Introduction
There were ~500,000 people with cervical cancer
worldwide in 2012, and in women, cervical cancer ranks second in
cancer occurrence and 15th in cancer mortality (1–3).
Currently, the pathogenesis of cervical cancer is not fully clear,
but human papillomavirus (HPV) infection is established to be the
most closely associated risk factor for cervical cancer occurrence.
However, high-risk HPV infection alone is not enough to induce
tumor progression (4).
Previous studies have revealed that HPV activates
the NF-κB pathway to promote expression of different anti-apoptosis
genes that induce cervical cancer development through inhibition of
cellular apoptosis (5–8). NF-κB an essential nuclear
transcription factor composed of RelA/p65, p50, p52 and NF-κB
family members (9). NF-κB pathway
activation is associated with the development of various tumors and
can promote tumor cell growth, proliferation and anti-apoptotic
effects to promote malignant transformation and tumor cell
metastases (10,11). NF-κB pathway activation is a common
mechanism used to promote tumor survival; the activated NF-κB
induces expression of various anti-apoptotic genes, such as the
cellular inhibitor of apoptosis protein (12), the X-linked inhibitor of apoptosis
protein (13) and Bcl-2 family
proteins (14). In addition, NF-κB
activation was shown to be present in cervical cancer (15), suggesting that NF-κB may have a
role in the development and progression of cervical cancer.
MicroRNAs (miRs) serve as markers of cervical cancer
progression (16), and it was
previously reported that miR-16 has an important role in the
regulation of cell apoptosis, proliferation, migration and invasion
of cervical cancer (17–19). In addition, miR-16 targets
inhibitor of nuclear factor κB kinase subunit β (Iκ-Bα) in
hepatocellular carcinoma (20),
and decreases NF-κB gene expression; thus, reducing the levels of
activated NF-κB (21). Therefore,
miR-16 has the important role of regulating the NF-κB signaling
pathway. On the other hand, long non-coding RNAs (lncRNAs) are
involved in different tumor processes (22) through regulation of target gene
expression at the transcriptional, post-transcriptional and
epigenetic levels (23–25). LncRNAs also at serve as precursors
of small interfering RNAs (siRNAs), miRs and piwi-interacting RNAs
(26,27), and can compete with miR as
competing endogenous RNAs (ceRNAs) to achieve inter-communication
and regulation (28–33). Furthermore, the lncRNA-plasmacytoma
variant translocation 1 (LncRNA PVT1) gene was reported to be a
biomarker of tumorigenesis and associated with the development of
cervical cancer (34–37) by competitively inhibiting miRs
(38,39).
Although no evidence currently exists to support the
hypothesis that the interaction between LncRNA PVT1 and miR-16
induces cervical cancer development, it is speculated that the
LncRNA PVT1 gene promotes cervical cancer development by inhibiting
miR expression and activating the NF-κB pathway. In the present
study, three known cervical cancer cell lines (HeLa, Ca Ski and
SiHa cells) and the H8 cell line (as an external control of three
cervical cancer cell lines) were selected as the experimental
models for in vitro experiments to investigate the effect
and molecular mechanisms of LncRNA PVT1 on cervical cancer
development.
Materials and methods
Cell culture
As it involved the use of human materials, the
present study was approved by the Ethics Committee of the
Affiliated Hospital of Qingdao University (Qingdao, China)
according to the experimental requirements of the school. HeLa, Ca
Ski, SiHa and H8 cells were purchased from the National
Infrastructure of Cell Line Resources of China. H8 cell line (the
human cervical epithelial immortalized cell line) was used as the
external control of the three cervical cancer cell lines, HeLa, Ca
Ski and SiHa. Each cell line was cultured in DMEM (Gibco; Thermo
Fisher Scientific, Inc.) containing 10% FBS (Gibco; Thermo Fisher
Scientific, Inc.) in 100 mm culture dishes (Corning Inc.) and
incubated in a 5% CO2. incubator at 37°C. The media was
changed every 3 days, and the cells were passaged once they reached
80% confluence. Additionally, 293T cells (purchased from the
National Infrastructure of Cell Line Resources of China) were used
in co-transfection experiment and cultured with the same
aforementioned treatment. To ensure cell activity, the cells from
passage 2–4 were used for further experiments.
Construction of LncRNA PVT1 and
3′-untranslated region (3′UTR) of NF-κB overexpression vectors and
siRNA of LncRNA PVT1
According to database sequences of the human LncRNA
PVT1 (National Center for Biotechnology Information reference
sequence: NR_003367.3), the pEGFP-N3 eukaryotic expression vector
(Beijing Tianyi Huiyuan Bioscience & Technology Inc.) carrying
the LncRNA PVT1 sequence was constructed via artificial nucleotide
synthesis at Tianyi Huiyuan Biotechnology Co. Ltd. Similarly,
according to the information on LncRNA PVT1 in the Ensembl genome
browser 96 database (reference number, ENSMUSG00000173039). The
sequences of the 3′UTR and the mutant of the 3′UTR of NF-κB gene
were obtained via artificial nucleotide synthesis at Beijing Tianyi
Huiyuan Bioscience & Technology Inc. and the expression plasmid
constructed. The siRNA (5′-GAGCUGCGAGCAAAGAUGU-3′) and control
siRNA (si-NC; 5′-GAUCGUACUAUAGCUUGUA-3′) of LncRNA PVT1 were
purchased from Guangzhou RiboBio Co., Ltd.
Cell transfection
Fugene6 transfection reagent (Roche Diagnostics) was
used according to the manufacturer's protocol. The overexpression
vector (final concentration 1 µg/ml) or siRNA of LncRNA PVT1 (final
concentration 50 nm) were transfected into HeLa, Ca Ski and SiHa
cells in 96-well plates or 100 mm dishes with 1×105
cells/ml and cultured for 48 or 60 h, respectively, and the
pEGFP-N3 empty vector or si-NC were also transfected with the
corresponding internal controls. Additionally, HeLa cells were
respectively treated with 5 µg/ml lipopolysaccharides (LPS;
Beyotime Institute of Biotechnology) plus the lncRNA PVT1 siRNA,
with pyrrolidinedithiocarbamate (PDTC; 50 µmol/l; Beyotime
Institute of Biotechnology) alone, or with PDTC (50 µmol/l) on the
basis of the lncRNA PVT1 overexpression treatment for 8 h to
activate or inhibit the NF-κB pathway, and then the cells were
continuously cultured for 60 h. In addition, using the Fugene6
transfection reagent, HeLa cells with 60% confluency were
transiently transfected with the miR-16 mimics (final concentration
100 nm) or miR-16 inhibitor (final concentration 50 nm). All cells
were collected for the experiments below following 60 h
treatment.
MTT assay
Following culture of the cells from the four cell
lines in 96-well plates with different treatments or no treatment
for 60 h, cell numbers were determined using the MTT assay. The
assay was conducted by adding 20 µl MTT (5 mg/ml) to each well of
the plates and incubating the plates for 4 h at 37°C. Following
removal of the supernatant, formazan crystals were dissolved in 200
µl dimethyl sulfoxide, and the absorbance was measured at 490 nm by
microplate reader (Thermo Fisher Scientific, Inc.). There were
eight replicate wells for each group to ensure the accuracy of the
experiments.
Flow cytometry (FCM)
Following treatment with or without the LncRNA-PVT1
siRNA for 48 h, HeLa cells were collected to use for cell cycle or
cell apoptosis analyses using a FACSCanto II flow cytometer
(Becton, Dickinson and Company). For the cell cycle analysis, cells
were centrifuged at 300 × g for 5 min at 4°C and washed with
ice-cold PBS following digestion with trypsin. The cells were then
mixed with 0.25% Triton X-100 and 5 µl propidium iodide (PI) and
incubated for 30 min at room temperature in the dark. Cells were
resuspended in 0.5 ml PBS and analyzed immediately.
For cellular apoptosis analysis, cells were digested
with trypsin, centrifuged at 300 × g for 5 min at 4°C, and washed
with ice-cold PBS. The cell pellets were resuspended in 100 µl of
Annexin V binding buffer, transferred to a 5 ml culture tube
containing 5 µl Annexin V-FITC, and mixed with 10 µl PI by using
the Annexin V-FITC Apoptosis Detection kit (Beyotime Institute of
Biotechnology). The tubes were gently vortexed and incubated for 15
min at room temperature in the dark. Subsequently, 300 µl binding
buffer was added and the cells were analyzed immediately.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from three cervical cancer
cells and H8 cells with different treatments using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocol. First-strand cDNA
was synthesized using a PrimeScript RT Master kit (Takara
Biotechnology Co., Ltd.) according to the manufacturer's protocol
(37°C for 15 min, 85°C for 5 sec and then stored at 4°C). The qPCR
was performed using SYBR Green reagents (Takara Biotechnology Co.,
Ltd.) and a 7500 Real-Time PCR System (Applied Biosystems; Thermo
Fisher Scientific, Inc.). Following initial denaturation for 30 sec
at 95°C, amplification was performed for 40 cycles (95°C for 5 sec
and 60°C for 32 sec). The specific primer sequences were as
follows: miR-16, forward 5′-TAGCAGCACGTAAATATTGGCG-3′; LncRNA PVT1,
forward 5′-TGCTCTAGAATCTGATGCACGTTCCACC-3′, reverse
5′-CCGGAATTCCTTAATTCTCCAATCTCAAAATAC-3′; NF-κB, forward
5′-GCATCCAGACCAACAACAACC-3′, reverse 5′-AGAGTTTCGGTTCACTCGGC-3′;
and cyclin D1 (CCND1), forward 5′-CAGATCATCCGCAAACACGC-3′, reverse
5′-AAGTTGTTGGGGCTCCTCAG-3′. Expression was normalized using β-actin
or U6 using the 2−ΔΔCq method (40). Primer sequences were as follows:
β-actin, forward 5′-GCTCGTCGTCGACAACGGCTC-3′, reverse
5′-CAAACATGATCTGGGTCATCTTCTC-3′; U6, forward
5′-CTCGCTTCGGCAGCACA-3′, reverse 5′-GCGAGCACAGAATTAATACGAC-3′. A
melting curve was constructed to verify the amplification of a
single PCR product. Experiments were performed in triplicate with
standard deviations of the Cq values not exceeding 0.5 on a
within-run basis.
Dual-luciferase reporter assay
Following the sequence alignment analysis of
NF-κB-3′UTR and miR-16, the pGL3-NF-κB-3′UTR reporter plasmid
(binding sites, UUAUAA), the corresponding mutant plasmid (AAGGCC)
and the empty plasmid (Beijing Tianyi Huiyuan Bioscience &
Technology Inc.) were constructed. Using the Fugene6 transfection
reagent, 293T cells with 60% confluency were transiently
transfected with the pGL3-NF-κB-3′UTR reporter plasmid (final
concentration 1 µg/ml) and miR-16 mimics (final concentration 100
nm) in the dual-luciferase reporter system, the corresponding
mutant plasmid and the empty plasmid were transfected as the
internal controls. After incubation for 36 h, firefly and
Renilla luciferase activities were measured and compared
using the dual-luciferase reporter assay kit (Promega Corporation)
by Thermo Scientific™ Fluoroskan Ascent™ FL microplate reader
(Thermo Fisher Scientific, Inc.) according to the manufacturer's
protocol.
Western blotting
Total cell lysates were extracted from HeLa cells
with the different treatments using a radioimmunoprecipitation
assay (RIPA) buffer (Beijing Solarbio Science & Technology Co.,
Ltd.) to detect the pNF-κB (p65), NF-κB (p65), Iκ-Bα, Bcl-2 and
CCND1 protein levels, and total protein levels in all samples were
assessed using a BCA Protein Assay kit (Beijing Solarbio Science
& Technology Co., Ltd.). After denaturation, equal amounts of
protein were loaded into each well of a 10–12.5% polyacrylamide gel
and subjected to sodium dodecyl sulphate polyacrylamide gel
electrophoresis (SDS-PAGE). The proteins were then transferred onto
polyvinylidene difluoride (PVDF) membranes, which were blocked with
5% skimmed milk (Sigma-Aldrich, Merck KGaA) at room temperature for
1 h on a shaker. Next, the membranes were incubated with primary
antibodies overnight at 4°C and subsequently washed three times for
30 min before being incubated with goat anti-rabbit IgG-HRP
(dilution 1:5,000, cat. no. ab205718; Abcam) at room temperature
for 1 h. The bound antibodies were visualized with the ECL Plus
Western Blotting Detection System (GE Healthcare Life Sciences).
The following monoclonal antibodies were used: Rabbit anti-pNF-κB
(p65) (65 kDa, dilution 1:10,000, cat. no. ab76302), rabbit
anti-NF-κB (p65) (65 kDa, dilution 1:1,000, cat. no. ab32536),
rabbi anti-Iκ-Bα (17 kDa, dilution 1:1,000, cat. no. ab178847),
rabbi anti-Bcl-2 (26 kDa, dilution 1:1,000, cat. no. Ab32124),
rabbi anti-CCND1 (34 kDa, dilution 1:10,000, cat. no. ab134175) and
rabbit anti-Tubulin antibody (55 kDa, dilution 1:5,000, cat. no.
ab59680) served as an internal protein loading control (Abcam).
Statistical analysis
All statistical analyses were performed using
one-way ANOVA followed by a Student-Newman-Keuls test (the
treatments with siRNA, siRNA plus LPS, overexpression, PDTC, or
PDTC plus overexpression, respectively) with the Statistical
Analysis Systems software (version 8.2; SAS Institute, Inc.) to
determine significance. The data are expressed as the mean ± SD
P<0.05 was considered to indicate a statistically significant
difference.
Results
LncRNA PVT1 induces an increase in
cervical cancer cell number
Compared with H8 cells, LncRNA PVT1 expression
levels were significantly increased in the three cervical cancer
cells (Fig. 1A), and the cell
numbers had also significantly increased (Fig. 1B). To further identify the effect
of LncRNA PVT1 on cervical cancer, HeLa, Ca Ski and SiHa cells were
transfected with either the LncRNA PVT1 overexpression vector or
siRNA for 60 h, and then cells were counted. Following transfection
with the overexpression vector, expression of the LncRNA PVT1 was
significantly increased in the three cervical cancer cells
(9–12-fold changes), and the knockdown efficiency of the LncRNA
PVT1 siRNA was also apparent (75–90%; Fig. 1C). The results also revealed that
cell numbers were significantly (P<0.05 or P<0.01) higher in
the three cervical cancer cell lines treated with the LncRNA PVT1
overexpression vector and significantly lower (P<0.01) in those
treated with siRNA compared with that of the corresponding internal
control cells (empty vector or si-NC; Fig. 1D). Given that LncRNA PVT1 had
similar effects on the cell numbers of the three cervical cancer
cell types, HeLa cells were used for subsequent further analysis in
the present study.
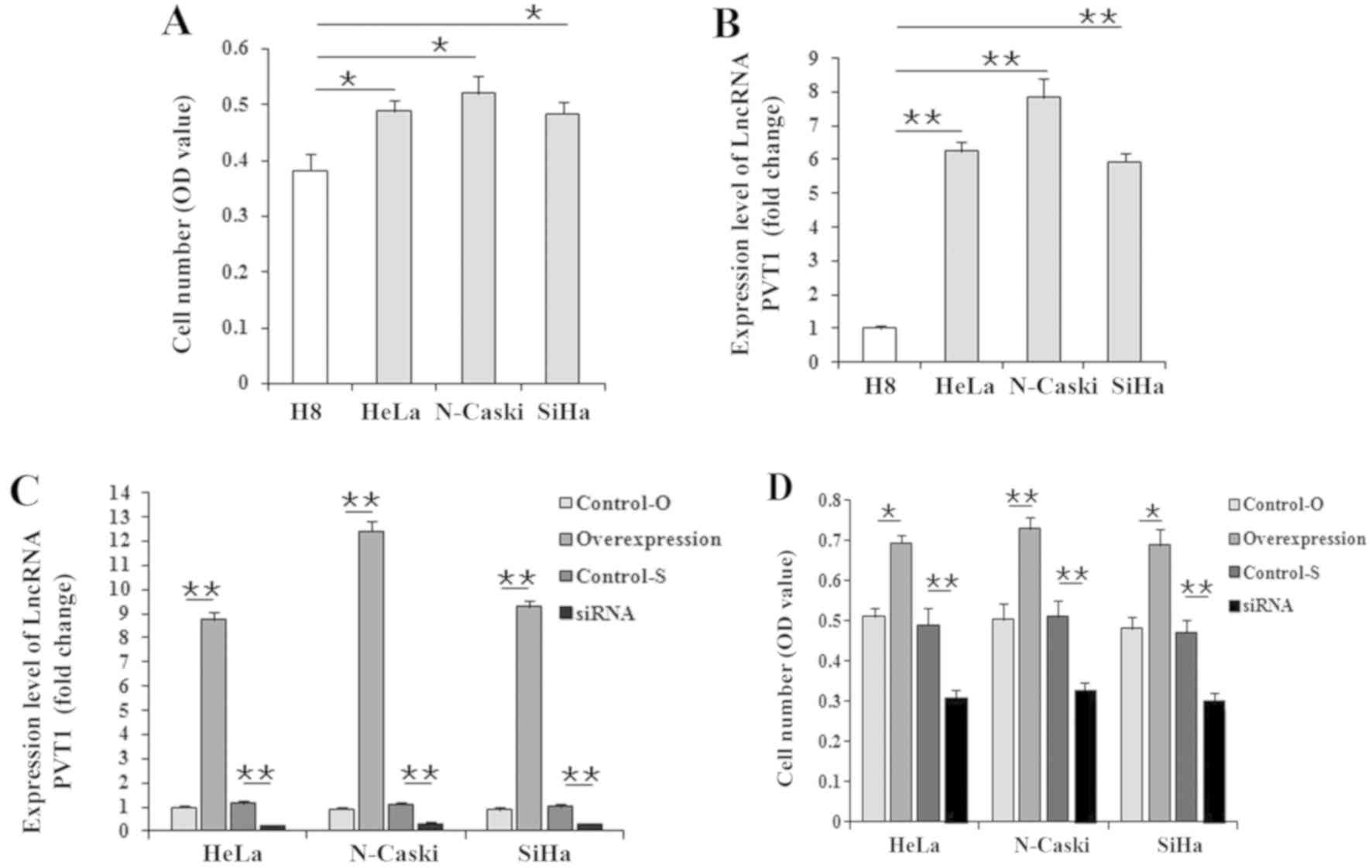 | Figure 1.Effect of LncRNA PVT1 on the cell
number of cervical cancer cell lines. After culturing for 60 h
in vitro, (A) the comparison of cell number, assessed with
MTT, and (B) LncRNA PVT1 expression, assessed with qPCR, between
normal cervical epithelial cells (H8) and cervical cancer cells
(HeLa, Ca Ski and SiHa) was performed. After culturing for 60 h
in vitro, (C) cell numbers and (D) LncRNA PVT1 expression of
three cervical cancer cells treated with LncRNA PVT1 overexpression
or siRNAs was performed. Data are presented as the mean ± standard
deviation; n=3 (qPCR) or 8 (MTT) *P<0.05; **P<0.01. OD,
optical density; LncRNA PVT1, long non-coding RNA plasmacytoma
variant translocation 1; qPCR, quantitative PCR; Control-O, vector
control; Control-S, negative control siRNA; siRNA, small
interfering RNA. |
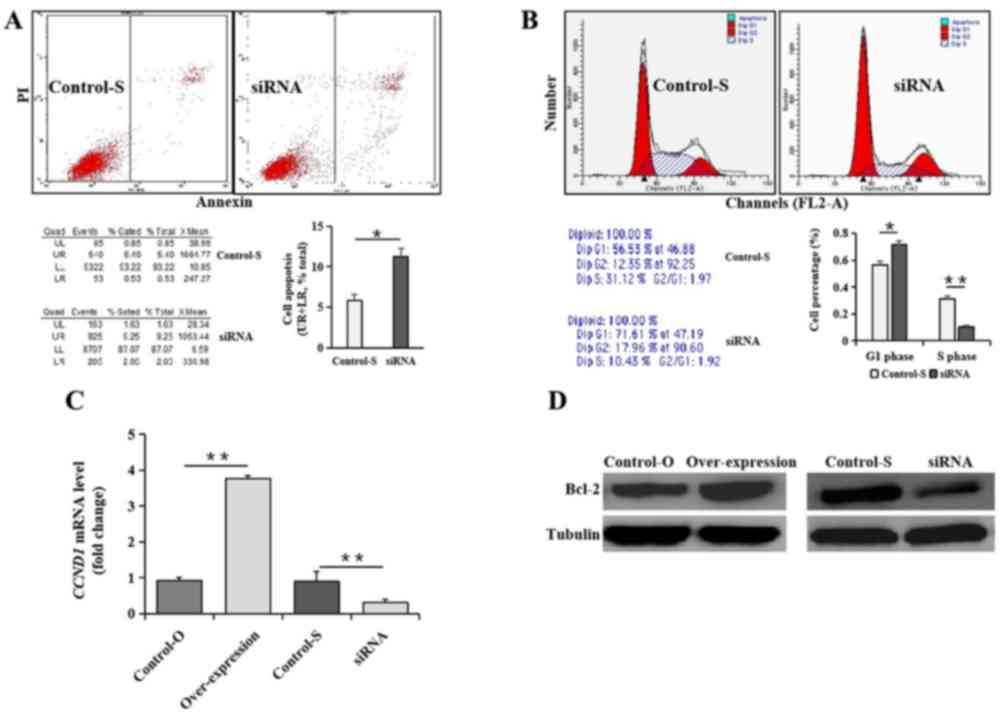
Furthermore, the FCM results revealed that cell
percentages in S phase were significantly lower (P<0.05) and in
the G1 phase were significantly higher (P<0.05;
Fig. 2A), and the apoptosis rate
was significantly higher (P<0.05; Fig. 2B) in HeLa cells with the
interference vector compared with that of untreated HeLa cells.
CCND1 mRNA and Bcl-2 protein expression was decreased in HeLa cells
treated with siRNA of LncRNA PVT1 compared with untreated HeLa
cells (P<0.01). However, CCND1 mRNA and Bcl-2 protein expression
was increased in HeLa cells with LncRNA PVT1 overexpression
compared with those of untreated HeLa cells (Fig. 2C and D).
LncRNA PVT1 activates the NF-κB
pathway in HeLa cells
As the NF-κB pathway has a role in cell apoptosis
and proliferation, whether LncRNA PVT1 could activate the NF-κB
pathway was investigated. The results revealed that both NF-κB
expression and activity levels were lower in HeLa cells with the
siRNA targeting LncRNA PVT1, and higher in HeLa cells with the
overexpression vector compared with that of untreated HeLa cells.
Similarly, Iκ-Bα levels changed in a manner opposite to that of
NF-κB activity in HeLa cells given the different treatments.
Furthermore, HeLa cells were co-treated with 5 µg/ml LPS (as an
activator of NF-κB) following transfection with siRNA LncRNA PVT1.
After 8 h of co-treatment, NF-κB activity recovered in the
untreated HeLa cell levels, but Iκ-Bα levels decreased again,
demonstrating an opposite effect to that NF-κB (Fig. 3A), which was accompanied by
corresponding changes in CCND1 and Bcl-2 expression (Fig. 3B). Furthermore, PDTC was used as a
specific inhibitor of NF-κB to investigate the specific mediating
action of NF-κB in the present study. When HeLa cells were treated
with PDTC for 8 h, the CCND1 and Bcl-2 expression levels exhibited
opposite effects to those observed following LPS treatment
(Fig. 3C). In addition, the cell
number of HeLa cells treated with both siRNA and LPS was
significantly increased (P<0.05) compared with that of the HeLa
cells treated with siRNA targeting LncRNA PVT1 only (Fig. 3D). However, the cell number of HeLa
cells was significantly decreased by treatment with PDTC
(P<0.05) compared with that in HeLa cells without PDTC, and a
significant decrease (P<0.05) was also observed in HeLa cells
treated with both PDTC and transfected with the LncRNA PVT1
overexpression vector compared with that in HeLa cells transfected
with the overexpression of LncRNA PVT1 only (Fig. 3D).
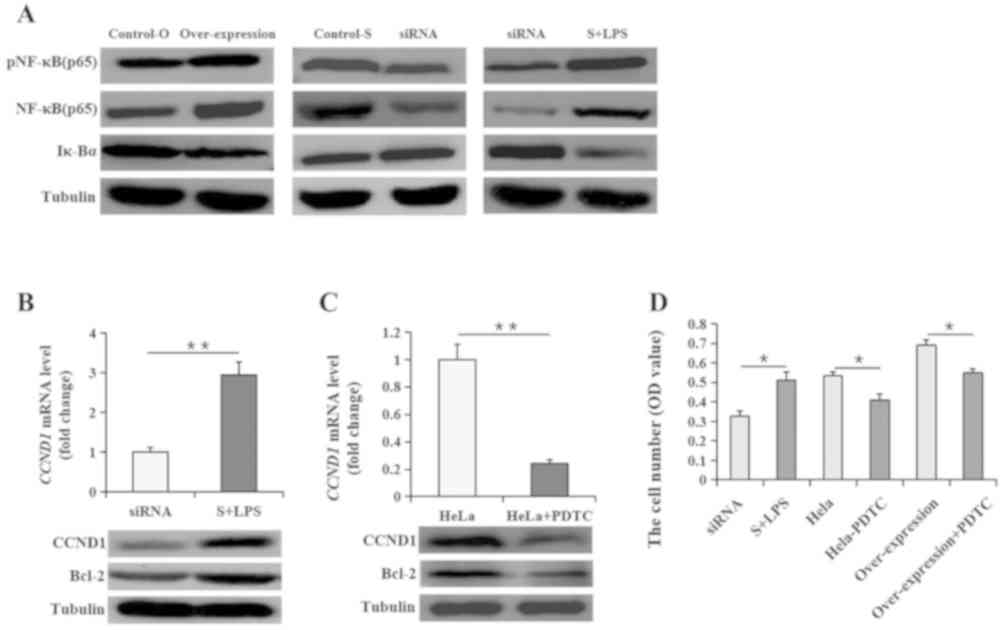 | Figure 3.NF-κB signaling pathway is involved
in the regulation of LncRNA PVT1 on cervical cancer cells. (A) The
changes of NF-κB (p65) expression and activity and Iκ-Bα expression
using western blotting in HeLa cells given different treatments.
(B) CCND1 mRNA and protein expression and Bcl-2 protein expression
in HeLa cells with LncRNA PVT1 siRNAs with or without LPS
treatment. (C) CCND1 mRNA and protein expression and Bcl-2 protein
expression in HeLa cells with PDTC. (D) HeLa cell number changes in
cultures with treatments of LncRNA PVT1 siRNAs with LPS, PDTC or
PDTC with LncRNA PVT1 overexpression were analyzed using an MTT
assay. Data are presented as the mean ± SEM; n=3 or 8. *P<0.05;
**P<0.01. LncRNA PVT1, long non-coding RNA plasmacytoma variant
translocation 1; Control-O, vector control; Control-S, negative
control siRNA; siRNA, small interfering RNA; S + LPS, siRNA +
lipopolysaccharide; p, phosphorylated; Iκ-Bα, inhibitor of nuclear
factor κB kinase subunit β; CCND1, cyclin D1; PDTC,
pyrrolidinedithiocarbamate; OD, optical density. |
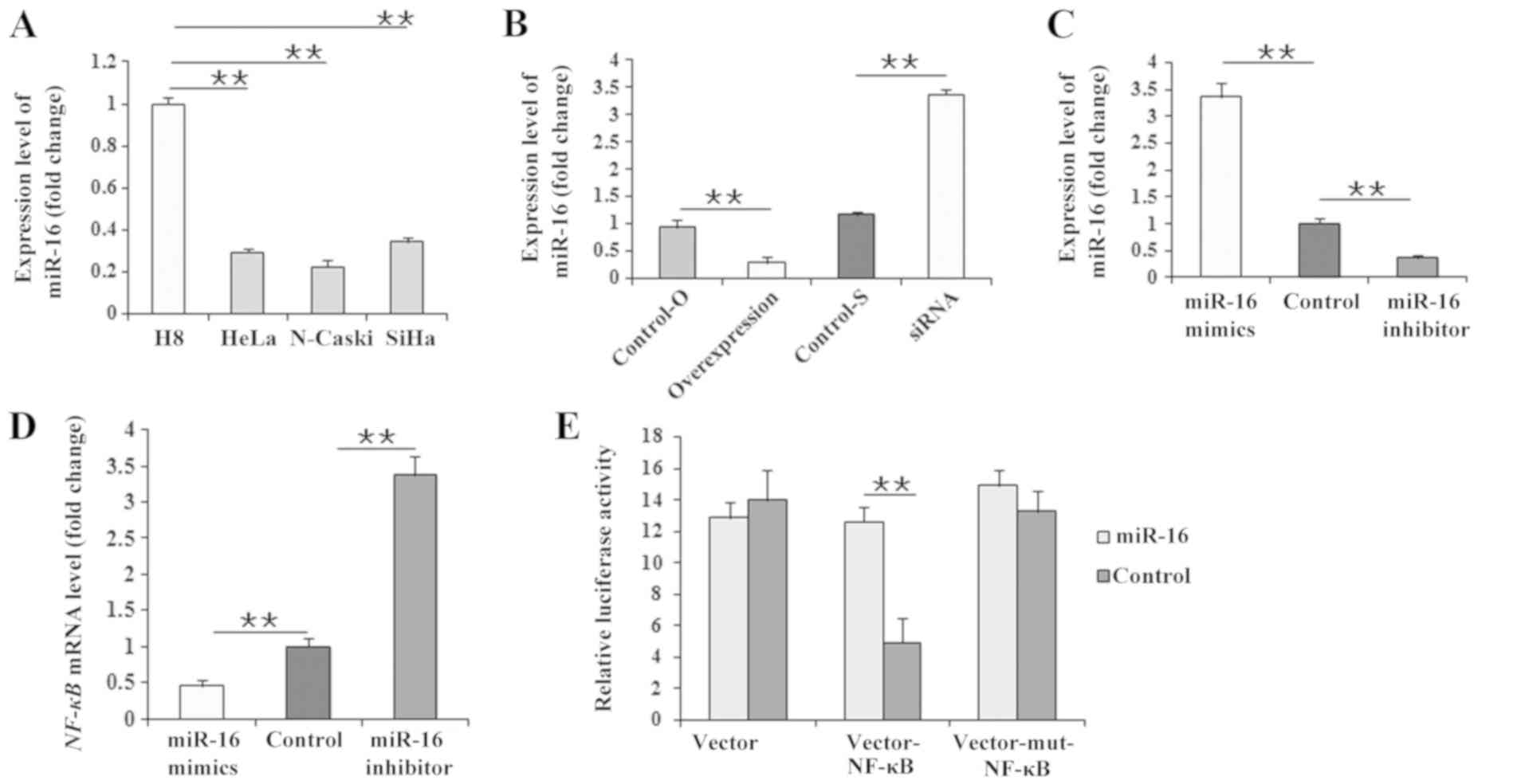
miR-16 is downregulated by LncRNA PVT1
and targets the NF-κB gene
According to the target regulation of miR-16 to
NF-κB, miR-16 expression levels were assessed in the present study.
Firstly, compared with H8 cells, miR-16 expression levels were
significantly lower in the three cervical cancer cell lines
(Fig. 4A). Subsequently, miR-16
expression levels were significantly reduced (P<0.01) in HeLa
cells treated with the LncRNA PVT1 overexpression vector compared
with HeLa cells transfected with the vector control (Fig. 4B). On the contrary, miR-16
expression levels were significantly upregulated (P<0.01) in
HeLa cells transfected with LncRNA PVT1 siRNA compared with the
control siRNA (Fig. 4B).
Subsequently, when the HeLa cells were transfected with miR-16
mimics and an miR-16 inhibitor (Fig.
4C), NF-κB expression levels were reduced by miR-16 mimics and
increased by miR-16 inhibitor (Fig.
4D). Considering the target regulation of miR-16 to NF-κB as
previous reported (41),
additional experiments were performed in order to investigate the
regulatory association between miR-16 and NF-κB in the present
study. The results of the dual-luciferase reporter assay revealed
that luciferase activity significantly increased in 293T cells
(P<0.01) following co-transfection with the miR-16 mimics and
the pEGFP-N3-3′UTR vector of the NF-κB gene (Fig. 4E), providing direct evidence that
miR-16 targets NF-κB mRNA.
Discussion
Cervical cancer causes considerable morbidity and
mortality in patients afflicted with this disease (1–3);
however, the pathogenesis of cervical cancer remains unclear. It is
known that the HPV infection is closely associated with the
occurrence of cervical cancer. However, simple high-risk HPV
infection is not enough to induce tumor progression (4). Considering previously reported
studies, it was speculated that lncRNA PVT1 may promote the
occurrence and development of cervical cancer via the NF-κB
pathway. As such, HeLa cells, a cervical cancer cell line, were
used to perform in vitro experiments that investigated the
effect of LncRNA PVT1 on the occurrence and development of cervical
cancer.
Using HeLa, Ca Ski and SiHa cells transfected with
LncRNA PVT1 overexpression or siRNAs, changes in cell numbers of
the three different cervical cancer cell lines were identified,
which were similar to that of LncRNA PVT1 expression. Given that
LncRNA PVT1 had a similar effect on cell numbers in all three cell
lines, HeLa cells were used for subsequent experiments in the
present study. Because of the change in cell numbers during the
cell cycle or as a result of apoptosis, FCM analysis was also
performed to define the effect of LncRNA PVT1 on HeLa cells, and
revealed that LncRNA PVT1 siRNA inhibited the G1/S phase
transition and promoted cellular apoptosis. CCND1 and Bcl-2 are the
important factors that positively regulate the cell cycle or cell
apoptosis (42,43). In the present study, the CCND1 mRNA
levels and Bcl-2 protein levels revealed results consistent with
that of the FCM results, which supported positive regulation of the
cell cycle and cellular apoptosis via these two factors. Therefore,
it can be assumed that LncRNA PVT1 promotes HeLa cell proliferation
by advancing the transition of the G1/S phase and
inhibiting cellular apoptosis. In order to assess the regulatory
role of the NF-κB pathway on preventing apoptosis and promoting
proliferation of the tumor cells (10,11,44),
an additional experiment was performed to detect NF-κB (p65)
activity in HeLa cells. As expected, the NF-κB (p65) activity in
HeLa cells was altered, which was similar to the change seen with
LncRNA PVT1 expression; however, Iκ-Bα activity exhibited the
opposite change. LPS is a known activator of the NF-κB signaling
pathway, and PDTC is the key inhibitor of the NF-κB signaling
pathway, and so it was verified that NF-κB pathway mediated LncRNA
PVT1 regulation in cervical cancer cells (45). HeLa cells were treated with LPS
based on the LncRNA PVT1 interference studies, for which both NF-κB
(p65) activity and cell numbers recovered. In addition, the
viability of HeLa cells overexpressing LncRNA PVT1 was reduced by
treatment with PDTC. These results confirmed that LncRNA PVT1
promoted the increase of cervical cancer cells via the NF-κB
pathway.
Based on a previous study regarding miR-16 in tumor
regulation (46), miR-16
expression was detected and significantly downregulated by LncRNA
PVT1. As LncRNAs can also compete with miRs, as ceRNA, to achieve
intercommunication and regulation (28–31),
it was hypothesized that a competitive relationship exists between
LncRNA PVT1 and miR-16 in HeLa cells. Thus, miR-16 mimics and an
inhibitor were used to investigate the regulatory relationship
between miR-16 and NF-κB. It was revealed that miR-16 and NF-κB
expression levels were contrary to one another, in that NF-κB
levels were significantly decreased after HeLa cells were
transfected with the miR-16 mimics, but were significantly
increased after being transfected with the miR-16 inhibitor. These
results indicated that miR-16 downregulated NF-κB expression. It
was previously reported that NF-κB activity was directly regulated
by miR-16, which targets the NF-κB gene (38). Therefore, a dual-luciferase
reporter assay was performed to provide direct evidence that miR-16
targets the NF-κB gene. Together, these findings expand the current
understanding of the underlying regulatory mechanism of LncRNA PVT1
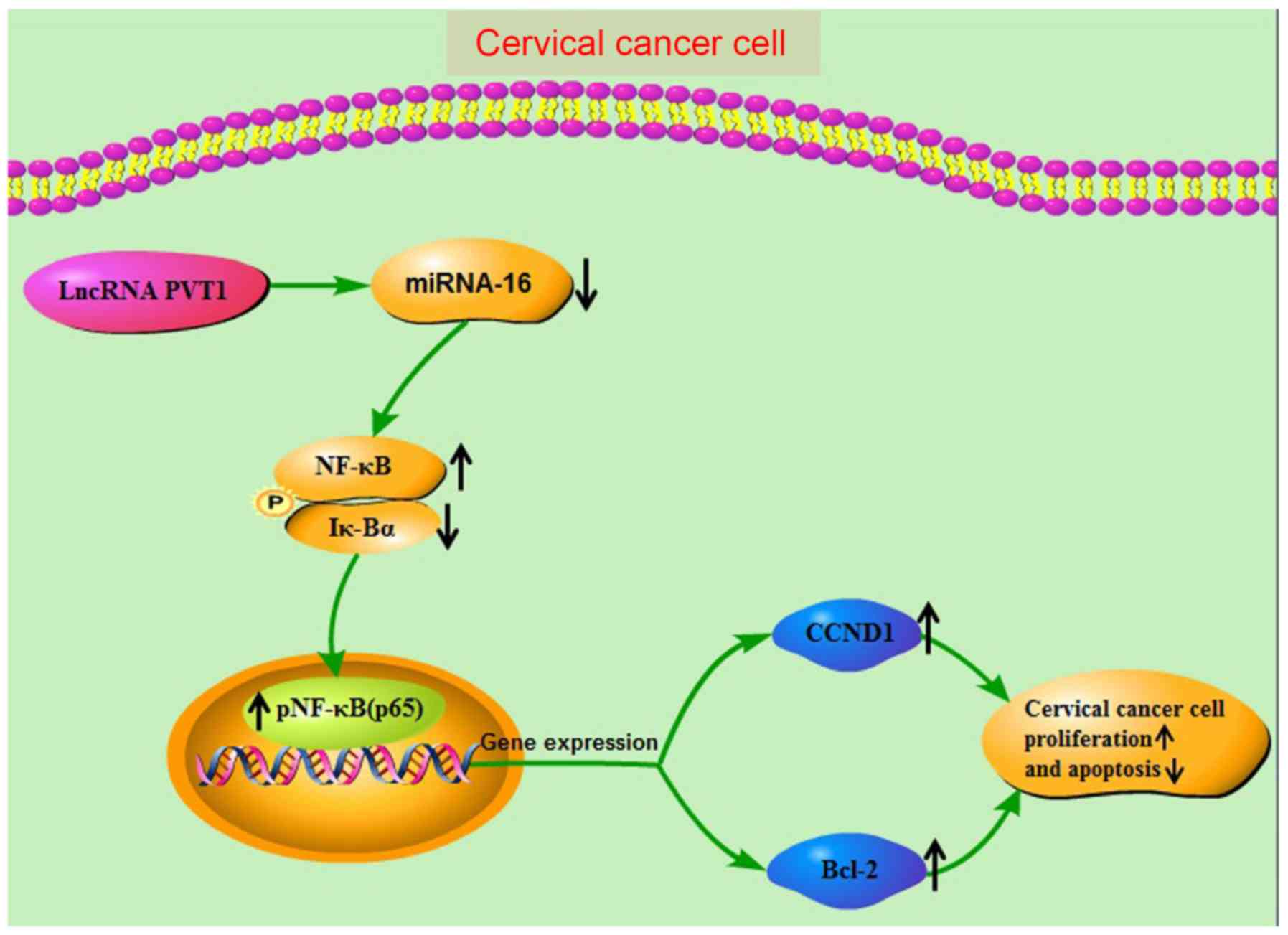
in promoting increases in HeLa cell numbers. Finally, Fig. 5 presents a proposed a molecular
regulatory network mediated by LncRNA PVT1 HeLa cells.
In summary, the data obtained in the present study
demonstrate that LncRNA PVT1 competitively inhibits miR-16, causing
an increase in the number of cervical cancer cells by promoting the
cell cycle and inhibiting cellular apoptosis via the NF-κB pathway,
thus promoting cervical cancer development. These results will add
to the theoretical basis of cervical cancer and provide a new
perspective for the treatment of cervical cancer.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
CW and HZ contributed to the design of the study and
performed the experiments, the interpretation of data and writing
of the manuscript. HY, LW, HC and JJ contributed to the
interpretation of data and writing of the manuscript. YW and AC
contributed to the design of the study. All authors submitted
comments on drafts and read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Joo YH, Jung CK, Sun DI, Park JO, Cho KJ
and Kim MS: High-risk human papillomavirus and cervical lymph node
metastasis in patients with oropharyngeal cancer. Head Neck.
34:10–14. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
den Boon JA, Pyeon D, Wang SS, Horswill M,
Schiffman M, Sherman M, Zuna RE, Wang Z, Hewitt SM, Pearson R, et
al: Molecular transitions from papillomavirus infection to cervical
precancer and cancer: Role of stromal estrogen receptor signaling.
Proc Natl Acad Sci USA. 112:E3255–E3264. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dalstein V, Riethmuller D, Prétet JL, Le
Bail Carval K, Sautière JL, Carbillet JP, Kantelip B, Schaal JP and
Mougin C: Persistence and load of high-risk HPV are predictors for
development of high-grade cervical lesions: A longitudinal French
cohort study. Int J Cancer. 106:396–403. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Nees M, Geoghegan JM, Hyman T, Frank S,
Miller L and Woodworth CD: Papillomavirus type 16 oncogenes
downregulate expression of interferon-responsive genes and
upregulate proliferation-associated and NF-kappaB-responsive genes
in cervical keratinocytes. J Virol. 75:4283–4296. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Spitkovsky D, Hehner SP, Hofmann TG,
Möller A and Schmitz ML: The human papillomavirus oncoprotein E7
attenuates NF-kappa B activation by targeting the Ikappa B kinase
complex. J Biol Chem. 277:25576–25582. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
James MA, Lee JH and Klingelhutz AJ: Human
papillomavirus type 16 E6 activates NF-kappaB, induces cIAP-2
expression, and protects against apoptosis in a PDZ binding
motif-dependent manner. J Virol. 80:5301–5307. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jia QP, Yan CY, Zheng XR, Pan X, Cao X and
Cao L: Upregulation of MTA1 expression by human papillomavirus
infection promotes CDDP resistance in cervical cancer cells via
modulation of NF-κB/APOBEC3B cascade. Cancer Chemother Pharmacol.
83:625–637. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gilmore TD: Introduction to NF-kappaB:
Players, pathways, perspectives. Oncogene. 25:6680–6684. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jiang N, Xie F, Guo Q, Li MQ, Xiao J and
Sui L: Toll-like receptor 4 promotes proliferation and apoptosis
resistance in human papillomavirus-related cervical cancer cells
through the Toll-like receptor 4/nuclear factor-κB pathway. Tumour
Biol. 39:10104283177105862017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Perkins ND: The diverse and complex roles
of NF-κB subunits in cancer. Nat Rev Cancer. 12:121–132. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Deveraux QL, Roy N, Stennicke HR, Van
Arsdale T, Zhou Q, Srinivasula SM, Alnemri ES, Salvesen GS and Reed
JC: IAPs block apoptotic events induced by caspase-8 and cytochrome
c by direct inhibition of distinct caspases. EMBO J. 17:2215–2223.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ono H, Iizumi Y, Goi W, Sowa Y, Taguchi T
and Sakai T: Ribosomal protein S3 regulates XIAP expression
independently of the NF-κB pathway in breast cancer cells. Oncol
Rep. 38:3205–3210. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chen QM and Tu VC: Apoptosis and heart
failure: Mechanisms and therapeutic implications. Am J Cardiovasc
Drugs. 2:43–57. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Prusty BK, Husain SA and Das BC:
Constitutive activation of nuclear factor-kB: Preferntial
homodimerization of p50 subunits in cervical carcinoma. Front
Biosci. 10:1510–1519. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
16
|
Pardini B, De Maria D, Francavilla A, Di
Gaetano C, Ronco G and Naccarati A: MicroRNAs as markers of
progression in cervical cancer: A systematic review. BMC Cancer.
18:6962018. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu J, Yang L, Zhang J, Zhang J, Chen Y,
Li K, Li Y, Li Y, Yao L and Guo G: Knock-down of NDRG2 sensitizes
cervical cancer Hela cells to cisplatin through suppressing Bcl-2
expression. BMC Cancer. 12:3702012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zubillaga-Guerrero MI, Alarcón-Romero Ldel
C, Illades-Aguiar B, Flores-Alfaro E, Bermúdez-Morales VH, Deas J
and Peralta-Zaragoza O: MicroRNA miR-16-1 regulates CCNE1 (cyclin
E1) gene expression in human cervical cancercells. Int J Clin Exp
Med. 8:15999–16006. 2015.PubMed/NCBI
|
|
19
|
Liu Z, Wu M, Shi H, Huang C, Luo S and
Song X: DDN-AS1-miR-15a/16-TCF3 feedback loop regulates tumor
progression in cervical cancer. J Cell Biochem. 120:10228–10238.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huang Y, Chen G, Wang Y, He R, Du J, Jiao
X and Tai Q: Inhibition of microRNA-16 facilitates the paclitaxel
resistance by targeting IKBKB via NF-κB signaling pathway in
hepatocellular carcinoma. Biochem Biophys Res Commun.
503:1035–1041. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang TQ, Lu XJ, Wu TF, Ding DD, Zhao ZH,
Chen GL, Xie XS, Li B, Wei YX, Guo LC, et al: MicroRNA-16 inhibits
glioma cell growth and invasion through suppression of BCL2 and the
nuclear factor-κB1/MMP9 signaling pathway. Cancer Sci. 105:265–271.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Qi F, Liu X, Wu H, Yu X, Wei C, Huang X,
Ji G, Nie F and Wang K: Long noncoding AGAP2-AS1 is activated by
SP1 and promotes cell proliferation and invasion in gastric cancer.
J Hematol Oncol. 10:482017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bai Y, Dai X, Harrison AP and Chen M: RNA
regulatory networks in animals and plants: A long noncoding RNA
perspective. Brief Funct Genomics. 14:91–101. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chen LL and Carmichael GG: Decoding the
function of nuclear long non-coding RNAs. Curr Opin Cell Biol.
22:357–364. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wierzbicki AT: The role of long non-coding
RNA in transcriptional gene silencing. Curr Opin Plant Biol.
15:517–522. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ben Amor B, Wirth S, Merchan F, Laporte P,
d'Aubenton- Carafa Y, Hirsch J, Maizel A, Mallory A, Lucas A,
Deragon JM, et al: Novel long nonprotein coding RNAs involved in
Arabidopsis differentiation and stress responses. Genome Res.
19:57–69. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ma X, Shao C, Jin Y, Wang H and Meng Y:
Long non-coding RNAs: A novel endogenous source for the generation
of dicer-like 1-dependent small RNAs in Arabidopsis thaliana. RNA
Biol. 11:373–390. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Salmena L, Poliseno L, Tay Y, Kats L and
Pandolfi PP: A ceRNA hypothesis: The Rosetta Stone of a hidden RNA
language. Cell. 146:353–358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Karreth FA, Tay Y, Perna D, Ala U, Tan SM,
Rust AG, DeNicola G, Webster KA, Weiss D, Perez-Mancera PA, et al:
In vivo identification of tumor-suppressive PTEN ceRNAs in an
oncogenic BRAF-induced mouse model of melanoma. Cell. 147:382–395.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tay Y, Kats L, Salmena L, Weiss D, Tan SM,
Ala U, Karreth F, Poliseno L, Provero P, Di Cunto F, et al:
Coding-independent regulation of the tumor suppressor PTEN by
competing endogenous mRNAs. Cell. 147:344–357. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cesana M, Cacchiarelli D, Legnini I,
Santini T, Sthandier O, Chinappi M, Tramontano A and Bozzoni I: A
long noncoding RNA controls muscle differentiation by functioning
as a competing endogenous RNA. Cell. 147:358–369. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang C, Han C, Zhang Y and Liu F: LncRNA
PVT1 regulate expression of HIF1α via functioning as ceRNA for
miR-199a-5p in non-small cell lung cancer under hypoxia. Mol Med
Rep. 17:1105–1110. 2018.PubMed/NCBI
|
|
33
|
Guo D, Wang Y, Ren K and Han X: Knockdown
of LncRNA PVT1 inhibits tumorigenesis in non-small-cell lung cancer
by regulating miR-497 expression. Exp Cell Res. 362:172–179. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yang JP, Yang XJ, Xiao L and Wang Y: Long
noncoding RNA PVT1 as a novel serum biomarker for detection of
cervical cancer. Eur Rev Med Pharmacol Sci. 20:3980–3986.
2016.PubMed/NCBI
|
|
35
|
Iden M, Fye S, Li K, Chowdhury T,
Ramchandran R and Rader JS: The lncRNA PVT1 Contributes to the
cervical cancer phenotype and associates with poor patient
prognosis. PLoS One. 11:e01562742016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Shen CJ, Cheng YM and Wang CL: LncRNA PVT1
epigenetically silences miR-195 and modulates EMT and
chemoresistance in cervical cancer cells. J Drug Target.
25:637–644. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang X, Wang G, Zhang L, Cong J, Hou J and
Liu C: LncRNA PVT1 promotes the growth of HPV positive and negative
cervical squamous cell carcinoma by inhibiting TGF-β1. Cancer Cell
Int. 18:702018. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Zhang S, Zhang G and Liu J: Long noncoding
RNA PVT1 promotes cervical cancer progression through
epigenetically silencing miR-200b. APMIS. 124:649–658. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gao YL, Zhao ZS, Zhang MY, Han LJ, Dong YJ
and Xu B: Long Noncoding RNA PVT1 facilitates cervical cancer
progression via negative regulating of miR-424. Oncol Res.
25:1391–1398. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang F, Yang L, Sun J, Zheng J, Shi L,
Zhang G and Cui N: Tumor suppressors microRNA-302d and microRNA-16
inhibit human glioblastoma multiforme by targeting NF-κB and FGF2.
Mol Biosyst. 13:1345–1354. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Wong L, Power N, Miles A and Tropepe V:
Mutual antagonism of the paired-type homeobox genes, vsx2 and
dmbx1, regulates retinal progenitor cell cycle exit upstream of
ccnd1 expression. Dev Biol. 402:216–228. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Valentin R, Grabow S and Davids MS: The
rise of apoptosis: Targeting apoptosis in hematologic malignancies.
Blood. 132:1248–1264. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hsieh SC, Hsieh WJ, Chiang AN, Su NW, Yeh
YT and Liao YC: The methanol-ethyl acetate partitioned fraction
from Chinese olive fruits inhibits cancer cell proliferation and
tumor growth by promoting apoptosis through the suppression of the
NF-κB signaling pathway. Food Funct. 7:4797–4803. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Xie S, Liu B, Fu S, Wang W, Yin Y, Li N,
Chen W, Liu J and Liu D: GLP-2 suppresses LPS-induced inflammation
in macrophages by inhibiting ERK phosphorylation and NF-κB
activation. Cell Physiol Biochem. 34:590–602. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Reid G, Pel ME, Kirschner MB, Cheng YY,
Mugridge N, Weiss J, Williams M, Wright C, Edelman JJ, Vallely MP,
et al: Restoring expression of miR-16: A novel approach to therapy
for malignant pleural mesothelioma. Ann Oncol. 24:3128–3135. 2013.
View Article : Google Scholar : PubMed/NCBI
|