Introduction
A number of previous studies have investigated the
central white matter (1–3), which is predominantly composed of
axons, which are surrounded by myelin. In the central nervous
system (CNS), myelin is synthesized by oligodendrocytes, which wrap
their membranes around axons to maintain saltatory conduction and
neuronal support. Like neurons, oligodendrocytes are highly
vulnerable to ischemic injury, which usually leads to loss and
disruption of the myelin sheath (4,5).
As a major source of new oligodendrocytes following
ischemic stress, oligodendrocyte precursor cells (OPCs) are
abundant throughout the gray and white matter of the adult brain
(6–9). Previous studies have shown that OPCs
are capable of rapidly dividing, migrating and eventually
differentiating into myelin forming oligodendrocytes in response to
local signals induced by stroke; this suggests that the adult brain
possesses the capacity for endogenous remyelination by providing
demyelinated areas with newly-formed mature oligodendrocytes
(10–12). However, the capacity for
spontaneous myelin repair is limited despite the abundance of OPCs,
this may be due to the maturation arrest of OPCs (13,14).
Therefore, a better understanding of the molecular mechanisms that
control the process of oligodendrocyte development is important to
elucidate potential therapeutic interventions for demyelinating
disorders.
Olig1 and Olig2, two closely related basic
helix-loop-helix transcription factors, have been identified and
shown to play an important role in the differentiation of
oligodendrocyte lineage cells from OPCs to mature oligodendrocytes
(15–17). Previous gain and loss-of-function
studies found that Olig2 is necessary for the initiation of
oligodendrocytes, whereas Olig1 is suggested to contribute to the
complete maturation of oligodendrocytes and to the repair of
demyelinated lesions (18–21). Olig2 is present in the nucleus of
oligodendrocyte at all developmental stages, while Olig1 is
translocated from the nucleus to the cytoplasm in OPCs during
normal myelinogenesis, and ultimately remains localized in the
cytoplasm. Following demyelination, Olig1 is translocated back to
the nucleus (22), suggesting a
nuclear function of Olig1 that is important for remyelination.
However, a detailed molecular mechanism of the translocation of
Olig1 remains to be established. Previous studies revealed that the
post-translation modification of proteins, particularly
phosphorylation and acetylation, regulates the subcellular location
of Olig1 (23,24). Additionally, Tonchev et al
(25) reported an increase in the
number of Olig1-immunopositive (Olig1+) cells in the subventricular
zone following ischemia. It is possible that both the intracellular
redistribution of Olig1 and regulation of its expression levels may
be required for the repair process, however, these mechanisms have
not been well studied in the middle cerebral artery occlusion
(MCAO) model. Exploring the temporal and spatial expression and
distribution of Olig1 will increase the understanding of the role
of Olig1 in oligodendrogenesis and facilitate the development of
novel therapeutic strategies for remyelination.
Ischemia acutely induces mature oligodendrocyte
damage, leading to the diffuse loss of myelin and axons, which is
associated with severe neurologic deficits (9). OPCs contribute to myelination in the
CNS and have received much attention regarding their role in
ischemic-associated injuries (10). During recovery from ischemia, a
significant increase in the number of OPCs is observed, and some of
them become mature myelinating oligodendrocytes, in peri-infarct
gray and white matter where sprouting axons are present (12). Transcriptional factor Olig1 was
detected in oligodendrocytes and OPCs of the CNS and regulated
oligodendrocyte lineage progression (18). Although previous studies have
demonstrated that the role of Olig1 in the development of OPCs
(17,22), the relationship between Olig1 and
OPCs development, and myelination, remains largely unknown. In the
present study, the expression patterns of Olig1, along with the
alteration of OPCs and myelin, were systematically observed in the
brain following ischemia insult (from 1 to 28 days) using
immunohistochemistry and western blot analysis. The effects of
Olig1 on the maturation of OPCs and the subsequent remyelination in
a rat focal ischemic model were discussed in detail. The present
study provided an insight for the restorative treatment of ischemia
or other demyelination disease that requires the manipulation of
endogenous oligodendrogenesis in order to enhance spontaneous brain
repair.
Materials and methods
Experimental animals
A total of 72 adult male Sprague-Dawley rats at age
6–8 weeks weighing 260–280 g were used in the present study and
randomly assigned to the ischemic group or the control group. The
rats were purchased from the Department of Experimental Animal
Sciences, Dalian Medical University, and housed under standard
laboratory conditions (temperature, 20–25°C; relative humidity,
50–70%) for 7 days before experimentation. The animals had free
access to food and water during the experiment, and were maintained
in natural day/night cycles. Efforts were made to minimize the
discomfort of the animals. All protocols were approved by Animal
Welfare Committee of Dalian Medical University and followed the
guidelines for Animal Care and Use adapted from the National
Institutes of Health.
Surgery
The rats were anesthetized with 80 mg 10% chloral
hydrate (300 mg/kg) by intraperitoneal injection, no signs of
peritonitis were observed in the animals. The middle cerebral
artery (MCA) was occluded as described by Longa et al
(26). After midline incision of
the neck skin, the right external carotid artery (ECA) was
carefully isolated from the surrounding nerves and fascia. Briefly,
a nylon monofilament suture of 0.26 mm diameter (with one end
rounded by heat and a diameter of 0.34 mm) was inserted into the
ECA and advanced into the internal carotid artery to obstruct the
origin of the right MCA. The suture was inserted 18–20 mm from the
bifurcation of the common carotid artery. The rectal temperature of
the animals was maintained at 37±0.5°C using a heat lamp during the
operation. After awakening, neurological deficits were evaluated
according to the method of Bederson et al (27). Rats without left forelimb paresis
or circling towards the left side were regarded as unsuccessful
models and were excluded from further study. The rats were
anesthetized with ether 90 min after MCAO and the occluding
filament was withdrawn. The rats in the control group were
subjected to isolation of their right carotid arteries only. At 1,
3, 7, 14 and 28 days after MCAO, the rats were anesthetized with 80
mg 10% chloral hydrate (300 mg/kg) followed by decapitation or by
perfusion (12 rats at each time-point and 12 total control
rats).
Immunohistochemistry staining
Rats were perfused with normal saline followed by 4%
paraformaldehyde (PFA) in 0.1 M phosphate buffer (PB, pH 7.4) for
20 min at 25°C. The brains were removed, post-fixed in 4% PFA for
24–48 h (4°C) and then transferred to PB with 30% sucrose (4°C).
Several days later, the brains were frozen in solid carbon dioxide
and then cut in 20-µm-thick serial sections using a cryostat
(Cryocut1800; Leica Microsystems, Inc.). The sections were mounted
on gelatin/chrome alum-coated glass slides.
The slides were processed for Olig1, NG2 or myelin
basic protein (MBP) using the avidin-biotin technique (Elite kit;
Vector Laboratories, Inc.), as described by Tu et al
(28). The sections were blocked
with 2% goat serum (OriGene Technologies, Inc.) for 30 min at 37°C
and then incubated overnight at 4°C in a humid chamber with the
following antibodies: Olig1 (1:400; MAB5540, Chemicon
International; Thermo Fisher Scientific, Inc.), NG2 (1:400; AB5320,
Chemicon International; Thermo Fisher Scientific, Inc.) or MBP
(1:200; BA0094; Wuhan Boster Biological Technology, Ltd.). After
washing in 0.01 M PBS three times, sections were incubated with
goat anti-mouse or goat anti-rabbit secondary antibodies conjugated
to HRP (1:100, SP9001 or SP9002, OriGene Technologies, Inc.) for 30
min at 37°Cand were finally processed with diaminobenzidine (1
mg/ml; 0.001% H2O2) for 2 min at 25°C.
Images were captured with a charge-coupled device
(CCD) spot light camera under ×100, ×200 and ×400 magnification
with light microscopy (BX51; Olympus Corporation). MetaMorph
software (v7.8; Molecular Devices, LLC) was used for the
quantification of cell number and the average intensity in the
region of the ischemic striatum.
For the analysis of Olig1 expression in normal rats,
3 sections in different brain areas (the cerebral cortex, corpus
callosum, and the striatum and hippocampus) were selected and 5
fields of each section at the same magnification were randomly
chosen to count Olig1 positive cells. The total number of
Olig1+ cells was obtained from three sections of each
brain area and then averaged for six rats.
An area of 1,400×1,200 µm in the ischemic striatum
was defined for counting Olig1 or NG2 positive cells at different
time-points after MCAO. The slides were visualized by light
microscope under ×100 magnification. Counting of the number of
Olig1 and NG2 cells was carried out blindly. In total, 4 slides
from each rat brain were obtained from 20 µm thick coronal sections
between 1.4 mm anterior and 0.4 mm posterior to the bregma. All
counts were pooled and results were expressed as the average number
of positive cells per rat. In total, 6 rats were studied per
group.
The average intensity of MBP-immunoreactivity in the
striatum of the ischemic side was calculated to identify the extent
of remyelination. The average intensity was defined as the
difference between the average gray value (mean density) within the
ischemic striatum and its background (29).
Western blot analysis
At 1, 3, 7, 14 and 28 days after reperfusion, the
animals were sacrificed and the levels of Olig1 and MBP were
determined. Western blot analysis was performed as described by
Jiang et al (30). Briefly,
the bilateral striatum of the brain were removed, quickly frozen in
liquid nitrogen and stored at −80°C for later use. Frozen tissues
were homogenized in homogenization buffer (50 mmol/liter Trisxbase,
2 mmol/liter EDTA, 40 mmol/liter NaF, 1 mmol/liter
phenylmethylsulfonyl fluoride). Protein concentrations were
determined using the bicinchoninic acid method. Samples containing
60 mg of protein were separated by 15% SDS-PAGE and transferred to
PVDF membranes. The blots were blocked with 5% non-fat milk for 1 h
at 25°C and incubated with mouse monoclonal anti-Olig1 antibody
(1:4,000; MAB5540; Chemicon International; Thermo Fisher
Scientific, Inc) or rabbit polyclonal anti-MBP antibody (1:1,000;
BA0094, Wuhan Boster Biological Technology, Ltd.) overnight at 4°C.
β-actin (1:8,000; sc-130065; Santa Cruz Biotechnology, Inc.) served
as an internal control. After washing three times with TBST,
membranes were incubated for 1 h at room temperature with goat
anti-mouse or goat anti-rabbit IgG-HRP (1:1,000; sc-2005 or
sc-2004; Santa Cruz Biotechnology, Inc.). Protein bands were
visualized using enhanced chemiluminescence reagents (Santa Cruz
Biotechnology, Inc.) and the membranes were exposed to
autoradiographic film for 1–5 min.
For the quantification of the western blotting
signals, the blots were scanned with ScanWizard 5.0 (Microtek
International, Inc.) and the density of the bands were measured
with the TotalLab version 1.0 software (TotalLab Ltd.). Values for
Olig1 or MBP were normalized to the intensity of β-actin.
Statistical analysis
The experiments were repeated three times. Data are
expressed as the mean ± SEM. Statistical analysis was performed
using GraphPad Prism (version 5.0; GraphPad Software, Inc.).
Differences between the control and various time-points were
determined using one-way ANOVA followed by Dunnett's post-hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Expression pattern of Olig1 protein in
control and ischemic rats
Fig. 1 shows the
expression pattern of Olig1 protein in control and ischemic brain
sections with immunohistochemical staining. Olig1-immunoreactive
cells were widely distributed throughout the gray and white matter
of the control adult brain, including the cerebral cortex, corpus
callosum, the striatum and hippocampus (Fig. 1A and B). Olig1-immunoreactivity in
the control rats was restricted predominantly to the cytoplasm and
rarely seen in the nuclei of oligodendroglial-like cells
(characterized by a small cell body, sparse cytoplasm and few
processes; Fig. 1C and D). After
focal ischemia, however, the nuclear localization of Olig1
significantly increased in the area surrounding lesions (Fig. 1E and F). This indicated that the
Olig1 protein was recruited to the nucleus following MCAO.
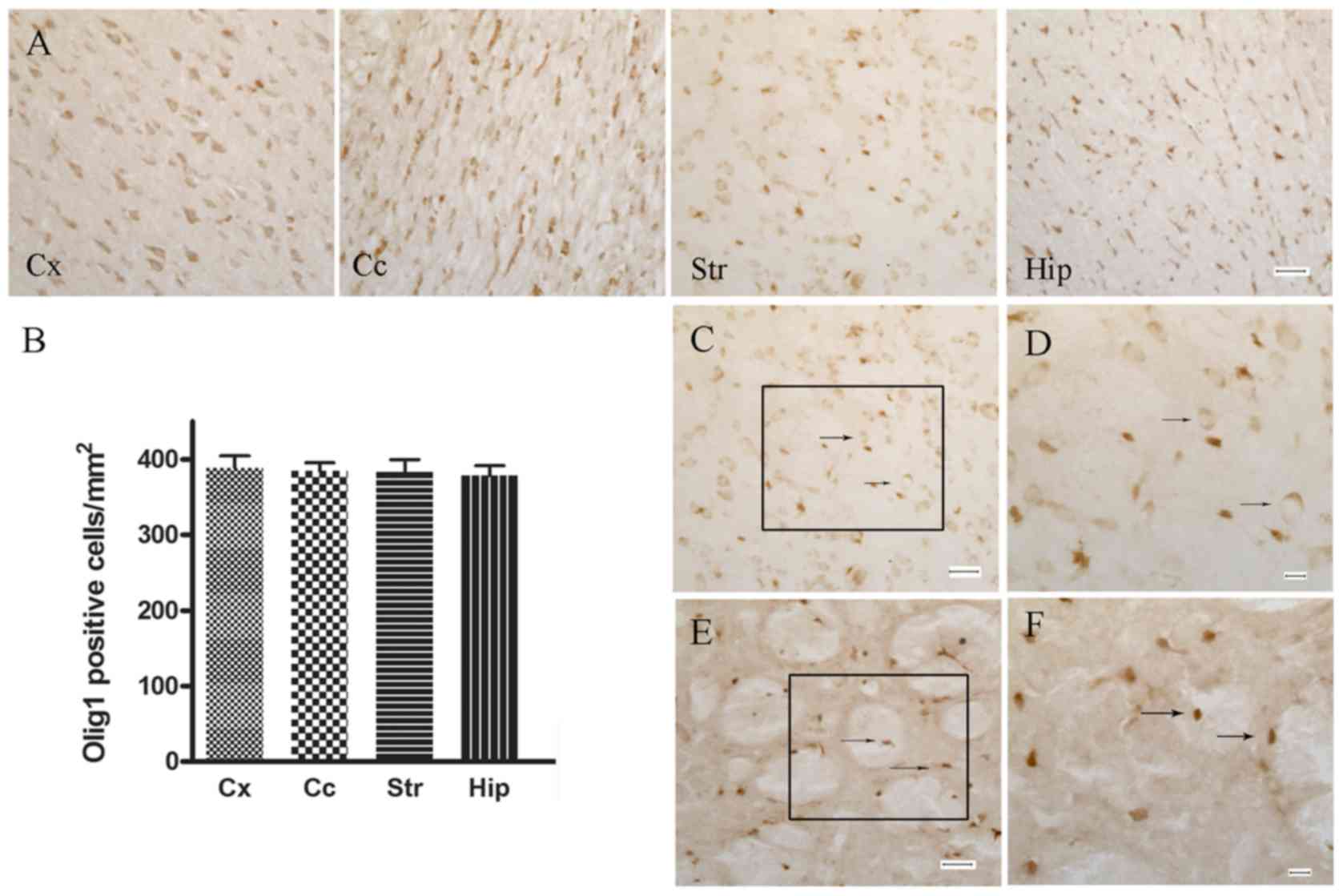 | Figure 1.Immunohistochemical staining of Olig1
in control and MCAO rats. (A) Distribution of Olig1 positive cells
in different regions of the brain. Scale bar, 30 µm. (B)
Statistical analysis of (A). (C) In normal rats, Olig1 was
predominantly located in the cytoplasm and rarely in the nuclei of
oligodendrocytes. Arrows indicate the cytoplasmic localization of
Olig1. Scale bar, 30 µm. (D) Higher magnification of black box in
(C). Scale bar, 10 µm. (E) MCAO rats exhibited extensive nuclear
localization of Olig1. Arrows indicate the nuclear localization of
Olig1. Scale bar, 30 µm. (F) Higher magnification of black box in
(E). Scale bar, 10 µm. Olig1, oligodendrocyte transcription factor
1; MCAO, middle cerebral artery occlusion; Cx, cortex; Cc, corpus
callosum; St, striatum; hip, hippocampus. |
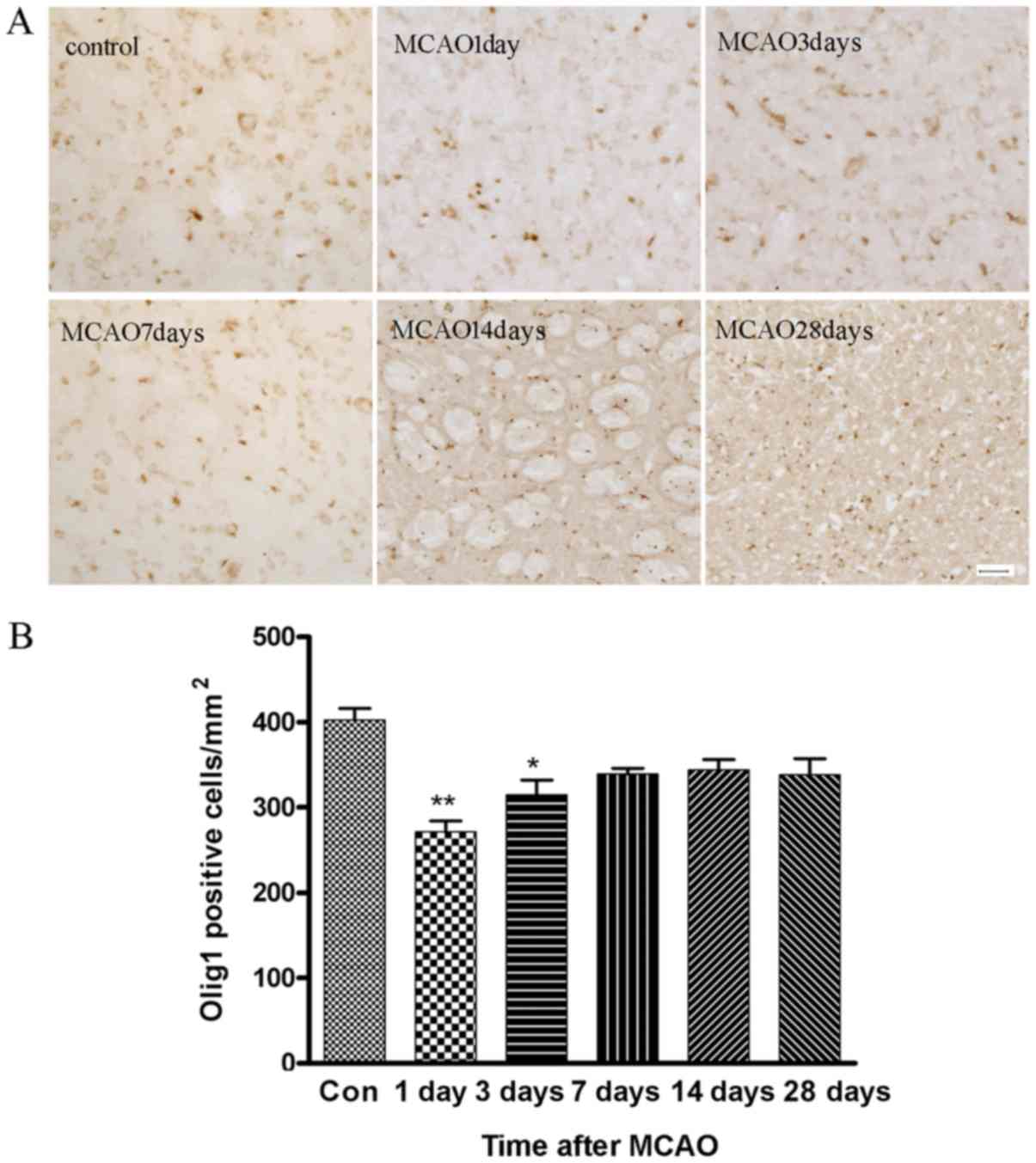
Time course of Olig1-immunoreactivity
after MCAO
Fig. 2A is a
representative example of the immunostaining for
Olig1-immunoreactivity in the right striatum on the sham-operation
day (control) and on days 1, 3, 7, 14 and 28 after MCAO. The
statistical results are shown in Fig.
2B. It was found that the number of Olig1-immunoreactive cells
were reduced at days 1 and 3 after MCAO, with lowest numbers
observed at day 1. The number of Olig1-immunoreactive cells
increased at day 7 and remained at this level for the next 4 weeks.
The ischemic side of MCAO rats exhibited extensive nuclear Olig1
staining, as observed by light microscopy, from day 1 to 28
following the operation. By contrast, the contralateral side showed
no evidence of Olig1 redistribution from the cytoplasm to the
nucleus in oligodendrocyte (data not shown), which indicated that
the presence of nuclear Olig1 is highly correlated with the active
lesions.
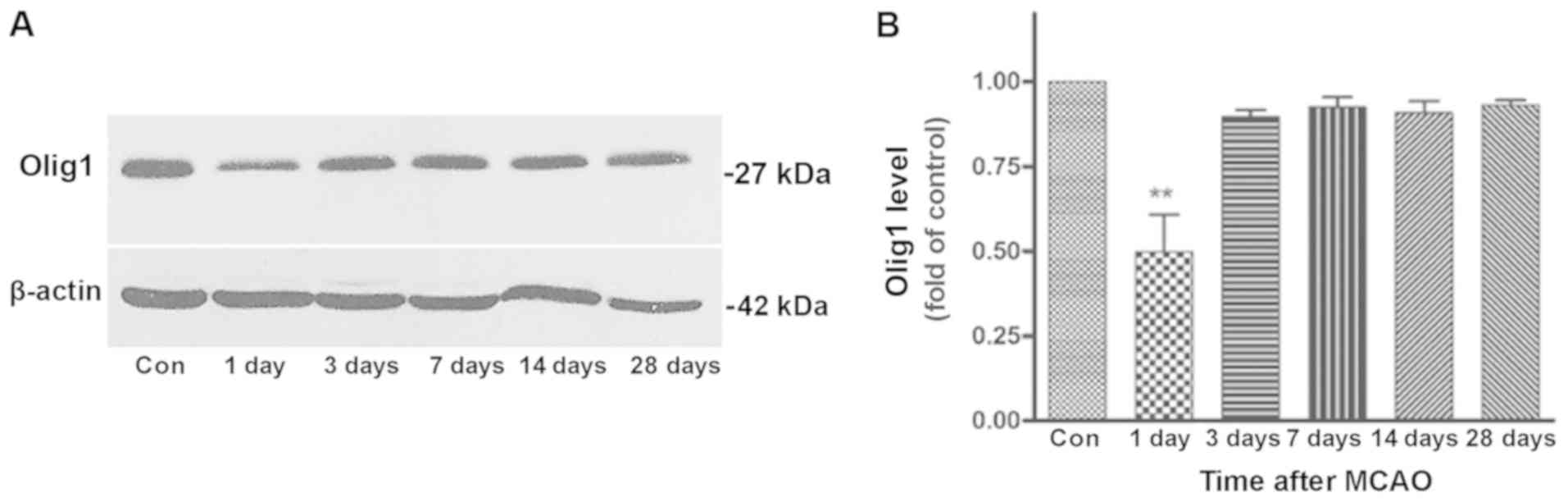
Western blotting was performed to analyze Olig1
expression. Results in the ischemic striatum at different
time-points (up to 28 days) are shown in Fig. 3A and B. Consistent with the
findings of the immunohistochemistry, Olig1 expression
significantly decreased at 1 day after MCAO, returned to a near
control level at day 3 and maintained this level throughout the
remaining period of the experiment. However, there was no
difference in the expression of Olig1 between control and MCAO rats
in the contralateral striatum at any time-point (Data not
shown).
Morphological changes of OPCs in the
ischemic striatum of the rats
Fig. 4A shows the
population of OPCs in the ischemic area, which was stained using an
NG2 antibody. The number of OPCs was uniformly distributed in the
white and gray matter of the control brain. These cells had small
bodies, round or oval, with multiple and highly branched processes
extending in all directions. In the brains sampled 7 days after
MCAO, the OPCs exhibited swollen cell bodies, with very heavy
staining, and many short and hypertrophied processes, indicating
OPCs were activated and entered mitosis. The number of NG2-positive
cells was quantified in the ischemic region at each of the
time-points (Fig. 4B). The number
of OPCs in the ischemic striatum significantly decreased at days 1
and 3, and recovered at day 7. The number of OPCs reached a peak at
day 14, and this level was maintained until day 28 after MCAO.
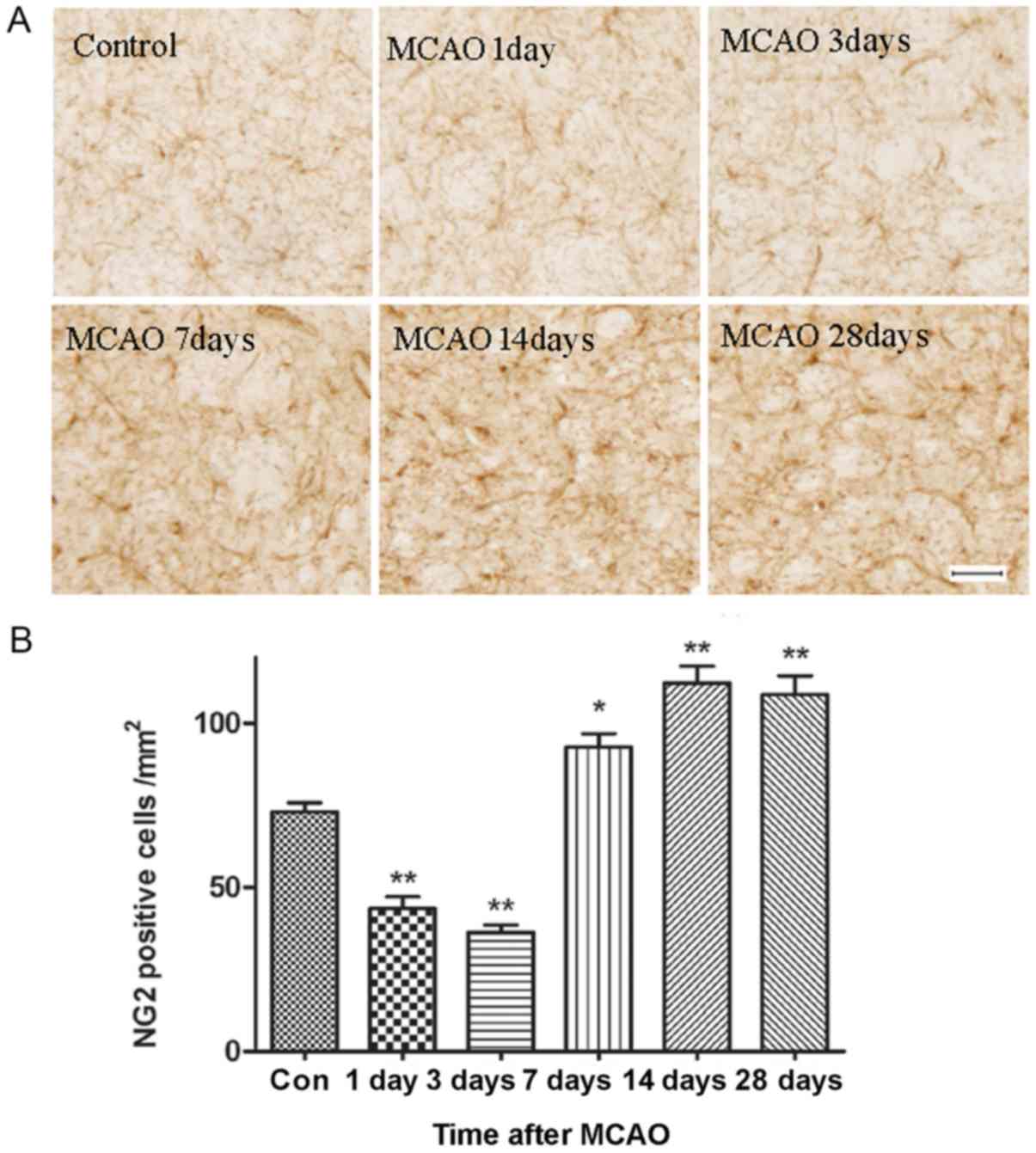 | Figure 4.Immunostaining of OPCs in the
ischemic area. (A) Representative images of sections stained with
anti-NG2, a marker of OPCs, at day 1, 3, 7, 14 and 28 after
reperfusion. Scale bar, 50 µm. There was a significant increase in
the number of OPCs and this was accompanied by morphological
changes in these cells from day 7 to 28 after reperfusion. (B)
Statistical analysis of the number of NG2 positive cells. n=6.
*P<0.05, **P<0.01 vs. Con. MCAO, middle cerebral artery
occlusion; Con, control; OPCs, oligodendrocyte precursor cells;
NG2, chondroitin sulfate proteoglycan 4. |
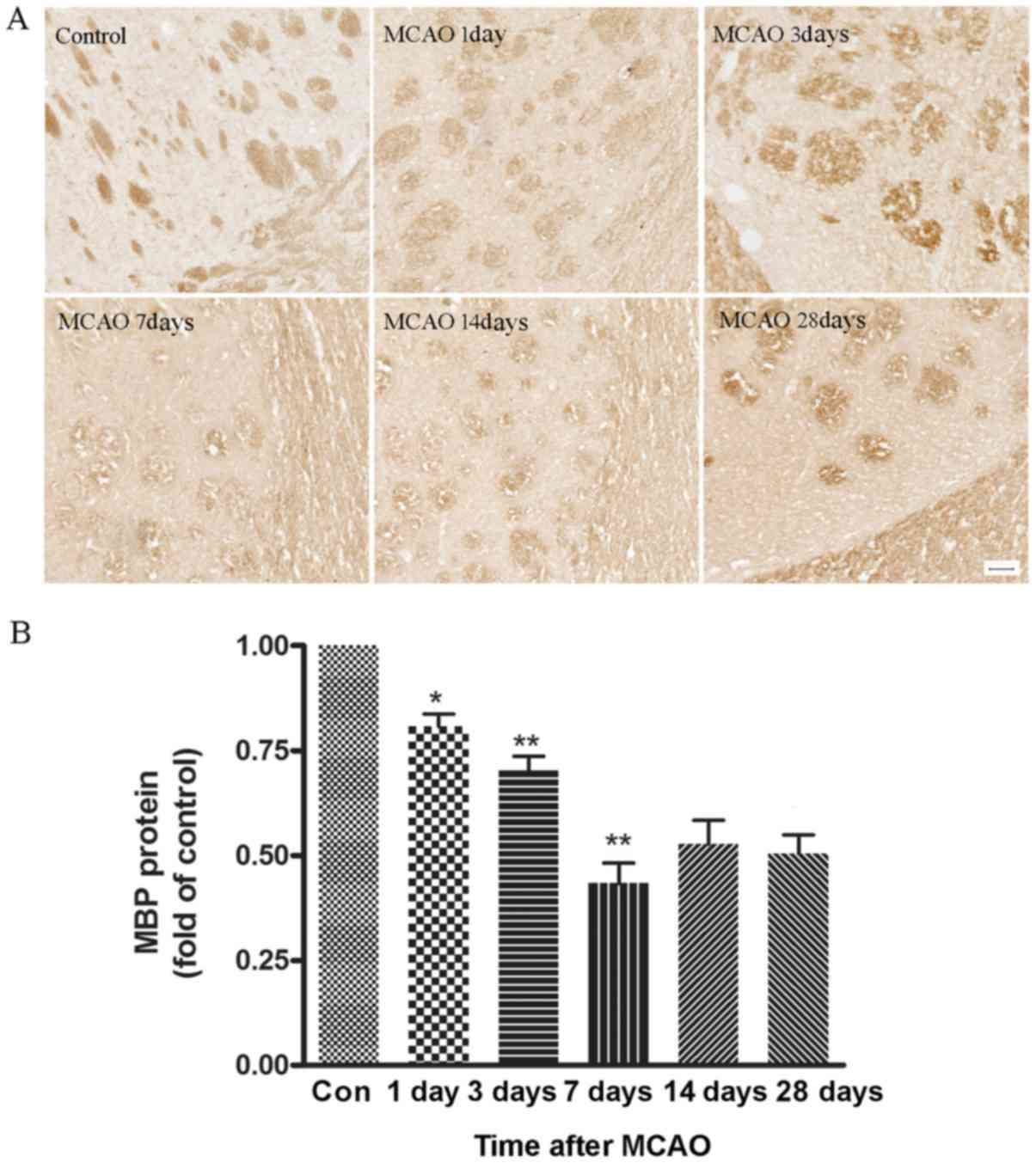
Changes in the expression of MBP in
brain tissues
To investigate the extent of demyelination in the
white matter of MCAO rats, the expression of MBP, a major
constituent of CNS myelin, was studied using immunohistochemistry
and western blotting. Representative images from the time course of
MBP staining in ischemic striatum is shown in Fig. 5A. Many myelinated fibers, which
were detected with anti-MBP antibody, were clearly visible in the
control cerebral striatum and each fiber tract could be easily
followed. By contrast, these fibers became obscure and spaces
between fiber tracts appeared during reperfusion following MCAO.
The ratio of MBP optical densities on the ischemic side was also
analyzed (Fig. 5B). The average
intensity of MBP staining in the ischemic group was decreased
gradually from day 1 to 7, and marginally recovered at days 14 and
28. However, the difference between days 14 and 28 was not
statistically significant (P>0.05).
Results of the western blotting and band density
analysis are shown in Fig. 6A and
B. MBP levels in the affected striatal tissues were measured at
days 1, 3, 7, 14 and 28 after MCAO. Compared with the control, the
average level of MBP progressively decreased from day 1 to 7,
reaching a minimum at day 7, then recovered at days 14 and 28.
Despite the increased expression of MBP at days 14 and 28, the MBP
expression level remained lower than in the control group. These
data suggested that myelin loss occurred at an early time-point (1
day) and advanced during ischemia.
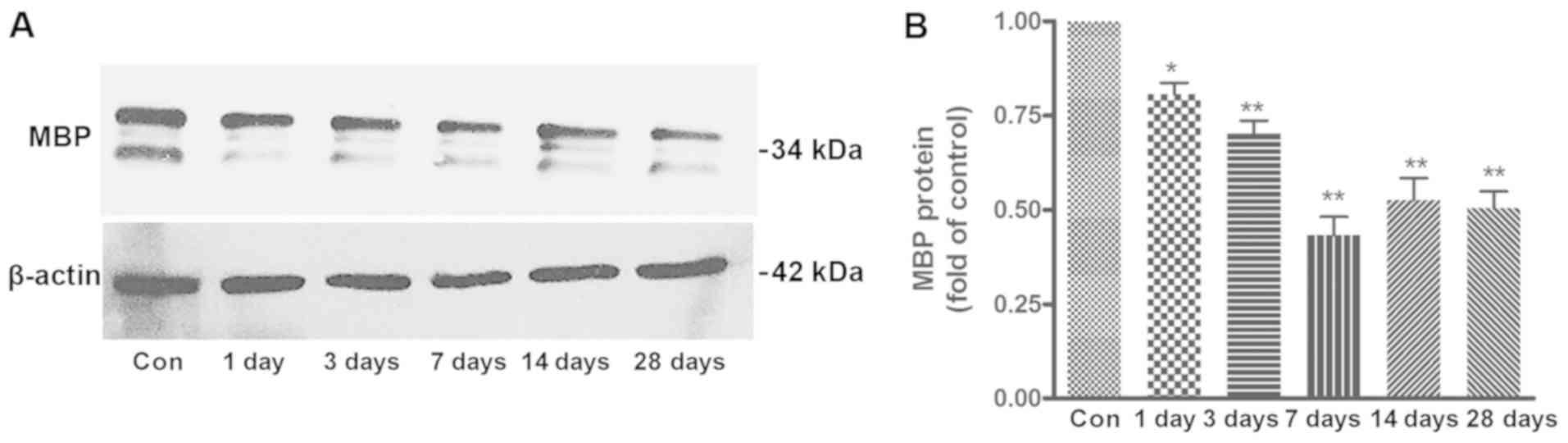 | Figure 6.Western blot analysis of MBP in the
ischemic striatum of MCAO rats. (A) MBP expression in the Con group
and at different time points (1, 3, 7, 14 and 28 days) after MCAO.
(B) Quantification of the results shown in (A). The expression of
MBP decreased from day 1 to 7, with the lowest expression at day 7.
MBP expression increased at days 14 and 28, however, it remained
lower than the control group. n=6. *P<0.05, **P<0.01 vs. Con.
MBP, myelin basic protein; MCAO, middle cerebral artery occlusion;
Con, control. |
Discussion
OPCs are defined as glial cells in the CNS and
constitute a stable population of quiescent cells that divide
infrequently (6). Remyelination is
a complicated and elaborate process, which is predominantly
mediated by endogenous OPCs (10).
In response to injury signals at sites of demyelination, OPCs in
the vicinity are activated. OPCs then proliferate and migrate into
the injured area and differentiate into oligodendrocytes, which
form new myelin sheaths wrapping the demyelinated axons (11,31–33).
This indicates that the adult brain possesses the capacity to
regenerate myelin by supplying mature oligodendrocytes that
originate from activated OPCs. Accordingly, the OPCs in the adult
brain are hypothesized to have the potential to repair damaged
brain tissue. Tanaka et al (12) showed that the upregulation in the
number of OPCs may contribute to an almost complete recovery of the
myelin in the peri-infarct area after ischemic insult. However,
previous studies demonstrated that remyelination is limited and
incomplete, although OPCs are present (13,14).
These inconsistent results indicate that it is necessary to further
explore the contribution of OPCs to myelin repair following
ischemia, with the aim of identifying potential targets for
clinical treatment.
In the present study, changes in OPCs and myelin
were observed systematically from day 1 to 28 after MCAO. The
findings of the present study revealed that OPCs stained with an
anti-NG2 antibody were abundant and homogenously distributed in the
white and gray matter of adult rat brains. These cells persist
quiescently and have small bodies with multiple, and highly
branched, processes under normal conditions. Consistent with
previous studies (12,34), we found that OPCs exhibited swollen
and hypertrophied cell bodies with many radiating thick processes
when the brain was subjected to ischemia insult, indicating OPCs
were activated and entered into the mitotic phase in response to
damage. This transition from a quiescent to an activated state
enabled OPCs to undergo differentiation, which is important to the
success of remyelination. In addition, these morphological changes
were accompanied by a significant increase in the number of OPCs.
As shown in the present study, the number of OPCs reduced
significantly between day 1 and 3 after reperfusion, and then
increased until day 28. A significant decrease in the population of
OPCs at the early stage following reperfusion suggested that OPCs
may be as vulnerable as neurons to ischemia. The ischemic striatum
demonstrated a gradual increase in the number of OPCs, possibly
because the activated OPCs migrated into the injury sites from the
outer pre-infarct area to substitute the loss of these cells.
Although a number of OPCs were recruited into the ischemic
striatum, whether they could differentiate terminally into mature
oligodendrocytes and form new myelin remains to be determined. MBP
was used as a specific marker of mature oligodendrocytes and myelin
sheath (35). It was found that
MBP expression decreased from day 1 to 7 following focal cerebral
ischemia, and marginally recovered at days 14 and 28 (Figs. 5 and 6), which indicated a loss of myelin at
day 1 and a continuous advancement of demyelination until day28.
Despite the late increase, the MBP expression level remained lower
than that of the control group, suggesting that the injured myelin
can only be partially repaired. The limited remyelination indicated
that OPCs maturation may be inhibited under ischemic condition.
This is supported by the fact that Olig1 is transported into the
cytoplasm following oligodendrogenesis (22,36,37);
however, in the present study, most of the Olig1 protein remained
in nucleus as late as 28 days after ischemia, suggesting that OPCs
maturation was hindered. The molecular mechanisms regulating OPC
differentiation and maturation under pathological conditions
require further investigation.
Olig1 is important for oligodendrocyte
differentiation from OPCs to mature oligodendrocytes and the repair
of demyelinated lesions (36,38).
The expression pattern of Olig1 and its effects on remyelination
were investigated. It was observed that Olig1 was located
predominantly in cells with an oligodendroglial morphology (small
process-bearing cells) and widely distributed homogeneously
throughout the white and gray matter of the adult brain. This is
consistent with other reports (16,39).
That the expression of Olig1 persists throughout the life of an
oligodendrocyte suggests a fundamental role for this gene in
oligodendrocyte maturation and/or survival (40). The fact that oligodendrocytes fail
to develop in Olig1 knockout mice supports this viewpoint (19,20).
Furthermore, the present study demonstrated that in the normal
brain, the Olig1 protein was cytoplasmic. According to the
subcellular localization of Olig1 at different developmental
stages, it was predicted that the small proportion of cells
containing nuclear Olig1 is likely to be the quiescent
undifferentiated progenitors, which are abundant and comprise 5–8%
the glial cell population in the normal brain (41). The function of these nuclear Olig1+
cells in the undamaged CNS is unclear, however, it may involve
spontaneous remyelination, as suggested in the human CNS during
aging (42,43). In the present study, when the brain
underwent ischemia, Olig1 displayed extensive nuclear localization
in the affected striatum, consistent with a previous report
(22). However, the meaning of the
dynamic redistribution of Olig1 is poorly understood. It was shown
that the phosphorylation of Olig1 regulated its nuclear-cytoplasmic
shuttling during oligodendrocyte development. Olig1 localized into
the nucleus to facilitate myelin gene expression, then Olig1
changed the phosphorylation state and translocated into the
cytoplasm to enhance membrane expansion and differentiate into
mature oligodendrocytes (24).
These findings imply that the cytoplasmic relocation of Olig1 may
contribute to the process by which OPCs mature into
oligodendrocytes. However, the detailed molecular mechanism of this
process requires further investigation.
For the first time in the present study, to the best
of our knowledge, a systematic and long-term observation of Olig1
expression after MCAO was performed. Using immunostaining it was
found that the number of Olig1+ cells decreased from day
1 to 3 after MCAO, and recovered to a near normal level until day
28. Western blotting analysis supported the results of the
immunostaining. The mechanism mediating the rapid reduction in the
number of Olig1+ cells at day 1 after ischemia is likely
to be complex. As Olig1 is predominantly expressed by OPCs and
myelinating oligodendrocytes (16,39),
the death of these cells within the lesion area may explain the
reduced number of Olig1+ cells, although it cannot be
excluded that the transcriptional downregulation of Olig1 may also
contribute to this. The increased number of Olig1+ cells
observed from day 7 to day 28 after ischemia may be due to the
recruitment and/or proliferation of Olig1+ OPCs at the
injury sites, which may partially compensate for Olig1+
cells death.
The transcription factor Olig1 is an important
regulator of OPCs and can promote the development of immature OPCs
to mature oligodendrocytes, and eventually contribute to the
migration of mature of oligodendrocytes to newly formed myelin
(44). The present study showed a
sharp decline in the expression of Olig1 and MBP at 1 day following
the ischemia insult. Subsequently, the expression of Olig1 was
restored to near normal levels from day 3 to 28, while MBP
expression decreased from day 3 to 7 and recovered marginally until
day 28.
Despite the partial recovery at the late stage of
ischemia, the expression of MBP expression failed to reach the
levels observed in the control group. The expression of MBP
increased at the late compared to the early stage, which is a
pattern similar to that of Olig1; this suggested that Olig1 has a
promoting role in the repair and regeneration of myelin. These
findings revealed that the downregulation of Olig1 in
hypoxic-ischemic brain tissue led to a decreased expression of some
proteins, such as MBP, that are required to form myelin, and thus
delayed the synthesis of myelin. However, despite the repair effect
of Olig1, myelin was not fully repaired to a normal level. Our
previous study revealed that the upregulation of Olig1 may enhance
the differentiation of OPCs into mature oligodendrocytes and
accelerate the kinetics of myelination after focal cerebral
ischemia (45), indicating that
the normal level of Olig1 is not sufficient for myelinogenesis
following ischemia. The effect of the upregulation of Olig1 on the
repair process following ischemia requires further
investigation.
Olig1 is an important regulator of myelin specific
genes, however, the changes of Olig1 expression did not parallel
those of MBP, leading to the speculation that Olig1 regulates the
expression of MBP along with other transcription factors. The
process governing remyelination is complex and orchestrated by a
network of transcriptional regulators; Olig1 as a single
transcription factor is not sufficient (46). It has been reported that increased
expression of the homeobox protein Nkx2.2 and Olig2 had an
important role in the differentiation of OPCs into remyelinating
oligodendrocytes (47). An in
vitro experiment showed that oligodendrocyte differentiation
initiated by Olig1 gene transfection did not lead to full
maturation, which highlighted requirement for Nkx2.2 (48). Moreover, other transcription
factors, such as Sox9, Sox10, Nkx2.2 and zinc finger protein 488,
may be involved in regulation (49–51).
It was found that Nkx2.2+/Olig2+ OPCs failed
to remyelinate in areas depleted of astrocytes, which suggested
astrocytes may produce a wide range of signaling molecules to
support differentiation (52,53).
However, glial scars composed of astrocytes may prevent
remyelinating cells from gaining access to demyelinated axons, so
it is not yet clear whether astrocytes promote or hinder the
process (54,55). In addition, the existence of myelin
inhibitory factor and the delayed clearance of myelin debris
surrounding demyelinated axons by macrophages also have inhibitory
effects on remyelination (56).
Processes governing remyelination rely not only on the intrinsic
determinants expressed in a specific spatial and temporal sequence,
but also on extracellular signals.
In conclusion, the present study investigated the
changes of Olig1 expression in MCAO rats and further explored the
effects of Olig1 on OPC maturation, and remyelination, using
immunostaining and western blotting. The present study has shown
for the first time, to the best of our knowledge, that Olig1 is
markedly decreased during the initial 24 h after demyelination
injury, and is subsequently returned to near control levels, which
are maintained up to day 28. Olig1 translocated from cytoplasm to
nucleus at the ischemia site. The number of OPCs increased from day
7 to 28 after ischemic insult, while the expression of MBP
decreased from day 1 to 7 and marginally increased at days 14 and
28. Despite the increase at the late time-points following MCAO,
the final levels of these proteins remained lower than the
corresponding levels in the control group; this suggested that
Olig1 has a limited ability to promote the repair of myelin. In the
present study it was found that Olig1 can promote myelination by
stimulating nuclear transcription, but this effect is insufficient
for the complete repair of the damaged myelin. Additional studies
are required to determine whether interventions based on Olig1
expression or stimulating nuclear Olig1 relocation into the
cytoplasm to promote endogenous remyelination would be efficient to
prevent and/or treat ischemic-related disease.
Acknowledgements
The authors would like to thank Professor Jie Li
(University of Cincinnati) for helpful corrections to the
manuscript. The authors would also like to thank Dr You Wan (The
Neuroscience Research Institute, Peking University) for technical
assistance.
Funding
The current study was supported by grants from The
National Natural Science Foundation of China (grant nos. 30570626,
81371355 and 81671191) and The Beijing Natural Science Foundation
(grant no. 7082028).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
HZ and YBZ conceived and designed the study. XYG and
ZHL performed the experiments. JWL, SPW and DXW analyzed the data
and drafted the manuscript. All authors have read and approved the
final manuscript.
Ethics approval and consent to
participate
The animal-related experiments were approved by
Animal Welfare Committee of Dalian Medical University and followed
the guidelines for Animal Care and Use adapted from NIH, USA.
Patients consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Marin MA and Carmichael ST: Stroke in CNS
white matter: Models and mechanisms. Neurosci Lett. 684:193–199.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Goldberg MP and Ransom BR: New light on
white matter. Stroke. 34:330–332. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Pantoni L, Garcia JH and Gutierrez JA:
Cerebral white matter is highly vulnerable to ischemia. Stroke.
27:1641–1647. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Back SA, Han BH, Luo NL, Chricton CA,
Xanthoudakis S, Tam J, Arvin KL and Holtzman DM: Selective
vulnerability of late oligodendrocyte progenitors to
hypoxia-ischemia. J Neurosci. 22:455–463. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Dewar D, Underhill SM and Goldberg MP:
Oligodendrocytes and ischemic brain injury. J Cereb Blood Flow
Metab. 23:263–274. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Nishiyama A: Polydendrocytes: NG2 cells
with many roles in development and repair of the CNS.
Neuroscientist. 13:62–76. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dawson MR, Polito A, Levine JM and
Reynolds R: NG2-expressing glial progenitor cells: An abundant and
widespread population of cycling cells in the adult rat CNS. Mol
Cell Neurosci. 24:476–488. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Eugenín-von Bernhardi J and Dimou L:
NG2-glia, more than progenitor cells. Adv Exp Med Biol. 949:27–45.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
van Tilborg E, de Theije CGM, van Hal M,
Wagenaar N, de Vries LS, Benders MJ, Rowitch DH and Nijboer CH:
Origin and dynamics of oligodendrocytes in the developing brain:
Implications for perinatal white matter injury. Glia. 66:221–238.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Keirstead HS and Blakemore WF: The role of
oligodendrocytes and oligodendrocyte progenitors in CNS
remyelination. Adv Exp Med Biol. 468:183–197. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nishiyama A, Komitova M, Suzuki R and Zhu
X: Polydendrocytes (NG2 cells): Multifunctional cells with lineage
plasticity. Nat Rev Neurosci. 10:9–22. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tanaka K, Nogawa S, Suzuki S, Dembo T and
Kosakai A: Upregulation of oligodendrocyte progenitor cells
associated with restoration of mature oligodendrocytes and
myelination in peri-infarct area in the rat brain. Brain Res.
989:172–179. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Franklin RJ: Why does remyelination fail
in multiple sclerosis? Nat Rev Neurosci. 3:705–714. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lubetzki C, Williams A and Stankoff B:
Promoting repair in multiple sclerosis: Problems and prospects.
Curr Opin Neurol. 18:237–244. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ross SE, Greenberg ME and Stiles CD: Basic
helix-loop-helix factors in cortical development. Neuron. 39:13–25.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou Q, Wang S and Anderson DJ:
Identification of a novel family of oligodendrocyte
lineage-specific basic helix-loop-helix transcription factors.
Neuron. 25:331–343. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Miller RH: Regulation of oligodendrocyte
development in the vertebrate CNS. Prog Neurobiol. 67:451–467.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jakovcevski I and Zecevic N: Olig
transcription factors are expressed in oligodendrocyte and neuronal
cells in human fetal CNS. J Neurosci. 25:10064–10073. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhou Q and Anderson DJ: The bHLH
transcription factors OLIG2 and OLIG1 couple neuronal and glial
subtype specification. Cell. 109:61–73. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lu QR, Sun T, Zhu Z, Ma N, Garcia M,
Stiles CD and Rowitch DH: Common developmental requirement for Olig
function indicates a motor neuron/oligodendrocyte connection. Cell.
109:75–86. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Takebayashi H and Nabeshima Y, Yoshida S,
Chisaka O, Ikenaka K and Nabeshima Y: The basic helix-loop-helix
factor olig2 is essential for the development of motoneuron and
oligodendrocyte lineages. Curr Biol. 12:1157–1163. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Arnett HA, Fancy SP, Alberta JA, Zhao C,
Plant SR, Kaing S, Raine CS, Rowitch DH, Franklin RJ and Stiles CD:
bHLH transcription factor Olig1 is required to repair demyelinated
lesions in the CNS. Science. 306:2111–2115. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Dai J, Bercury KK, Jin W and Macklin WB:
Olig1 Acetylation and nuclear export mediate oligodendrocyte
development. J Neurosci. 35:15875–15893. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Niu J, Mei F, Wang L, Liu S, Tian Y, Mo W,
Li H, Lu QR and Xiao L: Phosphorylated olig1 localizes to the
cytosol of oligodendrocytes and promotes membrane expansion and
maturation. Glia. 60:1427–1436. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tonchev AB, Yamashima T, Sawamoto K and
Okano H: Transcription factor protein expression patterns by neural
or neuronal progenitor cells of adult monkey subventricular zone.
Neuroscience. 139:1355–1367. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Longa EZ, Weinstein PR, Carlson S and
Cummins R: Reversible middle cerebral artery occlusion without
craniectomy in rats. Stroke. 20:84–91. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bederson JB, Pitts LH, Tsuji M, Nishimura
MC, Davis RL and Bartkowski H: Rat middle cerebral artery
occlusion: Evaluation of the model and development of a neurologic
examination. Stroke. 17:472–476. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tu H, Deng L, Sun Q, Yao L, Han JS and Wan
Y: Hyperpolarization-activated, cyclic nucleotide-gated cation
channels: Roles in the differentialelectrophysiological properties
of rat primary afferent neurons. J Neurosci Res. 76:713–722. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Luo H, Cheng J, Han JS and Wan Y: Change
of vanilloid receptor 1 expression in dorsal root ganglion and
spinal dorsal horn during inflammatory nociception induced by
complete Freund's adjuvant in rats. Neuroreport. 15:655–658. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang YQ, Xing GG, Wang SL, Tu HY, Chi YN,
Li J, Liu FY, Han JS and Wan Y: Axonal accumulation of
hyperpolarization-activated cyclic nucleotide-gated cation channels
contributes to mechanical allodynia after peripheral nerve injury
in rat. Pain. 137:495–506. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Song FE, Huang JL, Lin SH, Wang S, Ma GF
and Tong XP: Roles of NG2-glia in ischemic stroke. CNS Neurosci
Ther. 23:547–553. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Mandai K, Matsumoto M, Kitagawa K,
Matsushita K, Ohtsuki T, Mabuchi T, Colman DR, Kamada T and
Yanagihara T: Ischemic damage and subsequent proliferation of
oligodendrocytes in focal cerebral ischemia. Neuroscience.
77:849–861. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chamberlain KA, Nanescu SE, Psachoulia K
and Huang JK: Oligodendrocyte regeneration: Its significance in
myelin replacement and neuroprotection in multiple sclerosis.
Neuropharmacology. 110:633–643. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bu J, Banki A, Wu Q and Nishiyama A:
Increased NG2(+) glial cell proliferation and oligodendrocyte
generation in the hypomyelinating mutant shiverer. Glia. 4:51–63.
2004. View Article : Google Scholar
|
|
35
|
Luessi F, Kuhlmann T and Zipp F:
Remyelinating strategies in multiple sclerosis. Expert Rev
Neurother. 14:1315–1334. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Paes de Faria J, Kessaris N, Andrew P,
Richardson WD and Li H: New Olig1 null mice confirm a non-essential
role for Olig1 in oligodendrocyte development. BMC Neurosci.
15:122014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Qi Q, Zhang Y, Shen L, Wang R, Zhou J, Lü
H and Hu J: Olig1 expression pattern in neural cells during rat
spinal cord development. Neuropsychiatr Dis Treat. 12:909–916.
2016.PubMed/NCBI
|
|
38
|
Sabo JK, Heine V, Silbereis JC, Schirmer
L, Levison SW and Rowitch DH: Olig1 is required for noggin-induced
neonatal myelin repair. Ann Neurol. 81:560–571. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Lu QR, Yuk D, Alberta JA, Zhu Z, Pawlitzky
I, Chan J, McMahon AP, Stiles CD and Rowitch DH: Sonic
hedgehog-regulated oligodendrocyte lineage genes encoding bHLH
proteins in the mammalian central nervous system. Neuron.
25:317–329. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ligon KL, Kesari S, Kitada M, Sun T,
Arnett HA, Alberta JA, Anderson DJ, Stiles CD and Rowitch DH:
Development of NG2 neural progenitor cells requires Olig gene
function. Proc Natl Acad Sci USA. 103:7853–7858. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Dawson MR, Levine JM and Reynolds R:
NG2-expressing cells in the central nervous system: Are they
oligodendroglial progenitors? J Neurosci Res. 61:471–479. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Peters A and Sethares C: Is there
remyelination during aging of the primate central nervous system? J
Comp Neurol. 460:238–254. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Franklin RJ, Zhao C and Sim FJ: Ageing and
CNS remyelination. Neuroreport. 13:923–928. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Cheng T, Xue X and Fu J: Effect of Olig1
on the development of oligodendrocytes and myelination in a
neonatal rat PVL model induced by hypoxia-ischemia. Mol Med Rep.
11:2379–2386. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhao H, Gao XY, Wang DX and Zhang YB:
Effect of adenovirus-mediated gene transfer of Olig1 on
oligodendrocyte differentiation and remyelination in a rat model of
focal cerebral ischemia. Neural Regen Res. 4:862–867. 2009.
|
|
46
|
He L and Lu QR: Coordinated control of
oligodendrocyte development by extrinsic and intrinsic signaling
cues. Neurosci Bull. 29:129–143. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Fancy SP, Zhao C and Franklin RJ:
Increased expression of Nkx2.2 and Olig2 identifies reactive
oligodendrocyte progenitor cells responding to demyelination in the
adult CNS. Mol Cell Neurosci. 27:247–254. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Balasubramaniyan V, Timmer N, Kust B,
Boddeke E and Copray S: Transient expression of Olig1 initiates the
differentiation of neural stem cells into oligodendrocyte
progenitor cells. Stem Cells. 22:878–882. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Watanabe M, Hadzic T and Nishiyama A:
Transient upregulation of Nkx2.2 expression in oligodendrocyte
lineage cells during remyelination. Glia. 46:311–322. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Soundarapandian MM, Selvaraj V, Lo UG,
Golub MS, Feldman DH, Pleasure DE and Deng W: Zfp488 promotes
oligodendrocyte differentiation of neural progenitor cells in adult
mice after demyelination. Sci Rep. 1:22011. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Finzsch M, Stolt CC, Lommes P and Wegner
M: Sox9 and Sox10 influence survival and migration of
oligodendrocyte precursors in the spinal cord by regulating PDGF
receptor alpha expression. Development. 135:637–646. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Talbott JF, Loy DN, Liu Y, Qiu MS, Bunge
MB, Rao MS and Whittemore SR: Endogenous
Nkx2.2+/Olig2+ oligodendrocyte precursor
cells fail to remyelinate the demyelinated adult rat spinal cord in
the absence of astrocytes. Exp Neurol. 192:11–24. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Carmen J, Magnus T, Cassiani-Ingoni R,
Sherman L, Rao MS and Mattson MP: Revisiting the
astrocyte-oligodendrocyte relationship in the adult CNS. Prog
Neurobiol. 82:151–162. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Haindl MT, Köck U, Zeitelhofer-Adzemovic
M, Fazekas F and Hochmeister S: The formation of a glial scar does
not prohibit remyelination in an animal model of multiple
sclerosis. Glia. 67:467–481. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Williams A, Piaton G and Lubetzki C:
Astrocytes-friends or foes in multiple sclerosis? Glia.
55:1300–1312. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Kotter MR, Li WW, Zhao C and Franklin RJ:
Myelin impairs CNS remyelination by inhibiting oligodendrocyte
precursor cell differentiation. J Neurosci. 26:328–332. 2006.
View Article : Google Scholar : PubMed/NCBI
|