Introduction
Chronic myeloid leukemia (CML) is characterized by
the formation of the Philadelphia (Ph) chromosome, which occurs in
pluripotent hematopoietic stem cells (1). This translocation generates the BCR
activator of RhoGEF and GTPase (BCR)-ABL fusion gene that encodes
p210BCR-ABL protein (2). The oncoprotein exhibits constitutive
tyrosine kinase activity and serves a fundamental role in the
formation of CML (3). Imatinib, a
tyrosine kinase inhibitor (TKI), is the upfront treatment for
Ph+ CML (4,5). However, drug resistance is a major
reason for relapsed and refractory CML following the termination of
imatinib treatment (6), and a
number of mechanisms of resistance are independent of
p210BCR-ABL upregulation and mutation status (7,8).
Therefore, it is important to develop a technique to improve the
therapeutic effects of imatinib.
Homoharringtonine (HHT) is a plant alkaloid with
antitumor properties that is derived from trees of the genus
Cephalotaxus; it has been widely used in China for the
treatment of hematological malignancies since the 1970s (9,10).
HHT has served an important role in the treatment of CML, both
prior to the widespread use of TKIs, and at present following the
development of TKI resistance (11–13).
The FDA has approved HHT for CML refractory to TKIs (1). The anti-leukemic mechanism of HHT is
based on the inhibition of protein synthesis (10). HHT reduces p210BCR-ABL
protein expression level in BCR-ABL+ cells independently
of BCR-ABL mutational status (13–15).
HHT also has a synergistic relationship with imatinib in clinical
therapy (16), but the working
mechanism is poorly understood.
The zinc-finger protein, X-linked (ZFX) gene is on
the mammalian X chromosome and is a transcriptional regulator
involved in the maintenance of embryonic and hematopoietic stem
cells (17,18). Previous studies suggest that ZFX
serves a pivotal role in tumorigenesis of multiple types of cancer,
including lung cancer, gastric cancer, breast cancer, malignant
glioma and leukemia (19–23). Additionally, ZFX participates in
drug resistance in hepatocellular carcinoma (24,25).
The results of our previous study also demonstrated that ZFX may be
involve in the regulation of cell proliferation and imatinib
resistance in CML (26). Thus, the
present study investigated the effects and mechanisms of HHT
facilitating imatinib sensitivity in K562 human CML cells. The
results indicated that HHT may enhance the effects of imatinib on
CML cells by downregulating ZFX expression.
Materials and methods
Cell culture
K562 human CML cells were purchased from The Cell
Bank of Type Culture Collection of The Chinese Academy of Sciences.
Cells were cultivated in RPMI-1640 medium supplemented with 10% FBS
(both Gibco; Thermo Fisher Scientific, Inc.) in a humidified
atmosphere with 5% CO2 at 37°C. A range of
concentrations of imatinib (Selleck Chemicals) or HHT (Bio-Techne)
were added to the cells during experiments.
Transfection
To overexpress ZFX, the human ZFX sequence was
amplified and cloned into the pEGFP-C1 expression plasmid by
Shanghai GeneChem Co., Ltd. Cell transfection was performed by
electroporation. Typically, 7×106 cells and pEGFP-C1-ZFX
or empty vector were electroporated using a Bio-Rad Gene Pulser II
(Bio-Rad Laboratories, Inc.) with 250 V voltage and 950 µFd
electric capacity. Cells were subsequently resuspended in RPMI-1640
medium and cultured for 24–72 h.
Cell viability assay
Cell Counting Kit-8 (CCK-8; Dojindo Molecular
Technologies, Inc.) assay was used to examine cell viability
following different treatments. K562 cells were seeded in a 96-well
plate (8×103 cells/well), cultured for 24 h and treated
with different concentrations of imatinib or HHT for 24 or 48 h at
37°C. Subsequently, 10 µl CCK-8 solution was added to the plate and
the cells were incubated for 2 h at 37°C. Absorbance at 450 nm was
measured using a microplate reader (BioTek Instruments, Inc.). HHT
treatment or ZFX overexpression may affect cell viability;
therefore, the relative viability of drug-treated cells was
normalized to DMSO-treated cells to eliminate a false-positive
effect.
Colony formation assay
Drug-treated K562 cells were cultured in a two-layer
soft agar system as previously described (27). Single-cell suspensions were washed
with RPMI-1640 medium, enumerated and plated into a 12-well plate
(1 ml/well; 1×103 cells/ml). The feeder layer was
prepared with agar equilibrated at 42°C. Following incubation for
10 days at 37°C, the colonies (≥40 cells for each) were counted
under an inverted microscope (magnification, ×100; Olympus
Corporation).
Apoptotic assay
Apoptosis was detected using the Annexin
V-FITC/propidium iodide (PI) Apoptosis Detection kit (BD
Biosciences) according to the manufacturer's protocol. Stained
cells were analyzed using a flow cytometer (BD Biosciences), and
cells were separated into normal, early apoptotic, late apoptotic
and dead cells. The relative ratios of early apoptotic cells were
analyzed by FlowJo software (version 10; FlowJo LLC).
Phosphorylated-tyrosine (p-Tyr)
protein assay
Drug-treated K562 cells were fixed with 1%
paraformaldehyde (BD Biosciences) for 30 min and permeabilized with
3% saponin (BD Biosciences) for 1.5 h (both at room temperature).
Subsequently, the cells were stained using phycoerythrin-conjugated
p-Tyr (1:1,000; cat. no. 558008) or p-CRK like proto-oncogene,
adaptor protein (p-Crkl; 1:1,000; cat. no. 560788) antibodies (both
BD Biosciences). Following washing with PBS, cells were recovered
in 3% saponin and submitted to flow cytometric analysis (BD
Biosciences), and mean fluorescence intensity (MFI) was recorded by
FlowJo software (version 10) to observe the levels of p-Tyr and
p-Crkl proteins.
Western blot analysis
Cells were lysed using RIPA buffer supplemented with
a protease inhibitor cocktail (Sigma-Aldrich; Merck KGaA). The
protein concentration was determined using a bicinchoninic acid
assay, and equal amounts (20–30 µg) of total protein were separated
by 6–10% SDS-PAGE and transferred onto PVDF membranes, which were
then blocked with 5% skim milk for 2 h at room temperature. The
primary antibodies against ZFX (1:1,000; cat. no. ab115998; Abcam),
β-Actin (1:1,000; cat. no. ab8224; Abcam), PI3K (1:1,000; cat. no.
05–212; Merck KGaA), AKT (1:1,000; cat. no. 07-383; Merck KGaA) and
p-AKT (1:1,000; cat. no. 04-736; Merck KGaA) were used to incubate
the membranes overnight at 4°C. Following incubation with secondary
goat anti-mouse (1:2,000; cat. no. ab6789; Abcam) or goat
anti-rabbit (1:2,000; cat. no. ab6721; Abcam) antibody for 2 h at
room temperature, blots were visualized using ECL Detection Reagent
(cat. no. P0018; Beyotime Institute of Biotechnology) and analyzed
using Image Lab software (Bio-Rad Laboratories, Inc.).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was isolated from cells using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.) and
reverse transcribed with FastQuant RT kit (Tiangen Biotech Co.,
Ltd.). PCR was performed in triplicate with SuperReal PreMix Plus
(Tiangen Biotech Co., Ltd.) using the Real-Time PCR Detection
System (Roche Molecular Systems, Inc.). qPCR was conducted at 95°C
for 5 min, followed by 40 cycles at 95°C for 10 sec, 65°C for 20
sec and 72°C for 30 sec. The primer sequences for ZFX and β-Actin
were as follows: ZFX, forward 5′-GGCAGTCCACAGCAAGAAC-3′, reverse
5′-TTGGTATCCGAGAAAGTCAGAAG-3′; β-Actin, forward
5′-CTCCATCCTGGCCTCGCTGT-3′ and reverse 5′-GCTGTCACCTTCACCGTTCC-3′.
Relative mRNA levels were normalized to β-Actin expression using
the 2−ΔΔCq method (28).
Statistical analysis
SPSS 19.0 software (IBM Corp.) was used for
statistical analysis. Data are expressed as the mean ± SD.
Differences between two groups were assessed by Student's t-test.
Statistical differences among multiple groups were analyze by
one-way analysis of variance followed by a Bonferroni post hoc
test. Each experiment was repeated for at least three times.
P<0.05 was considered to indicate a statistically significant
difference.
Results
HHT facilitates imatinib sensitivity
in CML cells
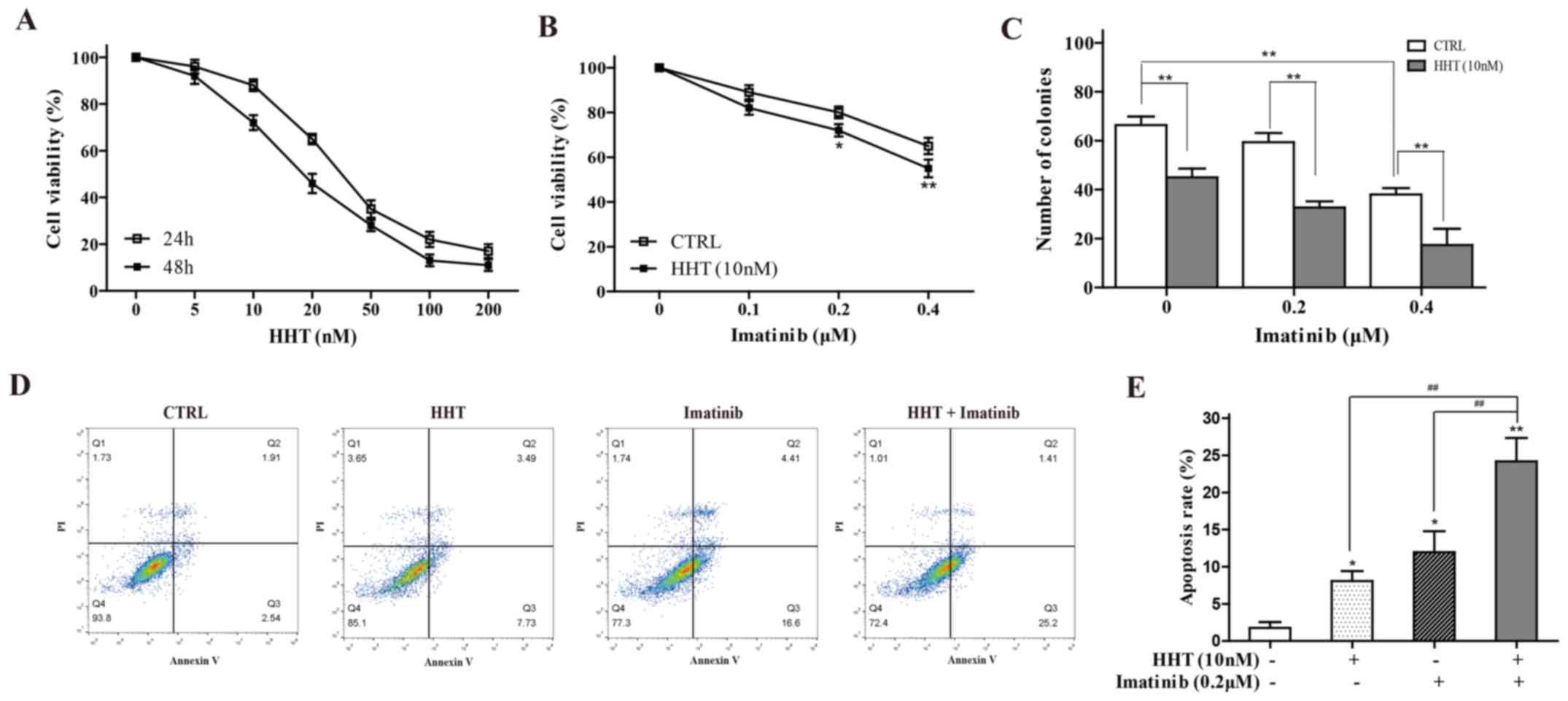
The effects of HHT on K562 cell viability were
tested by CCK-8 assay. Following treatment for 24 or 48 h, HHT
reduced cell viability in a dose- and a time-dependent manner
(Fig. 1A). A HTT concentration of
10 nM was selected for subsequent experiments, as this
concentration produced a small inhibitory effect at 24 h that would
still enable the detection of further sensitization effects. 24-h
co-treatment with 10 nM HHT and a range of concentrations of
imatinib resulted in significantly greater inhibition of cell
viability compared with imatinib alone (Fig. 1B). The results of a cloning
experiment demonstrated the additive effect of imatinib and HHT on
the reduced ability of K562 cells to form colonies compared with
either drug alone (Fig. 1C). In
addition, compared with HHT-alone or imatinib-alone groups, 24-h
co-treatment with HHT and imatinib significantly increased the
early apoptotic rate of K562 cells (Fig. 1D). An imatinib concentration of 0.2
µM imatinib was selected, as this concentration produced a small
inhibitory effect upon which further sensitization could be
observed, like for HTT.
Effects of HHT combined with imatinib
on tyrosine kinase activity in CML cells
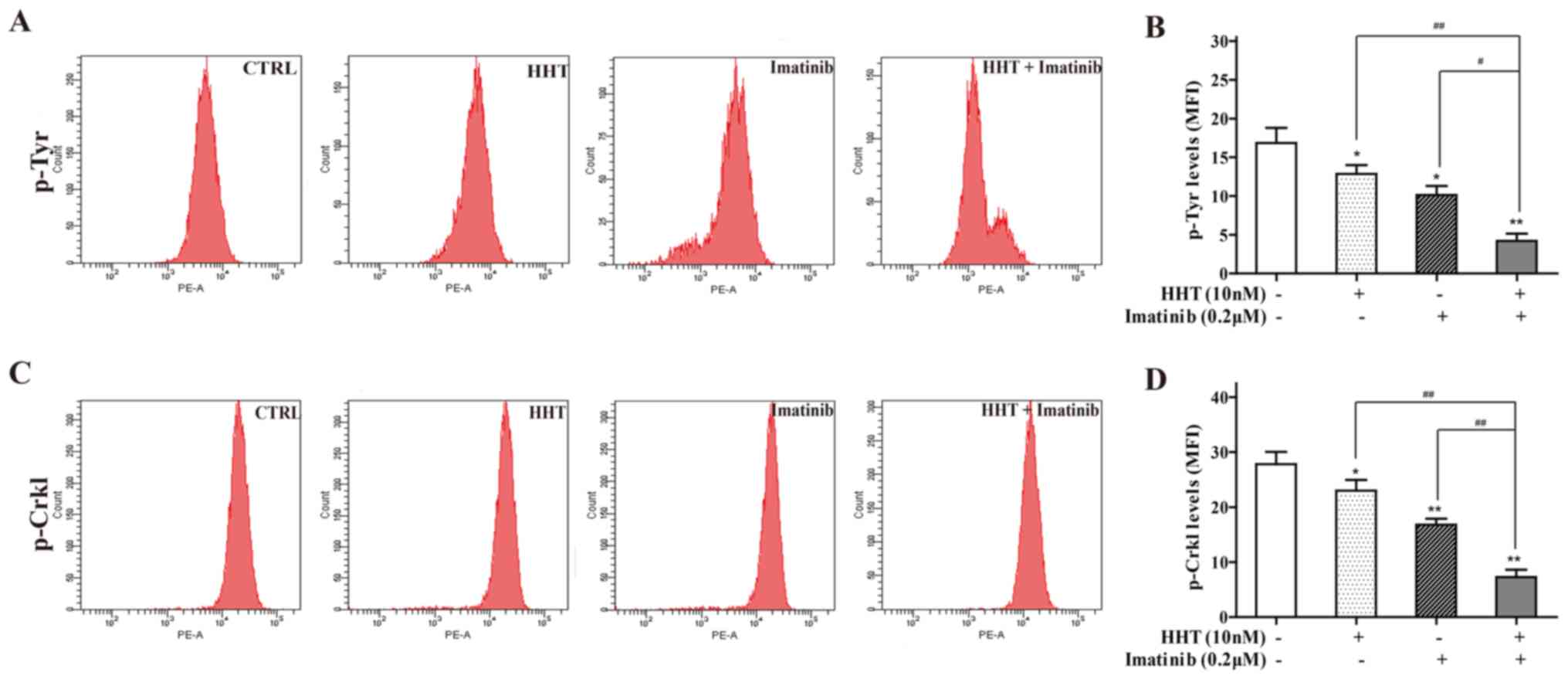
Tyrosine kinase activity may be reflected by p-Try
protein expression levels in BCR-ABL+ cells (29). Co-treatment of cells with HHT and
imatinib for 24 h significantly decreased the MFI of p-Tyr staining
compared with either treatment alone (Fig. 2A). p-Crkl, which has been reported
as an appropriate measure of tyrosine kinase activity in CML
(30), was also detected; the MFI
of p-Crkl staining in co-treated cells was significantly deceased
compared with the individual drug groups (Fig. 2B).
HHT regulates imatinib sensitivity
through suppression of PI3K/Akt pathway in CML cells
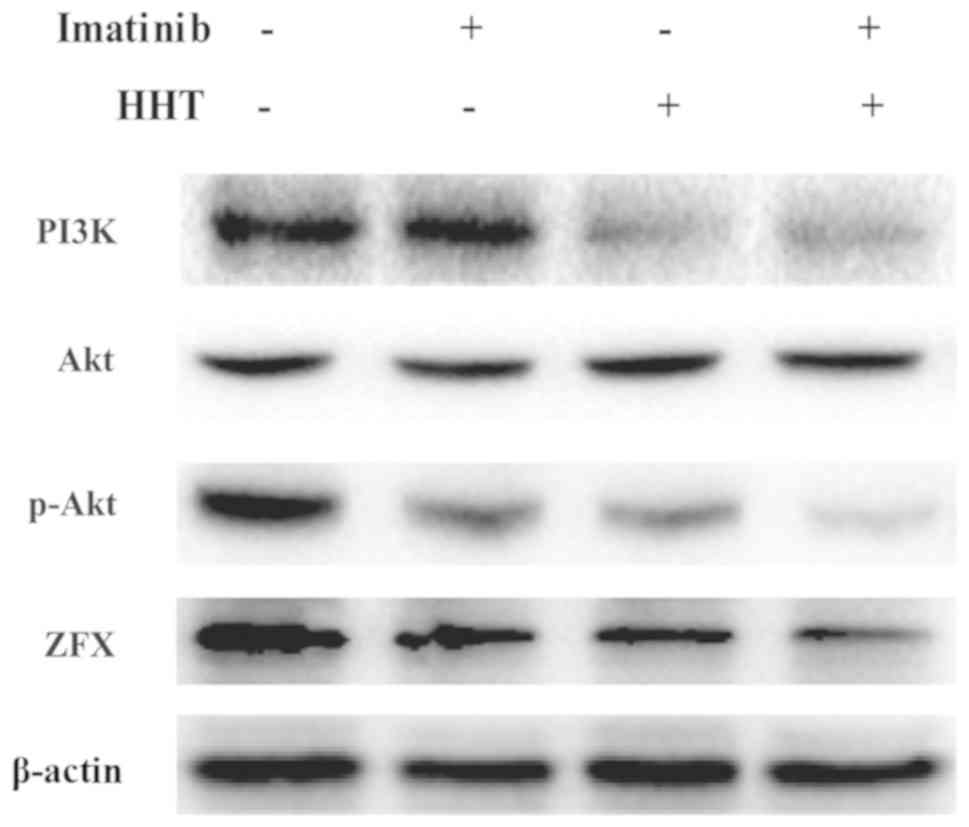
The abnormal activation of PI3K/Akt pathway results
in imatinib resistance in CML (31). Therefore, the expression levels of
PI3K, Akt and p-Akt were assessed. Compared with imatinib treatment
alone, co-treatment with HHT and imatinib for 24 h downregulated
the expression levels of PI3K and p-Akt in K562 cells (Fig. 3). However, no difference in total
Akt was detected. No notable difference in PI3K expression was
observed between the HHT and co-treatment groups.
HHT downregulates ZFX expression
levels in CML cells
The results of our previous study demonstrated that
ZFX silencing may inhibit CML cell growth through the PI3K/AKT
pathway (26). To test whether the
effect of HHT on CML cells was associated with ZFX expression,
RT-qPCR and western blot analyses were performed; the results
revealed that the mRNA and protein expression levels of ZFX were
dose-dependently decreased by HHT treatment for 24 h in K562 cells
(Fig. 4A and B). In addition, HHT
and imatinib co-treatment downregulated ZFX protein expression
levels (Fig. 3).
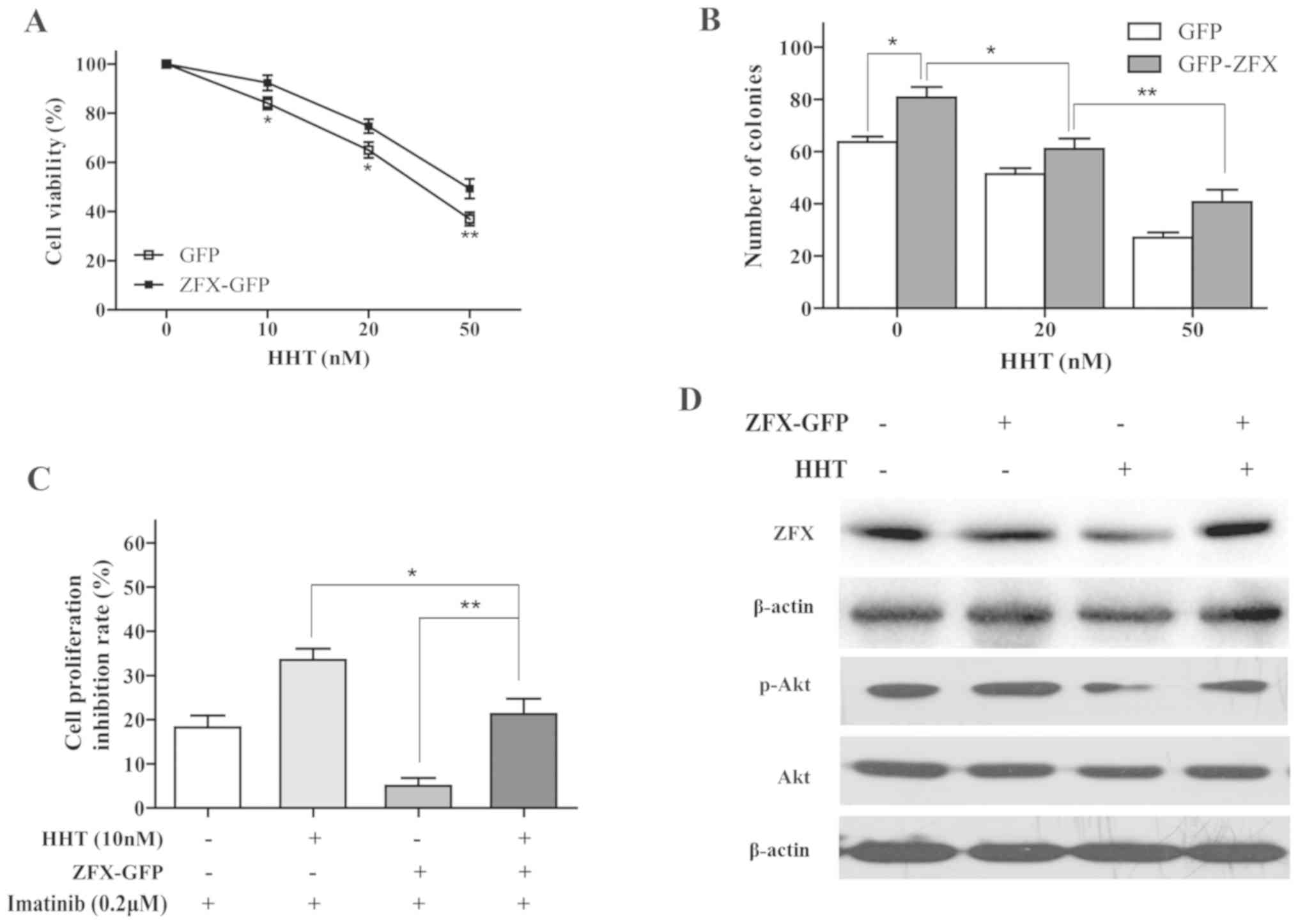
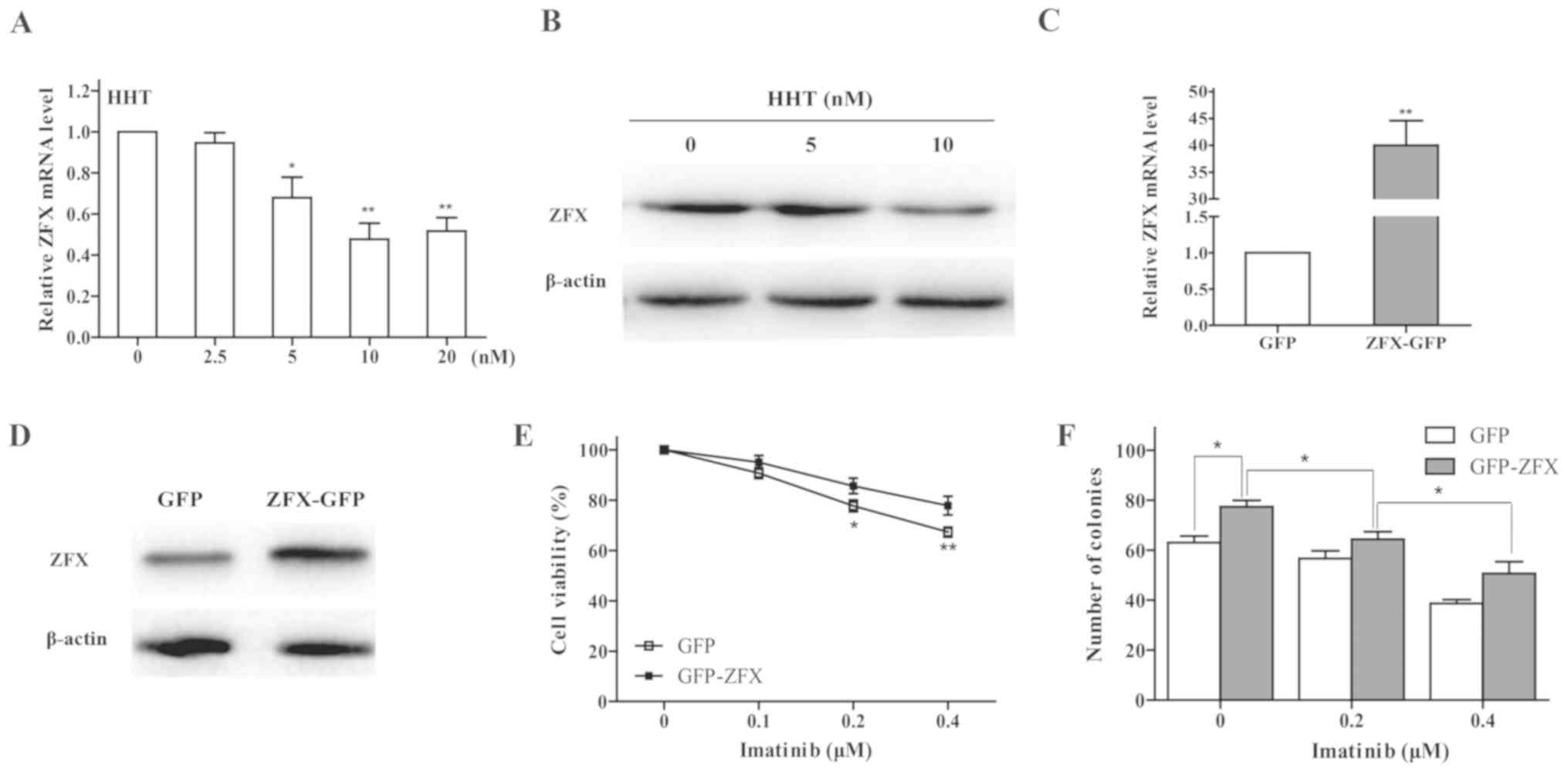 | Figure 4.Effects of ZFX on imatinib resistance
in chronic myeloid leukemia cells. (A) mRNA and (B) protein
expression levels of ZFX in K562 cells were determined by RT-qPCR
and western blotting, respectively, following treatment with HHT.
*P<0.05 and **P<0.01 vs. CTRL. (C) RT-qPCR and (D) western
blotting were used to examine ZFX mRNA and protein expression
levels, respectively, in K562 cells following transfection with
ZFX-GFP and empty GFP plasmid. **P<0.01 vs. GFP. (E) Viability
of K562 cells transfected with ZFX-GFP treated with imatinib for 24
h. *P<0.05, **P<0.01 vs. GFP. (F) K562 cell colony formation
following transfection with ZFX-GFP and treatment with imatinib for
10 days. *P<0.05. GFP, green fluorescent protein; HHT,
homoharringtonine; RT-qPCR, reverse transcription-quantitative PCR;
ZFX, zinc-finger protein, X-linked. |
Overexpression of ZFX induces imatinib
resistance in CML cells
To validate the effects of ZFX on CML cell response
to imatinib, K562 cells transfected with either ZFX-GFP or empty
GFP plasmids were incubated with imatinib for 24 h. ZFX mRNA and
protein expression levels were successfully increased following
transfection with the ZFX-GFP plasmid (Fig. 4C and D). The results of the CCK-8
assay indicated that overexpression of ZFX decreased the
sensitivity of K562 cells to imatinib (Fig. 4E). In addition, overexpression of
ZFX reversed the inhibitory effects of imatinib on colony formation
in K562 cells (Fig. 4F).
Overexpression of ZFX attenuates the
effects of HHT on CML cells
Overexpression of ZFX reversed the HHT-induced
inhibition of proliferation and clone formation in K562 cells
(Fig. 5A and B). Additionally, the
effects of HHT on imatinib sensitivity were attenuated by
overexpression of ZFX; the cell inhibition rate in the HHT + ZFX
group was significantly increased compared with that in the ZFX
group (Fig. 5C). ZFX
overexpression also reversed HHT-induced decrease of p-AKT
expression (Fig. 5D). Thus, the
data suggested that ZFX may participate in HHT-induced imatinib
sensitivity in CML cells.
Discussion
HHT treatment is effective for patients with CML and
may provide an effective treatment for patients with TKI-resistant
CML with BRC-ABL mutations (32,33).
Thus, co-treatment with HHT and imatinib may provide a novel
approach to improve the efficiency of CML treatment. The results of
the present study have demonstrated a potential mechanism of HHT in
enhancing the effect of imatinib on CML cells.
In the present study, HHT treatment increased CML
cell sensitivity to imatinib. Co-treatment with HHT and imatinib
resulted in decreases in K562 cell viability and in the number of
colonies formed compared with either treatment alone. In addition,
co-treatment induced apoptosis in K562 cells more effectively
compared with individual treatments. These results were consistence
with our previous study in imatinib-resistant CML cells (34). Therefore, HHT may facilitate
imatinib sensitivity by inducing apoptosis in CML.
HHT is a broad-spectrum protein TKI that inhibits
signaling protein phosphorylation by oncogenic proteins, such as
Janus kinase 2 V617F and p210BCR-ABL, thus blocking the
survival signaling pathways of leukemia cells (10,35).
Constitutive Try phosphorylation is the major characteristic of
BCR-ABL+ cells (1). In
the present study, flow cytometry was used to measure the p-Tyr and
p-Crkl expression levels, which were previously demonstrated to be
abnormally high in CML cells (30,36).
The results of the present study demonstrated that co-treatment
with HHT and imatinib decreased p-Tyr and p-Crkl expression levels
compared with individual drug treatment. HHT reduces
p210BCR-ABL expression in BCR-ABL+ cells
(37), which may partially explain
the additive interaction between HHT and imatinib.
The PI3K/AKT signaling pathway is essential for CML
cell viability, and may be an effective target for therapeutic
intervention in imatinib-resistant CML (3,31).
Previous studies have reported that HHT mediates myeloid cell
apoptosis by inhibiting the PI3K/AKT signaling pathway (13,38).
In the present study, PI3K and p-AKT expression levels were
decreased by co-treatment with HHT and imatinib compared with
either treatment alone. These data indicated that HHT may enhance
the effects of imatinib through synergistic inhibition of the
PI3K/AKT pathway.
To further examine the crucial molecules except for
p210BCR-ABL underlying the mechanisms via HHT enhances
imatinib sensitivity through the PI3K/AKT pathway, the role of ZFX,
which is a known mediator of biological function in cancer cells
(39–41), was investigated. ZFX mRNA and
protein expression levels were downregulated by HHT. Overexpression
of ZFX reversed imatinib- or HHT-induced inhibition of cell
viability. In addition, ZFX overexpression significantly weakened
HHT-induced imatinib sensitization and reversed the inhibitory
effect of HHT on p-AKT expression. Thus, ZFX may be responsible for
abolishing the sensitization of imatinib mediated by HHT.
In conclusion, the results of the present study
demonstrated that HHT may increase imatinib sensitivity of CML
cells through downregulation of ZFX expression, which leads to the
inhibition of PI3K/AKT pathway, thereby providing new insight into
the therapeutic strategy of CML treatment.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JW and BW drafted the manuscript. JW, BW, YS, XL and
YD collected, analyzed and interpreted the data. YL and CW
conceived and designed the present study. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Apperley JF: Chronic myeloid leukaemia.
Lancet. 385:1447–1459. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ren R: Mechanisms of BCR-ABL in the
pathogenesis of chronic myelogenous leukaemia. Nat Rev Cancer.
5:172–183. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Steelman LS, Pohnert SC, Shelton JG,
Franklin RA, Bertrand FE and McCubrey JA: JAK/STAT, Raf/MEK/ERK,
PI3K/Akt and BCR-ABL in cell cycle progression and leukemogenesis.
Leukemia. 18:189–218. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Druker BJ, Talpaz M, Resta DJ, Peng B,
Buchdunger E, Ford JM, Lydon NB, Kantarjian H, Capdeville R,
Ohno-Jones S and Sawyers CL: Efficacy and safety of a specific
inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid
leukemia. N Engl J Med. 344:1031–1037. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kantarjian HM, Cortes JE, O'Brien S, Giles
F, Garcia-Manero G, Faderl S, Thomas D, Jeha S, Rios MB, Letvak L,
et al: Imatinib mesylate therapy in newly diagnosed patients with
Philadelphia chromosome-positive chronic myelogenous leukemia: High
incidence of early complete and major cytogenetic responses. Blood.
101:97–100. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hochhaus A, Kreil S, Corbin AS, La Rosée
P, Müller MC, Lahaye T, Hanfstein B, Schoch C, Cross NC, Berger U,
et al: Molecular and chromosomal mechanisms of resistance to
imatinib (STI571) therapy. Leukemia. 16:2190–2196. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jabbour EJ, Cortes JE and Kantarjian HM:
Resistance to tyrosine kinase inhibition therapy for chronic
myelogenous leukemia: A clinical perspective and emerging treatment
options. Clin Lymphoma Myeloma Leuk. 13:515–529. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Binato R, Mencalha A, Pizzatti L, Scholl
V, Zalcberg I and Abdelhay E: RUNX1T1 is overexpressed in imatinib
mesylate-resistant cells. Mol Med Rep. 2:657–661. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Powell RG, Weisleder D and Smith CR Jr:
Antitumor alkaloids for Cephalataxus harringtonia: Structure and
activity. J Pharm Sci. 61:1227–1230. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lü S and Wang J: Homoharringtonine and
omacetaxine for myeloid hematological malignancies. J Hematol
Oncol. 7:22014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kantarjian HM, Talpaz M, Smith TL, Cortes
J, Giles FJ, Rios MB, Mallard S, Gajewski J, Murgo A, Cheson B and
O'Brien S: Homoharringtonine and low-dose cytarabine in the
management of late chronic-phase chronic myelogenous leukemia. J
Clin Oncol. 18:3513–3521. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Legros L, Hayette S, Nicolini FE, Raynaud
S, Chabane K, Magaud JP, Cassuto JP and Michallet M: BCR-ABL(T315I)
transcript disappearance in an imatinib-resistant CML patient
treated with homoharringtonine: A new therapeutic challenge.
Leukemia. 21:2204–2206. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang L, You LS, Ni WM, Ma QL, Tong Y, Mao
LP, Qian JJ and Jin J: β-Catenin and AKT are promising targets for
combination therapy in acute myeloid leukemia. Leuk Res.
37:1329–1340. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dong Y, Gao X, Zhao Y, Wei M, Xu L, Yang G
and Liu L: Semi-random mutagenesis profile of BCR-ABL during
imatinib resistance acquirement in K562 cells. Mol Med Rep.
16:9409–9414. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Fresno M, Jiménez A and Vázquez D:
Inhibition of translation in eukaryotic systems by harringtonine.
Eur J Biochem. 72:323–330. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tipping AJ, Mahon FX, Zafirides G, Lagarde
V, Goldman JM and Melo JV: Drug responses of imatinib
mesylate-resistant cells: Synergism of imatinib with other
chemotherapeutic drugs. Leukemia. 16:2349–2357. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Galan-Caridad JM, Harel S, Arenzana TL,
Hou ZE, Doetsch FK, Mirny LA and Reizis B: Zfx controls the
self-renewal of embryonic and hematopoietic stem cells. Cell.
129:345–357. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Harel S, Tu EY, Weisberg S, Esquilin M,
Chambers SM, Liu B, Carson CT, Studer L, Reizis B and Tomishima MJ:
ZFX controls the self-renewal of human embryonic stem cells. PLoS
One. 7:e423022012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang M, Xu S, Yue W, Zhao X, Zhang L,
Zhang C and Wang Y: The role of ZFX in non-small cell lung cancer
development. Oncol Res. 20:171–178. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wu S, Lao XY, Sun TT, Ren LL, Kong X, Wang
JL, Wang YC, Du W, Yu YN, Weng YR, et al: Knockdown of ZFX inhibits
gastric cancer cell growth in vitro and in vivo via downregulating
the ERK-MAPK pathway. Cancer Lett. 337:293–300. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhu Z, Li K, Xu D, Liu Y, Tang H, Xie Q,
Xie L, Liu J, Wang H, Gong Y, et al: ZFX regulates glioma cell
proliferation and survival in vitro and in vivo. J Neurooncol.
112:17–25. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yang H, Lu Y, Zheng Y, Yu X, Xia X, He X,
Feng W, Xing L and Ling Z: shRNA-mediated silencing of ZFX
attenuated the proliferation of breast cancer cells. Cancer
Chemother Pharmacol. 73:569–576. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Weisberg SP, Smith-Raska MR, Esquilin JM,
Zhang J, Arenzana TL, Lau CM, Churchill M, Pan H, Klinakis A, Dixon
JE, et al: ZFX controls propagation and prevents differentiation of
acute T-lymphoblastic and myeloid leukemia. Cell Rep. 6:528–540.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang S, Shu R, Yue M and Zhang S: Effect
of over-expression of Zinc-finger protein (ZFX) on self-renewal and
drug-resistance of hepatocellular carcinoma. Med Sci Monit.
22:3025–3034. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lai KP, Chen J, He M, Ching AK, Lau C, Lai
PB, To KF and Wong N: Overexpression of ZFX confers self-renewal
and chemoresistance properties in hepatocellular carcinoma. Int J
Cancer. 135:1790–1799. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu J, Wei B, Wang Q, Ding Y, Deng Z, Lu X
and Li Y: ZFX facilitates cell proliferation and imatinib
resistance in chronic myeloid leukemia cells. Cell Biochem Biophys.
74:277–283. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang J, Ikezoe T, Nishioka C, Udaka K and
Yokoyama A: Bcr-Abl activates AURKA and AURKB in chronic myeloid
leukemia cells via AKT signaling. Int J Cancer. 134:1183–1194.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Desplat V, Lagarde V, Belloc F, Chollet C,
Leguay T, Pasquet JM, Praloran V and Mahon FX: Rapid detection of
phosphotyrosine proteins by flow cytometric analysis in
Bcr-Abl-positive cells. Cytometry A. 62:35–45. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
La Rosée P, Holm-Eriksen S, Konig H,
Härtel N, Ernst T, Debatin J, Mueller MC, Erben P, Binckebanck A,
Wunderle L, et al: Phospho-CRKL monitoring for the assessment of
BCR-ABL activity in imatinib-resistant chronic myeloid leukemia or
Ph+ acute lymphoblastic leukemia patients treated with nilotinib.
Haematologica. 93:765–769. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Burchert A, Wang Y, Cai D, von Bubnoff N,
Paschka P, Müller-Brüsselbach S, Ottmann OG, Duyster J, Hochhaus A
and Neubauer A: Compensatory PI3-kinase/Akt/mTor activation
regulates imatinib resistance development. Leukemia. 19:1774–1782.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Visani G and Isidori A: Resistant chronic
myeloid leukemia beyond tyrosine-kinase inhibitor therapy: Which
role for omacetaxine. Expert Opin Pharmacother. 15:1–3. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cortes J, Lipton JH, Rea D, Digumarti R,
Chuah C, Nanda N, Benichou AC, Craig AR, Michallet M, Nicolini FE,
et al: Phase 2 study of subcutaneous omacetaxine mepesuccinate
after TKI failure in patients with chronic-phase CML with T315I
mutation. Blood. 120:2573–2580. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Wu JJ, Ding YH, Deng ZK, Shi YY, Lu XY and
Li YF: Effect of homoharringtonine combined with Imatinib on the
K562/G01 cells and its mechanism. Zhongguo Shi Yan Xue Ye Xue Za
Zhi. 25:80–84. 2017.(In Chinese). PubMed/NCBI
|
|
35
|
Tong H, Ren Y, Zhang F and Jin J:
Homoharringtonine affects the JAK2-STAT5 signal pathway through
alteration of protein tyrosine kinase phosphorylation in acute
myeloid leukemia cells. Eur J Haematol. 81:259–266. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Hamilton A, Alhashimi F, Myssina S,
Jorgensen HG and Holyoake TL: Optimization of methods for the
detection of BCR-ABL activity in Philadelphia-positive cells. Exp
Hematol. 37:395–401. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Chen Y, Hu Y, Michaels S, Segal D, Brown D
and Li S: Inhibitory effects of omacetaxine on leukemic stem cells
and BCR-ABL-induced chronic myeloid leukemia and acute
lymphoblastic leukemia in mice. Leukemia. 23:1446–1454. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Meng H, Yang C, Jin J, Zhou Y and Qian W:
Homoharringtonine inhibits the AKT pathway and induces in vitro and
in vivo cytotoxicity in human multiple myeloma cells. Leuk
Lymphoma. 49:1954–1962. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jiang H, Zhang L, Liu J, Chen Z, Na R,
Ding G, Zhang H and Ding Q: Knockdown of zinc finger protein
X-linked inhibits prostate cancer cell proliferation and induces
apoptosis by activating caspase-3 and caspase-9. Cancer Gene Ther.
19:684–689. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Li K, Zhu ZC, Liu YJ, Liu JW, Wang HT,
Xiong ZQ, Shen X, Hu ZL and Zheng J: ZFX knockdown inhibits growth
and migration of non-small cell lung carcinoma cell line H1299. Int
J Clin Exp Pathol. 6:2460–2467. 2013.PubMed/NCBI
|
|
41
|
Fang Q, Fu WH, Yang J, Li X, Zhou ZS, Chen
ZW and Pan JH: Knockdown of ZFX suppresses renal carcinoma cell
growth and induces apoptosis. Cancer Genet. 207:461–466. 2014.
View Article : Google Scholar : PubMed/NCBI
|