Introduction
Allergic rhinitis (AR) is a chronic nasal mucosal
inflammation characterized by infiltration and activation of a
number of immune cells (1,2). Type 2 helper T (Th2) cells and
regulatory T (Treg) cells play an important role in AR (3,4). A
deficiency of Treg cells and higher numbers of Th2 cells are the
key factors underlying the development of allergic inflammation of
the nasal mucosa (5). T cells can
regulate immune function by secreting cytokines and proinflammatory
molecules. Transforming growth factor (TGF)-β, interleukin (IL)-5
and IL-4 are typical cytokines secreted by Treg and Th2 cells.
Dendritic cells (DCs) are an important type of
antigen-presenting cell that regulate the activation and
differentiation of T cells. Antigen-activated DCs display
co-stimulatory molecules, which determine whether naïve T cells
differentiate into type 1 helper T (Th1), Th2 or Treg cells
(6,7). A previous study demonstrated that
mature DCs (mDCs) express high levels of the co-stimulatory
molecules T-lymphocyte activation antigen CD80 (CD80) and CD86,
which provide the signals that trigger the activation,
proliferation and differentiation of T cells by interacting with
CD28 (8). Despite sharing the same
T cell stimulatory receptor, CD80 and CD86 induce different DC:T
cell interactions through alternative pathways. CD80 may be more
potent in inducing an antitumor immune response compared with CD86,
while CD86 preferentially induces Th2-driven allergic responses
(9,10). Vermaelen and Pauwels (11) demonstrated that airway DCs in a
mouse model of asthma expressed a high level of CD86, but not CD80.
Consistent with this, an allergen challenge with ovalbumin (OVA) in
a murine model of airway inflammation led to the maturation of lung
DCs and increased expression of CD86 (12). However, it is unclear if the
upregulation of CD86 in DCs may play a critical role in the
development of AR.
DCs have been shown to be important in AR. The
number of DCs in the nasal mucosa of patients with AR were
dramatically reduced following intranasal corticosteroid therapy
(13,14). Moreover, KleinJan et al
(15) reported that DCs in the
nasal mucosa of symptomatic AR patients displayed a more mature
phenotype (expressing CD86). In previous studies on AR models, mDCs
have been shown to upregulate the expression of the Th2-cell
cytokines IL-4, IL-5 and IL-13, and induce eosinophilic
inflammation (15,16). However, the effects of the
knockdown of CD86 in DCs on the differentiation and cytokine
secretion by T cells in AR are not yet known.
RNA interference is an approach to gene silencing.
Small interfering RNAs (siRNAs) are small non-coding RNA molecules
that are complementary to the mRNA transcript of the target gene.
These small RNA molecules elicit the sequence-specific degradation
of a complementary mRNA target. Previous studies have reported the
effects of siRNA in animal models of AR (16–22).
These previous studies targeted CD40, signal transducer and
activator of transcription 6, neurokinin-1 receptor, C-C chemokine
receptor type 3, transient receptor potential cation subfamily M
member 8, phosphatidylinositol-3-kinase C2β and calcium
release-activated calcium channel protein 1. In the present study,
the effect of the siRNA-mediated silencing of CD86 in DCs on Treg
and Th2 cell cytokine production was examined in vitro.
Materials and methods
Clinical specimens
Patients with AR (n=39) and healthy control subjects
(n=36) participated in the present study. The patients were
diagnosed based on the criteria of the Initiative on Allergic
Rhinitis and its Impact on Asthma (23), including a characteristic history
of paroxysmal sneezing, watery rhinorrhea, nasal obstruction,
positive specific immunoglobulin E (IgE), a positive skin prick
test (SPT) and nasal examination. All patients were prohibited from
taking any medication within 4 weeks of the study. Patients with
infectious rhinitis, an occupational or drug-induced etiology, and
those with any complications, were excluded. Healthy control
subjects were selected based on the following criteria: No history
of allergic diseases, a negative SPT or specific IgE to common
allergens (total IgE levels <100 kU/liter). In the siRNA group,
DCs from patients with AR were treated with siRNA intervention. In
the non-siRNA group, DCs from patients with AR were not treated
with siRNA intervention. The healthy control group consisted of
healthy subjects. The present study was approved by the Ethics
Committee of Chongqing Medical University. Informed consent for all
subjects was obtained from parents or legal guardians. All
procedures strictly conformed to the principles outlined in the
Declaration of Helsinki.
Generation of DCs
A total of 10 ml blood was collected from each
subject. Peripheral blood mononuclear cells (PBMCs) were isolated
by means of Ficoll-Plaque Plus density gradient centrifugation at
800 × g for 30 min at 4°C. After trypan blue staining, the
viability (>95%) of PBMCs was detected by an exclusion assay
under an optical microscope. CD14+ monocytes were
isolated using magnetic separation (Miltenyi Biotec GmbH). Isolated
CD14+ cells were transferred into 96-well plates, at a
density of 1.0×106 cells/well in 0.1 ml of complete
medium, for 6 days to promote differentiation into immature DCs
(imDCs). The complete medium consisted of RPMI-1640 (R&D
Systems Inc.), 10% fetal calf serum (R&D Systems Inc.), 100
U/ml penicillin, 100 ng/ml streptomycin, 100 ng/ml recombinant
human granulocyte-macrophage colony-stimulating factor (PeproTech,
Inc.) and 50 ng/ml recombinant human IL-4 (PeproTech, Inc.). Half
the volume of the old media was removed and fresh media was added
every 48 h. After 6 days of culture, 100 ng/ml lipopolysaccharide
(LPS; Sigma-Aldrich; Merck KGaA) was added to the cells for 24 h.
The mDCs were obtained on day 7.
Transfection of DCs with CD86 siRNA in
a lentiviral vector
CD86 siRNA in a lentiviral vector was purchased from
Shanghai GenePharma Co., Ltd. The CD86 siRNA sequence was
AGACCACATTCCTTGGATT. The component sequence of the lentivirus
vector was human U6, multiple cloning sites, cytomegalovirus, green
fluorescent protein (GFP), simian virus 40 and neomycin. The
expression of GFP indicated a successful transfection. Preliminary
experiments to calculate the multiplicity of infection (MOI) were
carried out; an MOI of 20 corresponded to a transfection efficiency
of 60.2%, while an MOI of 10 corresponded to a transfection
efficiency of 43.5%. Transfections were performed when DCs reached
a confluency of 30–50%, according to the manufacturer's protocol.
imDCs were seeded in 96-well plates and were transfected with CD86
siRNA in a lentiviral vector at an MOI of 20 on day 3 of the
CD14+ cell culture. After 72 h, 100 ng/ml LPS was added
to the DCs for 24 h. To exclude the effect of transfection reagent
on CD86 expression, a preliminary experiment was carried out (data
not shown). CD86 expression was determined by flow cytometry
through PE-conjugated anti-human CD86 mAb as described below. The
results showed that transfection with an empty lentiviral vector
(Shanghai GenePharma Co., Ltd.) had no effect on CD86 expression
(data not shown). To determine if siRNA had been successfully
transfected into DCs, the expression of GFP was observed using
fluorescence microscopy at 6 h post transfection. The transfection
efficiency was determined by flow cytometry at 48 h after
transfection.
Flow cytometry
DCs were stained with phycoerythrin
(PE)-Cyanine5-conjugated anti-human leukocyte antigen-DR isotype
(HLA-DR) mouse antibody (mAb) (eBioscience; Thermo Fisher
Scientific, Inc.), PE-conjugated anti-human CD86 mAb (eBioscience;
Thermo Fisher Scientific, Inc.) and FITC-conjugated anti-human CD80
mAb (eBioscience; Thermo Fisher Scientific, Inc.). A minimum of
20,000 events were collected in each analysis. DCs were analyzed
using a FACScan flow cytometer (BD Biosciences). Further data
analysis was performed using CellQuest software (CellQuest Pro
5.2.1, BD Biosciences).
Co-culture with CD4+ T
cells
Peripheral CD4+ T cells were isolated
from PBMCs using human CD4 microbeads (Miltenyi Biotec GmbH).
Co-culture was performed with a DC/T cell ratio of 1:4 in 24-well
plates for 7 days without any stimulating factors. The culture
plate was incubated at 37°C in a humidified incubator with 5%
CO2. The supernatant and co-cultured cells were
collected for the following assays. The supernatant was collected
and stored at −80°C until cytokine levels were measured.
ELISA for IL-4, IL-5 and TGF-β1
The levels of IL-4 (cat. no. BMS225HS), IL-5 (cat.
no. BMS278INST) and TGF-β1 (cat. nos. BMS249/4BMS249/4TEN) in the
supernatant was detected using ELISA (eBioscience; Thermo Fisher
Scientific, Inc.), according to the manufacturer's instructions.
The limits of detection for Il-4, Il-5 and TGF-β1 were 0.1, 10 and
8.6 pg/ml, respectively.
Reverse transcription-quantitative
(RT-q)PCR analysis of forkhead box P3 (FOXP3) and GATA-binding
protein 3 (GATA-3) expression
Total RNA was isolated using a Total RNA Extraction
kit (BioTeke Corporation). RT was performed using a Prime Script RT
Reagent kit (Takara, Biotechnology Co., Ltd.), according to the
manufacturer's instructions. The sequences of the primers (Sangon
Biotech, Shanghai, China) were as follows: FOXP3 forward,
5′-AGGGACCAAGAAGTGAGGTTTC-3′ and reverse,
5′-TGGGGTTTGTGTTGAGTGAG-3′; GATA-3 forward,
5′-AGACCACCACAACCACACTCT-3′ and reverse,
5′-GATGCCTTCCTTCTTCATAGTCA-3′; β-actin forward,
5′-AGCGAGCATCCCCCAAAGTT-3′ and reverse, 5′-GGGCACGAAGGCTCATCATT-3′.
The mRNA levels of FOXP3 and GATA-3 were quantified using qPCR as
described previously (5). A brief
introduction is as follows. Reactions were heated to 95°C for 4 min
followed by 30 cycles of denaturation at 95°C for 45 sec, annealing
at 55°C (FOXP3), 58°C (GATA-3), 59°C (β-actin) for 5 sec, and
extension at 72°C for 10 min. All PCR reactions were performed in
duplicate. To confirm the specificity of the PCR reaction, PCR
products were analyzed with 3% agarose gel electrophoresis and
ethidium bromide staining, followed by visualization with
anultraviolet rays transilluminator.
Western blot analysis of FOXP3 and
GATA3 expression
Proteins were extracted using RIPA (Thermo Fisher
Scientific, Inc.) from control co-cultured cells or following
transfection with empty vector or CD86-siRNA containing vector. The
protein levels of FOXP3 and GATA-3 were determined by western blot
analysis as described previously (5). The dilution of FOXP3 monoclonal
antibody (cat. no. 14-7979-82, eBioscience; Thermo Fisher
Scientific, Inc.) was 1:1,000. The dilution of GATA3 Monoclonal
Antibody (cat. no. 66400-1-IG, eBioscience; Thermo Fisher
Scientific, Inc.) was 1:2,000. The dilution of Goat anti-Mouse IgG
(H+L) Cross-Adsorbed Secondary Antibody (cat. no. G-21040,
Invitrogen; Thermo Fisher Scientific, Inc.) was 1:2,000. The
incubation temperature of the primary antibody was 4°C and the
incubation duration was overnight. The incubation temperature of
the secondary antibody was room temperature and the incubation
duration was 1 h. Data were analyzed with Quantity One software,
version 4.52 (Bio-Rad Laboratories, Inc.).
Statistical analysis
Each experiment was performed a minimum of five
times. All results are presented as the mean ± SD. Data were
analyzed by one-way ANOVA followed by Bonferroni post-hoc test
using SPSS v.11.5 software (SPSS, Inc.). P<0.05 was considered
to indicate a statistically significant difference.
Results
Expression of mDC surface
molecules
The expression of CD80, CD86 and HLA-DR on mDCs was
analyzed using flow cytometry. There were no significant
differences in the expression of CD80 and HLA-DR on mDCs between
the AR and the healthy control groups (data not shown). However,
mDCs from the AR group exhibited a significantly higher expression
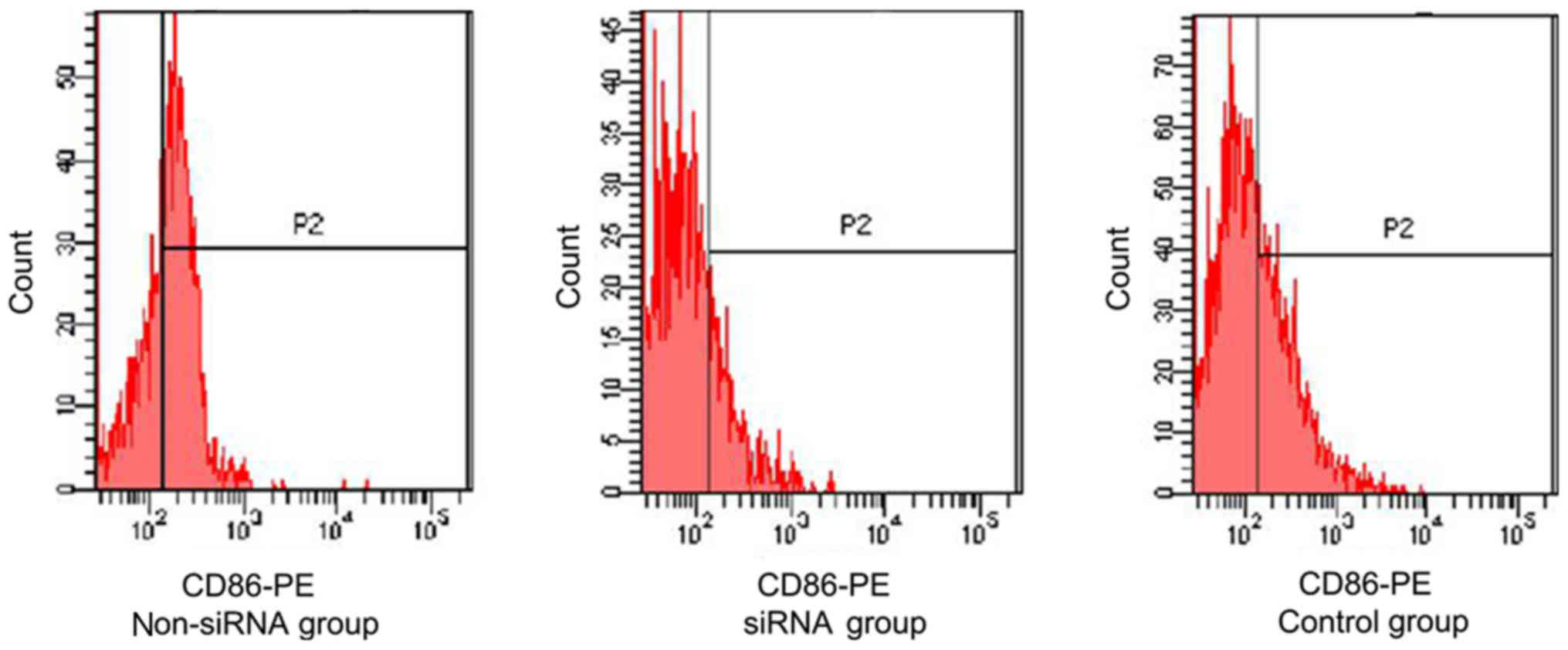
level of CD86 compared with the control group (P<0.05; Fig. 1). CD83 is an important surface
antigen expressed by mDCs. To verify that mDCs had been induced,
CD83 expression levels on mDCs were analyzed by flow cytometry in a
preliminary experiment. The mDCs were found to exhibit a
significantly higher CD83 expression level compared with the imDCs
(data not shown).
Effects of siRNA on surface molecule
expression in DCs
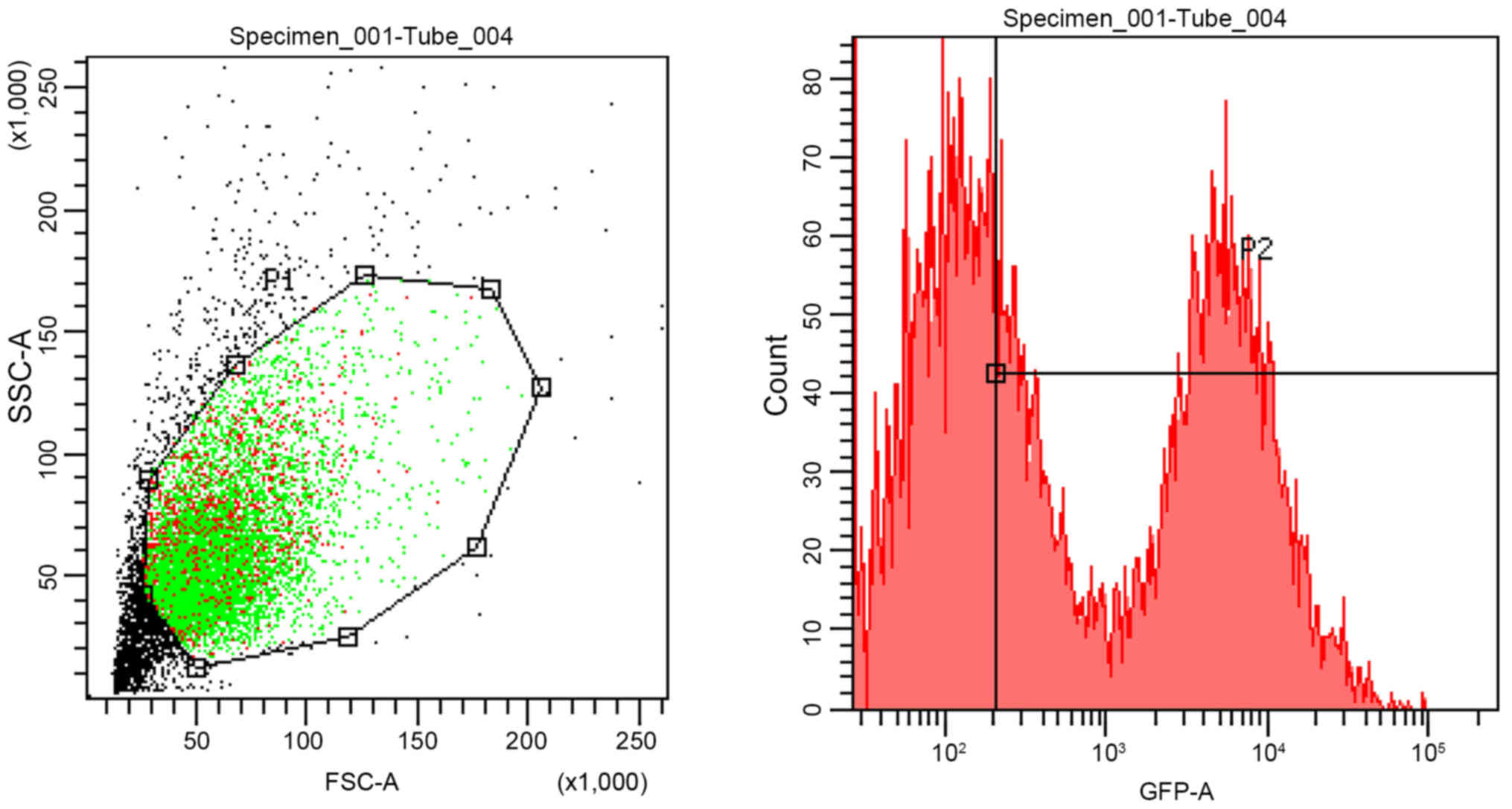
At 6 h after CD86 siRNA transfection, the efficiency
of transfection of the DCs was assessed by fluorescence microscopy
and the transfection efficiency was determined by flow cytometry.
DCs were successfully transfected with CD86 siRNA using a
lentiviral vector. As shown in Fig.
2, the transfection efficiency of CD86-siRNA was 60.2%, as
determined by assessing the number of GFP-positive cells. At 96 h
after transfection, the expression of CD86 on DCs was determined by
flow cytometry. CD86 expression levels were significantly lower in
the siRNA group (17.2±3.17) compared with those in the non-siRNA
(61.2±15.46) and control (37.6±5.93) groups. The difference was
statistically significant (P<0.05; Fig. 1). However, the CD80 expression
levels in the non-siRNA, siRNA and control groups were 85.45±17.81,
83.79±14.27 and 86.36±18.96%, respectively; the differences were
not significant (P>0.05; data not shown).
IL-4, IL-5 and TGF-β1 secretion by T
cells co-cultured with DCs
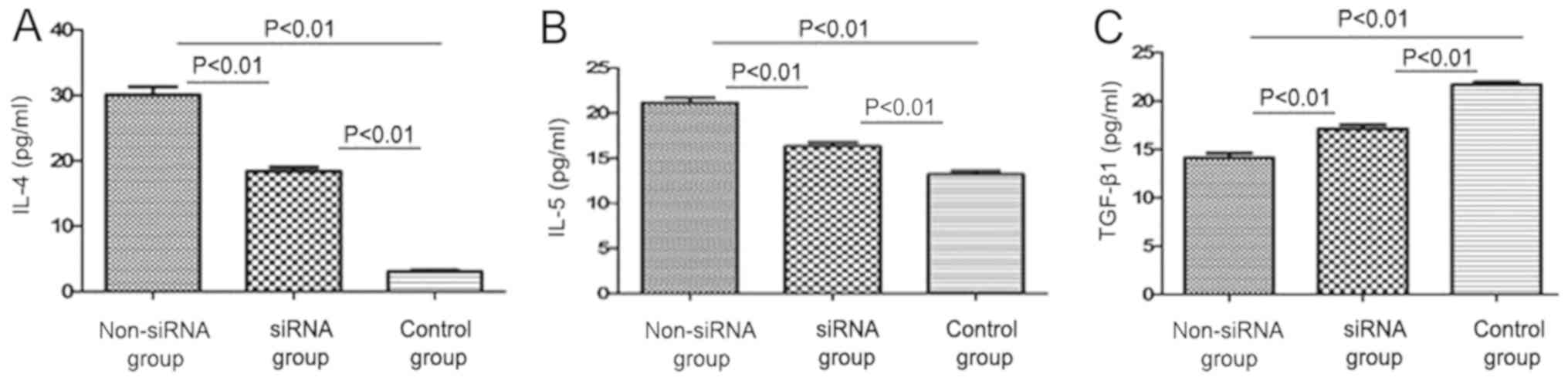
The expression levels of IL-4 and IL-5 were
significantly lower in the supernatant of the co-cultured cells in
the siRNA-treated group (19.07±0.58 and 15.9±0.51 pg/ml,
respectively) compared with those in the non-siRNA group
(30.23±1.37 and 22.04±0.78 pg/ml, respectively); the differences
were statistically significant (P<0.01; Fig. 3A and B). Moreover, IL-4 and IL-5
expression levels were significantly higher in the non-siRNA and
siRNA groups compared with those in the control group (P<0.01;
Fig. 3A and B). The expression of
TGF-β1 in the supernatant of the co-cultured cells was
significantly higher following transfection with siRNA targeting
CD86 (16.22±0.48 pg/ml) compared with that in the non-siRNA group
(14.27±0.51 pg/ml). The difference was statistically significant
(P<0.01; Fig. 3C).
FOXP3 and GATA3 mRNA expression by T
cells co-cultured with DCs
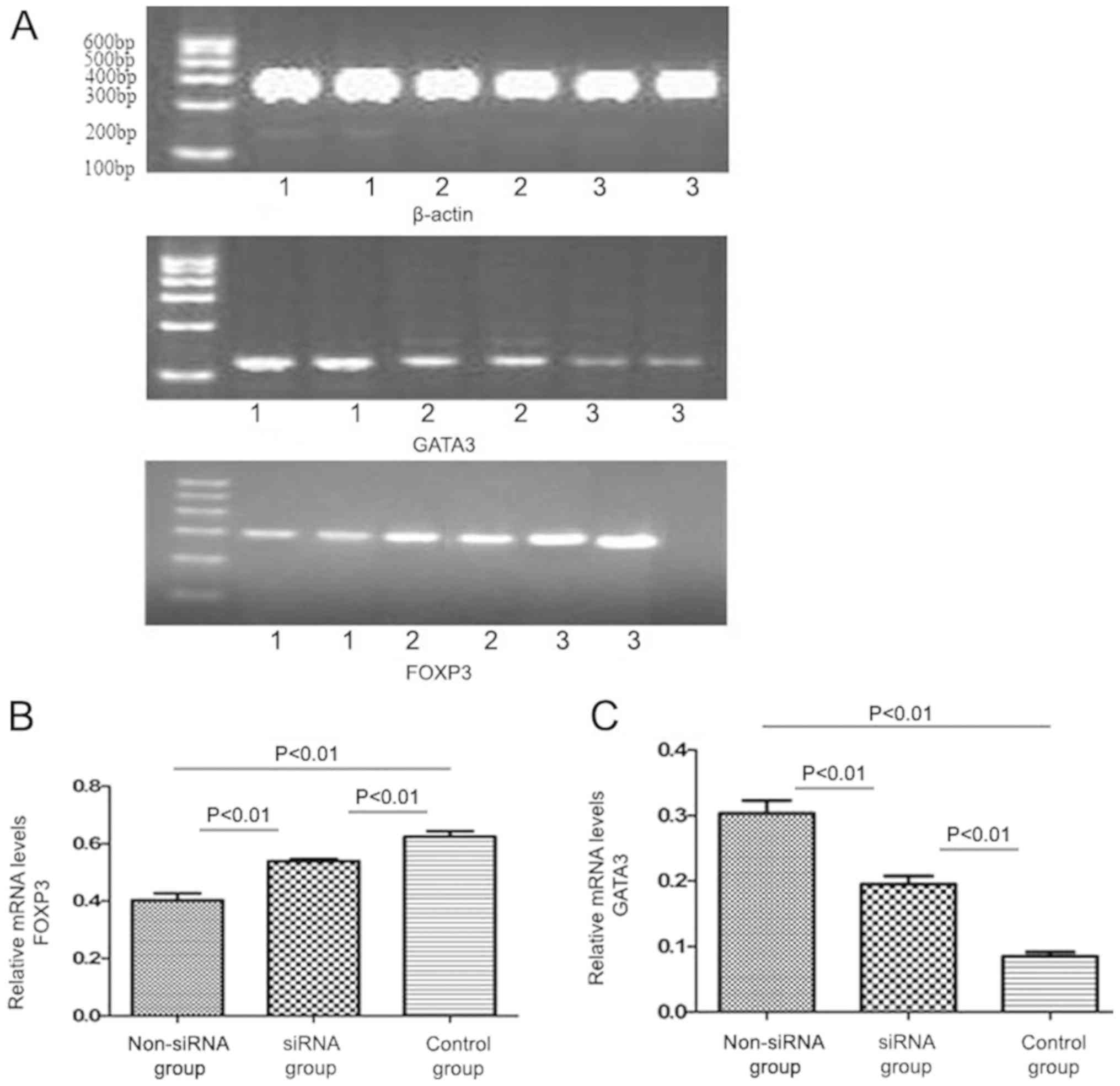
The expression level of FOXP3 mRNA was higher in the
siRNA-treated group (0.541±0.027) compared with that in the
non-siRNA group (0.412±0.035; P<0.01; Fig. 4), whereas GATA3 mRNA expression was
significantly lower in the siRNA group (0.196±0.013) compared with
that in the non-siRNA group (0.305±0.029). The difference was
statistically significant (P<0.01; Fig. 4).
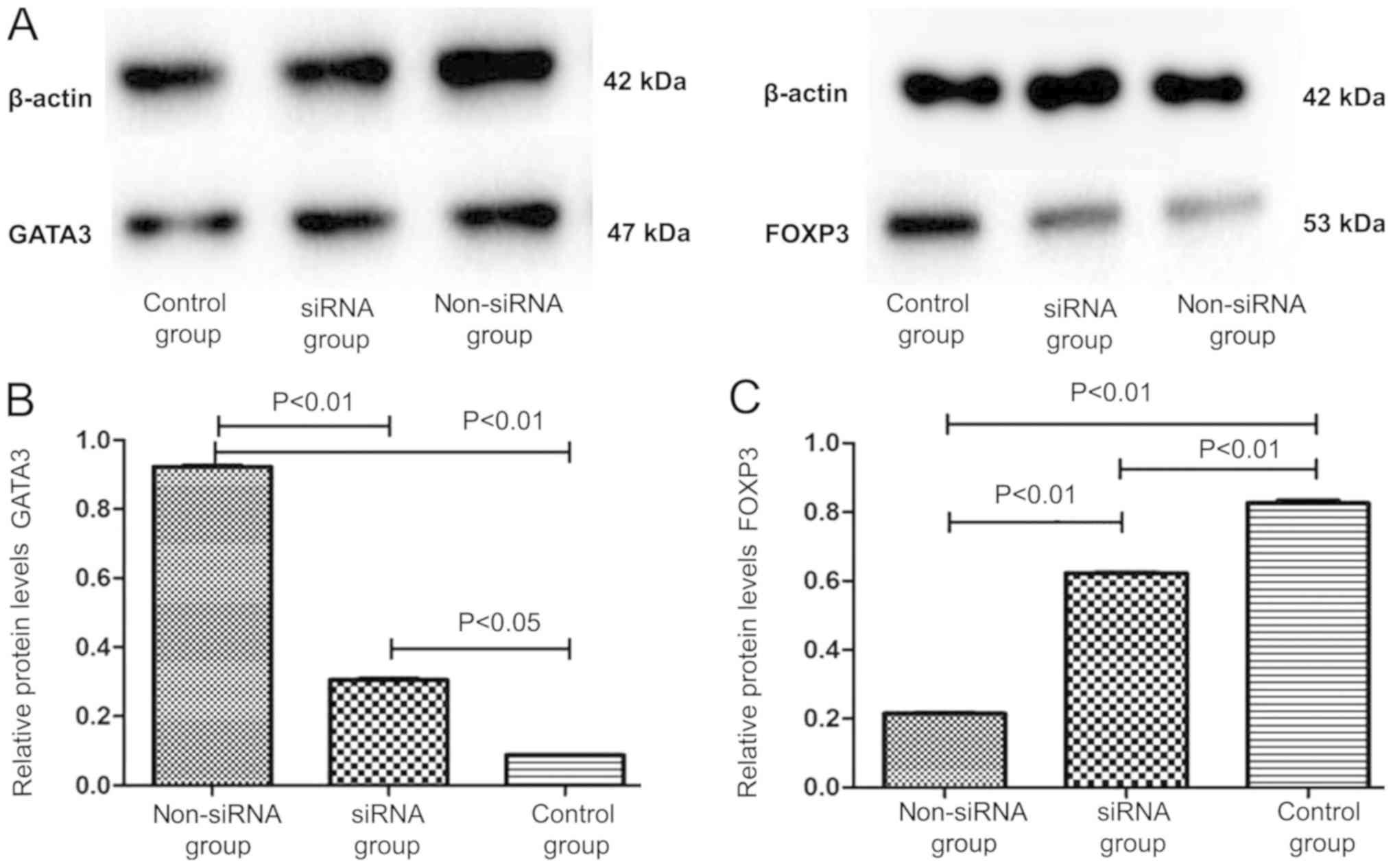
FOXP3 and GATA3 protein expression by
T cells co-cultured with DCs
The expression level of the FOXP3 protein was
considerably higher in the siRNA group (0.619±0.035) compared with
that in the non-siRNA group (0.217±0.021; P<0.01; Fig. 5). However, the GATA3 protein
expression level in the siRNA group (0.317±0.027) was significantly
lower compared with that in the non-siRNA group (0.917±0.046;
P<0.01; Fig. 5). These results
were consistent with those of the RT-qPCR analysis.
Discussion
In the present study, the mDCs of patients with AR
were found to express high levels of the co-stimulatory molecule
CD86. Following transfection with CD86 siRNA using a lentiviral
vector, the expression levels of CD86 on DCs were markedly reduced.
CD86 siRNA-transfected DCs induced Th2 cell deficiency and Treg
cell hyperactivity. These results indicated that CD86 plays a
potentially important role in the regulation of the Treg/Th2 cell
imbalance in allergic inflammation.
The co-stimulatory molecule CD86 is prominent in
allergic inflammation, including asthma and atopic dermatitis
(24–26). Despite sharing the same T cell
stimulatory receptor, CD86 appears to induce more potent allergic
responses compared with CD80. Wong et al (24) reported that steroid- and
non-steroid-treated asthmatic patients expressed higher levels of
plasma CD86, but not CD80, compared with control subjects. The
expression of CD86 on airway DCs was increased gradually following
continuous stimulation by allergens (11). In another animal model of asthma, a
continuous allergen challenge induced the maturation of lung DCs
and an increased expression of CD86 (12). It was demonstrated that T cell
activation is closely associated with increased levels of CD86, but
not CD80, on the surface of DCs during late-phase airway allergic
response using siRNA targeting CD86 (25). It has been shown that the local
application of CD86 siRNA targeting cutaneous DCs improved allergic
skin inflammation (26). In the
present study, mDCs from the peripheral blood of patients with AR
were found to express higher levels of CD86, but not CD80, compared
with the healthy control group. A similar observation in DCs was
described by KleinJan et al (15). DCs in the nasal mucosa of
symptomatic AR patients expressed higher levels of CD86, which was
associated with Th2-driven allergic responses. Together with the
study by KleinJan et al (15), the results of the present study
suggested that mDCs express high levels of CD86, which may play an
active role in allergic inflammation. Therefore, CD86 molecules on
DCs may represent a novel therapeutic target for AR.
The role of the co-stimulatory molecule CD86 in the
differentiation and activation of T cells was also investigated in
the present study. The data in the present study demonstrated that
T cells co-cultured with DCs produced higher levels of TGF-β1 and
lower levels of IL-4 and IL-5 in the siRNA group compared with
those in the non-siRNA and healthy control group. The expression of
GATA-3 was lower and that of FOXP3 higher at the mRNA and protein
levels in the siRNA-treated group compared with in the non-siRNA
and healthy control group. These results indicated that
CD86-silenced DCs may regulate the Treg/Th2 cell imbalance in
allergic inflammation. There are two aspects to the effect of
CD86-silenced DCs on T cells: Th2 deficiency and Treg
hyperactivity. CD86-silenced DCs decrease the number of IL-4- and
IL-5-producing Th2 cells. The presence of CD86 on DCs is required
for Th2 cell differentiation and activation (27). Previous studies using animal models
of asthma have demonstrated that anti-CD86 mAb treatment, but not
anti-CD80 mAb treatment, effectively inhibited antigen-specific IgE
and Th2 cytokine production (27–29).
In addition, the blockade of CD86 may suppress airway
hyper-responsiveness and Th2-driven allergic responses in allergic
mice (30). The present study also
demonstrated that CD86-silenced DCs did not promote T cell
activation or production of IL-4 and IL-5, whereas CD86-silenced
DCs increased the number of TGF-β1-producing Treg cells. CD86 plays
a crucial role in DC maturation (5). Immature or semi-mature DCs induce
immune tolerance (31,32). The mAb against CD3 on DCs increased
the number of antigen-specific Tregs in autoimmune diabetes
(33). Knockdown of CD40 using
siRNA in OVA-exposed DCs induced the generation of
allergen-specific Treg cells, which suppressed allergic responses
in vivo (16). The present
study demonstrated that the expression of FOXP3 at the mRNA or
protein level was higher in the CD86 siRNA-treated group compared
with that in the control group. These results suggested that the
effects of CD86-silenced DCs on allergic inflammation are closely
associated with the regulation of the Treg/Th2 cell imbalance.
Using CD86 siRNA-treated DCs to skew the immune response from Th2
cells towards Treg cells may be a viable therapeutic approach to
the treatment of AR.
To the best of our knowledge, the present study is
the first to introduce an effective method for inhibiting the
upregulation of CD86 on DCs from patents with AR using siRNA. An
advantage of siRNA is the ability to specifically knock down a
designated gene without directly affecting the expression of any
other mRNAs. CD86 expression on DCs decreased significantly
following siRNA treatment, whereas HLA-DR and CD80 expression
exhibited little or no change.
The present study had some limitations. For example,
Treg hyperactivity does not necessarily mean an increase in
function, it may also be due to an increase in the quantity of Treg
cells. The increased expression of FOXP3 at the mRNA and protein
level does not indicate amplification of the Treg function or
population; changes in function need to be verified using mixed
lymphocyte reaction experiments. Additionally, the data provided in
the present study are from in vitro experiments and no
disease model was used. These points will be addressed in future
studies.
In conclusion, the findings of the present study
suggested that mDCs from patients with AR express high levels of
the co-stimulatory molecule CD86, but not of CD80. CD86 expression
in DCs was specifically reduced using a lentiviral vector
expressing siRNA. CD86 siRNA-treated DCs altered the Treg/Th2 cell
balance. The findings from the present study indicated that CD86
may play an important role in the pathogenesis of allergic
inflammation and may be a novel target for RNA interference that
could be used in the treatment of AR.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation Project (grant no. 81500776) and the
Fundamental and Advanced Research Program of Chongqing (grant no.
cstc2015jcyjA10103).
Availability of materials and data
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
RS made substantial contributions to the
experimental design, generation and transfection of DCs, co-culture
of cells, acquisition of data and analysis of data. YY and ZG were
responsible for the collection of clinical specimens and the ELISA
to determine the expression levels of cytokines. CZ and XT were
responsible for flow cytometry. WK and PW carried out RT-qPCR and
western blotting analysis of FOXP3 and GATA3 expression levels. In
addition, XT was also involved in drafting the manuscript and
revising it critically for important intellectual content. All the
authors have read and approved the final version of this
manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of Chongqing Medical University. Informed consent was
obtained from the parents or legal guardians of all subjects. All
procedures strictly conformed to the principles outlined in the
Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Pilette C, Jacobson MR, Ratajczak C, Detry
B, Banfield G, VanSnick J, Durham SR and Nouri-Aria KT: Aberrant
dendritic cell function conditions Th2-cell polarization in
allergic rhinitis. Allergy. 68:312–321. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Skrindo I, Ballke C, Gran E, Johansen FE,
Baekkevold ES and Jahnsen FL: IL-5 production by resident mucosal
allergen-specific T cells in an explant model of allergic rhinitis.
Clin Exp Allergy. 45:1296–1304. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Akdis M, Verhagen J, Taylor A, Karamloo F,
Karagiannidis C, Crameri R, Thunberg S, Deniz G, Valenta R, Fiebig
H, et al: Immune responses in healthy and allergic individuals are
characterized by a fine balance between allergen-specific T
regulatory 1 and T helper 2 cells. J Exp Med. 199:1567–1575. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lee JH, Yu HH, Wang LC, Yang YH, Lin YT
and Chiang BL: The levels of CD4+CD25+ regulatory T cells in
paediatric patients with allergic rhinitis and bronchial asthma.
Clin Exp Immunol. 148:53–63. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sun R, Tang XY and Yang Y: Immune
imbalance of regulatory T/type 2 helper cells in the pathogenesis
of allergic rhinitis in children. J Laryngol Otol. 130:89–94. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mellman I and Steinman RM: Dendritic
cells: Specialized and regulated antigen processing machines. Cell.
106:255–258. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lambrecht BN and Hammad H: The role of
dendritic and epithelial cells as master regulators of allergic
airway inflammation. Lancet. 376:835–843. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lim TS, Goh JK, Mortellaro A, Lim CT,
Hämmerling GJ and Ricciardi-Castagnoli P: CD80 and CD86
differentially regulate mechanical interactions of T-cells with
antigen-presenting dendritic cells and B-cells. PLoS One.
7:e451852012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kuchroo VK, Das MP, Brown JA, Ranger AM,
Zamvil SS, Sobel RA, Weiner HL, Nabavi N and Glimcher LH: B7-1 and
B7-2 costimulatory molecules activate differentially the Th1/Th2
developmental pathways: Application to autoimmune disease therapy.
Cell. 80:707–718. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lenschow DJ, Ho SC, Sattar H, Rhee L, Gray
G, Nabavi N, Herold KC and Bluestone JA: Differential effects of
anti-B7-1 and anti-B7-2 monoclonal antibody treatment on the
development of diabetes in the nonobese diabetic mouse. J Exp Med.
181:1145–1155. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Vermaelen K and Pauwels R: Accelerated
airway dendritic cell maturation, trafficking, and elimination in a
mouse model of asthma. Am J Respir Cell Mol Biol. 29:405–409. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Gajewska BU, Swirski FK, Alvarez D, Ritz
SA, Goncharova S, Cundall M, Snider DP, Coyle AJ, Gutierrez-Ramos
JC, Stämpfli MR and Jordana M: Temporal-spatial analysis of the
immune response in a murine model of ovalbumin-induced airways
inflammation. Am J Respir Cell Mol Biol. 25:326–334. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Till SJ, Jacobson MR, O'Brien F, Durham
SR, KleinJan A, Fokkens WJ, Juliusson S and Löwhagen O: Recruitment
of CD1a+ Langerhans cells to the nasal mucosa in seasonal allergic
rhinitis and effects of topical corticosteroid therapy. Allergy.
56:126–131. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Holm A, Dijkstra M, Kleinjan A, Severijnen
LA, Boks S, Mulder P and Fokkens W: Fluticasone propionate aqueous
nasal spray reduces inflammatory cells in unchallenged allergic
nasal mucosa: Effects of single allergen challenge. J Allergy Clin
Immunol. 107:627–633. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
KleinJan A, Willart M, van Rijt LS,
Braunstahl GJ, Leman K, Jung S, Hoogsteden HC and Lambrecht BN: An
essential role for dendritic cells in human and experimental
allergic rhinitis. J Allergy Clin Immunol. 118:1117–1125. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Suzuki M, Zheng X, Zhang X, Zhang ZX,
Ichim TE, Sun H, Nakamura Y, Inagaki A, Beduhn M, Shunnar A, et al:
A novel allergen-specific therapy for allergy using CD40-silenced
dendritic cells. J Allergy Clin Immunol. 125:7372010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hosoya K, Satoh T, Yamamoto Y, Saeki K,
Igawa K, Okano M, Moriya T, Imamura O, Nemoto Y and Yokozeki H:
Gene silencing of STAT6 with siRNA ameliorates contact
hypersensitivity and allergic rhinitis. Allergy. 66:124–131. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang H, Zhang R, Wu J and Hu H: Knockdown
of neurokinin-1 receptor expression by small interfering RNA
prevents the development of allergic rhinitis in rats. Inflamm Res.
62:903–910. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhu XH, Liao B, Liu K and Liu YH: Effect
of RNA interference therapy on the mice eosinophils CCR3 gene and
granule protein in the murine model of allergic rhinitis. Asian Pac
J Trop Med. 7:226–230. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Cho Y, Jang Y, Yang YD, Lee CH, Lee Y and
Oh U: TRPM8 mediates cold and menthol allergies associated with
mast cell activation. Cell Calcium. 48:202–208. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Srivastava S, Cai X, Li Z, Sun Y and
Skolnik EY: Phosphatidylinositol-3-kinase C2β and TRIM27 function
to positively and negatively regulate IgE receptor activation of
mast cells. Mol Cell Biol. 32:3132–3139. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Y, Lin L and Zheng C: Downregulation
of Orai1 expression in the airway alleviates murine allergic
rhinitis. Exp Mol Med. 44:177–190. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Bousquet J, Khaltaev N, Cruz AA, Denburg
J, Fokkens WJ, Togias A, Zuberbier T, Baena-Cagnani CE, Canonica
GW, van Weel C, et al: Allergic rhinitis and its impact on asthma
(ARIA) 2008 update (in collaboration with the World Health
Organization, GA(2)LEN and AllerGen). Allergy. 63 (Suppl
86):S8–S160. 2008. View Article : Google Scholar
|
|
24
|
Wong CK, Lun SW, Ko FW, Ip WK, Hui DS and
Lam CW: Increased expression of plasma and cell surface
co-stimulatory molecules CTLA-4, CD28 and CD86 in adult patients
with allergic asthma. Clin Exp Immunol. 141:122–129. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Asai-Tajiri Y, Matsumoto K, Fukuyama S,
Kan-O K, Nakano T, Tonai K, Ohno T, Azuma M, Inoue H and Nakanishi
Y: Small interfering RNA against CD86 during allergen challenge
blocks experimental allergic asthma. Respir Res. 15:1322014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ritprajak P, Hashiguchi M and Azuma M:
Topical application of cream-emulsified CD86 siRNA ameliorates
allergic skin disease by targeting cutaneous dendritic cells. Mol
Ther. 16:1323–1330. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nakajima A, Watanabe N, Yoshino S, Yagita
H, Okumura K and Azuma M: Requirement of CD28-CD86 co-stimulation
in the interaction between antigen-primed T helper type 2 and B
cells. Int Immunol. 9:637–644. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tsuyuki S, Tsuyuki J, Einsle K, Kopf M and
Coyle AJ: Costimulation through B7-2 (CD86) is required for the
induction of a lung mucosal T helper cell 2 (TH2) immune response
and altered airway responsiveness. J Exp Med. 185:1671–1679. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Huh JC, Strickland DH, Jahnsen FL, Turner
DJ, Thomas JA, Napoli S, Tobagus I, Stumbles PA, Sly PD and Holt
PG: Bidirectional interactions between antigen-bearing respiratory
tract dendritic cells (DCs) and T cells precede the late phase
reaction in experimental asthma: DC activation occurs in the airway
mucosa but not in the lung parenchyma. J Exp Med. 198:19–30. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Crosby JR, Guha M, Tung D, Miller DA,
Bender B, Condon TP, York-DeFalco C, Geary RS, Monia BP, Karras JG
and Gregory SA: Inhaled CD86 antisense oligonucleotide suppresses
pulmonary inflammation and airway hyper-responsiveness in allergic
mice. J Pharmacol Exp Ther. 321:938–946. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Banchereau J and Steinman RM: Dendritic
cells and the control of immunity. Nature. 392:245–252. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lutz MB and Schuler G: Immature,
semi-mature and fully mature dendritic cells: Which signals induce
tolerance or immunity? Trends Immunol. 23:445–449. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
You S, Leforban B, Garcia C, Bach JF,
Bluestone JA and Chatenoud L: Adaptive TGF-beta-dependent
regulatory T cells control autoimmune diabetes and are a privileged
target of anti-CD3 antibody treatment. Proc Natl Acad Sci USA.
104:6335–6340. 2007. View Article : Google Scholar : PubMed/NCBI
|