Introduction
Diabetes mellitus (DM) is a metabolic disease that
is characterized by hyperglycemia (1). DM has multiple possible long-term
complications, including vascular lesions, neuropathy, retinopathy,
nephropathy and bone osteoporosis. DM was determined to affects the
skeletal system (2). Females with
DM have a three-fold higher risk of fracture when compared with
non-diabetic females (3). In
addition, fracture patients with diabetes require longer
hospitalization times than those without diabetes (4); however, the underlying mechanisms
associated with fracture risk in diabetes are yet to be completely
elucidated. Evidence has also demonstrated that DM accelerates
alveolar bone resorption (5). In
our previous study, rats subjected to experimental DM exhibited
more serious alveolar bone destruction when compared with the
normal group (6). This also
suggested that type 2 DM (T2DM) elevated the levels of tumor
necrosis factor-α, interleukin-1β and lipopolysaccharide in the
gingival crevicular fluid of rats (6). The enhanced levels of inflammatory
mediators contribute to an increase in osteoblast apoptosis,
decreasing their abundance (7).
Decreased activity of osteoblasts under diabetic conditions has
been discussed in numerous studies in both humans and animal models
(8,9); however, despite numerous studies that
have addressed this problem, the precise molecular mechanism
underling the effects of glucose in altering bone formation and
osteoblastic differentiation is yet to be elucidated.
Lentiviral vector technology has now become an
effective tool for therapeutic gene delivery (10–12).
Effectively, lentiviral vectors can infect dividing and
non-dividing cells then integrate exogenous genes or exogenous
short hairpin RNAs into a host chromosome, which culminates in the
stable and long-term expression of the target genes (13).
Hedgehog (Hh) signals serve an important role in
evolution and numerous biological events (14). The Hh gene family comprises at
least three members, including Sonic hedgehog (Shh), Desert
hedgehog and Indian hedgehog. Shh is involved in the formation of
organs and tissues; Shh proteins are involved in the same signaling
pathway. The activated Shh ligand binds to the transmembrane
protein receptor protein patched homolog 1 (PTCH), which results in
the release of the downstream protein smoothened (SMO). SMO
subsequently transduces signaling downstream which in turn
activates the transcription factors glioma-associated homologs.
Phosphorylated Gli enters the nucleus and activates target genes
(14). Without the Shh ligand,
PTCH binds to SMO and suppresses the activation of SMO, as well as
the associated genes (15). The
Shh pathway is involved in multiple developmental processes by
regulating cell differentiation (16). A previous study reported that Shh
induces osteoblastic differentiation and bone formation as
evidenced by an in vitro culture system and in vivo
transplantation experiments (17).
Additionally, it was demonstrated that by adding Shh to bone
mesenchymal stem cells (BMSCs), the differentiation of uncommitted
BMSCs to the osteoblast lineage was promoted (18). A previous study revealed that the
altered expression of Shh and bone morphogenic protein 4 (BMP4),
and their downstream genes are associated with variations of the
telencephalon in the embryos of diabetic mice (19). In addition, the study of Dunaeva
et al (20) demonstrated
that diabetes is associated with a functional inhibition of the Shh
signaling pathway.
These studies indicated that the Shh signaling
pathway was associated with osteoblastic differentiation and DM,
while DM could be associated with the skeletal system and
osteoblastic activity. We hypothesized that high glucose (HG)
levels altered the Shh signaling pathway in osteoblasts and
affected osteoblast functions. Therefore, in the present study, the
Shh pathway was induced and suppressed vial entiviral
vector-mediated Shh/small interfering (si)RNA gene transfer in
vitro; we also investigated whether in an in vivo animal
model, whether alterations in Shh expression may alter the effects
of HG on osteoblastic differentiation of BMSCs and new bone
formation.
Materials and methods
Statement of ethics
All animal experiments were conducted in strict
accordance with the principles of medical ethics and were approved
by Animal Care and Use Committee of The Second Affiliated Hospital
of Harbin Medical University (Harbin, China). All necessary permits
were obtained for the described field studies.
Isolation and culture of BMSCs
BMSCs were isolated from 3-week-old male
Sprague-Dawley rats (n=30, average weight of 80–100 g), which were
purchased from the Department of the Animal Experiment Center of
The Second Affiliated Hospital of Harbin Medical University. Their
housing conditions were as follows: 18–26°C, humidity 40–70%, fresh
air, 12 h light/dark cycle, and free access to food and water.
Briefly, rats were sacrificed, soaked in 75% ethanol for 10 min,
and the femurs and tibias were isolated from the soft tissues. The
proximal and distal ends of the femur and tibia were removed. The
exposed bone marrow cavity was flushed with 30 ml of Dulbecco's
modified Eagle's medium (DMEM; Hyclone; GE Healthcare Life
Sciences) containing 10% fetal bovine serum (FBS; Hyclone; GE
Healthcare Life Sciences), 100 U/ml penicillin and 100 µg/ml
streptomycin (Sigma-Aldrich, Merck KGaA). The cell suspension was
obtained by repeated rinsing through a 5-gauge needle, and was
placed in a centrifuge tube. The supernatant was discarded after
centrifugation at 1,000 × g for 10 min at room temperature. Cells
were then seeded at a density of 2×106 cells/ml in a 25
cm2 plastic culture flask in the low glucose (LG)-DMEM
containing 10% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin,
and incubated in 5% CO2 at 37°C. Following a 72-h
incubation, the unattached cells were removed by replacing the
medium and the remaining cells were considered to be BMSCs. When
the cells reached 90% confluence, they were trypsinized by 0.25%
trypsin and 0.02% ethylenediamine tetraacetic acid (Gibco; Thermo
Fisher Scientific, Inc.) for 2 min, and then passaged at a density
of 102 per cm2. The first-passage BMSCs were
cultured in 10% FBS, 100 U/ml penicillin and 100 µg/ml streptomycin
with DMEM containing different concentrations of glucose, 5%
CO2 at 37°C (LG-DMEM, 1,000 mg/l glucose; HG-DMEM, 4,500
mg/l glucose). The third passage generation cells were used for
further studies.
Transduction with lentiviral
vectors
Lentivirus gene transfer vectors fused with a green
fluorescent protein (GFP) sequence were constructed by Shanghai
GenePharma Co., Ltd. Lentiviral vectors can infect BMSCs then
integrate RNAs into a host chromosome. The targeting sequences for
Rattus norvegicus Shh (NM-017221) were
5′-GGTGCCAAGAAGGTCTTCTAC-3′, and the titer of Lenti-Shh (activate
Shh signaling), Lenti-siRNA (inhibit Shh signaling) and Lenti
(negative control) were all 1×108 UT/ml. The third
passage of BMSCs was plated in 24-well plates at a density of
0.5×105 cells/well in 0.5 ml medium per well prior to
transduction. Following a 24-h incubation the cells had grown to
30–50% confluence; 10% FBS, 100 U/ml penicillin and 100 µg/ml
streptomycin with the differing DMEM were removed and transductions
were performed at a multiplicity of infection (MOI) of 10 according
to a preliminary experiment. BMSCs were incubated in 10% FBS-DMEM
under eight different conditions: LG + Lenti-Shh, LG + Lenti-siRNA,
LG, LG + Lenti, HG + Lenti-Shh, HG + Lenti-siRNA, HG and HG +
Lenti. The volume of fresh medium was 0.5 ml per well. On the third
day, the culture medium was replaced with 10% FBS LG-DMEM or 10%
FBS HG-DMEM (1 ml/well), respectively. Every other day medium was
replenished, and it was suitable to proceed experiments until day
6. The transduction efficiency and the morphology of the BMSCs was
evaluated by two researchers independently via fluorescence and
phase contrast microscopes (Olympus Corporation) in 5–7 fields per
view (magnification, ×100).
Western blot analysis
Following transduction, cells were cultured in the
eight different types of media for 6 days, and were then lysed in
ice-cold radioimmunoprecipitation assay (Santa Cruz Biotechnology,
Inc.) and phenylmethyl sulfonyl fluoride lysis buffer (Santa Cruz
Biotechnology, Inc.) for 2 sec. The cells were subsequently scraped
from the plate with a cell scraper, and transferred to a 1.5 ml
microcentrifuge tube. The lysates were separated by centrifugation
at 12,000 × g for 5 min at 4°C. The supernatants were collected and
stored at −80°C until western blotting was performed. The protein
concentration was quantified with a bicinchoninic acid (BCA)
protein assay kit (Beyotime Institute of Biotechnology). The
samples were separated by 12% SDS-PAGE based on the molecular
weight of the target proteins (Shh, 19.45), and were then
transferred to a polyvinylidene difluoride membrane (EMD
Millipore). After blocking with 5% non-fat milk in TBST and 1X
Tris-buffered saline with 0.1% Tween-20, the membranes were
incubated with the following antibodies: Shh (C9C5) rabbit
monoclonal antibody (dilution 1:1,000; cat. no. 2207, Cell
Signaling Technology, Inc.) and anti-β-actin (dilution 1:1,000;
cat. no. 03-102, Sigma-Aldrich; Merck KGaA) at 4°C overnight. The
membranes were then incubated with horseradish
peroxidase-conjugated AffiniPure Goat Anti-Mouse IgG (H+L),
according to the origin of the primary antibodies, (dilution
1:5,000; cat. no. ZB-2305, OriGene Technologies, Inc.) for 1 h at
room temperature and were detected by using enhanced
chemiluminescence plus reagents (GE Healthcare Life Sciences)
according to the manufacturer's protocols; the emitted light was
captured on an X-ray film.
Alizarin red staining
The mineralization of the third passage BMSCs was
determined using Alizarin red staining (Sigma-Aldrich, Merck KGaA).
BMSCs were seeded in 24-well plates at a density of
5.0×104 cells/well. Following transduction, the eight
different media types were removed and replaced with osteogenic
medium containing 50 µg/ml ascorbic acid (Sigma-Aldrich; Merck
KGaA), 10 mmol/l β-glycerophosphate sodium (Sigma-Aldrich; Merck
KGaA), and 10% FBS LG-DMEM or 10% FBS HG-DMEM. The culture medium
was replaced every other day and staining was performed after three
weeks (21 days later, osteogenic-inducing medium can promote the
formation of mineralized nodules). Cells were fixed with 70%
ethanol for 1 h at room temperature, washed with PBS twice per well
and stained with 40 mM Alizarin red (pH 4.2, Sigma-Aldrich; Merck
KGaA) for 15 min at room temperature. The stained cells were rinsed
with dH2O four times to eliminate nonspecific staining,
and then observed using a light microscope (Nikon Corporation). We
randomly selected 5 fields of vision per sample for analysis
(magnification, ×100).
Alkaline phosphatase (ALP) activity
assay
Following transduction, the third passage cells in
24-well plates were treated with the osteogenic medium as described
above. The culture medium was changed every 2 days. Following 2
weeks of incubation in 5% CO2 at 37°C, ALP activity of
the BMSCs was detected using an ALP assay kit (Nanjing KeyGen
Biotech Co., Ltd.) according to the manufacturer's protocol. The
absorbance was recorded using a microplate absorbance reader
(Bio-Rad Laboratories, Inc.; Model-680) at 405 nm. The protein
concentration was measured with a BCA protein assay kit (Beyotime
Institute of Biotechnology). The ALP activity was normalized to the
total protein content.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
RNA samples for eight groups were analyzed to
measure the effects of high glucose on the expression of
osteogenesis-related genes in BMSCs. RT-qPCR was also used to
quantify the expression levels of the seven-transmembrane protein
SMO. On day 6 following transduction, total RNA was extracted using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), and the concentration of total RNA was measured with an
ultraviolet spectrophotometer (Thermo Fisher Scientific, Inc.).
Total RNA (300 ng) was reverse transcribed into cDNA using a Prime
Script™ RT Reagent kit (Takara Bio, Inc.) and RT was performed at
44°C for 60 min. Then, the mixture was incubated at 95°C for 5 min.
All of the primers for PCR are listed in Table I. qPCR was performed on a CFX-96
machine (Bio-Rad Laboratories, Inc.) using a SYBR PrimeScript EX
Taq PCR (Takara Bio, Inc.) according to the manufacturer's
protocols. All reactions were conducted in a 20 µl mixture
containing cDNA preparation (1 µl), 10 µl SYBR® Premix
Ex Taq™ (Tli RNaseH Plus), 1 µl of each primer and 7 µl
diethylpyrocarbonate water. The thermocycling conditions were 95°C
for 10 min, and then 40 cycles of 95°C for 20 sec and 60°C for 30
sec. A melting curve was acquired using 95°C for 15 sec, 60°C for
30 sec, and 95°C for 15 sec. The expression levels were normalized
to the expression of the housekeeping gene β-actin. Data were
analyzed using the 2−ΔΔCq method (21).
 | Table I.Primer sequences employed reverse
transcription-quantitative polymerase chain reaction. |
Table I.
Primer sequences employed reverse
transcription-quantitative polymerase chain reaction.
| Genes | Sequences
(5′-3′) | Size (bp) | Accession no. |
|---|
| β-actin | F:
CCCATCTATGAGGGTTACGC | 207 | NM_031144 |
|
| R:
TTTAATGTCACGCACGATTTC |
|
|
| BSP | F:
GATGAAAATGAGCAGGTCGTC | 95 | NM_012587 |
|
| R:
GCTTCTTCTCCGTTGTCTCCT |
|
|
| OPN | F:
AGGACAGCAACGGGAAGAC | 241 | M99252 |
|
| R:
TGAAACTCGTGGCTCTGATGT |
|
|
| BMP4 | F:
GCTCTGCTTTTCGTTTCTTCTT | 199 | NM_012827 |
|
| R:
TCCAGTAGTCGTGTGATGAGGT |
|
|
| SMO | F:
TGATGGCTGGAGTAGTGTGGT | 117 | NM_012807 |
|
| R:
TGAGCAGGTGGAAATAGGATG |
|
|
Animal experiments
A total of 60, 3-month-old male Sprague-Dawley rats
with average weight of 200–220 g, were obtained from the Department
of the Animal Experiment Center of The Second Affiliated Hospital
of Harbin Medical University. Their housing conditions were as
follows: 18–26°C, humidity 40–70%, fresh air, 12 h light/dark cycle
with free access to food and water. All animals were acclimatized
for at least 1 week. A total of 30 rats were fed an adequate high
fat (HF) diet (22) (34.5% fat,
17.5% protein, 48% carbohydrate; Beijing HFK Bio-Technology), while
the remaining animals (n=30) received normal chow for 4 weeks.
Then, the 30 rats on the HF diet were intraperitoneally injected
with streptozotocin (STZ; 30 mg/kg, Sigma-Aldrich; Merck KGaA)
dissolved in 0.1 mol/l citrate buffer, pH 4.5, to induce models of
diabetes. A diagnosis of diabetes was confirmed by measurement of
blood glucose randomly on the third and seventh days following STZ
administration. Rats were considered diabetic when the random blood
glucose levels were >11.1 mmol/l (23).
Rat tooth extraction model
The left incisors of the mandibular of 60 rats were
cut at the gingival level with a small diamond bur under anesthesia
(10% chloral hydrate, 300 mg/kg animal bodyweight, intraperitoneal
injection) at days 10, 8, 6, 4 or 2 following the induction of
diabetes; before this, mandibular incisor was fully extracted. On
day 0, the left incisors of the 60 animals were carefully extracted
(24,25). The animals were divided into four
groups: A normal group as a control (untreated post incisor
extraction, n=15), a normal group with MOI=10, 50 µl Lenti-Shh into
the tooth socket (n=15), a DM group with MOI=10, 50 µl Lenti-Shh
into the tooth socket (n=15), and a DM group (untreated post
incisor extraction, n=15). Microliter syringes (Shanghai GAOGE
Industrial and Trading Co., Ltd.) were used to inject Lenti-Shh
into the tooth socket. The extraction sockets of the teeth were
closed with periodontal dressing paste.
The rats were sacrificed at 8 weeks following tooth
extraction; the mandible was dissected and the samples were fixed
in 10% neutral buffered formalin at room temperature for 2 days.
Soft X-ray images of the mandibles were obtained via cabinet X-ray
systems (Faxitron, MC-20). To determine the alveolar ridge
reduction after tooth extraction, the distances between the highest
point of the mesial at the first molar to the lingual alveolar
ridge margin of the incisor were measured by an image analyzing
software (ImageJ; v2.1.4.7, National Institutes of Health) as
described previously (26). The
reduction rate was calculated according to the following formula:
(1-a/b) ×100% (a, the length after incisor extraction; b, the
length before incisor extraction). Subsequently, the left half of
the mandible was decalcified, specimens were decalcified in 10%
ethylene diamine tetraacetic acid for 4 weeks, dehydrated and then
embedded in paraffin. The paraffin serial sections in 6 µm were
obtained in the buccal and lingual direction and stained with
hematoxylin and eosin (HE) staining at room temperature for 1 h.
The histological sections were used to evaluate new bone formation
of the tooth socket in five randomly selected fields per view.
Histologic evaluation was performed at 100 magnification using a
light microscope (BX50, Olympus Corporation).
Statistical analysis
Statistical analyses were performed using Microsoft
Excel and SPSS 23.0 software (IBM Corp.). All of the data are
presented as the mean ± standard deviation. Statistical analysis
for differences between multiple groups was conducted using one-way
analysis of variance followed by a Tukey's post hoc test for
multiple comparisons. The experiments were conducted in triplicate.
P<0.05 was considered to indicate a statistically significant
difference.
Results
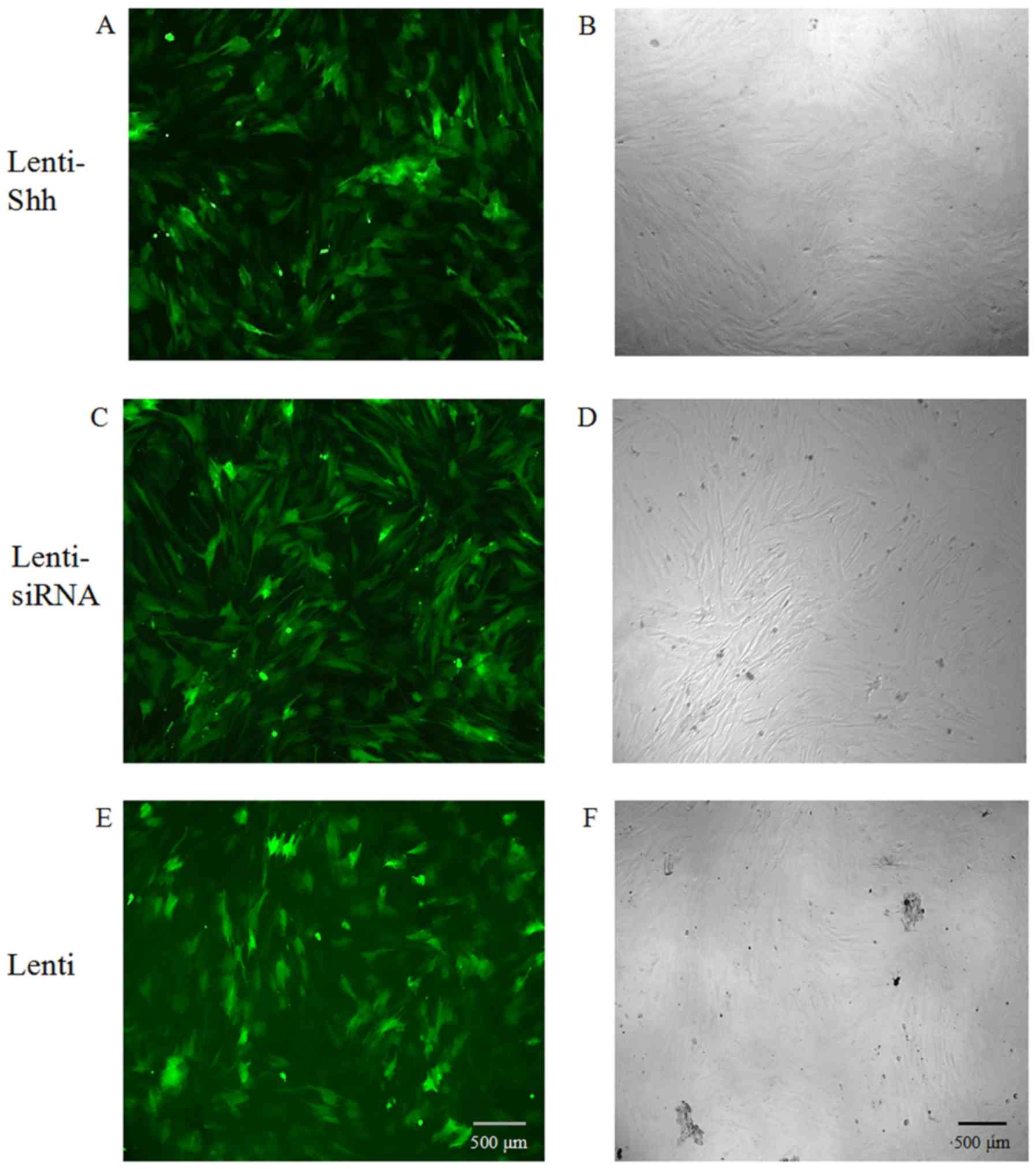
Transfection efficiency
As the lentiviral constructs were tagged with the
GFP reporter, the infection efficiency was examined using
fluorescence microscopy and phase contrast microscopy. At 72 h
following transduction, the BMSCs expressed GFP, suggesting that
the lentivirus had integrated into the genome of the BMSCs and the
infection efficiency was ~80% when the MOI was 10 (Fig. 1).
Efficiency of lentiviral-mediated
suppression or overexpression of Shh in BMSCs with LG-DMEM or
HG-DMEM
The present study performed western blotting in
order to determine the expression of Shh under LG-DMEM and HG-DMEM
treatment following transduction with Lenti-Shh (activate Shh
signaling), Lenti-siRNA (inhibit Shh signaling) and Lenti (negative
control), respectively. As presented in Fig. 2A-C, Shh protein expression was
markedly decreased in BMSCs when cultured in HG medium compared
with LG medium, which indicated that HG inhibited Shh protein
expression in BMSCs. In addition, Lenti-Shh treatment significantly
upregulated Shh expression in LG and HG medium compared with the
corresponding controls. These results suggested that following
transduction with Lenti-Shh, the decreased expression of Shh in
BMSCs under HG conditions could be reversed.
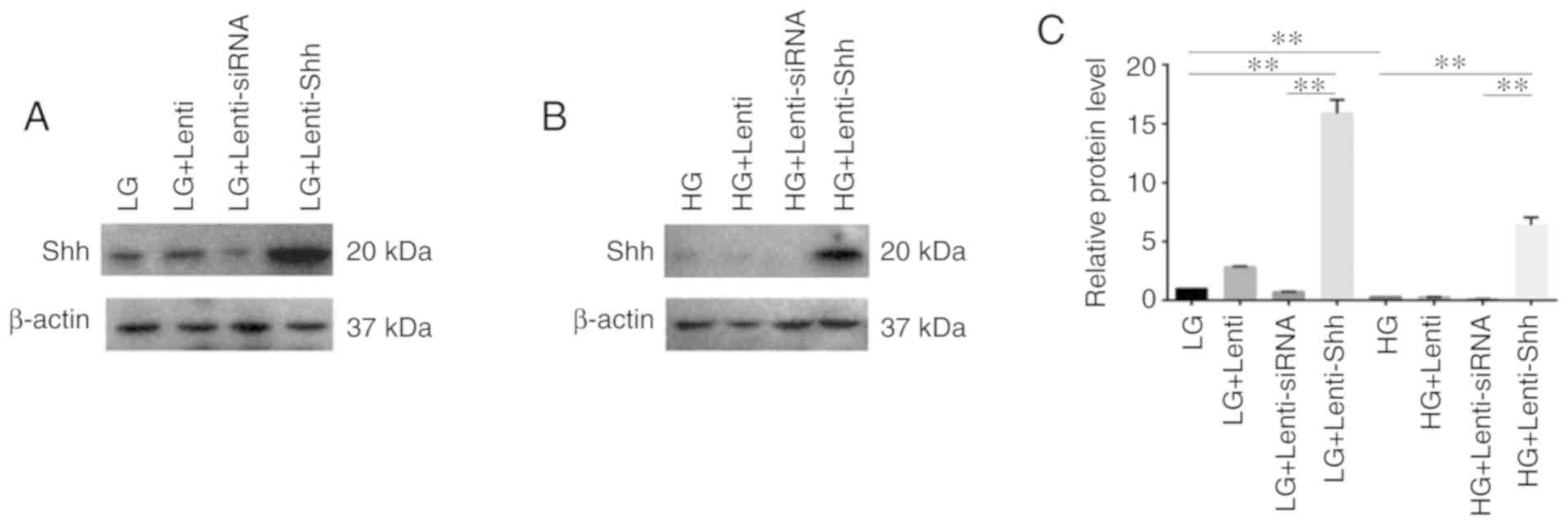 | Figure 2.Efficiency of lentiviral-mediated
suppression or overexpression of Shh in BMSCs with LG-DMEM or
HG-DMEM. (A and B) Expression of Shh protein and (C) relative
protein level of Shh in bone marrow stromal stem cells cultured in
eight different media conditions: LG, LG + Lenti, LG + Lenti-siRNA,
LG + Lenti-Shh, HG, HG + Lenti, HG + Lenti-siRNA and HG +
Lenti-Shh. **P<0.01. HG, high glucose; LG, low glucose; Lenti,
lentivirus; Shh, Sonic hedgehog; siRNA, small interfering RNA. |
Effect of HG on the expression levels
of SMO in BMSCs
Hh signaling in vertebrates is initiated by the
binding of secreted Hh proteins to the twelve-transmembrane protein
PTCH, which typically suppresses the activity of the
seven-transmembrane protein SMO (14). SMO in turn promotes the expression
of Hh target genes by the Gli family of transcription factors and
activates Hh signaling (15). The
present study performed RT-qPCR in order to quantify the expression
levels of SMO (Fig. 3), and the
results indicated that SMO expression was significantly decreased
in the HG group compared with LG group. Following transduction with
Lenti-Shh, the LG + Lenti-Shh and HG + Lenti-Shh groups exhibited
significantly upregulated SMO expression compared with the
corresponding controls. However, the levels of SMO were decreased
in the LG + Lenti-siRNA and HG + Lenti-siRNA groups; the levels SMO
expression in the LG + Lenti and HG + Lenti control groups were
similar to that of the LG and HG groups, respectively. These
results suggested that HG levels inhibited the expression of SMO;
however, following transduction with Lenti-Shh, the decreased
expression of SMO in BMSCs under HG conditions could be
reversed.
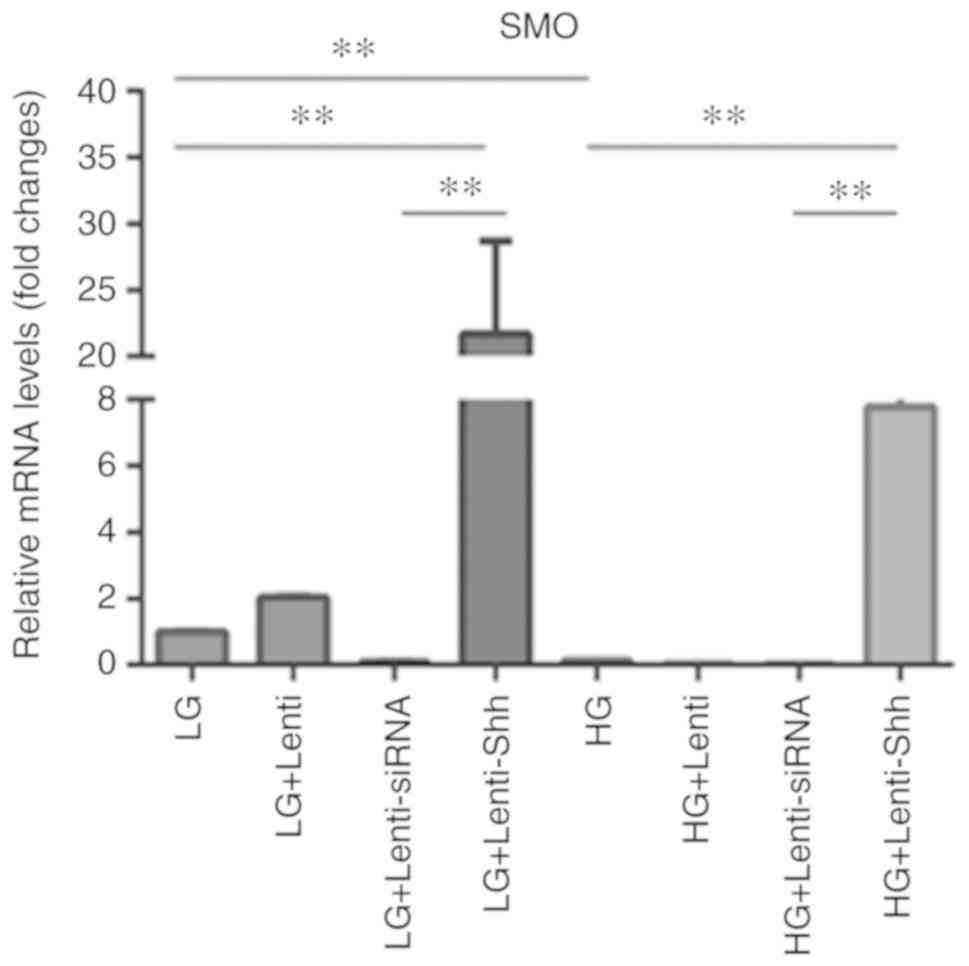 | Figure 3.Expression levels of SMO in bone
marrow stromal stem cells cultured under eight media conditions:
LG, LG + Lenti, LG + Lenti-siRNA, LG + Lenti-Shh, HG, HG + Lenti,
HG + Lenti-siRNA and HG + Lenti-Shh. **P<0.01. HG, high glucose;
LG, low glucose; Lenti, lentivirus; Shh, Sonic hedgehog; siRNA,
small interfering RNA; SMO, smoothened. |
Effect of high glucose and Shh pathway
on matrix mineralization of BMSCs
Osteoblast-secreted matrix is mineralized into
nodules (27). In the present
study immunohistochemical staining of mineralized nodules with
Alizarin red S after 21 days of culture was performed in 24-well
plates. The results presented in Fig.
4 demonstrated that matrix mineralization in the HG group was
decreased compared with the LG group. Following infection with
Lenti-Shh, a notable increase in mineralized nodules was observed
under LG and HG conditions, compared with LG and HG alone,
respectively. However, the LG + Lenti-siRNA and HG + Lenti-siRNA
groups exhibited markedly decreased matrix mineralization compared
with the other groups; the extent of staining in the LG + Lenti and
HG + Lenti control groups was similar to that of the LG and HG
groups, respectively. Taken together, these results indicated that
HG levels inhibited the matrix mineralization of BMSCs and this
negative effect could be reversed by activating the Shh pathway via
transduction with Lenti-Shh.
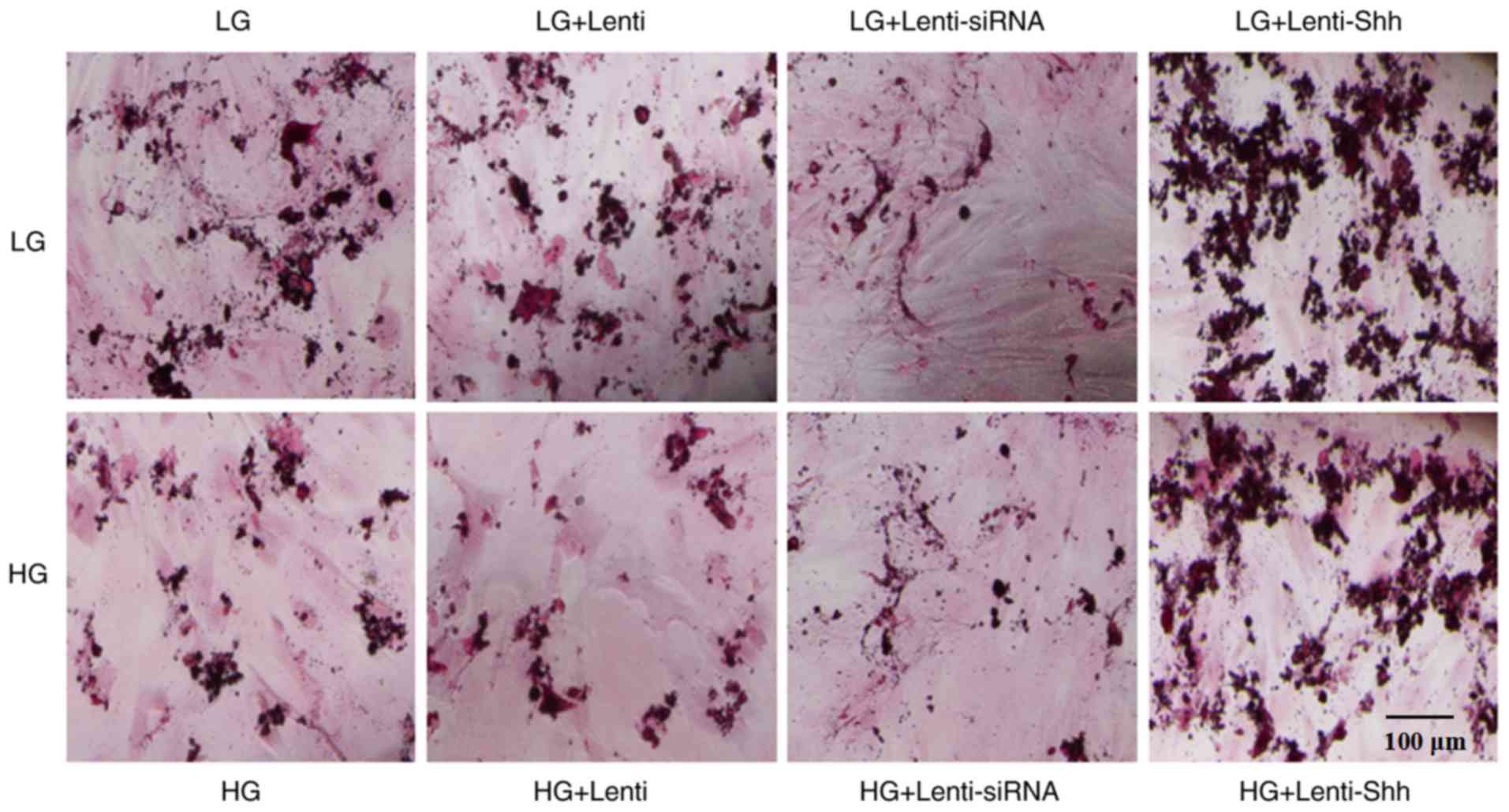 | Figure 4.Extent of matrix mineralization in
bone marrow stromal stem cells as determined by Alizarin red S
staining cultured under eight different media conditions: LG, LG +
Lenti, LG + Lenti-siRNA, LG + Lenti-Shh, HG, HG + Lenti, HG +
Lenti-siRNA and HG + Lenti-Shh. Scale bar, 100 µm. HG, high
glucose; LG, low glucose; Lenti, lentivirus; Shh, Sonic hedgehog;
siRNA, small interfering RNA. |
Effects of HG and Shh pathway on ALP
activity in BMSCs
The ALP activity levels of BMSCs cultured in eight
different media conditions were detected and are presented in
Fig. 5. The results demonstrated
that the ALP activity in the HG group was significantly reduced
compared with the LG group. Following transduction with Lenti-Shh,
the significantly increased ALP activity levels were detected under
both glucose conditions compared with the LG and HG groups,
respectively (P<0.05). Conversely, the LG + Lenti-siRNA and HG +
Lenti-siRNA groups exhibited notably decreased ALP activity levels
compared with the other groups compared with the corresponding
groups under the same glucose conditions. In addition, the ALP
activity levels of the LG + Lenti and HG + Lenti control groups
similar to the LG and HG groups, respectively. These results
indicated that HG concentrations inhibited the ALP activity of
BMSCs and this negative effect could be reversed by activating the
Shh pathway via transduction with Lenti-Shh.
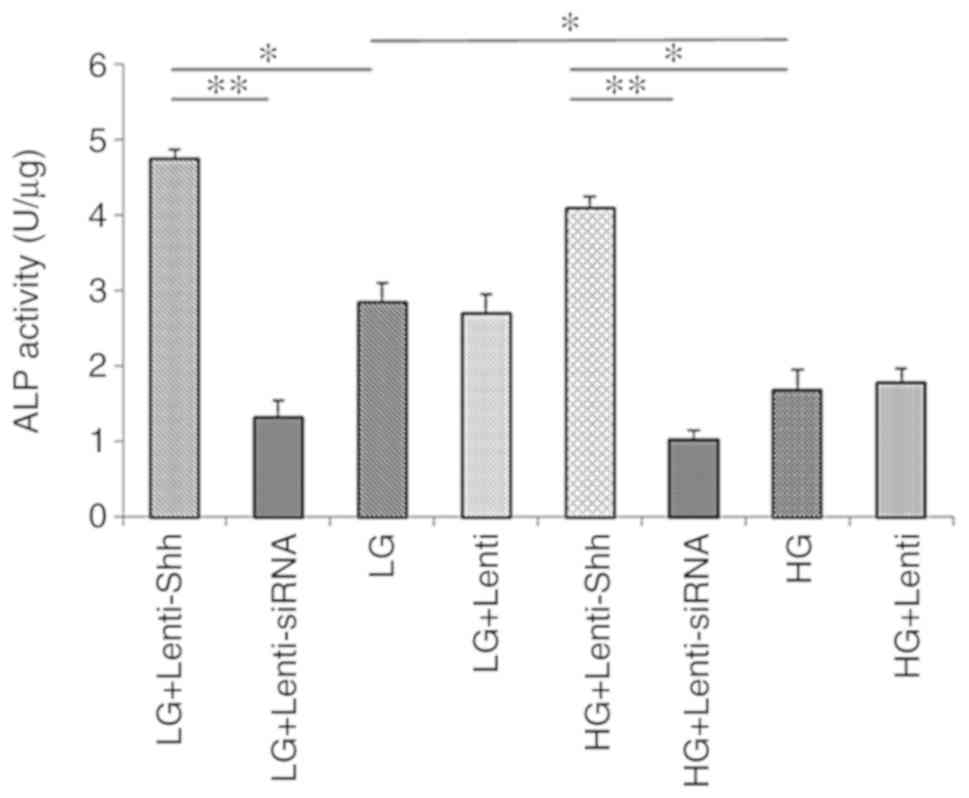 | Figure 5.ALP activity of bone marrow stromal
stem cellscultured in eight media conditions: LG + Lenti-Shh, LG +
Lenti-siRNA, LG, LG + Lenti, HG + Lenti-Shh, HG + Lenti-siRNA, HG
and HG + Lenti was analyzed on day 14. *P<0.05, **P<0.01.
ALP, alkaline phosphatase activity; HG, high glucose; LG, low
glucose; Lenti, lentivirus; Shh, Sonic hedgehog; siRNA, small
interfering RNA. |
Effects of HG on the expression of
osteogenesis-related genes in BMSCs and the role of Shh
protein
To provide further evidence as to whether a HG
inhibits the expression of osteogenesis-related genes in BMSCs and
to determine whether Lenti-Shh/siRNA could adjust gene expression,
the present study performed RT-qPCR to quantify their relative
expression levels. As presented in Fig. 6A-C, the expression levels of bone
sialoprotein (BSP), osteopontin (OPN) and BMP4 were significantly
downregulated in the HG group compared with the LG group, which
indicated that HG inhibited osteogenesis-related gene expression in
BMSCs. Following transduction with Lenti-Shh, the LG + Lenti-Shh
and HG + Lenti-Shh groups exhibited significantly increased BSP,
OPN and BMP4 expression levels compared with the LG and HG groups
respectively (P<0.05). Additionally, the LG + Lenti-siRNA and HG
+ Lenti-siRNA groups exhibited markedly decreased expression levels
compared with the other groups under the same glucose conditions.
The expression levels of the aforementioned genes in the LG + Lenti
and HG + Lenti control groups, were similar to those of the LG and
HG groups, respectively. These results suggested that the decreased
expression of BSP, OPN and BMP4 in BMSCs under HG conditions could
be reversed following transduction with Lenti-Shh. The in
vitro experiments were performed in triplicate.
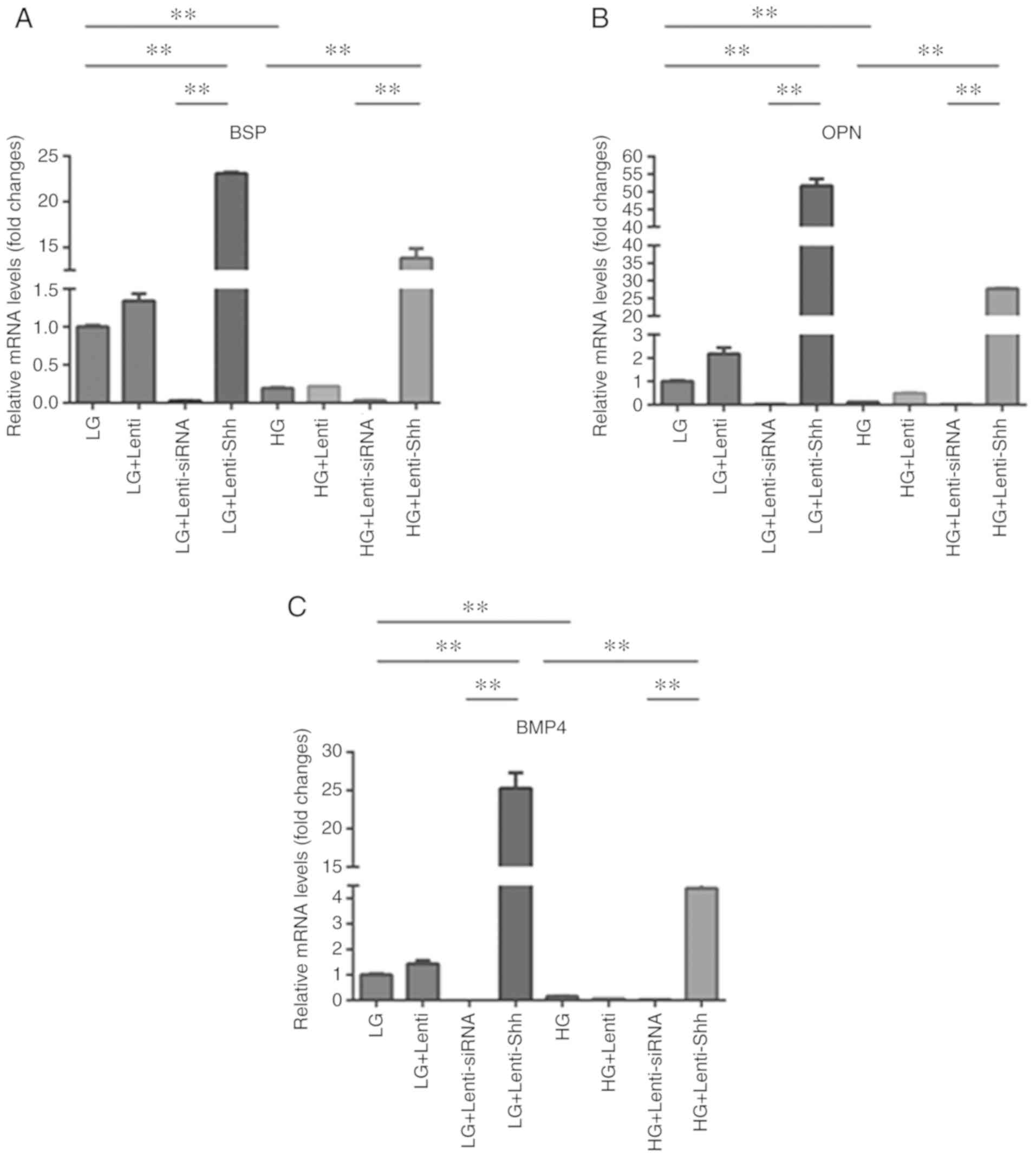 | Figure 6.Gene expression of bone marrow
stromal stem cells cultured in eight media conditions: LG, LG +
Lenti, LG + Lenti-siRNA, LG + Lenti-Shh, HG, HG + Lenti, HG +
Lenti-siRNA and HG + Lenti-Shh. The expression of (A) BSP, (B) OPN
and (C) BMP4 was quantified by reverse transcription-quantitative
polymerase chain reaction. **P<0.01. BMP4, bone morphogenic
protein 4; BSP, bone sialoprotein; OPN, osteopontin; HG, high
glucose; LG, low glucose; Lenti, lentivirus; Shh, Sonic hedgehog;
siRNA, small interfering RNA. |
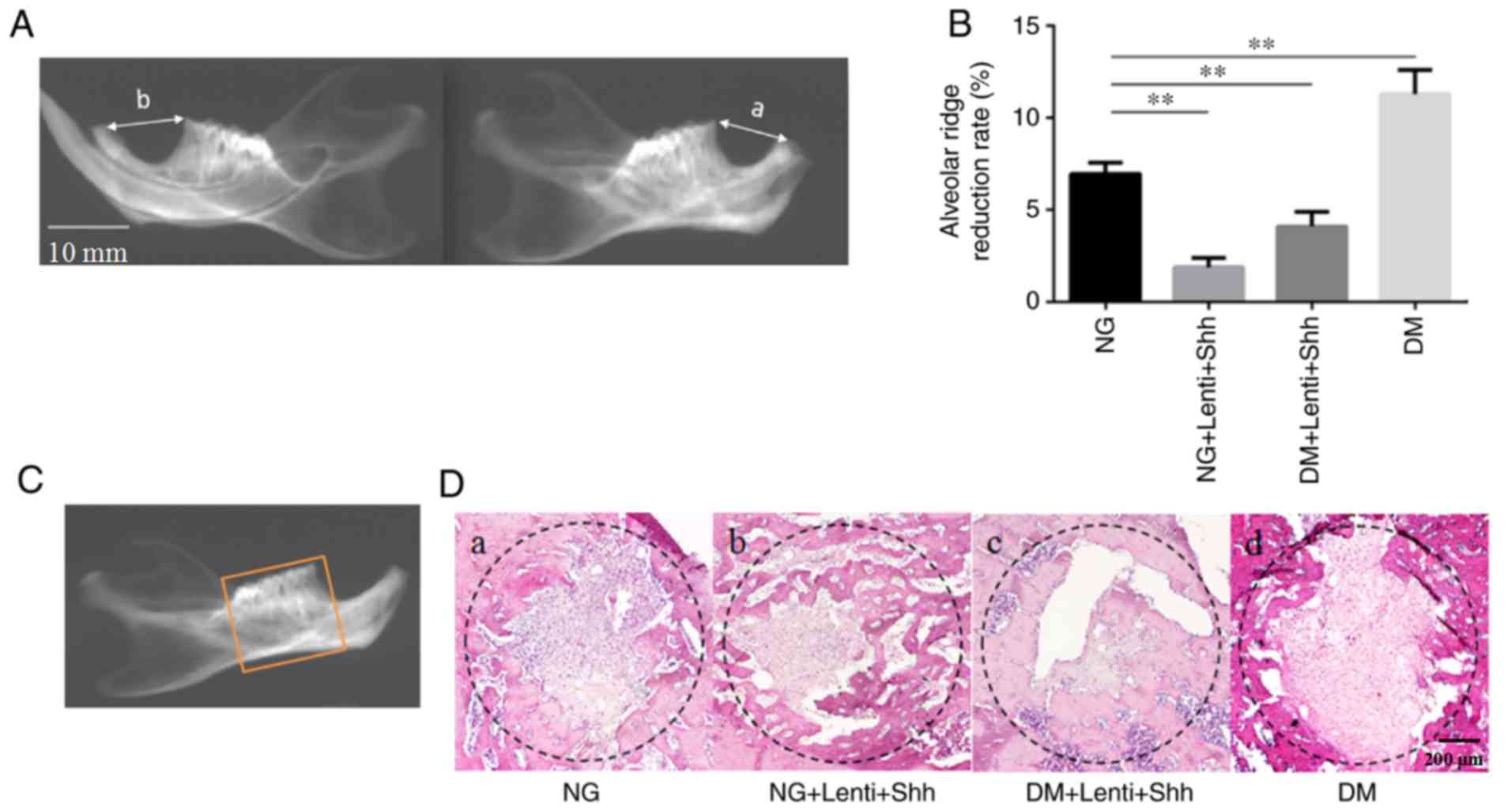
Histological analyses suggests that
Lenti-Shh enhances bone formation and reverses the adverse effect
of diabetes on tooth sockets in a rat model
The present study sought to confirm whether DM
alters the alveolar ridge absorption and the formation of new bones
in the tooth socket. The rate of alveolar ridge reduction was
measured among the four groups at 8 week (Fig. 7A and B). The results demonstrated
that the rate of alveolar ridge reduction was significantly
decreased in the normal group compared with the DM group. The
reduction rates of the normal group with Lenti-Shh and DM +
Lenti-Shh were significantly lower than the normal group. In
addition, the histological analysis of the socket revealed that
more fibrous connective tissue and reduced new bone formation were
detected in the DM group when compared with the normal group
(Fig. 7D-a and -d). Importantly,
more newly formed bone was detected in the normal group with
Lenti-Shh and DM +Lenti-Shh, than in the normal group (Fig. 7D-b and -c). New bone formation from
the edge of sockets towards the center and an increased abundance
of osteoclasts were evident in the Lenti-Shh treated groups. These
data suggest that diabetes inhibited the formation of new bones and
that Lenti-Shh may protect alveolar bone, while enhancing new bone
formation; thus, increasing the expression of Shh may reverse the
negative effect of diabetes.
Discussion
The present study investigated the effects of HG and
the Shh pathway on BMSC differentiation, and the expression of
osteogenesis-related genes. From cells transducing with Lenti-Shh
and Lenti-siRNA vectors, the results indicated that: i) Shh
signaling in osteoblasts was triggered by Lenti-Shh; ii) activation
of Shh signaling enhanced the expression of BMP4, BSP and OPN; iii)
HGmayimpair osteogenic differentiation; and iv) the inhibitory
effects of HG on the differentiation of BMSCs were reversed via
transduction with Shh lentiviral vectors. To verify these
observations in vivo, the present study used a rat tooth
extraction model, and found that: i) Lenti-Shh treatment resulted
in a revealed reductions of alveolar bone to a lesser extent; and
ii) significant bone formation inside the extraction socket when
compared with the normal and DM groups. Taken together, the results
of the present study demonstrated that osteogenic differentiation
was affected by the Shh pathway under HG conditions. Furthermore,
these effects could be controlled by way of activating Shh
signaling.
The Hh signaling pathway serves a key role in
numerous processes during embryonic development, as well as the
proliferation and differentiation of stem cells, and induces bone
formation (17,28,29).
Oliveira et al (30)
reported that activation of the Hh pathway increased the expression
of a panel of genes associated with the osteoblast phenotype
development in human mesenchymal stem cells. In addition, the Hh
pathway exerts a pivotal function in driving undifferentiated cells
to the osteoblast lineage (30).
In addition, several lines of evidence support the notion that
activation of the Hh pathway by purmorphamine increases the
transcription of numerous genes, including Gli1, PTCH and ALP;
however, ALP activity was induced in mouse embryonic mesoderm
fibroblasts and pre-osteoblast cell lines (31,32).
The results of the present study consistently demonstrated that
activation of Shh signaling via transduction with Lenti-Shh vectors
upregulated the expression of BMP4, BSP and OPN. The present study
also observed that the ALP activity and matrix mineralization of
BMSCs were increased by triggering Shh signaling. BMP4, BSP and OPN
are early transcription factors that are associated with osteoblast
differentiation (33). In
addition, ALP is involved in the formation and reconstruction of
bones (34–36). Therefore the aforementioned bone
related genes should be investigated further, in an attempt to
provide additional insight into the relationship between the Shh
pathway and HG levels.
The incidence of diabetes-associated bone metabolic
disorders is increasing (37). If
poorly controlled, the quality of life of patients could be
severely affected. A previous study demonstrated that patients with
T2DM had a 50% increased mortality and a 21% increased incidence of
hip fracture when compared with individuals without T2DM (38). To a certain extent, our previous
findings confirmed that the alveolar bone resorption was more
severe in rats with diabetes compared with non-diabetic rats
(4). Glucose in the
microenvironment markedly affects the gene regulation of cells. A
study reported that high levels of glucose inhibits the mRNA
expression of ALP and RUNX family transcription factor 2 in
periodontal ligament stem cells (39). The results of the present study
consistently demonstrated fewer mineralized nodules in the HG
groups, as well as ALP activity and the reduced mRNA expression of
osteogenic related genes when compared with LG groups. This is in
accordance with the findings that in the presence of HG
concentrations, osteoblasts undergo differentiation; however, with
a significant delay compared with control- or mannitol-treated
cells (40). Additionally, SMO
significantly impairs Shh-induced chemotaxis and is accompanied by
elevated expression levels of PTCH, which is a protein that
inhibits SMO activity (20). In
the present study, Shh protein and SMO gene expression were
decreased under HG conditions when compared with LG conditions.
This may be due to HG-induced downregulation of genes associated
with the Shh pathway. In addition the present study observed that,
following transduction with Lenti-Shh, the osteoblastic
differentiation of BMSCs was promoted, as were the expression of
Shh and SMO under HG and LG conditions. As hypothesized, the
activation of Shh signaling could prevent HG-mediated BMSCs
dysfunction thereby upregulating osteoblast differentiation;
however, this beneficial effect was attenuated following
transduction with Lenti-siRNA, which was accompanied with a
decrease in the expression of bone-related genes, ALP activity and
matrix mineralization deposition. As the results of the present
study demonstrated the high efficiency of lentiviral-mediated
overexpression and downregulation of Shh on osteoblastic
differentiation under HG conditions in BMSCs in vitro,
future studies should investigate and verify these effects in
animal models prior to use in clinical settings. The alveolar ridge
reduction rate was significantly higher in DM rats than the NG
rats, which indicated reduced bone formation in DM group. Following
treatment with Lenti-Shh, a significant loss in alveolar bone was
prevented in the NG + Lenti-Shh and DM + Lenti-Shh groups. These
results indicated that the activation of Shh signaling could
promote bone formation.
Due to its widespread role in embryonic and
postnatal development, Shh pathway activators have attracted a
great deal of attention as potential therapies (14). The study of Wang et al
(41) used an adenovirus that
expresses a secreted form of ShhN peptide to activate Hh signaling
in periosteal mesenchymal progenitors and observed robust bone
formation. Furthermore, in an additional study, a >90%
transduction efficiency at 1 week was recorded, and continued to
demonstrate stable expression for 8 weeks as identified in rat
BMSCs transduced with Lenti-CMV-EGFP (42). In the present study, we transduced
BMSCs with lentiviral vectors fused with GFP at an MOI of 10 and
>80% of the cells were GFP positive. Lentiviral vectors were
selected as gene delivery vehicles for research due to their
capacity to efficiently transduce non-dividing cells, shuttle large
genetic payloads and maintain stable long-term transgene
expression. It has been reported that Hh signaling could be
regulated by the presence of the Shh protein and GANT61 (43). However, proteins and chemicals
usually have other side effects on cells. The strong cytotoxicity
of GANT61 has a significant effect on cell survival (44,45),
which limits its ease of application in animal experiments. An
increasing amount of studies investigating bone tissue engineering
have investigated the application of cytokines, specific growth
factors and artificial scaffolds for promoting bone formation in
vivo via transducing signals to modulate cellular activities
(46,47). In the present study, the area of
new bone formation in the NG + Lenti-Shh and DM + Lenti-Shh groups
was larger than that of the NG and DM groups, respectively. This
indicated that Lenti-Shh transgene expression contributed to in
vivo bone mineralization and osteogenesis. Thus, lentiviral
transgene expression may be considered as an effective therapeutic
strategy for overcoming local bone defects, particularly for those
with diabetes.
In conclusion, the results of the present study are
in agreement with the hypothesis that HG alters the Shh pathway in
osteoblasts, which results in defects during osteoblastic
differentiation; the activation of Shh signaling could reverse
these deleterious effects and promote bone formation. Understanding
the mechanism underlying the effects of HG on osteoblastic
differentiation and matrix mineralization deposition may provide
novel insight into the possible therapeutic and prevention methods
of osteopenia associated with diabetes. Of note, further
investigation is warranted to validate the use of Lenti-Shh
transgene expression to ensure the safety and efficiency for human
use.
Acknowledgements
Not applicable.
Funding
The present study was supported by Natural Science
Foundation of China (grant no. 81570951), Natural Science
Foundation of China (grant no. 81500816), the Innovation Science
Foundation of Harbin Medical University (grant no. 2016LCZX10), and
Natural Science Foundation of Heilongjiang Province of China (grant
no. H2015103).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZLJ designed the study, performed the research,
analyzed data, and wrote the paper. BZ designed the in vitro
experiments and edited the manuscript. YL designed the in
vivo experiments and edited the manuscript. ZLJ, HJ, ZSL, MYL,
XFC, YYJ and HDB made substantial contributions to the analysis and
interpretation of data, and drafted the manuscript. All authors
reviewed the manuscript.
Ethics approval and consent to
participate
All animal experiments were conducted in strict
accordance with the principles of medical ethics and were approved
by Animal Care and Use Committee of the Second Affiliated Hospital
of Harbin Medical University (Harbin, China). All necessary permits
were obtained for the described field studies.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that there are no competing
interests.
References
|
1
|
de Carvalho GB, Dias-Vasconcelos NL,
Santos RKF, Brandão-Lima PN, da Silva DG and Pires LV: Effect of
different dietary patterns on glycemic control in individuals with
type 2 diabetes mellitus: A systematic review. Crit Rev Food Sci
Nutr. 1–12. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Marin C, Luyten FP, Van der Schueren B,
Kerckhofs G and Vandamme K: The impact of type 2 diabetes on bone
fracture healing. Front Endocrinol (Lausanne). 9:62018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ge Z, Liu ZZ, Kan J, Zhang JJ, Li SJ, Tian
NL, Ye F, Qian XS, Yang S, Chen MX, et al: Stent fracture is
associated with a higher mortality in patients with type-2 diabetes
treated by implantation of a second-generation drug-eluting stent.
Int J Cardiovasc Imaging. 33:1873–1881. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kayal RA, Siqueira M, Alblowi J, McLean J,
Krothapalli N, Faibish D, Einhorn TA, Gerstenfeld LC and Graves DT:
TNF-alpha mediates diabetes-enhanced chondrocyte apoptosis during
fracture healing and stimulates chondrocyte apoptosis through
FOXO1. J Bone Miner Res. 25:1604–1615. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Liu R, Bal HS, Desta T, Krothapalli N,
Alyassi M, Luan Q and Graves DT: Diabetes enhances periodontal bone
loss through enhanced resorption and diminished bone formation. J
Dent Res. 85:510–514. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Jiang ZL, Cui YQ, Gao R, Li Y, Fu ZC,
Zhang B and Guan CC: Study of TNF-α, IL-1β and LPS levels in the
gingival crevicular fluid of a rat model of diabetes mellitus and
periodontitis. Dis Markers. 34:295–304. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wittrant Y, Gorin Y, Woodruff K, Horn D,
Abboud HE, Mohan S and Abboud-Werner SL: High D(+)glucose
concentration inhibits RANKL-induced osteoclastogenesis. Bone.
42:1122–1130. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Serrano S, Mariñoso ML, Nacher M, Torres
A, Cuevas X, Loreta J, Munné A and Diez A: Modulation of osteoblast
activity by serum from diabetic and non-diabetic patients on
hemodialysis: A three-dimensional culture study. J Nephrol.
17:369–376. 2004.PubMed/NCBI
|
|
9
|
Liu C and Jiang D: High glucose-induced
LIF suppresses osteoblast differentiation via regulating
STAT3/SOCS3 signaling. Cytokine. 91:132–139. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Luo J, Sun MH, Kang Q, Peng Y, Jiang W,
Luu HH, Luo Q, Park JY, Li Y, Haydon RC and He TC: Gene therapy for
bone regeneration. Curr Gene Ther. 5:167–179. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Milone MC and O'Doherty U: Clinical use of
lentiviral vectors. Leukemia. 32:1529–1541. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Naldini L, Blömer U, Gallay P, Ory D,
Mulligan R, Gage FH, Verma IM and Trono D: In vivo gene delivery
and stable transduction of nondividing cells by a lentiviral
vector. Science. 272:263–267. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Naldini L, Blömer U, Gage FH, Trono D and
Verma IM: Efficient transfer, integration, and sustained long-term
expression of the transgene in adult rat brains injected with a
lentiviral vector. Proc Natl Acad Sci USA. 93:11382–11388. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kimura H, Ng JM and Curran T: Transient
inhibition of the Hedgehog pathway in young mice causes permanent
defects in bone structure. Cancer Cell. 13:249–260. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yuan X, Cao J, He X, Serra R, Qu J, Cao X
and Yang S: Ciliary IFT80 balances canonical versus non-canonical
hedgehog signalling for osteoblast differentiation. Nat Commun.
7:11018–11024. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang C, Shan S, Wang C, Wang J, Li J, Hu
G, Dai K, Li Q and Zhang X: Mechanical stimulation promote the
osteogenic differentiation of bone marrow stromal cells through
epigenetic regulation of Sonic Hedgehog. Exp Cell Res. 352:346–356.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kinto N, Iwamoto M, Enomoto-Iwamoto M,
Noji S, Ohuchi H, Yoshioka H, Kataoka H, Wada Y, Yuhao G, Takahashi
HE, et al: Fibroblasts expressing Sonic hedgehog induce osteoblast
differentiation and ectopic bone formation. FEBS Lett. 404:319–323.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ho JE, Chung EH, Wall S, Schaffer DV and
Healy KE: Immobilized sonic hedgehog N-terminal signaling domain
enhances differentiation of bone marrow-derived mesenchymal stem
cells. J Biomed Mater Res A. 83:1200–1208. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liao DM, Ng YK, Tay SSW, Ling EA and Dheen
ST: Altered gene expression with abnormal patterning of the
telencephalon in embryos of diabetic Albino Swiss mice.
Diabetologia. 47:523–531. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Dunaeva M, Voo S, van Oosterhoud C and
Waltenberger J: Sonic hedgehog is a potent chemoattractant for
human monocytes: Diabetes mellitus inhibits Sonic hedgehog-induced
monocyte chemotaxis. Basic Res Cardiol. 105:61–71. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Srinivasan K, Viswanad B, Asrat L, Kaul CL
and Ramarao P: Combination of high fat diet-fed and low-dose
streptozotocin-treated rat: A model for type 2 diabetes and
pharmacological screening. Pharmacol Res. 52:313–320. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ti Y, Xie GL, Wang ZH, Bi XL, Ding WY,
Wang J, Jiang GH, Bu PL, Zhang Y, Zhong M and Zhang W: TRB3 gene
silencing alleviates diabetic cardiomyopathy in a type 2 diabetic
rat model. Diabetes. 60:2963–2974. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li C, Shi C, Kim J, Chen Y, Ni S, Jiang L,
Zheng C, Li D, Hou J, Taichman RS and Sun H: Erythropoietin
promotes bone formation through Ephr inB2/EphB4 signaling. J Dent
Res. 94:455–463. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Elsubeihi ES and Heersche JN: Quantitative
assessment of post-extraction healing and alveolar ridge
remodelling of the mandible in female rats. Arch Oral Biol.
49:401–412. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Arai Y, Aoki K, Shimizu Y, Tabata Y, Ono
T, Murali R, Mise-Omata S and Wakabayashi N: Peptide-induced de
novo bone formation after tooth extraction prevents alveolar bone
loss in a murine tooth extraction model. Eur J Pharmacol.
782:89–97. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Reseland JE, Syversen U, Bakke I, Qvigstad
G, Eide LG, Hjertner O, Gordeladze JO and Drevon CA: Leptin is
expressed in and secreted from primary cultures of human
osteoblasts and promotes bone mineralization. J Bone Miner Res.
16:1426–1433. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ingham PW and McMahon AP: Hedgehog
signaling in animal development: Paradigms and principles. Genes
Dev. 15:3059–3087. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
McMahon AP, Ingham PW and Tabin CJ:
Developmental roles and clinical significance of hedgehog
signaling. Curr Top Dev Biol. 53:1–114. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Oliveira FS, Bellesini LS, Defino HL, da
Silva Herrero CF, Beloti MM and Rosa AL: Hedgehog signaling and
osteoblast gene expression are regulated by purmorphamine in human
mesenchymal stem cells. J Cell Biochem. 113:204–208. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu X, Walker J, Zhang J, Ding S and
Schultz PG: Purmorphamine induces osteogenesis by activation of the
hedgehog signaling pathway. Chem Biol. 11:1229–1238. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sinha S and Chen JK: Purmorphamine
activates the hedgehog pathway by targeting smoothened. Nat Chem
Biol. 2:29–30. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kasai T, Bandow K, Suzuki H, Chiba N,
Kakimoto K, Ohnishi T, Kawamoto S, Nagaoka E and Matsuguchi T:
Osteoblast differentiation is functionally associated with
decreased AMP kinase activity. J Cell Physiol. 221:740–749. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Jung WW: Protective effect of apigenin
against oxidative stress-induced damage in osteoblastic cells. Int
J Mol Med. 33:1327–1334. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Suh KS, Rhee SY, Jung WW, Kim NJ, Jang YP,
Kim HJ, Kim MK, Choi YK and Kim YS: Chrysanthemum zawadskii extract
protects osteoblastic cells from highly reducing sugar-induced
oxidative damage. Int J Mol Med. 32:241–250. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kawai S and Sugiura T: Characterization of
human bone morphogenetic protein (BMP)-4 and −7 gene promoters:
Activation of BMP promoters by Gli, a sonic hedgehog mediator.
Bone. 29:54–61. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Courties A, Berenbaum F and Sellam J: The
phenotypic approach to osteoarthritis: A look at metabolic
syndrome-associated osteoarthritis. Joint Bone Spine. Dec
22–2018.doi: 10.1016/j.jbspin.2018.12.005 (Epub ahead of print).
View Article : Google Scholar
|
|
38
|
Tebé C, Martinez-Laguna D, Moreno V,
Cooper C, Diez-Perez A, Collins GS and Prieto-Alhambra D:
Differential mortality and the excess rates of hip fracture
associated with type 2 diabetes: Accounting for competing risks in
fracture prediction matters. J Bone Miner Res. 33:1417–1421. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim SY, Lee JY, Park YD, Kang KL, Lee JC
and Heo JS: Hesperetin alleviates the inhibitory effects of high
glucose on the osteoblastic differentiation of periodontal ligament
stem cells. PLoS One. 8:e675042013. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Balint E, Szabo P, Marshall CF and Sprague
SM: Glucose-induced inhibition of in vitro bone mineralization.
Bone. 28:21–28. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Wang Q, Huang C, Zeng F, Xue M and Zhang
X: Activation of the Hh pathway in periosteum-derived mesenchymal
stem cells induces bone formation in vivo: Implication for
postnatal bone repair. Am J Pathol. 177:3100–3111. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Sugiyama O, An DS, Kung SP, Feeley BT,
Gamradt S, Liu NQ, Chen IS and Lieberman JR: Lentivirus-mediated
gene transfer induces long-term transgene expression of BMP-2 in
vitro and new bone formation in vivo. Mol Ther. 11:390–398. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Guan CC, Yan M, Jiang XQ, Zhang P, Zhang
XL, Li J, Ye DX and Zhang FQ: Sonic hedgehog alleviates the
inhibitory effects of high glucose on the osteoblastic
differentiation of bone marrow stromal cells. Bone. 45:1146–1152.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mazumdar T, DeVecchio J, Shi T, Jones J,
Agyeman A and Houghton JA: Hedgehog signaling drives cellular
survival in human colon carcinoma cells. Cancer Res. 71:1092–1102.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Pan D, Li Y, Li Z, Wang P and Liang Y: Gli
inhibitor GANT61 causes apoptosis in myeloid leukemia cells and
acts in synergy with rapamycin. Leuk Res. 36:742–748. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Lee SJ: Cytokine delivery and tissue
engineering. Yonsei Med J. 41:704–719. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Marei MK, Nouh SR, Saad MM and Ismail NS:
Preservation and regeneration of alveolar bone by tissue-engineered
implants. Tissue Eng. 11:751–767. 2005. View Article : Google Scholar : PubMed/NCBI
|