Introduction
Immune-related pancytopenia (IRP) is a type of
hemocytopenia exhibiting the following features: i) Hemocytopenia
or pancytopenia with a normal or higher percentage of reticulocytes
and/or neutrophils; ii) hyperplasia-bone marrow (BM) with a higher
percentage of nucleated erythroid cells in the sternum, with
erythroblastic islands that are easily observed; iii) good
responses to corticosteroids or high-dose intravenous
immunoglobulin (IVIg); iv) exclusion of other primary and secondary
hemocytopenia disorders; and v) positive result from the bone
marrow mononuclear cells (BMMNC)-Coombs test (1–3).
There may also be a humoral immune mechanism involved in the
pathogenesis of IRP.
Natural killer (NK) cells are a group of large
granule lymphocytes (4) and
different from T and B lymphocytes, NK cells can kill tumor cells
and virus-infected cells. They are core to innate immunity and are
adaptive immune regulators, playing important anti-infection and
anti-tumor roles, and eliminating exogenous cells (5,6). In
recent years, with the development of immunology and molecular
biology technology, biological cell immunotherapy has become the
fourth treatment option for cancer after surgery, radiotherapy and
chemotherapy. In view of the good clinical prospects of NK cell
immunotherapy for cancer, adoptive immunotherapy of NK cells has
gradually become a focus of research in recent years. As NK cells
only account for 10–15% of peripheral blood lymphocytes (7), it is difficult to meet the needs of
large-scale experiments and clinical cancer immunotherapy.
Therefore, in vitro amplification methods used to obtain a
large number of high-purity NK cells have become an important
focus. Researchers have investigated various amplification methods,
such as isolating NK cells from peripheral blood mononuclear cells
(PBMCs) using magnetic beads sorting (magnetic-activated
cell-sorting method; MACS), using recombinant interleukin (IL)-2
(rIL-2), recombinant IL-12 (rIL-12), recombinant IL-15 (rIL-15) and
recombinant IL-18 (rIL-18), or different combinations of these
factors, or using irradiated lethal K562 cells or HFWT cells to
stimulate the expansion of NK cells in PBMCs (8–10).
The present study aimed to further analyze changes in the quantity
of NK cells in patients with IRP. Different culture techniques were
also examined for the efficient amplification of human NK cells to
obtain an optimized method for inducing and amplifying NK cells, in
order to provide a foundation for the clinical application of NK
cell immunotherapy.
Materials and methods
Patients
Immune-related pancytopenia (IRP) is a type of
hemocytopenia exhibiting these following features: i) Hemocytopenia
or pancytopenia with a normal or higher percentage of reticulocytes
and/or neutrophils; ii) hyperplasia-bone marrow (BM) with a higher
percentage of nucleated erythroid cells in sternum, with
erythroblastic islands (EIs) that are easily observed; iii) good
responses to corticosteroids or high-dose intravenous
immunoglobulin (IVIg); iv) exclusion of other primary and secondary
hemocytopenia disorders; and v) positive result by BMMNC-Coombs
test (1). In total, 44 patients
with IRP who were successively diagnosed with IRP were enrolled at
The Hematology Department of General Hospital between September
2016 and July 2017. In the present study, patients were categorized
into two groups, IRP and remission IRP (R-IRP) groups. The present
study was approved by The Ethics Committee of the Hospital China.
The present study was conducted according to the Declaration of
Helsinki, and informed written consent was obtained from all
healthy controls and all patients or their guardians. According to
the protocols of the Ethic Committee for the Conduct of Human
Research, the diagnostic method of IRP was established, as
previously described (2). A total
of 23 healthy volunteers served as the normal control group, with
11 males and 12 females, with a median age of 28 (25–52) years. The
clinical characteristics of all patients are presented in Table I.
 | Table I.Clinical and laboratory indexes of
newly diagnosed patients with IRP and patients with IRP in
remission. |
Table I.
Clinical and laboratory indexes of
newly diagnosed patients with IRP and patients with IRP in
remission.
| Clinical
characteristics | IRP, n=23 | R-IRP, n=21 | P-value |
|---|
| Male/female | 12/11 | 11/10 | 0.656 |
| Age | 46 (8–72) | 40 (8–68) | 0.158 |
| HB, g/l | 77.5 (52–131) | 106.5 (100–156) |
<0.001a |
| RBC,
1012/l | 2.43 (1.33–3.90) | 3.35 (2.1–5.01) | 0.002a |
| Plt,
109/l | 38.5 (13–80) | 195.5 (110–300) |
<0.001a |
| WBC,
109/l | 3.52 (1.40–9.86) | 4.56
(2.19–17.58) | 0.109 |
| ANC,
109/l | 49.9
(17.2–90.4) | 50.8
(18.9–90.7) | 0.944 |
| Ret, % | 1.97
(0.66–3.97) | 2.46
(0.72–6.37) | 0.205 |
| LYMPH, % | 38.85
(7.10–76.6) | 44.5
(5.20–72.2) | 0.901 |
| LDH, U/l | 202 (125–331) | 209 (157–378) | 0.086 |
| IgG, g/l | 1,350
(581–1800) | 1,050
(702–1830) | 0.337 |
| IgM, g/l | 98.95
(47.5–260) | 117 (35.8–256) | 0.735 |
| ComplementC3,
g/l | 97.1
(46.4–131) | 101 (60.6–170) | 0.257 |
| ComplementC4,
g/l | 22.25
(9.02–35.6) | 27.5
(14.4–36.1) | 0.386 |
|
CD4+/CD8+ | 1.66
(0.5–5.57) | 1.42
(0.55–3.16) | 0.563 |
|
CD5+CD19+/CD19+ | 14.67
(0–39.28) | 6.98
(1.44–26.54) | 0.205 |
Flow cytometry
Fresh EDTA anticoagulant peripheral blood samples (2
ml in total) from each participant were placed in 6 marked fluid
centrifuge tubes (200 µl/tube). To each tube 10 µl PerCP-CD3 (1:20;
cat. no. 552851) and 10 µl phycoerythrin (PE)-VIO770-CD56 (1:20;
cat. no. 560842) were added, and 10 µl PE-IgG was added to
detection tube 1. Following 15 min incubation at 20°C in the dark,
hemolysis and washing, CD3-CD56+ cells were detected by
flow cytometry to determine the proportion of lymphocytes and the
percentages of NKG2-A/NKG2-B type II integral membrane protein
(NKG2A; 1:20; cat. no. 5170116477), NKG2-D type II integral
membrane protein (NKG2D; 1:20; cat. no. 4318869), CD158a (1:20;
cat. no. 27718) and natural killer cell p46-related protein (NKp46;
1:20; cat. no. 4255803), and the average fluorescence intensity of
CD3-CD56+ cells. All antibodies were purchased from BD
Pharmingen; BD Biosciences. At least 104−105
cells were acquired and analyzed by FACSCalibur flow cytometer (BD
Biosciences) and Cell Quest software version 6.0 (BD
Biosciences).
Isolation and purification of NK
cells
In total, 5 ml fresh human peripheral blood was
obtained from each patient with IRP. CD3-CD56+ cells
were freshly purified using NK cell MACS (Miltenyi Biotec, Inc.),
according to the manufacturer's protocol. Subsequently, cells were
detected using a multiparameter flow cytometer (BD Biosciences) and
analyzed using Cell Quest software (version 3.1; BD Biosciences).
The suspension containing NK cells was collected, the cells were
counted and the purity of the cells was measured by flow cytometry.
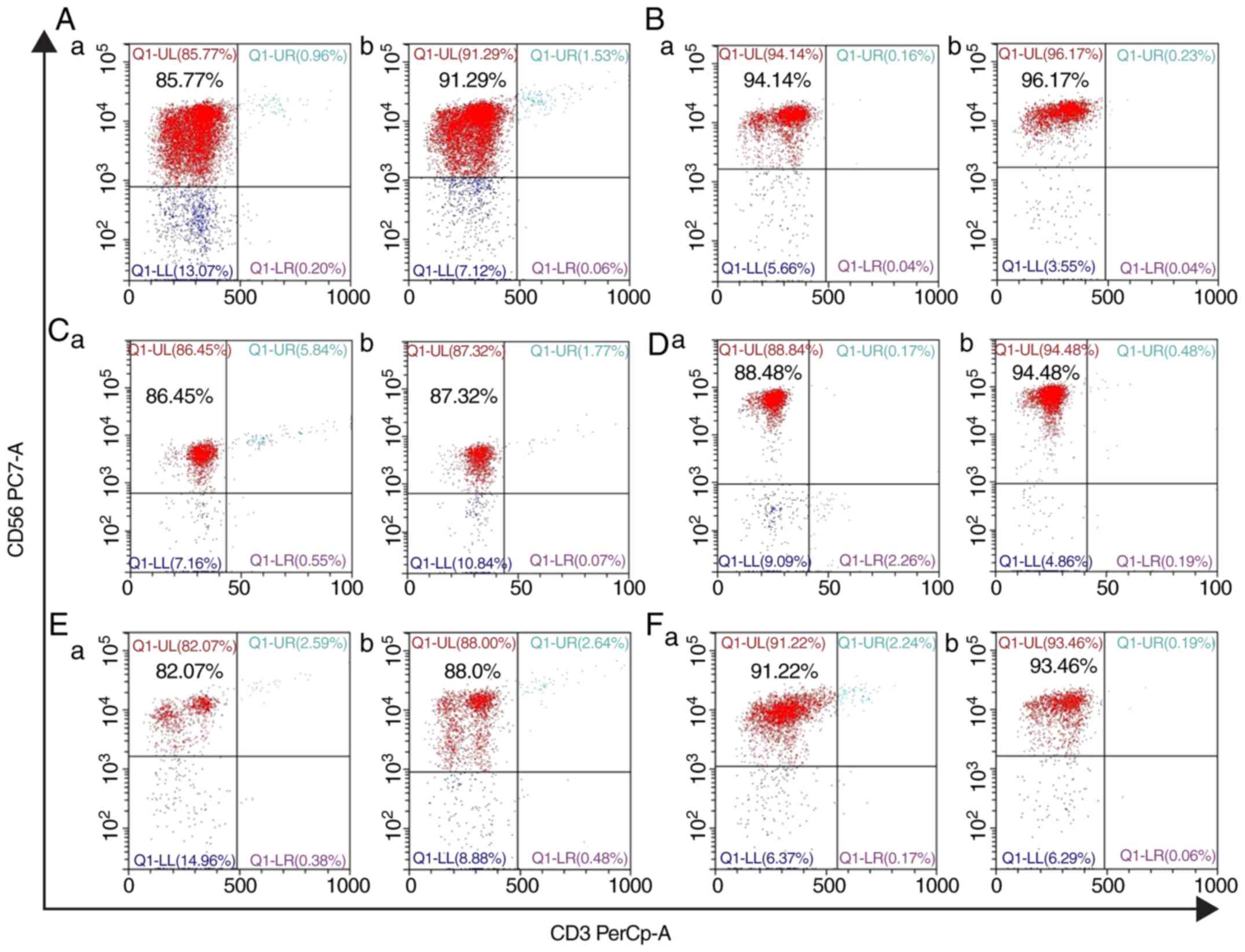
The NK cells were purified by MACS (Fig. 1). After centrifugation (300 × g for
5 min at 20°C, Tianjin Hao Yang Biological Products Technology Co.,
Ltd.), cells were precipitated and RPMI-1640 culture medium
(Beijing Solarbio Science & Technology Co., Ltd.) containing
20% FBS (Beijing Solarbio Science & Technology Co., Ltd.) was
used to adjust the cell concentration to 5×104
cells/ml.
Cultivation and amplification of NK
cells after purification
The purity of NK cells following MACS reached 90%.
Cells were suspended in RPMI-1640 culture medium containing 20% FBS
and the cell concentration was adjusted to 5×104/ml.
rIL-15 (Miltenyi Biotec, Inc.; 20 ng/ml) was added to the culture
medium containing rIL-2 (Miltenyi Biotec, Inc.; 500 IU/ml). NK cell
amplifiers (5 µl/ml/106 NK cells) was added to each well
of the 24-well culture plate (3524; Corning, Inc.). Cells were
cultured at a constant temperature of 37°C in a 5% CO2
incubator. The number of cells was then counted under a confocal
microscope (magnification, ×100; Olympus Corporation) every 3 days
for the first 6 days, and fresh medium and cytokines were added.
Subsequently, the number of cells was counted under a microscope
every 2 days for the last 4 days, and fresh medium and cytokines
were added. Cells were collected after 10 days of culture. A
confocal microscope (magnification, ×100; Olympus Corporation) was
used. In total, 10 µl cells were evenly placed on the cell counting
board (Watson Bio Lab). Then, the number of cells in 16 visual
fields were observed. If 10 µl were taken out of 1 ml, the number
of cells in 16 visual fields was multiplied by 104, and
the final number of cells was calculated according to the above
counting method.
Statistical analysis
The experiments were repeated 3 times. Data were
analyzed using Prism (version 7; GraphPad Software, Inc.) and SPSS
for Windows (version 24.0; IBM Corp.). For three independent
groups, one-way ANOVA was used with the S-N-K method used as a post
hoc test. For skewed distribution, the median is presented and was
analyzed using the rank-sum test. Correlation analysis was
performed using Pearson's correlation test. Non-normal distribution
data are represented as the median (four quantile interval).
P<0.05 was used to indicate a statistically significant
difference.
Results
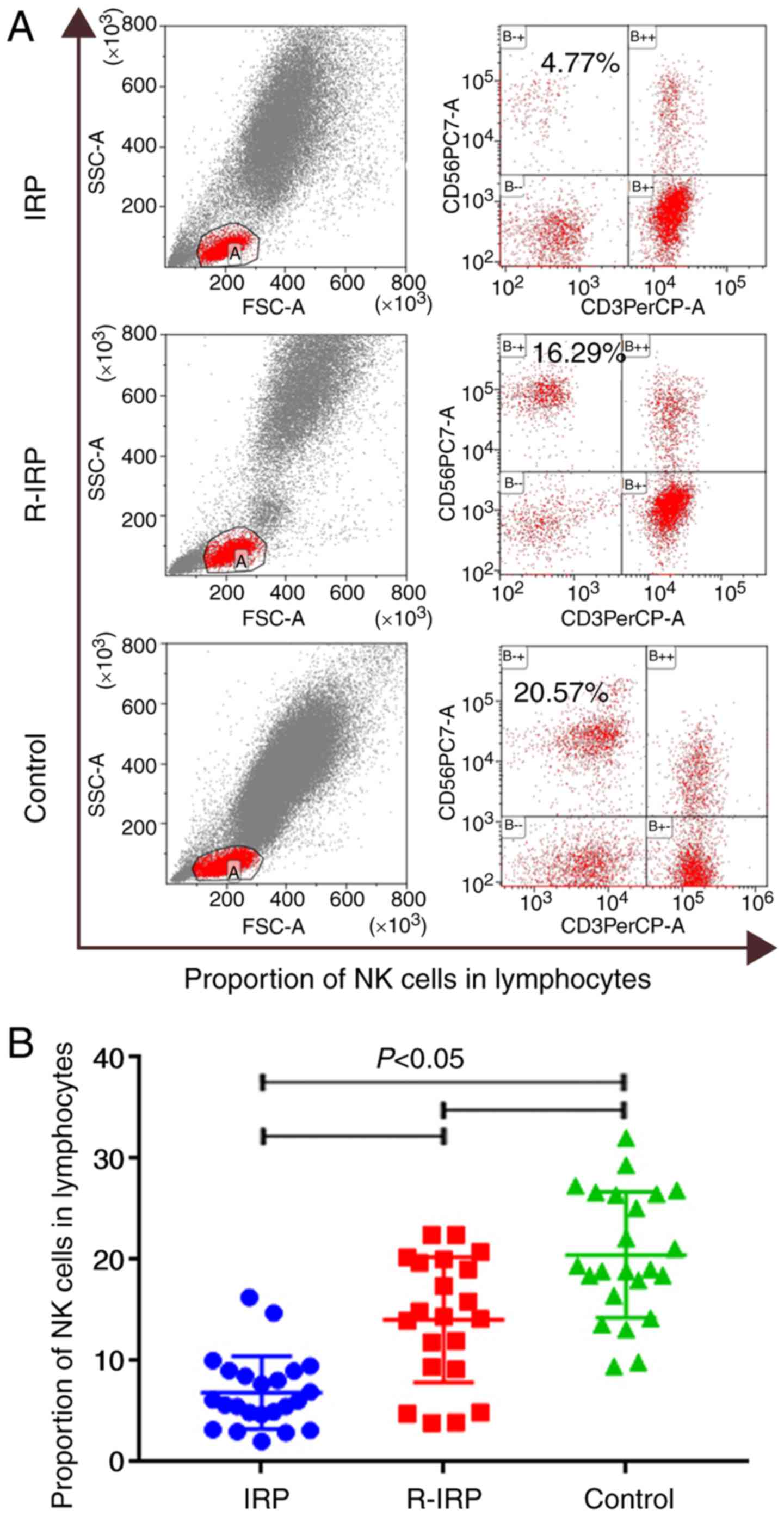
Percentage of NK cells in lymphocytes
of newly diagnosed patients with IRP is significantly
decreased
The percentage of NK cells in newly diagnosed
patients with IRP was significantly lower than the patients in
remission (P<0.01) and in healthy controls (P<0.0001).
Furthermore, the percentage of NK cells in patients in remission
was significantly lower than that in healthy controls (P=0.049;
P<0.05; Fig. 2).
Changes in purity before and after NK
cell culture
Before MACS, the proportion of NK cells in
lymphocytes accounted for 15.86%. The purity of NK cells was
≤94.14% before culture, but the purity of NK cells reached 96.17%
after culture (Fig. 1).
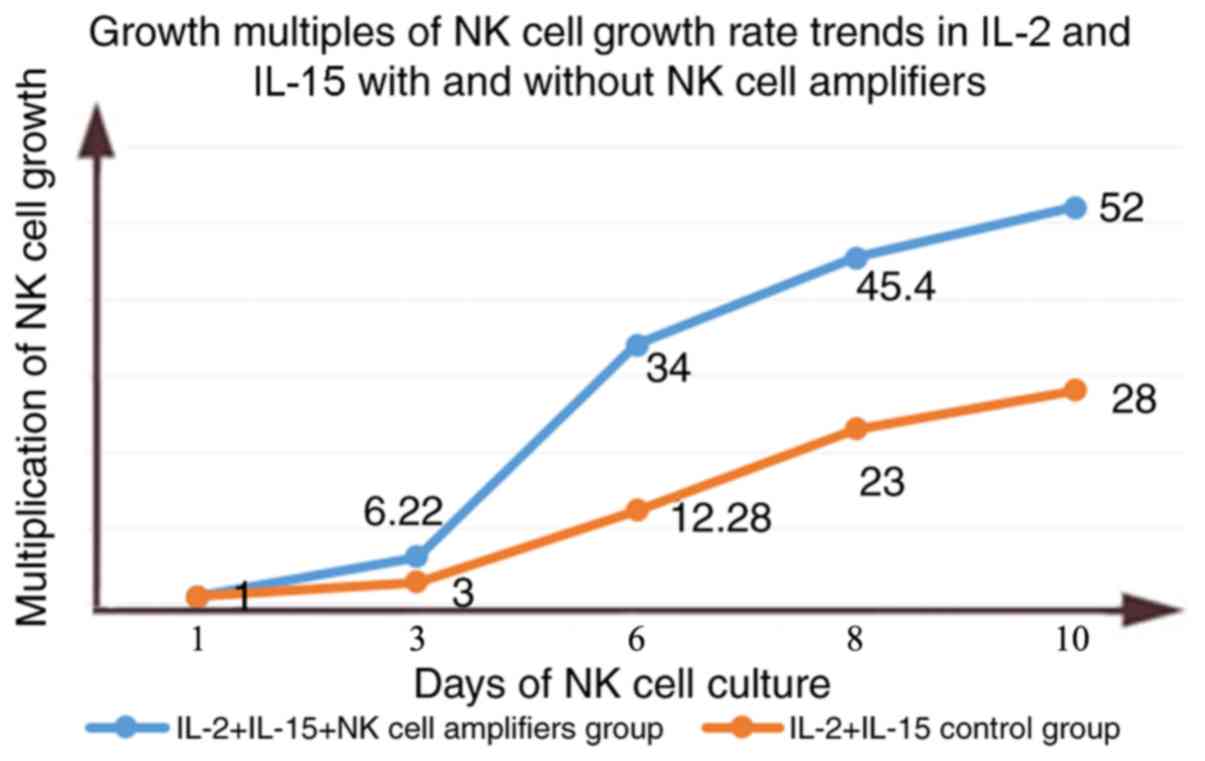
Effect of different cytokine
combinations on the human NK cell growth curve
NK cells were induced by RPMI-1640 culture medium
containing 20% FBS and different cytokines. The growth rate was
slow for the first 3 days; rIL-2+rIl-15 increased by ~3.5 times,
and the increase of rIL-2+rIL-15+NK cell amplifiers was ~5 times.
On day 6, the two groups of cells entered the logarithmic growth
phase, and the number of cells increased rapidly. At day 10, the
rIL-2+rIL-15 control group and the rIL-2+rIL-15+NK cell amplifiers
group were 28 times and 52 times, respectively, reaching the
maximum amplification of NK cells (Tables II and III). The highest multiple of
amplification of the rIL-2+rIL-15 control group and the
rIL-2+rIL-15+NK cell amplifying agent experimental group are shown
in Fig. 3. Overall, the
experimental group of the rIL-2+rIL-15+NK cell expansion agent was
the best for the purification of NK cells (Fig. 3).
 | Table II.Quantitative changes after
rIL-2+rIL-15 stimulation of NK cells. |
Table II.
Quantitative changes after
rIL-2+rIL-15 stimulation of NK cells.
|
| Number of NK
cells |
|---|
|
|
|
|---|
| Day | Sample 1 | Sample 2 | Sample 3 | Sample 4 | Sample 5 | Sample 6 |
|---|
| 1 |
9.6×104 |
2.2×104 |
6.6×104 |
1.6×104 |
9.3×104 |
3.3×104 |
| 3 |
2.4×105 |
6.468×104 |
1.02×105 |
2.944×104 |
2.79×105 |
1.293×105 |
| 6 |
4.032×105 |
1.77×105 |
3.036×105 |
1.324×105 |
1.142×106 |
3.828×105 |
| 8 |
1.01×106 |
4.312×105 |
7.062×105 |
3.104×105 |
2.13×106 |
7.095×105 |
| 10 |
1.98×107 |
5.192×105 |
1.036×106 |
4.384×105 |
2.6×106 |
9.075×105 |
 | Table III.Quantitative changes after
rIL-2+rIL-15+NK cell expansion agent stimulation of NK cells. |
Table III.
Quantitative changes after
rIL-2+rIL-15+NK cell expansion agent stimulation of NK cells.
|
| Number of NK
cells |
|---|
|
|
|
|---|
| Day | Sample 1 | Sample 2 | Sample 3 | Sample 4 | Sample 5 | Sample 6 |
|---|
| 1 |
9.6×104 |
2.2×104 |
6.6×104 |
1.6×104 |
9.3×104 |
3.3×104 |
| 3 |
3.36×105 |
5.17×104 |
3.597×105 |
7.74×104 |
5.784×105 |
1.716×105 |
| 6 |
1.07×106 |
2.09×105 |
5.676×105 |
1.484×105 |
3.162×106 |
7.128×105 |
| 8 |
2.55×106 |
2.99×105 |
1.12×106 |
2.784×105 |
4.22×106 |
1.237×106 |
| 10 |
2.68×106 |
3.762×105 |
1.35×106 |
3.68×106 |
4.836×106 |
1.56×106 |
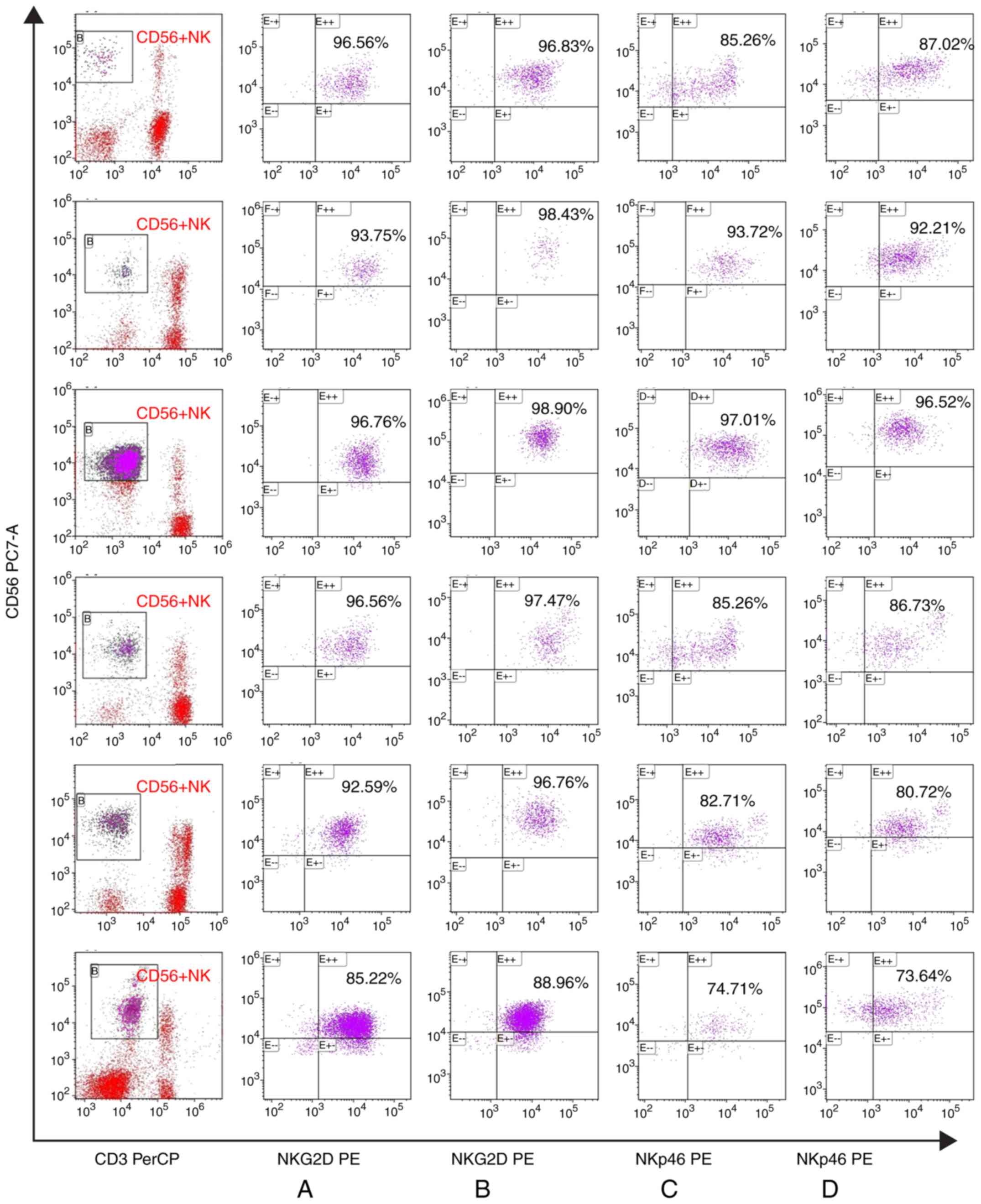
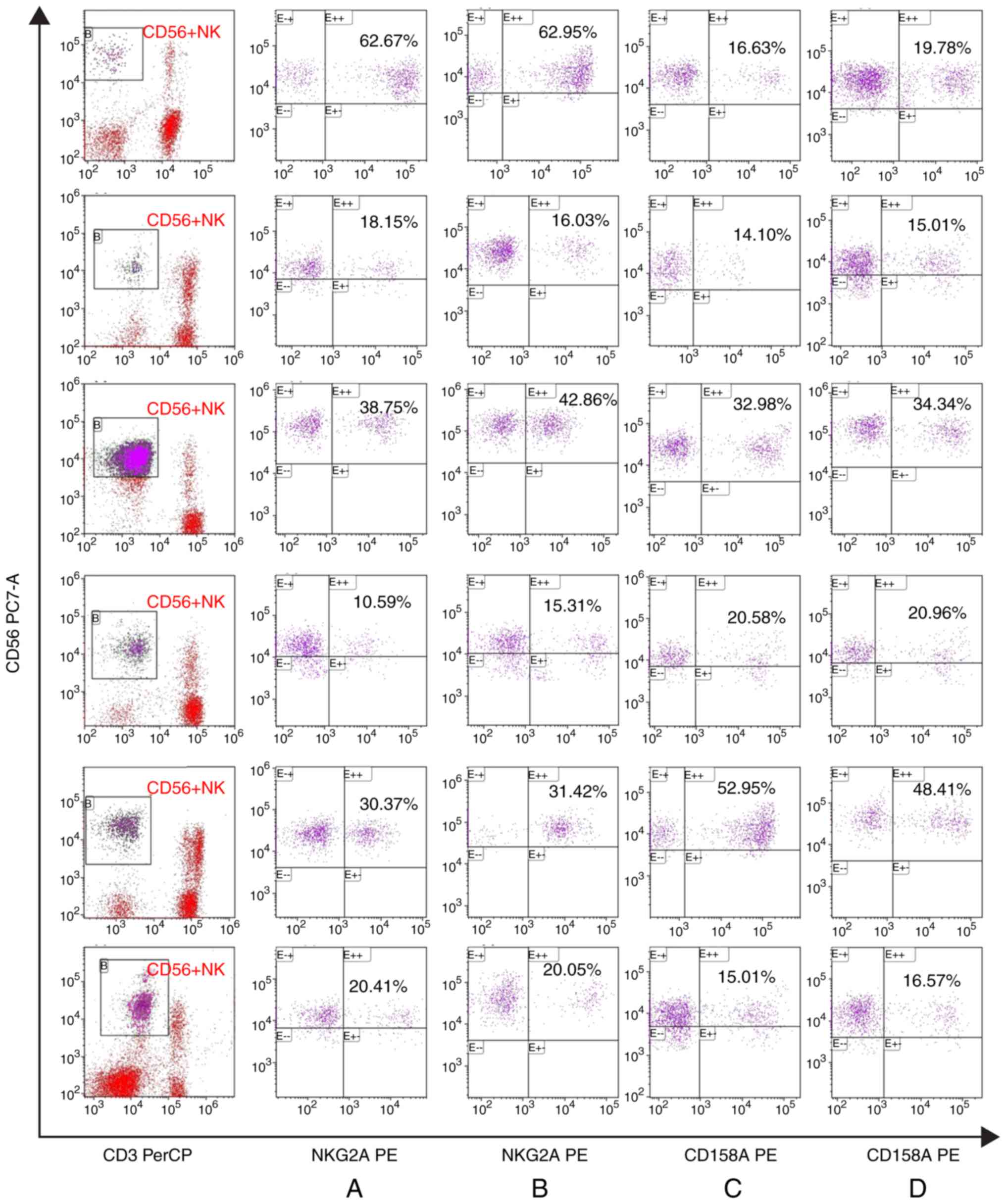
Changes to antibodies expressed by NK
cells before and after cell culture
There were no marked changes in the expression of
the activated receptor NKG2D and NKp46 in patients with IRP before
and after NK cell culture. Similarly, there were no marked changes
in the expression of the inhibitory receptors CD158a and NKG2A in
CD56+NK cells before and after NK cell culture (Figs. 4 and 5).
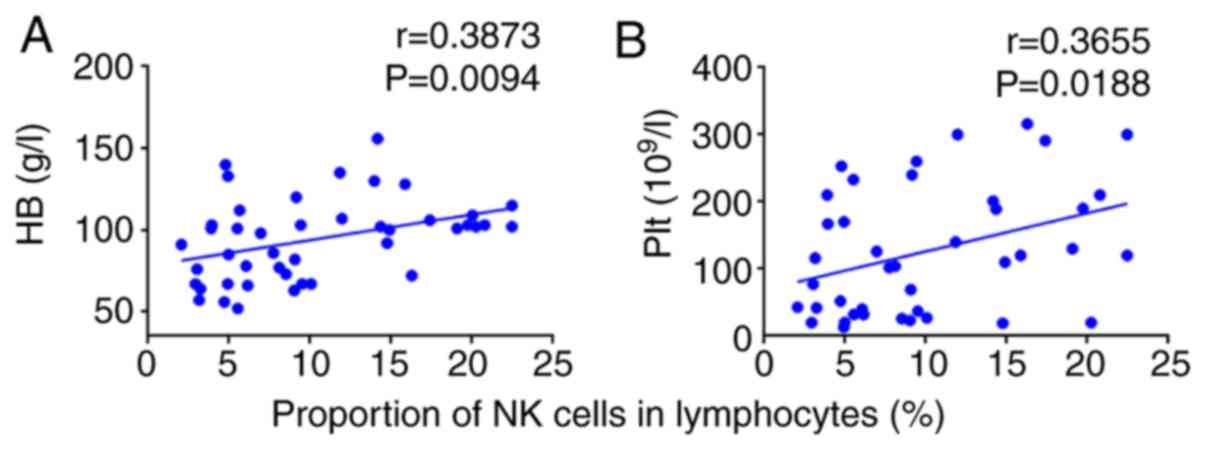
Correlation between the proportion of
NK cells in the lymphocytes of the patients and clinical
indicators
Correlations between the proportion of NK cells and
clinical and immune indices (CD4+/CD8+ and
CD5+CDl9+/CDl9+) were analyzed.
The proportion of NK cells in the lymphocytes of patients was
positively correlated with the hemoglobin count (r=0.3873;
P=0.0094; P<0.01; Fig. 6A). The
proportion of NK cells in the lymphocytes of patients was
positively correlated with the platelet count (r=0.3655; P=0.0188;
P<0.05; Fig. 6B).
The more severe the anemia and the lower the
platelet value, the lower the percentage of NK cells in patients
with IRP. Therefore, the proportion of NK cells is associated with
the severity of the disease.
Discussion
Following years of research, IRP is now recognized
as an immune-related disease. In our previous clinical studies
(11,12), patients with IRP were treated by
experimental administration of corticosteroids and/or high-dose γ
globulin; after 3 months of follow-up, all patients had responded
to treatment. Therefore, it was suggested that the incidence of
this disease might be related to abnormal humoral immunity in
patients (11,12). A previous in-depth study (11) of its pathogenic mechanism showed
that immunorelated haemocytopenia (IRP or BMMNC-Coombs
test-positive hemocytopenia) is an autoimmune disease where
autoantibodies target BM hemopoietic cells.
NK cells are a group of large granular lymphocytes
(13) that play an important
regulatory role in innate immunity and adaptive immunity, through
antibody-dependent cell-mediated cytotoxicity and NK cytotoxicity
(14,15). NK cells are an important part of
the innate immune system, particularly with respect to anti-viral
immunity and scavenging tumor cells, and can directly kill tumor
cells without specific antigen recognition (16,17).
In the present study, it was identified that the proportion of NK
cells in lymphocytes in newly diagnosed patients with IRP was
significantly decreased. It was hypothesized that that the
proportion of NK cells may be related to the immunopathogenesis of
IRP. Therefore, NK cells were isolated from patients with IRP, and
amplification and functional studies in vitro were
performed.
Isolated NK cells with high-purity cannot meet the
needs of further clinical studies as they only account for 10–15%
of peripheral blood lymphocytes (7). Therefore, it is particularly
important to rapidly and efficiently amplify large numbers of
high-purity NK cells. Therefore, researchers have studied different
amplification methods for NK cells. Carlens et al (18) applied CD3 monoclonal antibodies and
phytohemagglutinin to stimulate PBMCs, then added rIL-2 to amplify
cells; it was possible to obtain a large number of lymphocytes
(~193 times) in vitro, but the purity of NK cells was not
sufficiently high. Luhm et al (19) used feeding cells and cytokines to
amplify the purified NK cells obtained via MACS; the amplification
efficiency and NK cell purity were higher, but the feeding cells
must be involved in the culture system. Klingemann and Martinson
(20) and Li et al
(21) tried to establish a simple
and efficient NK cell expansion system in vitro, but only
produced cells of low purity and with low amplification efficiency.
In the present study, NK cell expansion agents were added and
promising results were identified. In our previous study, anti-CD3
was used to stimulate NK cells (22); however, it was mainly to measure
the function of NK cells, and the aim of the present study was to
primarily measure the number of NK cells. It is undeniable that
these studies met the needs of scientific research to a certain
extent; however, it was not possible to implement these methods in
clinical application. Based on the experiments mentioned above,
high-purity NK cells were isolated from the peripheral blood of 6
patients with IRP by MACS in the present study. Subsequently, an NK
cell culture system was used to expand cells in vitro, which
markedly reduced the cost of sorting NK cells.
In the present study, an NK cell expansion system
was designed, including RPMI-1640 culture medium containing 20%
FBS, rIL-2 and rIL-15, with or without an NK cell expansion
reagent. Li et al (23)
demonstrated that using rIL-2 combined with rIL-15 stimulation can
generate NK cells with high-purity, strong cytotoxic activity and
high amplification efficiency. Wang et al (24) expanded NK cells in vitro
using four methods, of which the combination of rIL-2 and rIL-15
was the simplest, and there was no significant difference in
amplification efficiency with other combinations. IL-2 is a
cytokine of the chemokine family, able to stimulate the
proliferation, killing activity, and cytokine production of NK
cells (25). The biological effect
of IL-15 is similar to that of IL-2, and it has synergistic effect
with IL-2 in stimulating the proliferation and activation of NK
cells (26). Therefore, the
amplification effects on NK cells of rIL-2 and rIL-15 combined with
or without NK cell expansion agents were compared.
To observe the amplification effect of NK cell
expansion agents on NK cells, the culture system was divided into
two groups according to different cytokine combinations: The
rIL-2+rIL-l5 group and the rIL-2+rIL-l5+NK cell amplification
group. The results demonstrated that after 10 days of culture,
compared with the control group, the aforementioned two groups can
promote the expansion of NK cells in vitro, and maintain the
high-purity of NK cells after amplification. An NK cell expansion
agent was added to the experimental group, and the proliferation
effect was markedly improved compared with the rIL-2+rIL-15 alone
group. The highest amplification rate was ×28 in the rIL-2+rIL-15
group, and the highest amplification rate was ×52 in the group with
the added expansion agent.
NKG2D and NKp46 (27,28)
are the activated receptors on NK cells, while NKG2A and CD158a are
the inhibitory receptors on CD56+NK cells; they all play an
important role in activating the killing function of NK cells. The
expression of the activated receptors NKG2D and NKp46 on NK cells
did not differ before and after cell culture. Similarly, there was
no difference in the expression of NKG2A and CD158a on NK cells.
This suggested that the function of NK cells after amplification
was similar to before culture. These results suggested that an NK
cell expansion agent can markedly promote the expansion of NK cells
in vitro, and can markedly enhance the efficiency of
amplification with rIL-2+rIL-15.
The immune status of patients with IRP was impaired,
including a decrease in NK cell numbers and suppression of their
protective function (29).
Therefore, NK cells from patients with IRP were screened for
amplification instead of those from healthy people. It may be safer
and more effective for expanded NK cells to be transfused back into
patients. In the present study, NK cells have not been transfused
back into patients; further experiments are required in the future.
In summary, it was possible to obtain many NK cells with
high-purity and a high proliferation rate from a small number of
high-purity NK cells by MACS sorting. The NK cells were
successfully amplified from incubation in a culture system
containing rIL-2 (500 U/ml), rIL-15 (20 ng/ml), an NK cell
expansion agent and RPMI-1640 (containing 20% FBS). Most
importantly, this culture amplification method is simple and
cost-effective, providing a good foundation for NK cells in the use
of tumor and adoptive immunotherapy.
Acknowledgements
Not applicable.
Funding
The present study was supported by The Tianjin
Chronic Disease Prevention and Control Science and Technology
Special Project (grant no. 16ZXMJSY00180), The Tianjin Natural
Science Foundation (grant no. JCQNJC11500) and The National Natural
Science Foundation of China (grant nos. 81400085, 81570106,
81600088, 81600093, 81800119 and 81700117).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
CL, LL and TC conceived and designed the study. YL,
BL and SD performed the experiments and were major contributors in
writing the manuscript. ZS analyzed and interpreted the data. RF
conceived the study, participated in its design and coordination,
and helped to draft the manuscript. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
The present study complied with the Declaration of
Helsinki and was approved by The Ethics Committee of Tianjin
Medical University General Hospital. Written informed consent was
obtained from all healthy controls and all patients or their
guardians.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Shao Y, Fu R, Liu H, Wang Y, Ding S, Wang
H, Li L and Shao Z: IgG autoantibody subclasses altered in
immuno-related hemocytopenia. Cell Immunol. 294:13–20. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fu R, Liu H, Wang Y, Liu H, He H, Chen J,
Wang H, Yu H, Ding K, Huang L, et al: Distinguishing immunorelated
haemocytopenia from idiopathic cytopenia of undetermined
significance (ICUS): A bone marrow abnormality mediated by
autoantibodies. Clin Exp Immunol. 177:412–418. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Li Y, Wang Y, Liu H, Ding K, Hao S, Shao
Y, Wang H, Chen J, Huang L, Shao Z and Fu R: Lower level of IL-35
and its reduced inhibition in Th17 cells in patients with bone
marrow mononuclear cells Coombs test-positive hemocytopenia. Mol
Med Rep. 17:2973–2981. 2018.PubMed/NCBI
|
|
4
|
Maghazachi AA: Role of chemokines in the
biology of natural killer cells. Curr Top Microbiol Immunol.
341:37–58. 2010.PubMed/NCBI
|
|
5
|
Carotta S: Targeting NK cells for
anticancer immunotherapy: Clinical and Preclinical Approaches.
Front Immunol. 7:1522016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Pittari G, Filippini P, Gentilcore G,
Grivel JC and Rutella S: Revving up natural killer cells and
cytokine-induced killer cells against hematological malignancies.
Front Immunol. 6:2302015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Purdy AK and Campbell KS: Natural killer
cells and cancer: Regulation by the killer cell Ig-like receptors
(KIR). Cancer Biol Ther. 8:2211–2220. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu YF, Zhang BH, Cen DY, Wei J and Chen C:
Expression of perforin in cord blood NK cells after IL-2/IL-15
stimulation and its relation with cytotoxicity. Zhongguo Shi Yan
Xue Ye Xue Za Zhi. 19:1015–1018. 2011.(In Chinese). PubMed/NCBI
|
|
9
|
Matikainen S, Paananen A, Miettinen M,
Kurimoto M, Timonen T, Julkunen I and Sareneva T: IFN-alpha and
IL-18 synergistically enhance IFN-gamma production in human NK
cells: Differential regulation of Stat4 activation and IFN-gamma
gene expression by IFN-alpha and IL-12. Eur J Immunol.
31:2236–2245. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Peng BG Liang LJ, He Q, Li J and Lu MD:
Selective expansion of human natural killer cells. Ai Zheng.
24:1287–1289. 2005.(In Chinese). PubMed/NCBI
|
|
11
|
Wang YH, Fu R, Dong SW, Liu H and Shao ZH:
Erythroblastic islands in the bone marrow of patients with
immune-related pancytopenia. PLoS One. 9:e951432014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu H, Fu R, Li L, Ding K, Wang Y, Wang H,
Zhang T, Wang G, Song J and Shao Z: Erythropoietin receptors and
IgG autoantibody expression on nucleated erythrocytes in some cases
of immuno-related pancytopenia. Clin Lab. 61:693–698. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Iannello A, Débbeche O, Samarani S,
Sabbagh S, Duval M and Ahmad A: Potential role of NK cells in
future anti-tumor immunotherapies. Med Sci (Paris). 23:502–508.
2007.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Vivier E, Tomasello E, Baratin M, Walzer T
and Ugolini S: Functions of natural killer cells. Nat Immunol.
9:503–510. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lünemann A, Lünemann JD and Münz C:
Regulatory NK-cell functions in inflammation and autoimmunity. Mol
Med. 15:352–358. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Forel JM, Chiche L, Thomas G, Mancini J,
Farnarier C, Cognet C, Guervilly C, Daumas A, Vély F, Xéridat F, et
al: Phenotype and functions of natural killer cells in
critically-ill septic patients. PLoS One. 7:e504462012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Farag SS, VanDeusen JB, Fehniger TA and
Caligiuri MA: Biology and clinical impact of human natural killer
cells. Int J Hematol. 78:7–17. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Carlens S, Gilljam M, Chambers BJ, Aschan
J, Guven H, Ljunggren HG, Christensson B and Dilber MS: A new
method for in vitro expansion of cytotoxic human
CD3−CD56+ natural killer cells. Hum Immunol.
62:1092–1098. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Luhm J, Brand JM, Koritke P, Höppner M,
Kirchner H and Frohn C: Large-scale generation of natural killer
lymphocytes for clinical application. J Hematother Stem Cell Res.
11:651–657. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Klingemann HG and Martinson J: Ex vivo
expansion of natural killer cells for clinical applications.
Cytotherapy. 6:15–22. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li Y, Huang SL, Wu YF, Wei J, Bao R and
Zhou DH: Expansion of CIK/NK cells from cord blood by using
different combinations of stem cell factor, FLT3 ligand and
interleukin 2, 7, 15 in vitro. Zhongguo Shi Yan Xue Ye Xue Za Zhi.
12:350–354. 2004.(In Chinese). PubMed/NCBI
|
|
22
|
Chen T, Liu C, Li L, Liu H, Wang T, Shao Z
and Fu R: CD56bright natural killer cells exhibit
abnormal phenotype and function in severe aplastic anemia. Int J
Lab Hematol. 41:353–363. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Li XH, Ma J, Wu XX, Wang FF, Li M, Da WM,
Yu L and Gao CJ: Study on ex vivo expansion of highly purified NK
cells from human peripheral blood and changes in their function.
Zhonghua Xue Ye Xue Za Zhi. 30:404–408. 2009.(In Chinese).
PubMed/NCBI
|
|
24
|
Wang XM, Li L, Yu JP, et al: Comparison of
four kinds of NK cell in vitro expansion methods. Chin J Cancer
Biotherapy. 336–341. 2013.(In Chinese).
|
|
25
|
Hutmacher C, Gonzalo Núñez N, Liuzzi AR,
Becher B and Neri D: Targeted delivery of IL2 to the tumor stroma
potentiates the action of immune checkpoint inhibitors by
preferential activation of NK and CD8+ T cells. Cancer
Immunol Res. 7:572–583. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Carson WE, Giri JG, Lindemann MJ, Linett
ML, Ahdieh M, Paxton R, Anderson D, Eisenmann J, Grabstein K and
Caligiuri MA: Interleukin (IL) 15 is a novel cytokine that
activates human natural killer cells via components of the IL-2
receptor. J Exp Med. 180:1395–1403. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jelenčić V, Šestan M, Kavazović I,
Lenartić M, Marinović S, Holmes TD, Prchal-Murphy M, Lisnić B, Sexl
V, Bryceson YT, et al: NK cell receptor NKG2D sets activation
threshold for the NCR1 receptor early in NK cell development. Nat
Immunol. 19:1083–1092. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Sandoval-Borrego D, Moreno-Lafont MC,
Vazquez-Sanchez EA, Gutierrez-Hoya A, López-Santiago R,
Montiel-Cervantes LA, Ramírez-Saldaña M and Vela-Ojeda J:
Overexpression of CD158 and NKG2A inhibitory receptors and
underexpression of NKG2D and NKp46 activating receptors on NK cells
in acute myeloid leukemia. Arch Med Res. 47:55–64. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yuan X, Fu R, Liu H, Wang YH, Li LJ, Liu
CY, Wang HL, Shao YY, Ding K, Chen J, et al: Quantities and
function of NK cells in patients with positive BMMNC-coombs test
and cytopenia. Zhonghua Xue Ye Xue Za Zhi. 37:393–398. 2016.(In
Chinese). PubMed/NCBI
|