Introduction
Cerebral aneurysm (CA) is one of the most common
clinical cerebrovascular diseases, occurring in 1–5% of the general
population (1). Most unruptured
CAs are thought to be asymptomatic or present with common symptoms,
such as chronic headache (2).
Anatomical features, demographic characteristics and medical
conditions were confirmed to be associated with the increasing risk
of rupture in CAs. Ruptured CAs are the most common cause of
subarachnoid hemorrhage (SAH). SAH affects 6–12 individual per
100,000 of the population each year and causes worldwide mortality
and morbidity. Approximately 50% of SAH patients succumb to the
disease, and half of the surviving patients suffer from
complications. Although microsurgical clipping and endovascular
coiling have become established therapies for CAs, effective
noninvasive medical therapies are still indispensable (3,4).
Consequently, it is crucial to discover novel effective medical
treatments to prevent CA growth and rupture.
Numerous studies have suggested that chronic
inflammation plays an important role in CA formation and
progression (5–9). Nuclear factor (NF)-κB-mediated
macrophage recruitment through the upregulation of monocyte
chemoattractant protein-1 (MCP-1) expression is an important
process in the inflammatory response. NF-κB also regulates the
expression of inflammation-associated genes, such as interleukin
(IL)-1β, inducible nitric oxide synthase (iNOS) and matrix
metalloproteinases (MMPs) (10).
IL-1β and iNOS may cause apoptosis in vascular smooth muscle cells,
leading to endothelial damage and disruption of the internal
elastic lamina (11). MMP-2 and
MMP-9 are involved in vessel wall remodeling occurring as a result
of collagen degradation and breakdown (12). Due to its vital role in CA, NF-κB
may serve as a therapeutic target for CA treatment.
Tanshinone IIA (Tan IIA) is a major component
isolated from a traditional Chinese medicine called Danshen
(Salvia miltiorrhiza Bunge), which is widely used for the
treatment of various diseases, such as cardiovascular diseases,
diabetes and cancer (13–15). Tan IIA was found to inhibit the
tumor necrosis factor (TNF)-α-induced increase in phospho-AKT and
NF-κB DNA-binding, leading to human aortic smooth muscle cell
migration and increased MMP-9 activity (16). However, the function of Tan IIA in
CA treatment has not yet been reported.
The main aim of the present study was to investigate
the role and possible mechanism of Tan IIA in CA formation in rat
models, and to provide potential clinical therapeutic approaches
for the suppression of CA formation and subarachnoid
hemorrhage.
Materials and methods
Induction of CAs in rats
All animal experiments were performed in accordance
with the Guide for the Care and Use of Laboratory Animals by the
National Institutes of Health. The handling procedures were
approved by the Institutional Review Board of Nanjing Medical
University. Male Sprague Dawley rats (n=6/group) aged 7 weeks were
used for CA induction, as previously described (17). All rats were housed on hard wood
chip bedding at 23°C and supplied with standard rat chow and water
ad libitum. The housing room followed a 12-h light/dark
cycle (lights on at 19:00 h). The rats underwent surgery under
general anesthesia with ketamine/xylazine [ketamine 80 mg/kg,
xylazine 10 mg/mg intraperitoneal (i.p.) injection], and the left
common carotid artery, which induced hemodynamic stress, and left
renal artery were ligated simultaneously. The treated rats were
then housed in standard facilities fed a high-salt diet containing
8% sodium chloride to induce systemic hypertension. Blood pressure
was measured by the tail-cuff method without anesthesia (Kent
Scientific Corp., Torrington, CT, USA). Rats were euthanized by
deep anesthesia (ketamine 240 mg/kg, xylazine 30 mg/kg, i.p.
injection) 4 weeks after CA induction, and then perfused
transcardially with phosphate-buffered saline followed by 4%
paraformaldehyde. The sacrifice of the rats after deep anesthesia
was confirmed by observing for the absence of movement, respiratory
and heartbeat activity for at least 3 min. Degenerative change
assessments of vascular walls were performed using Verhoeff-Van
Gieson staining. Aneurysm size and vascular wall thickness were
assessed by independent observers in a blinded manner. Macrophage
infiltration was examined by hematoxylin and eosin staining.
RNA isolation and
reverse-transcription quantitative polymerase chain reaction
(RT-qPCR)
Total RNA was extracted from homogenized tissues
using TRIzol reagent (Thermo Fisher Scientific, Inc., Waltham, MA,
USA), according to the manufacturer's instructions. cDNAs were
reversed transcribed from RNA samples using PrimeScript 1st Strand
cDNA Synthesis kit (Takara Bio, Inc., Otsu, Japan) and applied as
templates. RT-qPCR was performed using an ABI 7500 qPCR system
(Thermo Fisher Scientific, Inc.) with FastStart Universal SYBR
Green Master (Roche Diagnostics, Indianapolis, IN, USA), according
to the manufacturer's instructions. The cycling steps of the
reactions were 94°C for 15 sec, 60°C for 30 sec and 72°C for 30
sec. Each reaction was performed in triplicate in a final volume of
20 µl. RT-qPCR data were analyzed following the 2−ΔΔCq
method and normalized to GAPDH (18). Primers used were as followed: NF-κB
forward 5′-ACGATCTGTTTCCCCTCATC-3′ and reverse
5′-TGCTTCTCTCCCCAGGAATA-3′; MCP-1 forward
5′-CCTCCACCACTATGCAGGTCTC-3′ and reverse 5′-GCACGTGGATGCTACAGGC-3′;
MMP-2 forward 5′-CTGATAACCTGGATGCAGTCGT-3′ and reverse
5′-CCAGCCAGTCCGATTTGA-3′; MMP-9 forward 5′-TTCAAGGACGGTCGGTATT-3′
and reverse 5′-CTCGAGCCTAGACCCAACTTA-3′; GAPDH forward
5′-AAGAAGGTGGTGAAGCAGGC-3′ and reverse
5′-TCCACCACCCTGTTGCTGTA-3′.
Western blotting assay
Proteins were isolated from homogenized tissues that
were incubated with ice-cold RIPA lysis buffer containing 1 mM PMSF
for 10 min and centrifuged at 12,000 × g for 10 min at 4°C. Protein
concentration was measured by Pierce BCA Protein Assay kit (Thermo
Fisher Scientific, Inc.). Protein (40 mg) was separated using a 10%
SDS-PAGE gel and transferred onto 0.2 µm PVDF membranes (Roche
Diagnostics). Following blocking with 3% (w/v) BSA in TBST buffer
at room temperature, the membranes were incubated with primary
antibodies overnight at 4°C. HRP-conjugated secondary antibodies
were incubated with washed membranes for 1 h at room temperature.
Pierce ECL Western Blotting Substrate (Thermo Fisher Scientific,
Inc.) was used to detect the proteins. Antibodies used in this
assay were as follows: NF-κB (1:1,000, ab16502, Abcam, Cambridge,
USA), actin (1:5,000, ab179467, Abcam), Lamin B (1:10,000,
ab16048), MMP-9 (1:2,000, ab76003, Abcam) and GAPDH (1:5,000,
AC002, ABclonal Biotech Co., Ltd., Wuhan, China).
Cell culture
The murine macrophage RAW 264.7 cell line was
cultured in 6-well plates at a density of 1×106 cells
per well. Cells were maintained in DMEM with 10% (v/v) FBS, 100
IU/ml penicillin G, 100 µg/ml streptomycin, and 2 mM L-glutamine at
37°C in a humidified atmosphere containing 5% CO2. To
set up the Tan IIA-treated groups, the cells were treated the next
day with or without 10 µM Tan IIA (Merck KGaA, Darmstadt, Germany)
in DMSO for 2 h, and then stimulated with 1 µg/ml LPS or treated
with DMSO (control) for 24 h.
Cell proliferation and migration
assays
The viability of RAW 264.7 cells was assessed using
the Cell Counting Kit-8 kit (CCK-8, Dojindo, Japan). RAW 264.7
cells were plated at 1×104 in 96-well plates containing
6 parallel wells and treated with DMSO, LPS or LPS+Tan IIA. During
the following 72 h of incubation, 10 µl CCK-8 was added into each
well every 24 h. The plates were incubated with CCK-8 for 2 h, and
then the absorbance value was measured at 450 nm wavelength every
24 h using a microplate reader (PerkinElmer, Inc., Waltham, MA,
USA).
Next, 1×105 RAW 264.7 cells were
suspended into the upper chamber of the Transwell apparatus
(Corning Inc., Corning, NY, USA) in 100 ml serum-free medium, while
the bottom chamber contained medium with 10% FBS. Following
incubation for 24 h, the cells on the upper surface that did not
pass through the filter were removed using a moistened cotton swab.
The cells on the lower membrane surface were fixed with pure
methanol and stained with 0.1% crystal violet for 20 min. The
migrated cells were observed and counted using an inverted
microscope at ×100 magnification (Olympus Corporation, Tokyo,
Japan). Cell migration was expressed as a percentage of DMSO
control and results are shown as mean ± standard deviation from 3
independent experiments.
ELISA
Following the different treatments, supernatants of
the RAW 264.7 cells were collected for ELISA. Rat TNFα, Rat IL-6
and Rat MCP-1/CCL2 ELISA kits (Wuhan Boster Biological Technology,
Ltd., Wuhan, China) and Rat Pro IL1β ELISA kit (Wuhan Abebio
Science Co., Ltd., Wuhan, China) were used to measured TNFα, IL-6,
MCP-1 and IL-1β in the cell supernatants, respectively, according
to the manufacturer's instructions.
Gelatin zymography assay
To measure gelatinolytic activities of MMP-9 in the
treated cells, gelatin zymography assay was performed as previously
described (19). Briefly, protein
concentration was measured and protein (100 mg) was mixed with 4X
non-reducing sample buffer at room temperature for 10 min and
subjected to 10% SDS-PAGE containing 0.1% (w/v) gelatin. The
electrophoresis was performed at a constant voltage (125 V) for 90
min at 4°C. The gel was then incubated with 1X diluted renaturing
solution containing 2.5% (v/v) Trixon-100 solution for 30 min twice
at room temperature with gentle agitation. The gel was then washed
with 1X developing buffer for an additional 30 min at room
temperature, and the buffer was replaced with fresh developing
buffer and incubated at 37°C overnight. A staining solution was
used to stain the gel for at least 1 h and the gel was destained
with destaining solution until MMP-9 gelatinolytic activity
appeared as clearly transparent bands (92 kDa) against the blue
background.
Statistical analysis
All results are expressed as the mean ± standard
deviation from multiple independent experiments. The Student's
t-test was used to derive the significance between mean values of
two groups and one-way ANOVA with Tukey's honestly significant
difference (HSD) post hoc test was used in multiple comparisons.
Data analysis was performed with the statistical program GraphPad
Prism (GraphPad Software, Inc.). P<0.05 was considered to
indicate a statistically significant difference.
Results
Tan IIA suppresses CA formation in
rats
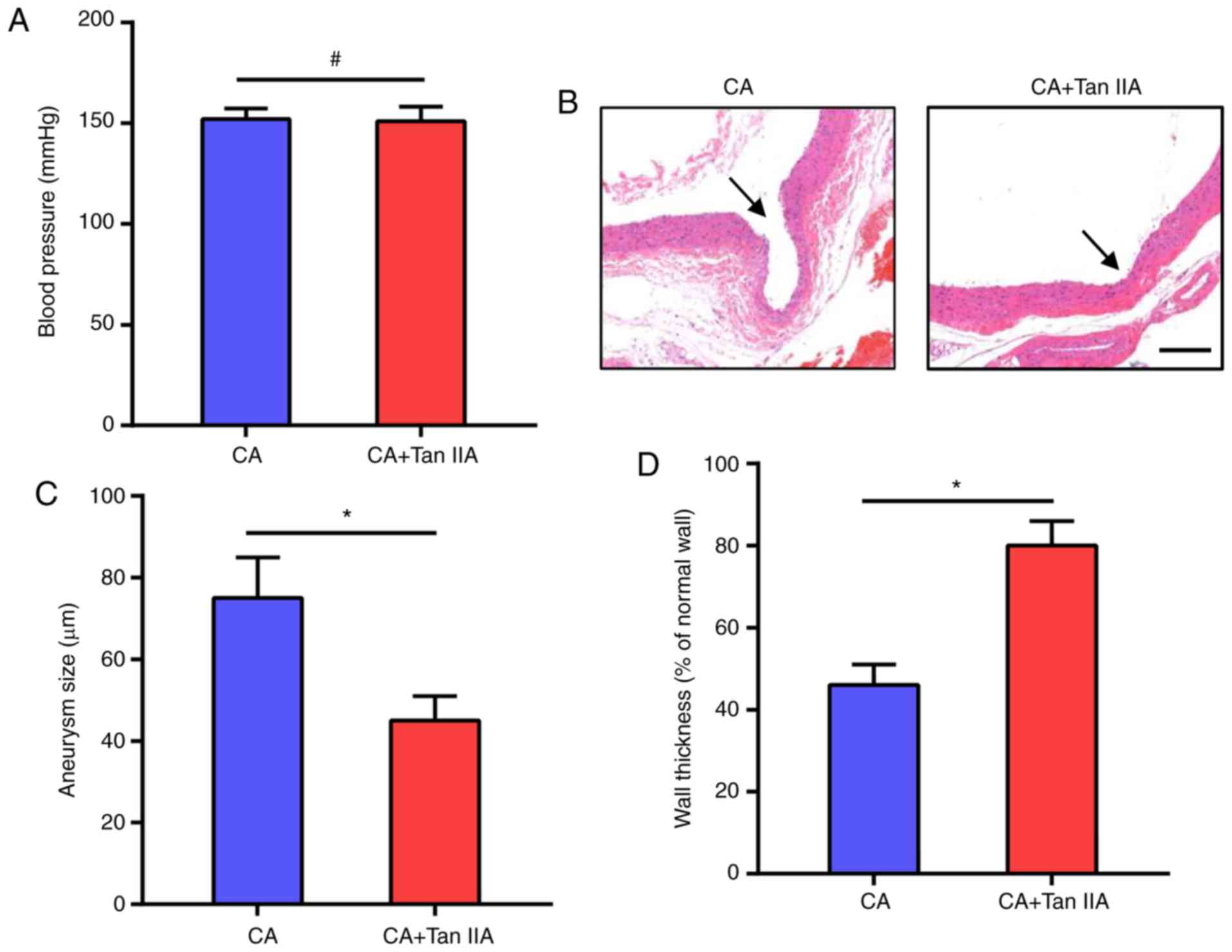
To investigate the effect of Tan IIA on CA
formation, Tan IIA was injected i.p. into rats for 4 weeks after CA
formation. The CAs in the rat models were induced by systemic
hypertension, thus we first examined the effect of Tan IIA on blood
pressure. As shown in Fig. 1A, Tan
IIA did not affect blood pressure which was increased in the rats
after the surgeries. The morphologic evaluation suggested that Tan
IIA treatment suppressed the growth of CAs in the rats (Fig. 1B). The CA+Tan IIA group exhibited a
significant decrease in aneurysm size, as compared with the CA
group (Fig. 1C). Tan IIA treatment
also increased wall thickness, as compared with the CA group
(Fig. 1D). These data suggest that
Tan IIA can inhibit CA formation in rat models.
Tan IIA reduces inflammatory response
in aneurysmal walls
As macrophage-mediated chronic inflammation plays a
vital role in CA formation, the effects of Tan IIA on macrophage
infiltration into the CAs were examined. It was found that the
CA+Tan IIA group exhibited less macrophage infiltration, as
compared with the CA group (Fig.
2A). As the NF-κB signaling network is essential for macrophage
infiltration, NF-κB and downstream MCP-1, MMP-2 and MMP-9 mRNA
levels in the aneurysmal walls were tested. The results indicated
that the expression levels of these genes were significantly
reduced in the CA+Tan IIA group, as compared with the CA group
(Fig. 2B-E). In addition, NF-κB
protein levels were decreased in the rats treated with Tan IIA
(Fig. 2F and G). These data
suggested that the NF-κB pathway in macrophages is the target of
Tan IIA in the suppression of CA formation.
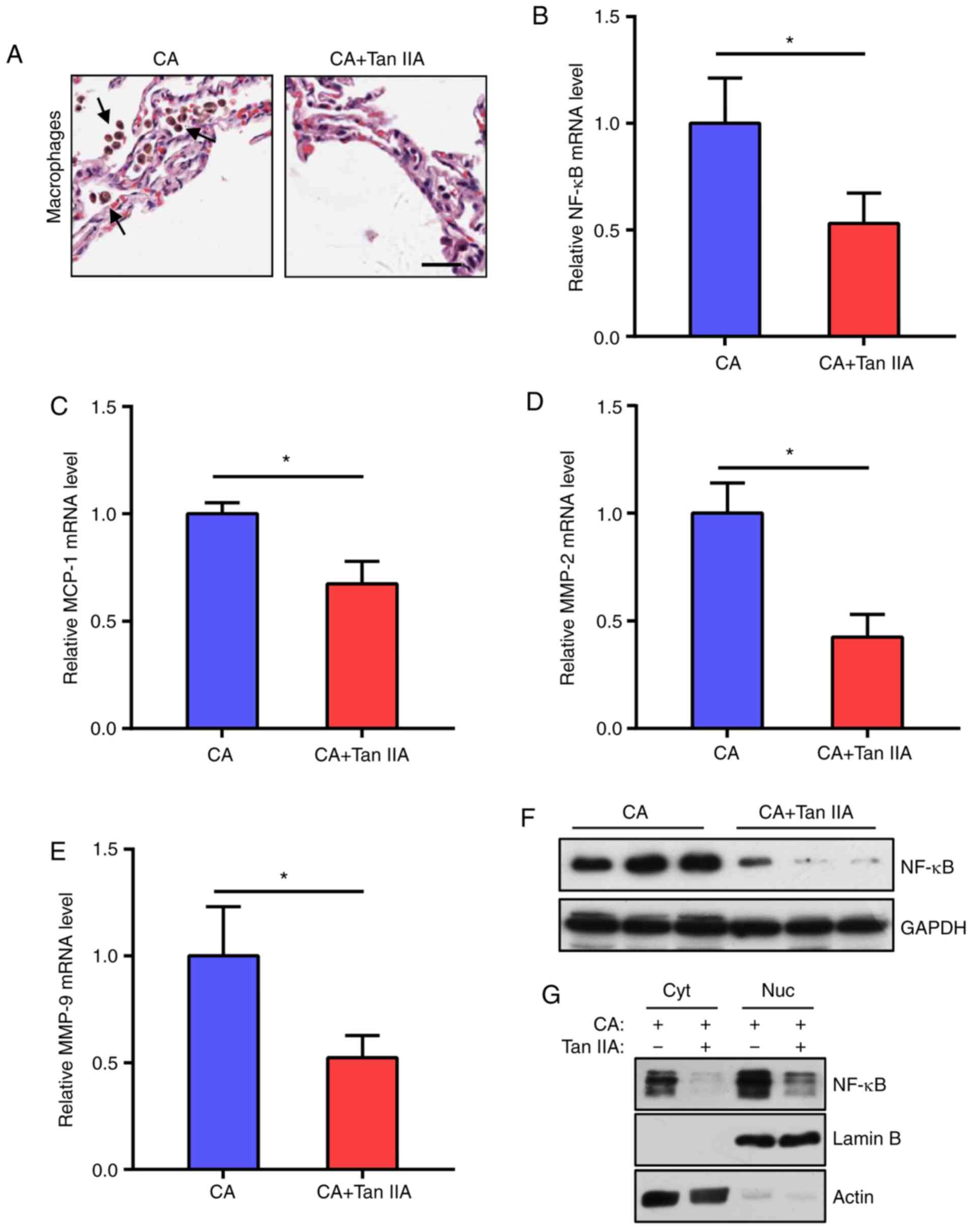 | Figure 2.Tan IIA reduces the inflammatory
response in aneurysmal walls. (A) Representative images of H&E
staining of aneurysmal walls. Arrows indicate macrophages. Scale
bar, 10 µm. (B-E) RT-qPCR analysis of mRNA expression levels of
NF-κB (B) MCP-1 (C) MMP-2 (D) and MMP-9 (E) normalized to GAPDH in
the CA and CA+Tan IIA groups. Data are presented as the mean ±
standard deviation (n=6/group), *P<0.05 vs. the CA group. (F)
Western blot analysis of NF-κB expression levels in the aneurysmal
walls of the CA and CA+Tan IIA groups (n=3/group). GAPDH served as
the loading control. (G) Western blot analysis of NF-κB
distribution in aneurysmal walls of the CA and CA+Tan IIA groups.
Cyt, cytoplasm, Nuc, nucleus. Lamin B served as the loading control
of the nucleus. Actin served as the loading control of the
cytoplasm. Tan IIA, tanshinone IIA; CA, cerebral aneurysm; RT-qPCR,
reverse transcription quantitative polymerase chain reaction;
NF-κB, nuclear factor-κB; MMP, matrix metalloproteinase. |
Tan IIA treatment ameliorates
inflammatory cytokine upregulation in LPS-stimulated RAW 264.7
cells
RAW 264.7 is an Abelson murine leukemia virus
transformed macrophage-like cell line derived from BALB/c mice and
is commonly used as an appropriate model of macrophages (20). LPS-stimulated RAW 264.7 murine
macrophage cells were used to evaluate the anti-inflammatory effect
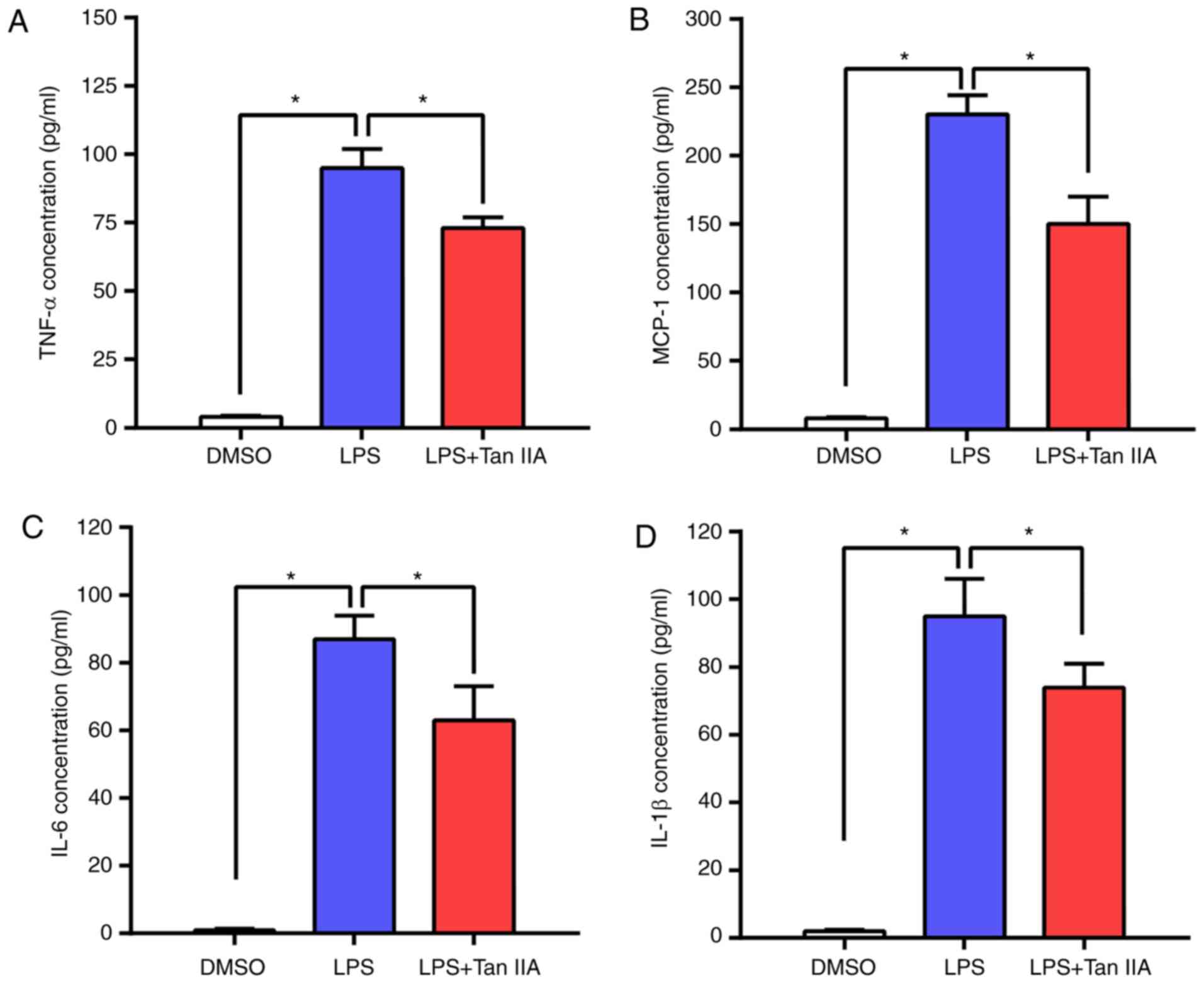
of Tan IIA. The concentrations of four main inflammatory cytokines
(TNFα, MCP-1, IL-6 and IL-1β) in RAW 264.7 cells were detected by
ELISA. As shown in Fig. 3A-D, LPS
led to an elevated production of these inflammatory cytokines in
RAW 264.7 cells, while Tan IIA treatment attenuated this effect.
These results indicated that Tan IIA can ameliorate the
inflammatory response triggered by LPS by reducing cytokine
production in RAW 264.7 cells.
Tan IIA restores the proliferation and
migration capacity of LPS-stimulated RAW 264.7 cells
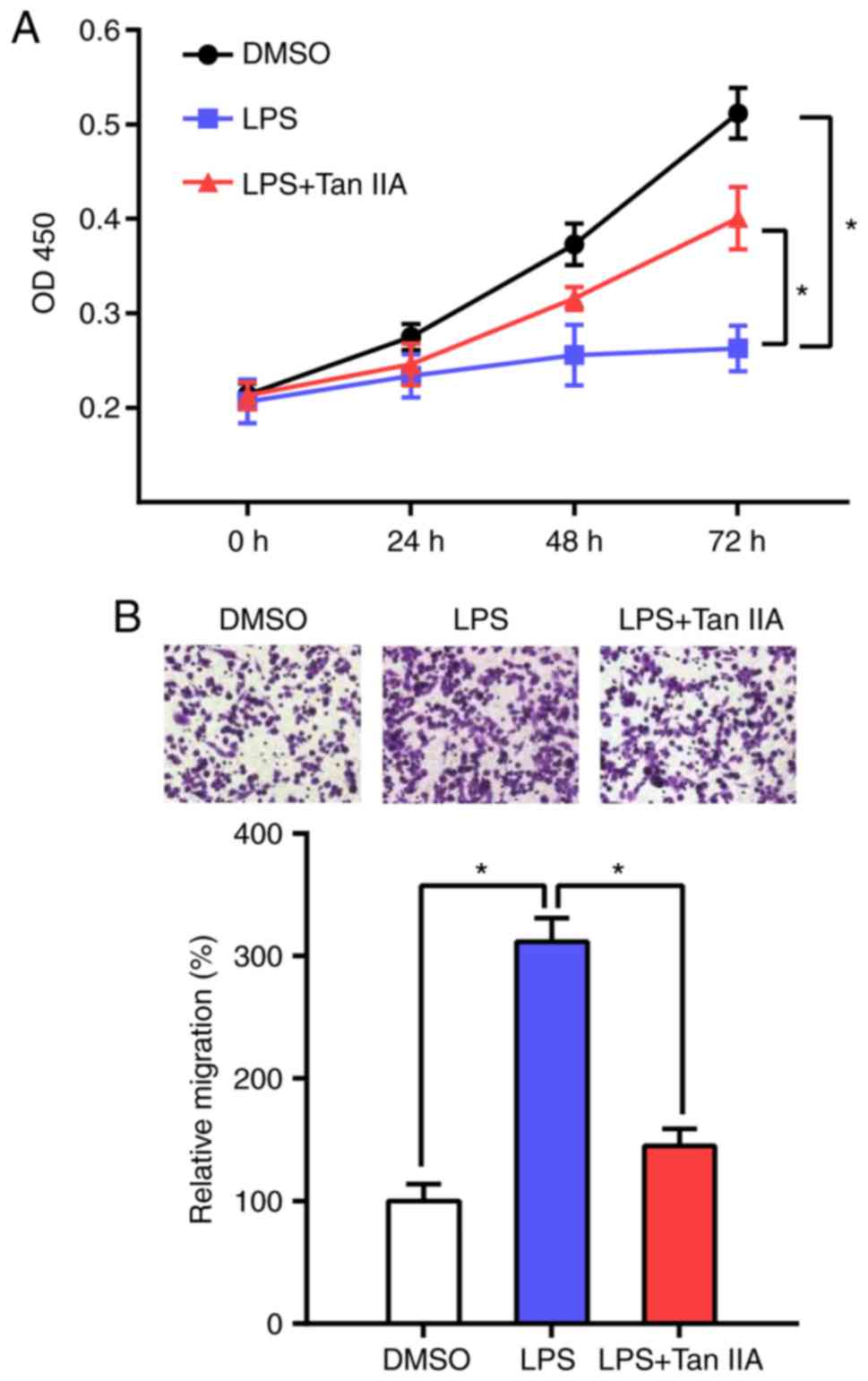
It has been reported that LPS stimulation induces
the differentiation of RAW 264.7 cells and inhibits their
proliferation (21). The present
results indicated that Tan IIA can reverse this process and
increase the proliferation of RAW 264.7 cells (Fig. 4A). Next, the effect of Tan IIA on
LPS-induced cell migration was determined by Transwell assay. The
results showed that the migration ability of RAW 264.7 cells was
markedly increased following LPS stimulation, whereas Tan IIA
treatment attenuated the effect of LPS on RAW 264.7 cell
migration.
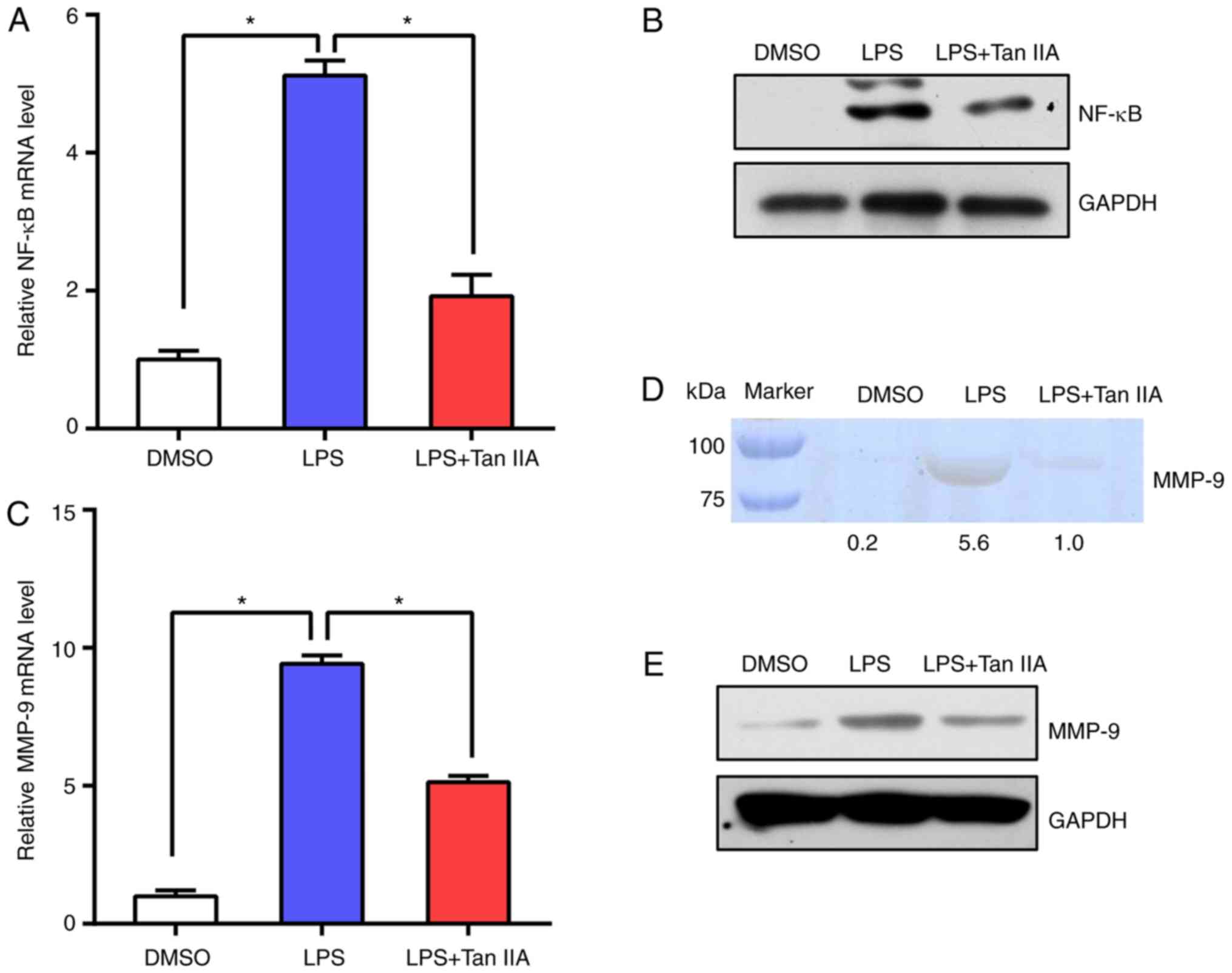
Subsequently, NF-κB mRNA and protein levels were
tested in RAW 264.7 cells. The results suggested that NF-κB
expression was upregulated following LPS stimulation and Tan IIA
treatment suppressed NF-κB activation (Fig. 5A and B). In addition, the levels of
MMP-9 in RAW 264.7 cells were detected by RT-qPCR and gelatin
zymography. The data indicated that the levels of MMP-9 were
notably induced by LPS, while Tan IIA treatment abolished the
effect of LPS on the upregulation of MMP-9 (Fig. 5C-E). These results suggested that
Tan IIA can suppress the NF-κB signaling pathway elicited by LPS in
RAW 264.7 cells.
Discussion
This is the first study to explore the function of
Tan IIA in CAs in rat models. In the present study, it was
demonstrated that Tan IIA treatment could inhibit CA formation in
rats through the suppression of macrophage infiltration. In
addition, Tan IIA was shown to exert an anti-inflammatory effect on
CAs, manifested by the suppression of NF-κB expression and
subsequent inflammatory activation in aneurysmal walls.
In rat models, increased hemodynamic stress at the
bifurcation sites of intracranial arteries was found to cause
prolonged inflammation and trigger formation and progression of
CAs, which is consistent with the disease pathogenesis in humans
(17,22). This model is widely used to explore
the mechanisms underlying human CA formation and growth. In the
past few decades, numerous studies have identified the presence of
inflammatory responses in CA lesions and have suggested that
inflammatory processes play a vital role in the pathogenesis of CAs
(5–9). NF-κB is a key transcription factor
regulating the expression of a series of inflammation-related
genes. Chronic inflammatory responses such as macrophage
infiltration, cytokine release and matrix metalloproteinase
expression caused by abnormally activated NF-κB promotes CA
formation and growth (12,23–25).
It has been reported that NF-κB p50 subunit deficiency or
inhibition of NF-κB activity with chimeric decoy oligonucleotides
may suppress both CA formation and progression (12), which indicates that the NF-κB
signaling pathway is a promising target for the clinical treatment
of CA.
Tan IIA is a diterpenoid naphthoquinone found in the
traditional Chinese medicine Danshen. It has diverse
pharmacological effects, including anti-inflammatory, antioxidant
and antibacterial, and is extensively used in China for the
treatment of cancer, liver and cardiovascular diseases and diabetes
(13–15). Although several studies have
investigated the function of Tan IIA in different animal models of
human diseases (26,27), the effect of Tan IIA on CA
formation and progression has not been reported. In the present
study, it was found that Tan IIA treatment prevented the process of
macrophage infiltration and degeneration of aneurysmal walls. This
effect was due to the inhibition of NF-κB and MCP-1 expression.
Similar findings were reported in a myocardial infarction rat model
(28). In addition, MMP-2 and MMP
−9 expressions were also decreased in the Tan IIA-treated group.
These MMPs are mainly secreted by infiltrated macrophages and play
a crucial role in tissue remodeling (29). In human CAs, MMP-2 and MMP-9
expression was found to be markedly elevated in the serum and
aneurysmal walls of patients (30). However, several studies have
demonstrated that MMP-2 does not contribute to CA formation
(31,32). Although there are some conflicting
studies concerning the function of MMPs in CA pathogenesis, the
results suggest that Tan II treatment reduces MMP-2 and MMP-9
levels and subsequently alleviates destruction in aneurysmal
walls.
Macrophages derived from blood monocytes play a
vital role in all stages of the inflammatory response (33). The LPS-stimulated RAW 264.7 murine
macrophage cell model is a common experimental macrophage model for
anti-inflammatory drug screening. LPS can induce NF-κB activation
and cytokine production in RAW 264.7 cells. The present data
demonstrated that Tan IIA treatment reduced cytokine levels and
NF-κB expression, indicating that Tan IIA has an anti-inflammatory
effect in RAW 264.7 cells. In addition, Tan IIA could reverse
LPS-induced RAW 264.7 cell differentiation, which was characterized
by decreased proliferation and increased migration. The expression
of MMP-9 was also affected by Tan IIA. These results are consistent
with the previous findings as discussed above.
In conclusion, the present findings suggest that Tan
IIA attenuates inflammatory responses in CA formation through
inhibition of NF-κB signaling and macrophage infiltration. These
findings may furnish novel therapeutic candidates for the treatment
of CAs.
Acknowledgements
Not applicable.
Funding
This study was supported by grants from the Jiangsu
Province Traditional Chinese Medicine Bureau (grant no. YB2017093)
and the Science and Technology Development Key Projects of Nanjing
Medical University (grant no. 2016NJMUZD026).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
JM, DH, GQ and NL designed and organized the study.
JM, DH, ZW, JZ and HL performed the experiments. JM, DH, ZL, XW and
YL analyzed and interpreted the data. GQ, ZL, XW and YL helped to
draft the introduction and results section of the paper. JM and NL
wrote and revised the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
All animal experiments were performed in accordance
with the Guide for the Care and Use of Laboratory Animals by the
National Institutes of Health. The handling procedures were
approved by the Institutional Review Board of Nanjing Medical
University
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Vlak MHM, Algra A, Brandenburg R and
Rinkel GJ: Prevalence of unruptured intracranial aneurysms, with
emphasis on sex, age, comorbidity, country, and time period: A
systematic review and meta-analysis. Lancet Neurol. 10:626–636.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baron EP: Headache, cerebral aneurysms,
and the use of triptans and ergot derivatives. Headache.
55:739–747. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bowles E: Cerebral aneurysm and aneurysmal
subarachnoid haemorrhage. Nurs Stand. 28:52–59. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
van Gijn J, Kerr RS and Rinkel GJ:
Subarachnoid haemorrhage. Lancet. 369:306–318. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pera J, Korostynski M, Krzyszkowski T,
Czopek J, Slowik A, Dziedzic T, Piechota M, Stachura K, Moskala M,
Przewlocki R and Szczudlik A: Gene expression profiles in human
ruptured and unruptured intracranial aneurysms: What is the role of
inflammation? Stroke. 41:224–231. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hosaka K and Hoh BL: Inflammation and
cerebral aneurysms. Transl Stroke Res. 5:190–198. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tuttolomondo A, Di Sciacca R, Di Raimondo
D, Pedone C, La Placa S, Pinto A and Licata G: Effects of clinical
and laboratory variables and of pretreatment with cardiovascular
drugs in acute ischaemic stroke: A retrospective chart review from
the GIFA study. Int J Cardiol. 151:318–322. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Di Raimondo D, Tuttolomondo A, Buttà C,
Miceli S, Licata G and Pinto A: Effects of ACE-inhibitors and
angiotensin receptor blockers on inflammation. Curr Pharm Des.
18:4385–4413. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Licata G, Tuttolomondo A, Corrao S, Di
Raimondo D, Fernandez P, Caruso C, Avellone G and Pinto A:
Immunoinflammatory activation during the acute phase of lacunar and
non-lacunar ischemic stroke: Association with time of onset and
diabetic state. Int J Immunopathol Pharmacol. 19:639–646. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Aoki T, Kataoka H, Shimamura M, Nakagami
H, Wakayama K, Moriwaki T, Ishibashi R, Nozaki K, Morishita R and
Hashimoto N: NF-kappaB is a key mediator of cerebral aneurysm
formation. Circulation. 116:2830–2840. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moriwaki T, Takagi Y, Sadamasa N, Aoki T,
Nozaki K and Hashimoto N: Impaired progression of cerebral
aneurysms in interleukin-1β-deficient mice. Stroke. 37:900–905.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Aoki T, Kataoka H, Morimoto M, Nozaki K
and Hashimoto N: Macrophage-derived matrix metalloproteinase-2 and
−9 promote the progression of cerebral aneurysms in rats. Stroke.
38:162–169. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhang M, Qian Y and Tang A: Research
progress of pharmacologic actions of TanshinoneIIA. Med
Recapitulate. 16:2661–2664. 2010.
|
|
14
|
Wang X: Progress of pharmacological
research and clinical application of Tanshinone II A. Guang Ming J
Chin Med. 26:1514–1517. 2011.
|
|
15
|
Hosaka K, Downes DP, Nowicki KW and Hoh
BL: Modified murine intracranial aneurysm model: Aneurysm formation
and rupture by elastase and hypertension. J Neurointerv Surg.
6:474–479. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Toth M, Sohail A and Fridman R: Assessment
of gelatinases (MMP-2 and MMP-9) by gelatin zymography. Methods Mol
Biol. 878:121–135. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li Y, Gu B, Liu J, Xiong X, Zhou C and Wu
G: Research progress of Tanshinone II A. Li ShiZhen Med Materia Med
Res. 21:1770–1772. 2010.
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jin UH, Suh SJ, Chang HW, Son JK, Lee SH,
Son KH, Chang YC and Kim CH: Tanshinone IIA from Salvia
miltiorrhiza BUNGE inhibits human aortic smooth muscle cell
migration and MMP-9 activity through AKT signaling pathway. J Cell
Biochem. 104:15–26. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hartley JW, Evans LH, Green KY, Naghashfar
Z, Macias AR, Zerfas PM and Ward JM: Expression of infectious
murine leukemia viruses by RAW264.7 cells, a potential complication
for studies with a widely used mouse macrophage cell line.
Retrovirology. 5:12008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Saxena RK, Vallyathan V and Lewis DM:
Evidence for lipopolysaccharide-induced differentiation of RAW264.7
murine macrophage cell line into dendritic like cells. J Biosci.
28:129–134. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jou LD, Lee DH, Morsi H and Mawad ME: Wall
shear stress on ruptured and unruptured intracranial aneurysms at
the internal carotid artery. AJNR Am J Neuroradiol. 29:1761–1767.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chalouhi N, Ali MS, Jabbour PM,
Tjoumakaris SI, Gonzalez LF, Rosenwasser RH, Koch WJ and Dumont AS:
Biology of intracranial aneurysms: Role of inflammation. J Cereb
Blood Flow Metab. 32:1659–1676. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Starke RM, Raper DM, Ding D, Chalouhi N,
Owens GK, Hasan DM, Medel R and Dumont AS: Tumor necrosis factor-α
modulates cerebral aneurysm formation and rupture. Transl Stroke
Res. 5:269–277. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Starke RM, Chalouhi N, Ali MS, Jabbour PM,
Tjoumakaris SI, Gonzalez LF, Rosenwasser RH, Koch WJ and Dumont AS:
The role of oxidative stress in cerebral aneurysm formation and
rupture. Curr Neurovasc Res. 10:247–255. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Tang FT, Cao Y, Wang TQ, Wang LJ, Guo J,
Zhou XS, Xu SW, Liu WH, Liu PQ and Huang HQ: Tanshinone IIA
attenuates atherosclerosis in ApoE(−/-) mice through
down-regulation of scavenger receptor expression. Eur J Pharmacol.
650:275–284. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ji X, Tan BK, Zhu YC, Linz W and Zhu YZ:
Comparison of cardioprotective effects using ramipril and DanShen
for the treatment of acute myocardial infarction in rats. Life Sci.
73:1413–1426. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ren ZH, Tong YH, Xu W, Ma J and Chen Y:
Tanshinone II A attenuates inflammatory responses of rats with
myocardial infarction by reducing MCP-1 expression. Phytomedicine.
17:212–218. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ota R, Kurihara C, Tsou TL, Young WL,
Yeghiazarians Y, Chang M, Mobashery S, Sakamoto A and Hashimoto T:
Roles of matrix metalloproteinases in flow-induced outward vascular
remodeling. J Cereb Blood Flow Metab. 29:1547–1558. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jin D, Sheng J, Yang X and Gao B: Matrix
metalloproteinases and tissue inhibitors of metalloproteinases
expression in human cerebral ruptured and unruptured aneurysm. Surg
Neurol. 68 (Suppl):S11–S16. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Nuki Y, Matsumoto MM, Tsang E, Young WL,
van Rooijen N, Kurihara C and Hashimoto T: Roles of macrophages in
flow-induced outward vascular remodeling. J Cereb Blood Flow Metab.
29:495–503. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Pannu H, Kim DH, Guo D, King TM, Van
Ginhoven G, Chin T, Chang K, Qi Y, Shete S and Milewicz DM: The
role of MMP-2 and MMP-9 polymorphisms in sporadic intracranial
aneurysms. J Neurosurg. 105:418–423. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Fujiwara N and Kobayashi K: Macrophages in
inflammation. Curr Drug Targets Inflamm Allergy. 4:281–286. 2005.
View Article : Google Scholar : PubMed/NCBI
|