Introduction
Alzheimer's disease (AD) is the most common type of
dementia; it is a degenerative disease of the central nervous
system and the main clinical features include progressive memory
loss, cognitive impairment and personality changes. According to
data released by the International Association of Alzheimer's
Disease in 2014, there were ~44,400,000 patients with AD globally,
with an annual increase of ~7,700,000 cases, or 1 case every 4 sec
(1). The characteristic
pathological changes of AD are the formation of the cerebral cortex
and senile plaques. The core component of senile plaques is a large
amount of fibrous amyloid β protein (Aβ) deposition. Aβ is mainly
concentrated in the cerebral cortex and in the hippocampus
(2). The neurotoxicity of natural
Aβ in vivo and in vitro has been demonstrated
previously (3,4); Aβ plaques may disrupt neuronal
excitability, induce axonal varicose veins and neurite rupture, and
significantly reduce spinal density (5).
Apolipoprotein E4 (APOE4) is located on chromosome
19 and is a genetic risk factor for AD development (6). Previous studies have reported that
APOE4 can induce calcium overload and neuronal death (7), which may lead to multiple spatial
memory and work memory damage (8,9) and
inhibit hippocampal synaptic plasticity (10). An association has been demonstrated
between APOE4 and Aβ plaque loading in the subcortical brain region
of patients with mild cognitive impairment. APOE4 enhances the
spontaneous fibrogenesis of Aβ peptides and accelerates the
aggregation of Aβ in vitro (11). Aβ protein metabolism produces a
variety of sequence fragments with neurotoxic effects; in
vivo, Aβ1-40 (80–90% of Aβ fragments) and
Aβ1-42 (10–20% of Aβ fragments) are two types of Aβ
protein that are abnormally metabolized by APP, both of which
coexist in senile plaques (12).
Histone deacetylase 6 (HDAC6) is a member of the
histone deacetylase family, which is comprised of five domains;
increased activity of HDAC6 is has been reported to occur in the
brain of AD patients (13,14). HDAC6 is a cytoplasmic deacetylase,
and previous studies have shown that reduction or inhibition of
HDAC6 in AD model mice can improve mouse memory (15). A previous study demonstrated that
Aβ-induced mitochondrial axonal transport injury can be rescued by
HDAC6 inhibitors in primary neurons (16). Another study using patient samples
indicated that the expression level of HDAC6 was elevated by 52% in
the cortex and 91% in the hippocampus, the learning and memory
center of the brain, in patients with AD (17,18).
Although HDAC6 has great potential as a target for AD therapy
(19), the effects of HDAC6 on Aβ
and APOE4 co-aggregation on the brain has not been fully
understood. In the present study, Aβ and APOE4 co-injection models
were established to determine the effects of HDAC6 expression on
learning and memory ability and brain injury induced by APOE4 and
Aβ1-40 co-aggregation.
Materials and methods
Animals and groups
All experiments were conducted in accordance with
the Guide for the Care and Use of Laboratory Animals of the
National Institutes of Health (20) and have been approved by the
Affiliated Yantai Yuhuangding Hospital of Qingdao University Animal
Ethics Committee. A total of 36 specific-pathogen-free grade
Sprague-Dawley male rats (age, 8–10 weeks; weight, 200±20 g) were
purchased from Beijing Weitong Lihua Experimental Animal Technology
Co., Ltd. [licence no. SCXK (Jing) 2012-0001]. Rats were provided
food and water ad libitum and housed in normal laboratory
conditions (temperature, 20±2°C; relative humidity, 40–45%; 12-h
light/dark cycle). The rats were divided into the following four
groups (n=9/group): i) Control group, which did not receive any
treatments; ii) sham group, which received that same surgical
procedure (described below) as the model group and were injected
with 2 µl sterile saline; iii) Model group, which received surgery
and were injected with APOE4 (AMS Biotechnology, Ltd.) +
Aβ1-40 (Beijing Zhongshan Jinqiao Biotechnology Co.,
Ltd.); and iv) HDAC6 group, which received surgery and were
injected with APOE4 + Aβ1-40 as well as HDAC6 inhibitor
(10 mg/kg; tubastatin A hydrochloride; cat. no. ab141415;
Abcam).
AD model establishment
Rats were anesthetized by intraperitoneal injection
of 1% pentobarbital sodium (40 mg/kg) and were fixed on a brain
stereotaxic instrument (Rui Wo De). The head and incisors were
fixed using the earbuds and the toothed rods. The rats were
maintained in the horizontal position, and the surgical area was
disinfected with lodophor disinfectant. The skin was separated from
the periosteum and blood vessels with a wet cotton swab. A ~2-cm
incision was made from the middle of the skull. The CA1 area of the
rat bilateral hippocampus was located as previously described
(21). A 5-µl microsyringe was
fixed on the positioning device holder. Holes were drilled in the
skull using the dental drill bilaterally at 3.0 mm posterior to
bregma, 2 mm from the skull midline, and the needle was inserted
4.0 mm at each site. The rats in the sham group were injected with
2 µl of sterile saline on each side. The bilateral hippocampus of
rats in APOE4 + Aβ1-40 group was slowly injected with 1
µl Aβ1-40 (10 µg/µl) and 1 µl APOE4 (1 µg/µl)
suspension. The needle was kept in the brain for 5 min to ensure
adequate dispersion, then the needle was slowly withdrawn. The
skull was blocked with dental cement, and the wound was sterilized
with sulfamethoxazole (Shanghai Guang Rui Biological Technology
Co., Ltd.) to prevent infection and sutured. The animal was kept in
a heated chamber until it recovered from anesthesia. At 2 weeks
post-surgery, the rats were examined in the Morris water maze
experiment.
Morris water maze
For the Morris water maze (130-cm diameter; Anhui
Zhenghua Biologic Apparatus Facilities Co., Ltd.) assay, a 15-cm
diameter transparent circular platform was placed in the circular
pool filled with warm water (25±1°C) at ~1.5 cm below the water
surface. The circular platform was placed in one of the four
quadrants in the pool. Rats were trained continuously, with two
sessions of four trials each day for 5 days, one in the morning and
one in the afternoon. In each session, rats were placed into the
water gently, successively in the east, west, south and north
quadrants of the maze, with the order remaining consistent for each
session. The length of time it took the rats, once placed into the
water, to find and climb the platform was recorded as the escape
latency. If the rats did not find the platform within 60 sec, they
were guided to the platform and allowed to stay on it for 15 sec;
after the 15 sec rest period, the next training session was
performed, and learning and memory data were recorded.
The platform was removed on day 6, and the rats
received four probe trials, in which they were placed into the
water in randomly selected quadrants. During these trials, the time
to first entry into the previous platform location (T1), time spent
in the target quadrant (T2) and number of times that they passed
through the previous platform location (N) were recorded; each test
lasted for 90 sec. T1, T2 and N for each animal were averaged
across the four trials. After the probe tests, the swimming line of
rats were examined after placing a visible platform within the
pool.
Hematoxylin and eosin (H&E)
staining
After the behavioral experiments, brain tissue was
removed, and the hippocampus was isolated and fixed in 4%
paraformaldehyde (Sangon Biotech Co., Ltd.) at 4°C overnight. The
samples were embedded in paraffin and cut into 4 µm sections. The
paraffin sections were dewaxed with xylene, dehydrated with serial
dilutions of ethanol, stained with hematoxylin for 10 min at room
temperature and eosin for 5 min at room temperature (Beijing
Solarbio Science & Technology Co., Ltd.), and rinsed in
distilled water for 30 sec. The sections were subsequently washed 2
times (1 min each) with 95% anhydrous and 3 times in xylene (5 min
each), and then mounted with Permount mounting medium (Thermo
Fisher Scientific, Inc.). Pathological changes were observed under
an optical microscope (magnification, ×200; Olympus Corporation)
and hippocampal neurons cells were counted using Aperio ImageScope
v11.1 software (Leica Microsystem GmbH); 5 fields per sample were
analyzed for assessment of pathological changes, and the data were
expressed as the cell number.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted from the hippocampus using a
TRIzol kit (Invitrogen; Thermo Fisher Scientific, Inc.). A
spectrophotometer was used to determine the purity and integrity of
RNA; an optical density (OD)260/OD280 of 1.8–2.0 indicated good RNA
purity. cDNA was synthesized by SuperScript III Reverse
Transcriptase (Thermo Fisher Scientific, Inc.). cDNA (2 µl) was
used for qPCR using a DyNAmo HS SYBR Green qPCR kit (Thermo Fisher
Scientific, Inc.). Primers were designed and synthesized by
Shanghai GenePharma Co., Ltd., and the sequences were as follows:
HDAC6, forward 5′-TGGCGGACTAGAAAGAGCCT-3′, reverse
5′-GAAGGGGTGACTGGGGATTG-3′; choline acetyltransferase (ChAT),
forward 5′-AGCCACTTTCAGTCAGTCGG-3′, reverse 5′-ACTCCAGAGTAGCA-3′;
β-actin, forward 5′-GTGGGGATAATGAACTTGCAG-3′, reverse
5′-GGAACCCCTGGTAGAACAGT-3′. The qPCR thermocycling conditions were:
95°C for 10 min; followed by 40 cycles at 95°C for 15 sec and 60°C
for 1 min. β-actin was used as the internal reference and the
relative mRNA expression levels were calculated by the
2−ΔΔCq method (22).
Immunohistochemistry
The hippocampal tissues were embedded in paraffin
and sectioned (6 µm) (23). The
sections were deparaffinized with xylene two times, and rehydrated
in a descending ethanol series. Endogenous peroxidase was inhibited
by incubating the sections for 30 min with 3%
H2O2. Antigen retrieval was performed using
citrate buffer at high temperature for 10 min. The sections were
subsequently blocked for 20 min in 5% BSA, and incubated with
anti-HDAC6 primary antibody (1:100; cat. no. ABIN1872957;
antibodies-online GmbH) overnight at 4°C. After rewarming, sections
were incubated with a horseradish peroxidase-conjugated goat
anti-rabbit IgG secondary antibody (cat. no. ab6721; Abcam) at 37°C
for 1 h; the sections were visualized with 3,3-diaminobenzidine
tetrahydrochloride as the chromogen (Beijing Solarbio Science &
Technology Co., Ltd.). The sections were subsequently dehydrated
and coverslipped with Permount mounting medium (Thermo Fisher
Scientific, Inc.). The slides were observed under a ×40 optical
microscope (Olympus Corporation) and positive-staining cells were
counted using Aperio ImageScope v11.1 software (Leica Biosystems
Imaging, Inc.); the data were expressed as the percentage of
positive cells out of the total number of cells counted.
Western blot assay
Total protein of the hippocampal tissues was
extracted using RIPA lysis buffer containing proteinase inhibitor
cocktail, and the concentration of protein was determined by
bicinchoninic acid assay. Proteins (50 µg) were separated by 10%
SDS-PAGE and then transferred onto PVDF membranes (EMD Millipore).
The membranes were blocked with 5% skimmed milk at 4°C overnight,
incubated with primary antibodies against HDAC6 (1:1,000; cat. no.
ab1440; Abcam); microtubule-associated protein tau (tau; 1:1,000;
cat. no. ab64193, Abcam), phosphorylated (p)-tau (phospho-Ser199;
1:1,000; cat. no. ab81268, Abcam); glycogen synthase kinase 3β
(GSK3β; 1:1,000; cat. no. 5676; Cell Signaling Technology, Inc.);
p-GSK3β (1:1,000; cat. no. 9322; Cell Signaling Technology, Inc.)
at 4°C overnight. After incubating with secondary antibody sheep
anti-rabbit IgG (1:5,000; cat. no. ab97095; Abcam) at 37°C for 1 h,
protein bands were visualized using the ECL chemiluminescence
system (Thermo Fisher Scientific, Inc.). Protein expression levels
were normalized to β-actin (1:2,000; cat. no. ab8227; Abcam) and
quantified by Image J software version 1.46 (NIH).
Statistical analysis
SPSS v20.0 statistical software (IBM Corp.) was used
for statistical analysis. All the data are expressed as the mean ±
SD. Comparisons between two groups was performed using Student's
t-test, and multiple groups were compared by one-way ANOVA followed
by Fisher's LSD post hoc test. P<0.05 was considered to indicate
a statistically significant difference.
Results
HDAC6 inhibition reduces the escape
latency of rats
The results of location navigation experiments
revealed no significant difference in the escape latency in rats
across days 1–4 (Table I).
However, the escape latency of the model group (26.19±10.23 sec)
and the HDAC6 group (20.86±10.02 sec) were significantly longer
compared with that of the control group at day 5 (14.65±8.01 sec;
P=0.026 and P=0.034, respectively). There was no significant
difference in escape latency on day 5 between the sham group and
the control group (P=0.109). The escape latency of HDAC6 group was
significantly decreased compared with that of the model group at
day 5 (P=0.043).
 | Table I.Escape latency period of the rats at
different time points. |
Table I.
Escape latency period of the rats at
different time points.
| Group (n=9
rats/group) | Day 1 (sec) | Day 2 (sec) | Day 3 (sec) | Day 4 (sec) | Day 5 (sec) |
|---|
| Control | 62.93±18.76 | 34.15±15.35 | 28.42±13.15 | 19.52±11.31 | 14.65±8.01 |
| Sham | 64.76±19.54 | 36.89±16.47 | 30.56±14.96 | 21.68±12.52 | 17.23±9.15 |
| Model | 72.76±21.43 | 63.39±15.97 | 34.96±15.42 | 28.86±11.98 |
26.19±10.23a |
| HDAC6 | 67.43±20.32 | 37.26±14.87 | 32.74±15.13 | 26.97±11.36 |
20.86±10.02a,b |
HDAC6 inhibition reduces the ability
of spatial memory of rats
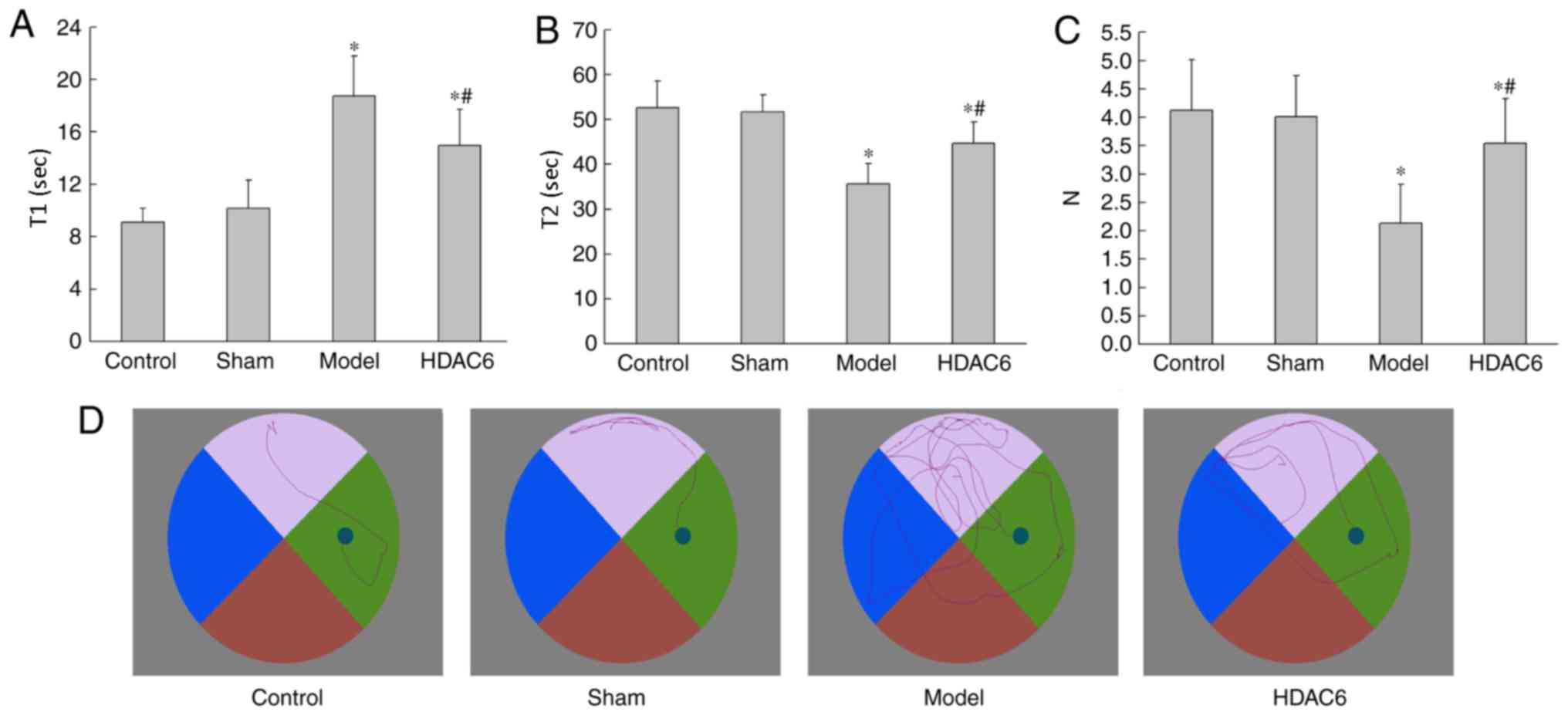
As demonstrated in Fig.
1, the T1 times of the model group and the HDAC6 group rats
were significantly increased compared with the control group rats
(P=0.019 and P=0.029, respectively; Fig. 1A). The T2 times of the model group
and the HDAC6 group rats were also significantly decreased compared
with the control group rats (P=0.032 and P=0.047, respectively;
Fig. 1B). Compared with the model
group, T1 and T2 in the HDAC6 group were significantly different
(P=0.033 and P=0.041, respectively). The N of the model and HDAC6
groups was significantly decreased compared with the control group
(P=0.021 and P=0.042, respectively; Fig. 1C). N in the HDAC6 group was
significantly increased compared with that in the model group
(P=0.036). However. compared with the model group, N was
significantly increased for the HDAC6 group (P=0.029; Fig. 1C). In the control and sham group,
the rats' swimming line and target were direct; they found the
platform in a short time and distance. In the model group, the
rats' swimming lines were disordered, meaning they found the
platform in a longer time and distance (Fig. 1D). The results showed that APOE4 +
Aβ1-40 co-injection could decrease the ability of
spatial memory of rats, and that inhibition of HDAC6 activity could
attenuate the short-term effects on spatial learning ability.
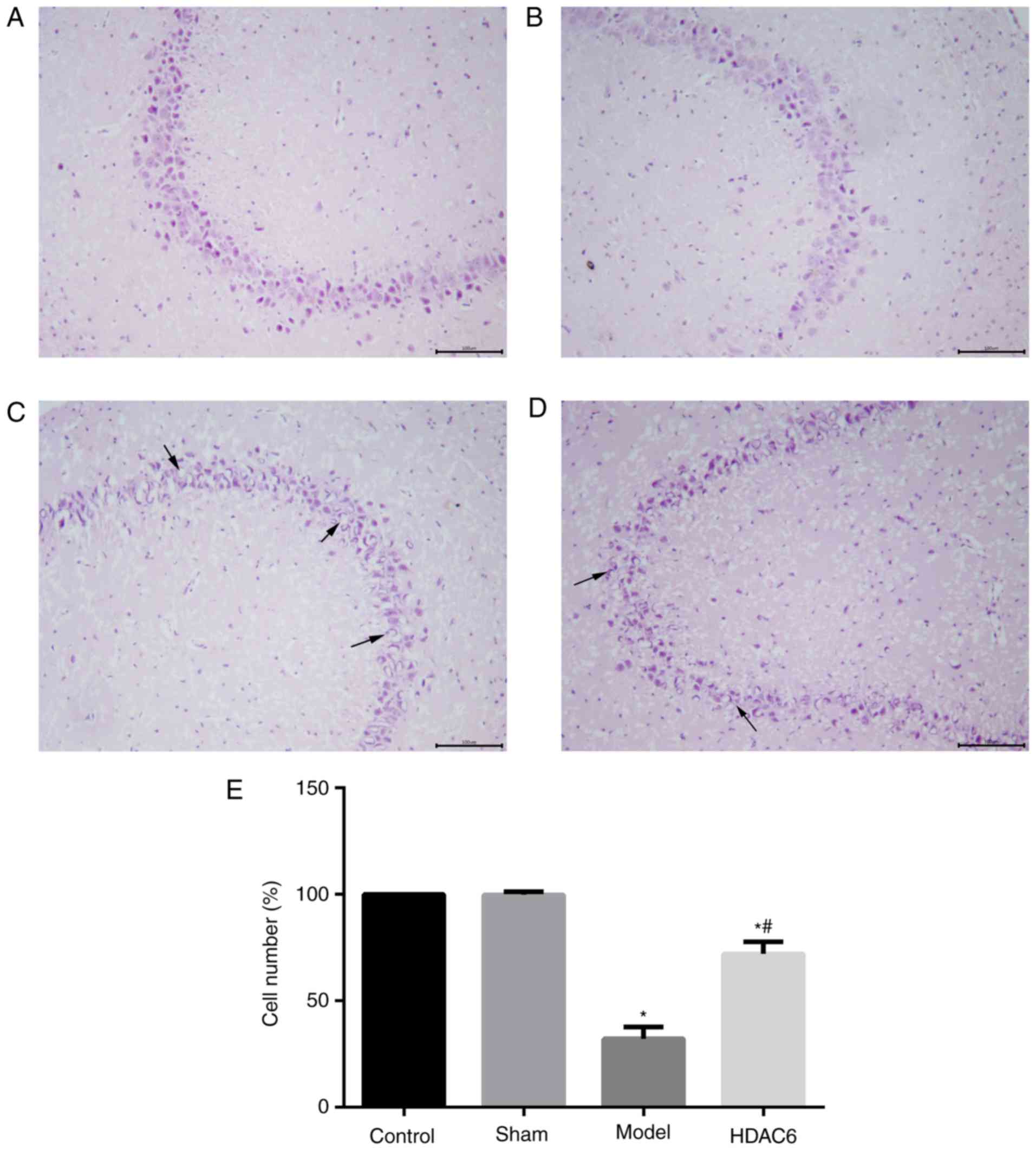
Hippocampal histopathological
changes
H&E staining results demonstrated that the
control group hippocampus organizational structure was clear and
complete, the cells were arranged neatly close, cytoplasm rich,
comprising dense surrounding cells without edema (Fig. 2A). Similar morphology was observed
in sham group hippocampus (Fig.
2B); the number of hippocampal neurons was not significantly
different between the sham group and the control group (Fig. 2E). Hippocampal tissues from model
group rats were incomplete, sparse, edematous and irregular, and
the number of the neurons were significantly reduced compared with
the control group (Fig. 2C and E).
The structures of some of the hippocampal neuron cells in the HDAC6
group were partially incomplete, and the number of cells was
significantly increased compared with that in the model group
(Fig. 2D-E). The results indicated
that APOE4 and Aβ1-40 co-injection may lead to the
development of hippocampal cell dysplasia, and that inhibition of
HDAC6 activity may reduce the damage of hippocampal tissues in
model rats.
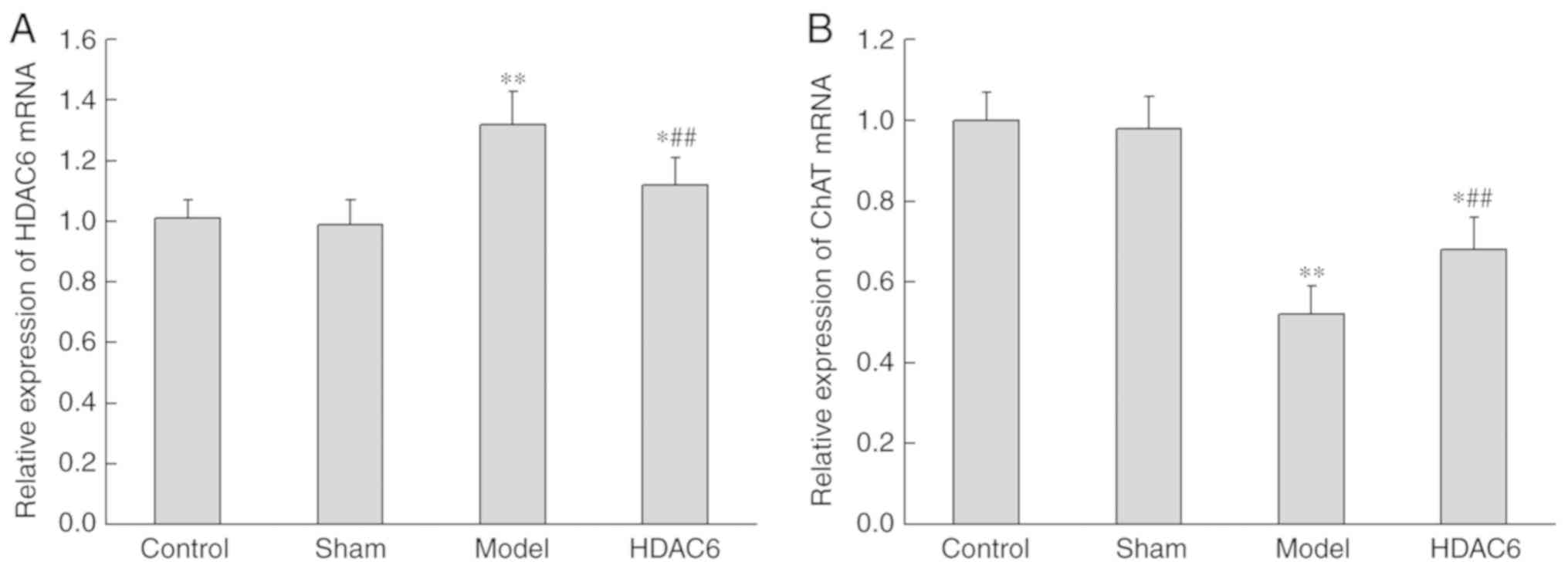
HDAC6 inhibition increases the
expression of ChAT mRNA in hippocampus
The effects of APOE4 + Aβ1-40 injection
on the expression levels of HDAC6 and ChAT mRNA were detected by
RT-qPCR. Compared with the control group, the expression of HDAC6
in the model group was significantly increased (P=0.009; Fig. 3A), whereas the expression of ChAT
mRNA was significantly decreased (P=0.001; Fig. 3B). Subsequently, in model rats
treated with HDAC6 inhibitor, the expression of HDAC6 was
significantly decreased (P=0.008) and the expression of ChAT mRNA
was significantly increased (P=0.003).
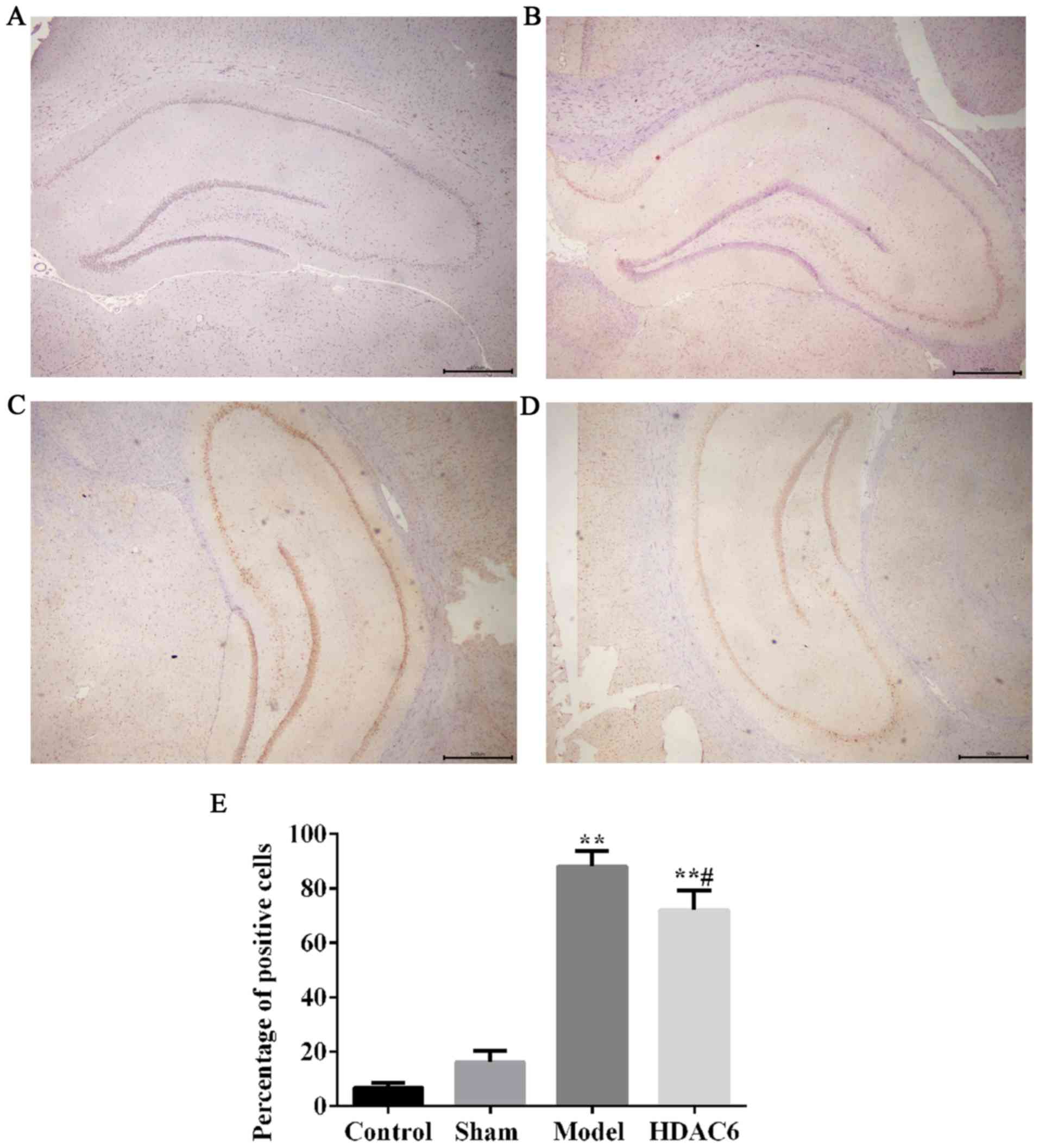
Changes in HDAC6 protein
expression
Immunohistochemistry results demonstrated only a few
HDAC6-positive cells were observed in the hippocampus in the
control group and in the sham group (Fig. 4A and B, respectively). The number
of HDAC6-positive cells was higher in the model group (Fig. 4C) as well as in the HDAC6 group
(Fig. 4D) compared with the
control group, and this increase was significant (Fig. 4E). The percentage of HDAC6-positive
cells in the HDAC6 group was significantly lower compared with the
model group rats (Fig. 4E);
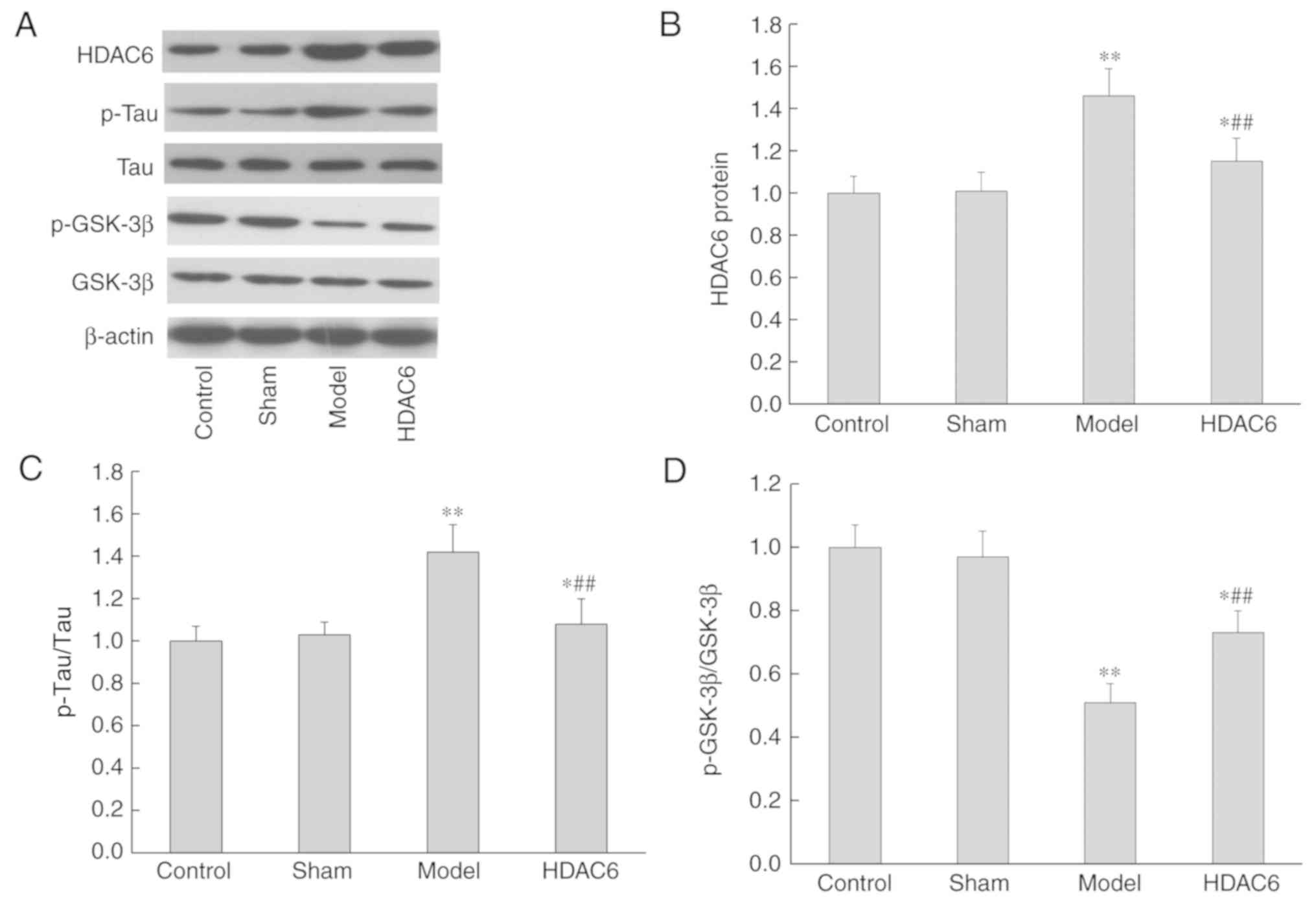
similar result were observed in the western blotting analyses
(Fig. 5A and B).
Changes in tau and GSK-3β protein
phosphorylation
Compared with the control group, the expression of
p-tau protein in the model and in the HDAC6 groups were
significantly increased (P=0.0082 and P=0.025, respectively;
Fig. 5A and C), whereas the
expression of p-GSK-3β protein was significantly decreased
(P=0.0042 and P=0.019, respectively; Fig. 5A and D). However, in the HDAC6
inhibitor group, the phosphorylation of tau protein was
significantly decreased (P=0.0076; Fig. 5B and D), whereas the
phosphorylation of GSK-3β protein was significantly increased
(P=0.031; Fig. 5A and D) compared
with the model group. The results indicated that inhibition of
HDAC6 expression may decrease the damage of APOE4 and
Aβ1-40 co-aggregation on the brain tissue of rats, and
this mechanism may be related to the reduction of p-tau protein
content and the increase in GSK-3β protein phosphorylation
levels.
Discussion
The etiology and pathogenesis of AD are not yet
clear. There are many factors involved, including Aβ deposition,
tau hyperphosphorylation, autophagy injury, synaptic transduction,
abnormal mitochondria and energy metabolism, abnormal insulin
signal transduction pathway, inflammatory injury and oxidative
stress (23). Results from the
present study indicate that HDAC6 inhibition may have a protective
effect on the spatial cognition and learning ability of the model
rats, and this mechanism may be related to the hyperphosphorylation
of tau protein and dysregulation of the cholinergic system in the
hippocampus. The interaction of HDAC6, tau protein and the
cholinergic system still needs to be further studied. The effects
of Aβ and hyperphosphorylated tau proteins, and their further
aggregation and polymerization in AD have received substantial
attention in the scientific community. The tau protein is a
phosphorus-containing glycoprotein that is encoded by a single gene
located on chromosome 17q21, it is a microtubule-associated protein
(24). Tau hyperphosphorylation
promotes its own integrated oligomers, which can further form
neurofibrillary tangles that have significant neurotoxic effects
and may affect the formation and stability of microtubules,
resulting in axonal transport obstacles (25). The decrease of microtubule
stability is an early pathological feature of AD. In addition, tau
oligomers may also mediate Aβ toxicity (26). A previous study has reported that
HDAC6 and tau proteins affect the stability of microtubules
(27). HDAC6 can combine with the
tau protein in vitro and in vivo (28), and promotes the phosphorylation of
tau protein (27). The HDAC6
inhibitor can reverse microtubule damage induced by tau
hyperphosphorylation (29). HDAC6
inhibitors may reduce the phosphorylation of tau protein by
indirect action with GSK3β. In the present study, the expression
level of p-tau protein was decreased, and the expression of
p-GSK-3β protein was increased following HDAC6 inhibition, which
suggested that HDAC6 inhibitors might inhibit the phosphorylation
of tau protein by increasing the level of GSK3β to protect the
stability of microtubules.
The damage of the cholinergic system in the
hippocampus is directly related to the pathogenesis of AD (30). ChAT is a key enzyme in the
synthesis of cholinergic neurotransmitters acetylcholine, which is
synthesized in cholinergic neurons (31). As such, the expression of ChAT mRNA
was investigated to determine the effects of HDAC6 on the damage of
cholinergic system in hippocampus. The results demonstrated that
the expression of ChAT mRNA was increased following HDAC6
inhibition, which suggested that HDAC6 may affect the function of
the cholinergic system by inhibiting the expression of ChAT
resulting in AD damage.
In recent years, studies have revealed that HDAC6
expression was increased in the hippocampus and cortex of AD
patients (27,32). However, the reasons for this
increase and the mechanism are still controversial. Several studies
indicated that HDAC6 overexpression may be one of the causes of AD
pathogenesis of neurodegenerative disease (27,32,33).
It has also been reported that overexpression of HDAC6 may be the
result of AD lesions and serve a neuroprotective role in (34,35).
Results from the present study were consistent with this. Aβ and
APOE4 co-injection models were reported to be better to simulate AD
lesions (36). Therefore, the
present study established a Aβ1-40 and APOE4
co-injection model, which was subsequently treated with an HDAC6
inhibitor, to explore the role of HDAC6 in AD. It was hypothesized
that the overexpression of HDAC6 may affect the progression of AD
by affecting key enzymes, such as ChAT, in the choline system and
microtubule stability. HDAC6 mRNA and protein expression levels
were reduced in model rats treated with the HDAC6 inhibitor; it was
suggested that the decrease in the expression level of HDAC6 may be
caused by the activation of ChAT, tau and GSK3β, and that there may
be an interaction feedback loop between them. Whether the specific
mechanism is in line with this assumption requires further
confirmation studies.
In conclusion, the present study demonstrated that
inhibition of HDAC6 expression may improve the cognitive function
of rats following Aβ1-40 and APOE4 co-aggregation. The
results suggested that HDAC6 may be a reliable target for AD
therapy.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YD, AK, QT and ZY designed the study; YD, AK, QT and
ZY performed the experiments; YD, AK and QT performed data
analysis; YD, AK, QT and ZY contributed pathological analysis; YD
and AK interpreted the data; YD, AK and QT acquired the samples;
YD, AK, QT and YZ wrote the manuscript. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
This study was approved by the Ethics Committee of
the Affiliated Yantai Yuhuangding Hospital of Qingdao University
(Yantai, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Alzheimer's Disease International, .
Dementia statistics. 2013.
|
|
2
|
Selkoe DJ and Hardy J: The amyloid
hypothesis of Alzheimer's disease at 25 years. EMBO Mol Med.
8:595–608. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sala Frigerio C and De Strooper B:
Alzheimer's disease mechanisms and emerging roads to novel
therapeutics. Annu Rev Neurosci. 39:57–79. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yamin G, Ono K, Inayathullah M and Teplow
DB: Amyloid beta-protein assembly as a therapeutic target of
Alzheimer's disease. Curr Pharm Des. 14:3231–3246. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Marin MA, Ziburkus J, Jankowsky J and
Rasband MN: Amyloid-β plaques disrupt axon initial segments. Exp
Neurol. 281:93–98. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thangavel R, Bhagavan SM, Ramaswamy SB,
Surpur S, Govindarajan R, Kempuraj D, Zaheer S, Raikwar S, Ahmed
ME, Selvakumar GP, et al: Co-expression of glia maturation factor
and apolipoprotein E4 in Alzheimer's disease brain. J Alzheimers
Dis. 61:553–560. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Qiu Z, Crutcher KA, Hyman BT and Rebeck
GW: ApoE isoforms affect neuronal N-methyl-D-aspartate calcium
responses and toxicity via receptor-mediated processes.
Neuroscience. 122:291–303. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bour A, Grootendorst J, Vogel E, Kelche C,
Dodart JC, Bales K, Moreau PH, Sullivan PM and Mathis C:
Middle-aged human apoE4 targeted-replacement mice show retention
deficits on a wide range of spatial memory tasks. Behav Brain Res.
193:174–182. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Yin JX, Turner GH, Lin HJ, Coons SW and
Shi J: Deficits in spatial learning and memory is associated with
hippocampal volume loss in aged apolipoprotein E4 mice. J
Alzheimers Dis. 27:89–98. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Chen Y, Durakoglugil MS, Xian X and Herz
J: ApoE4 reduces glutamate receptor function and synaptic
plasticity by selectively impairing ApoE receptor recycling. Proc
Natl Acad Sci USA. 107:12011–12016. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
van Bergen JM, Li X, Hua J, Schreiner SJ,
Steininger SC, Quevenco FC, Wyss M, Gietl AF, Treyer V, Leh SE, et
al: Colocalization of cerebral iron with amyloid beta in mild
cognitive impairment. Sci Rep. 6:355142016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
LaFerla FM, Green KN and Oddo S:
Intracellular amyloid-beta in Alzheimer's disease. Nat Rev
Neurosci. 8:499–509. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kuhlmann N, Wroblowski S, Knyphausen P, de
Boor S, Brenig J, Zienert AY, Meyer-Teschendorf K, Praefcke GJ,
Nolte H, Krüger M, et al: Structural and mechanistic insights into
the regulation of the fundamental Rho regulator RhoGDIα by lysine
acetylation. J Biol Chem. 291:5484–5499. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fukada M, Hanai A, Nakayama A, Suzuki T,
Miyata N, Rodriguiz RM, Wetsel WC, Yao TP and Kawaguchi Y: Loss of
deacetylation activity of Hdac6 affects emotional behavior in mice.
PLoS One. 7:e309242012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen S, Owens GC, Makarenkova H and
Edelman DB: HDAC6 regulates mitochondrial transport in hippocampal
neurons. PLoS One. 5:e108482010. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Choi H, Kim HJ, Kin J, Kim S, Yang J, Lee
W, Park Y, Hyeon SJ, Lee DA, Ryu H, et al: Increased acetylation of
Peroxiredoxin1 by HDAC6 inhibition leads to recovery of Aβ-induced
impaired axonal transport. Mol Neurodegener. 12:232017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Odagiri S, Tanji K, Mori F, Miki Y, Kakita
A, Takahashi H and Wakabayashi K: Brain expression level and
activity of HDAC6 protein in neurodegenerative dementia. Biochem
Biophys Res Commun. 430:394–399. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhang L, Sheng S and Qin C: The role of
HDAC6 in Alzheimer's disease. J Alzheimers Dis. 33:283–295. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Majid T, Griffin D, Criss Z, Jarpe M and
Pautler RG: Pharmocologic treatment with histone deacetylase 6
inhibitor (ACY-738) recovers Alzheimer's disease phenotype in
amyloid precursor protein/presenilin 1 (APP/PS1) mice. Alzheimers
Dement (N Y). 1:170–181. 2015.PubMed/NCBI
|
|
20
|
National Research Council (US) Institute
for Laboratory Animal Research, . Guide for the Care and Use of
Laboratory AnimalsNational Academies Press; Washington, DC:
1996
|
|
21
|
Paxinos G and Watson C: The Rat Brain in
Stereotaxic Coordinates6th. Hard cover edition. Academic Press; San
Diego, CA: 2007
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jiang S, Nandy P, Wang W, Ma X, Hisa J,
Wang C, Wang Z, Niu M, Siedlak S, Torres S, et al: Mfn2 ablation
causes an oxidative stress response and eventual neuronal death in
the hippocampus and cortex. Mol Neurodegener. 13:52018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stutzmann GE: The pathogenesis of
Alzheimers disease is it a lifelong ‘calciumopathy’?
Neuroscientist. 13:546–559. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Neve RL, Harris P, Kosik KS, Kurnit DM and
Donlon TA: Identification of cDNA clones for the human
microtubule-associated protein, tau, and chromosomal location of
the genes for tau and microtubule-associated protein 2. Mol Brain
Res. 1:271–280. 1986. View Article : Google Scholar
|
|
26
|
Alonso A, Zaidi T, Novak M, Grundke Iqbal
I and Iqbal K: Hyperphosphorylation induces self-assembly of tau
into tangles of paired helical filaments/straight filaments. Proc
Natl Acad Sci USA. 98:6923–6928. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Lasagna-Reeves CA, Castillo-Carranza DL,
Guerrero-Muoz MJ, Jackson GR and Kayed R: Preparation and
characterization of neurotoxic tau oligomers. Biochemistry.
49:10039–10041. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ding H, Dolan PJ and Johnson GV: Histone
deacetylase 6 interacts with the microtubule-associated protein
tau. J Neurochem. 106:2119–2130. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Noack M, Leyk J and Richter-Landsberg C:
HDAC6 inhibition results in tau acetylation and modulates tau
phosphorylation and degradation in oligodendrocytes. Glia.
62:535–547. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Xiong Y, Zhao K, Wu J, Xu Z, Jin S and
Zhang YQ: HDAC6 mutations rescue human tau-induced microtubule
defects in Drosophila. Proc Natl Acad Sci USA. 110:4604–4609. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Orta-Salazar E, Cuellar-Lemus CA,
Díaz-Cintra S and Feria-Velasco AI: Cholinergic markers in the
cortex and hippocampus of some animal species and their correlation
to Alzheimer's disease. Neurologia. 29:497–503. 2014.(In English,
Spanish). View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li Q, Chen M, Liu H, Yang L and Yang G:
Expression of APP, BACE1, AChE and ChAT in an AD model in rats and
the effect of donepezil hydrochloride treatment. Mol Med Rep.
6:1450–1454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Espallergues J, Teegarden SL, Veerakumar
A, Boulden J, Challis C, Jochems J, Chan M, Petersen T, Deneris E,
Matthias P, et al: HDAC6 regulates glucocorticoid receptor
signaling in serotonin pathways with critical impact on stress
resilience. J Neurosci. 32:4400–4416. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Selenica ML, Benner L, Housley SB, Manchec
B, Lee DC, Nash KR, Kalin J, Bergman JA, Kozikowski A, Gordon MN
and Morgan D: Histone deacetylase 6 inhibition improves memory and
reduces total tau levels in a mouse model of tau deposition.
Alzheimers Res Ther. 6:122014. View
Article : Google Scholar : PubMed/NCBI
|
|
35
|
Simões-Pires C, Zwick V, Nurisso A,
Schenker E, Carrupt PA and Cuendet M: HDAC6 as a target for
neurodegenerative diseases: What makes it different from the other
HDACs? Mol Neurodegener. 8:72013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Wu M, He Y, Zhang J, Yang J and Qi J:
Co-injection of Aβ1-40 and ApoE4 impaired spatial memory and
hippocampal long-term potentiation in rats. Neurosci Lett.
648:47–52. 2017. View Article : Google Scholar : PubMed/NCBI
|