Introduction
Atopic dermatitis (AD) is a chronic inflammatory
skin disease. Clinical observations used to diagnose AD include
chronic dermatitis, pruritus, facial and extensor eczema in infants
and children and flexural eczema in adults (1–3). The
prevalence of AD is increasing annually.
S100A8 and S100A9 are members of the S100 family
proteins, having constitutive or inducible expressions in
neutrophils and monocytes/macrophages (4). These proteins are included in
damage-associated molecular pattern (DAMP), and perform various
functions, including control of cell motility, protein
phosphorylation for cell activation, and calcium homeostasis for
tumor progression or suppression through Toll-like receptor 4
(TLR4) (4,5). High levels of S100 calcium binding
protein A8 (S100A8) and S100A9 are characteristic of several
inflammatory conditions, such as chronic inflammatory bowel
disease, rheumatoid arthritis, cystic fibrosis, psoriasis and AD
(6,7).
Cytokine dysregulation is one of the most important
pathogenic mechanisms associated with AD. Interleukin-6 (IL-6),
IL-8, and monocyte chemoattractant protein-1 (MCP-1) are essential
cytokines involved in the induction and alleviation of AD (8). In addition, defects in skin barrier
function are critical for the development of AD (9). Disruption of skin barrier proteins,
including filaggrin, loricrin and involucrin, can make it easier
for allergens or antigens to permeate the skin (9). Such defects may be the result of an
increase in cytokines or may result in increased production of
inflammatory cytokines, including IL-1, IL-2, IL-4, IL-6, IL-8,
tumor necrosis factor-α (TNF-α), and MCP-1. These cytokines and
chemokines may both induce and alleviate AD (8,9).
Based on the above, this study was performed to
investigate whether S100A8 and S100A9 are associated with
alteration of cytokine secretion and skin barrier proteins.
Materials and methods
Reagents
TLR4 inhibitor CLI-095 (TLR4i), protein kinase δ
(PKCδ) inhibitor (rottlerin), p38 mitogen-activated protein kinase
(MAPK) inhibitor (SB202190), MEK inhibitor (PD98059) and nuclear
factor-κB (NF-κB) inhibitor (BAY-11-7085) were obtained from
Calbiochem (Merck KGaA). Antibodies against p38 MAPK (cat. no.
9212), phospho-p38 MAPK (cat. no. 9211), phospho-extracellular
signal regulated kinase 1/2 (ERK1/2; cat. no. 9101), rabbit IgG-HRP
(cat. no. 7074), and mouse IgG-HRP (cat. no. 7076) were acquired
from Cell Signaling Technology, Inc. Anti-ERK2 (cat. no. sc-154)
and anti-TLR4 (cat. no. sc-10741) antibodies were obtained from
Santa Cruz Biotechnology, Inc.
Production of recombinant S100A8 and
S100A9 proteins
In our previous report, the cDNA of human S100A8 and
S100A9 was cloned into pET28 expression vector (Merck KGaA)
(10). Recombinant S100A8 and
S100A9 expression was induced with 1 mM isopropyl
β-D-thiogalactoside in E. coli BL21 (DE3; Merck KGaA).
Subsequently, bacteria were centrifuged at 5,000 × g for 10 min at
4°C and the pellet was lysed in BugBuster Protein Extraction
reagent (Merck KGaA). A spectrophotometer (Thermo Fisher
Scientific, Inc.) was used for measuring protein concentration in
the lysates.
Cell culture
Human keratinocytic HaCaT cells (Addexbio; cat. no.
T0020001) were cultured in Dulbecco's modified Eagle's medium
supplemented with 10% heat-inactivated fetal bovine serum (cat. no.
12484-010; Gibco; Thermo Fisher Scientific, Inc.), penicillin (100
U/ml), and streptomycin (100 µg/ml). The cultured cells were
maintained at sub-confluency in a 95% air, 5% CO2
humidified atmosphere at 37°C. An Annexin V-fluorescein
isothiocyanate (FITC) apoptosis detection kit (BD Biosciences) and
an RF500 flow cytometer (Sysmex Corporation) were used to evaluate
the cytotoxicity.
ELISA
HaCaT cells were treated with S100A8 or S100A9 for
6, 12, 24 and 48 h, followed by centrifugation of the supernatant
at 5,000 × g for 10 min at 4°C. The concentrations of IL-6, IL-8
and MCP-1 in the cell supernatant were measured with a sandwich
ELISA using OptEIA™ Set human IL-6 (cat. no. 555220), IL-8 (cat.
no. 555244) and MCP-1 (cat. no. 555179; all BD Biosciences)
according to the manufacturer's protocol.
Western blotting
HaCaT cells were treated with S100A8 or S100A9 for
24 h, and the cells were harvested and lysed with lysis buffer
(TransLab). The homogenate was centrifuged at 12,000 × g for 15 min
at 4°C, and the supernatant was collected as total lysate. Protein
concentration of the lysate was measured by a protein assay kit
(Thermo Scientific, Inc.). Following separation of the protein
samples (50 mg/lane) by 10% SDS-PAGE, the transferred
nitrocellulose membranes were incubated with primary (1:1,000) and
secondary antibodies as described in the reagents section (1:3,000)
for 1 h at room temperature, and developed by using an enhanced
chemiluminescence detection system (Amersham; GE Healthcare,
Chicago, IL, USA). The same blot was stripped and re-probed with
anti-ERK2 or anti-β-actin antibodies as described in the reagents
section used as an internal control.
NF-κB p65 transcription factor
assay
The DNA-binding activity of NF-κB was assessed by
using EZ-Detect™ transcription factor kits for NF-κB p65 (Pierce;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol. DNA-binding specificity was evaluated by using wild type
(5′-CACAGTTGAGGGGACTTTCCCAGGC-3′) or mutant NF-κB oligonucleotides
(5′-CACAGTTGAGGCCACTTTCCCAGGC-3′). Chemiluminescent detection was
performed using a luminometer (Thermo Fisher Scientific, Inc.).
Statistical analysis
All data (n=38) are presented as the mean ± standard
deviation. Data were analyzed by using a paired Student's t-test
for two-group comparisons and a one-way analysis of variance with
Tukey's post hoc tests for comparisons of more than two groups.
Analyses were performed using the SPSS statistical software
package, version 10.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
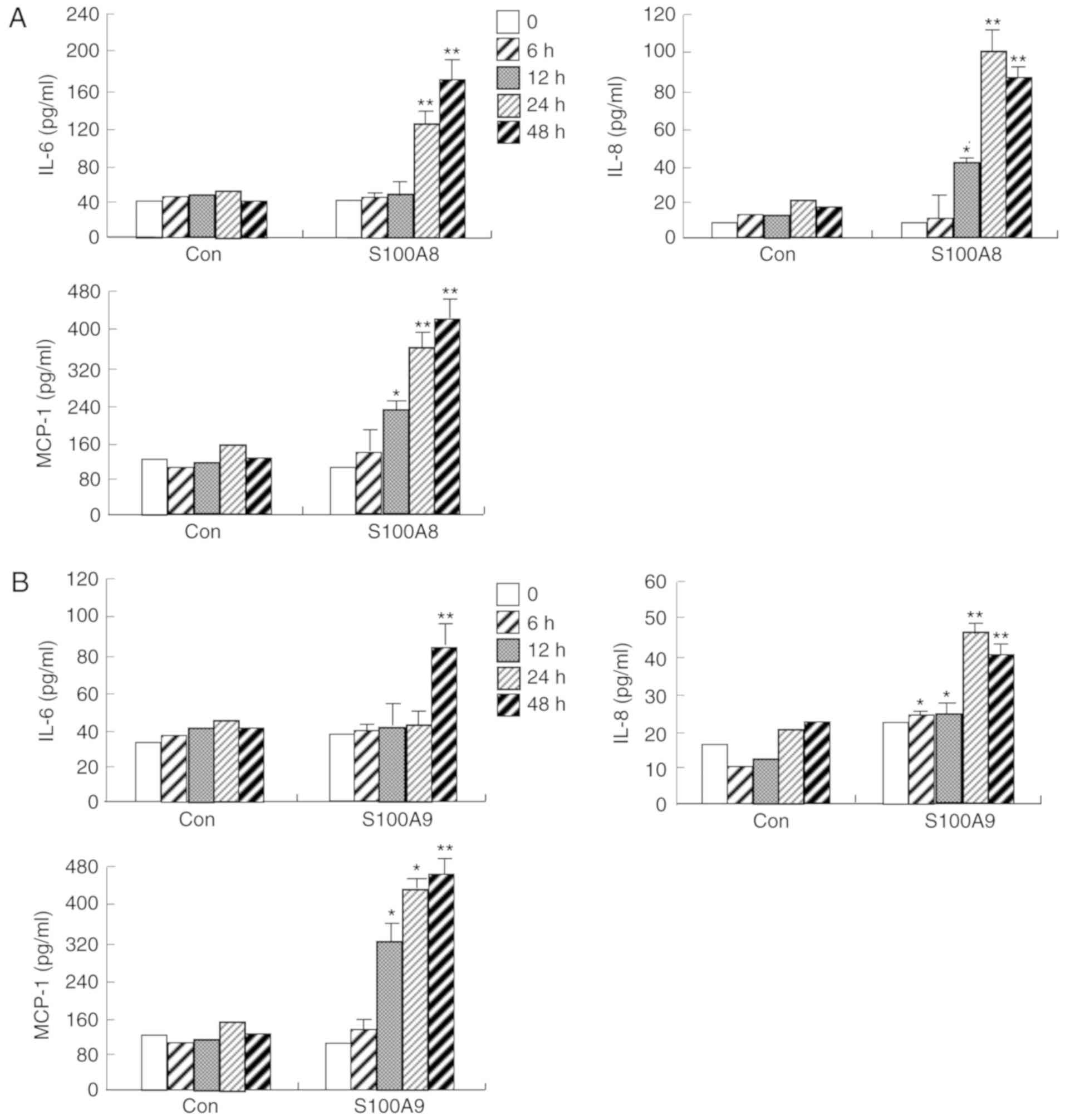
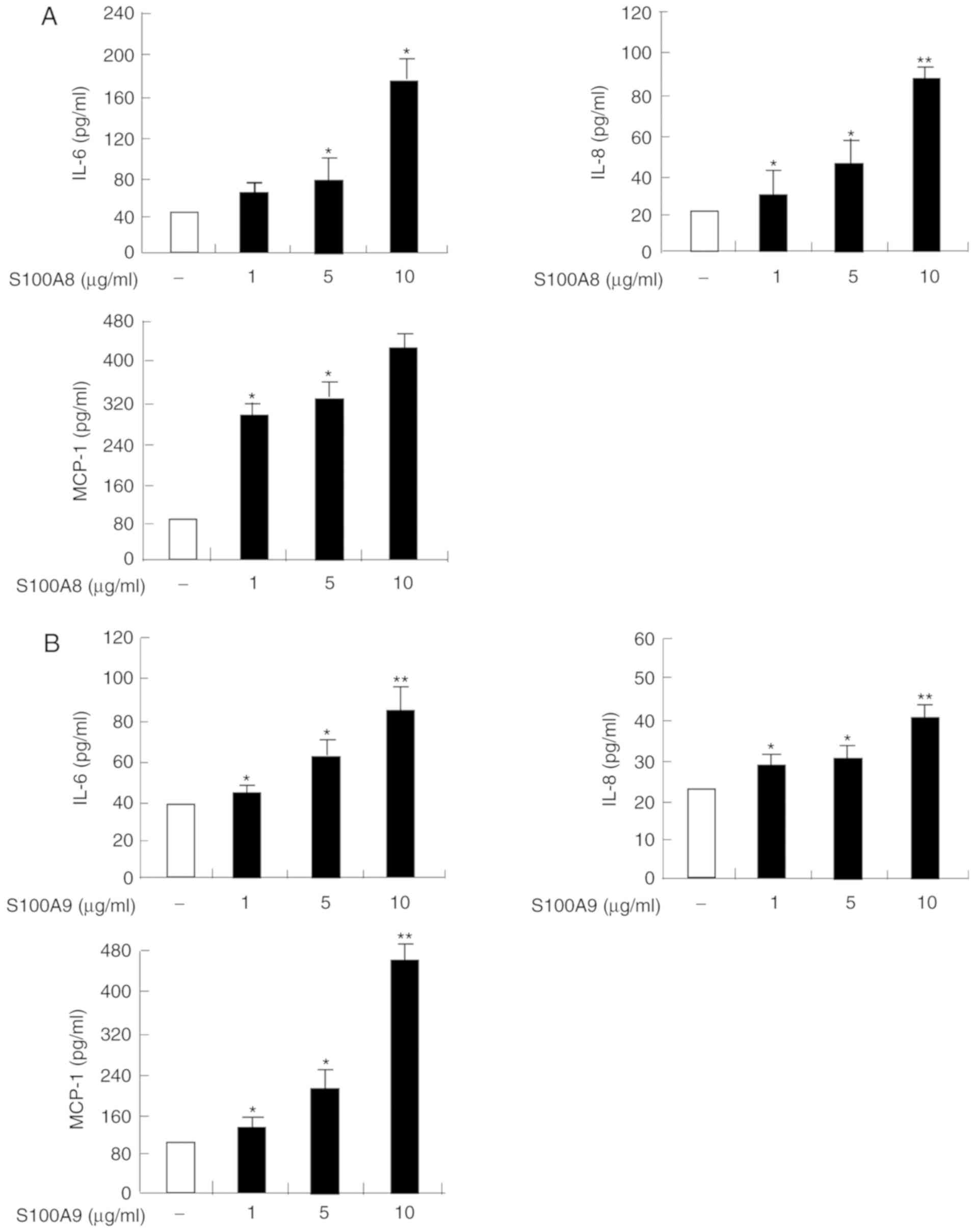
S100A8 and S100A9 induce expression of
IL-6, IL-8, and MCP-1 in HaCaT cells, which is associated with
TLR4, PKCδ, ERK, p38 MAPK and NF-κB signaling pathways
The protein expression levels of IL-6, IL-8 and
MCP-1 were significantly increased in a time and dose-dependent
manner following S100A8 or S100A9 treatment, in HaCaT cells
(Figs. 1 and 2). To evaluate the signaling mechanism(s)
induced by S100A8 and S100A9 treatment, alteration of the cytokine
expression was evaluated by pretreatment with protein-specific
inhibitors prior to S100A8 and S100A9 stimulation. As demonstrated
in Fig. 3, the expression levels
of IL-6, IL-8, and MCP-1 increased by S100A8 or S100A9 treatment,
were significantly reduced by specific inhibitors, including TLR4i,
rottlerin, PD98059, SB203580 and BAY-11-7085. These results
confirmed that TLR4, PKCδ, ERK, p38 MAPK and NF-κB signaling
pathways are involved in S100A8 or S100A9-induced secretion of
IL-6, IL-8 and MCP-1.
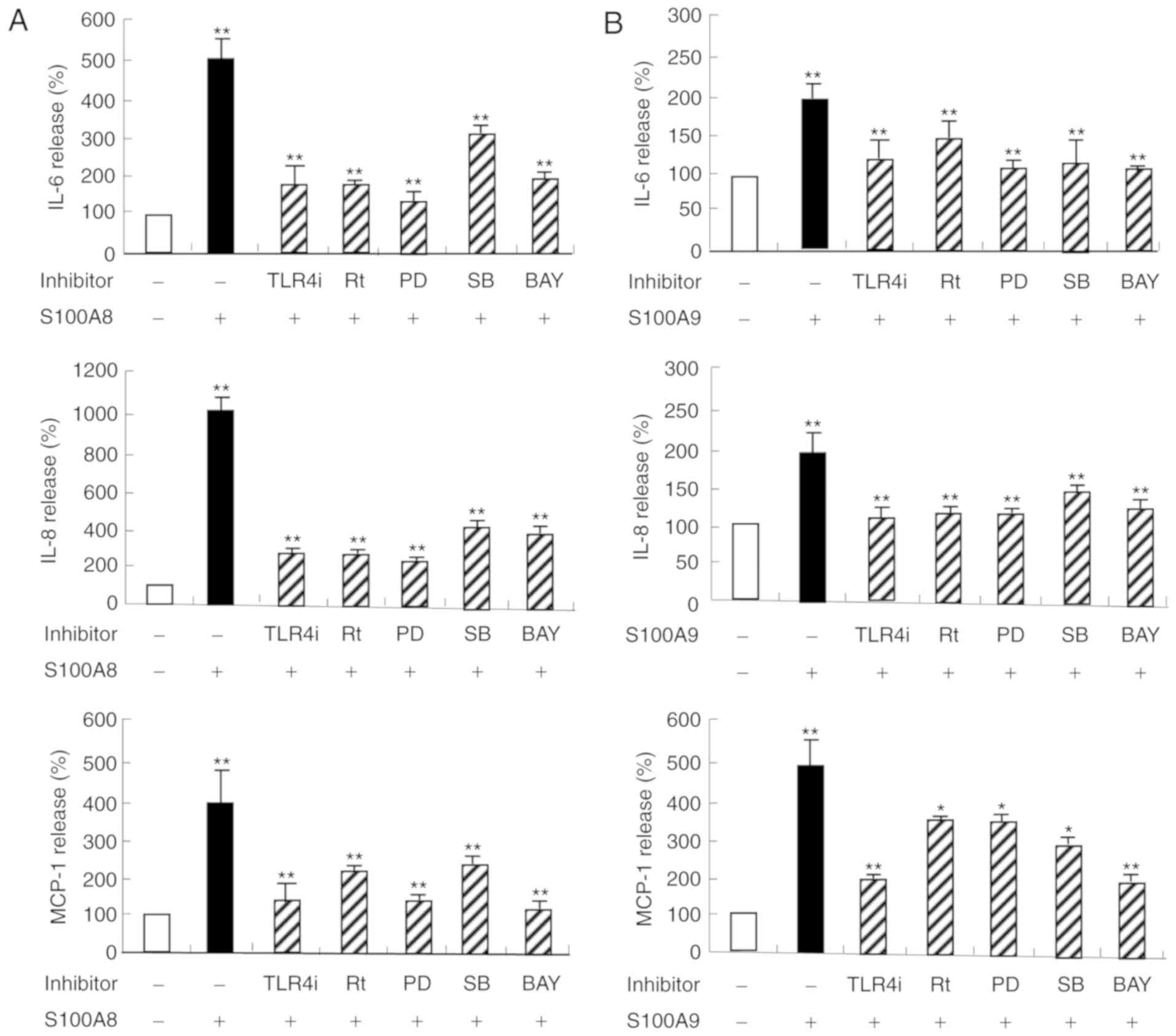 | Figure 3.HaCaT cells were pretreated for 1 h
with and without 5 µM TLR4i, 5 µM Rt, 10 µM PD, 10 µM SB or 2 µM
BAY, followed by incubation with (A) S100A8 or (B) S100A9 for 48 h.
Data are expressed as the mean ± standard deviation. *P<0.05 and
**P<0.01 vs. the control. IL, interleukin; S100, calcium binding
protein; TLR, toll-like receptor; TLR4i, CLI-095; Rt, rottlerin;
PD, PD98059; SB, p38 mitogen-activated protein kinase inhibitor;
BAY, BAY-11-7085; MCP, monocyte chemoattractant protein. |
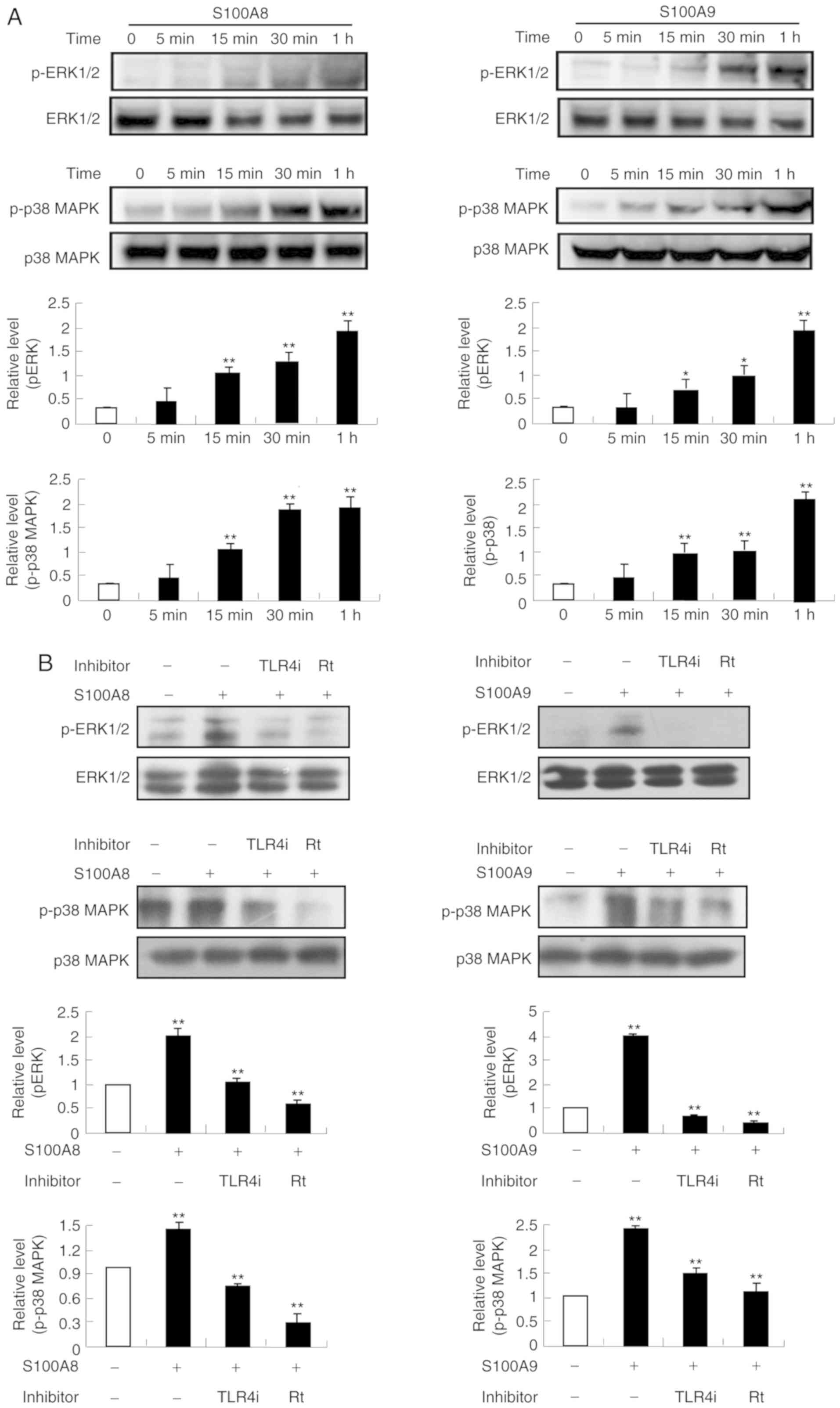
S100A8 and S100A9 induce activation of
ERK, p38 MAPK and NF-κB signaling pathways
Since the signaling inhibitors blocked cytokine
release induced by S100A8 and S100A9, whether S100A8 and S100A9
induce activation of MAPK and NF-κB signaling pathways was
examined. Following stimulation with S100A8 and S100A9,
phosphorylation of ERK and p38 MAPK was induced, reaching maximal
phosphorylation levels at 1 h (Fig.
4A). Additionally, ERK and p38 MAPK activation induced by
S100A8 and S100A9 was blocked by TLR4i and rottlerin (Fig. 4B). These results indicated that
S100A8 and S100A9 activate ERK and p38 MAPK signaling pathways via
TLR4 and rottlerin. As shown in Fig.
5, both S100A8 and S100A9 NF-κB activity in a time-dependent
manner, and this activation was suppressed by TLR4i, rottlerin,
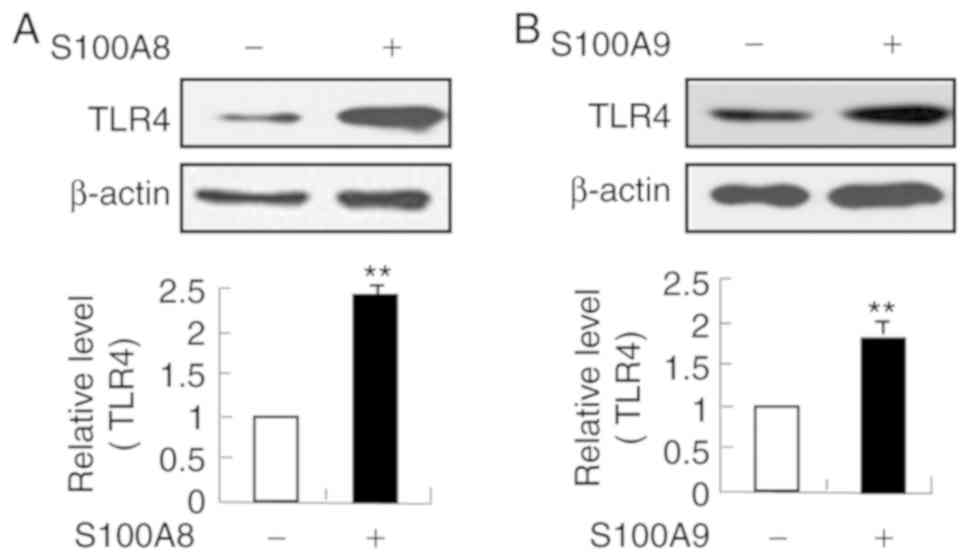
PD98059 and SB202190. In addition, TLR4 expression was upregulated
by S100A8 and S100A9 (Fig. 6).
These results indicated that S100A8 and S100A9 may activate NF-κB
via TLR4, PKCδ, ERK and p38 MAPK signaling pathways.
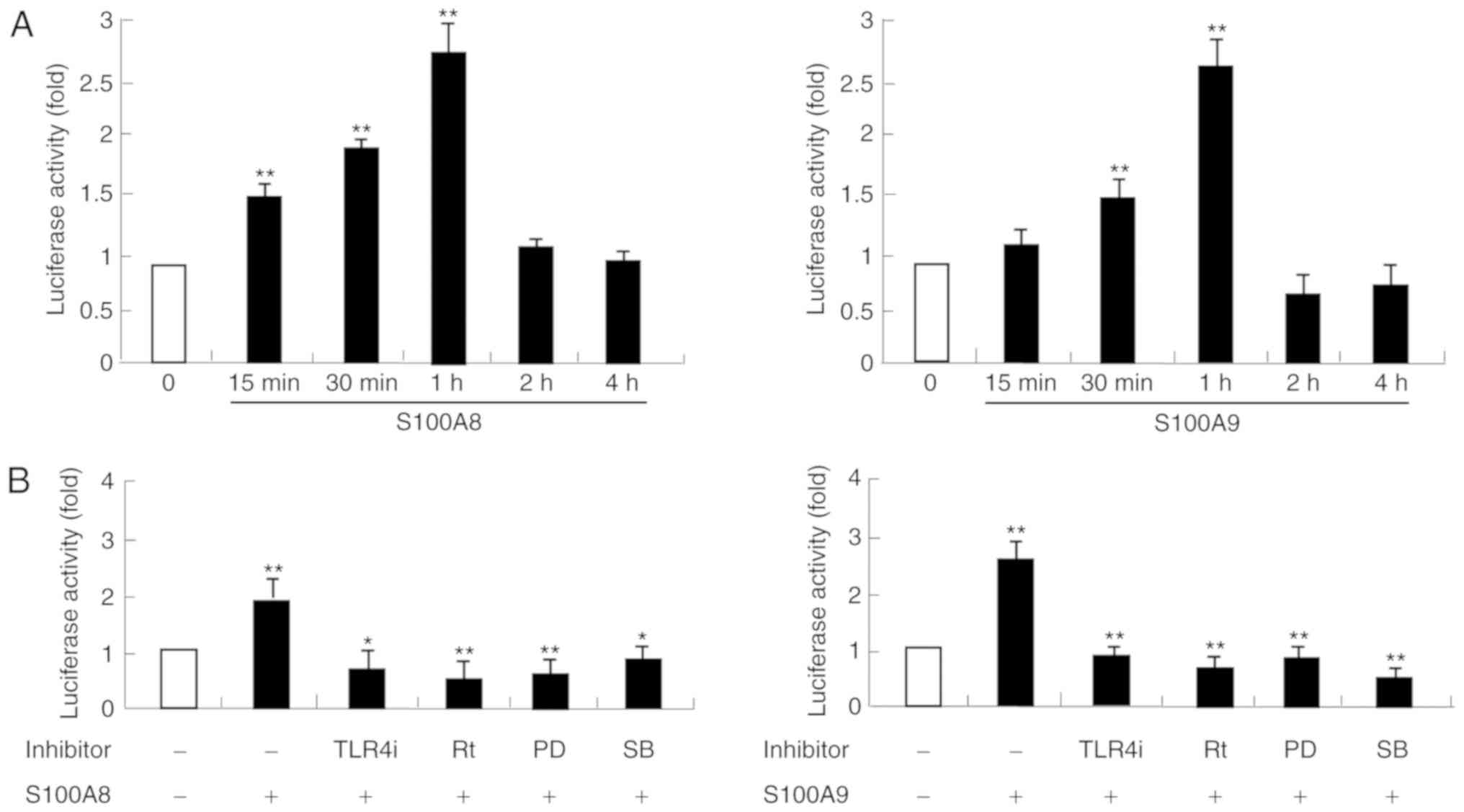 | Figure 5.S100A8 and S100A9 induced NF-κB
activation. (A) HaCaT cells were incubated with S100A8 and S100A9
(10 µg/ml) for the indicated times. (B) HaCaT cells were pretreated
for 1 h with and with 5 µM TLR4i, 5 µM Rt, 10 µM PD or 10 µM SB,
followed by incubation with S100A8 or S100A9 (10 µg/ml) for 1 h and
NF-κB in the lysates was measured by luciferase activity assay.
Data are expressed as the mean ± standard deviation. *P<0.05 and
**P<0.01 S100A8/A9-treated group vs. the control and
inhibitor-treated group vs. S100A8/A9-treated group. NF-κB, nuclear
factor-κB; S100, calcium binding protein; TLR, toll-like receptor;
TLR4i, CLI-095; Rt, rottlerin; PD, PD98059; SB, p38
mitogen-activated protein kinase inhibitor. |
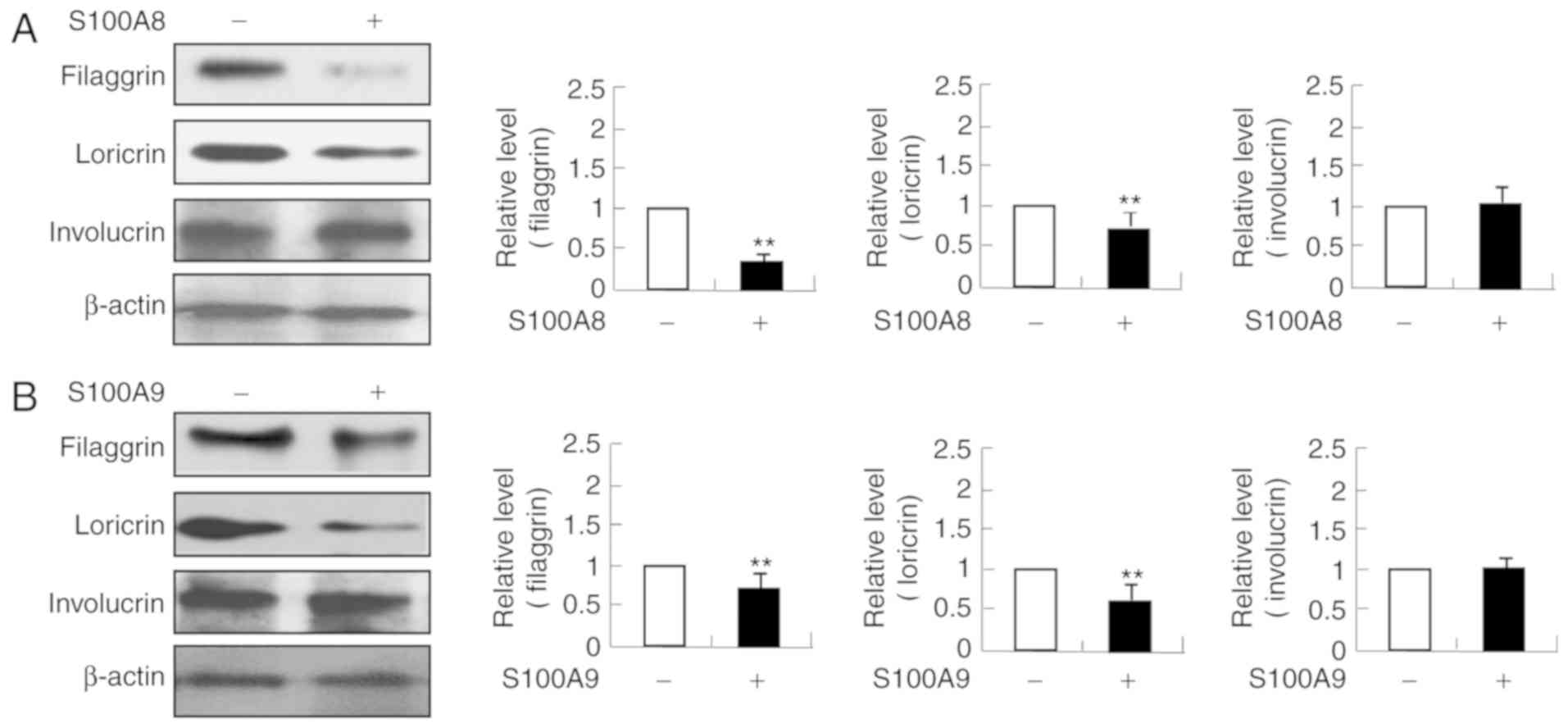
Filaggrin and loricrin expression
levels are decreased by S100A8 and S100A9
As skin barrier proteins are important in the
pathogenesis of AD, whether S100A8 and S100A9 alter the expression
of filaggrin, loricrin and involucrin was examined. The expression
levels of filaggrin and loricrin were decreased following
stimulation with S100A8 or S100A9; whereas involucrin expression
was not altered (Fig. 7). These
results indicated that S100A8 and S100A9, in addition to regulating
cytokine production, may also regulate skin barrier proteins
expression.
Discussion
The S100 proteins have calcium-binding capacity and
can induce a variety of inflammatory responses. S100A8 and S100A9
are produced by neutrophils, monocytes, macrophages, and
keratinocytes and their expression levels are increased under
inflammatory conditions (4). IL-1α
is produced by epithelial cells and acts autonomously on those
cells to induce the expression of S100A9 and cellular
differentiation (11). Decreased
hydration induces the expression of S100A8 and S100A9, resulting in
an increase in hypertrophic scarring (12). In addition, S100A8 and S100A9
levels are increased in lesional skin of patients with AD (13,14).
In the present study, it was examined whether S100A8 and S100A9,
which are involved in AD, alter the expression levels of cytokines
and skin barrier proteins. S100A8 and S100A9 increased the
expression of IL-6, IL-8, and MCP-1 in human keratinocytes. MCP-1
is a member of the C-C chemokine family and a potent chemotactic
factor in monocytes that can modulate the migration and invasion of
macrophages, memory T cells and natural killer cells (8). Additionally, MCP-1 is implicated in a
variety of inflammatory diseases, including asthma, rheumatoid
arthritis and chronic idiopathic urticarial (8). Furthermore, increased expression of
MCP-1 contributes to the proinflammatory environment in AD
(8,15). IL-6 serves essential roles in the
transition from acute to chronic inflammation. IL-8 secreted by
macrophages is associated with inflammatory conditions. The complex
interaction of these cytokines with chemokines may evoke AD
pathogenesis (15). In addition,
immunological reactions induced by S100 proteins include
intracellular and extracellular signaling. The human keratinocytic
HaCaT cell has low basal endogenous and exogeneous expression of
S100A8 and S100A9 (16). Although
it is expected that inflammatory cytokines induce intracellular
expression or secretion of S100A8 and S100A9, the exact stimuli are
still unknown and needs to be investigated further.
Defects in skin barrier function are an essential
feature of the development of AD (17). Although filaggrin, loricrin, and
involucrin are three key proteins in skin barrier functions, S100A8
and S100A9 treatment was effective only on the expressions of
filaggrin and loricrin with no effect on involucrin expression.
These results indicated that S100A8 and S100A9 may have specific
inhibitory effects on skin barrier proteins. IL-6 is negatively
associated with filaggrin expression, and decreased expression of
filaggrin elicits expression of IL-8 and MCP-1 (18,19).
Since skin barrier proteins are associated with cytokine secretion,
there may be several mechanisms involved in the increase of
cytokine secretion and the decrease of filaggrin and loricrin
expressions induced by S100A8 and S100A9. Further studies are
required to investigate the S100A8- and S100A9-associated
mechanisms affecting the expression and function of skin barrier
proteins, such as filaggrin and loricrin, and cytokine release.
MAPK is a typical signaling transduction pathway
that transduces extracellular stimulation from the cell membrane to
the intracellular nucleus (20).
MAPK, which is activated by receptors that bind with growth
hormones, cytokines and stressors, is involved in a variety of
cellular functions, including cell proliferation, differentiation,
and apoptosis (21). In the
current study, S100A8 and S100A9 induced activation of ERK and p38
MAPK. Both ERK (ERK1/2) and p38 MAPK have important roles in
cytokine secretion and allergic diseases, including AD (21,22).
c-Jun N-terminal kinase (JNK) is an important MAPK associated with
filaggrin expression (17).
Further studies are required to investigate the association between
the alteration of skin barrier proteins and JNK activation. In
addition, the present results demonstrated that TLR4 is elevated
following S100A8 and S100A9 treatment. It can be hypothesized that
the increased TLR4 expression levels due to S100A8 and S100A9
treatment enhance cytokine secretion induced by S100A8 and S100A9
via TLR4; this positive feedback loop may result in alleviation of
AD.
In summary, S100A8 and S100A9 promote cytokine
production, which is critical in the inflammation response, and
decrease filaggrin and loricrin expression levels. These
observations indicate that S100A8 and S100A9 may be important
molecules in the pathogenesis and progression of AD.
Acknowledgements
Not applicable
Funding
This study was supported by the BK21 plus program
through the National Research Foundation (NRF) funded by the
Ministry of Education of Korea and by Sysmex Korea Inc. (grant no.
19-001).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
MJK wrote manuscript, performed the experiments, and
analyzed the data. MAI and JSL performed the experiments and
analyzed the data. JYM, DHK, and AG interpreted the data. ISK
conceived the study, designed the experiments, and reviewed the
paper.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Spergel JM and Paller AS: Atopic
dermatitis and the atopic march. J Allergy Clin Immunol. 112 (6
Suppl):S118–S127. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Bieber T: Atopic dermatitis. N Engl J Med.
358:1483–1494. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim EH, Lee JS, Lee NR, Baek SY, Kim EJ,
Lee SJ and Kim IS: Regulation of constitutive neutrophil apoptosis
due to house dust mite allergen in normal and allergic rhinitis
subjects. PLoS One. 9:e1058142014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Goyette J and Geczy CL:
Inflammation-associated S100 proteins new mechanisms that regulate
function. Amino Acids. 41:821–842. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kim IS and Lee JS: Anti-apoptotic Effects
of house dust mite. S100A8 and S100A9 on spontaneous apoptosis of
neutrophils in coculture with immune cells and in the presence of T
helper cytokines. Biomed Sci Lett. 21:122–125. 2015. View Article : Google Scholar
|
|
6
|
Nacken W, Roth J, Sorg C and Kerkhoff C:
S100A8/S100A9: Myeloid representatives of the S100 protein family
as prominent players in innate immunity. Microsc Res Tech.
60:569–580. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Foell D and Roth J: Proinflammatory S100
proteins in arthritis and autoimmune disease. Arthritis Rheum.
50:3762–3771. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kim IS, Kim DH, Yun CY and Lee JS: A
(S)-(+)-decursin derivative,
(S)-(+)-3-(3,4-dihydroxy-phenyl)-acrylic acid
2,2-dimethyl-8-oxo-3,4-dihydro-2H,8H-pyrano[3,2-g]-chromen-3-yl-ester,
attenuates the development of atopic dermatitis-like lesions in
NC/Nga mice. Mol Biol Rep. 40:2541–2548. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kim IS, Kim MJ, Shin DH, Son KH, Park HY
and Lee JS: Arazyme inhibits cytokine expression and upregulates
skin barrier protein expression. Mol Med Rep. 8:551–556. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kim DH, Choi E, Lee JS, Lee NR, Baek SY,
Gu A, Kim DH and Kim IS: House dust mite allergen regulates
constitutive apoptosis of normal and asthmatic neutrophils via
Toll-like receptor 4. PLoS One. 10:e01259832015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bando M, Zou X, Hiroshima Y, Kataoka M,
Ross KF, Shinohara Y, Nagata T, Herzberg MC and Kido J: Mechanism
of interleukin-1α transcriptional regulation of S100A9 in a human
epidermal keratinocyte cell line. Biochim Biophys Acta.
1829:954–962. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhong A, Xu W, Zhao J, Xie P, Jia S, Sun
J, Galiano RD, Mustoe TA and Hong SJ: S100A8 and S100A9 are induced
by decreased hydration in the epidermis and promote fibroblast
activation and fibrosis in the dermis. Am J Pathol. 186:109–122.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jin S, Park CO, Shin JU, Noh JY, Lee YS,
Lee NR, Kim HR, Noh S, Lee Y, Lee JH and Lee KH: DAMP molecules
S100A9 and S100A8 activated by IL-17A and house-dust mites are
increased in atopic dermatitis. Exp Dermatol. 23:938–941. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sakaguchi M, Yamamoto M, Miyai M, Maeda T,
Hiruma J, Murata H, Kinoshita R, Winarsa Ruma IM, Putranto EW,
Inoue Y, et al: Identification of an S100A8 receptor neuroplastin-β
and its heterodimer formation with EMMPRIN. J Invest Dermatol.
136:2240–2250. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kim IS, Song GY, Kim DH, Cho SH, Yun CY
and Lee JS: Effect of
(E)-2-(3,4-dimethoxyphenyl)-4-oxo-4H-chromen-7-yl-3-(3,4-dimethoxyphenyl)
acrylate on the development of atopic dermatitis-like lesions. Life
Sci. 91:338–344. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Benedyk M, Sopalla C, Nacken W, Bode G,
Melkonyan H, Banfi B and Kerkhoff C: HaCaT keratinocytes
overexpressing the S100 proteins S100A8 and S100A9 show increased
NADPH oxidase and NF-kappaB activities. J Invest Dermatol.
127:2001–2011. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cha KJ, Im MA, Gu A, Kim DH, Lee D, Lee
JS, Lee JS and Kim IS: Inhibitory effect of Patrinia scabiosifolia
Link on the development of atopic dermatitis-like lesions in human
keratinocytes and NC/Nga mice. J Ethnopharmacol. 206:135–143. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sakai T, Hatano Y, Zhang W, Fujiwara S and
Nishiyori R: Knockdown of either filaggrin or loricrin increases
the productions of interleukin (IL)-1α, IL-8, IL-18 and granulocyte
macrophage colony-stimulating factor in stratified human
keratinocytes. J Dermatol Sci. 80:158–160. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ilves T, Tiitu V, Suttle MM, Saarinen JV
and Harvima IT: Epidermal expression of filaggrin/profilaggrin is
decreased in atopic dermatitis: Reverse association with mast cell
tryptase and IL-6 but not with clinical severity. Dermatitis.
26:260–267. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Chang L and Karin M: Mammalian MAP Kinase
signaling cascades. Nature. 410:33–40. 2011.
|
|
21
|
Park JH, Kim MS, Jeong GS and Yoon J:
Xanthii fructus extract inhibits TNF-α/IFN-γ-induced Th2-chemokines
production via blockade of NF-κB, STAT1 and p38-MAPK activation in
human epidermal keratinocytes. J Ethnopharmacol. 171:85–93. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Lee NR, Park BS, Kim SY, Gu A, Kim DH, Lee
JS and Kim IS: Cytokine secreted by S100A9 via TLR4 in monocytes
delays neutrophil apoptosis by inhibition of caspase 9/3 pathway.
Cytokine. 86:53–63. 2016. View Article : Google Scholar : PubMed/NCBI
|