Introduction
Alzheimer's disease (AD) is a multifactorial
neurodegenerative disorder that mostly affects the elderly.
Prevalence studies have revealed that >35 million people are
suffering from AD worldwide, and this number is predicted to reach
>100 million by the year 2050, if new preventive or
neuroprotective therapies do not emerge (1). The neuropathology of AD is
characterized by extracellular deposition of amyloid β (Aβ)
plaques, and intracellular neurofibrillary tangles and loss of
neurons in the brain (2). Although
the mechanisms of neuronal cell death in AD still remain unknown,
the deposition of Aβ has been reported to be neurotoxic both in
vitro and in vivo, involving reactive oxygen species
(ROS) generation, inflammation, and an increase in intracellular
Ca2+ (3–8). A large number of studies have
confirmed that the excessive production of Aβ itself leads to
Aβ-induced free radical generation and elevated oxidative stress,
leading to cell death (9). Thus,
one promising preventive or therapeutic intervention in AD may be
to attenuate or suppress oxidative stress-dependent, Aβ-mediated
cytotoxicity.
Plants are a major component of diets that possess
neuroprotective effects, including antioxidant and
anti-inflammatory effects, and can improve memory and cognitive
functions (10–13). The most important advantages of the
medicinal use of plants are their minimal side effects and their
relatively low cost, as compared to synthetic medicines. According
to the World Health Organization, ~80% of the world's population
currently uses medicinal plants for their primary healthcare
(14), and the current trend is to
conduct investigations into plant-based medicines. Therefore,
searching for plants that can attenuate oxidative stress might be a
useful strategy for preventing and/or treating Aβ-induced
neurotoxicity. Oroxylum indicum (L.), also known as ‘broken
bones plant’, ‘Indian trumpet flower’, ‘Shyonaka’ and ‘Midnight
horror’, belongs to the Bignoniaceae family, which is widely
distributed in tropical countries, such as India, Taiwan, Cambodia,
Laos, Myanmar, Indonesia, Malaysia, Vietnam, Nepal, China, the
Philippines and Thailand (15).
Oroxylum indicum has been used in traditional herbal
medicine in Asian countries for the treatment of various diseases
over several centuries (16).
Studies have indicated that almost all parts of the plant possesses
medicinal properties (15), mainly
antioxidant, anti-inflammatory, anticancer and immunomodulatory
properties. Other effects, such as antibacterial and
gastro-protective, have also been reported. The principal active
components of this plant are the flavonoids chrysin, oroxylene A
and baicalein (15,17). Other secondary metabolites, such as
triterpene, carboxylic acid, ursolic acid, glycosides, tannins,
alkaloids and terpenoids, have also been identified. Although many
medicinal properties of Oroxylum indicum have been
demonstrated, the effects of Oroxylum indicum extract on
Aβ-induced oxidative stress have not, to our knowledge, been
investigated. The aim of the present study was to investigate the
protective effect of Oroxylum indicum (L.) fruit pod extract
against Aβ25-35-induced oxidative stress and injury in SH-SY5Y
cells. The mechanisms underlying its neuroprotection were also
investigated. Phenolic compounds and flavonoids have been shown to
be very good antioxidants (18),
thus their concentration in Oroxylum indicum extract was
also determined. The fruit pod of Oroxylum indicum was
selected for the present study, since this part is more readily
edible than other parts (17).
Aβ25–35 was chosen because this fragment is an active toxic
fragment of Aβ 1–42 peptides (19), and it has been reported that
Aβ25–35 and Aβ 1–42 have similar effects in inducing neuronal cell
death and neuritic atrophy (20,21).
SH-SY5Y cells were selected as a model, since they are commonly
used in AD research and differentiated SH-SY5Y cells have displayed
properties similar to those of mature neurons (22).
Materials and methods
Chemicals and antibodies
SH-SY5Y cell line was purchased from the American
Type Culture Collection (Manassas, VA, USA; catalog number
CRL-2266). Cell culture reagents, including penicillin/streptomycin
were from Thermo Fisher Scientific, Inc. (Waltham, MA, USA).
DMEM/F12 medium was from Thermo Fisher Scientific, Inc. and fetal
bovine serum (FBS) from Gemini Bio-Products (West Sacramento, CA,
USA). Non-essential amino acids and all-trans retinoic acid (RA)
were from Merck KGaA (Darmstadt, Germany). The ROS detection kit
was also obtained from Merck KGaA. The catalase activity kit was
from Biovision Inc. (Milpitas, CA, USA), and the superoxide
dismutase (SOD) activity kit from Cayman Chemical Company (Ann
Arbor, MI, USA). The lactate dehydrogenase (LDH) kit, together with
Aβ25–35 and 3-(3,4-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium
bromide (MTT) were from Merck KGaA. The LDH assay kit was from
Thermo Fisher Scientific, Inc., and the caspase 3/7 activity kit
was from Promega Corporation (Madison, WI, USA). Antibodies against
total- and phosphor(p)-Akt, and p-cAMP-responsive element binding
protein (CREB) were from Cell Signaling Technology, Inc., (Danvers,
MA, USA). Secondary anti-rabbit or anti-mouse antibodies and ECL
detection kits were from GE Healthcare (Chicago, IL, USA). RIPA
buffer, protease and phosphatase inhibitor cocktail, calcein-AM and
quercetin were obtained from Merck KGaA. The BCA protein assay was
from Thermo Fisher Scientific, Inc.
Plant material and extraction
Fruits of Oroxylum indicum were collected
from Maha Sarakham Province, Thailand, in July 2016. Species
identification was performed by members of the Applied Thai
Traditional Medicine Department, Faculty of Medicine, Mahasarakham
University. A specimen was deposited at the herbarium at the
Faculty of Science, Mahasarakham University (specimen no.
MSUT_7234). An ethanolic extract of Oroxylum indicum was
prepared by drying the fruits, then weighing and chopping them, and
macerating them in 95% (v/v) ethanol for 7 days at room temperature
(RT). The extract was then filtered, concentrated using a rotary
evaporator and lyophilized. The % yield of extract was 12.89% per
dry weight of Oroxylum indicum fruits.
Determination of total flavonoid
content
The total flavonoid content of Oroxylum
indicum crude extract was determined by the aluminum chloride
colorimetric method. In brief, 100 µl of 1 mg/ml Oroxylum
indicum crude extract was mixed with 0.9 ml flavonoid mixture
(10% aluminum hydroxide, 1 M potassium acetate; dilution, 0.1, 0.1
and 4.3 ml). The mixture was incubated for 30 min at RT in the
dark, and the absorbance intensity was measured at 450 nm. The
total flavonoid content was calculated from a calibration curve and
the result was expressed as mg rutin equivalent per g dry
weight.
Determination of total phenol
content
The total phenolic content of the Oroxylum
indicum extract was determined using the Folin-Ciocalteu
method. In brief, 100 µl of 1 mg/ml Oroxylum indicum crude
extract was mixed thoroughly with 0.45 ml Folin-Ciocalteu reagent
for 5 min, followed by the addition of 0.45 ml of 60 g/l sodium
bicarbonate. The mixture was kept in the dark for a further 1 h at
RT, and the absorbance intensity was measured at 650 nm. The total
phenolic content was calculated from the calibration curve, and the
results were expressed as mg of gallic acid equivalent per g dry
weight.
Cell culture
Human neuroblastoma SH-SY5Y cells were maintained in
DMEM/F12 medium supplemented with 10% FBS, 1%
penicillin-streptomycin and 1% non-essential amino acids at 37°C in
a humidified atmosphere containing 5% CO2. The culture
medium was changed every 3 days. Cells were plated at an
appropriate density according to each experiment. Cells were
differentiated with 10 µM all-trans retinoic acid over 6 days prior
to treatments. At the beginning of each experiment, the culture
medium in each well was completely removed and replaced with fresh
medium containing Aβ with or without Oroxylum extract.
Preparation of Aβ25–35 stock
solution
Aβ25–35 peptide was dissolved in deionized distilled
water as a 1 mM stock solution and incubated at 37°C for 3 days.
The solution was aliquoted into 1-ml tubes, kept at −20°C and
thawed for subsequent use.
Cell viability assay
The in vitro cytotoxicity of Aβ25–35 and
Oroxylum indicum were determined using the MTT assay.
SH-SY5Y cells were plated in 96-well plates at a density of
1×104 cells/well and cultured as described above. Cells
were treated with various concentrations of Aβ25–35 (10–30 µM) and
Oroxylum indicum (0–100 µg/ml) in 1% FBS for 24 h. To
determine whether Oroxylum indicum protects against
Aβ-induced neurotoxicity, cells were treated with Aβ25–35 with or
without of Oroxylum indicum extract in 1% FBS for 24 h.
Following 24 h of treatment, the medium was removed and replaced
with MTT reagent at a final concentration of 0.5 mg/ml. Cells were
then incubated for 4 h at 37°C in 5% CO2 in an
incubator. Following incubation, MTT reagent was aspirated and 100
µl dimethyl sulfoxide was added to dissolve the insoluble purple
formazan product. Absorbance was determined at 570 nm using a
Synergy-4 plate reader (BioTek Instruments, Inc Winooski, VT, USA).
Results were expressed as a percentage of the control.
Intracellular ROS assay
Cells were seeded at a density of 1×104
cells/well in 96-well plates and cultured as described above. After
24 h, intracellular ROS levels were measured using the fluorescent
probe 2′,7′-dichlorofluorescein, as previously described (13). Data were expressed as the
percentage of ROS relative to untreated controls.
Analysis of cell injury by the LDH
assay
Cells were plated at a density 1×104
cells/well in 96-well plates. Cells were then cultured and treated
as described above. Following 24 h of treatment, Aβ-induced cell
injury was measured by determining how much of the intracellular
enzyme LDH had been released into the culture medium. Culture
medium (100 µl) was collected from each well and transferred to a
new 96 well plate, and 100 µl reaction mixture was added to each
well and incubated for 30 min at 37°C. Absorbance was measured at
492 nm using a microplate reader. The quantity of LDH released was
then expressed as a percentage of the untreated control.
Determination of catalase (CAT)
activity assay
Cells were plated at a density 1×105
cells/well in 6-well plates. Overnight cultured cells were treated
as described above. Following 24 h of treatment, cells were
harvested with a rubber policeman and collected by centrifugation
(2,000 × g for 10 min at 4°C). The cell pellets were homogenized in
cold assay buffer and centrifuged at 10,000 × g for 15 min at 4°C.
The supernatant was also collected for the assay. Catalase activity
was determined using a commercially available assay kit (Biovision
Inc.), according to the manufacturer's instructions.
SOD activity assay
Cells (1×105 cells/well) were plated in
6-well plates and were then cultured and treated as described
above. Following 24 h of treatment, cells were harvested as
described for the CAT activity assay. Cell pellets were then
homogenized in 20 mM cold HEPES buffer, and centrifuged at 1,500 ×
g for 5 min at 4°C. The supernatant was also collected for the
assay. Superoxide dismutase activity was measured using an assay
kit from Cayman Chemical, according to the manufacturer's
instructions. Results were expressed as a percentage of the
untreated control.
Western blotting
Cells (1×105 cells/well) were plated in
6-well plates, and then cultured and treated as described above.
Following treatment, cells were collected and total protein
concentration was determined using a BCA kit. Equal amounts of
proteins were separated by 4–20% SDS-polyacrylamide gel and
transferred onto nitrocellulose membranes. The membranes were then
incubated with primary antibodies against Bcl-2 (1:1,000), p-Akt,
total Akt (1:1,000), p-CREB (1:1,000) and actin (1:5,000) overnight
at 4°C. The membranes were then washed with TBST (Tris-buffered
saline, 0.1% Tween 20), and probed with secondary antibody
conjugated with HRP for 1 h at RT. Protein bands were detected
using an enhanced chemiluminescence detection kit, and results were
expressed as a fold change of the untreated control.
Detection of caspase-3/7 activity in
cell culture
Caspase-3/7 activity was measured using
Caspase-Glo® 3/7 kits from Promega Corporation,
according to the manufacturer's instructions. Briefly, the
caspase-GloR 3/7 buffer and lyophilized caspase-GloR 3/7 substrate
were equilibrated at RT. The contents of the caspase-GloR 3/7
buffer were transferred into the bottle containing caspase-GloR3/7
substrate. Equal volumes of reaction mixture were added to samples
and incubated for 30 min-2 h prior to the luminescence measurement.
Results were expressed as a percentage of the untreated
control.
Statistical analysis
All data are expressed as the mean ± SEM from at
least three independent experiments performed in triplicate.
Multiple comparisons of data were evaluated by one-way ANOVA
followed by Bonferroni post-hoc test. A P<0.05 was considered to
indicate a statistically significant difference.
Results
Effects of Aβ25–35 on the viability of
differentiated and undifferentiated SH-SY5Y cells
Since the SH-SY5Y cell line shares only a few
properties with mature neurons (23), it is important to differentiate
these cells with retinoic acid, so that they are comparable to
in vivo models. Undifferentiated SH-SY5Y cells have been
used as a model of cytotoxicity in several studies (24–26),
but the comparative cytotoxic effects of Aβ on cell survival of
RA-differentiated and undifferentiated SH-SY5Y cells has not yet
been reported. Therefore, the purpose of this study was to compare
the in vitro cytotoxicity of Aβ25–35 between differentiated
and undifferentiated SH-SY5Y cells. Both differentiated and
undifferentiated cells were treated with various concentrations of
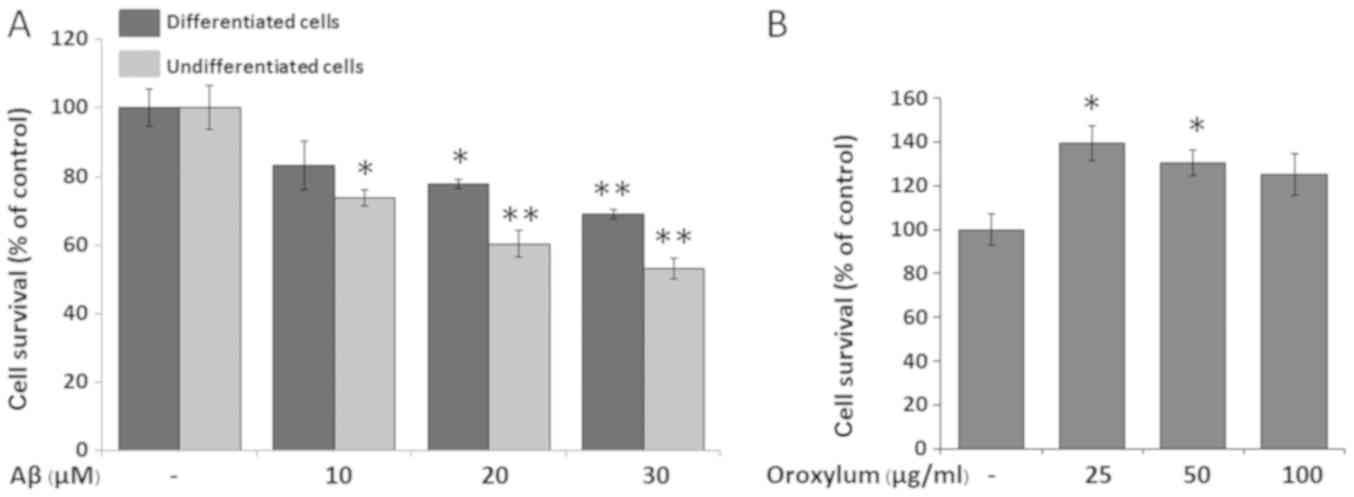
Aβ25–35 (0–30 µM). As shown in Fig.
1A, Aβ25–35 treatments were toxic to both cell groups,
beginning at 10 µM for undifferentiated cells and 20 µM for
differentiated cells. Although undifferentiated SH-SY5Y cells were
more susceptible to Aβ25–35 than differentiated cells, the
differentiated cells possess more neuron-like properties, including
neurite outgrowth and morphological changes of neurons in the
brain. Therefore, RA-differentiated SH-SY5Y cells were selected for
subsequent assays, and Aβ25–35 was used at a concentration of 20
µM.
Effects of Oroxylum indicum on the
viability of SH-SY5Y cells
To determine whether Oroxylum indicum has any
effect on the viability of SH-SY5Y cells, the cells were treated
with various concentrations of the extract (0–100 µg/ml). The
results indicated that Oroxylum indicum extract at
concentrations of 25 and 50 µg/ml increased cell viability
(139.45±7.89 and 130.61±5.83% of control value, respectively) and
that concentrations of up to 100 µg/ml were non-toxic to SH-SY5Y
cells (Fig. 1B). Therefore, the
highest non-toxic concentrations of Oroxylum indicum (50 and
100 µg/ml) were used in subsequent assays.
Oroxylum indicum protected SH-SY5Y
cells against Aβ25-35-induced cytotoxicity
To determine the effect of Oroxylum indicum
on Aβ25-35-induced cytotoxicity, SH-SY5Y cells were challenged with
20 µM Aβ25–35 in the presence or absence of 50 and 100 µg/ml
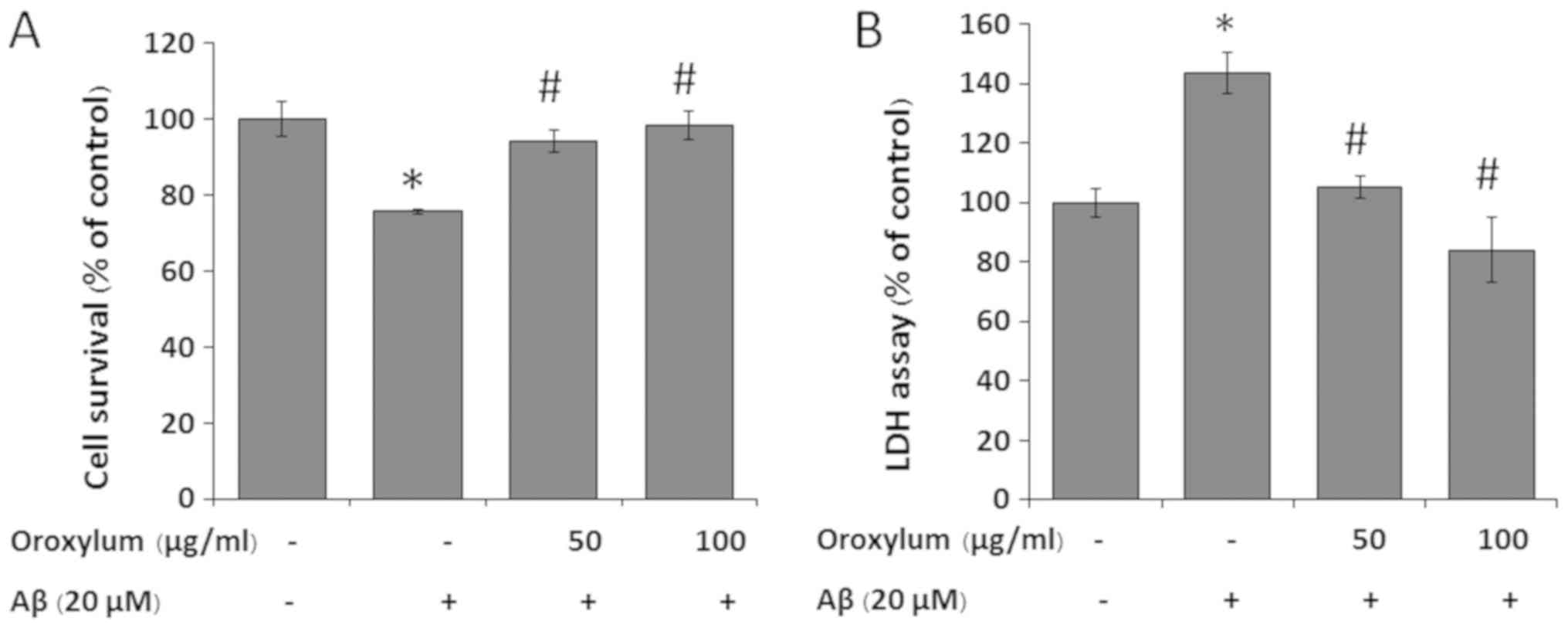
Oroxylum indicum extract for 24 h. As shown in Fig. 2A, treatment of SH-SY5Y cells with
20 µM Aβ25–35 for 24 h induced cytotoxicity, as demonstrated by a
cell viability reduction to 76.83±0.67%, when compared with the
control group. When the cells were treated with Oroxylum
indicum extract at concentrations of 50 and 100 µg/ml, cell
viability was restored to 94.14±2.79 and 98.35±3.74%, respectively,
indicating a concentration-dependent cytoprotective effect.
To further confirm the cytoprotective effect of
Oroxylum indicum, LDH, another indicator of cell toxicity,
was also examined. The results were similar to those determined by
the MTT assay. The exposure of SH-SY5Y cells to 20 µM Aβ25–35
resulted in a 1.45-fold increase in LDH release in the medium, when
compared to that in the control group. Treatment of cells with 50
and 100 µg/ml Oroxylum indicum extract reduced
Aβ25-35-induced LDH release in a concentration-dependent manner
(Fig. 2B).
Oroxylum indicum inhibited
Aβ25-35-induced intracellular accumulation of ROS
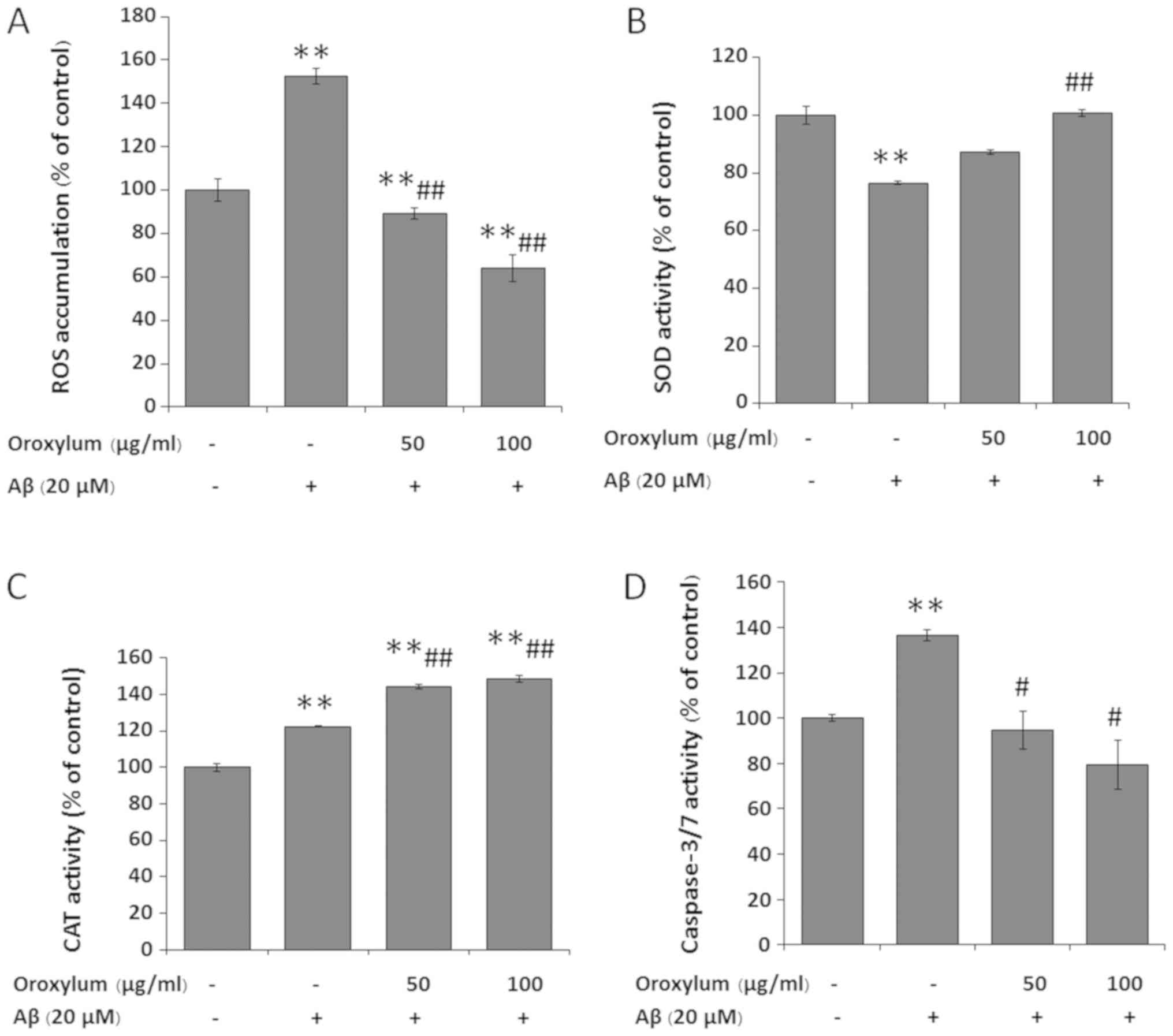
ROS play a critical role in Aβ-dependent cell death.
Therefore, in the present study, the effect of Oroxylum
indicum on Aβ25-35-induced intracellular ROS production was
examined. SH-SY5Y cells exposed to Aβ25–35 for 24 h exhibited
elevated ROS levels (152.4±3.53%, as compared to the control group)
(Fig. 3A). When the cells were
treated with Oroxylum indicum extract at concentrations of
50 and 100 µg/ml, there was a significant and
concentration-dependent inhibition of Aβ25-35-induced intracellular
ROS levels (89.17±2.46 and 64.25±6.21%, respectively).
Oroxylum indicum increased the
activity of anti-oxidative enzymes challenged by Aβ25-35
To determine whether the cytoprotective effect of
Oroxylum indicum is associated with the activity of
anti-oxidative enzymes, SOD and CAT activity was determined.
Following exposure to Aβ25–35 for 24 h, SOD activity was
significantly decreased to 76.48±0.6% of the control value.
Treatment of cells with 50 and 100 µg/ml Oroxylum indicum
extract for 24 h restored SOD activity to 87.30±0.81 and
100.76±1.19%, respectively (Fig.
3B). Following exposure to Aβ25-35, CAT activity was
significantly increased to 122.45±0.029% of the control value. CAT
activity following treatment with Oroxylum indicum extract
was significantly increased beyond that of the Aβ treatment and
control groups (144.15 and 148.4%, respectively) (Fig. 3C).
Oroxylum indicum reduced
Aβ25-35-induced caspase-3/7 activity
It has been reported that Aβ-induced neuronal cell
death through the activation of the caspase pathway (27–31).
To study the protective mechanism of Oroxylum indicum,
caspase-3/7 activity was measured. Following Aβ25–35 treatment,
caspase-3/7 activity significantly increased to levels that were
1.4-fold higher than that of the control group. However, the
induction of caspase-3/7 activity was blocked in the presence of
Oroxylum indicum (Fig.
3D)
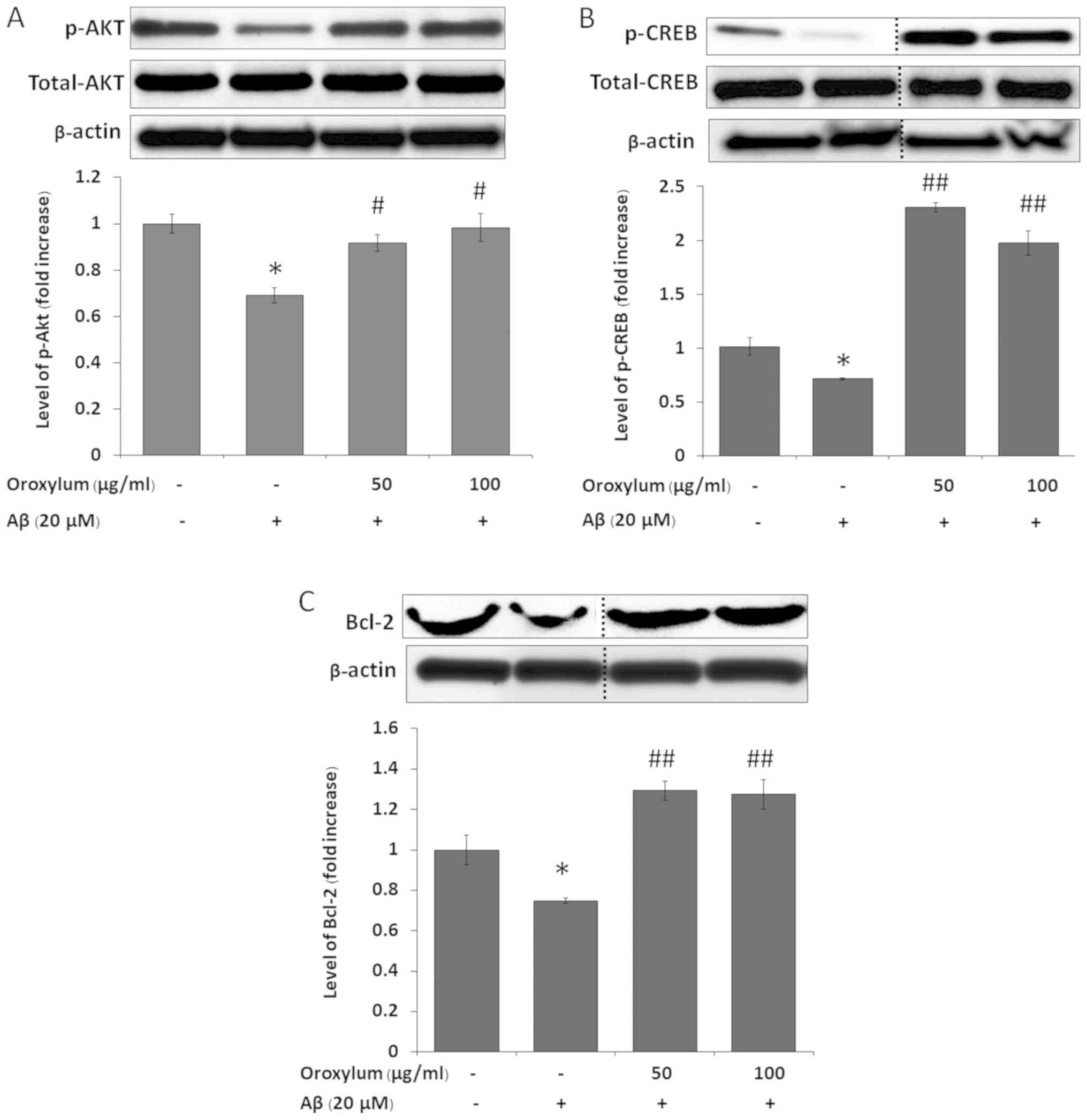
Oroxylum indicum enhanced the
phosphorylation of Akt
The activation of Akt has been associated with the
inhibition of the apoptotic cleavage of caspases, as well as the
promotion of neuronal survival (32–35).
We therefore hypothesized that Oroxylum indicum can modulate
the signaling of Akt. The results showed that treatment with
Aβ25–35 for 15 min significantly decreased p-Akt, as compared to
the untreated control (Fig. 4A).
Oroxylum indicum treatments significantly increased the
phosphorylation of Akt, as compared to Aβ25–35 treatment.
Oroxylum indicum enhanced the
phosphorylation of CREB
It has been reported that one element of the
downstream signaling of Akt is CREB the transcription factor that
regulates neuronal survival (35).
We therefore examined whether CREB phosphorylation increased in
SH-SY5Y cells following treatment with Aβ25–35 in the presence of
Oroxylum indicum. When cells were treated with Aβ25–35 for 1
h, CREB phosphorylation was significantly decreased, when compared
with the untreated control. The phosphorylation of CREB was
significantly increased following treatment with Oroxylum
indicum, when compared to both the Aβ25–35 treatment and
control groups (Fig. 4B).
Oroxylum indicum enhanced Bcl-2
expression
The transcription factor CREB has been identified as
a positive regulator of Bcl-2 (an apoptosis suppressor gene)
expression (36,37). Therefore, these results
demonstrated that Aβ25–35 ameliorated Bcl-2 expression, as compared
with the control group. Treatment with Oroxylum indicum
extract for 24 h caused a significant increase in Bcl-2 expression,
as compared to the Aβ25–35 group (Fig.
4C).
Total phenolic and flavonoid content
of extracts from fruit pot of Oroxylum indicum
The Oroxylum indicum fruit extract was
standardized using colorimetric methods to quantify the total
phenolic content using gallic acid as a standard, and the total
flavonoid content using rutin as a standard. The values obtained
were 10.50±0.68 and 17.08±0.85 mg/g of dried extract, respectively
(Table I).
 | Table I.Total phenolic and flavonoid content
of Oroxylum indicum fruit extract. |
Table I.
Total phenolic and flavonoid content
of Oroxylum indicum fruit extract.
| Total
phenolic/flavonoid content | Concentrations
(mg/g) |
|---|
| Total phenolic
contenta | 30.50±0.68 |
| Total flavonoid
contentb | 17.08±0.85 |
Discussion
Accumulation of amyloid plaques in the brains of AD
patients has been reported to induce cytotoxicity mediated through
the generation of ROS and elevated oxidative stress (9). Therefore, the attenuation or
suppression of this oxidative stress-dependent, Aβ-mediated
cytotoxicity may be a promising strategy for preventive or
therapeutic intervention in AD. Recently, natural antioxidants from
medicinal and edible plants have attracted considerable attention
as promising agents for reducing the risk of oxidative
stress-induced neurological diseases. In the present study,
RA-differentiated SH-SY5Y cells were selected as a model, since
they displayed properties similar to those of mature neurons. It
was demonstrated that Aβ25-35-treated cells exhibited increased ROS
production, which was consistent with previous reports (24,38,39).
Treatment with Oroxylum indicum extract inhibited Aβ-induced
ROS production in a concentration-dependent manner, indicating an
antioxidant effect of Oroxylum indicum. Phenolic and
flavonoid compounds in plants are reported to be very good
antioxidants. Phenolics are effective hydrogen donors, while
flavonoids act as scavengers of various oxidizing species, such as
hydroxyl radicals, peroxy radicals and the superoxide anion
(40). Therefore, it was
reasonable to determine the quantities of these phytochemical
classes in Oroxylum indicum. In the present study, it was
demonstrated that the total phenolic and flavonoid content of
Oroxylum indicum was 10.50±0.68 and 17.08±0.85 mg/g,
respectively. Although Oroxylum indicum extract contained
only low levels of phenolics and flavonoids, a marked decrease in
ROS production was observed following treatment with the extract,
suggesting that there is another antioxidative defense mechanism
that can eliminate ROS. Under normal physiological conditions, ROS
production is balanced by endogenous cellular antioxidant systems,
including the cooperative action of SOD and CAT (41). SOD is the first line of defense
against free radicals, its ROS-metabolizing activity occurring due
to catalytic dismutation of the superoxide anion radical
(O2•−) into O2 and
H2O2. H2O2 is then
converted into O2 and H2O by CAT, another
major primary antioxidant defense component (41). It was demonstrated herein that
treatment with Aβ25–35 decreases SOD activity, which was consistent
with a previous report (42). A
Aβ25–35 treatment-induced SOD activity reduction in cultured cells
is possibly due to it metabolites or a direct toxic effect of
Aβ25-35; however, unknown factors other than severe cell damage may
also cause this effect. Of note, an increase in CAT activity was
observed following exposure of cells to Aβ25-35, which might have
been either a direct induction or a compensatory mechanism against
Aβ insult. When cells are treated with Oroxylum indicum, SOD
and CAT activity is increased, suggesting that Oroxylum
indicum may stimulate cells to increase SOD and CAT expression,
leading to an increase in their enzymatic activity in order to cope
with Aβ-induced ROS production or may result from it metabolites.
Therefore, the mechanism(s) through which Oroxylum indicum
attenuates ROS production may be due to its phenolic and flavonoid
content and/or its ability to increase SOD and CAT enzyme
activity.
It has been reported that Aβ-induced neurotoxicity
is related to ROS generation and caspase activation (27–31),
so we next investigated the effects of Oroxylum indicum on
the activation of caspases-3/7, which are known to be effector
caspases. The protective effect of Oroxylum indicum against
Aβ-induced cytotoxicity was also determined. Treatment with
Oroxylum indicum not only attenuated Aβ-induced caspase-3/7
activity, but also protected SH-SY5Y cells against Aβ-induced
cytotoxicity, as determined by the MTT assay. The MTT assay was
selected, since it has repeatedly been shown to be a very sensitive
indicator of Aβ-induced cell death (43). The protective effect of Oroxylum
indicum was further confirmed by LDH assay, another indicator
of cell toxicity. The results reported above demonstrated that
treatment with Oroxylum indicum reduced Aβ-induced LDH
release. This finding indicated that Oroxylum indicum
extract can protect SH-SY5Y cells against Aβ-induced cell
injury.
The PI3K/Akt pathway is a key signal transduction
pathway that mediates cell growth and promotes cell survival
(44). The activation of Akt can
phosphorylate CREB (45).
Phosphorylated CREB is then translocated to the nucleus, where it
upregulates the anti-apoptotic protein Bcl-2 (45). As Akt is an upstream signal that
regulates p-CREB, and the phosphorylation process is rapid during
protein translation, which occurs several hours following
treatment, p-Akt and p-CREB were detected at 15 min and 1 h,
respectively, and Bcl-2 at 24 h following treatment. Exposure of
SH-SY5Y cells to Aβ decreased p-Akt. This finding was consistent
with a previous report, which showed that intraneuronal Aβ
accumulation leads to a decrease in the levels of phosphor-Akt
(46). Exposure of SH-SY5Y cells
to Aβ also decreased CREB phosphorylation, as well as the protein
expression of Bcl-2. However, treatment with Oroxylum
indicum increased the phosphorylation of Akt and CREB, as well
as the expression of Bcl-2 protein. These results suggested that
the activation of Akt/CREB/Bcl-2 pathway also plays a critical role
in Oroxylum indicum-induced neuronal protective effects
against Aβ insult.
To the best of our knowledge, there is no published
study regarding the effects of Oroxylum indicum on Aβ
exposure in neuronal cells. The present study was the first to show
that Oroxylum indicum extract protects SH-SY5Y cells against
Aβ25-35-induced cell injury. Although a cell line was used in this
study, several studies have demonstrated the effects of Oroxylum
indicum extract in vivo, including its protective
effects against paracetamol-induced liver damage in experimental
rats and cisplatin-induced renal injury in Wistar male albino rats,
its hepatoprotective activity against CCl4-induced liver damage in
rats, as well as its anti-central nervous system-depressant
activity in an animal model (47–51).
We hope that this finding will encourage further investigations
into the activity of Oroxylum indicum in AD and other
neurodegenerative diseases.
In conclusion, the results of the present study
suggested that Oroxylum indicum extract protects SH-SY5Y
cells against Aβ25-35-induced injury, at least partly, by
inhibiting oxidative stress, increasing SOD and CAT activity,
attenuating caspase 3/7 activity and promoting cell survival
pathways, such as Akt/CREB/Bcl-2 (Fig.
5). The present data suggested that Oroxylum indicum
could be useful in the prevention of Aβ-induced neurotoxicty in AD
and related neurodegenerative diseases. Further studies into the
activity of Oroxylum indicum extract in vivo are now
required.
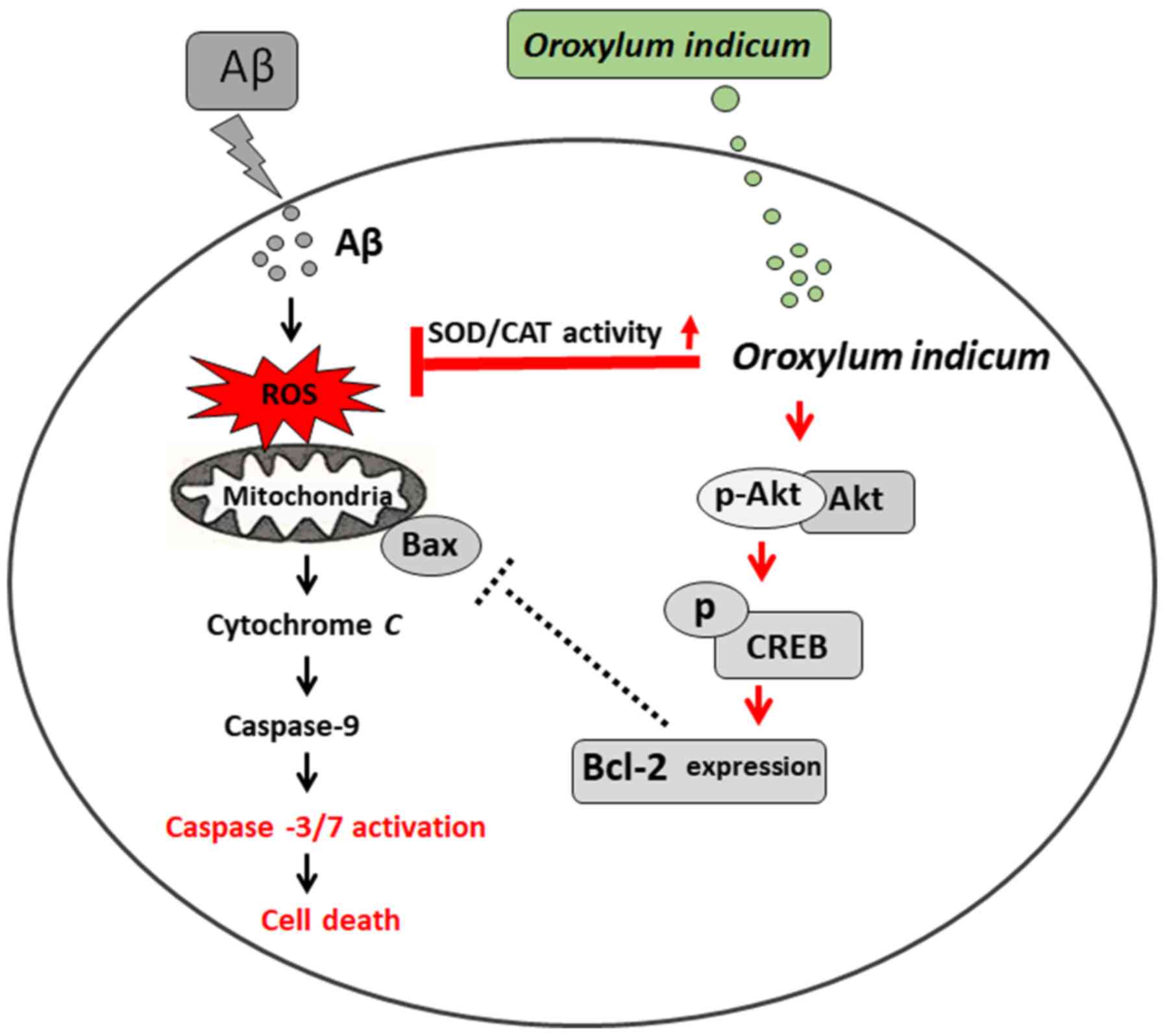 | Figure 5.Hypothetical scheme illustrating
signaling pathways through which Oroxylum indicum protects
cells against Aβ-induced cell injury. It has been reported that Aβ
causes ROS accumulation (52–53).
ROS accumulation can induce the release of cytochrome c through
several mechanisms. One mechanism is associated with the Bcl-2
family of proteins (Bcl-2, Bcl-Xl, Bax and Bid) (54). Once cytochrome c is released, it
can bind to caspase-9 to form a complex, which subsequently
activates caspases-3/7 (and other caspases) and eventually causes
cell death (54). Oroxylum
indicum also increases SOD and CAT activity, decreases ROS
production and decreases caspases-3/7 activity. Increases in SOD
and CAT activity might contribute to inhibition of ROS production.
If ROS production decreases, the activation of caspases-3/7 may
decrease. It has been reported that the Akt/CREB/Bcl-2 pathway is
important in promoting cell survival (45). Oroxylum indicum also
increases the phosphorylation of Akt and CREB, and increases
expression of the anti-apoptotic protein Bcl-2. Increasing
phosphorylation of Akt might contribute to an increase in the
phosphorylation of CREB, resulting in an increase in Bcl-2.
Therefore, activation of the Akt/CREB/Bcl-2 pathway might also play
a critical role in Oroxylum indicum-induced neuronal
protective effects against Aβ insult. Information in red front
represents finding from the current study. Aβ, Amyloid-β; ROS,
reactive oxygen species; SOD, superoxide dismutase; CAT, catalase;
CREB, cAMP-responsive element binding protein. |
Acknowledgements
Not applicable.
Funding
This study was financially supported by a grant from
Mahasarakham University Faculty of Medicine and George M. Leader
Family Funds.
Availability of data and materials
All the data generated and analyzed in the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
NM was responsible for the conception and design of
the study, as well as the acquisition of data and drafting of the
manuscript. NM and BB performed the experiments. NM, JRC and SYL
were responsible for data analysis and interpretation, and revising
the manuscript. The final version of the manuscript has been read
and approved by all authors.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Querfurth HW and LaFerla FM: Alzheimer's
disease. N Engl J Med. 362:329–344. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Paulson JB, Ramsden M, Forster C, Sherman
MA, McGowan E and Ashe KH: Amyloid plaque and neurofibrillary
tangle pathology in a regulatable mouse model of Alzheimer's
disease. Am J Pathol. 173:762–772. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Butterfield DA, Drake J, Pocernich C and
Castegna A: Evidence of oxidative damage in Alzheimer's disease
brain: Central role for amyloid beta-peptide. Trends Mol Med.
7:548–554. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Butterfield DA, Griffin S, Munch G and
Pasinetti GM: Amyloid beta-peptide and amyloid pathology are
central to the oxidative stress and inflammatory cascades under
which Alzheimer's disease brain exists. J Alzheimers Dis.
4:193–201. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Butterfield DA, Swomley AM and Sultana R:
Amyloid beta-peptide (1–42)-induced oxidative stress in Alzheimer
disease: Importance in disease pathogenesis and progression.
Antioxid Redox Signal. 19:823–835. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Bharadwaj PR, Dubey AK, Masters CL,
Martins RN and Macreadie IG: Abeta aggregation and possible
implications in Alzheimer's disease pathogenesis. J Cell Mol Med.
13:412–421. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gray CW and Patel AJ: Neurodegeneration
mediated by glutamate and beta-amyloid peptide: A comparison and
possible interaction. Brain research. 691:169–179. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ueda K, Shinohara S, Yagami T, Asakura K
and Kawasaki K: Amyloid beta protein potentiates Ca2+
influx through L-type voltage-sensitive Ca2+ channels: A
possible involvement of free radicals. J Neurochem. 68:265–271.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Muthaiyah B, Essa MM, Chauhan V and
Chauhan A: Protective effects of walnut extract against amyloid
beta peptide-induced cell death and oxidative stress in PC12 cells.
Neurochem Res. 36:2096–2103. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mohd Sairazi NS, Sirajudeen KN, Asari MA,
Muzaimi M, Mummedy S and Sulaiman SA: Kainic acid-induced
excitotoxicity experimental model: protective merits of natural
products and plant extracts. Evid Based Complement Alternat Med.
2015:9726232015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Solanki I, Parihar P, Mansuri ML and
Parihar MS: Flavonoid-based therapies in the early management of
neurodegenerative diseases. Adv Nutr. 6:64–72. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Venkatesan R, Ji E and Kim SY:
Phytochemicals that regulate neurodegenerative disease by targeting
neurotrophins: A comprehensive review. Biomed Res Int.
2015:8140682015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nootchanat MPC, Chalisa LC and Walaiporn
T: Okra (Abelmoschus esculentus Linn) inhibits
lipopolysaccharide-induced inflammatory mediators in BV2 microglial
cells. Tropical J Pharmaceutical Res. 16:1285–1295. 2017.
View Article : Google Scholar
|
|
14
|
World Health Organization: General
Guidelines for Methodologies on Research and Evaluation of
Traditional Medicine. WHO; Geneva, Switzerland: 2000
|
|
15
|
Lawania RD, Mishra A and Gupta R: Oroxylum
indicum: A Review. Pharm J. 2:304–310. 2010.
|
|
16
|
Narisa K, Wilkinson JM and Cavanagh H:
Cytotoxic effect of four thai edible plants on mammalian cell
proliferation. Thai Pharm Health Sci J. 1:189–195. 2006.
|
|
17
|
Jiwajinda S, Santisopasri V, Murakami A,
Kim OK, Kim HW and Ohigashi H: Suppressive effects of edible thai
plants on superoxide and nitric oxide generation. Asian Pac J
Cancer Prev. 3:215–223. 2002.PubMed/NCBI
|
|
18
|
Diaz P, Jeong SC, Lee S, Khoo C and
Koyyalamudi SR: Antioxidant and anti-inflammatory activities of
selected medicinal plants and fungi containing phenolic and
flavonoid compounds. Chin Med. 7:262012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yan SD, Fu J, Soto C, Chen X, Zhu H,
Al-Mohanna F, Collison K, Zhu A, Stern E, Saido T, et al: An
intracellular protein that binds amyloid-beta peptide and mediates
neurotoxicity in Alzheimer's disease. Nature. 389:689–695. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kaminsky YG, Tikhonova LA and Kosenko EA:
Critical analysis of Alzheimer's amyloid-beta toxicity to
mitochondria. Front Biosci (Landmark Ed). 20:173–197. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hughes E, Burke RM and Doig AJ: Inhibition
of toxicity in the beta-amyloid peptide fragment beta-(25–35) using
N-methylated derivatives: A general strategy to prevent amyloid
formation. J Biol Chem. 275:25109–25115. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Liu YQ, Jia MQ, Xie ZH, Liu XF, Hui Y and
Zheng XL: Arrestins contribute to amyloid beta-induced cell death
via modulation of autophagy and the alpha7nAch receptor in SH-SY5Y
cells. Sci Rep. 7:34462017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Shipley MM, Mangold CA and Szpara ML:
Differentiation of the SH-SY5Y human neuroblastoma cell line. J Vis
Exp. 531932016.PubMed/NCBI
|
|
24
|
Wang Y, Miao Y, Mir AZ, Cheng L, Wang L,
Zhao L, Cui Q, Zhao W and Wang H: Inhibition of
beta-amyloid-induced neurotoxicity by pinocembrin through Nrf2/HO-1
pathway in SH-SY5Y cells. J Neurol Sci. 368:223–230. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu XY, Wang LX, Chen Z and Liu LB:
Liraglutide prevents beta-amyloid-induced neurotoxicity in SH-SY5Y
cells via a PI3K-dependent signaling pathway. Neurol Res.
38:313–319. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sarkar B, Dhiman M, Mittal S and Mantha
AK: Curcumin revitalizes Amyloid beta (25–35)-induced and
organophosphate pesticides pestered neurotoxicity in SH-SY5Y and
IMR-32 cells via activation of APE1 and Nrf2. Metab Brain Dis.
32:2045–2061. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Deshpande A, Mina E, Glabe C and Busciglio
J: Different conformations of amyloid beta induce neurotoxicity by
distinct mechanisms in human cortical neurons. J Neurosci.
26:6011–6018. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
He Y, Cui J, Lee JC, Ding S, Chalimoniuk
M, Simonyi A, Sun AY, Gu Z, Weisman GA, Wood WG and Sun GY:
Prolonged exposure of cortical neurons to oligomeric amyloid-β
impairs NMDA receptor function via NADPH oxidase-mediated ROS
production: Protective effect of green tea
(−)-epigallocatechin-3-gallate. ASN Neuro. 3:e000502011.PubMed/NCBI
|
|
29
|
Sponne I, Fifre A, Koziel V, Oster T,
Olivier JL and Pillot T: Membrane cholesterol interferes with
neuronal apoptosis induced by soluble oligomers but not fibrils of
amyloid-beta peptide. FASEB J. 18:836–838. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shelat PB, Chalimoniuk M, Wang JH,
Strosznajder JB, Lee JC, Sun AY, Simonyi A and Sun GY: Amyloid beta
peptide and NMDA induce ROS from NADPH oxidase and AA release from
cytosolic phospholipase A2 in cortical neurons. J Neurochem.
106:45–55. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pate KM, Rogers M, Reed JW, van der Munnik
N, Vance SZ and Moss MA: Anthoxanthin polyphenols Attenuate Aβ
oligomer-induced neuronal responses associated with alzheimer's
disease. CNS Neurosci Ther. 23:135–144. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Allan LA, Morrice N, Brady S, Magee G,
Pathak S and Clarke PR: Inhibition of caspase-9 through
phosphorylation at Thr 125 by ERK MAPK. Nat Cell Biol. 5:647–654.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Hermann C, Assmus B, Urbich C, Zeiher AM
and Dimmeler S: Insulin-mediated stimulation of protein kinase Akt:
A potent survival signaling cascade for endothelial cells.
Arterioscler Thromb Vasc Biol. 20:402–409. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kitazumi I and Tsukahara M: Regulation of
DNA fragmentation: The role of caspases and phosphorylation. FEBS
J. 278:427–441. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bonni A, Brunet A, West AE, Datta SR,
Takasu MA and Greenberg ME: Cell survival promoted by the Ras-MAPK
signaling pathway by transcription-dependent and -independent
mechanisms. Science. 286:1358–1362. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Pugazhenthi S, Miller E, Sable C, Young P,
Heidenreich KA, Boxer LM and Reusch JE: Insulin-like growth
factor-I induces bcl-2 promoter through the transcription factor
cAMP-response element-binding protein. J Biol Chem.
274:27529–27535. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wilson BE, Mochon E and Boxer LM:
Induction of bcl-2 expression by phosphorylated CREB proteins
during B-cell activation and rescue from apoptosis. Mol Cell Biol.
16:5546–5556. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Hwang S, Lim JW and Kim H: Inhibitory
effect of lycopene on amyloid-β-induced apoptosis in neuronal
cells. Nutrients. 9(pii): E8832017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wang H, Xu Y, Yan J, Zhao X, Sun X, Zhang
Y, Guo J and Zhu C: Acteoside protects human neuroblastoma SH-SY5Y
cells against beta-amyloid-induced cell injury. Brain Res.
1283:139–147. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ratty AK and Das NP: Effects of flavonoids
on nonenzymatic lipid peroxidation: Structure-activity
relationship. Biochem Med Metab Biol. 39:69–79. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Olsvik PA, Kristensen T, Waagbo R,
Rosseland BO, Tollefsen KE, Baeverfjord G and Berntssen MH: mRNA
expression of antioxidant enzymes (SOD, CAT and GSH-Px) and lipid
peroxidative stress in liver of Atlantic salmon (Salmo salar)
exposed to hyperoxic water during smoltification. Comp Biochem
Physiol C Toxicol Pharmacol. 141:314–323. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Tong Y, Bai L, Gong R, Chuan J, Duan X and
Zhu Y: Shikonin protects PC12 cells against β-amyloid
peptide-induced cell injury through antioxidant and antiapoptotic
activities. Sci Rep. 8:262018. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Behl C, Davis JB, Lesley R and Schubert D:
Hydrogen peroxide mediates amyloid beta protein toxicity. Cell.
77:817–827. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Shaw RJ and Cantley LC: Ras, PI(3)K and
mTOR signalling controls tumour cell growth. Nature. 441:424–430.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pugazhenthi S, Nesterova A, Sable C,
Heidenreich KA, Boxer LM, Heasley LE and Reusch JE: Akt/protein
kinase B up-regulates Bcl-2 expression through cAMP-response
element-binding protein. J Biol Chem. 275:10761–10766. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Magrane J, Rosen KM, Smith RC, Walsh K,
Gouras GK and Querfurth HW: Intraneuronal beta-amyloid expression
downregulates the Akt survival pathway and blunts the stress
response. J Neurosci. 25:10960–10969. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Sastry AVS, Girija Sastry V, Mallikarjun P
and Srinivas K: Chemical and pharmacological evaluation of aqueous
extract of root bark of ‘Oroxylum indicum’ vent. Int J Pharm
Technol. 3:1796–1806. 2011.
|
|
48
|
Sreedevi Adikay, Usha Rani U and Bharathi
Koganti: Protective effect of ethanolic extract of Oroxylum indicum
against cisplatin-induced acute renal failure. Int J Pharm Therap.
2:48–53. 2011.
|
|
49
|
Tenpe R, Aman U, Burle S and Yeole YG: In
vitro antioxidant and preliminary hepatoprotective activity of
Oroxylum indicum Vent leaf extracts. Pharmacologyonline. 1:35–43.
2009.
|
|
50
|
Bichitra Nanda Tripathy, Panda SK, Sahoo
S, Mishra SK and Nayak L: Phytochemical analysis and
hepatoprotective effect of stem bark of Oroxylum indicum (L) Vent.
On carbon tetrachloride induced hepatotoxicity in rat. Int J Pharma
Biol Arc. 2:1714–1717. 2011.
|
|
51
|
Kevalkumar R, Rathod, Rashmi CA, Miloni JK
and Tejas HG: Evaluation of effect of Oroxylum indicum leaves on
central nervous system with special emphasis on epilepsy. J
Chemical Pharm Res. 8:680–685. 2016.
|
|
52
|
Varadarajan S, Yatin S, Aksenova M and
Butterfield DA: Review: Alzheimer's amyloid beta-peptideassociated
free radical oxidative stress and neurotoxicity. J Struct Biol.
130:184–208. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Butterfield DA: Beta-Amyloid-associated
free radical oxidative stress and neurotoxicity: Implications for
Alzheimer's disease. Chem Res Toxicol. 10:495–506. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Redza-Dutordoir M and Averill-Bates DA:
Activation of apoptosis signalling pathways by reactive oxygen
species. Biochim Biophys Acta. 1863:2977–2992. 2016. View Article : Google Scholar : PubMed/NCBI
|