. 1A). No significant
difference was detected in FAM99A expression between placental
tissues from patients with early onset and late onset PE (Fig. 1A).
Effects of FAM99A on the proliferation
and migration of HTR-8/SVneo cells
The significant downregulation of FAM99A in
placental tissues from women with severe PE suggested a possible
role for FAM99A in PE. To examine the potential function of FAM99A
in vitro, HTR-8/SVneo trophoblast cells were used in the
present study. Transfection with pcDNA3.1-FAM99A increased the
expression levels of FAM99A in HTR-8/SVneo cells compared with in
cells transfected with pcDNA3.1 (Fig.
1B). si-FAM99A transfection significantly suppressed the
expression levels of FAM99A in HTR-8/SVneo cells compared with
transfection with si-NC (Fig. 1C).
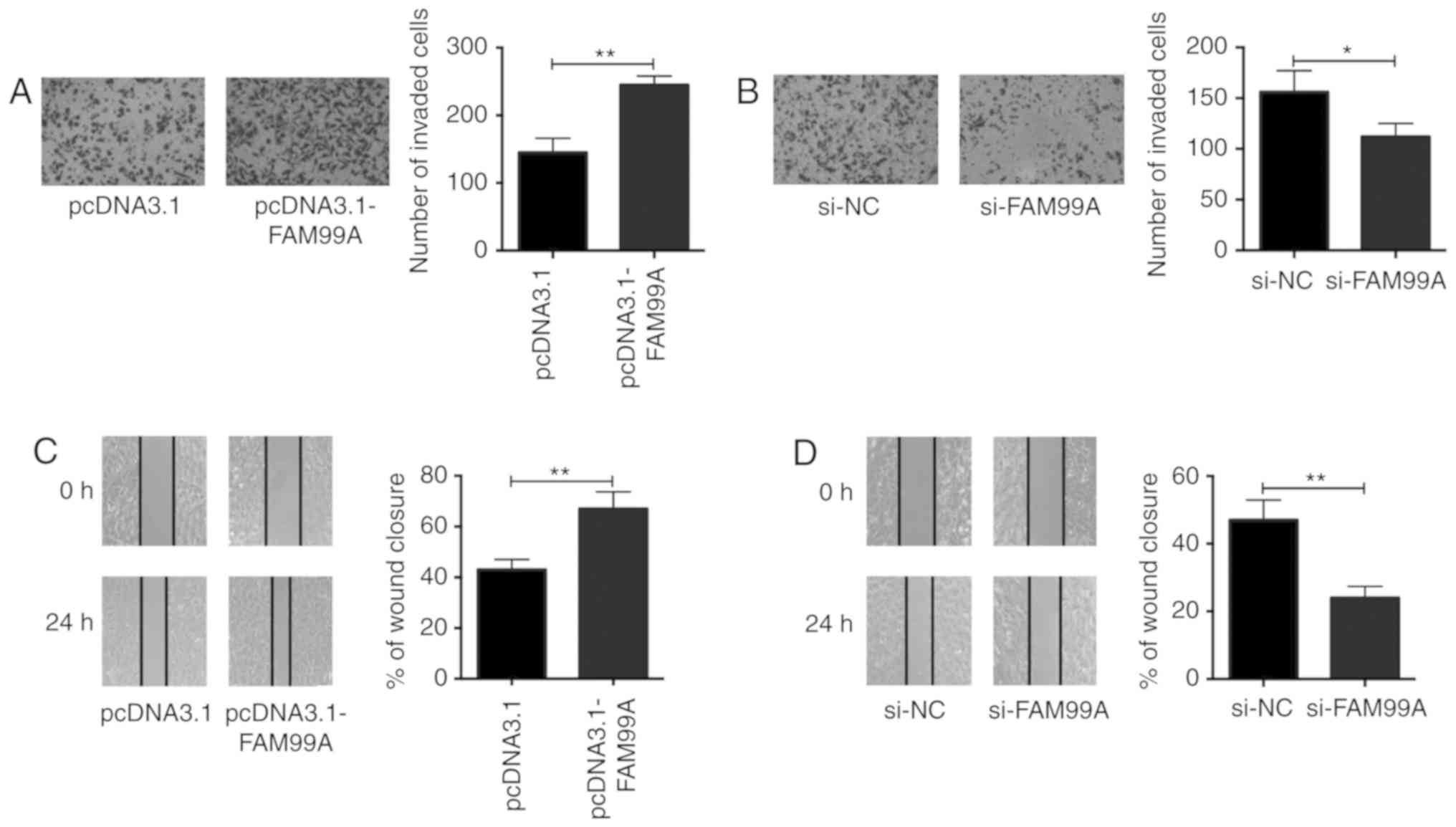
In vitro functional assays, including cell invasion and
wound healing assays, were performed to evaluate the invasive and
migratory abilities of HTR-8/SVneo cells. The cell invasion assay
revealed that FAM99A overexpression promoted invasion of
HTR-8/SVneo cells (Fig. 2A),
whereas knockdown of FAM99A suppressed invasion of HTR-8/SVneo
cells (Fig. 2B). In addition, the
wound healing assay demonstrated that overexpression of FAM99A
accelerated wound closure (Fig.
2C); however, knockdown of FAM99A suppressed wound closure in
HTR-8/SVneo cells (Fig. 2D).
Effects of FAM99A on HTR-8/SVneo cell
apoptosis
To assess the effects of FAM99A on HTR-8/SVneo cell
apoptosis, flow cytometry was performed to measure cell apoptotic
rate. The results revealed that overexpression of FAM99A decreased
the apoptotic rate of cells (Fig.
3A), whereas knockdown of FAM99A increased the apoptotic rate
of HTR-8/SVneo cells (Fig. 3B). In
addition, the effects of FAM99A on cell apoptosis-associated
protein expression were determined by western blot analysis. The
results demonstrated that FAM99A overexpression decreased cleaved
caspase-3, cleaved caspase-9 and Bax protein expression, and
increased Bcl-2 protein expression in HTR-8/SVneo cells (Fig. 3C). Knockdown of FAM99A had the
opposite effects on cleaved caspase-3, cleaved caspase-9, Bcl-2 and
Bax protein expression in HTR-8/SVneo cells (Fig. 3D). Furthermore, overexpression of
FAM99A decreased caspase-3 activity in HTR-8/SVneo cells (Fig. 3E), whereas knockdown of FAM99A
increased caspase-3 activity in HTR-8/SVneo cells (Fig. 3F).
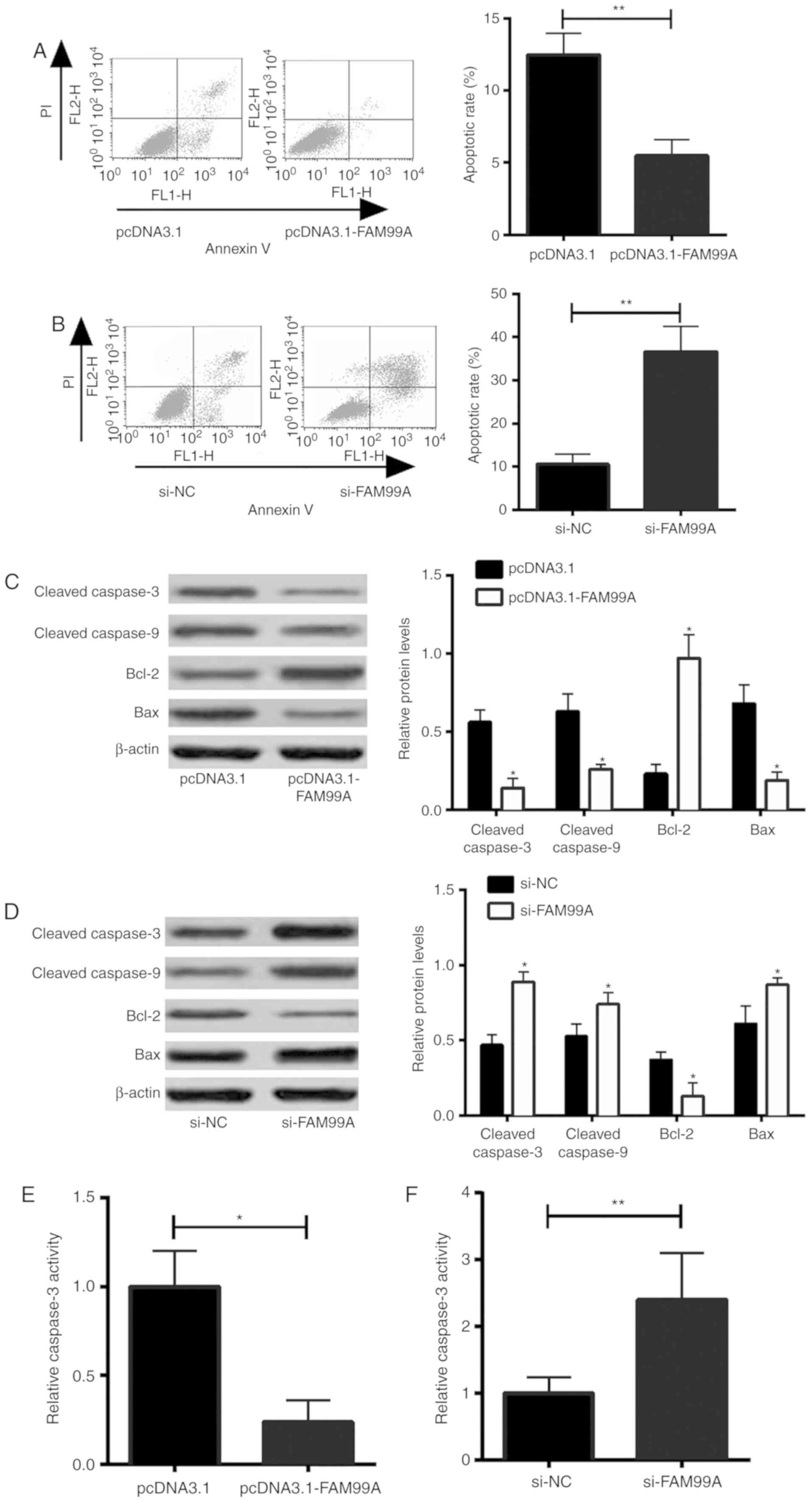 | Figure 3.Effects of FAM99A on HTR-8/SVneo cell
apoptosis. Apoptotic rate of HTR-8/SVneo cells transfected with (A)
pcDNA3.1 or pcDNA3.1-FAM99A, and (B) si-NC or si-FAM99A-transfected
HTR-8/SVneo cells was determined by flow cytometry. Cleaved
caspase-3, caspase-9, Bcl-2 and Bax protein expression in
HTR-8/SVneo cells transfected with (C) pcDNA3.1 or pcDNA3.1-FAM99A,
and (D) si-NC or si-FAM99A was determined by western blot analysis.
Caspase-3 activity of HTR-8/SVneo cells transfected with (E)
pcDNA3.1 or pcDNA3.1-FAM99A, and (F) si-NC or si-FAM99A was
determined using a caspase-3 activity assay kit. n=3. *P<0.05,
**P<0.01 vs. controls. FAM99A, family with sequence similarity
99 member A; NC, negative control; PI, propidium iodide; si, small
interfering RNA. |
Effects of FAM99A on Wnt/β-catenin
signaling in HTR-8/SVneo cells
To assess the role of FAM99A in Wnt/β-catenin
signaling activities, a TOP-FLASH luciferase assay was performed.
Overexpression of FAM99A increased TOP-FLASH activity (Fig. 4A), whereas knockdown of FAM99A
suppressed TOP-FLASH activity in HTR-8/SVneo cells (Fig. 4B), suggesting the enhanced effects
of FAM99A on the Wnt/β-catenin signaling activity. In addition,
β-catenin, glycogen synthase kinase (GSK)-3β, DKK-1 and c-myc
protein expression levels were determined by western blot analysis.
As shown in Fig. 4C,
overexpression of FAM99A increased the protein expression levels of
β-catenin, dickkopf WNT signaling pathway inhibitor 1 (DKK-1) and
c-myc, and decreased the protein expression levels of GSK-3β in
HTR-8/SVneo cells. Conversely, knockdown of FAM99A decreased the
protein expression levels of β-catenin, DKK-1 and c-myc, and
upregulated GSK-3β protein expression (Fig. 4D).
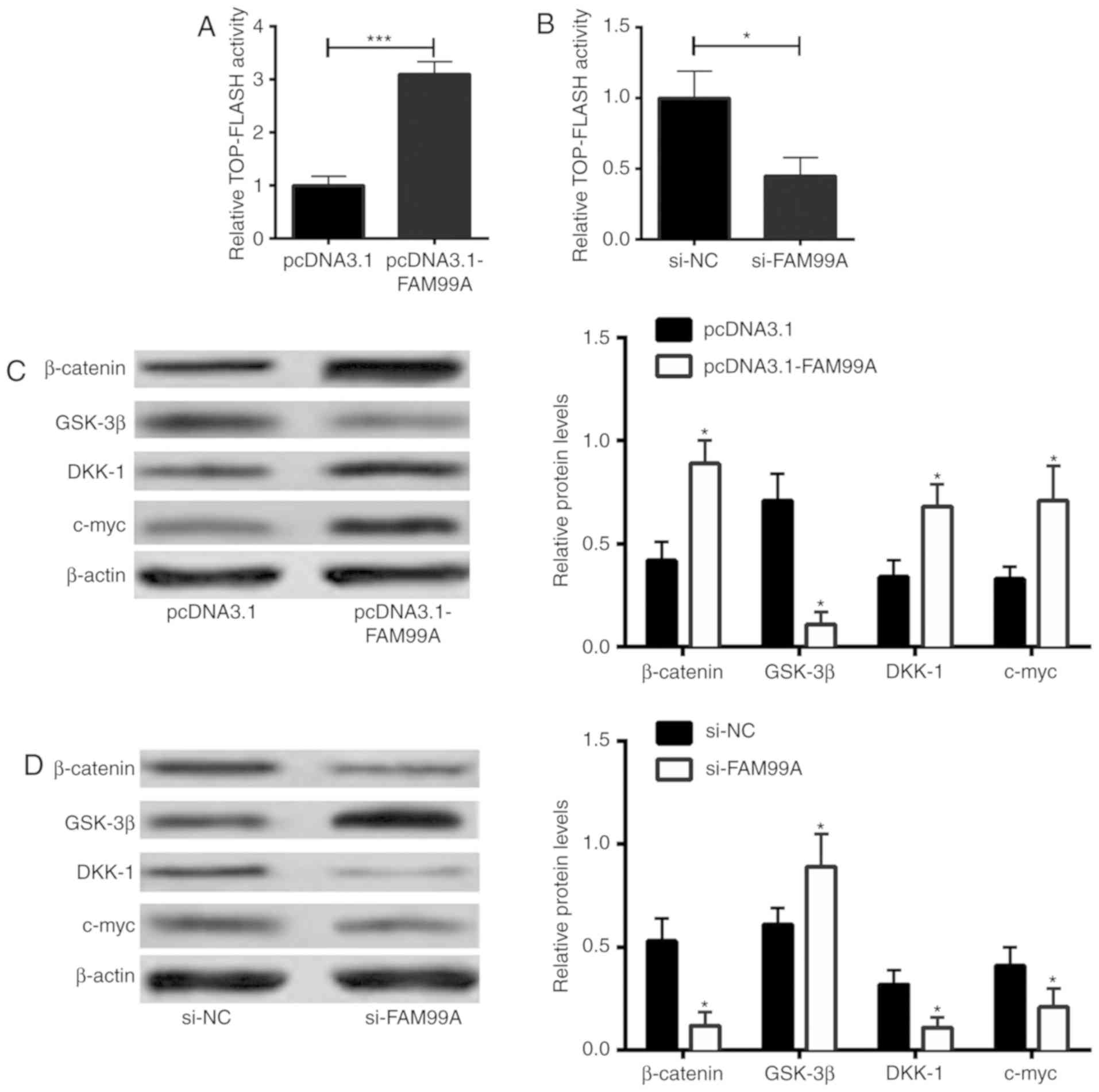 | Figure 4.Effects of FAM99A on Wnt/β-catenin
signaling in HTR-8/SVneo cells. Wnt/β-catenin signaling activity of
HTR-8/SVneo cells transfected with (A) pcDNA3.1 or pcDNA3.1-FAM99A,
and (B) si-NC or si-FAM99A was determined using the TOP-FLASH
luciferase assay. β-catenin, GSK-3β, DKK-1 and c-myc protein
expression levels in (C) pcDNA3.1- or pcDNA3.1-FAM99A-transfected,
and (D) si-NC- or si-FAM99A-transfected HTR-8/SVneo cells were
determined by western blot analysis. n=3. *P<0.05 and
***P<0.001 vs. controls. DKK-1, dickkopf WNT signaling pathway
inhibitor 1; FAM99A, family with sequence similarity 99 member A;
GSK-3β, glycogen synthase kinase-3β; NC, negative control; PI,
propidium iodide; si, small interfering RNA. |
Discussion
Recently, accumulating evidence has indicated that
lncRNAs may serve essential roles in PE development and progression
(19). The present findings
revealed that dysregulation of FAM99A could affect trophoblast cell
invasion, migration and apoptosis, suggesting a potential role for
FAM99A in the occurrence and development of PE.
Previous studies have identified associations
between lncRNAs and the pathogenesis of PE. Among these lncRNAs,
SPRY4-IT1, Uc.187 and replication protein A-interacting protein are
upregulated in placental tissues from women with severe PE, and
these lncRNAs serve suppressive roles in trophoblast cell invasion
and migration (13,20,21).
By contrast, downregulated lncRNAs (MEG3, MALAT1, H19, ATB, taurine
up-regulated 1 and plasmacytoma variant translocation 1) in
placental tissues from women with PE have been identified to
enhance trophoblast cell invasion and migration (11,12,22–25).
To date, little is known regarding the role of FAM99A in the
pathogenesis of PE. A previous study reported that FAM99A rs7131362
is associated with maternal circulating clinically relevant
triglyceride concentrations early in pregnancy (26). Petry et al (27) demonstrated that fetal FAM99A
rs1489945 is associated with maternal mean arterial blood pressure.
Microarray analysis also indicated that FAM99A is downregulated in
the placental tissues from patients with PE (14). In the present study, FAM99A was
significantly downregulated in the placental tissues from women
with PE compared with in those from healthy pregnancies, thus
suggesting that a decreased level of FAM99A may be associated with
the progression and development of PE. Previous studies have
identified that impairment of spiral artery remodeling contributes
to the pathogenesis of PE (28),
and impaired EVTs serve a key role in the pathogenesis of PE
(29). In the present study, in
vitro functional assays revealed that overexpression of FAM99A
promoted trophoblast cell invasion and migration, and inhibited
apoptosis, whereas downregulation of FAM99A suppressed trophoblast
cell invasion and migration, and induced apoptosis. These results
suggested that downregulation of FAM99A in the placental tissues
may exert a suppressive effect on trophoblasts.
The Wnt/β-catenin pathway functions to modulate
essential biological processes, such as cell invasion, migration,
apoptosis and proliferation, and it belongs to the canonical
Wnt-signaling pathway (30).
Dysregulation of the Wnt/β-catenin signaling pathway has been
reported to serve a key role in various types of human disease,
particularly the development of human cancer (31,32).
Previous evidence has indicated that abnormal activation of
Wnt/β-catenin signaling may be associated with the pathogenesis of
PE. Zhuang et al (33)
reported that the staining intensity of β-catenin is decreased in
placental tissues from women with PE. In addition, oxidative
stress-induced C/EBPβ inhibits the activities of Wnt/β-catenin
signaling, which subsequently contributes to the pathogenesis of PE
(34). A study using different
activators of Wnt/β-catenin signaling revealed that Wnt/β-catenin
signaling is closely related to trophoblast cell differentiation
(35). In addition, decreased
expression of WNT2 in the villi of patients with unexplained
recurrent spontaneous abortion may cause trophoblast cell
dysfunction via suppressing Wnt/β-catenin signaling (36). On this basis, the present study
further examined the effects of FAM99A on Wnt/β-catenin signaling
activity. The results demonstrated that overexpression of FAM99A
increased Wnt/β-catenin signaling activity, whereas knockdown of
FAM99A decreased Wnt/β-catenin signaling activity, suggesting that
the effects of FAM99A on the biological behaviors of trophoblasts
may involve modulation of Wnt/β-catenin signaling.
The present study has several limitations. Firstly,
the expression of FAM99A in the human placenta throughout gestation
was not known. Future studies in which the expression profile of
FAM99A in the peripheral blood from patients with PE is monitored
throughout gestation are required to confirm the role of FAM99A in
the pathogenesis of PE. Secondly, the timing of caesarean section
varied among the patients and this may have contributed to the
differential expression of FAM99A. Thirdly, as various factors may
affect the expression of FAM99A in clinical samples, such as fetal
distress and pre-labor rupture of membranes, caution should be
applied in interpreting the results. Future studies may investigate
these factors in order to verify the present findings regarding the
role of FAM99A in PE.
In conclusion, the present results indicated that
the lncRNA FAM99A was downregulated in placental tissues from
patients with PE. Downregulation of FAM99A may lead to suppressed
cell invasion and migration, and increased cell apoptosis in
trophoblasts, which may impair the process of spiral artery
remodeling. The biological effects of FAM99A on trophoblasts may
involve modulation of Wnt/β-catenin signaling.
Acknowledgements
The authors would like to thank Dr L. Zhang
(Department of Biomedical Sciences, Xi'an Medical University) for
his support in performing statistical analysis.
Funding
The present study was supported by The Northwest
Women and Children's Hospital.
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
YC designed the study. TH, YQ and YL performed the
experiments, analyzed the data and wrote the manuscript. JW and RH
performed the statistical analysis. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
All experiments were approved by the Ethics
Committee of The Northwest Women and Children's Hospital, and
written informed consent was obtained from all the recruited
subjects.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Kurtz WS, Glueck CJ, Hutchins RK, Sisk RA
and Wang P: Retinal artery and vein thrombotic occlusion during
pregnancy: Markers for familial thrombophilia and adverse pregnancy
outcomes. Clin Ophthalmol. 10:935–938. 2016.PubMed/NCBI
|
|
2
|
Powe CE, Levine RJ and Karumanchi SA:
Preeclampsia, a disease of the maternal endothelium: The role of
antiangiogenic factors and implications for later cardiovascular
disease. Circulation. 123:2856–2869. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gao L, Qi HB, Kamana KC, Zhang XM, Zhang H
and Baker PN: Excessive autophagy induces the failure of
trophoblast invasion and vasculature: Possible relevance to the
pathogenesis of preeclampsia. J Hypertens. 33:106–117. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Salomon C, Yee SW, Mitchell MD and Rice
GE: The possible role of extravillous trophoblast-derived exosomes
on the uterine spiral arterial remodeling under both normal and
pathological conditions. Biomed Res Int. 2014:6931572014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Saito S and Nakashima A: A review of the
mechanism for poor placentation in early-onset preeclampsia: The
role of autophagy in trophoblast invasion and vascular remodeling.
J Reprod Immunol. 101-102:80–88. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hung TH, Chen SF, Lo LM, Li MJ, Yeh YL and
Hsieh TT: Increased autophagy in placentas of intrauterine
growth-restricted pregnancies. PLoS One. 7:e409572012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sharp AN, Heazell AE, Crocker IP and Mor
G: Placental apoptosis in health and disease. Am J Reprod Immunol.
64:159–169. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wieczorek E and Reszka E: mRNA, microRNA
and lncRNA as novel bladder tumor markers. Clin Chim Acta.
477:141–153. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Freedman JE and Miano JM; National Heart,
Lung, and Blood Institute Workshop Participants, : Challenges and
opportunities in linking long noncoding RNAs to cardiovascular,
lung, and blood diseases. Arterioscler Thromb Vasc Biol. 37:21–25.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Tian L, Zheng F, Li Z, Wang H, Yuan H,
Zhang X, Ma Z, Li X, Gao X and Wang B: miR-148a-3p regulates
adipocyte and osteoblast differentiation by targeting
lysine-specific demethylase 6b. Gene. 627:32–39. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Y, Zou Y, Wang W, Zuo Q, Jiang Z,
Sun M, De W and Sun L: Down-regulated long non-coding RNA MEG3 and
its effect on promoting apoptosis and suppressing migration of
trophoblast cells. J Cell Biochem. 116:542–550. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen H, Meng T, Liu X, Sun M, Tong C, Liu
J, Wang H and Du J: Long non-coding RNA MALAT-1 is downregulated in
preeclampsia and regulates proliferation, apoptosis, migration and
invasion of JEG-3 trophoblast cells. Int J Clin Exp Pathol.
8:12718–12727. 2015.PubMed/NCBI
|
|
13
|
Zuo Q, Huang S, Zou Y, Xu Y, Jiang Z, Zou
S, Xu H and Sun L: The Lnc RNA SPRY4-IT1 modulates trophoblast cell
invasion and migration by affecting the epithelial-mesenchymal
transition. Sci Rep. 6:371832016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He X, He Y, Xi B, Zheng J, Zeng X, Cai Q,
Ouyang Y, Wang C, Zhou X, Huang H, et al: LncRNAs expression in
preeclampsia placenta reveals the potential role of LncRNAs
contributing to preeclampsia pathogenesis. PLoS One. 8:e814372013.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Peng L, Liu Z, Xiao J, Tu Y, Wan Z, Xiong
H, Li Y and Xiao W: MicroRNA-148a suppresses epithelial-mesenchymal
transition and invasion of pancreatic cancer cells by targeting
Wnt10b and inhibiting the Wnt/β-catenin signaling pathway. Oncol
Rep. 38:301–308. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tranquilli AL, Brown MA, Zeeman GG, Dekker
G and Sibai BM: The definition of severe and early-onset
preeclampsia. Statements from the international society for the
study of hypertension in pregnancy (ISSHP). Pregnancy Hypertens.
3:44–47. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Graham CH, Hawley TS, Hawley RG,
MacDougall JR, Kerbel RS, Khoo N and Lala PK: Establishment and
characterization of first trimester human trophoblast cells with
extended lifespan. Exp Cell Res. 206:204–211. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
McAninch D, Roberts CT and Bianco-Miotto
T: Mechanistic insight into long noncoding RNAs and the placenta.
Int J Mol Sci. 18:E13712017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zou Y, Jiang Z, Yu X, Sun M, Zhang Y, Zuo
Q, Zhou J, Yang N, Han P, Ge Z, et al: Upregulation of long
noncoding RNA SPRY4-IT1 modulates proliferation, migration,
apoptosis, and network formation in trophoblast cells HTR-8SV/neo.
PLoS One. 8:e795982013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Song X, Rui C, Meng L, Zhang R, Shen R,
Ding H, Li J, Li J and Long W: Long non-coding RNA RPAIN regulates
the invasion and apoptosis of trophoblast cell lines via complement
protein C1q. Oncotarget. 8:7637–7646. 2017.PubMed/NCBI
|
|
22
|
Zuckerwise L, Li J, Lu L, Men Y, Geng T,
Buhimschi CS, Buhimschi IA, Bukowski R, Guller S, Paidas M and
Huang Y: H19 long noncoding RNA alters trophoblast cell migration
and invasion by regulating TβR3 in placentae with fetal growth
restriction. Oncotarget. 7:38398–38407. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu X, Chen H, Kong W, Zhang Y, Cao L, Gao
L and Zhou R: Down-regulated long non-coding RNA-ATB in
preeclampsia and its effect on suppressing migration,
proliferation, and tube formation of trophoblast cells. Placenta.
49:80–87. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu Y, Ge Z, Zhang E, Zuo Q, Huang S, Yang
N, Wu D, Zhang Y, Chen Y, Xu H, et al: The lncRNA TUG1 modulates
proliferation in trophoblast cells via epigenetic suppression of
RND3. Cell Death Dis. 8:e31042017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Xu H, Sun Q, Lu L, Luo F, Zhou L, Liu J,
Cao L, Wang Q, Xue J, Yang Q, et al: MicroRNA-218 acts by
repressing TNFR1-mediated activation of NF-κB, which is involved in
MUC5AC hyper-production and inflammation in smoking-induced
bronchiolitis of COPD. Toxicol Lett. 280:171–180. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Petry CJ, Koulman A, Lu L, Jenkins B,
Furse S, Prentice P, Matthews L, Hughes IA, Acerini CL, Ong KK and
Dunger DB: Associations between the maternal circulating lipid
profile in pregnancy and fetal imprinted gene alleles: A cohort
study. Reprod Biol Endocrinol. 16:822018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Petry CJ, Sanz Marcos N, Pimentel G, Hayes
MG, Nodzenski M, Scholtens DM, Hughes IA, Acerini CL, Ong KK, Lowe
WL Jr and Dunger DB: Associations between fetal imprinted genes and
maternal blood pressure in pregnancy. Hypertension. 68:1459–1466.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Freitag N, Tirado-González I, Barrientos
G, Herse F, Thijssen VL, Weedon-Fekjær SM, Schulz H, Wallukat G,
Klapp BF, Nevers T, et al: Interfering with Gal-1-mediated
angiogenesis contributes to the pathogenesis of preeclampsia. Proc
Natl Acad Sci USA. 110:11451–11456. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Palei AC, Spradley FT, Warrington JP,
George EM and Granger JP: Pathophysiology of hypertension in
pre-eclampsia: A lesson in integrative physiology. Acta Physiol
(Oxf). 208:224–233. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hoppler S and Kavanagh CL: Wnt signalling:
Variety at the core. J Cell Sci. 120:385–393. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Krishnamurthy N and Kurzrock R: Targeting
the Wnt/beta-catenin pathway in cancer: Update on effectors and
inhibitors. Cancer Treat Rev. 62:50–60. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Nusse R and Clevers H: Wnt/β-catenin
signaling, disease, and emerging therapeutic modalities. Cell.
169:985–999. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhuang B, Luo X, Rao H, Li Q, Liu X and Qi
H: Expression and significance of SATB1 and wnt/β-catenin signaling
molecule in the placenta of preeclampsia. Zhonghua Fu Chan Ke Za
Zhi. 50:283–290. 2015.(In Chinese). PubMed/NCBI
|
|
34
|
Zhuang B, Luo X, Rao H, Li Q, Shan N, Liu
X and Qi H: Oxidative stress-induced C/EBPβ inhibits β-catenin
signaling molecule involving in the pathology of preeclampsia.
Placenta. 36:839–846. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kumar P, Thirkill TL, Ji J, Monte LH and
Douglas GC: Differential effects of sodium butyrate and lithium
chloride on rhesus monkey trophoblast differentiation. PLoS One.
10:e01350892015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang Y, Wang Y, Liang Q, Yao L, Gu S and
Bai X: MiR-338-5p promotes inflammatory response of fibroblast-like
synoviocytes in rheumatoid arthritis via targeting SPRY1. J Cell
Biochem. 118:2295–2301. 2017. View Article : Google Scholar : PubMed/NCBI
|