Introduction
Breast cancer is the second leading cause of
cancer-related mortality in women in the United States, with annual
255,180 diagnosed cases and 41,070 deaths reported in 2017
(1). Triple-negative breast cancer
(TNBC), which accounts for ~15% of all breast cancers, represents a
collection of cancers that do not express estrogen receptor (ER),
progesterone receptor (PR) and erb-b2 receptor tyrosine kinase 2
(HER2) (2). Given the lack of
expression of ER, PR and HER2, the effective treatment options for
patients with TNBC are typically limited to cytotoxic therapies.
However, the effect of chemotherapy is often diminished by the
development of drug resistance (2).
Acquired drug resistance refers to the scenario
wherein patients that are initially sensitive to chemotherapy
eventually develop resistance during treatment (2,3).
Exploring how cancer cells can eliminate the damaging effect of
chemotherapeutic drugs and how to improve drug sensitivity are
important strategies for treating cancer. Therefore, novel
chemotherapy strategies must be developed. Combining
chemotherapeutics with traditional Chinese medicine can result in
cooperative effects, reduction in the required doses of
chemotherapeutics, and consequently low drug toxicity, decreased
side effects and reduced drug resistance (4,5).
Gambogic acid (GA), which is the main active
ingredient of gamboge, is a brownish to orange dry resin that is
secreted from Garcinia hanburyi, a plant widely found in
nature. Previous studies (6–8) have
revealed that GA has potent antitumor effects on TNBC cells in
vitro and in vivo. In addition, GA could reverse
docetaxel resistance in gastric cancer, cisplatin resistance in
lung cancer, doxorubicin resistance in breast and ovarian cancers,
5-fluorouracil resistance in colorectal cancer, and multidrug
resistance in epithelial cancer (9–12).
However, the effect of GA on paclitaxel-resistant TNBC remains
unknown, and the mechanisms by which GA induces anticancer effects
remain to be elucidated.
In the present study, the results revealed that GA
treatment inhibited proliferation and induced apoptosis of
paclitaxel-resistant TNBC cells through the sonic hedgehog (SHH)
signaling pathway. The current study reported that GA overcame drug
resistance and may therefore serve as a combination treatment for
TNBC therapy.
Materials and methods
Cell culture and establishment of
paclitaxel-resistant cell lines
The human TNBC cell lines MDA-MB-231 and MDA-MB-468
were obtained from The Cell Bank of Type Culture Collection of the
Chinese Academy of Sciences (Shanghai, China) and cultured in DMEM
medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with
10% FBS (HyClone; GE Healthcare Life Sciences) in a humidified
incubator at 37°C with 5% CO2. To establish
paclitaxel-resistant cell lines, MDA-MB-231 and MDA-MB-468 cells
were cultured with 1 µM paclitaxel (Sigma-Aldrich; Merck KGaA) for
60 days. The medium with 1 µM paclitaxel was changed every 3 days.
GA (Key Laboratory of Carcinogenesis and Intervention, China
Pharmaceutical University, Nanjing, China) was dissolved in DMSO
(Sigma-Aldrich; Merck KGaA) and stored at −20°C.
Cell proliferation assay via real-time
cell impedance analysis (RTCA)
For RTCA, the xCELLigence system (Roche Applied
Science) was applied to dynamically monitor cell proliferation
rates. The assay was executed according to the manufacturer's
instructions. The impedance was indicated as cell index. RTCA
software, supplied by the manufacturer, was used to analyze the
measurements.
Cell viability assay
Cell viability was examined by the Cell Counting
Kit-8 (CCK8) assay (Beyotime Institute of Biotechnology). Briefly,
MDA-MB-231 and MDA-MB-468 cells were trypsinized and seeded at
3,000 cells/well in a 96-well plate. After culturing for the
indicated time (0, 24, 48 and 72 h), 10 µl of CCK-8 reagent was
added into each well and incubated at 37°C. After 3 h, the
absorbance of each well was measured using a Multiskan MK3
spectrophotometer set at a wavelength of 450 nm.
Colony formation assay
Paclitaxel-resistant MDA-MB-231 and MDA-MB-468 cells
(400 cells/well) were seeded in 6-well plates. After 1 week of
culture, the colonies were fixed with methanol at room temperature
for 20 min, stained with 0.1% crystal violet (Beyotime Institute of
Biotechnology) for 20 min, and the images of the stained colonies
were captured using a CKX41 light microscope (Olympus Corporation).
The number of colonies was counted from the images.
Flow cytometry
The apoptotic rate of cells was examined via the
Annexin V and propidium iodide (PI) double-staining method,
according to the manufacturer's instructions (Nanjing KeyGen
Biotech Co., Ltd.). The stained cells were immediately analyzed via
flow cytometry using ModFit LT 3.0 (Verity Software House,
Inc.).
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA from cells was isolated using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.), according to
the manufacturer's protocol. cDNA was synthesized from 1 µg total
RNA using a PrimeScript RT Reagent kit with gDNA Eraser (Takara
Biotechnology Co., Ltd.), according to the manufacturer's
instructions. qPCR was performed using SYBR Premix Ex Taq (Takara
Biotechnology Co., Ltd.). The thermocycling conditions were: 5 min
at 95°C, followed by 40 cycles of 30 sec at 95°C, 60 sec at 60°C
and 30 sec at 72°C; 1 sec at 99°C; 15 sec at 59°C; 1 sec at 95°C;
followed by cooling to 40°C. β-actin was used as the internal
reference control. Relative fold changes in mRNA expression were
calculated using the formula 2−ΔΔCq (13). The primer sequences were: SHH,
forward 5′-CCCAATTACAACCCCGACATC-3′ and reverse
5′-TCACCCGCAGTTTCACTCCT-3′; patched 1 (PTCH1), forward
5′-TGAGACTGACCACGGCCTG-3′ and reverse 5′-ACCCTCAGTTGGAGCTGCTTC-3′;
GLI family zinc finger 1 (GLI1), forward
5′-AGGGCTGCAGTAAAGCCTTCA-3′ and reverse 5′-CTTGACATGTTTTCGCAGCG-3′;
and β-actin, forward 5′-GATCATTGCTCCTCCTGAGC-3′ and reverse
5′-ACTCCTGCTTGCTGATCCAC-3′.
Western blot analysis
The protein expression levels of SHH, PTCH1, GLI1,
Bcl-2, BAX, and cleaved caspase-3 were analyzed via western blot
assay. Total proteins were extracted from tissues using the T-PER
Tissue Protein Extraction Reagent (Pierce; Thermo Fisher
Scientific, Inc.). Protein concentrations were determined using a
bicinchoninic acid (BCA) Protein Assay Kit (Pierce; Thermo Fisher
Scientific, Inc.). Proteins (20 µg) were separated by SDS-PAGE (10%
gels) and transferred to polyvinylidene fluoride membranes (EMD
Millipore). Membranes were blocked at room temperature with 5%
non-fat milk for 1 h and incubated at 4°C overnight with the
following antibodies: SHH (1:200; cat. no. ab19897; Abcam), PTCH1
(1:200; cat. no. ab53715; Abcam), GLI1 (1:200; cat. no. ab49314;
Abcam), Bcl-2 (1:500; cat. no. ab196495; Abcam), cleaved caspase-3
(1:500; cat. no. ab2302; Abcam), BAX (1:500; cat. no. ab53154;
Abcam) and β-actin (1:500; cat. no. ab8227; Abcam), then treated
with a Horseradish peroxidase-labeled goat anti-rabbit secondary
antibody (1:1,000; cat. no. ab150077; Abcam) for 2 h at room
temperature. The protein bands were visualized using an enhanced
chemiluminescence system (Beyotime Institute of Biotechnology).
β-actin served as a loading control for normalization.
Immunohistochemistry
The expression of SHH in the xenograft tumors was
detected as described previously (4), using an anti-SHH antibody (1:200;
cat. no. ab19897; Abcam). The tumors were rinsed in PBS, followed
by fixation with 3% neutral formalin for 24 h at room temperature.
Paraffin-embedded sections of tumor tissue (4 µm thick) were
deparaffinized in xylene, rehydrated via graded alcohol solutions,
blocked in methanol containing 3% hydrogen peroxide for 10 min at
room temperature, and then incubated with SHH antibody at 4°C
overnight. Following rinsing with PBS solution, biotinylated goat
anti-rabbit serum IgG (1:2,000; cat. no. ab64256; Abcam) was used
as secondary antibody for 2 h at room temperature and streptavidin
peroxidase complex reagent was applied for 1 h at room temperature.
Finally, the sections were incubated in a 3,3′-diaminobenzidine
solution at room temperature for 10 min and then counterstained
with hematoxylin for 3 min at room temperature. Ten randomly
selected visual fields per section were examined under a light
microscope in order to evaluate the SHH expression.
Animal studies
All experiments involving animals were approved by
the Animal Care and Welfare Committee of WeiFang People's Hospital
(permit no. WF2016032702). Female BALB⁄c nude mice (n=24; age, 4
weeks; weight, 20–25 g) were procured from the Laboratory Animal
Center of YangZhou University (YangZhou, China) and maintained
under specific pathogen-free conditions (26–28°C, air pressure
difference 10–20 kPa, 10-h light/14-h dark cycle, food and water
provided ad libitum). Animal health and behavior were
monitored every day.
Paclitaxel-resistant MDA-MB-231 (5×106
cells/mouse, suspended in 200 µl normal saline) were subcutaneously
injected into the right flanks of athymic nude mice. Seven days
post-injection, the mice were divided into four groups (n=6 per
group) and subjected to different treatments as follows: Group 1,
saline control administration; group 2, 5 mg/kg paclitaxel
administration; group 3, 2 mg/kg GA administration; group 4, 2
mg/kg GA + 5 mg/kg paclitaxel combined administration. The
intravenous administrations were done once every other day for 14
days. Tumor volume (mm3) was calculated every 3 days
using the formula V=0.5× length × width2. All mice were
sacrificed by cervical dislocation after 14 days of treatments.
Tumors were collected and photographed at 2 weeks after
treatments.
Statistical analysis
All experiments were performed in triplicate. Unless
otherwise indicated, the data were presented as mean ± SD.
Statistical significance was determined using SPSS 13.0 (SPSS,
Inc.). Differences between two groups were assessed using Student's
t-test (two-tailed). Data from more than two groups were analyzed
using one-way ANOVA followed by Tukey's test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Establishment of the
paclitaxel-resistant TNBC cells
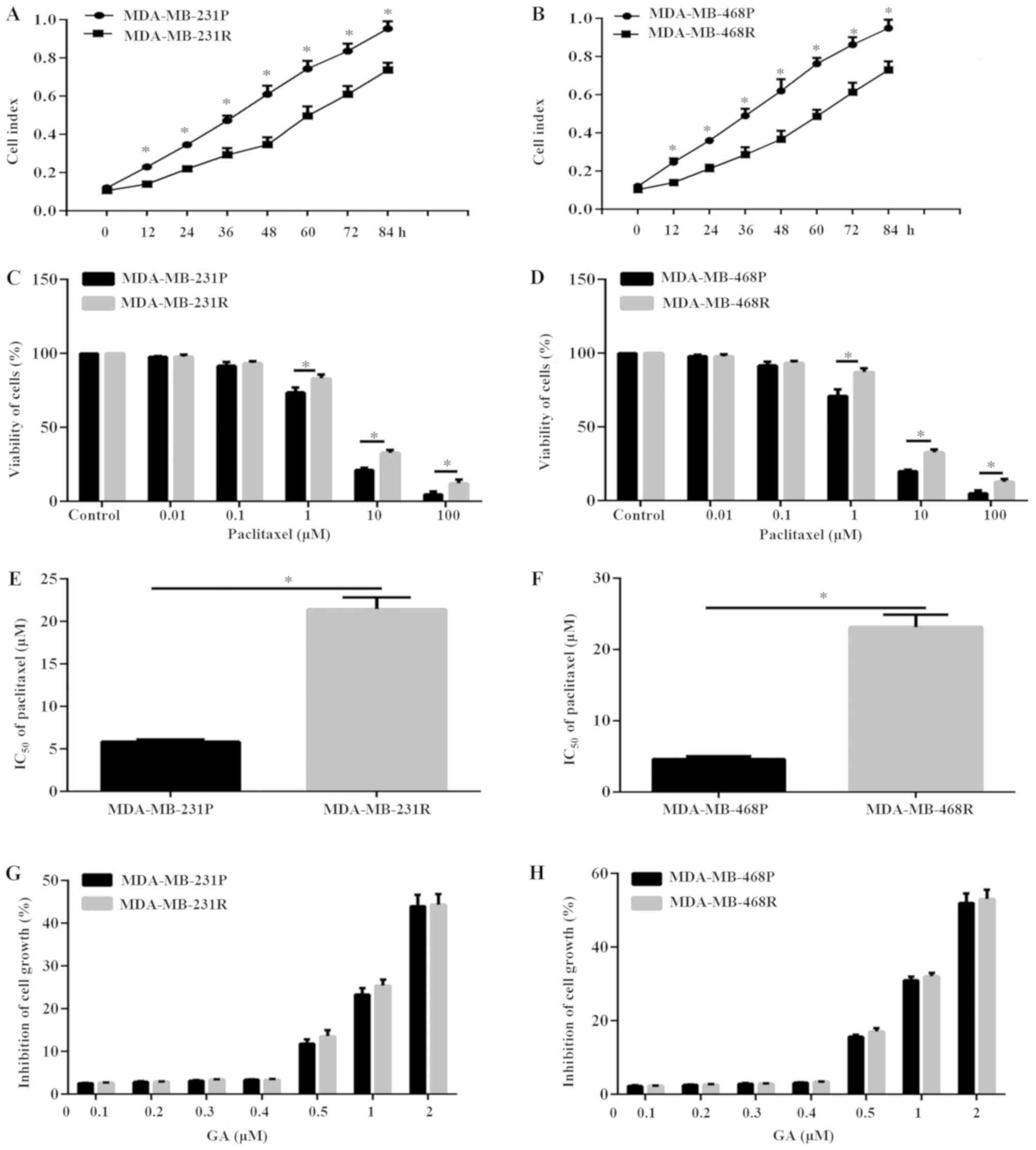
To explore the potential anticancer effect of GA on
paclitaxel-resistant TNBC, the TNBC cell lines MDA-MB-231 and
MDA-MB-468 were cultured with 1 µM paclitaxel for 60 days in order
to establish paclitaxel-resistant cells (termed thereafter
MDA-MB-231R and MDA-MB-468R, respectively). MDA-MB-231R and
MDA-MB-468R cells grew significantly slower compared with the
drug-sensitive parental cells (termed MDA-MB-231P and MDA-MB-468P,
respectively; Fig. 1A and B). In
order to confirm the establishment of paclitaxel-resistant TNBC
cells, the sensitivity to paclitaxel of MDA-MB-231 cells and
MDA-MB-468 cells was analyzed. Results of CCK-8 assay demonstrated
that the sensitivity to paclitaxel of MDA-MB-231R and MDA-MB-468R
cells was significantly reduced compared with the MDA-MB-231P and
MDA-MB-468P cells, respectively (Fig.
1C and D). In addition, The IC50 of paclitaxel in
MDA-MB-231R and MDA-MB-468R cells increased by ~4-fold and ~5-fold,
respectively, compared with their parental cells (Fig. 1E and F). The dose of 5 µM
paclitaxel was selected for further experiments, which was
approximately the IC50 of paclitaxel in the parental
MDA-MB-231P and MDA-MB-468P cells.
To investigate the potential cytotoxic and
antiproliferation effects of GA, MDA-MB-231 and MDA-MB-468,
parental and resistant, cells were cultured with different
concentrations of GA for 8 h. The results illustrated that
treatment with GA at concentrations >0.4 µM induced significant
inhibition of MDA-MB-231 and MDA-MB-468 cell numbers, in both the
parental and resistant cell lines, in a dose-dependent manner
(Fig. 1G and H). To avoid the
inhibitory effects of GA, the non-inhibitory GA concentration of
0.2 µM was selected for subsequent experiments in the present
study.
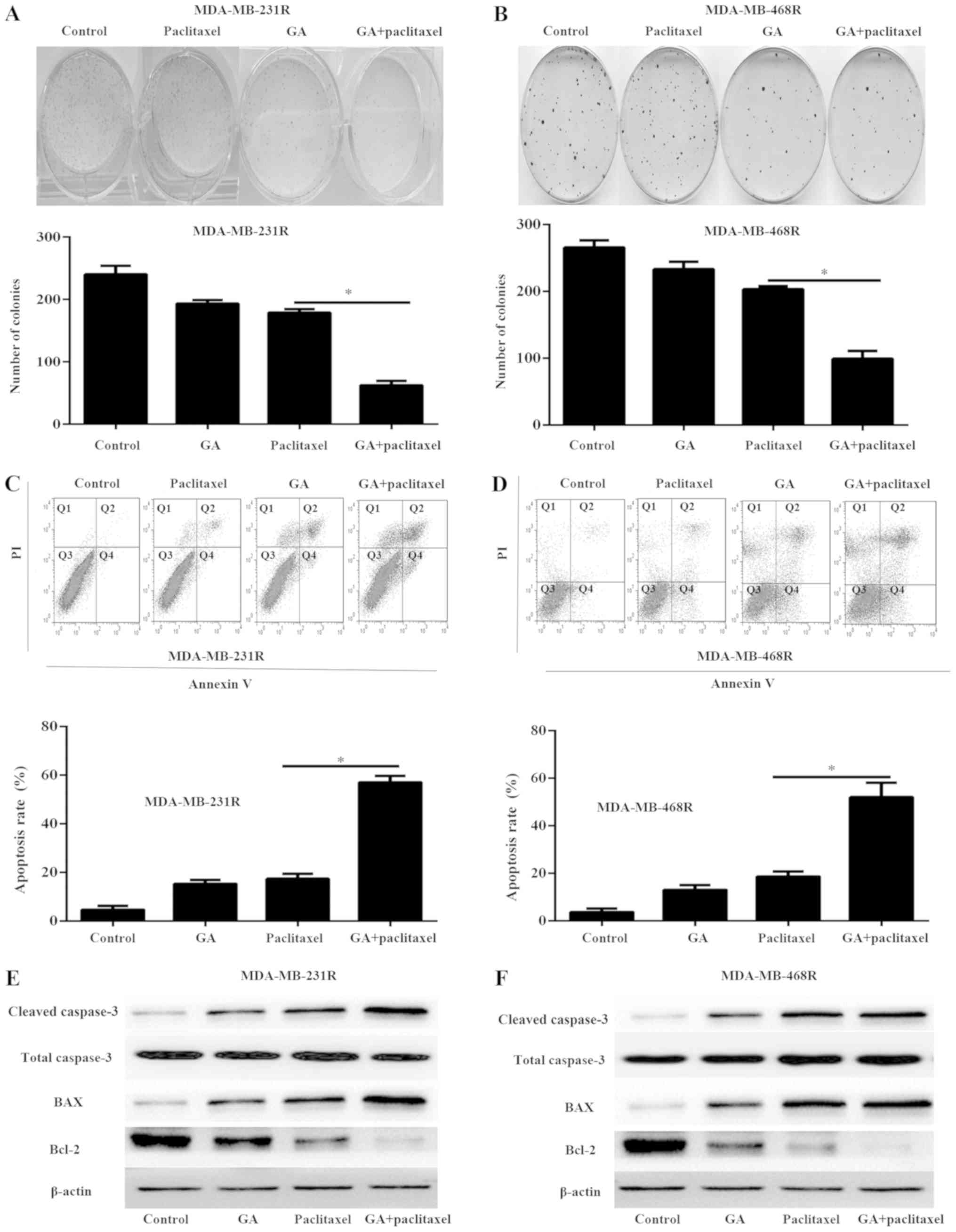
Combination of paclitaxel and GA
inhibits proliferation and induces apoptosis
MDA-MB-231R and MDA-MB-468R cells were treated with
5 µM paclitaxel and/or 0.2 µM GA in the subsequent experiments. To
investigate the anticancer effect of GA and paclitaxel on
MDA-MB-231R and MDA-MB-468R cells, colony formation assays were
performed (Fig. 2A and B).
Treatment of MDA-MB-231R and MDA-MB-468R cells with paclitaxel or
GA alone only weakly inhibited cell colony formation. However, the
combination treatment resulted in fewer colonies compared with
paclitaxel or GA treatment alone (Fig.
2A and B). In addition, the cell apoptosis rate was determined
by flow cytometry analysis. The combination of paclitaxel and GA
resulted in increased apoptosis compared with paclitaxel or GA
treatment alone (Fig. 3C and D).
Furthermore, western blot analysis was used to detect the
expression of cleaved caspase-3, Bcl-2 and BAX in the MDA-MB-231R
and MDA-MB-468R cells following the indicated treatments. The
results demonstrated that the combination treatment significantly
increased the expression of cleaved caspase-3 and BAX and decreased
the expression of Bcl-2 than either agent alone (Fig. 2E and F). These results demonstrated
that GA enhanced the cytotoxicity effects of paclitaxel in
resistant TNBC cells.
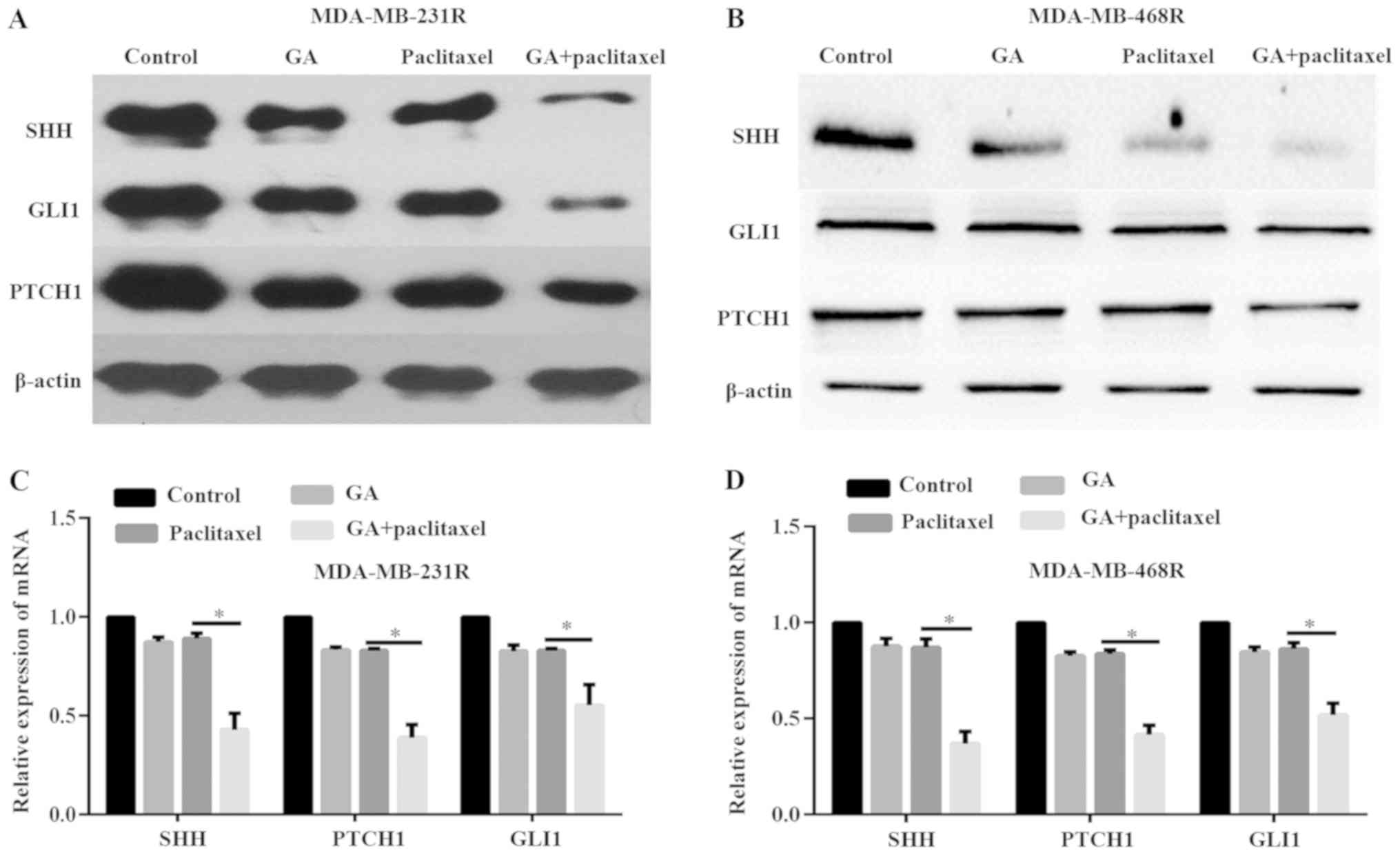
GA sensitizes MDA-MB-231R and
MDA-MB-468R cells to paclitaxel by inhibiting the SHH pathway in
vitro
A previous study has reported that the SHH signaling
pathway is associated with paclitaxel resistance in breast cancer
(14). To investigate the
underlying mechanisms of GA reducing MDA-MB-231R and MDA-MB-468R
cell drug resistance, the expression levels of SHH and its target
genes GLI1 and PTCH1 were determined both at the protein and mRNA
level, via western blot and qPCR analyses, respectively. The
results demonstrated that treatment of MDA-MB-231R and MDA-MB-468R
cells with paclitaxel or GA alone only weakly decreased the
expression of SHH, GLI1 and PTCH1, both at the protein and mRNA
level (Fig. 3A-D). However, the
combination of paclitaxel and GA significantly inhibited the
expression of SHH, GLI1 and PTCH1 compared to either agent alone
(Fig. 3A-D). These data indicated
that combination of GA with paclitaxel enhanced the antitumor
effects of paclitaxel in resistant TNBC cells through inactivation
of the SHH signaling pathway in vitro.
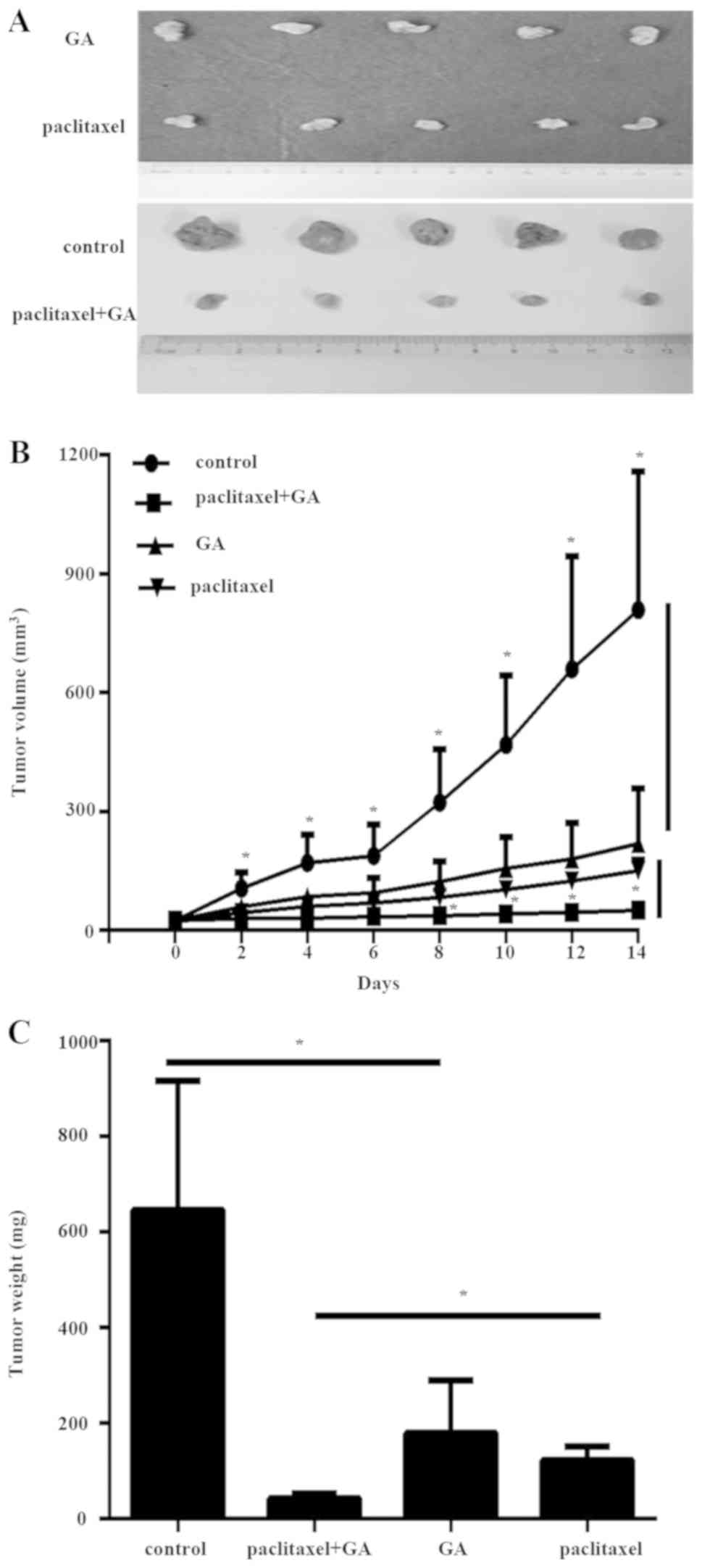
GA increases the sensitivity to
paclitaxel in resistant TNBC cells in vivo
Next, the effect of GA on the sensitivity toward
paclitaxel of MDA-MB-231R cells was investigated in vivo,
via a mouse model. After 14 days of treatments, the tumors were
removed and photographed (Fig.
4A). The volume and weight measurements of the excised
xenograft tumors revealed that combination of GA with paclitaxel
resulted in significantly reduced tumor growth (Fig. 4B and C). These data indicated that
GA significantly enhanced the antitumor effect of paclitaxel in
paclitaxel-resistant TNBC cells.
GA sensitizes TNBC to paclitaxel
through inhibiting the SHH pathway in vivo
To explore the potential mechanism of GA enhancing
drug sensitivity in vivo, immunohistochemistry and qPCR
analyses were performed on the xenograft tumor tissues. The results
revealed that paclitaxel or GA treatment alone only weakly
inhibited the expression of SHH in the tumor tissues (Fig. 5A and B). However, the combination
paclitaxel and GA treatment significantly decreased the expression
of SHH in the xenograft tumors compared with paclitaxel alone
(Fig. 5A and B), suggesting that
GA sensitized TNBC cells to paclitaxel via inhibiting the SHH
pathway in vivo.
To further investigate the mechanism of the
combination treatment on tumor growth, the protein expression
levels of cleaved caspase-3, Bcl-2 and BAX were detected by western
blotting. In accordance with the results of the in vitro
experiments, the combination of paclitaxel and GA markedly enhanced
the expression levels of cleaved caspase-3 and BAX and decreased
the expression levels of Bcl-2 in the xenograft tumors, compared
with either agent alone (Fig.
5C).
Discussion
Drug resistance is a serious problem that leads to
therapeutic failure in breast cancer. The mechanisms underlying
drug resistance are poorly understood and overcoming drug
resistance is an important endeavor that must be achieved in order
to increase the overall survival of patients with cancer. Natural
plant products, such as GA, have been extensively investigated for
their potential to reverse drug resistance, which would be
beneficial in the success of chemotherapy treatments. In the
present study, the mechanism by which GA overcomes drug resistance
was investigated in TNBC.
GA is a candidate drug that has been approved by the
China Food and Drug Administration for a phase II clinical trial in
solid tumor therapy (15).
Previous studies showed that GA could induce apoptosis in a broad
range of human cancers (4,6,7,16,17).
Furthermore, GA treatment combined with chemotherapeutic drugs
resulted in a synergistic effect on the chemotherapeutic efficacy
against drug-resistant cancer cells (9–12).
Therefore, GA could possibly overcome the paclitaxel resistance in
TNBC by promoting apoptosis.
To explore the potential of GA to overcome
paclitaxel resistance in TNBC cells, the present study first
established the paclitaxel-resistant TNBC cancer cell lines
MDA-MB-231R and MDA-MB-468R. Compared with paclitaxel alone, the
combined application of paclitaxel and GA synergistically reduced
the colony formation abilities of MDA-MB-231R and MDA-MB-468R
cells. The results of flow cytometry and western blot analyses
demonstrated that the additive effect of GA to paclitaxel was
accompanied by an enhanced apoptosis. Similarly, other previous
studies have also reported that GA combined with other
chemotherapeutic drugs could enhance the apoptosis rate of drugs in
a broad range of cancer cells (9–12).
In summary, the present study demonstrated that GA significantly
decreased the cell viability and enhanced the cells apoptosis of
MDA-MB-231R and MDA-MB-468R cells induced by paclitaxel. These data
indicated that GA could increase the sensitivity to paclitaxel in
paclitaxel-resistant TNBC.
The SHH signaling pathway is crucial for regulating
various cell processes, such as proliferation, cell growth,
survival, inflammatory response and apoptosis (18). Enhanced activation of the SHH
pathway is linked to the development and progression of several
types of cancer and to chemotherapy resistance (18). A recent study reported that the
inactivation of the SHH signaling pathway is involved in the
success of chemotherapy-induced apoptosis in breast cancer
(14). In the present study, the
results indicated that GA might enhance the drug sensitivity of
paclitaxel in human breast cancer by inactivating the SHH signaling
pathway.
Apoptosis constitutes a fundamental intrinsic
mechanism underlying tumor suppression, and the resistance of
apoptosis is a well-established aspect of cancer (19). The activation of the SHH pathway
promotes cell survival, upregulates Bcl-2 and downregulates cleaved
caspase-3 and BAX, which are key regulators of apoptosis (14). In the present study, the results
demonstrated that GA and paclitaxel treatment significantly
increased the expression of cleaved caspase-3 and BAX and decreased
the expression of bcl-2, via modulating the activation of the SHH
signaling pathway. These findings suggested that GA reversed drug
resistance in TNBC cells by inhibiting the SHH signaling pathway,
indicating that GA could be a potentially useful natural therapy
for overcoming drug resistance in vitro. A limitation of the
present study is that only the SHH pathway was investigated in
regards to the role of GA in paclitaxel-resistant TNBC. Future
studies will investigate the potential involvement of the other
signaling pathways in GA paclitaxel sensitization.
In the present study, a xenograft
paclitaxel-resistant TNBC tumor model was generated in nude mice
through subcutaneous inoculation of MDA-MB-231R cells, in order to
evaluate the effect of GA on the drug sensitivity and the SHH
signaling pathway in vivo. As expected from the in
vitro results, the combination of paclitaxel and GA
significantly reduced the tumor size and inactivated the SHH
signaling pathway in the xenograft tumors. In addition, the
combination of paclitaxel and GA significantly enhanced the
expression of cleaved caspase-3 and BAX and reduced the expression
of Bcl-2 in the xenograft tumors, compared with either treatment
alone. These results were consistent with the present findings
in vitro.
In conclusion, the present results revealed that GA
treatment inhibited proliferation and induced apoptosis in
paclitaxel-resistant TNBC cells, by inhibiting the SHH signaling
pathway in vitro and in vivo. These findings
indicated that GA may be a promising adjuvant drug for the therapy
of paclitaxel-resistant TNBC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data analyzed during the present study are
included in this published article.
Authors' contributions
YW and YT conceived and designed the experiments.
YW, YS and YT conducted all of the experiments. YW and YT wrote and
revised the manuscript. All authors read and approved the final
manuscript.
Ethics approval and consent to
participate
All experiments involving animals were approved by
the Animal Care and Welfare Committee of WeiFang People's Hospital
(permit no. WF2016032702).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Holohan C, Van Schaeybroeck S, Longley DB
and Johnston PG: Cancer drug resistance: An evolving paradigm. Nat
Rev Cancer. 13:714–726. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
3
|
Zahreddine H and Borden KL: Mechanisms and
insights into drug resistance in cancer. Front Pharmacol. 4:282013.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang F, Zhang W, Guo L, Bao W, Jin N, Liu
R, Liu P, Wang Y, Guo Q and Chen B: Gambogic acid suppresses
hypoxia-induced hypoxia-inducible factor-1a/vascular endothelial
growth factor expression via inhibiting phosphatidylinositol
3-kinase/Akt/mammalian target protein of rapamycin pathway in
multiple myeloma cells. Cancer Sci. 105:1063–1070. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Suzuki R, Kang Y, Li X, Roife D, Zhang R
and Fleming JB: Genistein potentiates the antitumor effect of
5-Fluorouracil by inducing apoptosis and autophagy in human
pancreatic cancer cells. Anticancer Res. 34:4685–4692.
2014.PubMed/NCBI
|
|
6
|
Qi Q, Lu N, Wang XT, Gu HY, Yang Y, Liu W,
Li C, You QD and Guo QL: Anti-invasive effect of gambogic acid in
MDA-MB-231 human breast carcinoma cells. Biochem Cell Biol.
86:386–395. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li C, Qi Q, Lu N, Dai Q, Li F, Wang X, You
Q and Guo Q: Gambogic acid promotes apoptosis and resistance to
metastatic potential in MDA-MB-231 human breast carcinoma cells.
Biochem Cell Biol. 90:718–730. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li D, Song XY, Yue QX, Cui YJ, Liu M, Feng
LX, Wu WY, Jiang BH, Yang M, Qu XB, et al: Proteomic and
bioinformatic analyses of possible target-related proteins of
gambogic acid in human breast carcinoma MDA-MB-231 cells. Chin J
Nat Med. 13:41–51. 2015.PubMed/NCBI
|
|
9
|
Wang S, Wang L, Chen M and Wang Y:
Gambogic acid sensitizes resistant breast cancer cells to
doxorubicin through inhibiting P-glycoprotein and suppressing
survivin expression. Chem Biol Interact. 235:76–84. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang X, Deng R, Lu Y, Xu Q, Yan M, Ye D
and Chen W: Gambogic acid as a non-competitive inhibitor of
ATP-binding cassette transporter B1 reverses the multidrug
resistance of human epithelial cancers by promoting ATP-binding
cassette transporter B1 protein degradation. Basic Clin Pharmacol
Toxicol. 112:25–33. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang J and Yuan Z: Gambogic acid
sensitizes ovarian cancer cells to doxorubicin through ROS-mediated
apoptosis. Cell Biochem Biophys. 67:199–206. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang W, Zhou H, Yu Y, Li J, Li H, Jiang
D, Chen Z, Yang D, Xu Z and Yu Z: Combination of gambogic acid with
cisplatin enhances the antitumor effects on cisplatin-resistant
lung cancer cells by downregulating MRP2 and LRP expression. Onco
Targets Ther. 9:3359–3368. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
He M, Fu Y, Yan Y, Xiao Q, Wu H, Yao W,
Zhao H, Zhao L, Jiang Q, Yu Z, et al: The hedgehog signalling
pathway mediates drug response of MCF-7 mammosphere cells in breast
cancer patients. Clin Sci. 129:809–822. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chi Y, Zhan XK, Yu H, Xie GR, Wang ZZ,
Xiao W, Wang YG, Xiong FX, Hu JF, Yang L, et al: An open-labeled,
randomized, multicenter phase IIa study of gambogic acid injection
for advanced malignant tumors. Chin Med J (Engl). 126:1642–1646.
2013.PubMed/NCBI
|
|
16
|
Huang GM, Sun Y, Ge X, Wan X and Li CB:
Gambogic acid induces apoptosis and inhibits colorectal tumor
growth via mitochondrial pathways. World J Gastroenterol.
21:6194–6205. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yang Y, Sun X, Yang Y, Yang X, Zhu H, Dai
S, Chen X, Zhang H, Guo Q, Song Y, et al: Gambogic acid enhances
the radiosensitivity of human esophageal cancer cells by inducing
reactive oxygen species via targeting Akt/mTOR pathway. Tumour
Biol. 37:1853–1862. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Rimkus TK, Carpenter RL, Qasem S, Chan M
and Lo HW: Targeting the sonic hedgehog signaling pathway: Review
of smoothened and GLI inhibitors. Cancers. 8(pii): E222016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Noble P, Vyas M, Al-Attar A, Durrant S,
Scholefield J and Durrant L: High levels of cleaved caspase-3 in
colorectal tumour stroma predict good survival. Br J Cancer.
108:2097–2105. 2013. View Article : Google Scholar : PubMed/NCBI
|