Introduction
Cases of pancreatic cancer account for 3% of all new
cancer diagnoses; however this disease the fourth leading cause of
cancer-associated mortality due to its highly aggressive nature
(1). With the increasing incidence
of pancreatic cancer, this disease is predicted to be the second
most common cause of cancer-associated mortality by 2030 (2). Pancreatic ductal adenocarcinoma
(PDAC), which develops from the exocrine pancreas, is the most
common type of pancreatic cancer and accounts for 95% of pancreatic
cancer cases (3). Surgical
resection is the only treatment that prolongs survival; however,
the 5-year survival rates are low even after curative resection
(4–6).
Transforming growth factor-β (TGF-β) signaling has a
central role in the development of multiple cancers, including PDAC
(7,8). The activation of TGF-β signaling
promotes epithelial-mesenchymal transition (EMT) to mediate
metastasis of PDAC tumors (9),
which is a major cause of failure in treatment and the high
mortality rate among patients with PDAC. TGF-β signaling is
involved in the pathogenesis of PDAC through interactions with
multiple regulatory molecules, such microRNAs (miRNAs) (10). Interactions between TGF-β signaling
and long non-coding RNAs (lncRNAs) in PDAC have not been well
investigated. LINC01638 is a recently characterized lncRNA with an
oncogenic role in triple breast cancer (11). LINC01638 lncRNA promotes the
development and progression of triple-negative breast cancer by
activating metadherin-Twist1 signaling through the inhibition of
speckle-type POZ protein-mediated c-Myc degradation (11); however, the role of LINC01638
lncRNA in other diseases is unknown. A preliminary microarray
analysis was performed and revealed that LINC01638 lncRNA was
upregulated in PDAC, indicating the potential role of LINC01638
lncRNA in PDAC. The findings of the present study indicated that
LINC01638 may upregulate TGF-β to regulate the migration and
invasion of PDAC cells.
Materials and methods
Patients and specimens
A total of 102 patients with PDAC were diagnosed and
treated in West China Hospital (Chengdu, China) between March 2016
and March 2018. The current study included 46 of the 102 patients
according to inclusion and exclusion criteria. Inclusion criteria
were as follows: i) Patients were diagnosed with PDAC via biopsies;
ii) patients were willing to donate adjacent healthy tissue
biopsies; iii) patients fully understood the experimental protocol
and were willing to participate. Exclusion criteria were as
follows: i) Patients with other malignancies; ii) patients with
chronic diseases; iii) patients that had received treatment for any
disease within 3 months prior to sampling. The study also included
38 healthy volunteers from whom samples were collected at the
aforementioned hospital during the same time period to serve as the
control group. Controls were age- and sex-matched to the patient
group. Volunteers with a family history of malignancy were
excluded. No significant differences in age and sex were observed
between the two groups. Basic information is presented in Table I. This study was approved by the
ethics committee of West China Hospital. All participants signed
informed consent and fully understood the experimental
protocol.
 | Table I.Basic information of the patient and
control groups. |
Table I.
Basic information of the patient and
control groups.
|
| Sex |
|
|
|---|
|
|
|
|
|
|---|
| Groups | Male | Female | Age range
(years) | Average age
(years) |
|---|
| Patient | 25 | 21 | 25-68 | 45.5±4.9 |
| Control | 20 | 18 | 24-69 | 46.1±5.4 |
Specimen collection
Blood (5 ml) was extracted from each participant 1
day after admission before breakfast. Blood samples were used to
prepare plasma. Briefly, blood was centrifuged at 1,200 × g in EDTA
tubes at room temperature for 20 min, and the supernatant (plasma)
was collected. Tumor tissues and adjacent healthy tissues within 2
cm around tumors were collected from 3 different sites in each PDAC
patient through fine needle biopsy. All samples were kept in liquid
nitrogen prior to use.
Reverse transcrttpion-quantitative
polymerase chain reaction
TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) was used to extract
total RNA with all operations performed in strict accordance with
manufactuer's instructions. cDNA was synthesized using SuperScript
IV Reverse Transcriptase (Thermo Fisher Scientific, Inc.) under the
following conditions: 25°C for 5 min, 55°C for 30 min and 75°C for
10 min. SYBR® Green Real-Time PCR Master Mixes (Thermo
Fisher Scientific, Inc.) was used to prepare all PCR rection
systems. PCR reaction conditions were: 1 min at 95°C, followed by
40 cycles of 12 sec at 95°C and 40 sec at 58.5°C. Primers used in
PCR reactions were: 5′-AATACATCAGCACTGTTGCCTTT-3′ (forward)
5′-CTCCATACATACATCTCCAAAAAGT-3′ (reverse) for LINC01638 lncRNA;
5′-GACCTCTATGCCAACACAGT-3′ (forward) and 5′-AGTACTTGCGCTCAGGAGGA-3′
(reverse) for β-actin. Multiple endogenous controls (such as 18S
rRNA) were used and consistent results were obtained. Cq valus were
processed using 2−ΔΔCq method (12). Expression level of LINC01638 lncRNA
was normalized to the sample with the lowest expression level,
which was set to 1. All PCR products were sequenced and correct
products were obtained.
ELISA
Plasma levels of TGF-β1 were measued using Human
TGF-β1 Quantikine ELISA kit (cat. no. DB100B; R&D Systems,
Inc., Minneapolis, MN, USA). All operations were performed in
strict according with manufactuer's instructions.
Cell lines and cell culture
Human PDAC cell line PL45 (ATCC®
CRL-2558™) and normal pancreas duct cell line hTERT-HPNE
(ATCC® CRL-4023™) were purchased from the American Type
Culture Collection (Manassas, VA, USA). Cell culture was performed
using Dulbecco's Modified Eagle's medium (DMEM; cat. no. 30-2002;
ATCC) contaning 10% fetal bovine serum (FBS; Sangon Biotech Co.,
Ltd.). Cells were cultured at 37°C with 5% CO2.
Transfection was performed using LINC01638 lncRNA expression or
negative control (NC) empty vectors (pcDNA3.1), and LINC01638
lncRNA shRNA
(5′-TGCTGTTGACAGTGAGCGCCTCTAGAATGTGCTACAATTATAGTGAAGCCACAGATGTATAATTGTAGCACATTCTAGAGTTGCCTACTGCCTCGGA-3′)
and NC shRNA
(5′-CCGGCAACAAGATGAAGAGCACCAACTCGAGTTGGTGCTCTTCATCTTGTTGTTTTT-3′)
vectors (BLOCK-iT™) provided by GeneCopoeia, Inc. (Rockville, MD,
USA). Lipofectamine® 3000 reagent (Thermo Fisher
Scientific, Inc.) was used to transfect vectors (10 nM) into the
two cell lines (5×104 cells). All operations were
performed according to the instructions of the kit. LINC01638
lncRNA expression levels were measured by RT-qPCR at 12 h after
transfection. Overexpression >200% and knockdown <50%
compared with control cells (untransfected) and negative control
cells (transfected with empty vectors) were achieved before
subsequent experiments. For TGF-β1 treatment experiments, cells
were treated with 10, 20 and 40 ng/ml exogenous TGF-β1
(Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at 37°C with 5%
CO2 for 24 h prior to subsequent experiments.
In vitro cell migration and invasion
assay
Cells were harvested at 24 h following transfection
and serum free cell suspensions with a cell density of
4×104 cells/ml were prepared. Cell migration and
invasion abilities were evaluated using Transwell migration and
invasion assays. For the migration assay, the upper chamber was
filled with 4×103 cells in 0.1 ml cell suspension
(prepared using DMEM without serum). The lower chamber was filled
with RPMI-1640 medium (Thermo Fisher Scientific, Inc.) supplemented
with 20% FBS (Sigma-Aldrich; Merck KGaA). The chamber was
maintained at 37°C for 12 h and the membranes were collected and
stained with 0.5% crystal violet (Sigma-Aldrich; Merck KGaA) for 20
min at room temperature. The same protocol was used for the
invasion assay, with Matrigel (cat. no. 356234; EMD Millipore,
Billerica, MA, USA) used to coat the upper chamber prior to use.
Migrating and invading cells were observed under an optical
microscope (magnification, ×40; 5 random fields per membrane).
Western blot analysis
Cell lysis buffer (cat. no. P0013K; Beyotime
Institute of Biotechnology, Haimen, China) was used to extract
total protein with all procedures performed in strict accordance
with the instructions of the kit. Bicinchoninic acid assay was
performed to measure protein concentrations. Following denaturing,
protein samples (30 µg/lane) were subjected to SDS-PAGE on 10%
gels. Gel transfer to polyvinylidene difluoride membranes was
performed. Then, membranes were blocked in 5% non-fat milk for 2 h
at room temperature, followed by incubation with rabbit anti-human
primary antibodies of TGF-β1 (1:1,500; cat. no. ab92486; Abcam) and
β-actin (1:1,500; cat. no. ab8227; Abcam) overnight at 4°C. The
following day, membranes were further incubated with goat
anti-rabbit IgG-horseradish peroxidase secondary antibody (1:1,300;
cat. no. MBS435036; MyBioSource, Inc., San Diego, CA, USA) at room
temperature for 2 h. Signals were developed using ECL™ Blotting
Reagents (cat. no. RPN2109; Sigma-Aldrich; Merck KGaA).
Densitometry analysis was performed using Image J software (v1.6;
National Institutes of Health, Bethesda, MD, USA).
Statistical analysis
GraphPad Prism 6 software (GraphPad Software, Inc.,
La Jolla, CA, USA) was used to process all data. All experiments
were performed three times and data were presented as the mean ±
standard deviation. Data were compared by unpaired t-test (between
two groups) and one-way analysis of variance followed by Tukey's
test (among multiple groups). Comparisons between tumor tissues and
adjacent healthy tissues were performed by paired t-test. Receiver
operating characteristic (ROC) curve analysis was performed to
evaluate the diagnostic value of plasma LINC01638 for PDAC, with
patients with PDAC as true positive cases and healthy controls as
true negative cases. Correlation between plasma levels of LINC01638
and TGF-β1 was analyzed by Pearson correlation analysis. P<0.05
was considered to indicated a statistically significant
difference.
Results
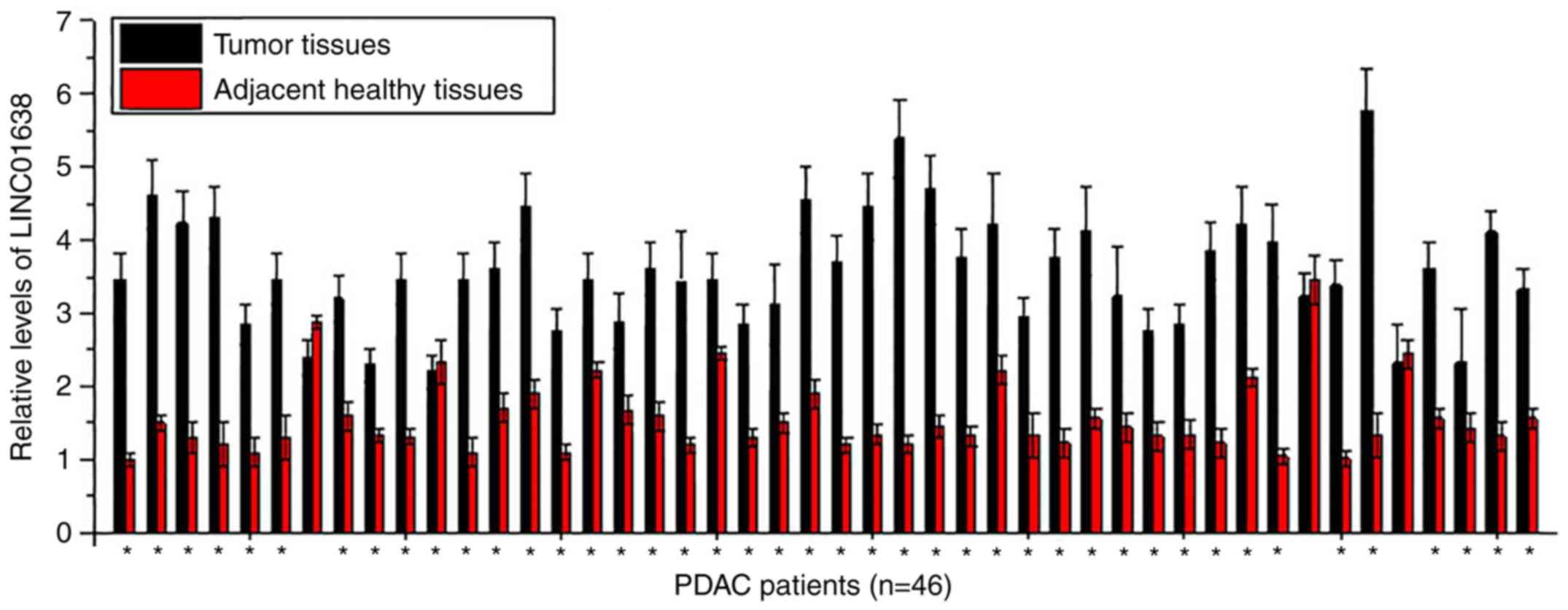
LINC01638 is significantly upregulated
in tumor tissues compared with adjacent healthy tissues in the
majority of patients with PDAC
Expression of LINC01638 in tumor tissues and
adjacent healthy tissues of 46 patients with PDAC was detected by
RT-qPCR. As presented in Fig. 1,
significantly upregulated expression levels of LINC01638 in tumor
tissues than in adjacent healthy tissues were detected in 43 of 46
patients (samples from 3 sites per tissue were analyzed),
indicating the potential role of LINC01638 overexpression in
PDAC.
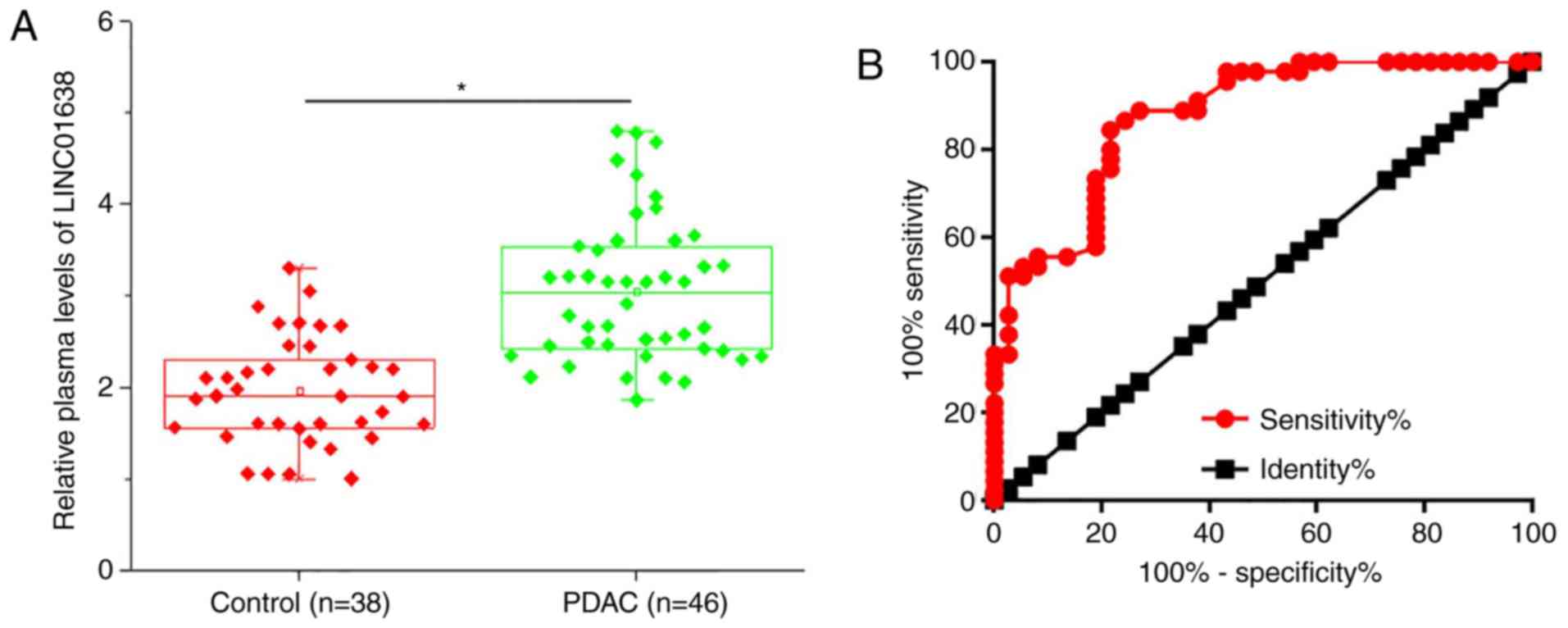
Upregulation of plasma LINC01638
distinguishes patients with PDAC from healthy controls
Plasma levels of LINC01638 in 46 patients with PDAC
and 38 healthy controls were measured by RT-qPCR. As presented in
Fig. 2A, the plasma levels of
LINC01638 were significantly higher in patients with PDAC than in
healthy controls (P<0.05). ROC curve analysis was performed to
evaluate the diagnostic value of plasma LINC01638 for PDAC
(Fig. 2B). The area under the
curve was 0.8760, with 95% confidence interval of 0.8022–0.9497 and
standard error of 0.03762 (P<0.0001). Therefore, plasma lncRNA
LINC01638 is a potential diagnostic marker for PDAC.
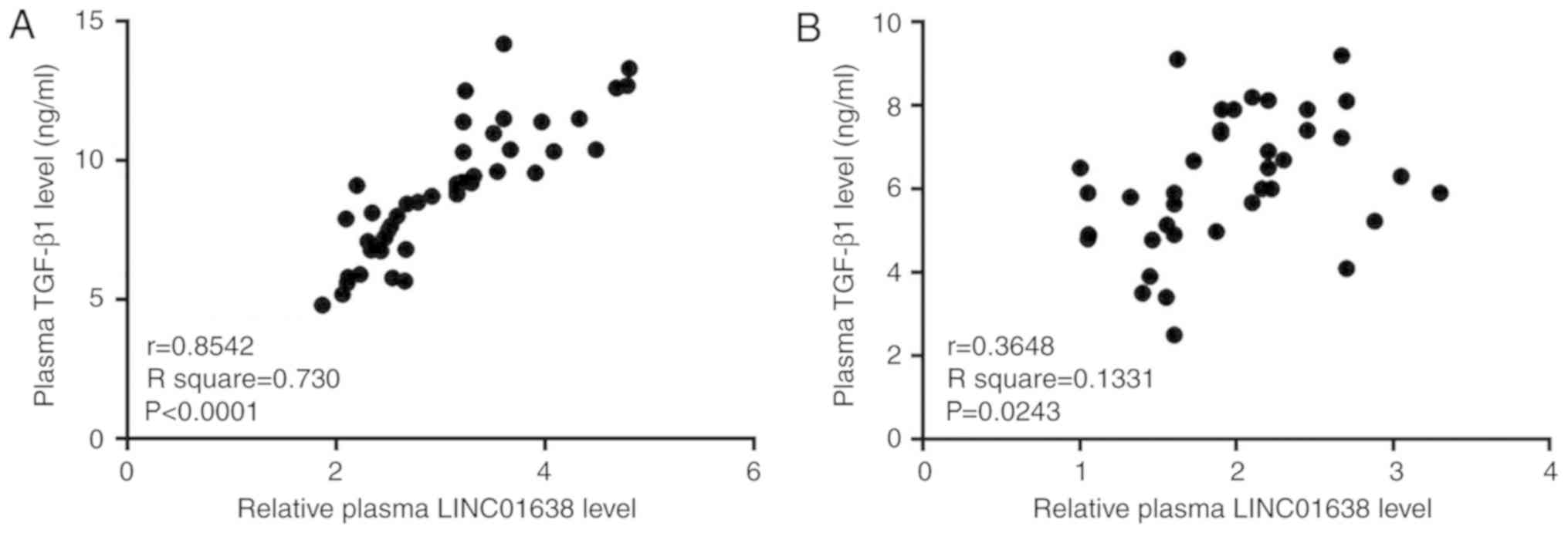
Plasma levels of LINC01638 and TGF-β1
are positively correlated in patients with PDAC, but not in healthy
controls
Correlations between the plasma levels of LINC01638
and various cancer-associated factors were analyzed by Pearson
correlation analysis. A significant positive correlation between
plasma levels of LINC01638 and TGF-β1 was observed in patients with
PDAC (R2=0.730; P<0.05, Fig. 3A). In addition, a significant
correlation (R2=0.1331; P<0.05) between the plasma
levels of LINC01638 and TGF-β1 was also observed in healthy
controls (Fig. 3B); however, the
correlation between the two factors was notably stronger in
patients with PDAC, based on the greater R2 value.
Correlation between LINC01638 and other selected factors were not
significant (data not shown). These data indicated the potential
association between LINC01638 and TGF-β1 in PDAC.
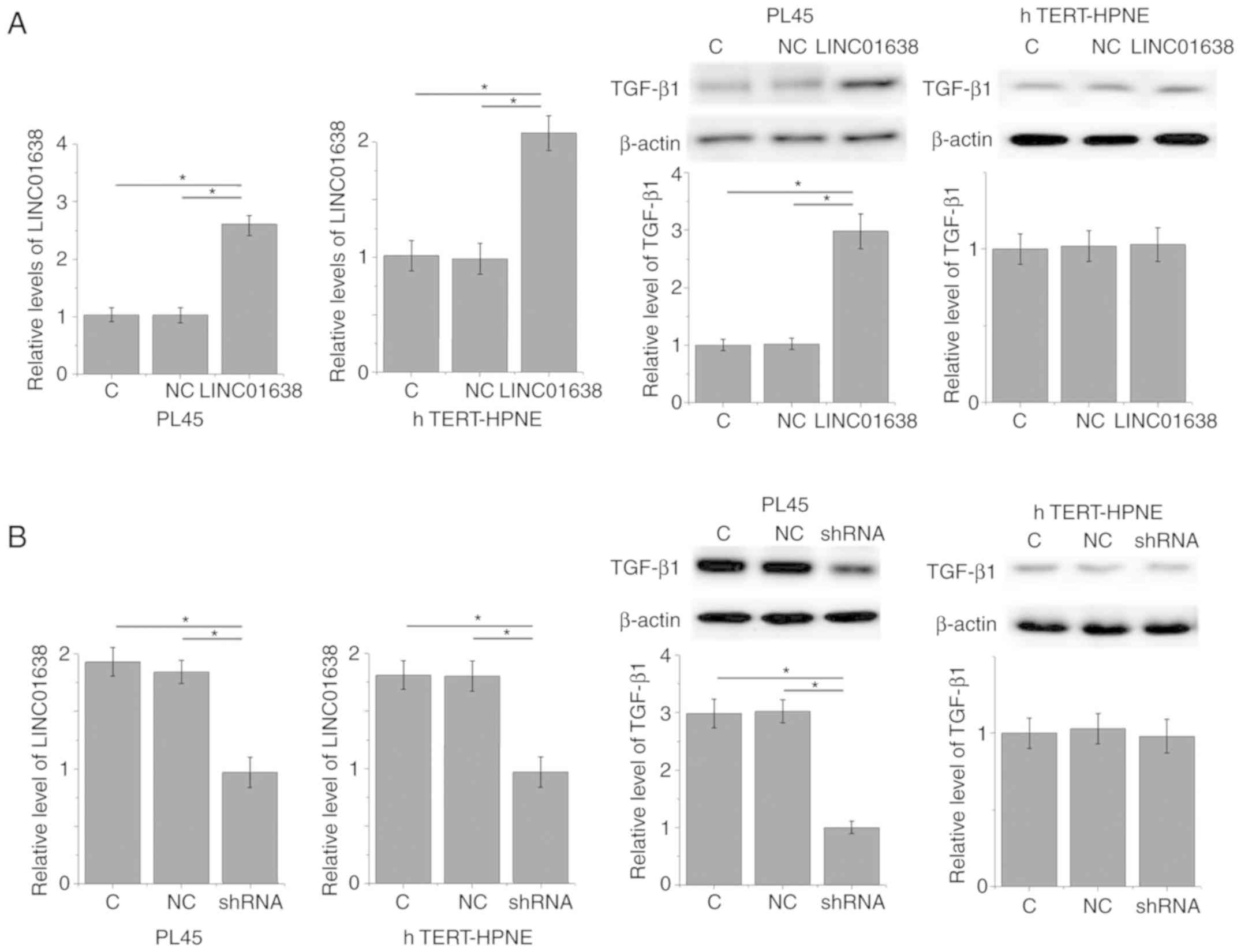
LINC01638 regulates TGF-β1 expression
in PDAC cells
The positive correlation between plasma levels of
LINC01638 and TGF-β1 in patients with PDAC indicated the potential
interactions between LINC01638 and TGF-β1. To further analyze this
interaction, LINC01638 expression vectors and LINC01638 shRNA
vectors were transfected into a PDAC cell line, PL45, and normal
pancreas duct cell line, hTERT-HPNE. Compared with untransfected
control cells and negative control cells, LINC01638 overexpression
led to significantly upregulated expression of TGF-β1 in PL45 PDAC
cells (P<0.05; Fig. 4A);
however, LINC01638 overexpression did not upregulate expression of
TGF-β1 in the hTERT-HPNE normal pancreas duct cell line. By
contrast, LINC01638 shRNA knockdown led to significantly
downregulated expression of TGF-β1 in cells in the PDAC cell line,
PL45 (P<0.05; Fig. 4B);
however, LINC01638 knockdown did not alter expression of TGF-β1 in
the hTERT-HPNE normal pancreas duct cell line. In addition,
treatment with exogenous TGF-β1 at concentrations of 10, 20 and 40
ng/ml did not significantly affect the expression of LINC01638 in
the two cell lines (data not shown). Therefore, LINC01638 is likely
to be an upstream activator of TGF-β1 in PDAC.
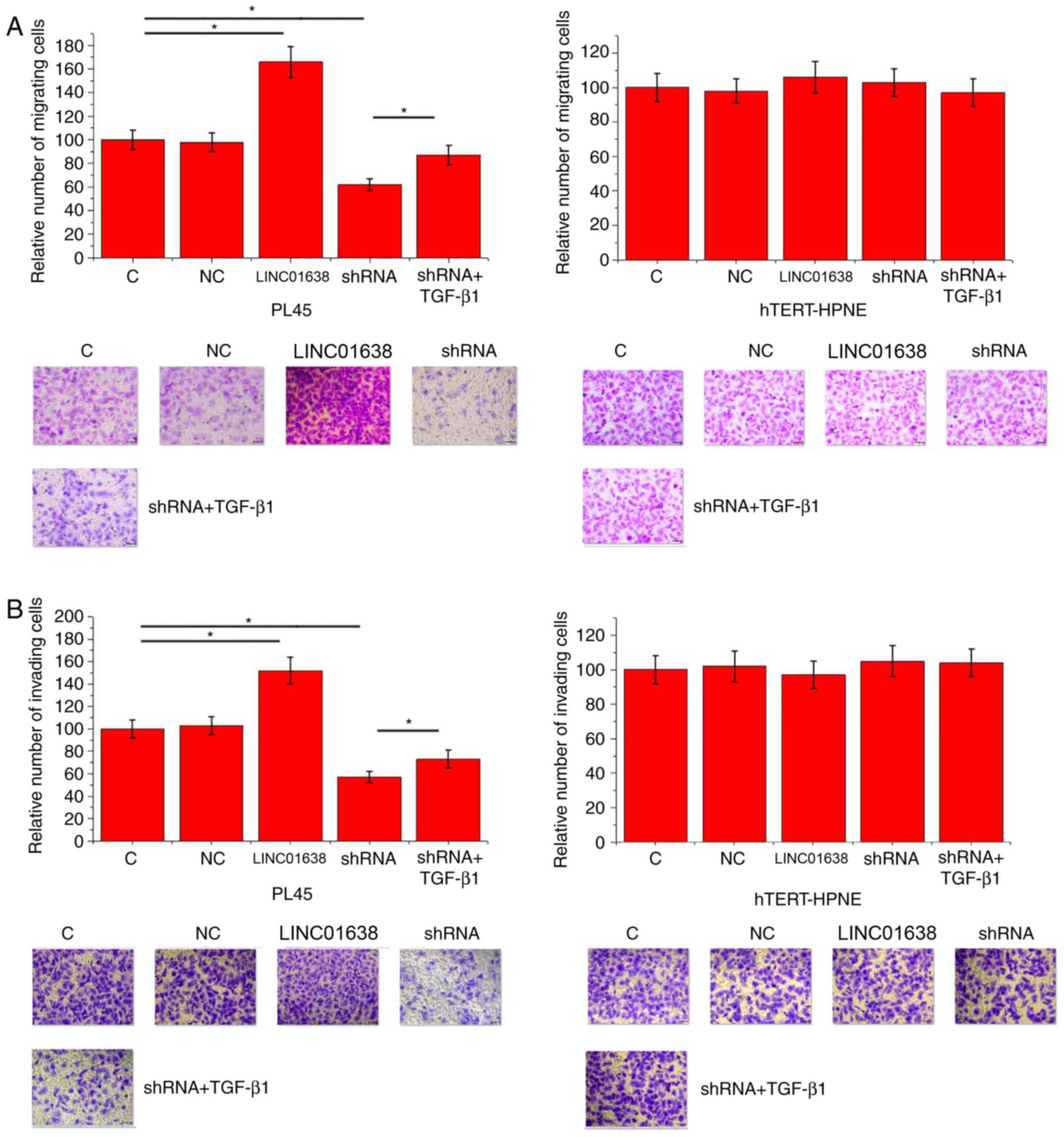
LINC01638 regulates PDAC cell
migration and invasion via TGF-β1
Compared with control cells and negative control
cells, LINC01638 overexpression significantly promoted migration
(Fig. 5A) and invasion (Fig. 5B) of cells of the PL45 cells
(P<0.05), but did not alter the migration of hTERT-HPNE normal
pancreas duct cells. By contrast, LINC01638 shRNA knockdown
inhibited migration (Fig. 5A) and
invasion (Fig. 5B) of cells of
PL45 PDAC cells (P<0.05), but did not alter the invasion of
hTERT-HPNE normal pancreas duct cells. In addition, treatment with
exogenous TGF-β1 (10 ng/ml) significantly attenuated the inhibitory
effects of LINC01638 shRNA silencing on cancer cell migration
(Fig. 5A) and invasion (Fig. 5B; P<0.05). Thus, LINC01638 may
regulate cancer cell migration and invasion via TGF-β1 in PDAC.
Discussion
LINC01638 is a recently identified lncRNA with a
characterized oncogenic role in triple breast cancer (11). The current study investigated the
role of LINC01638 in the pathogenesis of PDAC. The findings
revealed that the action of LINC01638 in PDAC is, at least
partially, mediated via the activation of TGF-β signaling.
Previous studies have demonstrated that the
development and progression of PDAC is accompanied by changes in
the expression pattern of numerous lncRNAs (13,14).
lncRNAs that are upregulated or downregulated in PDAC indicates
their functionality in this disease. It has been reported that the
lncRNA TMED11P is downregulated in patients with PDAC compared with
healthy tissues, and the reduced expression level of lncRNA TMED11P
is associated with accelerated progression and poor prognosis of
this disease (15). In another
study, lncRNA AFAP1-AS1 was reported to be upregulated in PDAC,
which supports its role as an oncogenic lncRNA in the pathogenesis
of this disease (16).
Upregulation of LINC01638 has been reported in triple-negative
breast cancer tissues compared with paired healthy tissue (11), while its expression pattern in
other disease is unknown. In the current study, LINC01638 was
upregulated in PDAC tissues compared with paired healthy tissues in
the majority of patients with PDAC, indicating the role of
LINC01638 as an oncogenic lncRNA in this disease.
Changes of circulating molecules provide guidance
for the diagnosis of human diseases (17). Circulating lncRNAs, such as lncRNA
MALAT (18), also have potential
application for the diagnosis of PDAC. In the present study, plasma
levels of LINC01638 were significantly higher in patients with PDAC
compared with healthy controls. In effect, upregulation of plasma
LINC01638 sensitively and specifically distinguished patients with
PDAC from healthy controls; therefore, detecting changes in plasma
LINC01638 may provide guidance for the diagnosis of PDAC. However,
the expression of LINC01638 may also been affected in other human
diseases, which may affect the diagnostic specificity; therefore,
multiple approaches should be combined to reduce false
positives.
TGF-β is a key mediator involved in the development
of multiple human diseases (19).
TGF-β interacts with multiple signaling molecules, including
lncRNAs (20,21); however, the crosstalk between TGF-β
signaling and lncRNAs in PDAC is not fully established. The data in
the present study suggest that LINC01638 may be an activator of
TGF-β1 that promotes the migration and invasion of PDAC cells.
However, treatment with downstream TGF-β1 only partially reverse
the inhibitory effects of LINC01638 shRNA silencing on cancer cell
migration and invasion, indicating the involvement of other
pathways in LINC01638-mediated regulation of PDAC cell migration
and invasion. The molecular mechanism of the interactions between
TGF-β1 and LINC01638 remains unknown. Future studies will attempt
to elucidate this molecular mechanism and explore the downstream
effectors of TGF-β signaling in the regulation of EMT.
In conclusion, LINC01638 was demonstrated to be
upregulated during the development of PDAC. LINC01638 is involved
in the regulation of migration and invasion of PDAC cells, at least
partially, via regulation of TGF-β1.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HL and WH designed the experiments. HL, JY and LZ
performed experiments. ML, SL and DY collected and analyzed the
data. WH drafted the manuscript. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the West China Hospital. All patients and healthy
volunteers provided written informed consent prior to their
inclusion within the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Rahib L, Smith BD, Aizenberg R, Rosenzweig
AB, Fleshman JM and Matrisian LM: Projecting cancer incidence and
deaths to 2030: The unexpected burden of thyroid, liver, and
pancreas cancers in the United States. Cancer Res. 74:2913–2921.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gallmeier E and Gress TM: Pancreatic
ductal adenocarcinoma. Internist (Berl). 59:805–822. 2018.(In
German). View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferrone CR, Pieretti-Vanmarcke R, Bloom
JP, Zheng H, Szymonifka J, Wargo JA, Thayer SP, Lauwers GY,
Deshpande V, Mino-Kenudson M, et al: Pancreatic ductal
adenocarcinoma: Long-term survival does not equal cure. Surgery.
152 (3 Suppl 1):S43–S49. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Adamska A, Domenichini A and Falasca M:
Pancreatic ductal adenocarcinoma: Current and evolving therapies.
Int J Mol Sci. 18(pii): E13382017. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ishiwata T: Pancreatic ductal
adenocarcinoma: Basic and clinical challenges for better prognosis.
J Carcinog Mutagene. S9:0052013.
|
|
7
|
Principe DR, Doll JA, Bauer J, Jung B,
Munshi HG, Bartholin L, Pasche B, Lee C and Grippo PJ: TGF-β:
Duality of function between tumor prevention and carcinogenesis. J
Natl Cancer Inst. 6:djt3692014. View Article : Google Scholar
|
|
8
|
Hesler RA, Huang JJ, Starr MD, Treboschi
VM, Bernanke AG, Nixon AB, McCall SJ, White RR and Blobe GC:
TGF-β-induced stromal CYR61 promotes resistance to gemcitabine in
pancreatic ductal adenocarcinoma through downregulation of the
nucleoside transporters hENT1 and hCNT3. Carcinogenesis.
37:1041–1051. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Thakur AK, Nigri J, Lac S, Leca J, Bressy
C, Berthezene P, Bartholin L, Chan P, Calvo E, Iovanna JL, et al:
TAp73 loss favors Smad-independent TGF-β signaling that drives EMT
in pancreatic ductal adenocarcinoma. Cell Death Differ.
23:1358–1370. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhu Z, Xu Y, Zhao J, Liu Q, Feng W, Fan J
and Wang P: miR-367 promotes epithelial-to-mesenchymal transition
and invasion of pancreatic ductal adenocarcinoma cells by targeting
the Smad7-TGF-β signalling pathway. Br J Cancer. 112:1367–1375.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Luo L, Tang H, Ling L, Li N, Jia X, Zhang
Z, Wang X, Shi L, Yin J, Qiu N, et al: LINC01638 lncRNA activates
MTDH-Twist1 signaling by preventing SPOP-mediated c-Myc degradation
in triple-negative breast cancer. Oncogene. 37:6166–6179. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhou Y, Gong B, Jiang ZL, Zhong S, Liu XC,
Dong K, Wu HS, Yang HJ and Zhu SK: Microarray expression profile
analysis of long non-coding RNAs in pancreatic ductal
adenocarcinoma. Int J Oncol. 48:670–680. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fu XL, Liu DJ, Yan TT, Yang JY, Yang MW,
Li J, Huo YM, Liu W, Zhang JF, Hong J, et al: Analysis of long
non-coding RNA expression profiles in pancreatic ductal
adenocarcinoma. Sci Rep. 6:335352016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhu Z, Zhou J, Zhang Z and Li D: Decreased
expression of lncRNA TMED11P is correlated with progression and
prognosis in pancreatic ductal adenocarcinoma. Int J Clin Exp
Pathol. 9:10550–10556. 2016.
|
|
16
|
Ye Y, Chen J, Zhou Y, Fu Z, Zhou Q, Wang
Y, Gao W, Zheng S, Zhao X, Chen T and Chen R: High expression of
AFAP1-AS1 is associated with poor survival and short-term
recurrence in pancreatic ductal adenocarcinoma. J Transl Med.
13:1372015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Poruk KE, Blackford AL, Weiss MJ, Cameron
JL, He J, Goggins M, Rasheed ZA, Wolfgang CL and Wood LD:
Circulating tumor cells expressing markers of tumor-initiating
cells predict poor survival and cancer recurrence in patients with
pancreatic ductal adenocarcinoma. Clin Cancer Res. 23:2681–2690.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu JH, Chen G, Dang YW, Li CJ and Luo DZ:
Expression and prognostic significance of lncRNA MALAT1 in
pancreatic cancer tissues. Asian Pac J Cancer Prev. 15:2971–2977.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Blobe GC, Schiemann WP and Lodish HF: Role
of transforming growth factor β in human disease. N Engl J Med.
342:1350–1358. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yuan JH, Yang F, Wang F, Ma JZ, Guo YJ,
Tao QF, Liu F, Pan W, Wang TT, Zhou CC, et al: A long noncoding RNA
activated by TGF-β promotes the invasion-metastasis cascade in
hepatocellular carcinoma. Cancer Cell. 25:666–681. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Li Z, Dong M, Fan D, Hou P, Li H, Liu L,
Lin C, Liu J, Su L, Wu L, et al: lncRNA ANCR down-regulation
promotes TGF-β-induced EMT and metastasis in breast cancer.
Oncotarget. 8:67329–67343. 2017.PubMed/NCBI
|