Introduction
Fungal and bacterial infections cause serious
problems in medicine, the environment and the food industry.
Candida albicans causes vaginal candidiasis, which affects
75 million women every year (1).
Staphylococcus aureus, the most common pathogen in human
purulent infections, is responsible for food poisoning,
osteomyelitis, and chronic and recurrent infections that are
difficult to prevent using antibiotics (2). Antibiotic resistance in bacteria,
such as S. aureus and Pseudomonas aeruginosa, is
becoming increasingly prevalent and serious (3). Escherichia coli can cause
gastrointestinal or urethral tract infections in humans and animals
under certain conditions. A range of existing biocides, including
antibiotics (4), metal particles
(silver or tin), titanium oxide (5) and quaternary ammonium salts (6), have been banned owing to their
toxicity (7). Researchers are
therefore investigating natural broad-spectrum antibacterial agents
that may be used to reduce the problem of drug resistance (8–10).
Plant-derived compounds, including tannins, flavonoids, alkaloids
and peptides have been reported to exhibit therapeutic action
against bacterial infection (11–13),
indicating their utility as antibacterial substances. Antibacterial
drugs are classified according to four mechanism of action: i)
interference with cell wall synthesis; ii) causing damage to the
plasma membrane; iii) inhibiting bacterial protein synthesis; and
iv) affecting nucleic acid metabolism.
The rhizome of the orchid Gastrodia elata
Blume, has been used medicinally in Asian countries for >2,000
years (14,15) owing to its various pharmacological
actions, including anti-epilepsy (16), anti-convulsion (15), anti-inflammation (17,18)
and anti-tetanus (16). Among its
active ingredients, the polypeptides have received wide attention.
G. elata protein extracts have been reported to have
significant antifungal activity and antibacterial activity against
gram-positive bacteria, and exhibits little hemolytic activity on
rabbit red blood cells (19). In
our previous study, the antimicrobial activities of
polypeptide-enriched G. elata extracts (GEP) were
demonstrated using the agar diffusion method (20) and its effects on C. albicans
were confirmed in a mouse model of vulvovaginal candidiasis.
However, the antimicrobial mechanism of action of GEP has, to the
best of our knowledge, not yet been reported.
The present study aimed to investigate the possible
mechanism of GEP-mediated antimicrobial efficacy on C. albicans,
E. coli, S. aureus and P. aeruginosa by determining its
effect on cell walls, cell membranes and the levels of
intracellular and extracellular proteins. Data from the present
study suggested that the antimicrobial effects of GEP may be
related to the modulation of membrane integrity and intracellular
energy metabolism.
Materials and methods
Preparation of GEP and molar mass
distribution analysis
In the Changbai Mountain region (Jilin, China),
G. elata has been planted artificially, and is an economic
species. In the present study, the cultured G. elata
rhizomes were purchased from Taobao and stored at room temperature.
The rhizomes of G. elata were crushed and extracted for 2 h
in saline at 80°C, the resulting solution was then filtered. Papain
(Shanghai Yuanye Biological Technology Co., Ltd.) was added to the
collected filtrate to give a final concentration of 0.32 g/liter.
The resulting solution was heated at 60°C for 30 min (to trigger
papain-mediated hydrolysis) and then heated in a water bath at
100°C for a further 40 min (to inactivate the papain).
Subsequently, the solution was passed through a 10 kDa
ultrafiltration membrane (Sartocon 2 PES; Sartorius AG), and the
resulting filtrate was concentrated by rotary evaporation at 60°C
with a rotation speed of 10 rpm until the extract was determined to
have a concentration of 22.5 g/l using the bicinchoninic acid (BCA)
method.
The molar mass distribution of GEP was determined
using gel permeation chromatography (Wyatt Technology) equipped
with two serial OHpak polymer matrix phase columns (OHpak SB-806
and 803; 8.0×300 mm; Shodex). The samples were eluted using a 0.02%
aqueous solution of NaN3, at a flow-rate of 1 ml/min at 40°C.
Calculations of the average molar masses [number average molar mass
(Mn), weight-average molar mass (Mw), z-average molar mass (Mz)]
and dispersity index (Mw/Mn) were calculated using Omi SEC v4.7
software (Malvern Panalytical).
Strains and culture condition
In total, four microbial strains, C. albicans
(cat. no. ATCC 10231), E. coli (cat. no. 8099), S.
aureus (cat. no. ATCC 6538) and P. aeruginosa (cat. no.
ATCC 27853) were supplied by the Guangdong Microbial Culture
Center. The bacterial strains were cultured in lysogeny broth (10
g/l peptone, 5 g/l yeast extract and 10 g/l NaCl) at 37°C with
shaking at 150 rpm for 20 h. C. albicans was cultured in
Sabouraud Dextrose Broth (Qingdao Hope Bio-Technology Co., Ltd.) at
28°C with shaking at 150 rpm for 20 h.
Determination of the minimum
inhibitory concentration (MIC)
After culturing for 20 h, suspensions with a final
concentration of 1×106 colony-forming units (CFU)/ml
were collected. For each microbial strain, 100 µl aliquots of
prepared suspension were added to 96-well plates and 100 µl
aliquots containing different concentrations of GEP diluted in PBS
were added to the wells (1.127, 2.254, 4.508, 6.762, 9.016, 11.270
and 13.524 mg/ml of GEP for C. albicans and 1.803, 2.254,
2.705, 3.156, 3.606, 4.057 and 4.508 mg/ml of GEP for E. coli,
S. aureus and P. aeruginosa); 100 µl of PBS was added to
the negative control wells and 100 µl of 75% ethanol (analytical
pure) was added to the positive control wells. The plates were
cultured for 24 h at 37°C (bacterial strains) or 28°C (C.
albicans), and 20 µl of 0.2% triphenyltetrazolium chloride
(Sigma-Aldrich; Merck KGaA) was added to the bacterial suspensions
and 20 µl of 5 mg/ml MTT was added to the fungal suspensions, as
previously described (21,22). The plates were incubated for a
further 4 h in darkness. MIC was defined as the minimum
concentration of GEP that completely inhibited cell viability, as
shown by the absence of formazan crystal formation. After
observation, formazan crystals were dissolved using DMSO and were
measured at 490 nm for the three bacterial strains and 570 nm for
C. albicans. The same results were obtained from analysis
under a microscope.
Construction of growth curves
In total, 20 ml of bacterial or fungal suspensions
(1×106 CFU/ml) was mixed with 20 ml of 2X MIC GEP (1X
MIC treated group) or PBS (Control group), and cultured at the
aforementioned temperatures. The optical density at 600 nm was
determined using 1 ml of mixture using a Synergy HT Multi-detection
microplate reader (Omega Bio-Tek, Inc.) at 0, 1, 2, 4, 6, 8 and 10
h for E. coli and P. aeruginosa, and 0, 1, 2, 4, 6,
8, 12, 16 and 20 h for C. albicans and S. aureus.
Scanning electron microscopy (SEM)
analysis
The bacterial and fungal suspensions at a final
density of 5×105 CFU/ml mixed with GEP (1X MIC treatment
group) or an equal volume of PBS (Control group) were incubated for
16 h at 37°C (bacterial strains) or 28°C (C. albicans).
After centrifugation at 604 × g at room temperature for 5 min and
washing with PBS three times, the collected precipitates were fixed
with 2.5% (v/v) glutaraldehyde for 2 h at 4°C, dehydrated by an
ascending series of ethanol washes (30–100%), freeze-dried and
gold-sputtered. Samples were observed by SEM using a Hitachi X650
(Hitachi, Ltd.).
Determination of optical density (OD)
at 260 nm to detect DNA and RNA
The bacterial and fungal suspensions (final density,
5×105 CFU/ml) mixed with GEP (1X MIC treatment group) or
PBS (Control group) were incubated for 0 or 2 h. At each time
point, 1 ml of mixture was removed, centrifuged at 9,660 × g at
room temperature for 10 min and the resulting supernatant passed
through a microporous filter (0.22 µm; EMD Millipore). The optical
density of the resulting supernatant was measured at 260 nm using a
microplate reader (23).
Determination of the activities of
extracellular enzymes
In the preliminary experiments to determine
extracellular alkaline phosphatase (AKP) activity, samples were
collected from the supernatants of each strain after 1, 2, 4, 8 and
10 h 1X MIC GEP or PBS Control treatment. AKP activity was detected
using an AKP assay kit (cat. no. A059-1; Nanjing Jiancheng
Bioengineering Institute). According to the preliminary results
(Fig. S1), a 2 h incubation was
selected for the subsequent experiments.
In the preliminary experiments, the extracellular
β-galactosidase activity was detected in the cultured medium after
1, 2, 4, 8 and 10 h 1X MIC GEP or PBS Control treatment. According
the preliminary results (Fig.
S2), a 4 h incubation was selected for the subsequent
experiments. Samples (100 µl) were added to 400 µl 0.05 mol/l
O-nitrophenyl-β-D-galactopyranoside (ONPG; Sigma-Aldrich; Merck
KGaA) and the mixture was incubated in a water bath at 37°C for 40
min. The resulting mixture was immediately combined with 500 µl 0.5
mol/l sodium bicarbonate and the optical density at 420 nm was
measured using a microplate reader. The formula for the calculation
of β-galactosidase activity is as follows (24,25):
β-Galactosidase activity (U/ml)=(OD420 × A)/(B × C ×
0.0045) where A represents the volume of the system (ml), B
represents the incubation time (min), C represents the sample
volume (ml) and 0.0045 represents the molar extinction coefficient
of ONPG (ml/nmol).
Determination of the activities of
intracellular enzymes
After incubation for 10 h, the treated bacteria and
fungi were collected by centrifugation at 604 × g at room
temperature for 5 min and washed with PBS. Intracellular proteins
were extracted using a bacterial protein extraction kit (cat. no.
BB-3123; Best Science) or yeast total protein extraction kit (cat.
no. BB-3125; Best Bio Science). The concentration of the protein
samples was determined using the BCA method.
The activities of intracellular malate dehydrogenase
(MDH; cat. no. A021-2), succinate dehydrogenase (SDH; cat. no.
A022), ATPase (cat. no. A095-1),
Na+/K+-ATPase (cat. no. A070-2) and
Ca2+-ATPase (cat. no. A070-4) were determined according
to the instructions for the commercial kits (all from Nanjing
Jiancheng Bioengineering Institute).
Statistical analysis
All data are presented as the mean ± SD and
represent six independent experiments; each experiment was
performed in triplicate. Statistical significance was determined
using two-tailed Student's t-tests using SPSS Statistics v24 (IBM
Corp.) for Windows. P>0.05 was considered to indicate a
statistically significant difference.
Results
Molar mass distribution of GEP
Mw/Mn is defined as the polydispersity index,
representing the dispersion of the molecular weight of GEP. The
smaller the polydispersity index value, the more uniform in molar
mass the sample is. The Mw/Mn of GEP was determined to be 4.820,
which suggested that the molecular weight of GEP is moderately
uniform (Table I).
 | Table I.The molar mass distribution of
GEP. |
Table I.
The molar mass distribution of
GEP.
| Parameter | GEP |
|---|
| Mw/Mn | 4.820 |
| Mn (g/mol) |
1.102×104 |
| Mw (g/mol) |
5.310×104 |
| Mz (g/mol) |
3.142×105 |
MICs of GEP against the four microbial
strains
GEP had the highest MIC value for C. albicans
(5.635 mg/ml) and the lowest MIC value for S. aureus (1.127
mg/ml) (Table II). The MIC values
of GEP against E. coli and P. aeruginosa were 1.803
and 1.352 mg/ml, respectively. These data suggested that
gram-negative bacteria (E. coli, P. aeruginosa) and
gram-positive bacteria (S. aureus) are more sensitive to GEP
than fungi (C. albicans).
 | Table II.MICs of polypeptide-enriched
Gastrodia elata extracts against four microbial strains. |
Table II.
MICs of polypeptide-enriched
Gastrodia elata extracts against four microbial strains.
| Microbial
strain | MIC (mg/ml) |
|---|
| Candida
albicans | 5.635 |
| Escherichia
coli | 1.803 |
| Staphylococcus
aureus | 1.127 |
| Pseudomonas
aeruginosa | 1.352 |
Effects of GEP on the growth of the
four microbial strains
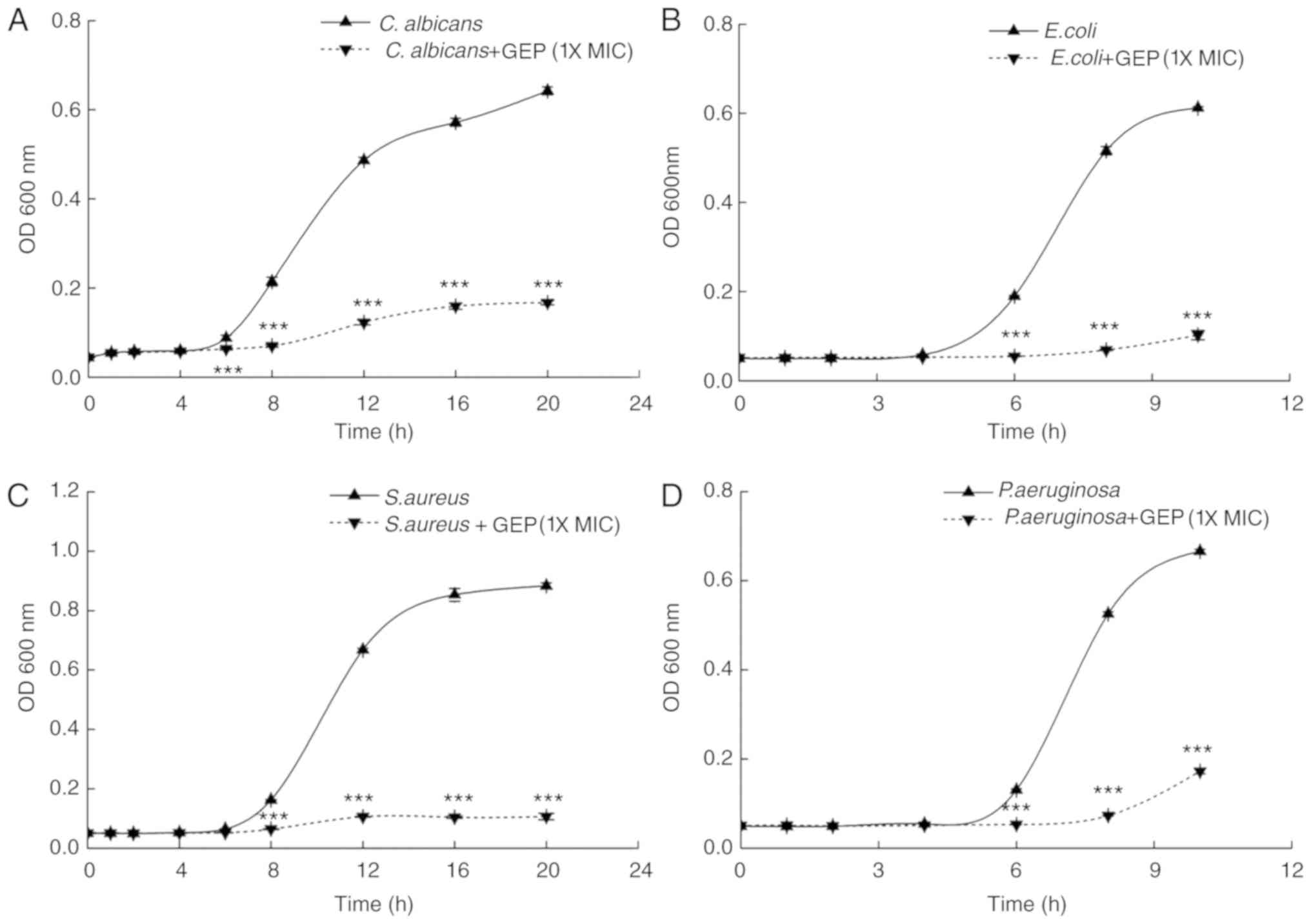
Growth curves were constructed from 0 to 10 or 20 h
for the different strains to investigate the effects of GEP at 1X
MIC on antimicrobial properties over time (26). Compared with the respective
PBS-treated Control groups, the number of cells was significantly
lower following treatment with GEP (1X MIC), which suggested that
GEP inhibits the growth of C. albicans, E. coli, S. aureus
and P. aeruginosa (Fig. 1);
GEP almost completely inhibited the growth of E. coli
(Fig. 1B) and S. aureus
(Fig. 1C).
Effects of GEP on cell wall
integrity
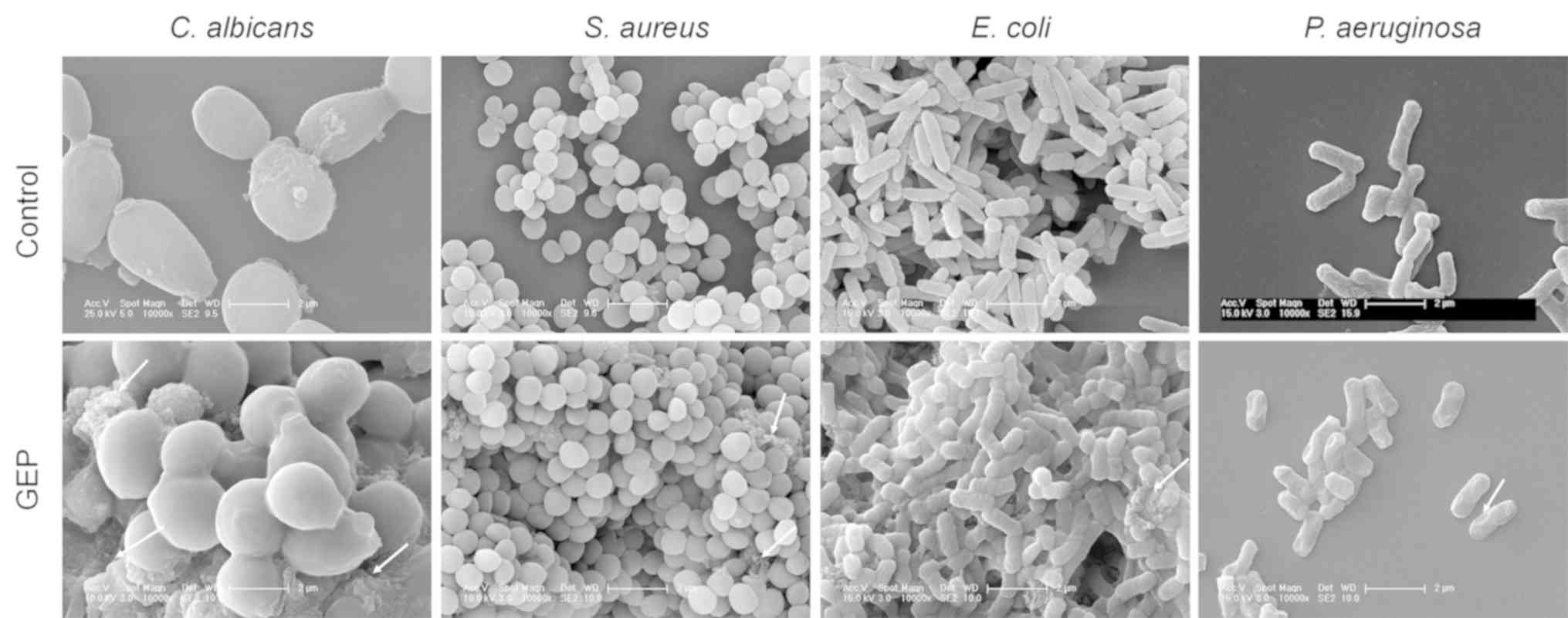
To investigate the morphology and the integrity of
the cell wall of the four microbial strains, SEM was performed and
the activity of AKP was determined. PBS-treated C. albicans
and S. aureus cells had a smooth and plump sphere shape,
whereas following 16 h of GEP treatment vesication or the formation
of irregular protruding structures were observed on the cell
surface (Fig. 2). For E.
coli and P. aeruginosa, compared with the control group,
cell-surface damage, such as uneven spots and curves, was apparent
in the GEP-treated groups (Fig.
2). Some visible cell debris was observed in the GEP-treated
E. coli and S. aureus (Fig. 2).
AKP, a phosphatase found between the cell wall and
cell membrane, serves as an index for cell wall permeability
(27). Compared with the Control
group, GEP (1X MIC) treatment for 2 h caused a 43.9-fold
(P<0.001), 10.0-fold (P<0.001), 4.4-fold (P<0.001) and
8.8-fold (P<0.001) increase in the extracellular AKP activity
for C. albicans, E. coli, S. aureus and P.
aeruginosa, respectively (Fig.
3), which suggested that GEP treatment resulted in damage and
disruption to the cell wall.
Effects of GEP on cell membrane
permeability
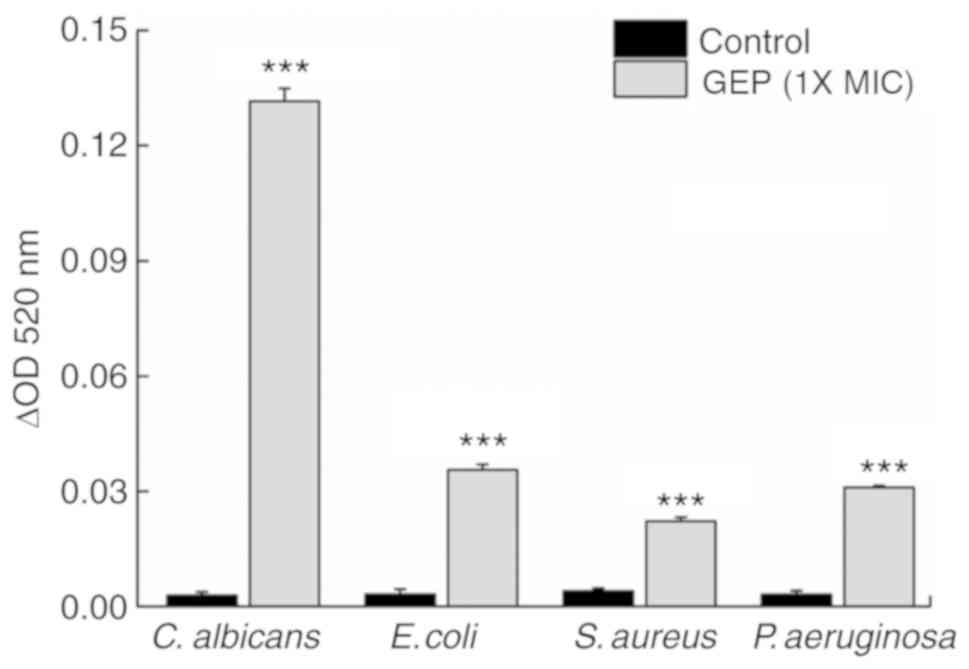
β-Galactosidase is an intracellular enzyme that
leaks from cells following damage to the cell membrane; therefore,
β-galactosidase can be used as an indicator of cell membrane
integrity (25). Compared with the
Control group, treatment with GEP (1X MIC) for 4 h resulted in a
28.3 (P<0.001), 7.6 (P<0.001), 8.1 (P<0.01) and 7.1%
(P<0.01) increase in extracellular β-galactosidase activity in
the samples of C. albicans, E. coli, S. aureus and P.
aeruginosa, respectively (Fig.
4A).
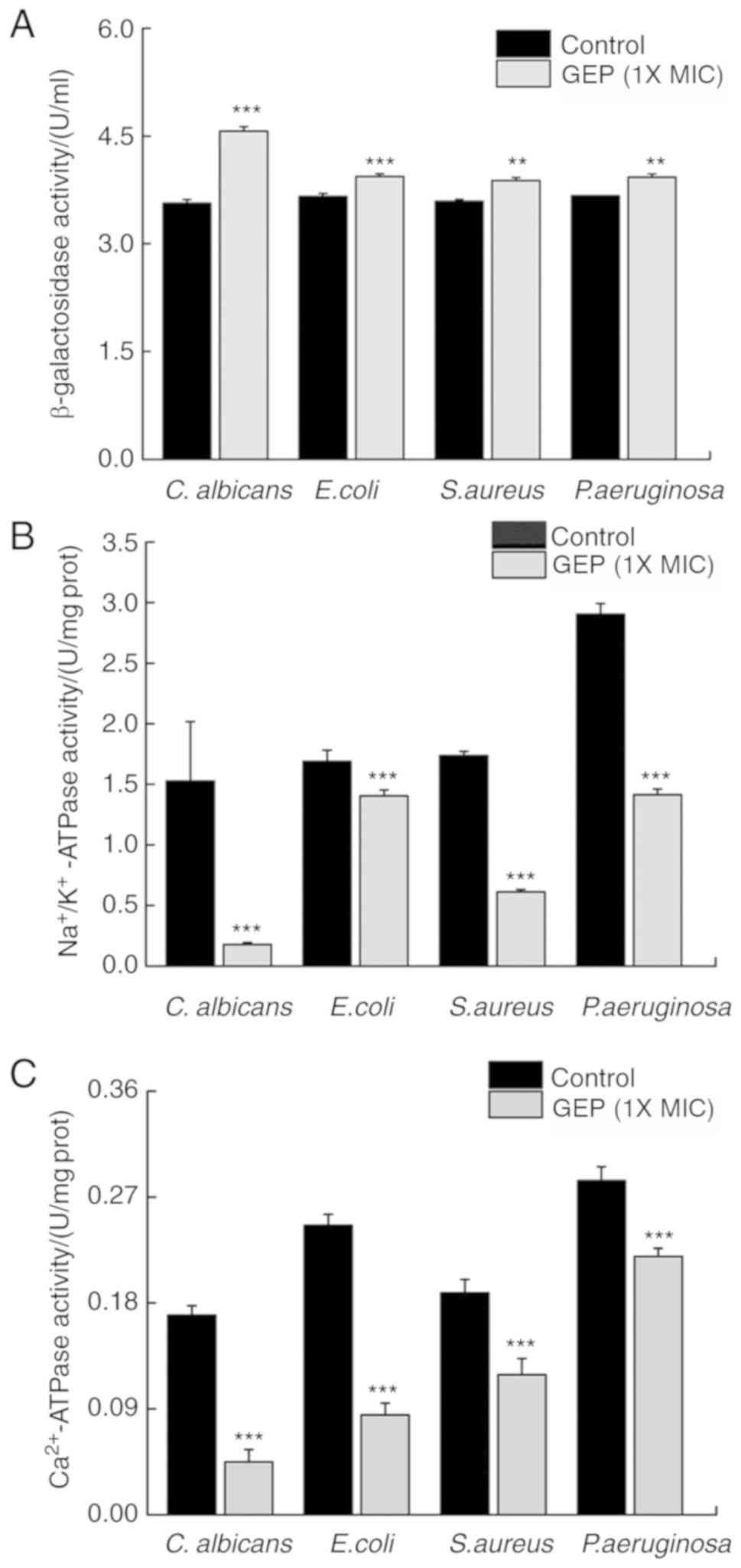
Ion pumps, such as the
Na+/K+-ATPase and the Ca2+-ATPase,
are important membrane proteins that regulate cell membrane
permeability (28). Compared with
the Control group, treatment with GEP (1X MIC) resulted in a
decrease in the activities of Na+/K+-ATPase
(Fig. 4B) and
Ca2+-ATPase (Fig. 4C)
of 88.3 (P<0.001) and 73.5% (P<0.001), respectively, for
C. albicans; 16.9 (P<0.001) and 65.5% (P<0.001),
respectively, for E. coli; 64.7 (P<0.001) and 36.9%
(P<0.001), respectively, for S. aureus; and 51.3
(P<0.001) and 22.7% (P<0.001) for P. aeruginosa. These
findings suggested that GEP damaged the cell membranes of these
microbial strains.
Effects of GEP on the leakage of
genetic material
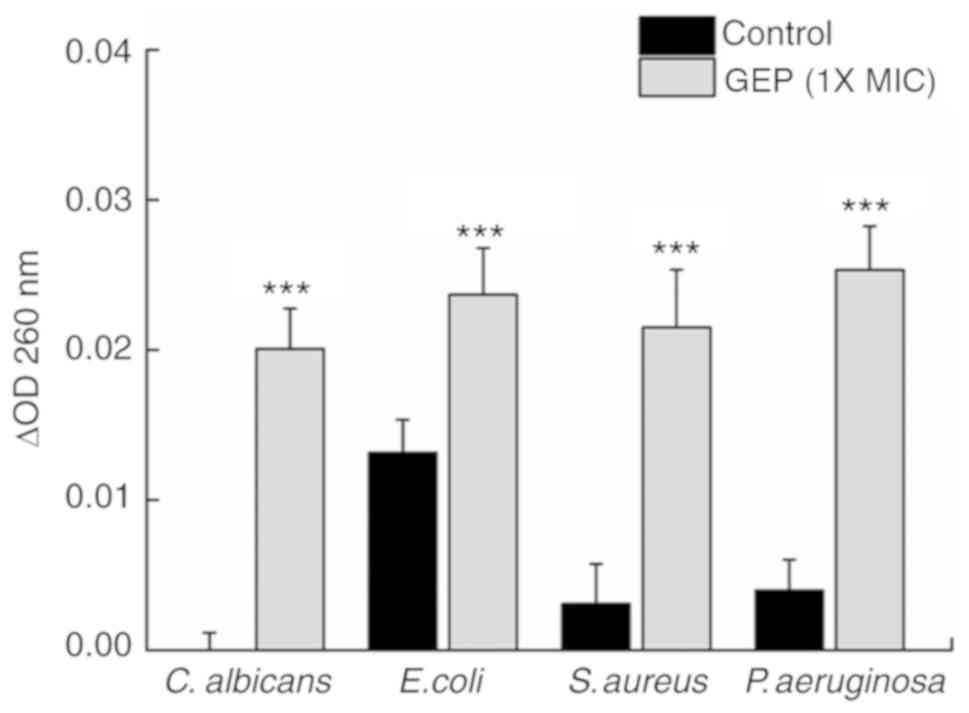
A high OD260 may be detected following
the leakage of nucleotides (25).
Compared with the Control group, GEP (1X MIC) treatment caused a
590% (P<0.001) increase in the OD260 between 0 and 2
h in S. aureus and a 530% (P<0.001) increase for P.
aeruginosa (Fig. 5); a 100
(P<0.001) and 80% (P<0.01) increase in the OD was observed
for C. albicans and E. coli, respectively. These
results indicated that GEP may cause leakage of genetic material
from these microorganisms.
Effects of GEP on intracellular enzyme
activity
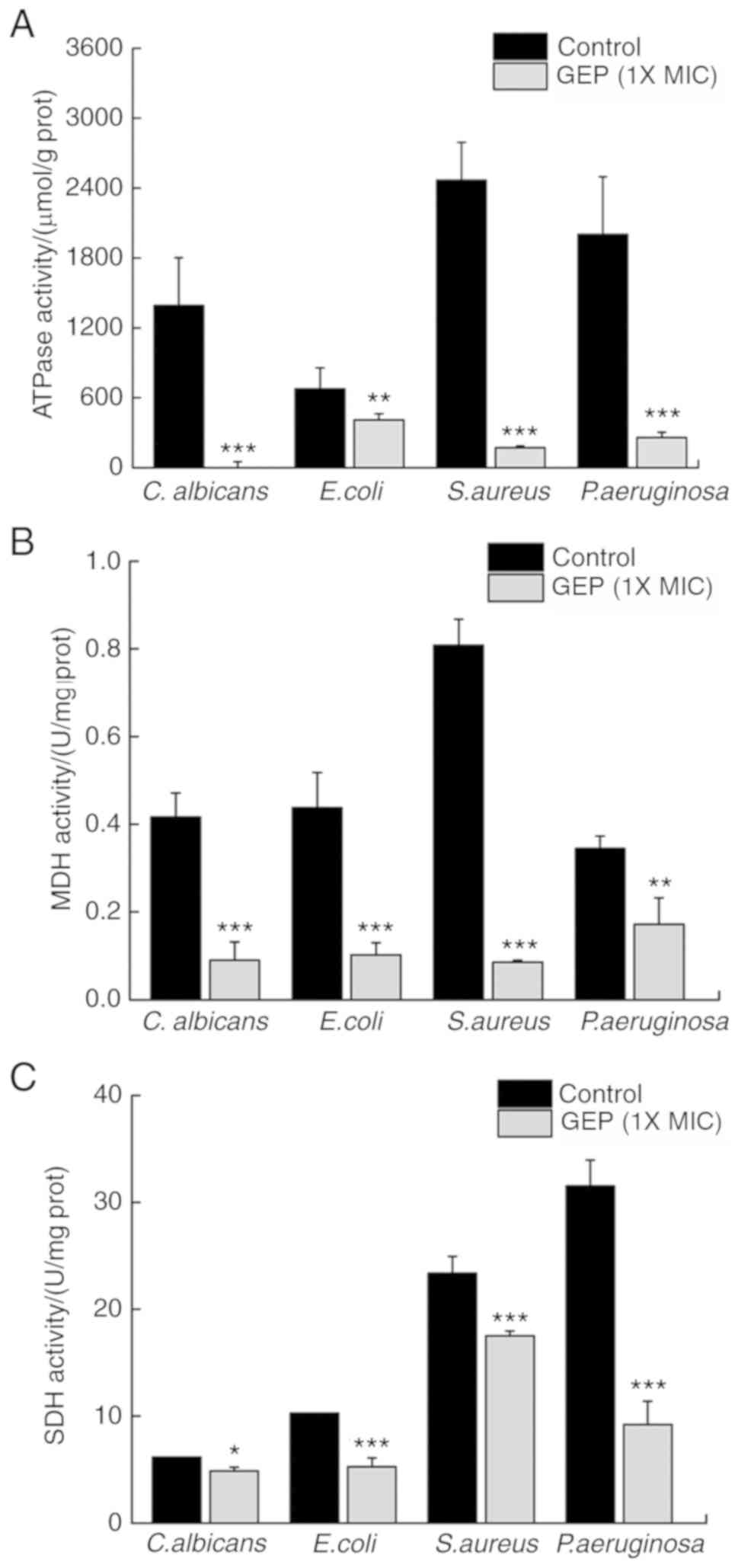
SDH, MDH and ATPases serve important roles in
microbial energy metabolism. ATPases hydrolyze ATP and provide
energy to drive other chemical reaction in the cell (29). Compared with the Control group, GEP
(1X MIC) treatment reduced the activity of ATPases by 100.0
(P<0.001), 39.4 (P<0.01), 93.0 (P<0.001) and 87.1%
(P<0.001) in C. albicans, E. coli, S. aureus and P.
aeruginosa, respectively (Fig.
6A).
MDH affects biosynthesis through its coenzyme
NADP+ (29); SDH is an
important marker of the health of microbial energy metabolism.
Compared with the Control group, GEP (1X MIC) treatment reduced the
activity of MDH by 78.4 (P<0.001), 76.6 (P<0.001), 89.4
(P<0.001) and 50.2% (P<0.01) in C. albicans, E. coli, S.
aureus and P. aeruginosa, respectively (Fig. 6B). GEP treatment suppressed the
activity of SDH by 21.1 (P<0.05), 48.6 (P<0.001), 25.1
(P<0.001) and 70.7% (P<0.001) in C. albicans, E. coli, S.
aureus and P. aeruginosa, respectively (Fig. 6C). These data suggested that GEP
treatment disturbed energy metabolism in these microorganisms.
Discussion
The antimicrobial activity of GEP against fungi and
gram-positive bacteria has been reported previously (19,20).
Our previous study demonstrated the antimicrobial activities of GEP
using the agar diffusion method (20) and are now investigating the
therapeutic efficacy of GEP against vulvovaginal candidiasis in
mice (30). In the present study,
it was demonstrated that the effects of GEP may derive from the
damage caused to cell walls and membranes, triggering the leakage
of genetic material and disrupting cellular metabolism.
The SEM results demonstrated that GEP damaged cell
walls and membranes, leading to changes in cell morphology and cell
death These changes may result in the loss of intracellular
material (31). Various
antibacterial agents act primarily on the cell membrane (32), which is a selectively permeable
barrier that enables the normal growth of bacteria (23). Leakage of intracellular substances
serves as an indicator of membrane integrity. AKP is present
between the cell wall and the cell membrane (33,34).
Damage to the cell wall increases cell permeability, leading to the
leakage of AKP (27). The enhanced
extracellular AKP levels within 2 h of treatment with GEP are
consistent with the SEM results, and further suggested that GEP
damaged cell walls and membranes.
β-Galactosidase is an important microbial enzyme
that hydrolyzes lactose into galactose and glucose to produce
energy, and provide a source of carbon (35). In the present study, it was
demonstrated that GEP may regulate energy metabolism in bacteria
and fungi. In addition to enhancing the extracellular activities of
β-galactosidase, GEP directly suppressed the intracellular activity
levels of SDH and MDH in the four microbial strains. The
tricarboxylic acid (TCA) cycle is the metabolic pathway linking the
three major classes of nutrients (sugars, lipids and amino acids)
and the main energy production mode of the four microbial strains
examined. MDH catalyzes the reversible conversion of malic acid and
oxaloacetic acid in the last step of the TCA cycle, influencing ATP
production (36,37). SDH provides electrons for the
respiratory chain. Decreased SDH activity inhibits mitochondrial
electron transport and oxidative respiration, resulting in ATP
consumption, thus hindering the TCA cycle and energy metabolism
(37,38).
ATPases hydrolyzes ATP to release energy (23). As previously reported, the
inhibition of the ATPase activity of Ca2+-ATPase and
Na+/K+-ATPase leads to an overload of
intracellular Ca2+ and Na+, the inhibition of
energy metabolism, a reduction in ATP production and an imbalance
of osmotic pressure, which causes cell swelling and apoptosis
(38). The data from the present
study suggested that these changes in energy metabolism may be one
of the reasons for the antimicrobial efficacy of GEP.
In the present study, the possible mechanisms
underlying the antimicrobial activities of GEP were investigated,
and the results indicated that GEP may disrupt energy metabolism,
especially the TCA cycle, and may also be able to damage microbial
cell walls and membranes, causing the leakage of genetic
material.
Supplementary Material
Supporting Data
Acknowledgements
Not applicable.
Funding
The present study was funded by The Special Projects
of the Cooperation between Jilin University and Jilin Province
(grant no. SXGJXX2017-1) and The Industrial Technology Research and
Development Projects from Development and Reform Commission of
Jilin Province (grant no. 2019C050-8).
Availability of data and materials
All data generated and analyzed during this study
are included in this published article.
Authors' contributions
DW and HB designed the experiments. FK, XC, SZ, RW,
XZ and YM performed the experiments. FK, XC, SZ and RW contributed
to data analysis and proofreading. DW, FK and XC wrote the paper.
DW and HB revised the paper.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Wilson D: Candida albicans. Trends
Microbiol. 27:188–189. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wang Y, Bojer MS, George SE, Wang Z,
Jensen PR, Wolz C and Ingmer H: Inactivation of TCA cycle enhances
Staphylococcus aureus persister cell formation in stationary
phase. Sci Rep. 8:108492018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Babii C, Bahrin LG, Neagu AN, Gostin I,
Mihasan M, Birsa LM and Stefan M: Antibacterial activity and
proposed action mechanism of a new class of synthetic tricyclic
flavonoids. J Appl Microbiol. 120:630–637. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hanna H, Benjamin R, Chatzinikolaou I,
Alakech B, Richardson D, Mansfield P, Dvorak T, Munsell MF,
Darouiche R, Kantarjian H and Raad I: Long-term silicone central
venous catheters impregnated with minocycline and rifampin decrease
rates of catheter-related bloodstream infection in cancer patients:
A prospective randomized clinical trial. J Clin Oncol.
22:3163–3171. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sunada K, Watanabe T and Hashimoto K:
Studies on photokilling of bacteria on TiO2 thin film. J
Photochem Photobiol A Chem. 156:227–233. 2003. View Article : Google Scholar
|
|
6
|
Klibanov AM: Permanently microbicidal
materials coatings. J Mater Chem. 17:2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Oger PC, Piesse C, Ladram A and Humblot V:
Engineering of antimicrobial surfaces by using temporin analogs to
tune the biocidal/antiadhesive effect. Molecules. 24:8142019.
View Article : Google Scholar :
|
|
8
|
Cardon S, Sachon E, Carlier L, Drujon T,
Walrant A, Alemán-Navarro E, Martínez-Osorio V, Guianvarc'h D,
Sagan S, Fleury Y, et al: Peptidoglycan potentiates the membrane
disrupting effect of the carboxyamidated form of DMS-DA6, a
Gram-positive selective antimicrobial peptide isolated from
Pachymedusa dacnicolor skin. PLoS One. 13:e02057272018.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Burt S: Essential oils: Their
antibacterial properties and potential applications in foods-a
review. Int J Food Microbiol. 94:223–253. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gibbons S: Anti-staphylococcal plant
natural products. Nat Prod Rep. 21:263–277. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Khan MI, Ahhmed A, Shin JH, Baek JS, Kim
MY and Kim JD: Green tea seed isolated saponins exerts
antibacterial effects against various strains of gram positive and
gram negative bacteria, a comprehensive study in vitro and in vivo.
Evid Based Complement Alternat Med. 2018:34861062018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Liu M, Yang K, Wang J, Zhang J, Qi Y, Wei
X and Fan M: Young astringent persimmon tannin inhibits
methicillin-resistant Staphylococcus aureus isolated from
pork. LWT. 100:48–55. 2019. View Article : Google Scholar
|
|
13
|
Fernebro J: Fighting bacterial
infections-future treatment options. Drug Resist Updat. 14:125–139.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matias M, Silvestre S, Falcão A and Alves
G: Gastrodia elata and epilepsy: Rationale and therapeutic
potential. Phytomedicine. 23:1511–1526. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu J, Wu B, Tang C and Zhao J: Analytical
techniques and pharmacokinetics of gastrodia elata blume and
its constituents. Molecules. 22(pii): E11372017.PubMed/NCBI
|
|
16
|
Ojemann LM, Nelson WL, Shin DS, Rowe AO
and Buchanan RA: Tian ma, an ancient Chinese herb, offers new
options for the treatment of epilepsy and other conditions.
Epilepsy Behav. 8:376–383. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang H, Zhang R, Qiao Y, Xue F, Nie H,
Zhang Z, Wang Y, Peng Z and Tan Q: Gastrodin ameliorates
depression-like behaviors and up-regulates proliferation of
hippocampal-derived neural stem cells in rats: Involvement of its
anti-inflammatory action. Behav Brain Res. 266:153–160. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hwang SM, Lee YJ, Kang DG and Lee HS:
Anti-inflammatory effect of Gastrodia elata rhizome in human
umbilical vein endothelial cells. Am J Chin Med. 37:395–406. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chen C, Li X, Li J, Xu Y, Jing X, Wu S,
Liu X and Zhang X: Purification and characterization of an
antimicrobial protein from Gastrodia elata Blume tubers.
Trop J Pharm Res. 17(9)2018. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang X, Lu J, Chen Y, Zhao Y, Tong P, Teng
L and Wang D: Optimization of the extraction of gastrodia
elata protein and its antibacterial. J Chin Inst Food Sci
Technol. 18:176–128. 2018.
|
|
21
|
Kifer D, Mužinić V and Klarić MŠ:
Antimicrobial potency of single and combined mupirocin and
monoterpenes, thymol, menthol and 1,8-cineole against
Staphylococcus aureus planktonic and biofilm growth. J
Antibiot (Tokyo). 69:689–696. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Silva JP, Peres AR, Paixão TP, Silva AS,
Baetas AC, Barbosa WL, Monteiro MC and Andrade MA: Antifungal
activity of hydroalcoholic extract of chrysobalanus icaco
against oral clinical isolates of candida species.
Pharmacognosy Res. 9:96–100. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sun XH, Zhou TT, Wei CH, Lan WQ, Zhao Y,
Pan YJ and Wu Vivian CH: Antibacterial effect and mechanism of
anthocyanin rich Chinese wild blueberry extract on various
foodborne pathogens. Food Control. 94:155–161. 2018. View Article : Google Scholar
|
|
24
|
Wang J, Ma M, Yang J, Chen L, Yu P, Wang
J, Gong D, Deng S, Wen X and Zeng Z: In vitro antibacterial
activity and mechanism of monocaprylin against escherichia
coli and staphylococcus aureus. J Food Prot.
81:1988–1996. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shen S, Zhang T, Yuan Y, Lin S, Xu J and
Ye H: Effects of cinnamaldehyde on Escherichia coli and
Staphylococcus aureus membrane. Food Control. 47:196–202.
2015. View Article : Google Scholar
|
|
26
|
Su R, Li T, Fan D, Huang J, Zhao J, Yan B,
Zhou W, Zhang W and Zhang H: The inhibition mechanism of
ϵ-polylysine against Bacillus cereus emerging in surimi gel
during refrigerated storage. J Sci Food Agric. 99:2922–2930. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
He N, Wang P, Wang P, Ma C and Kang W:
Antibacterial mechanism of chelerythrine isolated from root of
Toddalia asiatica (Linn) Lam. BMC Complement Altern Med.
18:2612018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Li X, He C, Song L, Li T, Cui S, Zhang L
and Jia Y: Antimicrobial activity and mechanism of Larch bark
procyanidins against Staphylococcus aureus. Acta Biochim
Biophys Sin (Shanghai). 49:1058–1066. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li T, Yang SR, Chen M, Song LY and He CF:
Antibacterial mechanism of ginger mix-fried magnolia bark extract
against escherichia coli and staphylococcus aureus.
Modern Food Sci Technol. 32:84–92. 2016.
|
|
30
|
Cai X, Kong F, Wang R, Zhai S, Guan X,
Zhang G and Wang D: Candida albicans vaginitis in a murine
model is reduced by polypeptide-enriched Gastrodia elata
extracts. Future Microbiol. 14:839–846. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bajpai VK, Al-Reza SM, Choi UK, Lee JH and
Kang SC: Chemical composition, antibacterial and antioxidant
activities of leaf essential oil and extracts of Metasequioa
glyptostroboides Miki ex Hu. Food Chem Toxicol. 47:1876–1883.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Eom SH, Lee DS, Jung YJ, Park JH, Choi JI,
Yim MJ, Jeon JM, Kim HW, Son KT, Je JY, et al: The mechanism of
antibacterial activity of phlorofucofuroeckol-A against
methicillin-resistant Staphylococcus aureus. Appl Microbiol
Biotechnol. 98:9795–9804. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Cui H, Zhang C, Li C and Lin L:
Antimicrobial mechanism of clove oil on Listeria
monocytogenes. Food Control. 94:140–146. 2018. View Article : Google Scholar
|
|
34
|
Guo L, Zhang F, Wang X, Chen H, Wang Q,
Guo J, Cao X and Wang L: Antibacterial activity and action
mechanism of questin from marine Aspergillus flavipes HN4-13
against aquatic pathogen Vibrio harveyi. 3 Biotech.
9:142019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Watson AL and Chiu NHL: Fluorometric
cell-based assay for β-galactosidase activity in probiotic
gram-positive bacterial cells-Lactobacillus helveticus. J
Microbiol Methods. 128:58–60. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bhatt DK and Bano M: Modulation of
tricarboxylic acid cycle dehydrogenases during hepatocarcinogenesis
induced by hexachlorocyclohexane in mice. Exp Toxicol Pathol.
61:325–332. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Li Y, Shao X, Xu J, Wei Y, Xu F and Wang
H: Tea tree oil exhibits antifungal activity against Botrytis
cinerea by affecting mitochondria. Food Chem. 234:62–67.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Fu Y, Jia FB, Wang J, Song M, Liu SM, Li
YF, Liu SZ and Bu QW: Effects of sub-chronic aluminum chloride
exposure on rat ovaries. Life Sci. 100:61–66. 2014. View Article : Google Scholar : PubMed/NCBI
|