Introduction
Lung cancer is the main reason for cancer-associated
mortality, causing >1.37 million cases of mortality globally
annually (1,2). In China, the number of lung cancer
cases has reached 800,000, and 700,000 cases of mortality have been
caused by lung cancer in 2017. The 5-year survival rate of lung
cancer is as low as ~18%, due to the fact that the majority of
patients are in the middle or late stage when they are admitted for
hospitalization and thus have lost the opportunity for surgical
treatment (3). Non-small cell lung
cancer (NSCLC) constitutes 85% of all lung cancer cases. The
initiation and development of NSCLC is attributed to the aberrant
expression of proto-oncogenes and tumor-suppressive genes, which
result in tumor cell growth, metastasis and eventually tumor
progression (1).
MicroRNAs (miRNAs/miRs) are short endogenous
non-protein-coding RNAs that are ~22 nucleotides in length. miRNAs
serve notable functions in regulating human gene expression by base
pairing to the 3′ untranslated region (3′UTR) of the target mRNA
(4). Various miRNAs are involved
in a diverse range of cellular processes, including cell
proliferation, apoptosis, development and differentiation (5). Increasing evidence demonstrates that
the dysregulation of miRNA expression is associated with the
development and progression of various cancer types including NSCLC
(6–10).
miR-4500, which is 16 nucleotides in length and is
located in chromosome 13, was discovered by high-throughput
sequencing technology (11). One
previous study demonstrated that miR-4500 was downregulated in
NSCLC lung tissues compared with their non-tumor counterparts, and
that the low expression of miR-4500 promoted tumor growth by
targeting lin-28 homolog B (LIN28B) and NRAS proto-oncogene, GTPase
(NRAS) (12). Using a
bioinformatic algorithm, signal transducer and activator of
transcription proteins 3 (STAT3) was predicted to be a novel
potential target of miR-4500 (13).
STAT3 has been demonstrated to be crucial in tumor
development and cancer-associated inflammation (14). STAT3 may prevent apoptosis and
promotes cell proliferation and angiogenesis (15). A number of miRNAs are known to
interact with the 3′-UTR of STAT3 mRNA and thereby negatively
regulate STAT3 (16–19). For example, miR-17, miR-20a and
miR-106b control lung epithelial branching morphogenesis through
the downregulation of STAT3 and mitogen-activated protein kinase 14
protein levels and followed by the regulation of E-Cadherin
distribution (20). miR-9600
suppresses tumor progression and promotes paclitaxel sensitivity in
NSCLC through altering STAT3 expression in NSCLC cell lines
(18).
The present study initially investigated the
expression of miR-4500 and STAT3 in NSCLC tissues and the human
NSCLC cell lines A549 and H1975. Then, the function of miR-4500 in
cell proliferation, migration, invasion and apoptosis in
vitro was examined. Furthermore, the molecular mechanism of
miR-4500 in tumor progression was studied. The results of the
present study suggested that miR-4500 may serve a regulatory role
in NSCLC progression, and may be a novel strategy and prognostic
marker for the diagnosis and prognosis of NSCLC.
Materials and methods
Human tissue samples
The present study was ethically approved by the
Ethics Committee of the First People's Hospital of Changzhou
(Changzhou, China). Clinical samples (NSCLC tissues and adjacent
normal tissues) were collected from 40 patients with NSCLC (average
age, 57; female to male ratio, 17:23; 11 patients aged <60 years
old and 29 patients aged ≥60 years old) who received surgery at The
First People's Hospital of Changzhou (Changzhou, China) between
July 2015 and November 2017 subsequent to obtaining written
informed consent. All patient diagnoses of NSCLC were confirmed
based on a pathological assay, and none of the patients received
any prior cancer treatment.
Cell culture and transfection
Human NSCLC cell lines A549 and H1975 and a human
normal lung cell line (16HBE), obtained from the Type Culture
Collection of the Chinese Academy of Sciences (Shanghai, China),
were cultured in RPMI-1640 medium (Hyclone; GE Healthcare Life
Sciences, Logan, UT, USA) in the presence of 10% fetal bovine serum
(FBS; Biowest, Riverside, MO, USA) and 1% penicillin/streptomycin
(Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) in an
incubator at 37°C in 5% CO2. miR-4500 mimics, miRNA
mimics-negative control (NC), miR-4500 inhibitor and miRNA
inhibitor NC were purchased from Shanghai GenePharma Co., Ltd.
(Shanghai, China). Small interfering RNA (siRNA) against human
STAT3 mRNA and the control siRNA were synthesized by Guangzhou
RiboBio Co., Ltd. (Guangzhou, China). Transfection was performed
using Lipofectamine 2000 Reagent (Invitrogen; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol.
Briefly, A549 and H1975 cells were seeded in 6-well plates at a
density of 5.0×103 cells/well transfected with miRNA
(100 nM) or siRNA (50 nM) when the cells reached 60–70% confluence.
Subsequently, cells were cultured with fresh medium containing 10%
FBS for 48 h prior to further experiments.
The target sequences of the siRNA used are as
follows: siRNA-STAT3 sense, 5′-CCAGTCAGTGACCAGGCAGAAG-3′ and
antisense, 5′-GCACGTACTCCATCGCTGACA-3′.
RNA isolation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from tissues and cells using
Trizol (Invitrogen; Thermo Fisher Scientific, Inc.) and reverse
transcribed using a Moloney murine leukemia virus reverse
transcriptase (Promega Corporation, Madison, WI, USA) at 37°C for
30 min. miRNA was extracted using the miRcute miRNA Isolation kit
(Tiangen Biotech Co., Ltd., Beijing, China). cDNA was synthesized
using the One Step PrimeScript miRNA cDNA Synthesis kit according
to the manufacturer's protocol (Takara Bio, Inc., Otsu, Japan). The
expression levels of miR-4500 and STAT3 mRNA were determined using
RT-qPCR using the SYBR ExScript RT-qPCR kit according to the
manufacturer's protocol (Takara Biotech Co., Ltd.) in the ABI 7500
Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific,
Inc.) with U6 and GAPDH functioning as endogenous controls,
respectively. The primers used for qPCR were as follows: STAT3
forward, 5′-ATCACGCCTTCTACAGACTGC-3′ and reverse,
5′-CATCCTGGAGATTCTCTACCACT-3′; GAPDH forward,
5′-CCACTCCTCCACCTTTGAC-3′ and reverse 5′-ACCCTGTTGCTGTAGCCA-3′; and
U6 forward, 5′-CTCGCTTCGGCAGCACA-3′ and reverse,
5′-AACGCTTCACGAATTTGCGT-3′. The reaction system consisting of a
total of 20 µl of volume was as follows: 1 µl cDNA, 10 µl SYBR
Premix EX Taq, 1 µl each of the primers (10 µM) and 7 µl
ddH2O. The thermocycling conditions were as follows:
95°C for 5 min, followed by 40 cycles of 95°C for 10 sec and 60°C
for 30 sec. Melting curve analysis was performed at the end of each
PCR cycle to confirm that only one product was amplified and
detected. All fold changes were calculated using the comparative Cq
(2−ΔΔCq) method using U6 or GAPDH for normalization
(21).
Western blotting
Total protein was extracted from transfected cells
using radioimmunoprecipitation assay buffer (Beijing Solarbio
Science & Technology Co., Ltd., Beijing, China). Protein
concentration was measured with a Bicinchoninic acid Protein assay
kit (Beyotime Institute of Biotechnology, Haimen, China). An equal
amount of protein (50 µg) per lane was separated using 10% SDS-PAGE
gel and blotted onto polyvinylidene fluoride membranes. The
membrane was blocked using phosphate buffered saline (PBS) with
Tween-20 (0.1% Tween in PBS) and probed with primary antibodies for
STAT3 (1:1,000; cat. no. ab32124; Abcam, Cambridge, MA, USA), BCL2
apoptosis regulator (Bcl-2; 1:1,000; cat. no. ab32124; Abcam), BCL2
associated X, apoptosis regulator (Bax; 1:1,000; cat. no. ab32503;
Abcam), cleaved-caspase-3 (1:1,000; cat. no. ab13847; Abcam),
cleaved-caspase-9 (1:1,000; cat. no. ab13847; Abcam), matrix
metalloproteinase (MMP)-2 (1:1,000; cat. no. ab37150; Abcam), MMP-9
(1:1,000; cat. no. ab73734; Abcam) and GAPDH (1:5,000; cat. no.
G8795; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) at 4°C
overnight. The membranes were then incubated with the appropriate
horseradish peroxidase-conjugated anti-rabbit secondary antibody
(1:5,000; cat. no. ab6858; Abcam) at room temperature for 1 h. The
membranes were visualized using an enhanced chemiluminescent kit
(Beyotime Institute of Biotechnology, Beijing, China). The
immunoreactive bands were scanned using a densitometer, and the
gray value of the bands were calculated automatically using ImageJ
software version k 1.45 (National Institutes of Health, Bethesda,
MD, USA).
Cell proliferation assay
Cell proliferation of A549 and H1975 was detected
using the Cell Counting Kit-8 (CCK-8; Wuhan Boster Biological
Technology, Ltd., Wuhan, China) according to the manufacturer's
protocol. A colony formation assay and EdU assay were performed to
confirm the CCK-8 assay results. For the EdU assay, cells were
serum-starved for 24 h, prior to treatment. EdU was added to the
culture 2 h prior to cell collection and detected using the
Click-it assay kit (Thermo Fisher Scientific, Inc.). For the colony
formation assay, cells were seeded in a 6-well plate at a density
of 1,000 cells/well 24 h after transfection. Cells were incubated
for 2 weeks and the clones were fixed at room temperature for 20
min using methanol and stained with 0.1% crystal violet for 4 h at
room temperature, and counted under a light microscope.
Cell apoptosis assay
The apoptosis of A549 and H1975 cells was detected
using flow cytometry analysis. A fluorescein isothiocyanate
(FITC)-Annexin V/propidium iodide (PI) Apoptosis Detection kit (BD
Pharmingen; BD Biosciences, Franklin Lakes, NJ, USA) was used
according to the manufacturer's protocol. All cells were collected
48 h post-transfection and stained using Annexin V-FITC (5 ml) and
PI (5 ml) according to the manufacturer's protocol. Flow cytometer
(Beckman Coulter, Inc., Brea, CA, USA) was used for cell apoptosis
determination and analyzed using FlowJo 10.06 software (FlowJo LLC,
Ashland, OR, USA). All analyses were performed in triplicate.
Wound healing assay
A549 and H1975 cells were seeded in 6-well plates
and then incubated with 10 µg/ml mitomycin C for 2 h. Wounds were
created using a 200 µl sterile pipette tips when the density
reached ~80%. Photographs were obtained at 24 h following wound
generation under a light microscope (magnification, ×200).
Transwell invasion assay
A549 and H1975 cells were plated in the upper
Transwell chamber (Corning Incorporated, Corning, NY, USA) that was
pre-coated with Matrigel (Growth factor reduced; BD Biosciences),
at a density of 4×104 cells/well in 100 µl DMEM (with
0.5% FBS). The bottom chambers of the Transwell chamber were filled
with DMEM with 20% FBS. After 24 h at 37°C, fixed cells were washed
twice with PBS and fixed with 4% paraformaldehyde for 30 min at
room temperature. Subsequently, cells were stained with 0.1%
crystal violet for 30 min at room temperature. The migration and
invasion cells were counted and imaged under a light microscope
(magnification, ×200).
Luciferase reporter assay
The primers used for pGL3-STAT3-3′UTR construction
were as follows: Sense, 5′-GGTACCGTGGCCTGCCAGTTGCAGA-3′ and
antisense, 5′-AAGCTTCTCAGTCGTATCTTTCTG-3′. PCR products were cloned
into the pGL3-basic vector using KpnI/HindIII sites. The mutant
version of this construct (pGL3-STAT3-mut-3′UTR) carrying 4 base
pair substitutions in the miR-4500 target sites was obtained by
site-directed mutagenesis using the QuikChange Site-Directed
Mutagenesis kit (Agilent Technologies, Inc., Santa Clara, CA, USA)
according to the manufacturer's protocol. The A549 and H1975 cells
were seeded at a concentration of 5.0×103 cells/well
onto 24-well plates and co-transfected with either miR-4500 mimics
or miR-NC and pGL3-STAT3-3′UTR or pGL3-STAT3-mut-3′UTR using
Lipofectamine 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) for
36 h at 37°C. After 36 h, luciferase activity was measured using
the Dual-Glo Luciferase Assay System (Promega Corporation). Renilla
luciferase was used to normalize the luciferase activity.
Statistical analysis
All results are reported as the mean ± standard
deviation and at least three independent replicates were performed.
GraphPad software 5.0 (GraphPad Software, Inc., La Jolla, CA, USA)
was used to perform the statistical analysis. Pearson's correlation
analysis was used to assess the correlation between STAT3 mRNA
expression and miR-4500 levels. Differences between two groups were
calculated using a Student's t-test. Differences between multiple
groups were analyzed using a one-way analysis of variance with
Tukey's post hoc test. P<0.05 was considered to indicate a
statistically significant difference.
Results
STAT3 is a putative target of miR-4500
in NSCLC
Bioinformatics algorithms [TargetScan software
(http://www.targetscan.org/)] (22) implied that there was putative
miR-4500 target site in the 3′-UTR of STAT3 (Fig. 1). Considering that STAT3 may be one
of the key oncogenic drivers in NSCLC, the present study further
investigated the association between miR-4500 and STAT3.
miR-4500 expression is negatively
correlated with STAT3 in NSCLC tissues and cell lines
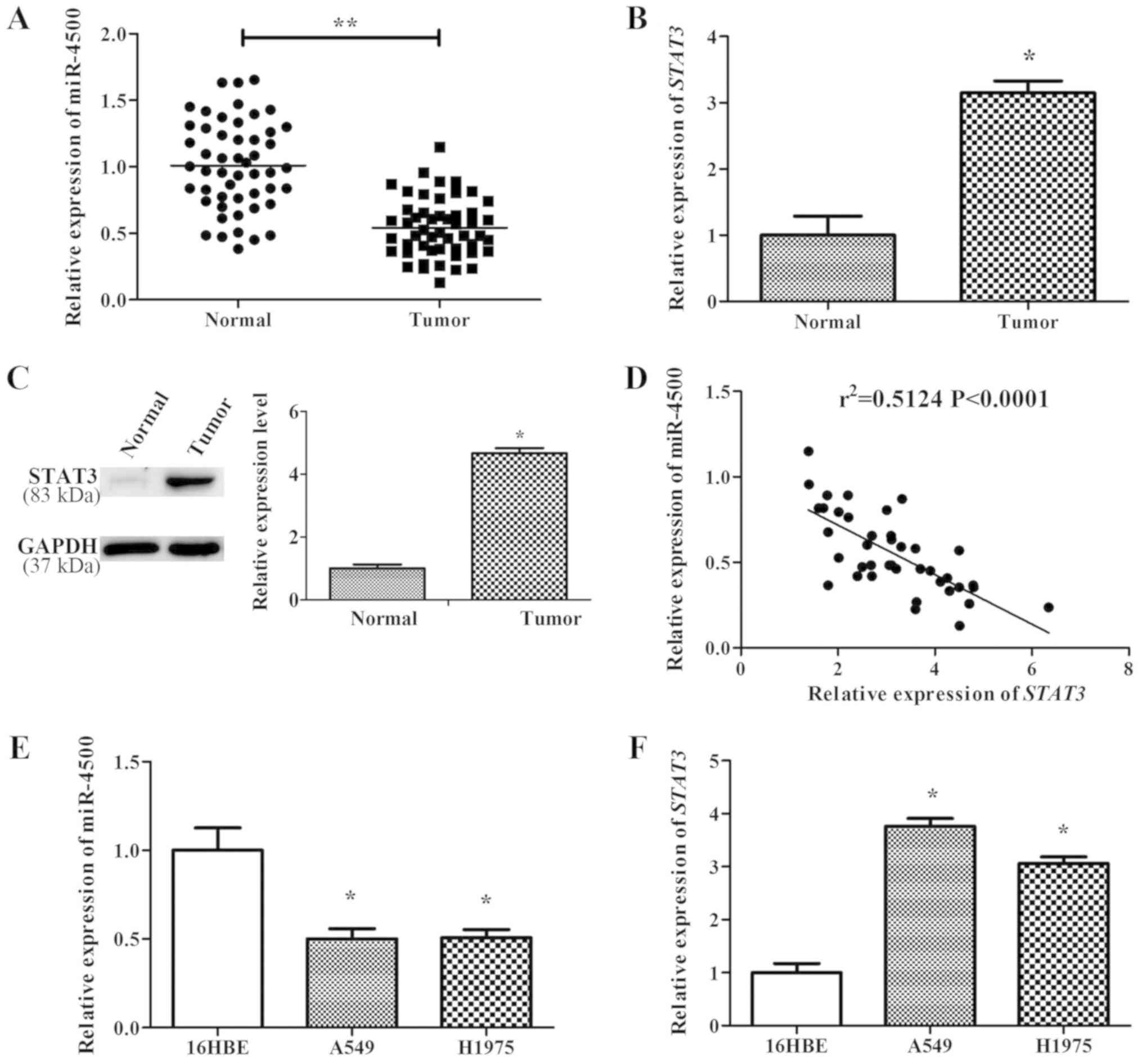
miR-4500 and STAT3 expression level were studied in
40 human NSCLC lung tissues. RT-qPCR results indicated that the
expression levels of miR-4500 in NSCLC lung tissues were
significantly lower compared with normal tissues (P<0.01;
Fig. 2A). Conversely, STAT3 was
significantly upregulated in NSCLC lung tissues on the mRNA and
protein levels compared with normal tissues (P<0.05; Fig. 2B and C). Additionally, Pearson's
correlation analysis was used to assess the correlation between
STAT3 mRNA expression and miR-4500 levels in NSCLC lung tissues,
and the results indicated that the expression levels of STAT3 mRNA
and miR-4500 were significantly negatively correlated
(r2=0.5124, P<0.0001; Fig. 2D). Furthermore, NSCLC cell lines
(A549 and H1975) also exhibited significantly lower miR-4500
expression levels and higher STAT3 expression levels compared with
a normal lung cell line, 16HBE (P<0.05; Fig. 2E and F).
miR-4500 directly targets STAT3 in
NSCLC
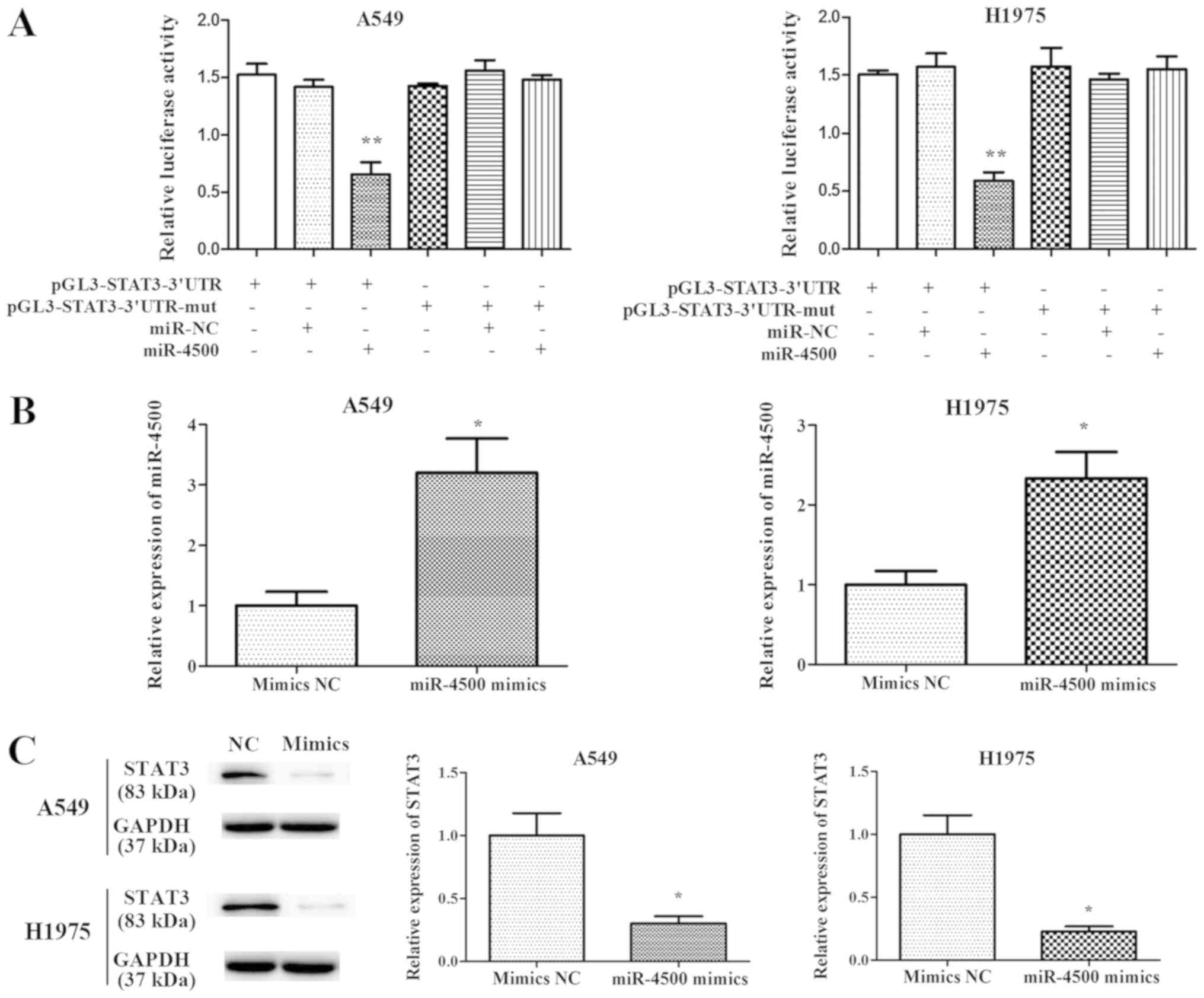
To validate the interaction between STAT3 and
miR-4500, luciferase reporter assays were performed. The results
indicated that the overexpression of miR-4500 significantly
decreased the luciferase activity of wild type STAT3-3′-UTR
compared with mutated STAT3-3′-UTR (P<0.01), indicating that
miR-4500 targeted the 3′-UTR of STAT3 directly in NSCLC (Fig. 3A).
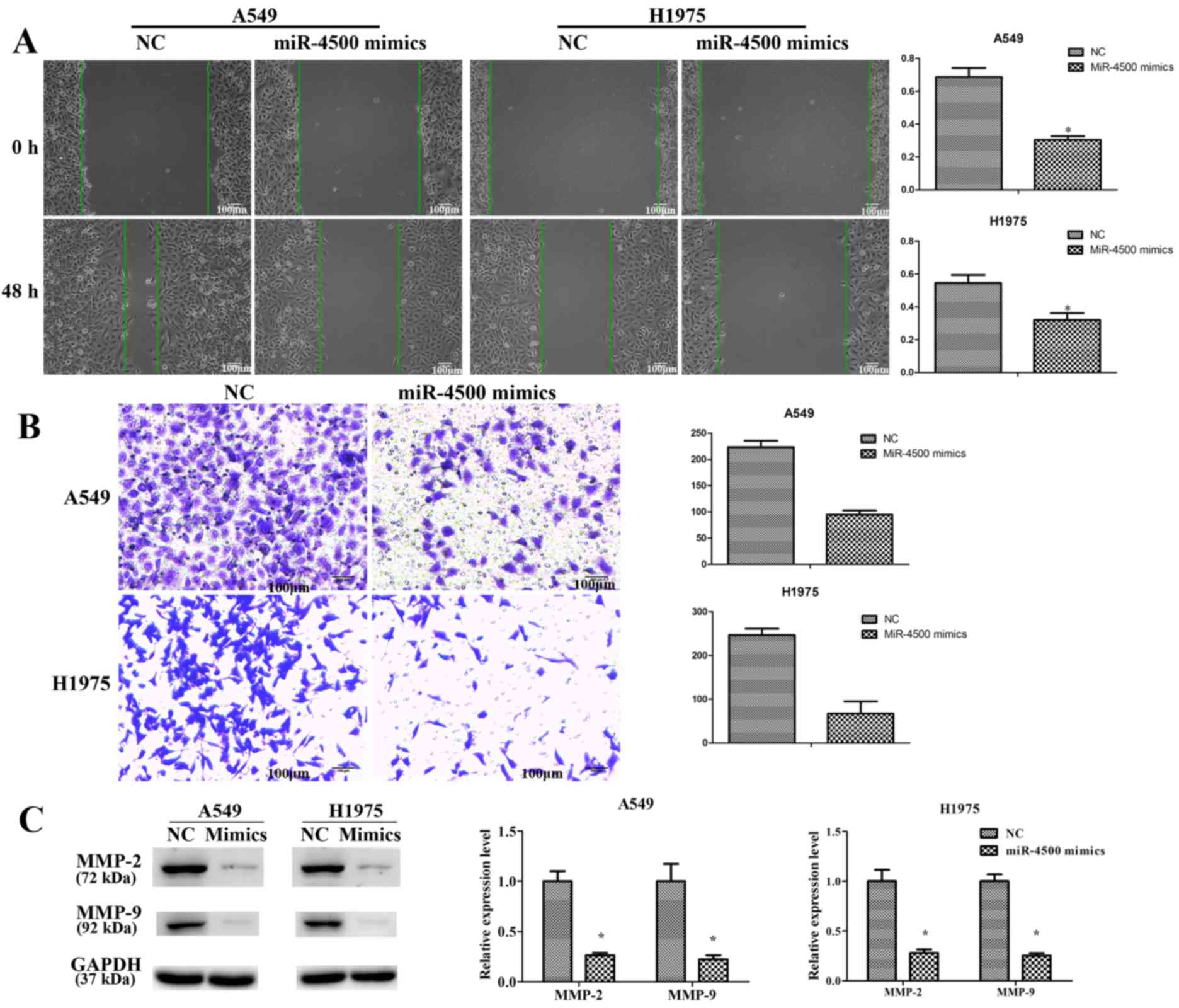
miR-4500 suppresses NSCLC cell
proliferation and invasion and induces apoptosis in vitro
In order to investigate the function of miR-4500 in
cell proliferation and apoptosis, miR-4500 was overexpressed in
A549 and H1975 cells by transfecting the cells with miR-4500
mimics. RT-qPCR results (Fig. 3B)
indicated that a 2–4 fold significant increase in miR-4500 mRNA
levels were expressed subsequent to transfection with mimics
compared with the negative control (P<0.05). STAT3 expression
was significantly decreased in A549 and H1975 cells following
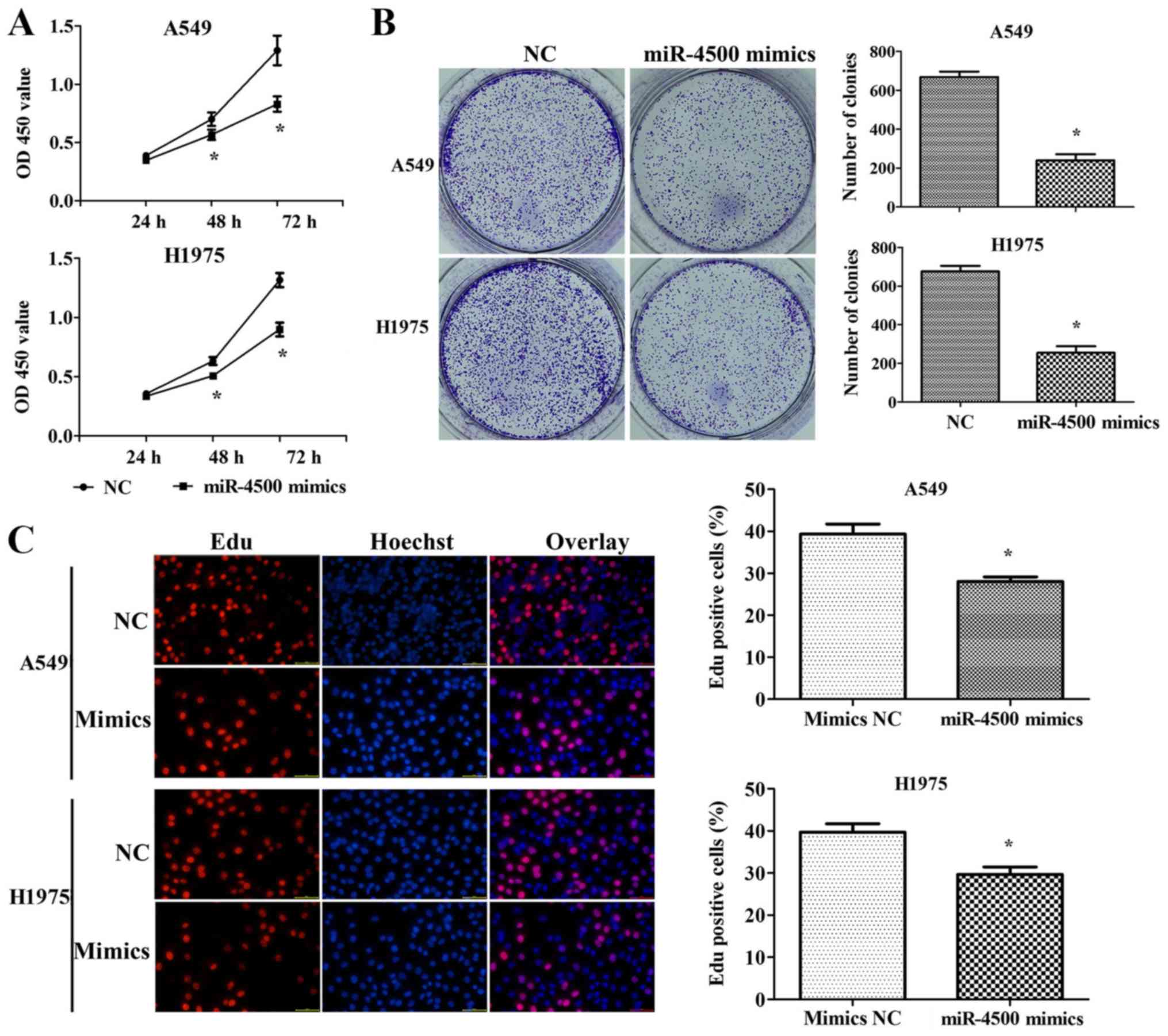
transfection with miR-4500 mimics (P<0.05; Fig. 3C). A CCK-8 assay indicated that
miR-4500 mimics inhibited cell proliferation significantly when
compared with the negative control at the 48 and 72 h mark
(P<0.05; Fig. 4A). The same
conclusion was derived from the colony formation assay (P<0.05;
Fig. 4B) and the EdU assay
(P<0.05; Fig. 4C). Furthermore,
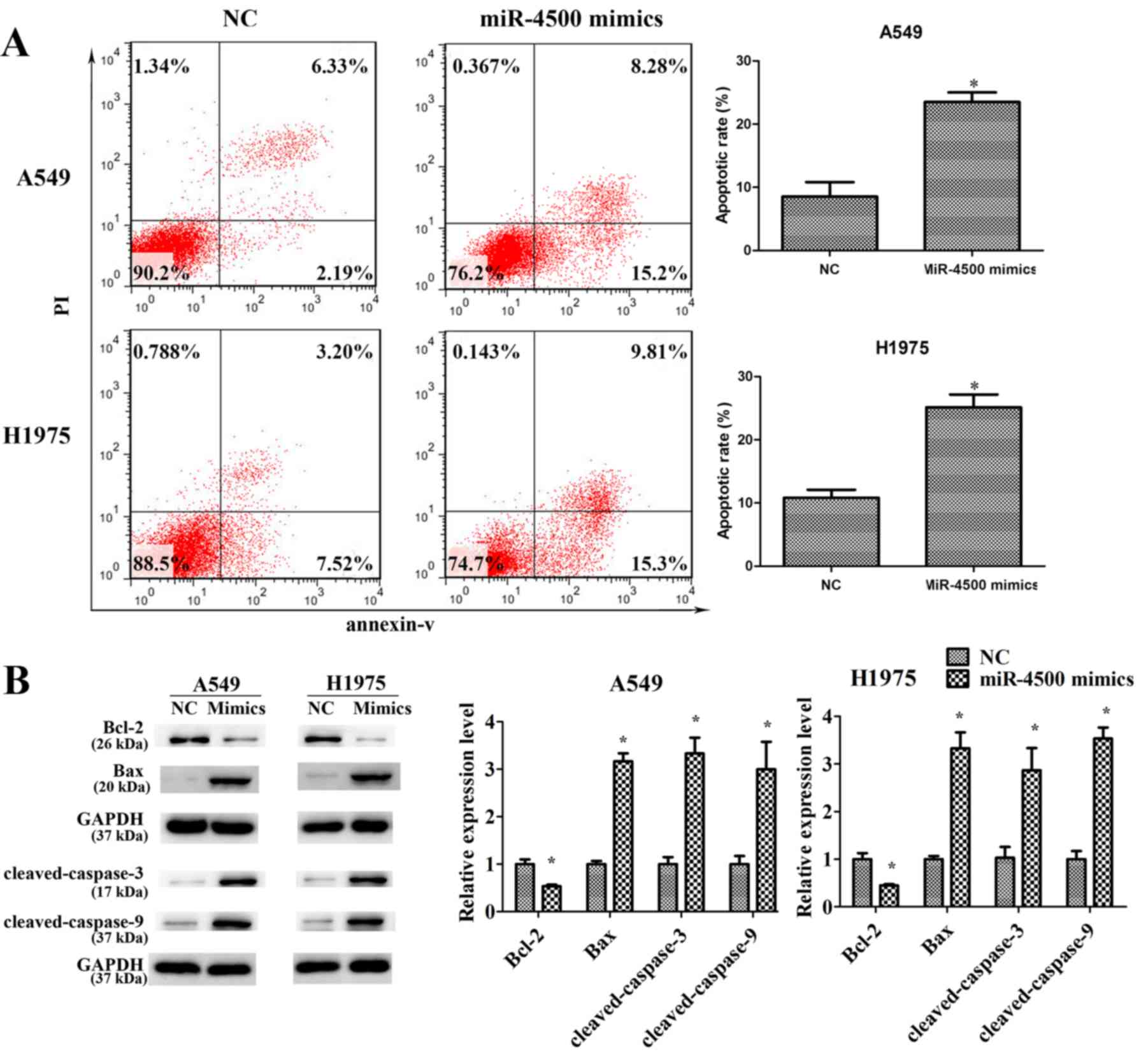
miR-4500 mimics significantly promoted cell apoptosis in A549 and
H1975 cells compared with the negative control (P<0.05), with
the anti-apoptotic protein Bcl2 significantly downregulated and
pro-apoptosis proteins (Bax, cleaved-caspase-3 and
cleaved-caspase-9) significantly upregulated compared with the
negative control (P<0.05; Fig.
5).
A previous study has demonstrated the function of
miR-4500 in NSCLC cell proliferation and apoptosis, with no data on
cell migration and invasion (12).
Therefore, a wound healing assay and a Transwell assay were
performed in order to study the function of miR-4500 in NSCLC cell
migration and invasion. The results revealed a significant positive
effect of miR-4500 in migration and invasion inhibition compared
with the NC group (P<0.05; Fig. 6A
and B). MMPs participate in cancer cell invasion by degrading
the extracellular matrix (23,24).
Western blotting revealed that the protein expression levels of
MMP-2 and MMP-9 were significantly inhibited in A549 and H1975
cells subsequent to transfection with miR-4500 mimics compared with
the NC group (P<0.05; Fig.
6C).
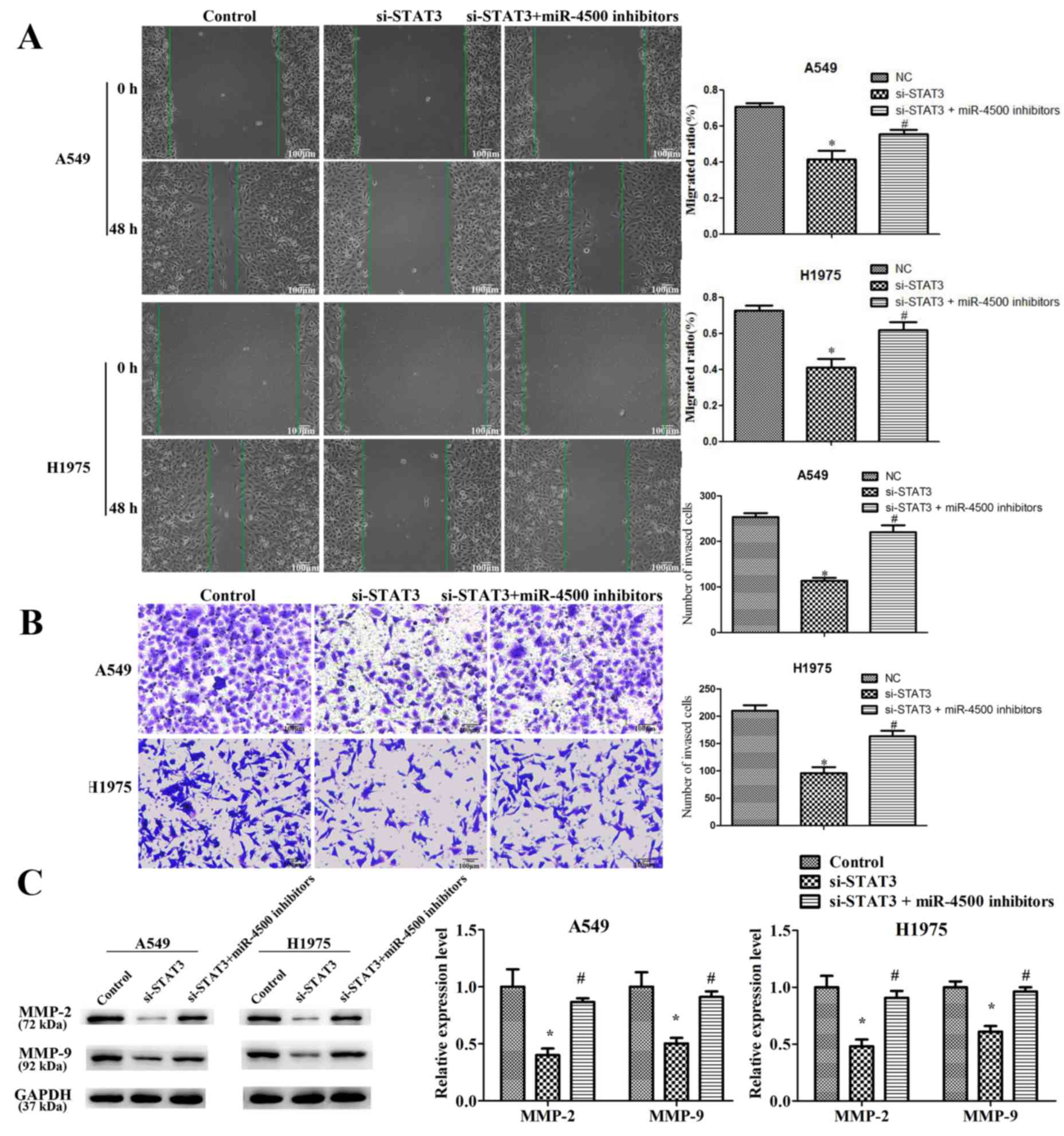
miR-4500 suppresses NSCLC cell
proliferation, migration and invasion and induces apoptosis
partially by downregulating STAT3 in vitro
To identify the function of STAT3 in
miR-4500-suppressed NSCLC cell progression, STAT3 expression was
successfully significantly knocked down in A549 and H1975 cells by
transfection with STAT3-targeted siRNA (P<0.05; Fig. 7A). It was revealed that STAT3
downregulation significantly suppressed cell proliferation
(P<0.05), migration (P<0.05) and invasion (P<0.05) and
significantly induced apoptosis (P<0.05) compared with the
control. However, miR-4500 inhibitors partially restored the
effects of si-STAT3 on cell growth and migration in A549 and H1975
cells (P<0.05; Figs. 7B-D and
8). This data implies that
miR-4500 suppresses NSCLC cell proliferation, migration and
invasion and induces apoptosis partially by downregulating STAT3
in vitro.
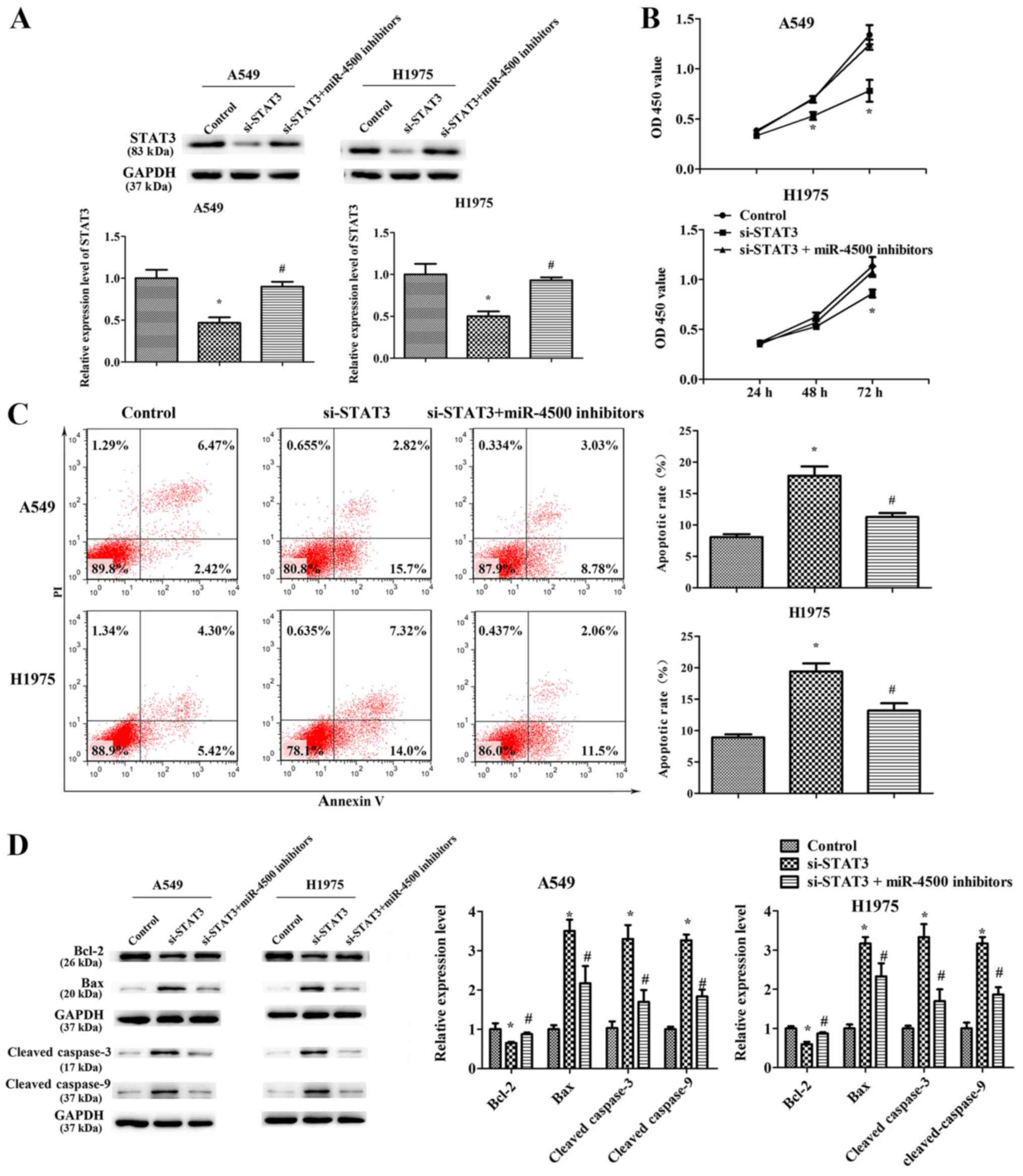 | Figure 7.miR-4500 suppresses non-small cell
lung cancer cell proliferation and induces apoptosis partially by
downregulating STAT3 in vitro. (A) Western blotting
determined the protein expression levels of STAT3 in A549 and H1975
cells following transfection with si-STAT3 or NC or a combination
of si-STAT3 and miR-4500 inhibitors. (B) Cell Counting Kit-8 assays
and (C) an apoptosis flow cytometry assay of A549 and H1975 cells
following transfection with si-STAT3 or NC or a combination of
si-STAT3 and miR-4500 inhibitors. (D) Western blotting determined
the protein expression levels of Bax, Bcl-2, cleaved-caspase-3 and
cleaved-caspase-9 protein in A549 and H1975 cell lines transfected
with si-STAT3 or NC or a combination of si-STAT3 and miR-4500
inhibitors. All data were presented as the mean ± standard
deviation and each experiment was performed in triplicate.
*P<0.05 vs. the control. #P<0.05 vs. the si-STAT3
group. miR, microRNA; STAT3, signal transducer and activator of
transcription 3; si-, small interfering RNA; OD, optical density;
Bcl-2, BCL2 apoptosis regulator; Bax, BCL2 associated X, apoptosis
regulator; NC, negative control; PI, propidium iodide. |
Discussion
miRNAs control various processes, including cellular
growth, proliferation, differentiation, regulation of the cell
cycle, aging, apoptosis, metabolism and neuronal patterning. An
increasing number of miRNAs have been demonstrated to be associated
with the development and progression of different types of cancer
(25–29). All this indicates the potential
function of miRNAs as novel diagnostic or prognostic
biomarkers.
The let-7 family is one of the earliest identified
miRNA families, originally determined to serve a function in the
timing of larval development in Caenorhabditis elegans
(30). The members of let-7 are
highly expressed in normal lung tissues and have been identified to
negatively control multiple different oncogenes (31). miRNA-4500 is one of the let-7
family members, and has been reported to be downregulated in NSCLC,
consequently promoting tumor growth by targeting LIN28B and NRAS
(32).
The present study confirmed the lower expression of
miR-4500 in NSCLC tissues and cells compared with normal control
tissues and cells. Cell lines A549 and H1975 were selected to study
the role of miR-4500 in tumorigenesis. Results indicate that
miR-4500 suppressed cell proliferation, migration, invasion and
promoted apoptosis in vitro.
STAT3 is a well-known oncogenic gene in NSCLC
(33–35). The constitutive activation of STAT3
is a common feature in NSCLC, and has also been proposed to serve a
notable function in tumor resistance to conventional and targeted
small-molecule therapies (36–38).
STAT3 suppresses cancer cell apoptosis via the inactivation of
either extrinsic or intrinsic apoptotic pathways (39,40).
Meanwhile, STAT3 promotes NSCLC cell proliferation and angiogenesis
(12,13).
Bioinformatic algorithms (TargetScan) implied that
there was a putative miR-4500 target site in the 3′-UTR of STAT3.
The present study revealed that the expression of STAT3 is
negatively correlated with miR-4500 expression in the tissues of 40
patients with NSCLC. Furthermore, targeting miR-4500 and STAT3 was
confirmed using a luciferase reporter assay. Additionally, the
tumor inhibition effect of si-STAT3 in A549 and H1975 lines may be
partially restored using a miR-4500 inhibitor, which suggests that
miR-4500 may suppress tumor progression in NSCLC by regulating
STAT3. In order to confirm this, further studies will also be
performed in the future in cells overexpressing STAT3 and
miR-4500.
Altogether, miR-4500, that was demonstrated to be
significantly downregulated in NSCLC tissues and cell lines, may
suppress NSCLC cell proliferation, migration and invasion, and
promote apoptosis by regulating STAT3 expression, indicating that
miR-4500 may be a tumor suppressor and a potential therapeutic
target in NSCLC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and material
The datasets used during the present study are
available from the corresponding author on reasonable request.
Authors' contributions
ZL and ZZ were major contributors in sample
collection, performing the experiments, data interpretation and
preparing the paper. HB, QZ, SZ, LZ and XZ participated in sample
collection, performing the experiments and data interpretation. JZ
produced the experimental design and revised the manuscript. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Written informed consent was obtained from each
patient and the present study was ethically approved by the Ethics
Committee of the First People's Hospital of Changzhou (Changzhou,
China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Inamura K: Lung Cancer: Understanding its
molecular pathology and the 2015 WHO classification. Front Oncol.
7:1932017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McIntyre A and Ganti AK: Lung cancer-A
global perspective. J Surg Oncol. 115:550–554. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2016. CA Cancer J Clin. 66:7–30. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Deng S, Calin GA, Croce CM, Coukos G and
Zhang L: Mechanisms of microRNA deregulation in human cancer. Cell
Cycle. 7:2643–2646. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bartel DP: MicroRNAs: Genomics,
biogenesis, mechanism, and function. Cell. 116:281–297. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Calin GA and Croce CM: MicroRNA signatures
in human cancers. Nat Rev Cancer. 6:857–866. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fang C, Chen YX, Wu NY, Yin JY, Li XP,
Huang HS, Zhang W, Zhou HH and Liu ZQ: MiR-488 inhibits
proliferation and cisplatin sensibility in non-small-cell lung
cancer (NSCLC) cells by activating the eIF3a-mediated NER signaling
pathway. Sci Rep. 7:403842017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li T, Ding ZL, Zheng YL and Wang W:
MiR-484 promotes non-small-cell lung cancer (NSCLC) progression
through inhibiting Apaf-1 associated with the suppression of
apoptosis. Biomed Pharmacother. 96:153–164. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lin L, Tu HB, Wu L, Liu M and Jiang GN:
MicroRNA-21 regulates non-small cell lung cancer cell invasion and
chemo-sensitivity through SMAD7. Cell Physiol Biochem.
38:2152–2162. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yin J, Wang M, Jin C and Qi Q: miR-101
sensitizes A549 NSCLC cell line to CDDP by activating caspase
3-dependent apoptosis. Oncol Lett. 7:461–465. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Jima DD, Zhang J, Jacobs C, Richards KL,
Dunphy CH, Choi WW, Au WY, Srivastava G, Czader MB, Rizzieri DA, et
al: Deep sequencing of the small RNA transcriptome of normal and
malignant human B cells identifies hundreds of novel microRNAs.
Blood. 116:e118–e127. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang L, Qian J, Qiang Y, Huang H, Wang C,
Li D and Xu B: Down-regulation of miR-4500 promoted non-small cell
lung cancer growth. Cell Physiol Biochem. 34:1166–1174. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lin Q, Zheng H, Xu J, Zhang F and Pan H:
LncRNA SNHG16 aggravates tumorigenesis and development of
hepatocellular carcinoma by sponging miR-4500 and targeting STAT3.
J Cell Biochem. Feb 18–2019.(Epub ahead of print) Doi:
10.1002/jcb.28440.
|
|
14
|
Yu H, Pardoll D and Jove R: STATs in
cancer inflammation and immunity: A leading role for STAT3. Nat Rev
Cancer. 9:798–809. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Harada D, Takigawa N and Kiura K: The role
of STAT3 in non-small cell lung cancer. Cancers (Basel). 6:708–722.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Liu J, Fei D, Xing J and Du J:
MicroRNA-29a inhibits proliferation and induces apoptosis in
rheumatoid arthritis fibroblast-like synoviocytes by repressing
STAT3. Biomed Pharmacother. 96:173–181. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shan D, Shang Y and Hu T: MicroRNA-411
inhibits cervical cancer progression by directly targeting STAT3.
Oncol Res. 27:349–358. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun CC, Li SJ, Zhang F, Zhang YD, Zuo ZY,
Xi YY, Wang L and Li DJ: The novel miR-9600 suppresses tumor
progression and promotes paclitaxel sensitivity in non-small-cell
lung cancer through altering STAT3 expression. Mol Ther Nucleic
Acids. 5:e3872016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Haghikia A, Hoch M, Stapel B and
Hilfiker-Kleiner D: STAT3 regulation of and by microRNAs in
development and disease. JAKSTAT. 1:143–150. 2012.PubMed/NCBI
|
|
20
|
Carraro G, El-Hashash A, Guidolin D,
Tiozzo C, Turcatel G, Young BM, De Langhe SP, Bellusci S, Shi W,
Parnigotto PP and Warburton D: miR-17 family of microRNAs controls
FGF10-mediated embryonic lung epithelial branching morphogenesis
through MAPK14 and STAT3 regulation of E-Cadherin distribution. Dev
Biol. 333:238–250. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wan LY, Woo CS, Turner PC, Wan JM and
El-Nezami H: Individual and combined effects of Fusarium toxins on
the mRNA expression of pro-inflammatory cytokines in swine jejunal
epithelial cells. Toxicol Lett. 220:238–246. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Koller D and Friedman N: Probabilistic
Graphical Models: Principles and techniques-adaptive computation
and machine learning. Probabilistic graphical models-principles and
techniques. 2009.
|
|
23
|
Du WW, Ling F, Li M, Yang X, Liang Y, Peng
C, Qian W, O'Malley YQ, Askeland RW, Sugg SL, et al: MicroRNA
miR-24 enhances tumor invasion and metastasis by targeting PTPN9
and PTPRF to promote EGF signaling. J Cell Sci. 126:1440–1453.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Li S, Li Z, Guo F, Qin X, Liu B, Lei Z,
Song Z, Sun L, Zhang HT, You J and Zhou Q: miR-223 regulates
migration and invasion by targeting Artemin in human esophageal
carcinoma. J Biomed Sci. 18:242011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hong L, Han Y, Zhang H, Zhao Q and Qiao Y:
miR-210: A therapeutic target in cancer. Expert Opin Ther Targets.
17:21–28. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Xia B, Li H, Yang S, Liu T and Lou G:
MiR-381 inhibits epithelial ovarian cancer malignancy via YY1
suppression. Tumour Biol. 37:9157–9167. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Wang C, Chen Q, Li S, Li S, Zhao Z, Gao H,
Wang X, Li B, Zhang W, Yuan Y, et al: Dual inhibition of PCDH9
expression by miR-215-5p up-regulation in gliomas. Oncotarget.
8:10287–10297. 2017.PubMed/NCBI
|
|
28
|
Danza K, De Summa S, Pinto R, Pilato B,
Palumbo O, Merla G, Simone G and Tommasi S: MiR-578 and miR-573 as
potential players in BRCA-related breast cancer angiogenesis.
Oncotarget. 6:471–483. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Xu HY, Wang Y, Zhang H and Xu J: The long
non-coding RNA UCA1, as a prognostic biomarker for high grade
serous ovarian carcinoma. Eur J Gynaecol Oncol. 38:883–889.
2017.
|
|
30
|
Reinhart BJ, Slack FJ, Basson M,
Pasquinelli AE, Bettinger JC, Rougvie AE, Horvitz HR and Ruvkun G:
The 21-nucleotide let-7 RNA regulates developmental timing in
Caenorhabditis elegans. Nature. 403:901–906. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Johnson CD, Esquela-Kerscher A, Stefani G,
Byrom M, Kelnar K, Ovcharenko D, Wilson M, Wang X, Shelton J,
Shingara J, et al: The let-7 microRNA represses cell proliferation
pathways in human cells. Cancer Res. 67:7713–7722. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Yu FY, Tu Y, Deng Y, Guo C, Ning J, Zhu Y,
Lv X and Ye H: MiR-4500 is epigenetically downregulated in
colorectal cancer and functions as a novel tumor suppressor by
regulating HMGA2. Cancer Biol Ther. 17:1149–1157. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Song L, Turkson J, Karras JG, Jove R and
Haura EB: Activation of Stat3 by receptor tyrosine kinases and
cytokines regulates survival in human non-small cell carcinoma
cells. Oncogene. 22:4150–4165. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Alvarez JV, Greulich H, Sellers WR,
Meyerson M and Frank DA: Signal transducer and activator of
transcription 3 is required for the oncogenic effects of
non-small-cell lung cancer-associated mutations of the epidermal
growth factor receptor. Cancer Res. 66:3162–3168. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Looyenga BD, Hutchings D, Cherni I,
Kingsley C, Weiss GJ and Mackeigan JP: STAT3 is activated by JAK2
independent of key oncogenic driver mutations in non-small cell
lung carcinoma. PLoS One. 7:e308202012. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Gao SP, Mark KG, Leslie K, Pao W, Motoi N,
Gerald WL, Travis WD, Bornmann W, Veach D, Clarkson B, et al:
Mutations in the EGFR kinase domain mediate STAT3 activation via
IL-6 production in human lung adenocarcinomas. J Clin Invest.
117:3846–3856. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yin Z, Zhang Y, Li Y, Lv T, Liu J and Wang
X: Prognostic significance of STAT3 expression and its correlation
with chemoresistance of non-small cell lung cancer cells. Acta
Histochem. 114:151–158. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
You S, Li R, Park D, Xie M, Sica GL, Cao
Y, Xiao ZQ and Deng X: Disruption of STAT3 by niclosamide reverses
radioresistance of human lung cancer. Mol Cancer Ther. 13:606–616.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Epling-Burnette PK, Liu JH,
Catlett-Falcone R, Turkson J, Oshiro M, Kothapalli R, Li Y, Wang
JM, Yang-Yen HF, Karras J, et al: Inhibition of STAT3 signaling
leads to apoptosis of leukemic large granular lymphocytes and
decreased Mcl-1 expression. J Clin Invest. 107:351–362. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Ivanov VN, Bhoumik A, Krasilnikov M, Raz
R, Owen-Schaub LB, Levy D, Horvath CM and Ronai Z: Cooperation
between STAT3 and c-jun suppresses Fas transcription. Mol Cell.
7:517–528. 2001. View Article : Google Scholar : PubMed/NCBI
|