Introduction
Hyperuricemia is a common metabolic disease caused
by a disorder of purine metabolism in humans (1). In the majority of mammals, uric acid
(UA) is converted to the more soluble form allantoin by urate
oxidase (Uox), which is removed from the body. However, the
Uox gene has undergone mutational silencing during the
evolution of humans and hominoid apes; thus, humans are vulnerable
to hyperuricemia (2).
Hyperuricemia has been a global public health concern with an
increasing incidence and prevalence in recent years (3,4).
Hyperuricemia is a major risk factor for gout (5). In total, 10–20% patients with
hyperuricemia may progress to gout (5). In addition, studies have suggested
that hyperuricemia may also contribute to the development and
pathogenesis of metabolic and systemic pathologic diseases,
including diabetes, metabolic syndrome, hypertension,
atherosclerosis and chronic kidney disease (6,7).
The intestine is the primary organ responsible for
the clearance of UA and one third of UA in the blood is removed
through the intestines (8,9). Certain patients with hyperuricemia
present with gastrointestinal symptoms; however, the precise
effects of hyperuricemia on the intestines remain unclear. A
previous study showed that hyperuricemia is associated with an
increased risk of colorectal carcinoma (10). Additionally, it was documented that
supplementing exogenous UA via an intra-intestinal injection
induces the influx of heterophils into the intestines of rabbits
(11). Nevertheless, supplementing
exogenous UA in experimental animals within a short time does not
mimic human hyperuricemia adequately as the majority of cases of
hyperuricemia in humans are sustained for at least a few years. In
our previous study, a mouse model of hyperuricemia was established
by knocking out the Uox gene (12). The Uox-knockout (KO) mice
spontaneously developed hyperuricemia (serum UA concentration >7
mg/dl) and survived ≤62 weeks (12). Therefore, we employed this model to
investigate the effects of hyperuricemia effects on the intestines.
In the present study, intestinal barrier function and intestinal
permeability was assessed in the hyperuricemia mouse model, and the
serum and intestinal tissue levels of UA, tumor necrosis factor
(TNF)-α and interleukin (IL)-6, as well as serum uremic toxins were
measured. We aimed to investigate intestinal barrier dysfunction in
a mouse model of hyperuricemia, and further understand the
underlying mechanisms.
Materials and methods
Animal model
In our previous study, a hyperuricemia mouse model
was generated by knocking out the Uox gene using the
transcription activator-like effector nuclease technique (12). The male Uox-KO mice and
their wild-type (WT) counterparts (n=8 in each group) were used in
the present study. The mice from 4 weeks (weight, 13–15 g) to 24
weeks (weight, 28–32 g) after birth were maintained individually in
a temperature-controlled room at 22–24°C and 40–60% relative
humidity under a 12-h light/dark cycle with ad libitum
access to food and water. Blood and urine samples of all the
animals were collected in the morning. The blood samples were
centrifuged at 1,511 × g for 15 min at 4°C to obtain the serum. The
mice were euthanized at 24 weeks after birth using CO2
with 25% volume displacement per minute, and mortality was
confirmed by observing respiration and by using the corneal
reflection method. The intestines were harvested, and a section of
the intestinal tissues were cut and washed with cold normal saline
to remove blood; after drying on filter papers at room temperature,
the intestinal samples were weighed, then immediately homogenized
on ice. The homogenates were centrifuged at 5,000 × g for 10 min at
4°C to obtain the supernatants. All procedures were performed with
the approval of The Animal Research Ethics Committee of The
Affiliated Hospital of Qingdao University (approval no.
AHQU20140612A).
Measurement of UA
Serum UA levels were analyzed in the mice from 4 to
24 weeks, and the levels of UA in the urine and the supernatant of
the intestinal homogenate were measured at 24 weeks of age. The
levels of UA in serum, urine and supernatant were measured using a
UA determination kit R-i/ii (Beijing Leadman Biochemistry Co.,
Ltd.). The detection process was completed with an automatic
biochemical analyzer (Toshiba Corporation, Minato, Japan) according
to the manufacturer's protocols.
Histological examination
Intestinal samples were fixed by immersion in 10%
neutral formalin at room temperature for 48 h, and subsequently
embedded in paraffin. The paraffin block of tissue was sliced into
4-µm-thick sections, and stained with hematoxylin and eosin for 5
min at room temperature. Alcian blue staining was performed as
described previously; the sections were stained with Alcian blue
for 30 min at room temperature (13). Pathological changes in the
intestine were observed under a light microscope (magnification,
×400; Axiovert 200; Zeiss AG) and evaluated by an experienced
pathologist.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from intestinal tissue using
RNAiso Plus (Takara Bio, Inc.) and was reverse-transcribed into
cDNA using a PrimeScript RT reagent kit with gDNA Eraser (Takara
Bio, Inc.), according to the manufacturer's protocols. qPCR
reaction was performed in a 25 µl reaction volume, using TB Green
Premix Ex Taq reagent (Takara Bio, Inc.) using a fluorescent PCR
device (CFX96, Bio-Rad Laboratories, Inc.). The thermocycling
conditions were: i) 95°C For 30 sec; and ii) 40 cycles of 95°C for
5 sec and 60°C for 30 sec. The primer sequences were as follows:
Zona occludens-1 (ZO-1) forward, 5′CCACCTCTGTCCAGCTCTTC-3′,
reverse, 5′CACCGGAGTGATGGTTTTCT-3; Occludin forward,
5′CCTCCAATGGCAAAGTGAAT-3′, reverse, 5′CTCCCCACCTGTCGTGTAGT-3, and
β-actin forward, 5′CTCCCTGGAGAAGAGCTATGA-3 and reverse,
5′GGCATAGAGGTCTTTACGGATG-3′. All samples were run in triplicate in
a single 96-well reaction plate. The relative expression levels
were analyzed using the 2−ΔΔCq method (14,15).
Immunohistochemistry detection of
tight junction proteins
Immunohistochemistry for ZO-1 and occludin in
intestinal tissue was performed as described previously (16). Briefly, the intestinal tissues from
the Uox-KO mice and the WT mice were fixed and embedded in
paraffin as aforementioned, and 4-µm sections were cut from the
paraffin blocks. The sections were dewaxed in xylene (Beijing
Zhongshan Golden Bridge Biotechnology Co., Ltd.; OriGene
Technologies, Inc.) and antigen retrieval was performed using 10
mmol/l citrate buffer (pH 6.0; Beijing Zhongshan Golden Bridge
Biotechnology Co., Ltd.; OriGene Technologies, Inc.) at 120°C for 3
min. Endogenous peroxidases activity were inhibited with 3%
hydrogen peroxide for 10 min at room temperature. Then, the
sections were incubated at 4°C overnight using rabbit anti-ZO-1
(1:400; cat. no. bs-1329R; BIOSS Biotech Co., Ltd) and rabbit
anti-occludin (1:300; cat. no. A2601; ABclonal Biotech Co., Ltd.).
As a negative control, sections were incubated with PBS alone.
Subsequently, the sections were incubated with a goat-anti-rabbit
immunoglobulin G secondary antibody (1:500; cat. no. PV9001;
Beijing Zhongshan Golden Bridge Biotechnology Co., Ltd.; OriGene
Technologies, Inc.) at room temperature for 1 h and stained with
diaminobenzidine (OriGene Technologies, Inc.) at room temperature
for 5 min. The sections were counterstained with hematoxylin at
room temperature for 5 min and analysis was conducted with an
optical microscope (magnification, ×400). The immunoreactive
positive signals appeared brown.
Western blotting analysis of tight
junction proteins
Western blotting was performed as previously
described (17). Briefly, total
proteins were extracted from intestinal tissues using a protein
lysis buffer (50 mM Tris-HCl, 150 mM NaCl, 1% Triton X-100, 0.5%
sodium deoxycholate, 0.1% SDS; Applygene) and the protein
concentration was measured using a bicinchoninic acid assay kit
(Pierce; Thermo Fisher Scientific, Inc.). A total of 30 µg of
protein from each sample were resolved using a 10% SDS-PAGE gel and
transferred to a polyvinylidene difluoride membrane (EMD
Millipore), and blocked using 5% nonfat milk at room temperature
for 1 h. Membranes were incubated with primary antibodies at 4°C
overnight: Rabbit anti-ZO-1 (1:1,000; cat. no. 5406, Cell Signaling
Technology, Inc.), rabbit anti-occludin (1:1,000; cat. no. A2601;
ABclonal Biotech Co., Ltd.) and anti-GAPDH (1:1,000; cat. no. 8884;
Cell Signaling Technology, Inc.). Subsequently, the membranes were
incubated at 4°C with a horseradish peroxidase-conjugated secondary
antibody (1:10,000; cat. no. 7074; Cell Signaling Technology, Inc.)
for 1 h. An FR-980 Biological Image Analysis System (Furi) was used
to detect immunoreactive bands and densitometry analysis was
performed using ImageJ (version 1.8.0; National Institutes of
Health).
Quantification of intestinal
permeability
Diamine oxidase (DAO), D-lactate (D-LAC), and
endotoxin levels in the serum are commonly regarded as indirect
indicators of intestinal permeability (18). The concentrations of serum DAO,
D-LAC and endotoxin were determined using ELISA kits (DAO ELISA
Kit, cat. no. CSB-E10090m, Cusabio Technology LLC; D-lactate ELISA
Kit, cat. no. JL48176, Shanghai Future Industrial Limited By Share
Ltd.; endotoxin ELISA Kit, cat. no. CSB-E13066m, Cusabio Technology
LLC), according to the manufacturer's protocols.
Analysis of serum uremic toxins
The levels of serum creatinine (SCR) and blood urea
nitrogen (BUN) in serum samples (200 ul) were measured using an
automatic biochemical analyzer (Toshiba Corporation, Minato, Japan)
to assess kidney function. The detection of indoxyl sulfate (IS)
and p-cresol sulfate (PCS) levels in the serum were performed using
a mouse indoxyl sulfate ELISA Kit and p-cresol sulfate ELISA Kit
(cat. no. JL44175 and cat. no. JL48074, Shanghai Future Industrial
Limited By Share Ltd.) according to the manufacturer's
protocols.
ELISA measurement of cytokines in the
serum and intestinal tissues
The concentrations of TNF-α and IL-6 in the serum
and intestinal tissues were quantified using the mouse TNF-α ELISA
kit and mouse IL-6 ELISA kit, respectively (cat. nos. RK00027 and
RK00008, ABclonal Biotech Co., Ltd.) according to the
manufacturer's protocols.
Statistical analysis
All data were statistically analyzed using SPSS 21
(IBM Corp.) and GraphPad Prism 6 (GraphPad Software, Inc.). All
values were presented as the mean ± standard error of the mean.
Experiments were performed in triplicate. Quantitative data was
assessed using independent-sample t-tests or one-way analysis of
variance followed by a Tukey's post-hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
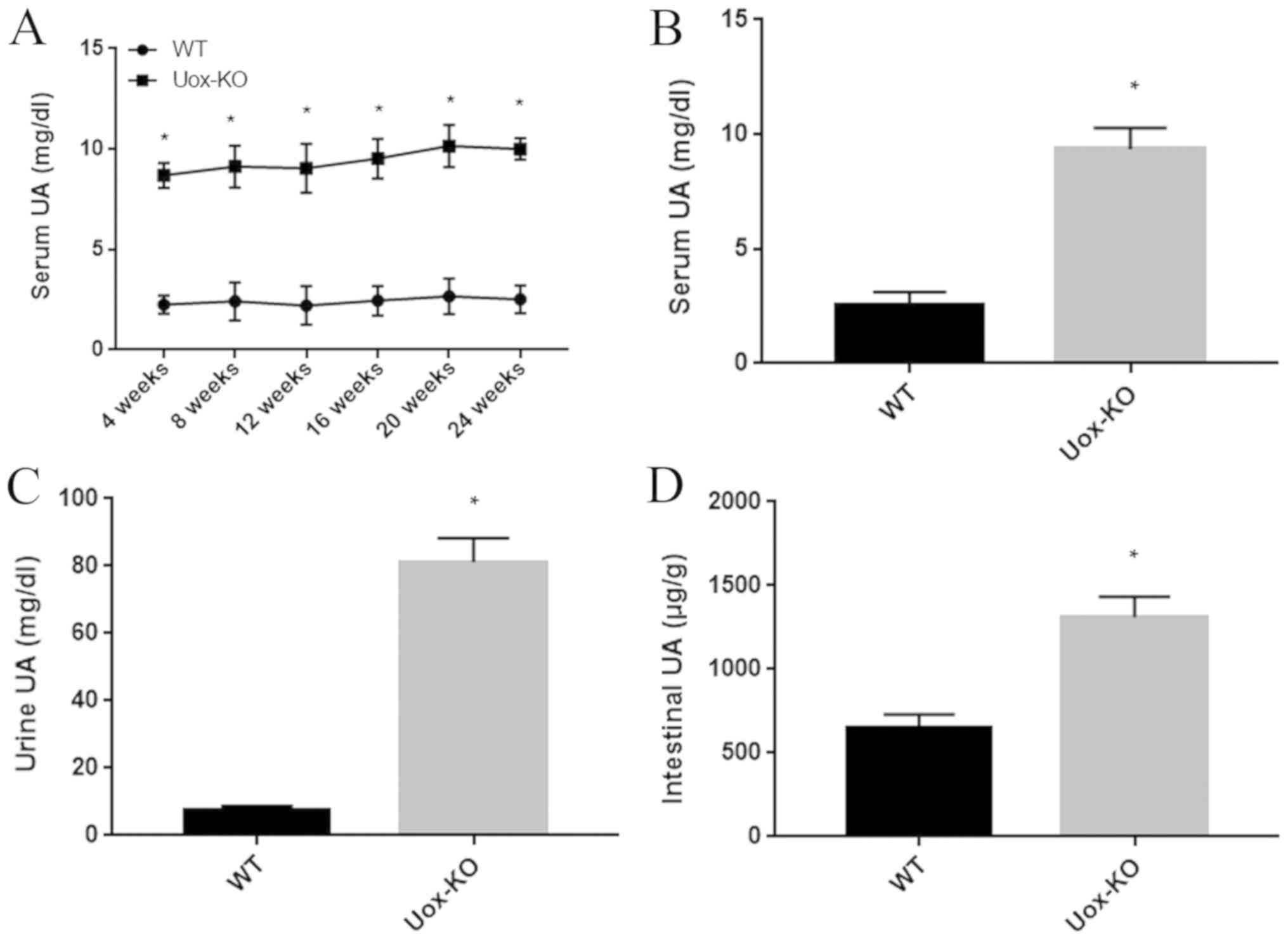
UA levels are elevated in the serum,
urine and intestinal tissues of the Uox-KO mice
The Uox-KO mice had significantly elevated
serum UA levels between 8.89–10.15 mg/dl; however, the levels
appeared to be stable and 3–4 fold higher compared with WT mice
(Fig. 1A and B). The urinary UA
concentration in Uox-KO mice was ~80 mg/dl, ~10 fold higher
compared with the WT littermate controls (Fig. 1C). The UA concentration in the
intestinal tissues was ~1,310 µg/g in the hyperuricemic mice, which
was significantly increased than that of the control group
(Fig. 1D).
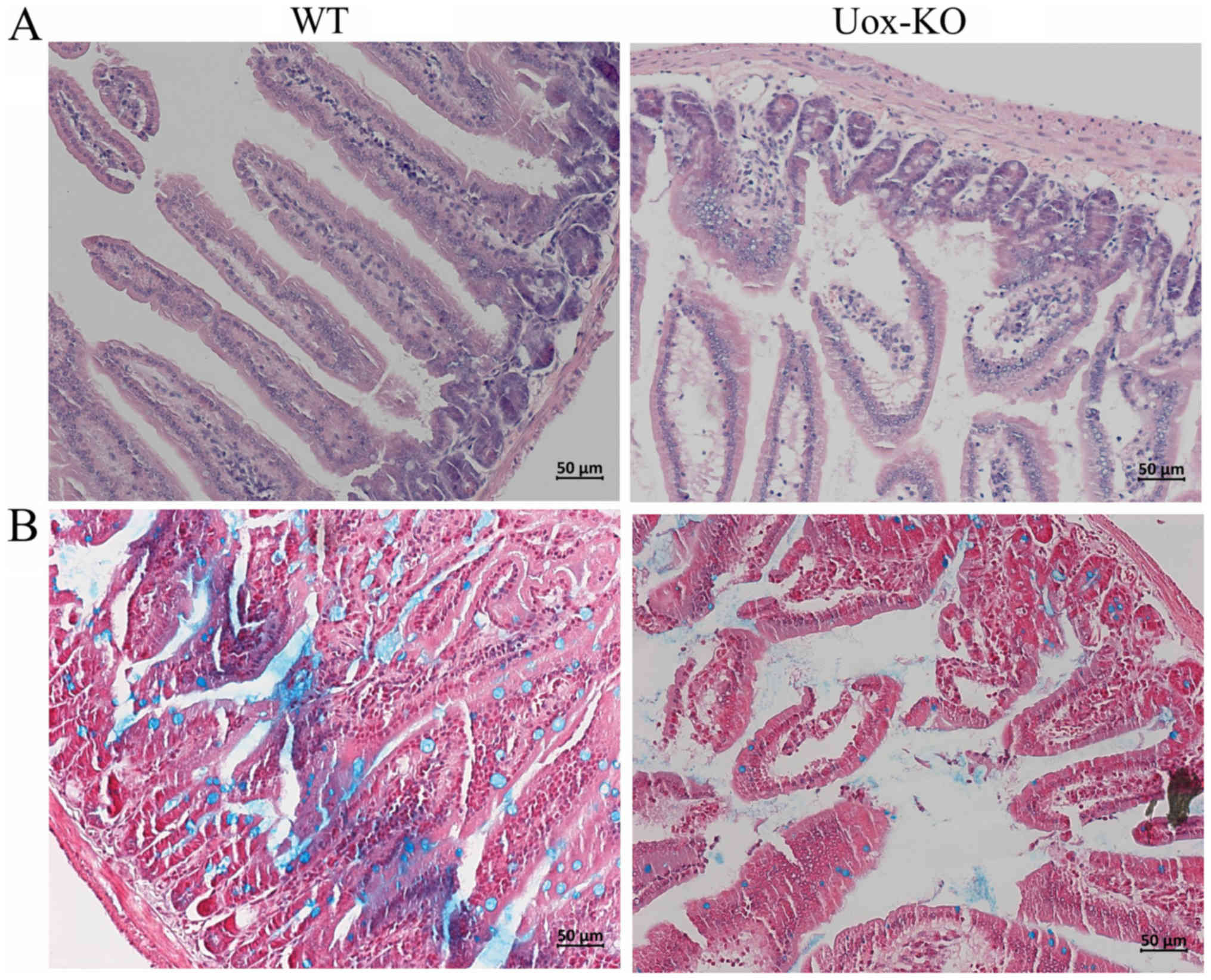
Histological observation of intestinal
damage in the Uox-KO mice
Using light microscopy, regional epithelial
shedding, fewer and fractured villi in the intestine of the
Uox-KO mice were observed. Furthermore, mucosal and
submucosal edema were observed in the Uox-KO mice.
Conversely, the intestinal epithelial structure was intact, and no
notable pathological changes were detected in the controls
(Fig. 2A). Additionally,
Uox-KO mice exhibited a notable reduction in mucins when
compared with the WT mice as determined using alcian blue staining
(Fig. 2B). These histological
results suggested that the structure of the intestinal epithelium
was disrupted in Uox-KO mice.
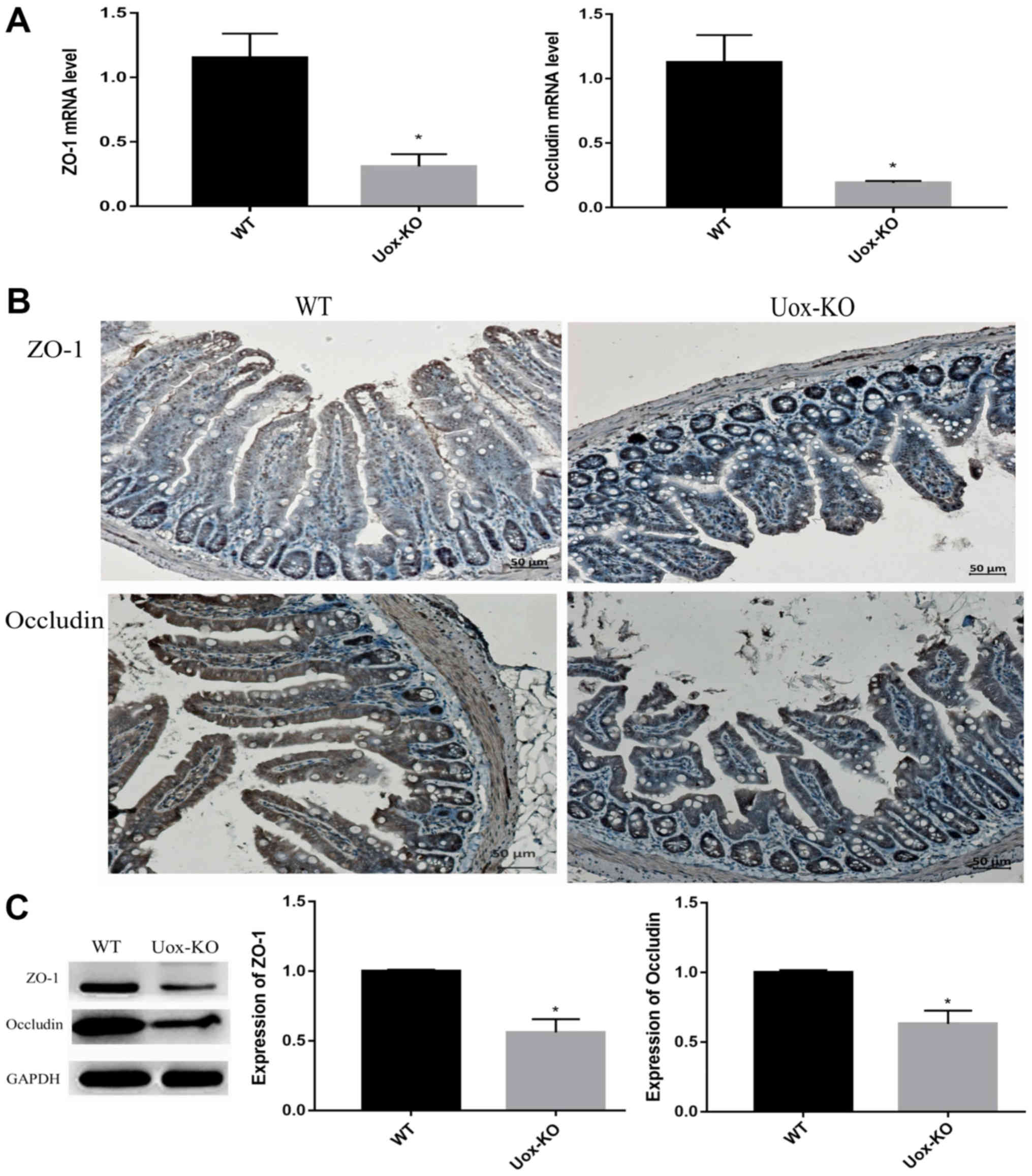
Expression of tight junction proteins
is downregulated in Uox-KO mice
ZO-1 and occludin are considered to be the most
important tight junction proteins, and contribute to the intestinal
mucosal barrier function (19).
Therefore, the mRNA and protein expression levels of ZO-1 and
occluding were measured in the intestinal tissues. The results of
RT-qPCR revealed that the mRNA expression levels of ZO-1 and
occluding were significantly downregulated in the Uox-KO
mice compared with the control (Fig.
3A). Consistent with the mRNA expression results, the protein
expression levels of ZO-1 and occluding in the intestinal tissues
were also downregulated in the Uox-KO mice compared with the
control as determined by western blotting (Fig. 3C). Immunohistochemistry
demonstrated that the degree of positive staining for ZO-1 and
occludin observed in the Uox-KO mice was markedly reduced
compared with the control (Fig.
3B).
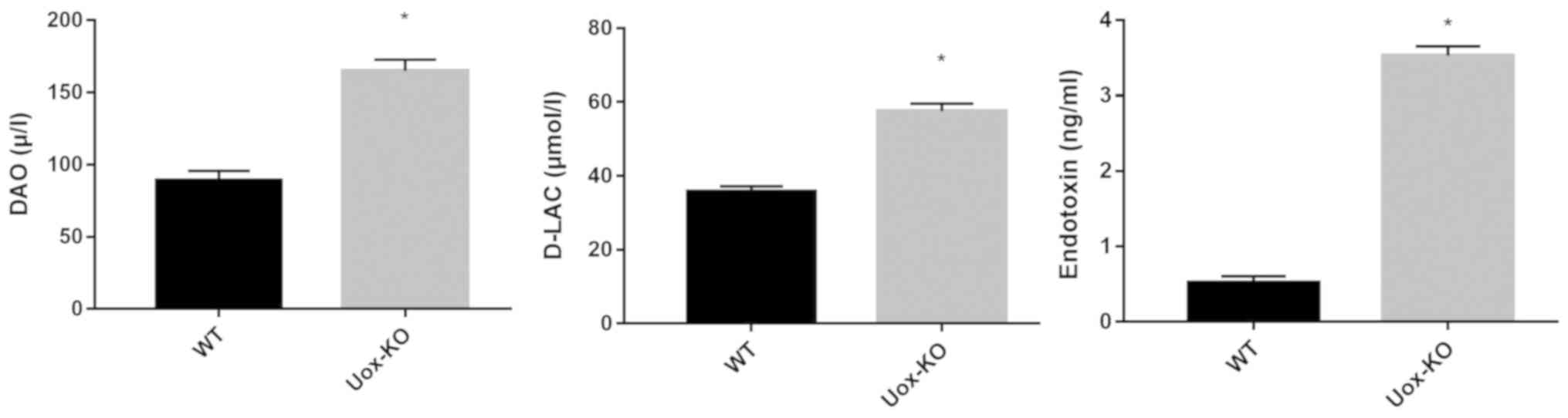
Intestinal permeability is increased
in the Uox-KO mice
Impairments in the intestinal barrier were
determined by intestinal permeability. Serum DAO, D-lactate and
endotoxin levels were measured as markers of intestinal
permeability. The results revealed that the serum levels of DAO,
D-LAC and endotoxin were significantly increased in the
Uox-KO mice compared with the WT mice (P<0.05; Fig. 4).
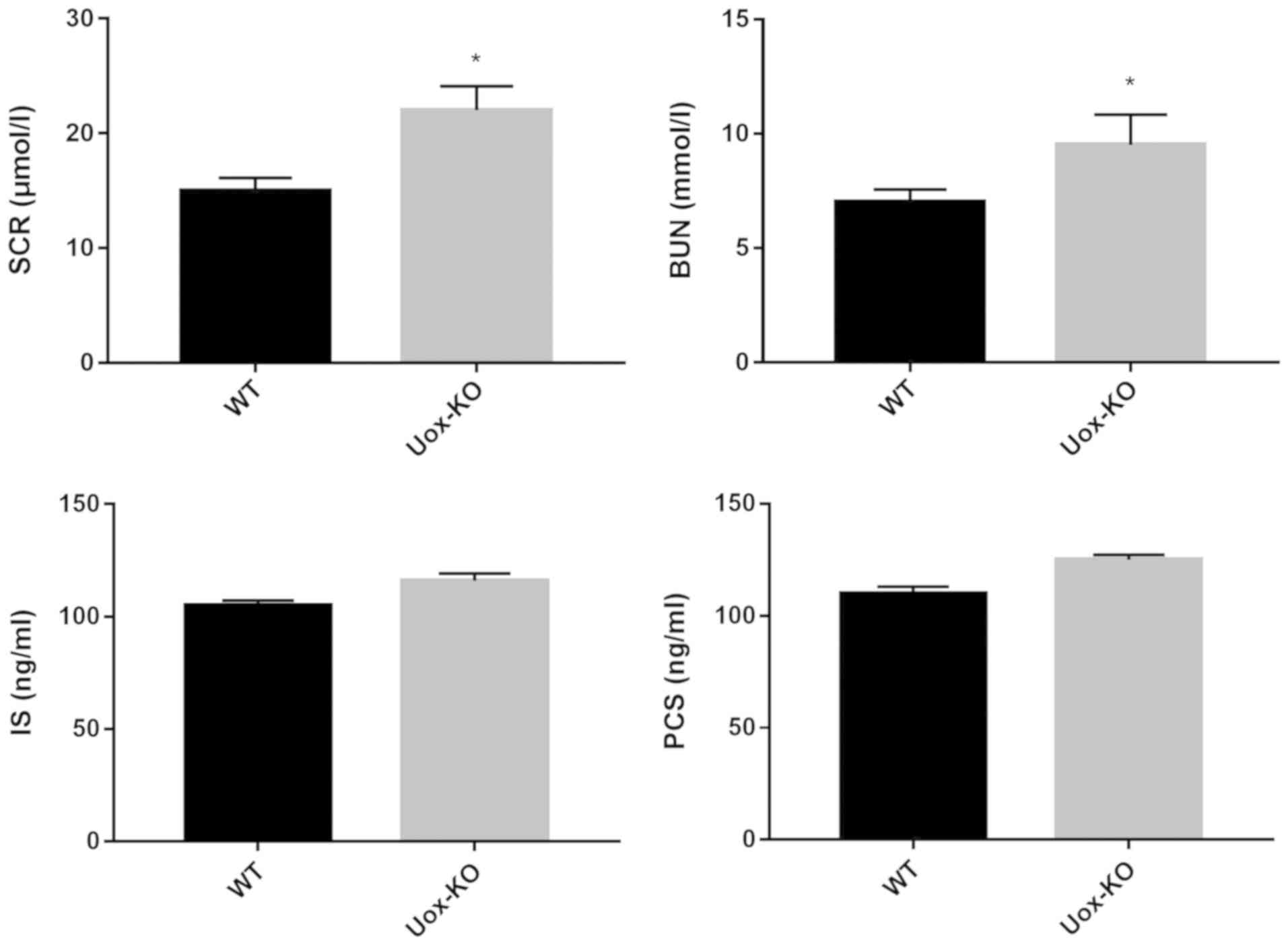
Levels of uremic toxin and cytokines
are increased in the Uox-KO mice
To determine the potential underlying mechanism of
intestinal barrier dysfunction in hyperuricemia, the serum uremic
levels and cytokine levels were measured in the Uox-KO and
WT mice. As presented in Fig. 5,
the serum uremic toxins levels of SCR and BUN were significantly
increased in Uox-KO mice compared with the control, whereas
the levels of IS and PCS exhibited marked increases in the
Uox-KO mice (Fig. 5). Thus,
it is unlikely that the extent of damage observed in the
hyperuricemic mice resulted from the slight increases in IS and
PCS.
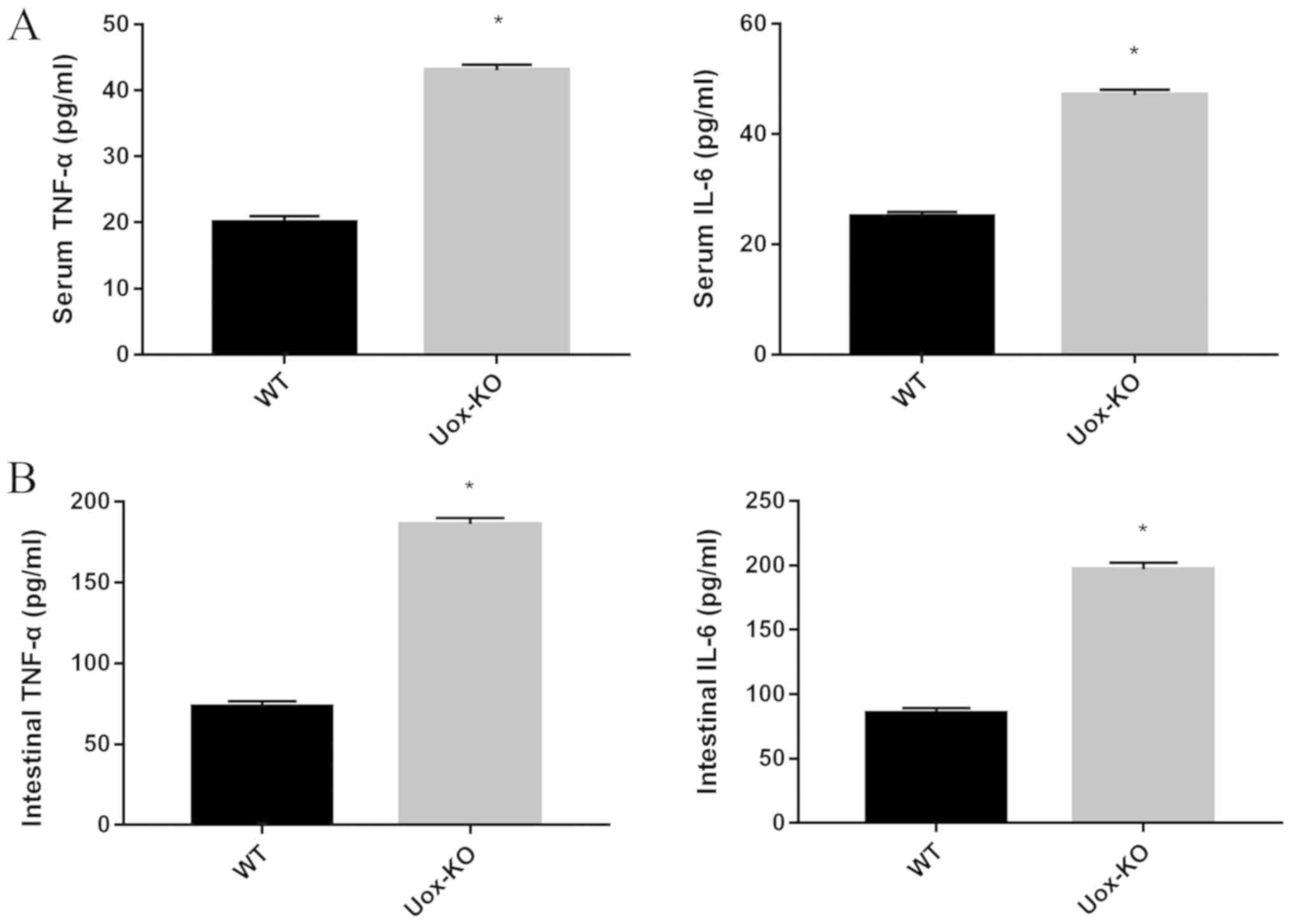
Inflammatory cytokines are known to contribute to
intestinal damage (20). The
levels of TNF-α and IL-6 in the serum and the intestinal
homogenates were significantly higher in the Uox-KO mice
compared with the WT mice (Fig.
6). The data indicated that the intestinal mucosal barrier
damage in the hyperuricemic mice may be associated with the
induction of the inflammatory response.
Discussion
Numerous studies have confirmed that elevated serum
UA levels contribute to the damage of multiple organs, including
the kidneys and heart, and joints (5–7).
Furthermore, a recent study indicated that UA has a role in
impairment of the integrity of vascular endothelial cells (21). Notably, the structure and
physiological characteristics between the vascular and intestinal
epithelium are homologous and similar; however, few studies have
addressed the possible disruptive effects of hyperuricemia on the
intestine. In the present study, the Uox-KO (hyperuricemic)
mice exhibited impairments in the intestinal barrier, and
pathological changes in the intestines were observed, including
sparse intestinal villi, mucosal and submucosal edema, suggesting
that hyperuricemia may have interfered with the epithelial
integrity of the intestine, contributing to intestinal damage. The
findings of the present study may improve current knowledge
regarding the intestinal damage caused by hyperuricemia; however,
damage to other organs under these conditions requires further
investigation.
Hyperuricemic mice models are generally constructed
by promoting purine synthesis, inhibiting UA degradation and
supplementing exogenous UA, either alone or in combination
(22,23). However, the UA levels in these
models usually fluctuate by a large margin (23). Therefore, these models may not
suited for mimicking hyperuricemia in humans. In the present study,
a mouse model of hyperuricemia was established by knocking out the
Uox gene. Our previous study revealed that these mice
exhibited a stably increased level of UA and had developed the
symptoms of compromised renal function, hypertension, aberrant
glycometabolism and lipometabolism, which are similar to those
observed in patients with hyperuricemia (12). In the present study, impaired
intestinal barrier function was also observed in these gene
deficient mice. Collectively, these data suggest that the mouse
model may be a more suitable model investigating hyperuricemia and
its related complications.
The importance of the intestinal epithelium for
excretion has been demonstrated (24). Approximately one-third of the
circulating UA is cleared via urate transporters in the intestinal
enterocytes (25). The intestinal
epithelium and the apical junctional complex constitute the
intestinal barrier, a dynamic interface between the internal and
external environments, which serves a crucial role in the
maintenance of intestinal homeostasis (26,27).
In the present study, intestinal epithelial damage was observed in
Uox-KO mice as detected by histological examination. Tight
junctions between the intestinal epithelial cells are involved in
the maintenance of the intestinal mucosal barrier function
(28). ZO-1 and occludin are
considered to be two of the primary tight junction proteins
(19). In the present study, a
significant decrease in the expression of ZO-1 and occluding in the
intestine of Uox-KO mice was reported compared with the
control. The mucus layer on the epithelial surface is also an
essential component of the intestinal barrier (29). The thickness of the mucus layer was
also decreased in hyperuricemic mice compared with the WT mice, as
determined by alcian blue staining. These results suggested that
intestinal mucosal barrier function may have been damaged in the
hyperuricemic mice.
Impairments in the intestinal mucosal barrier
manifest as increased intestinal permeability (30,31).
DAO is an intracellular enzyme in the intestinal epithelium,
whereas D-LAC and endotoxin are bacterial metabolites produced by
the intestinal flora (32). When
the permeability of the intestine is abnormally increased as a
result of some sort of disruption, DAO, D-LAC and endotoxin in the
lumen easily pass through the intestinal mucosa and into the
peripheral blood (32,33). In the present study, increases in
the serum levels of DAO, D-LAC and endotoxins were observed in the
hyperuricemic mice compared with the controls, suggesting enhanced
intestinal permeability in the hyperuricemic mice.
The present study suggested that the intestinal
barrier was damaged and intestinal permeability was increased in
the hyperuricemia mouse model; however, the exact mechanism
underlying injury had not been determined. Previous studies
demonstrated a high concentration of uremic toxins, including IS,
PCS and advanced glycation end products could result in the
destruction of the intestinal barrier structure in end-stage renal
disease (34–36). Additionally, chronic renal failure
induced by 5/6 nephrectomy results in increased levels of uremic
toxins which damages the intestinal mucosal barrier in rats
(37). Our previous study
indicated that kidney impairment was similarly detected in the
hyperuricemic Uox-KO mice, in which collapsed and necrotic
nephrons and focal tubulointerstitial fibrosis were observed
(12). In order to determine
whether intestinal injury was caused by kidney damage in the
hyperuricemic mice, the levels of uremic toxins, such as IS and PCS
in the serum were measured. The results indicated that their serum
levels were markedly increased in the hyperuricemic group compared
with the controls. The effects of notably increased uremic toxin
levels on intestinal damage may not fully account for the observed
intestinal injury in these models.
Numerous studies have demonstrated that the
increased production of inflammatory cytokines has a negative
impact on intestinal mucosal barrier and epithelial function
(20,38). For example, Xin et al
(39) showed that impaired
intestinal barrier in a rat model of chronic obstructive pulmonary
disease was associated with the intestinal inflammatory response,
in which elevated levels of the cytokines interferon-γ, TNF-α and
IL-8 were observed. In vitro experiments have also revealed
that TNF-α may result in structural changes to the tight junctions
by decreasing occludin and ZO-1 expression (40). In humans, patients with
hyperuricemia were found to have higher levels of inflammatory
cytokine levels in the serum compared with the healthy controls
(41). Similar results were
observed in rabbits, in which the addition of exogenous UA to the
intestinal lumen promoted an intestinal inflammatory reaction
(11). In the present study,
elevated production of the inflammatory cytokines TNF-α and IL-6 in
the serum and intestinal homogenates were detected in the
hyperuricemic mice. These results suggest there was a positive
association between the increased levels of inflammatory cytokines
and intestinal epithelial tight junction dysfunction. Therefore,
hyperuricemia may affect intestinal barrier function through the
initiation of inflammatory responses in the intestine. Further
studies are required to determine the precise mechanism underlying
intestinal mucosal barrier damage in hyperuricemic mice.
In conclusion, the present study demonstrated the
presence of a damaged intestinal barrier and enhanced intestinal
permeability in a mouse model of hyperuricemia. The impaired
intestinal barrier function may be associated with the inflammatory
process induced by UA. The findings of the present study may
provide novel insight into organ damage a consequence of
hyperuricemia.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81502025), the
Natural Science Foundation of Shandong Province (grant no.
ZR2018MH004) and China's Post-doctoral Science Fund (grant no.
2018M632631).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YG and ZT designed the study. YG, HL and YY
performed the experiments. HL, ZL, CL, YC and CJ contributed to
data analysis. YG and HL drafted the manuscript. YG, YY, and ZT
revised the manuscript. All authors have given approval to the
final version of the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Animal
Research Ethics Committee of the Affiliated Hospital of Qingdao
University and the approval number was AHQU20140612A.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Mandal AK and Mount DB: The molecular
physiology of uric acid homeostasis. Annu Rev Physiol. 77:323–345.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Álvarez-Lario B and Macarrón-Vicente J:
Uric acid and evolution. Rheumatology. 49:2010–2015. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Liu R, Han C, Wu D, Xia X, Gu J, Guan H,
Shan Z and Teng W: Prevalence of hyperuricemia and gout in mainland
China from 2000 to 2014: A systematic review and meta-analysis.
Biomed Res Int. 2015:7628202015. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Trifiro G, Morabito P, Cavagna L,
Ferrajolo C, Pecchioli S, Simonetti M, Bianchini E, Medea G,
Cricelli C, Caputi AP and Mazzaglia G: Epidemiology of gout and
hyperuricaemia in Italy during the years 2005–2009: A nationwide
population-based study. Ann Rheum Dis. 72:694–700. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Robinson PC: Gout-an update of aetiology,
genetics, co-morbidities and management. Maturitas. 118:67–73.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Johnson RJ, Bakris GL, Borghi C, Chonchol
MB, Feldman D, Lanaspa MA, Merriman TR, Moe OW, Mount DB and
Sanchez Lozada LG: Hyperuricemia, acute and chronic kidney disease,
hypertension, and cardiovascular disease: Report of a scientific
workshop organized by the national kidney foundation. Am J Kidney
Dis. 71:851–865. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bonakdaran S and Kharaqani B: Association
of serum uric acid and metabolic syndrome in type 2 diabetes. Cur
Diabetes Rev. 10:113–117. 2014. View Article : Google Scholar
|
|
8
|
Yun Y, Yin H, Gao Z, Li Y, Gao T, Duan J,
Yang R, Dong X, Zhang L and Duan W: Intestinal tract is an
important organ for lowering serum uric acid in rats. PLoS One.
12:e01901942017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu X, Li C, Zhou P and Jiang T: Uric acid
transporters hiding in the intestine. Pharm Biol. 54:3151–3155.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fini MA, Elias A, Johnson RJ and Wright
RM: Contribution of uric acid to cancer risk, recurrence and
mortality. Clin Transl Med. 1:162012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Crane JK and Mongiardo KM:
Pro-inflammatory effects of uric acid in the gastrointestinal
tract. Immunol Invest. 43:255–266. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lu J, Hou X, Yuan X, Cui L, Liu Z, Li X,
Ma L, Cheng X, Xin Y, Wang C, et al: Knockout of the urate oxidase
gene provides a stable mouse model of hyperuricemia associated with
metabolic disorders. Kidney Int. 93:69–80. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lindén SK, Florin TH and McGuckin MA:
Mucin dynamics in intestinal bacterial infection. PLoS One.
3:e39522008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Bustin SA, Benes V, Garson JA, Hellemans
J, Huggett J, Kubista M, Mueller R, Nolan T, Pfaffl MW, Shipley GL,
et al: The MIQE guidelines: Minimum information for publication of
quantitative real-time PCR experiments. Clin Chem. 55:611–622.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Dvorak K, Higgins A, Palting J, Cohen M
and Brunhoeber P: Immunohistochemistry with Anti-BRAF V600E (VE1)
mouse monoclonal antibody is a sensitive method for detection of
the BRAF V600E mutation in colon cancer: Evaluation of 120 cases
with and without KRAS mutation and literature review. Pathol Oncol
Res. 25:349–359. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang Y, Yang F, Xue J, Zhou X, Luo L, Ma
Q, Chen YF, Zhang J, Zhang SL and Zhao L: Antischistosomiasis liver
fibrosis effects of chlorogenic acid through IL-13/miR-21/Smad7
signaling interactions in vivo and in vitro. Antimicrob Agents
Chemother. 61(pii): e01347–16. 2017.PubMed/NCBI
|
|
18
|
Zhao Y, Qin GX, Sun Z, Che D, Bao N and
Zhang X: Effects of soybean agglutinin on intestinal barrier
permeability and tight junction protein expression in weaned
piglets. Int J Mol Sci. 12:8502–8512. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Song C and Zhao X: Uric acid promotes
oxidative stress and enhances vascular endothelial cell apoptosis
in rats with middle cerebral artery occlusion. Biosci Rep. 38(pii):
BSR201709392018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Shen L: Tight junctions on the move:
Molecular mechanisms for epithelial barrier regulation. Ann NY Acad
Sci. 1258:9–18. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang H, Zhang Q, Niu Y, Zhang X and Lu R:
Surface-layer protein from Lactobacillus acidophilus NCFM
attenuates tumor necrosis factor-α-induced intestinal barrier
dysfunction and inflammation. Int J Biol Macromol. 136:27–34. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Morisaki T: Animal models for abnormal
purine metabolism. Nihon Rinsho. 61 (Suppl 1):S482–S486. 2003.(In
Japanese).
|
|
23
|
Zhu Y, Peng X and Ling G: An update on the
animal models in hyperuricaemia research. Clin Exp Rheumatol.
35:860–864. 2007.
|
|
24
|
Sorensen LB: Role of the intestinal tract
in the elimination of uric acid. Arthritis Rheum. 8:694–706. 1965.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Matsuo H, Tsunoda T, Ooyama K, Sakiyama M,
Sogo T, Takada T, Nakashima A, Nakayama A, Kawaguchi M, Higashino
T, et al: Hyperuricemia in acute gastroenteritis is caused by
decreased urate excretion via ABCG2. Sci Rep. 6:310032016.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ruan Z, Liu S, Zhou Y, Mi S, Liu G, Wu X,
Yao K, Assaad H, Deng Z, Hou Y, et al: Chlorogenic acid decreases
intestinal permeability and increases expression of intestinal
tight junction proteins in weaned rats challenged with LPS. PLoS
One. 9:e978152014. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xu R, Lei Y, Shi J, Zhou YJ, Chen YW and
He ZJ: Effects of lactadherin on plasma D-lactic acid and small
intestinal MUC2 and claudin-1 expression levels in rats with
rotavirus-induced diarrhea. Exp Ther Med. 11:943–950. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Marchiando AM, Shen L, Graham WV, Edelblum
KL, Duckworth CA, Guan Y, Montrose MH, Turner JR and Watson AJ: The
epithelial barrier is maintained by in vivo tight junction
expansion during pathologic intestinal epithelial shedding.
Gastroenterology. 140:1208–1218.e1-e2. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Swidsinski A, Loening-Baucke V, Theissig
F, Engelhardt H, Bengmark S, Koch S, Lochs H and Dörffel Y:
Comparative study of the intestinal mucus barrier in normal and
inflamed colon. Gut. 56:343–350. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Van Spaendonk H, Ceuleers H, Witters L,
Patteet E, Joossens J, Augustyns K, Lambeir AM, De Meester I, De
Man JG and De Winter BY: Regulation of intestinal permeability: The
role of proteases. World J Gastroenterol. 23:2106–2123. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Odenwald MA and Turner JR: Intestinal
permeability defects: Is it time to treat? Clin Gastroenterol
Hepatol. 11:1075–1083. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Meng Y, Zhang Y, Liu M, Huang YK, Zhang J,
Yao Q, Zhao YL and Xiong JJ: Evaluating intestinal permeability by
measuring plasma endotoxin and diamine oxidase in children with
acute lymphoblastic leukemia treated with high-dose methotrexate.
Anticancer Agents Med Chem. 16:387–392. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Honzawa Y, Nakase H, Matsuura M and Chiba
T: Clinical significance of serum diamine oxidase activity in
inflammatory bowel disease: Importance of evaluation of small
intestinal permeability. Inflamm Bowel Dis. 17:E23–E25. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Lau WL, Kalantar-Zadeh K and Vaziri ND:
The Gut as a source of inflammation in chronic kidney disease.
Nephron. 130:92–98. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Vaziri ND, Yuan J, Rahimi A, Ni Z, Said H
and Subramanian VS: Disintegration of colonic epithelial tight
junction in uremia: A likely cause of CKD-associated inflammation.
Nephrol Dial Transplant. 27:2686–2693. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Adesso S, Ruocco M, Rapa SF, Piaz FD,
Raffaele Di Iorio B, Popolo A, Autore G, Nishijima F, Pinto A and
Marzocco S: Effect of indoxyl sulfate on the repair and intactness
of intestinal epithelial cells: Role of reactive oxygen Species'
release. Int J Mol Sci. 20(pii): E22802019. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Vaziri ND, Zhao YY and Pahl MV: Altered
intestinal microbial flora and impaired epithelial barrier
structure and function in CKD: The nature, mechanisms, consequences
and potential treatment. Nephrol Dial Transplant. 31:737–746. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Luissint AC, Parkos CA and Nusrat A:
Inflammation and the intestinal barrier: Leukocyte-epithelial cell
interactions, cell junction remodeling, and mucosal repair.
Gastroenterology. 151:616–632. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xin X, Dai W, Wu J, Fang L, Zhao M, Zhang
P and Chen M: Mechanism of intestinal mucosal barrier dysfunction
in a rat model of chronic obstructive pulmonary disease: An
observational study. Exp Ther Med. 12:1331–1336. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wang F, Graham WV, Wang Y, Witkowski ED,
Schwarz BT and Turner JR: Interferon-gamma and tumor necrosis
factor-alpha synergize to induce intestinal epithelial barrier
dysfunction by up-regulating myosin light chain kinase expression.
Am J Pathol. 166:409–419. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhou Y, Xu GQ, Pu ZY, et al: Relationship
between oxidative stress and inflammatory response in young
patients with primary hyperuricemia. China J Modern Med.
97:e131082017.
|