Introduction
Stroke is a leading cause of mortality and adult
disability worldwide (1). In an
ischaemic stroke, blood supply to the brain is notably decreased,
resulting in dysfunction of the brain (2). However, few advances in stroke
therapy have been made to further improve patient outcomes. The
post-stroke angiogenesis of brain endothelial cells is important
for rehabilitation (3–5).
Motor skill training, including willed movement, can
lead to structural and functional plasticity of neurons in the
motor cortex, which will enhance brain health and motor function
(6). Willed movement, a type of
voluntary motor training, is characterized by serving attention to
a goal and making efforts to achieve it (7). During willed movement therapy, food
motivation is always utilized to induce activities of the body and
limbs. Willed movement training is capable of inducing long-term
depression in rats with focal cerebral ischaemia in a protein
interacting with C kinase (PICK)1-dependent manner (8). In addition, willed movement training
can reduce brain damage after stroke through upregulation of
synaptic plasticity-associated genes (8–10).
Ischaemic stroke results from the lack of blood
supply to the brain, which can lead to brain dysfunction (11). Hypoxia-inducible factor-1 (HIF-1)
is essential for the adaptive response of cells under hypoxic
conditions by transcriptionally activating its downstream target
genes (such as vascular epithelial growth factor (VEGF), matrix
metalloproteinase (MMP)s, Flt-1, angiogenin (Ang)-1), which are
implicated in many diseases, including stroke (12,13).
HIF-1 is a heterodimer comprised of O2-regulated HIF-1α
and constitutively expressing HIF-1β, and it binds to
hypoxia-responsive elements upstream of hypoxia-regulated genes
(14,15). The HIF-1 signaling pathway improves
the blood supply to cerebral tissue and reduces hypoxia-induced
ischaemic injury (16). Notably,
hypoxia can induce cell migration and endothelial tube formation of
phenotypic processes in angiogenesis, which therefore facilitates
the rehabilitation of patients with focal cerebral ischaemia
(17,18). Thus, HIF-1α induction and
accumulation is a highly promising therapeutic strategy for
cerebral ischaemia.
In the present study, the effect of willed movement
on the motor deficit, cell proliferation, and angiogenesis of rat
brains with focal ischaemia was investigated. The present results
revealed that HIF-1α transcriptional activity is essential for
willed movement to alleviate hypoxia injury. In summary, the
present study demonstrated that HIF-1α is required for willed
movement to contribute to the cerebral rehabilitation of rats after
ischaemic stroke and provides a novel strategy for treating
patients after ischaemic stroke by accumulating HIF-1α.
Materials and methods
Animals
Male Sprague-Dawley rats (n=122, weight, 250–280 g;
age, 60 days) were purchased from the Experimental Animal Centre of
Central South University and were employed in the present study.
These rats were housed in cages at room temperature and had free
access to food and water with 12-h dark/light cycles. All
experiments were performed according to guidelines for the care and
use of animals and were approved by the animal ethics committees of
Central South University.
MCAO model
Focal cerebral ischaemia was induced by middle
cerebral artery occlusion (MCAO) according to Hirayama and Koizumi
(19). In brief, rats were
anesthetized by intraperitoneal injection with 10% chloral hydrate
(300 mg/kg body weight). A mid-line incision in the neck of the rat
was made to expose the common and external carotid arteries and
ligated them. Specifically, the internal carotid artery was closed
by an artery clamp. Subsequently, a 6-0 silicon rubber-coated nylon
monofilament (RWD Life Science Co., Ltd.) was inserted into the
right internal carotid artery. Insertion was stopped after
occlusion for 90 min. The animals were reanaesthetized and
reperfused by withdrawal of the monofilament. After surgery, the
rats were kept for ~2 h in a warm box heated by lamps to maintain
body temperature. Finally, the common carotid artery was
permanently ligated, and the neck wound was closed.
Training design
All rats with neurological deficits were randomly
divided into the following four groups 24 h post-MCAO: i) MCAO,
where rats were housed in standard cages after MCAO (n=6); ii)
willed movement (WM), where rats were housed in a ladder-containing
cage 24 h after MCAO surgery and had to climb the ladder or walls
of the apparatus to reach food and water (n=6); iii) environmental
modification (EM), where rats were housed in the same environment
as the WM group but could get water and food without climbing the
ladder (n=6); iv) common rehabilitation (CR), where rats were
housed in the rolling-grid training cage 24 h after MCAO surgery
and were trained twice a day (9:00 am and 4:00 pm) for 15 min each
time (n=6).
Neurobehavioural assessment
Neurological evaluation was performed on days 0, 3,
7, 15 and 30 after excision using rats with a modified neurological
deficit score (20). The rats were
trained for 15 days before sacrifice, and those that died during
training were excluded. Specifically, A total of 50 rats did not
recover from anaesthesia or died after the MCAO operation. All rats
were randomly allocated to groups for training, as aforementioned.
Neurological scores were defined as follows: 0, no deficit; 1,
flexion of the contralateral forelimb upon lifting the entire
animal by the tail; 2, decrease in thrust towards the contralateral
plane; and 3, circling to the contralateral side.
Triphenyltetrazolium chloride (TTC)
staining
The cerebral infarction area was examined in six
animals per group (n=24 for each replicate, the experiments were
performed three times) for 15 days post-training. Rats were
anaesthetized by intraperitoneal injection with 1% sodium
pentobarbital (40 mg/kg) and decapitated. The whole brains were
frozen at −20°C for 20 min. The tissues were cut into 2-mm-thick
coronal sections and then stained with 2% TTC solution at 37°C for
30 min. The stained slides were immersed with 4% paraformaldehyde
at 4°C overnight. The infarct area on each TTC-stained section was
measured using ImageTool 2.0 software (University of Texas Health
Science Center) and calculated as the infarct area × thickness (2
mm).
Cell lines and primary cerebral
endothelial cell isolation
Human umbilical vein endothelial cells (HUVECs) were
purchased from American Type Culture Collection and grown in
vascular cell basal medium (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 5 ng/ml VEGF (Sigma-Aldrich; Merck KGaA) at 37°C,
5% CO2. For primary cerebral endothelial cell isolation
(3), brains from rats of each
group 30 days post-training were removed and then stored in
Dulbecco's Modified Eagle's Medium/F12 (Gibco; Thermo Fisher
Scientific, Inc.) until further use. The tissues were washed with
PBS several times and minced and incubated with Liberase Blendzyme
2 (Roche Diagnostics GmbH) at a concentration of 0.625 Wu/ml at
37°C for 2 h. Subsequently, the cell suspension was washed with PBS
and centrifuged at 228 × g for 5 min at 25°C. Subsequently, the
cells were plated in dishes coated with collagen type I (BD
Biosciences) and grown in Endothelial Cell Growth Basal Medium
(EBM) complete medium (Lonza Group Ltd). Cells were cultured at
37°C, 5% CO2. After 24 h, non-adherent cells were
removed and seeded into new collagen type I-coated dish with EBM
media. The cultured cells were passaged at a ratio of 1:4 every 14
days. To mimic focal ischemia in vitro, HUVEC were treated
with 500 µM CoCl2 (Shanghai Aladdin Biochemical
Technology Co., Ltd.) treatment for 24 h.
Cell proliferation assay
The isolated primary cerebral endothelial cells from
all four groups were examined via MTT analysis. The cells were
counted and plated into 96-well plates for 24 h at 37°C. Then 0.1
mg/ml MTT was added to cells at 37°C for 3 h. The cells were lysed
in DMSO at room temperature for 30 min. Finally, the absorbance was
measured at 490 nm by a microplate reader (Bio-Tek China).
In vitro vascular tube formation
assay
To examine the ability of endothelial tube formation
in vitro, 24-well plates were coated with 10 µl of Matrigel
(BD Biosciences) as previously described (21) and 2×104 HUVECs or
primary cerebral endothelial cell were seeded. The tube length was
measured using MetaMorph Microscopy Automation and Image Analysis
Software (version 1.0; Molecular Devices, LLC). The images were
taken and processed by an inverted microscope. In total, >5
randomly selected fields of view were analyzed.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted using TRIzol (Thermo Fisher
Scientific, Inc.). Briefly, the cells were lysed with TRIzol
buffer, and then 200 µl chloroform (100%) was added to the mixture,
which was vortexed for 15 sec at 25°C. The resulting solution was
centrifuged at 17,366 × g for 15 min at 4°C. The supernatant was
harvested and mixed with an equivalent volume of isopropanol. The
resultant mixture was centrifuged at 17,366 × g for 10 min at 4°C.
The supernatant was removed and 75% ethanol was added to wash the
pellet and centrifuged at 17,366 × g for 10 min at 4°C. Ethanol was
discarded and the pellet was left to dry, then used 20–30 µl
DEPC-treated H2O (Invitrogen; Thermo Fisher Scientific,
Inc.) to elute the RNA pellet.
Total RNA (1 µg) was used to perform reverse
transcription according to the manufacturer's protocol (PrimeScript
Kit; Takara Bio, Inc.). For quantitative PCR, SYBR Green
(QuantiFast SYBR Green PCR Kit; Qiagen GmbH) was used as the probe
dye and the signal was detected according to the manufacturer's
protocol. GAPDH was used as the internal control. The following
primers were used: HIF-1α-Forward, CGCAAGTCCTCAAAGCACAG and
HIF-1α-Reverse, TCTGTTTGGTGAGGCTGTCC; VEGF-Forward,
TTTCTGCTGTCTTGGGTGCA and VEGF-Reverse, CCAGGGTCTCGATTGGATGG;
Ang-1-Forward, GCCTGATCTTACACGGTGCT and Ang-1-Reverse,
GCCTGATCTTACACGGTGCT; MMP2-Forward, GATCTACTCAGCCAGCACCC and
MMP2-Reverse, ACGACGGCATCCAGGTTATC; MMP9-Forward,
TACTCGACCTGTACCAGCGA and MMP9-Reverse, ATGCCATTCACGTCGTCCTT;
GAPDH-Forward, GAGTCAACGGATTTGGTCGT and GAPDH-Reverse,
TTGATTTTGGAGGGATCTCG. The thermocycling conditions were the
following: Initial PCR activation step at 95°C for 15 min, followed
by 40 cycles of denaturation at 94°C for 15 sec, annealing at 53°C
for 30 sec, and elongation at 72°C for 30 sec. Cq values were used
for quantification using the 2−∆∆Cq method (22).
Western blotting
In total, 1×106 cells of each group were
harvested and washed with 1×PBS once. Subsequently, 2×SDS loading
buffer (Dalian Meilun Biotech Co., Ltd.) was used to lyse cells.
The lysates were boiled at 95°C for 10 min and centrifuged at
16,172 × g for 1 min at 4°C. Approximately 50 µg of total protein
was loaded onto a 10% SDS-polyacrylamide gel and resolved at 120 V
for 0.5–1 h at 25°C. Subsequently, proteins were transferred onto
polyvinylidene fluoride membranes at 300 mA for 2–3 h at 4°C. The
membrane was blocked with 5% non-fat milk in 1×TBS with 0.1% Tween
(TBST) for 1 h at 25°C, then incubated with the corresponding
primary antibodies at 4°C overnight. The following day, the
membrane was washed with 1×TBST 3 times and incubated with
secondary antibodies at room temperature for 1 h. Finally, the
membrane was incubated with enhanced chemiluminescence reagent
(7Sea Biotech) and exposed using the Bio-Rad ChemiDoc Touch Imaging
System. The following antibodies were used in the present study:
Anti-HIF-1α (1:1,000; cat. no. ab51608; Abcam), anti-VEGF (1:1,000;
cat. no. ab52917; Abcam), anti-Ang-1 (1:1,000; cat. no. ab95230;
Abcam), anti-MMP-2 (1:1,000; cat. no. 87809; Cell Signaling
Technology), anti-MMP-9 (1:1,000; cat. no. 3852; Cell Signaling
Technology Inc.). The secondary antibody horseradish
peroxidase-conjugated goat anti-rabbit immunoglobulin G (cat. no.
A0208; 1:5,000; Beyotime Institute of Biotechnology) was incubated
with the membranes at room temperature for 1 h.
Small interference (si)RNA
transfection
SiRNAs (scrambled and siHIF-1α) were synthesized by
GenePharma and diluted to 20 µM as stock. To transfect cells,
siRNAs were diluted to 50 nM and were mixed with RNAiMAX reagent
(Thermo Fisher Scientific, Inc.) for 20 min at 25°C, and the
mixture was added to cultured cells. The sequence of siHIF-1α was
GCTCCCAATGTCGGAGTTT. Cells were harvested 48 h after
transfection.
Statistical analysis
GraphPad Prism (version 6.0; GraphPad Software,
Inc.) was used for statistical analysis. Each experiment was
performed three times, and all values are presented as the mean ±
standard deviation. Comparison of two groups was performed using
the two-tailed unpaired Student's t-test. Comparison of multiple
groups was performed using one-way analysis of variance followed by
Tukey's post-hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
CR, WM and EM reduce motor
deficit
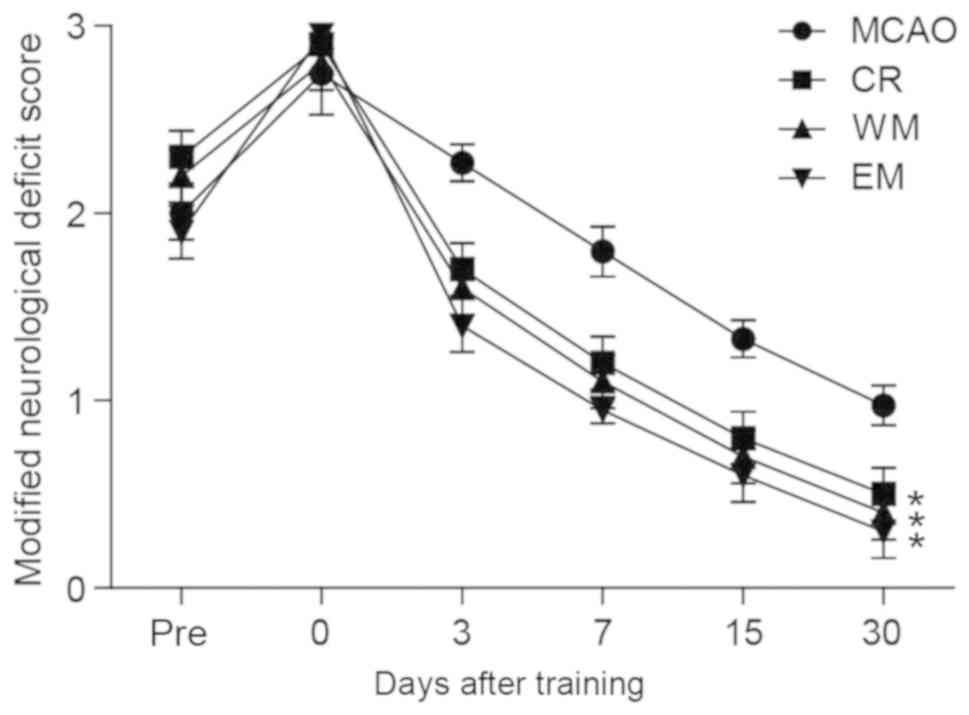
The neurological defect score in the MCAO, CR, WM
and EM groups was measured first (n=6 per group). The results
showed that rats in the CR, WM and EM groups exhibited a
significantly lower score compared with the MCAO group. However,
the groups CR, WM, and EM did not display significant differences
from each other (Fig. 1).
Willed movement improves neurological
rehabilitation of brains with ischaemia
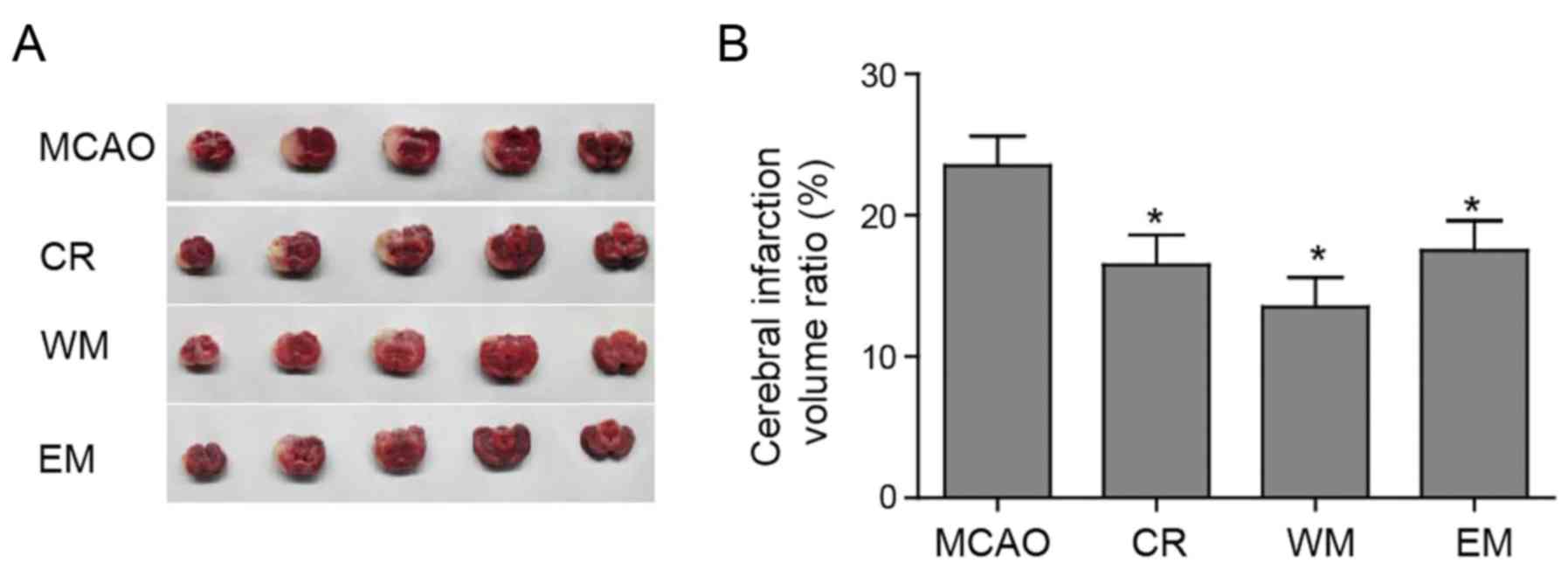
To investigate whether willed movement therapy
decreased the focal ischaemia damage, TTC staining of cerebral
tissue was performed to assess the infarctions. Representative
tissues correspond to different coronary sections per group as
presented in Fig. 2A. The
unstained areas in Fig. 2A are
infarcted tissues, and the red areas are normal tissues (Fig. 2A). In the four groups, different
volumes of infarcted tissue areas were observed. Compared with the
MCAO group, the CR, WM and EM groups displayed statistically
significant decreases in the volume of cerebral infarcted tissue
(Fig. 2B). The volume of cerebral
infarction tissue in the WM group was not significantly lower than
the other two training groups (Fig.
2B). In addition, there was no significant difference between
CR and EM group.
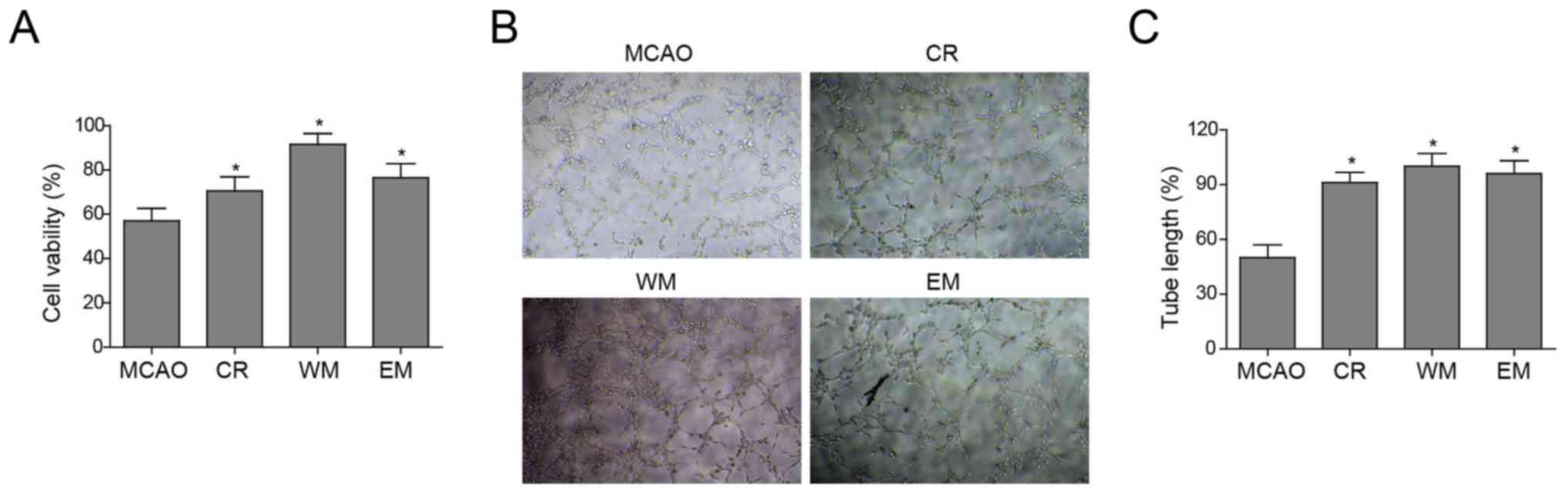
Willed movement promotes cell survival
and angiogenesis of ischaemic endothelial cells
To investigate the molecular mechanism by which the
three forms of exercise improved neurological rehabilitation,
primary cerebral endothelial cells were isolated from rats
subjected to CR, WM and EM and cultured. Subsequently, the MTT
method was performed to examine cell viability in the MCAO, CR, WM
and EM groups. The endothelial cells of the MCAO group showed the
lowest viability (Fig. 3A).
To investigate whether these therapies improved tube
formation of cerebral endothelial cells, cells of the CR, WM and EM
groups possessed a stronger ability for tube formation (evaluated
by total tube length) in vitro compared with the MCAO group.
Cells of the CR, WM and EM groups showed no significant differences
from each other (Fig. 3B). The
tube length of vessels formed in the MCAO group was half of that in
the other three groups, while no length disparity appeared between
the WM, EM and CR groups (Fig.
3C). Taken together, these results demonstrated that CR, EM,
and WM increased cell viability and enhanced angiogenesis of
cerebral endothelial cells compared with the MCAO group.
As angiogenesis has been associated with some
classical genes (12), and
angiogenesis-associated gene expression levels in the MCAO, CR, WM
and EM groups were investigated next. RT-qPCR was employed to
detect the mRNA expression levels of VEGF, Ang-1, MMP-2 and MMP-9
in cerebral endothelial cells of all four groups. The CR, EM and WM
groups showed elevated mRNA expression levels of all four genes
compared with MCAO (Fig. 4A).
Additionally, the protein expression levels of these four
angiogenesis genes were also investigated. Notably, the protein
expression levels of these genes only increased in the WM group
compared with the MCAO group, whereas the CR and EM groups showed
no difference in their expression levels (Fig. 4B), suggesting that the protein
expression level was regulated at the post-transcriptional
level.
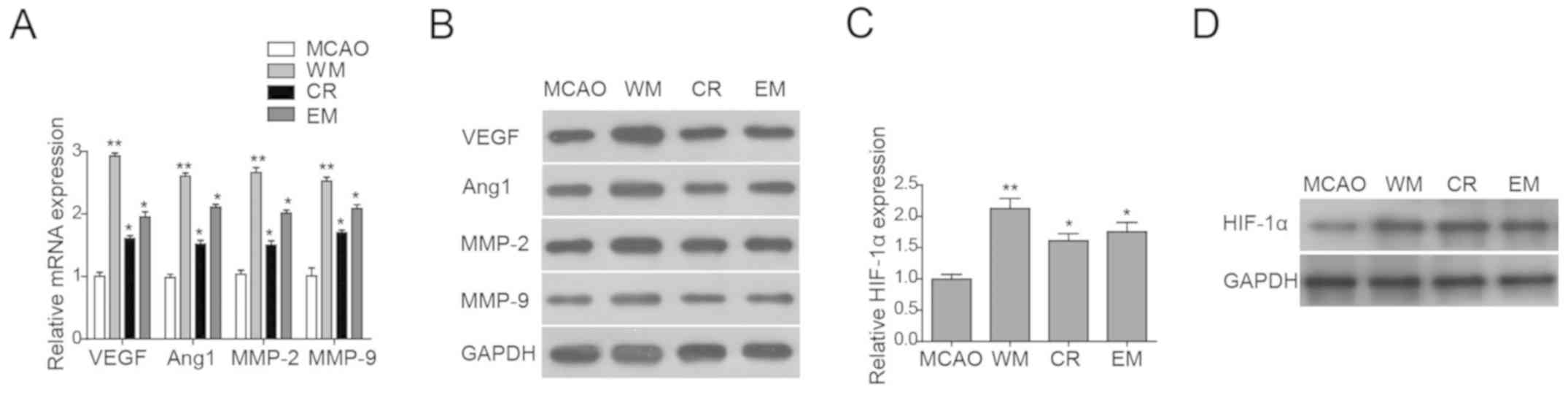 | Figure 4.Willed movement upregulates HIF-1α and
angiogenesis-associated genes. (A) RT-qPCR analysis and (B) western
blot analysis of angiogenesis-associated genes in primary cerebral
vascular endothelial cells isolated from all four groups of rats.
(C) RT-qPCR analysis of HIF-1α and (D) western blot analysis of
HIF-1α in primary cerebral vascular endothelial cells isolated from
all four groups of rats. Data are presented as the mean ± standard
deviation. *P<0.05, **P<0.01 vs. MCAO. Ang, angiogenin; CR,
common rehabilitation; EM, environmental modification; HIF,
hypoxia-inducible factor; MCAO, middle cerebral artery occlusion;
MMP, matrix metalloproteinase; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; VEGF,
vascular endothelial growth factor; WM, willed movement. |
Willed movement upregulates
HIF-1α
HIF-1α has an important role in angiogenesis and
endothelial cell survival (18).
The expression levels of HIF-1α in endothelial cells of the MCAO,
CR, WM and EM groups were investigated. RT-qPCR and western blot
analyses were performed to determine the HIF-1α mRNA and protein
expression levels, respectively. HIF-1α mRNA and protein expression
levels were higher in endothelial cells of the CR, WM and EM groups
compared with the MCAO (Fig. 4C and
D). In conclusion, the present study suggested that CR, WM and
EM may promote cell proliferation and angiogenesis and upregulation
of angiogenesis-associated genes and HIF-1α. However, the direct
effects of CR, WM and EM on angiogenesis and cell proliferation
require further investigation.
Hypoxia induces
angiogenesis-associated genes and HIF-1α expression
To mimic focal ischaemia in vitro, we
generated a HUVEC-based hypoxia model using CoCl2
treatment in culture. First, whether the expression of VEGF, Ang-1,
MMP-2 and MMP-9 was affected in hypoxic cells was investigated by
RT-qPCR and western blot analysis. It was found that these genes
were induced by hypoxia (Fig. 5A and
B). HIF-1α was gradually upregulated when cells were treated by
hypoxia and peaked at 12 h after hypoxia treatment (Fig. 5C and D). Taken together, the
present results suggested that hypoxia upregulated the expression
levels of HIF-1α and its downstream genes.
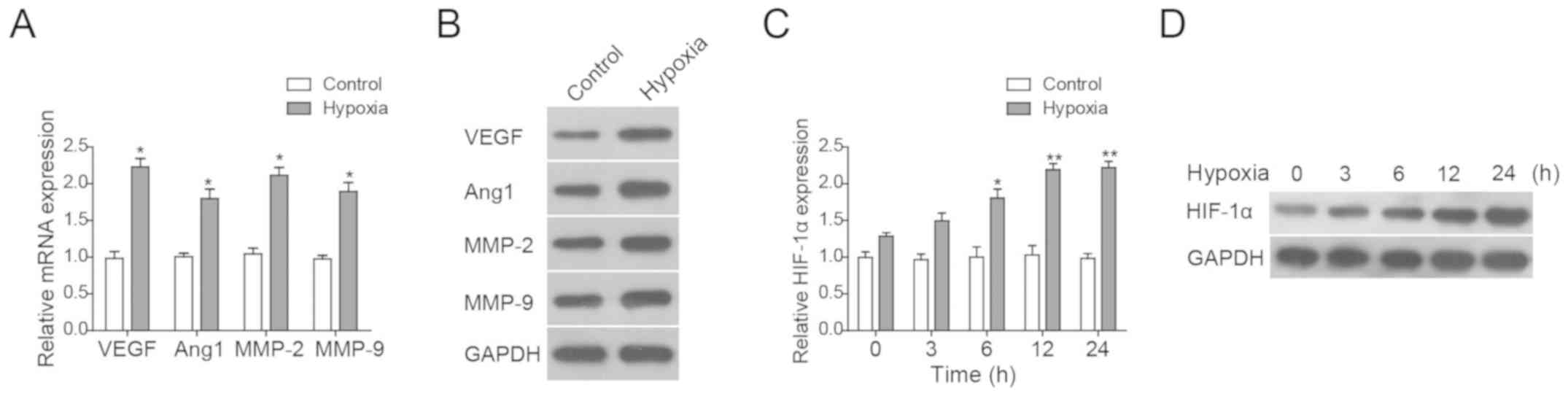 | Figure 5.Hypoxia induces
angiogenesis-associated genes and HIF-1α. (A) RT-qPCR analysis and
(B) western blot analysis of angiogenesis-associated molecules in
HUVECs under normoxic and hypoxic conditions. (C) RT-qPCR analysis
and (D) western blot analysis of HIF-1α in HUVECs under normoxic
and hypoxic conditions. All data are presented as the mean ±
standard deviation. *P<0.05, **P<0.01 vs. MCAO. Ang,
angiogenin; CR, common rehabilitation; EM, environmental
modification; HIF, hypoxia-inducible factor; MCAO, middle cerebral
artery occlusion; MMP, matrix metalloproteinase; RT-qPCR, reverse
transcription-quantitative polymerase chain reaction; VEGF,
vascular endothelial growth factor; WM, willed movement. |
HIF-1α is necessary for
hypoxia-induced angiogenesis of HUVECs
To confirm the role of HIF-1α in angiogenesis of
cerebral endothelial cells, HIF-1α-knockdown HUVECs using siRNA
against HIF-1α was constructed. RT-qPCR analysis showed the mRNA
expression levels of HIF-1α was notably reduced in HIF-1α-knockdown
HUVECs compared with the negative control (Fig. 6A). Subsequently, the effect of
HIF-1α on hypoxia-induced angiogenesis was investigated. The
results demonstrated that angiogenesis was potentiated in cells
under hypoxic conditions. However, HIF-1α depletion reduced the
in vitro tube formation in cells under hypoxia and
significantly shortened the tube length (Fig. 6B). Together, these data
demonstrated that HIF-1α is essential for hypoxia-enhanced
angiogenesis of cerebral endothelial cells. Finally, whether HIF-1α
depletion impacted on angiogenesis-associated gene expression
levels under hypoxic conditions was investigated. The results
showed that hypoxia induced the expression of VEGF, Ang-1, MMP-2
and MMP-9 at the mRNA and protein levels, whereas HIF-1α knockdown
reversed the increased expression of these genes (Fig. 6C and D). Collectively, these
findings demonstrated that HIF-1α serves a key role in in
vitro tube formation of endothelial cells under hypoxic
conditions, but this needs to be verified with future studies.
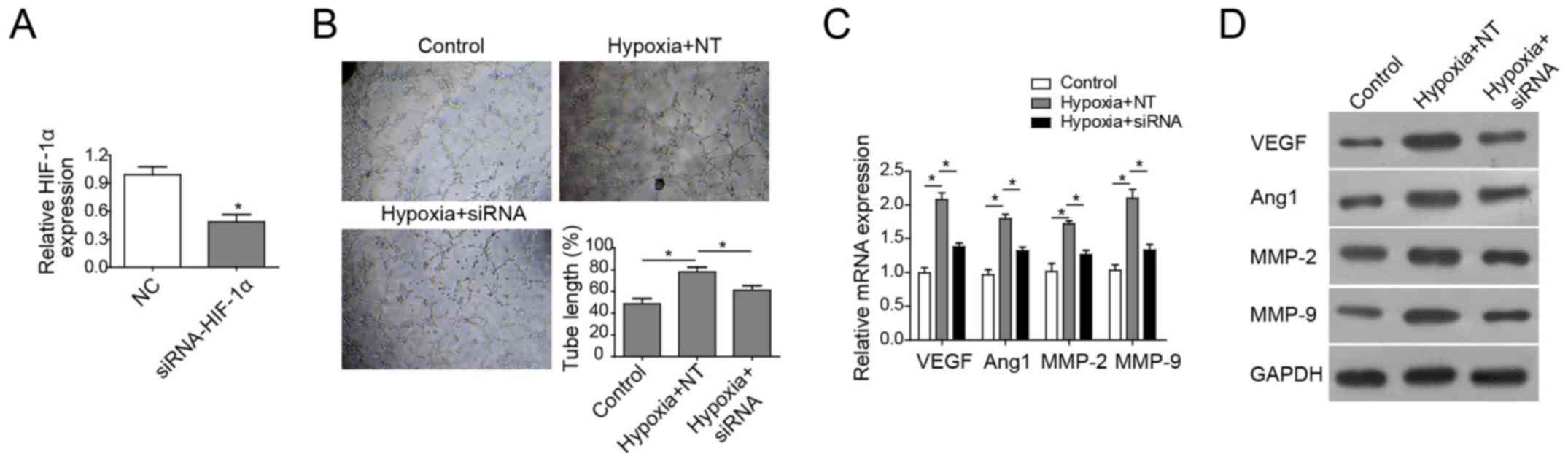 | Figure 6.HIF-1α is necessary for
hypoxia-induced angiogenesis in HUVECs. (A) RT-qPCR analysis of
HIF-1α in HUVECs transfected with scrambled siRNA or siRNA against
HIF-1α. (B) In vitro vascular tube formation assay of HUVECs
under normoxia and hypoxia. Cells were transfected with scrambled
siRNA or siRNA against HIF-1α. (C) RT-qPCR analysis and (D) western
blot analysis of angiogenesis-associated genes in HUVECs
transfected with scrambled or siRNA against HIF-1α. All data are
presented as the mean ± standard deviation. *P<0.05 vs. control.
Ang, angiogenin; CR, common rehabilitation; EM, environmental
modification; HIF, hypoxia-inducible factor; MCAO, middle cerebral
artery occlusion; MMP, matrix metalloproteinase; NC, normal
control; RT-qPCR, reverse transcription-quantitative polymerase
chain reaction; si, small interference; VEGF, vascular endothelial
growth factor; WM, willed movement; NT, negative control siRNA;
siRNA, small interfering RNA. |
Discussion
In the present study, exercise therapies,
particularly willed movement, were protective interventions in a
rat model of ischaemic stroke. Specifically, neurological behaviour
assessment and TTC staining showed that willed movement
significantly attenuated the injury caused by focal cerebral
ischaemia and reperfusion. Furthermore, the three therapies used in
the present study, promoted proliferation and in vitro tube
formation of brain vascular endothelial cells via upregulation of
HIF-1α and its downstream genes, including VEGF, Ang-1, MMP-2 and
MMP-9. In human umbilical vein endothelial cells (HUVECs), it was
demonstrated that HIF-1α induced the expression of VEGF, Ang-1,
MMP-2 and MMP-9 under hypoxic conditions. The present findings
suggest that willed movement may contribute to angiogenesis of
endothelial cells by upregulating HIF-1α, but this needs to be
verified in the future.
In stroke research, the key goal is to prevent
neuronal cell death and improve recovery (23). However, few therapeutic targets
have emerged. HIF-1α serves an important role in neurological
outcomes after ischaemic stroke via regulation of its downstream
genes that promote cell survival and angiogenesis (24). Previous studies have shown that
HIF-1α is induced in ischaemic brains (25). HIF-1α prevents apoptosis via
blockage of cytochrome c release, PARP cleavage and caspase
activation (24,26). Furthermore, HIF-1α can suppress p53
activation and thus maintain cell survival (27). However, Baranova et al
(28) and Helton et al
(29) reported that HIF-1α exerts
distinct roles in neuronal injuries following ischaemia. Baranova
et al (28) found that
HIF-1α knockdown increased tissue damage and decreased the survival
rate of MCAO rats, which indicated HIF-1α was neuroprotective. By
contrast, Helton et al (29) observed HIF-1α knockout alleviated
ischaemic damage, indicating HIF-1α may induce tissue damage in the
ischaemic brain. One explanation for this discrepancy could be that
Baranova used a mild ischaemic model (30 min ischaemia with
unilateral common carotid artery occlusion), whereas Helton used a
severe ischaemic model (75 min ischaemia with bilateral occlusion).
Together, the two studies support the idea that HIF-1α may lead to
cell death in severe ischaemia and promote cell survival after mild
ischaemic insults.
Willed movement training has been suggested to
upregulate some genes (such as, synaptophysin, AMPA and PICK1) that
are involved in synaptic plasticity and transmission in the brain
of rats undergoing MCAO (2,8,12,13).
However, the detailed molecular mechanism of how willed movement
training induces the expression of these genes remains unclear. In
the present study, WM upregulated HIF-1α and induced its target
angiogenesis-associated genes. Additionally, the mechanism of
willed movement-activated HIF-1α expression also requires further
investigation. To address this question, whether intracellular
signaling pathways are activated by willed movement and thus
activate the transcription of HIF-1α need to be investigated. To
systemically identify targets essential for HIF-1α-affected
rehabilitation of patients after ischaemic stroke is another issue,
which can be addressed by ChIP-seq and RNA-seq approaches.
In summary, the present findings emphasized the role
of willed movement in promoting HIF-1α expression, which in turn
enhanced angiogenesis and improved neurological rehabilitation of
brains with focal ischaemia. The present findings provide novel
insights into the mechanism of the effect of hypoxia on ischaemic
stroke and suggest HIF-1α accumulation as a strategy for treating
patients following stroke. However, all experiments were performed
in vitro and in rat cerebral tissue, therefore whether
similar results can be obtained using WM to patients remains to be
investigated.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ZZ conceived the study, and performed data and
statistical analysis. LZ and ZZ conceived and designed the study.
XR performed the literature search, experimental studies and
clinical studies. WZ performed data acquisition and edited the
manuscript. LZ drafted the manuscript. All authors read and
approved the final manuscript, revising it critically for important
intellectual content.
Ethics approval and consent to
participate
All animal experiments were performed according to
guidelines for the care and use of animals and were approved by the
animal ethics committees of Central South University.
Patient consent for publication
Not applicable.
Competing interests
Authors declare that they have no competing
interests.
References
|
1
|
Zhang JH, Li JK, Ma LL and Lou JY: RNA
interference-mediated silencing of S100B improves nerve function
recovery and inhibits hippocampal cell apoptosis in rat models of
ischemic stroke. J Cell Biochem. 119:8095–8111. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Nie J, Yang X, Tang Q, Shen Q and Li S:
Willed-movement training reduces brain damage and enhances synaptic
plasticity related proteins synthesis after focal ischemia. Brain
Res Bull. 120:90–96. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Navone SE, Marfia G, Nava S, Invernici G,
Cristini S, Balbi S, Sangiorgi S, Ciusani E, Bosutti A, Alessandri
G, et al: Human and mouse brain-derived endothelial cells require
high levels of growth factors medium for their isolation, in vitro
maintenance and survival. Vasc Cell. 5:102013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yu QJ, Tao H, Wang X and Li MC: Targeting
brain microvascular endothelial cells: A therapeutic approach to
neuroprotection against stroke. Neural Regen Res. 10:1882–1891.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Guell K and Bix GJ: Brain endothelial cell
specific integrins and ischemic stroke. Exp Rev Neurother.
14:1287–1292. 2014. View Article : Google Scholar
|
|
6
|
Waterland JC: The supportive framework for
willed movement. Am J Phys Med. 46:266–279. 1967.PubMed/NCBI
|
|
7
|
Tang Q, Yang Q, Hu Z, Liu B, Shuai J, Wang
G, Liu Z, Xia J and Shen X: The effects of willed movement therapy
on AMPA receptor properties for adult rat following focal cerebral
ischemia. Behav Brain Res. 181:254–261. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tang Q, Tan L, Yang X, Shen Q, Huang X,
Wang G, Chen H, Nie J, Li S and Wu L: Willed-movement training
reduces motor deficits and induces a PICK1-dependent LTD in rats
subjected to focal cerebral ischemia. Behav Brain Res. 256:481–487.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Volk L, Kim CH, Takamiya K, Yu Y and
Huganir RL: Developmental regulation of protein interacting with C
kinase 1 (PICK1) function in hippocampal synaptic plasticity and
learning. Proc Natl Acad Sci USA. 107:21784–21789. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Clem RL, Anggono V and Huganir RL: PICK1
regulates incorporation of calcium-permeable AMPA receptors during
cortical synaptic strengthening. J Neurosci. 30:6360–6366. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Moretti A, Ferrari F and Villa RF:
Pharmacological therapy of acute ischaemic stroke: Achievements and
problems. Pharmacol Ther. 153:79–89. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thirusangu P, Vigneshwaran V, Ranganatha
VL, Vijay Avin BR, Khanum SA, Mahmood R, Jayashree K and Prabhakar
BT: A tumoural angiogenic gateway blocker, Benzophenone-1B
represses the HIF-1α nuclear translocation and its target gene
activation against neoplastic progression. Biochem Pharmacol.
125:26–40. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lu Y, Wang B, Shi Q, Wang X, Wang D and
Zhu L: Brusatol inhibits HIF-1 signaling pathway and suppresses
glucose uptake under hypoxic conditions in HCT116 cells. Sci Rep.
6:391232016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sharp FR and Bernaudin M: HIF1 and oxygen
sensing in the brain. Nat Rev Neurosci. 5:437–448. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hirota K and Semenza GL: Regulation of
angiogenesis by hypoxia-inducible factor 1. Crit Rev Oncol Hematol.
59:15–26. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sun Y, He W and Geng L: Neuroprotective
mechanism of HIF-1α overexpression in the early stage of acute
cerebral infarction in rats. Exp Ther Med. 12:391–395. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Phillips PG, Birnby LM and Narendran A:
Hypoxia induces capillary network formation in cultured bovine
pulmonary microvessel endothelial cells. Am J Physiol.
268:L789–L800. 1995.PubMed/NCBI
|
|
18
|
Krishnamachary B, Berg-Dixon S, Kelly B,
Agani F, Feldser D, Ferreira G, Iyer N, LaRusch J, Pak B, Taghavi P
and Semenza GL: Regulation of colon carcinoma cell invasion by
hypoxia-inducible factor 1. Cancer Res. 63:1138–1143.
2003.PubMed/NCBI
|
|
19
|
Hirayama Y and Koizumi S:
Hypoxia-independent mechanisms of HIF-1α expression in astrocytes
after ischemic preconditioning. Glia. 65:523–530. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li Y, Xu L, Zeng K, Xu Z, Suo D, Peng L,
Ren T, Sun Z, Yang W, Jin X and Yang L: Propane-2-sulfonic acid
octadec-9-enyl-amide, a novel PPARα/γ dual agonist, protects
against ischemia-induced brain damage in mice by inhibiting
inflammatory responses. Brain, Behav Immun. 66:289–301. 2017.
View Article : Google Scholar
|
|
21
|
Kuo CH, Chen PK, Chang BI, Sung MC, Shi
CS, Lee JS, Chang CF, Shi GY and Wu HL: The recombinant lectin-like
domain of thrombomodulin inhibits angiogenesis through interaction
with Lewis Y antigen. Blood. 119:1302–1313. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chassagnon IR, McCarthy CA, Chin YK,
Pineda SS, Keramidas A, Mobli M, Pham V, De Silva TM, Lynch JW,
Widdop RE, et al: Potent neuroprotection after stroke afforded by a
double-knot spider-venom peptide that inhibits acid-sensing ion
channel 1a. Proc Natl Acad Sci USA. 114:3750–3755. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Piret JP, Lecocq C, Toffoli S, Ninane N,
Raes M and Michiels C: Hypoxia and CoCl2 protect HepG2 cells
against serum deprivation- and t-BHP-induced apoptosis: A possible
anti-apoptotic role for HIF-1. Exp Cell Res. 295:340–349. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhang L, Luo X, Chen F, Yuan W, Xiao X,
Zhang X, Dong Y, Zhang Y and Liu Y: LncRNA SNHG1 regulates
cerebrovascular pathologies as a competing endogenous RNA through
HIF-1α/VEGF signaling in ischemic stroke. J Cell Biochem.
119:5460–5472. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sasabe E, Tatemoto Y, Li D, Yamamoto T and
Osaki T: Mechanism of HIF-1alpha-dependent suppression of
hypoxia-induced apoptosis in squamous cell carcinoma cells. Cancer
Sci. 96:394–402. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Li J, Zhang X, Sejas DP, Bagby GC and Pang
Q: Hypoxia- induced nucleophosmin protects cell death through
inhibition of p53. J Biol Chem. 279:41275–41279. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Baranova O, Miranda LF, Pichiule P,
Dragatsis I, Johnson RS and Chavez JC: Neuron-specific inactivation
of the hypoxia inducible factor 1 alpha increases brain injury in a
mouse model of transient focal cerebral ischemia. J Neurosci.
27:6320–6332. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Helton R, Cui J, Scheel JR, Ellison JA,
Ames C, Gibson C, Blouw B, Ouyang L, Dragatsis I, Zeitlin S, et al:
Brain-specific knock-out of hypoxia-inducible factor-1alpha reduces
rather than increases hypoxic-ischemic damage. J Neurosci.
25:4099–4107. 2005. View Article : Google Scholar : PubMed/NCBI
|