Introduction
Patients with gastric erosions have slower
evacuation of the stomach and hypotonus of the stomach (1), as well as gastric inflammation and
decreased gastric motility (2,3).
Gastric diseases, such as achlorhydria, are well known to affect
nutrient absorption of factors, including iron (4). Nutrient digestion causes movement of
the stomach, including gastric secretions and gastric shaking
(5). Hormones regulate the
physiological functions of the stomach, such as gastric secretions
and motility (6).
Ghrelin is one of the hormones that is produced by
gastric cells, and is an orexigenic hormone as it increases
appetite (7). Endocrine cells of
the stomach regulate appetite through the hypothalamus and vagal
afferent nerve fibers (8). Ghrelin
has an important role in the regulation of food intake, stimulating
food administration in humans and regulating energy metabolites
(6,7). As such, ghrelin has been investigated
as a target to treat obesity (9).
The release of ghrelin may increase stomach movement according to
the aforementioned studies.
β-hydroxybutyric acid/β-hydroxybutyrate (β-HB)
regulates hormone synthesis and release, including of growth
hormone-releasing hormone in the hypothalamus (10). Sun et al (11) demonstrated that the
intracerebroventricular infusion of β-HB for 28 days significantly
decreased the body weight in high-fat fed rats, although β-HB is
similar to glucose as it provides energy for the brain in suckling
rats (12). Nowroozi-Asl et
al (13) reported that ghrelin
and β-HB are sensitive indicators of energy balance. Poggioli et
al (14) found that γ-HB
increased gastric motility in a rat model. However, to the best of
our knowledge, it is not known whether β-HB has an effect on
gastric motility, which would affect food intake and digestion. The
relationship among β-HB, ghrelin and gastric inflammation remains
unclear. In the present study, the effect of β-HB and ghrelin on
the motility of GASMCs, and inflammation in GASMCs, was
investigated.
Materials and methods
GASMC separation and
identification
In total, two Sprague Dawley rats (one male and one
female), aged 6 weeks (200±10 g), were purchased from Guangdong
Medical Laboratory Animal Center. Rats were kept in cages at
22°C±3°C with a stable humidity (50±10%) and a 12 h; light/dark
cycle. The rats had free access to food and water. The gastric
antrum was removed and D-Hanks medium (Beijing Solarbio Science and
Technology Co., Ltd.) was used to wash the gastric antrum three
times. The gastric antrum was cut into pieces; these pieces were
digested using type II collagenase (Gibco; Thermo Fisher
Scientific, Inc.) dissolved in M199 basic medium (Gibco; Thermo
Fisher Scientific, Inc.) for 30 min in a 37°C water bath. D-Hanks
was added to resuspend the precipitate after removal of the type II
collagenase and was agitated for 10 min. The mixed solution was
centrifuged at 2,000 × g for 5 min at room temperature. The
supernatant was discarded and M199, supplemented with 20% FBS
(Gibco; Thermo Fisher Scientific, Inc.) and 1% 10,000 U/ml
penicillin-10,000 µg/ml streptomycin (Gibco; Thermo Fisher
Scientific, Inc.), was used to resuspend and cultured the cells.
The cell solution was passed through a size 200 mesh screen
(Sigma-Aldrich; Merck KGaA). Animal experiments were approved by
the Institutional Animal Care and Use Committee of Southwest
University Hospital (no. 2017110853n).
Immunofluorescence was used to identify GASMCs.
Cells (5×104 cells/ml) were seeded into 35 mm plates and
4% paraformaldehyde was used to fix the cells at 4°C for 10 min. An
antibody against α-smooth muscle actin (1:100; cat. no. 19245; Cell
Signaling Technology, Inc.) was incubated with cells for 2 h at the
room temperature. TBST was used to wash cells three times. A
secondary antibody conjugated with Alexa Fluor® 594
(1:1,000; cat. no. 8889; Cell Signaling Technology, Inc) was
incubated with the cells for 2 h at the room temperature. PBS was
used to wash the cells. DAPI (5 µg/ml dissolved in PBS;
Sigma-Aldrich; Merck KGaA) was used to stain the cells for 4 min at
25°C and were then washed with PBS. The cells were observed using a
fluorescence microscope (Olympus Corporation).
Treatment with reagents
Ghrelin (10−10, 10−9,
10−8 and 10−7 mol/l; Sigma-Aldrich; Merck
KGaA) was dissolved in PBS and diluted in culture medium. β-HB
(0.5, 1, 5 and 10 mmol/l; MedChemExpress, LLC) was dissolved in
DMSO and diluted in culture medium. In some experiments
10−8 mol/Ghrelin and 5 mmol/l β-HB were combined to
treat cells.
Cell viability and transfection
GASMCs (4×103 cells/well) were seeded
into 96-well plates. After cells were treated different
concentrations of β-HB and Ghrelin as aforementioned for 24, 48 and
72 h, the medium was discarded. Cell Counting Kit-8 (CCK-8;
Sigma-Aldrich; Merck KGaA) was diluted with M199 basic medium
(1:9). In total, 10 µl CCK-8 solution was added to each well and
incubated with the cells for 1 h in a 37°C incubator. Absorbance
was determined at a wavelength of 450 nm using a Multiskan
microplate reader (Thermo Fisher Scientific, Inc.).
For transfections, 50 nM small interfering (si)RNA
against GHS-R (5′-CTGAAGGCATCTTTCACTACG-3′) or a negative control
siRNA (5′-CAGUACUUUUGUGUAGUACAA-3′) were mixed with
Lipofectamine® (Invitrogen; Thermo Fisher Scientific,
Inc.) and diluted in M199 basic medium. This solution was incubated
with cells for 3 h; the culture medium was then replaced, and cells
were cultured for a further 48 h.
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from GASMCs using
TRIzol® (Invitrogen; Thermo Fisher Scientific, Inc.),
according to the manufacturer's instructions and centrifuged at
15,000 × g for 15 min at 4°C. RT was carried out using an Arcturus™
RiboAmp™ HS PLUS cDNA Kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.), according to the manufacturer's protocol. RT was
conducted at 42°C for 15 min and 95°C for 3 min.
The primers used are listed in Table I and were synthesized by Sangon
Biotech Co., Ltd. qPCR was carried out using the PCR Taq Master Mix
(MedChemExpress, LLC) using the following conditions: 95°C for 2
min, followed by 40 cycles of 95°C for 20 sec, 52°C for 50 sec and
72°C for 25 sec. GAPDH was used as an internal reference for qPCR.
The relative mRNA expression was analyzed using the
2−ΔΔCq method (15).
 | Table I.Primers used in reverse
transcription-quantitative PCR. |
Table I.
Primers used in reverse
transcription-quantitative PCR.
| Name | Forward
(5′-3′) | Reverse
(5′-3′) |
|---|
| MYL9 |
CACCAGAAGCCAGATGTCC |
TTGAAAGCCTCCTTAAACTCC |
| MLCK |
GGAATTCCATATGAAGACCCCTGTGCCTGAGAAG |
CAGCCTCTAAGATCCCTGCC |
| RhoA |
GTAGAGTTGGCTTTAT |
CACTCCGTCTTGGTCTT |
| ROCK-1 |
AGTCTGTGGCAATGTGTGAG |
CTTCAAGCCGACTAACAGTG |
| GHS-R |
CCTGCTTCACCACCTTCTTG |
CCAAAAGGGTCATCATCTCT |
| TNF-α |
GCGACGTGGAACTGGCAGAAG |
GGTACAACCCATCGGCTGGCA |
| IL-6 |
ACGCTAGTCCTCCACGAT |
GGTTGTTTAACATTGCCTTT |
| MnSOD |
GGCCAAGGGCGCTGTTACAA |
CTGACCGAGCGTGGCTAC |
| Cu/ZnSOD |
GTTCCGAGGCCGCGCGT |
GTCCCCATATTGATGGAC |
| Catalase |
AGTGAGAGAAGTTAGAAAAAAGAA |
CAACTAACACAAATACCAAACT |
| GAPDH |
AATGTGTCCGTCGTGGATCTGA |
GATGCCTGCTTCACCACCTTCT |
Reactive oxygen species (ROS)
assay
GASMCs (4×106 cells/ml) were seeded into
75 mm plates. After the cells were treated with reagents as
aforementioned for 48 h, the cells were digested using 0.25%
trypsin-EDTA (Gibco; Thermo Fisher Scientific, Inc.) for 15 min.
Cells were resuspended in PBS following centrifugation at 1,000 × g
at room temperature for 3 min. A Total Reactive Oxygen Species
(ROS) Assay kit (Invitrogen; Thermo Fisher Scientific, Inc.) was
used, according to the manufacturer's protocol. Cells were analyzed
using a flow cytometer (Invitrogen; Thermo Fisher Scientific, Inc.)
and analysis software (FlowJo version 10.0; BD Biosciences) to
determine the level of fluorescence.
Western blot analysis
Proteins were extracted from GASMCs using cell lysis
buffer (Invitrogen; Thermo Fisher Scientific, Inc.) at 4°C for 2 h
and centrifuged at 13,000 × g at 4°C for 15 min. The bicinchoninic
acid method was used to determine the protein concentration.
Protein were separated by 10% SDS-PAGE. Proteins (30 µg/lane) were
then transferred to PVDF membranes. Membranes were blocked using 5%
milk solution for 2–3 h at room temperature. Primary antibodies
(Table II) were obtained from
Abcam and incubated with membranes at 4°C for 12 h. A horseradish
peroxidase-conjugated secondary antibody (1:2,000; cat. no. ab7090;
Abcam) was incubated with membranes for 2–3 h at room temperature.
An ECL™ western blotting reagents kit (Sigma-Aldrich; Merck KGaA)
was used to visualize protein bands and films were used to detect
the signal in a dark room. Densitometry analysis was performed
using ImageJ (Version 5.0; National Institutes of Health).
 | Table II.Primary antibodies used in western
blotting. |
Table II.
Primary antibodies used in western
blotting.
| Name | Item number | Weight | Dilution |
|---|
| MYL9 | ab191393 | 20 kDa | 1:1,000 |
| MLCK | ab232949 | 211 kDa | 1:1,000 |
| RhoA | ab187027 | 22 kDa | 1:5,000 |
| ROCK-1 | ab156284 | 158 kDa | 1:1,000 |
| GHS-R | ab85104 | 40 kDa | 1:500 |
| MnSOD | ab13533 | 25 kDa | 1:5,000 |
| Cu/ZnSOD | ab13498 | 19 kDa | 1:1,000 |
| Catalase | ab16731 | 60 kDa | 1:2,000 |
| GAPDH | ab181602 | 37 kDa | 1:10,000 |
Statistical analysis
All experiments were independently performed at
least three times and all values are presented as the mean ± SD.
The data were analyzed using one-way ANOVA and significant
differences were analyzed using Tukey's post hoc test using SPSS
19.0 (IBM, Corp). P<0.05 was considered to indicate a
statistically significant difference.
Results
Effects of β-HB on cell viability and
ROS levels in GASMCs
GASMCs isolated from rat gastric antrum had a normal
shape (Fig. 1A). Treatment with
0–10 mmol/l β-HB had a mild inhibitory effect on the viability of
GASMCs (Fig. 1B). β-HB stimulated
the production of ROS in GASMCs (Fig.
1D and E). Therefore, β-HB effected cell viability and ROS
levels in GASMCs in a dose-dependent manner.
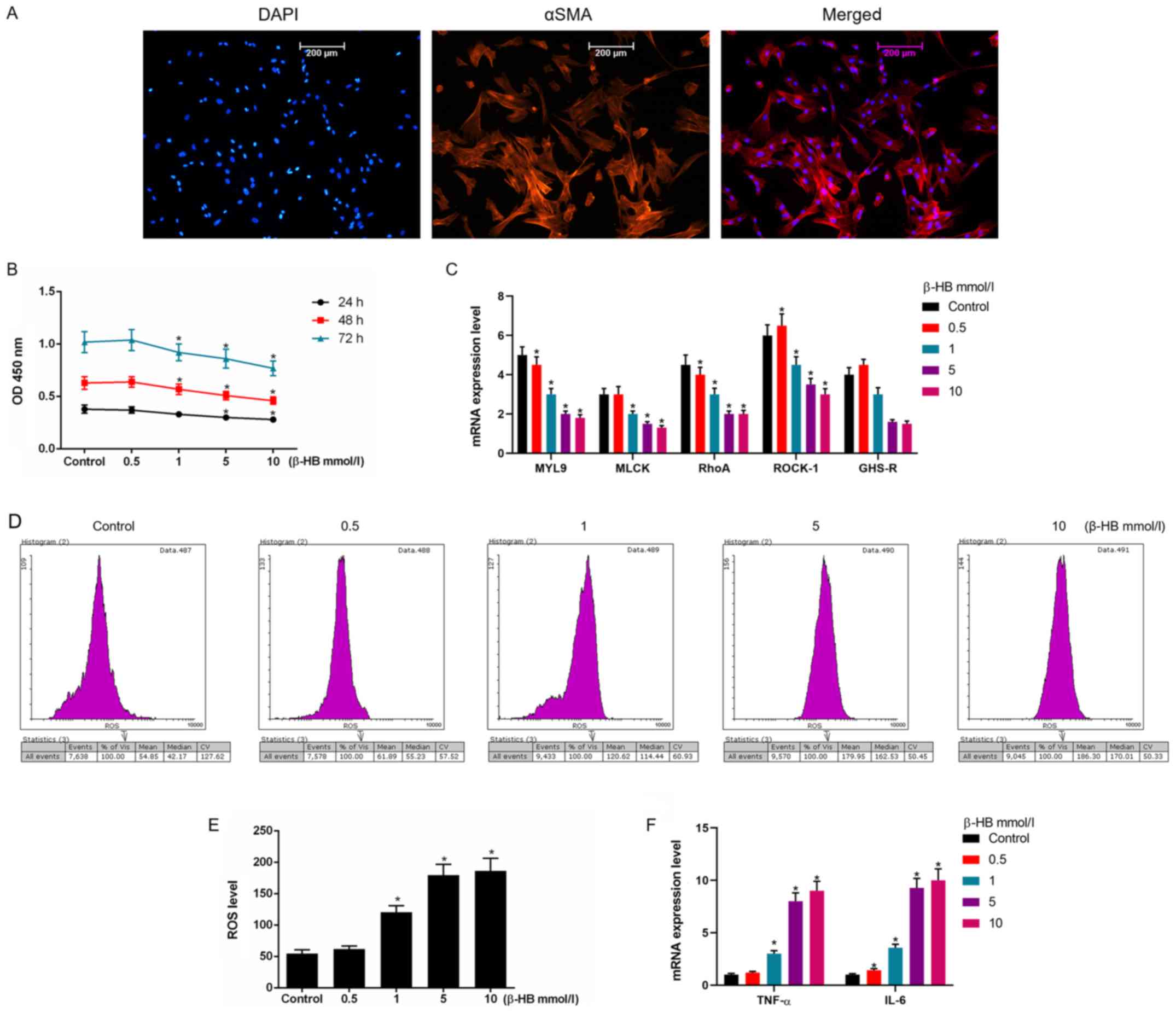 | Figure 1.Effects of β-HB on the expression of
MYL9, MLCK, RhoA, ROCK-1, GHS-R, ROS, TNF-α and IL-6 in rat GASMCs.
(A) GASMCs were extracted from rat gastric antrum. GASMCs were
stained with DAPI and an α-smooth muscle actin antibody. (B) GASMCs
were treated with 0.5–10 mmol/l β-HB for 24, 48 and 72 h. The Cell
counting Kit-8 assay was used to determine cell viability. GASMCs
were treated with 0.5–10 mmol/l β-HB for 48 h. (C) RT-qPCR was used
to determine the expression of MYL9, MLCK, RhoA, ROCK-1 and GHS-R.
(D) GASMCs were treated with 0.5–10 mmol/l β-HB for 48 h. An ROS
assay kit was used to determine the ROS level using flow cytometry.
(E) Quantification of ROS levels. (F) GASMCs were treated with
0.5–10 mmol/l β-HB for 48 h. The expression levels of TNF-α and
IL-6 were determined using RT-qPCR. ANOVA was used to analyze the
differences. *P<0.05 vs. respective control. Β-HB,
β-hydroxybutyric acid; GASMCs, gastric antral smooth muscle cells;
MYL9, myosin regulatory light polypeptide 9; MLCK, myosin light
chain kinase; RhoA, transforming protein RhoA; ROCK-1,
Rho-associated protein kinase-1; GHS-R, growth hormone secretagogue
receptor; ROS, reactive oxygen species; TNF-α, tumor necrosis
factor-α; IL-6, interleukin-6; RT-qPCR, reverse
transcription-quantitative PCR; OD, optical density. |
Effects of β-HB on the expression of
myosin regulatory light polypeptide (MYL) 9, myosin light chain
kinase (MLCK), transforming protein RhoA (RhoA), Rho-associated
protein kinase-1 (ROCK-1), growth hormone secretagogue receptor
(GHS-R), tumor necrosis factor (TNF)-α and interleukin (IL)-6 in
GASMCs
β-HB inhibited the expression of MYL9, MLCK, RhoA,
ROCK-1 and GHS-R. Higher concentrations of β-HB were found to have
stronger inhibitory effects on the mRNA expression of these factors
(Fig. 1C). By contrast, β-HB
stimulated the expression of TNF-α and IL-6 in GASMCs (Fig. 1F), suggesting that β-HB could
induce inflammation in GASMCs.
Effects of ghrelin on cell viability
and the expression of MYL9, MLCK, RhoA, ROCK-1 and GHS-R in
GASMCs
Low concentrations of ghrelin were found to have no
effect on the viability of GASMCs after 24 and 48 h (Fig. 2A). However, these low
concentrations of ghrelin promoted the expression of MYL9, RhoA and
ROCK-1 at 48 h (Fig. 2B). Ghrelin
(10−8 mol/l) promoted the expression of MLCK and
10−9 mol/l ghrelin increased the mRNA expression of
GHS-R (Fig. 2B). There was no
significant effect on the expression of MYL9, MLCK, RhoA, ROCK 1
and GHS R in GASMCs with 10−10 mol/l of ghrelin.
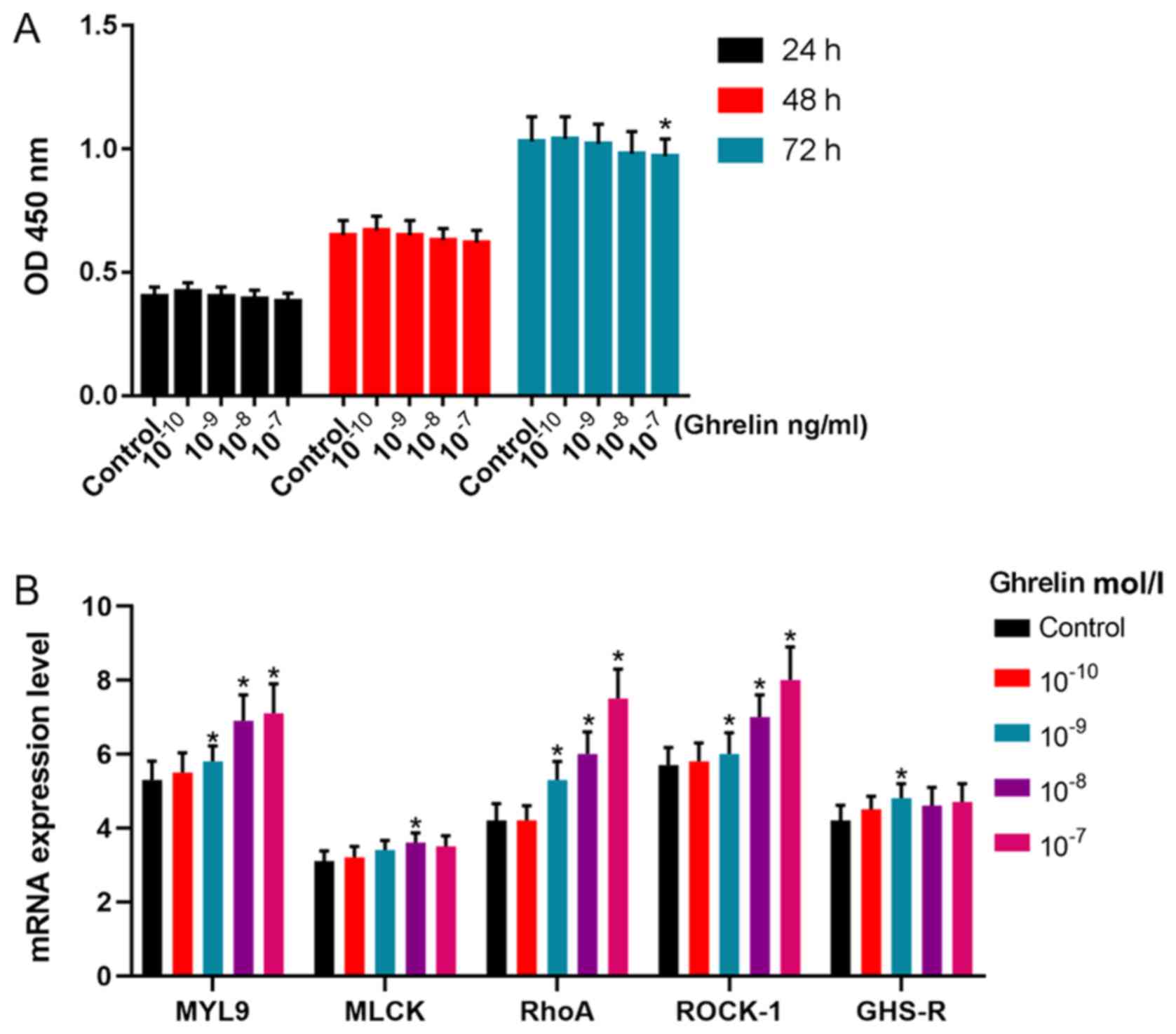 | Figure 2.Effects of ghrelin on the expression
of MYL9, MLCK, RhoA, ROCK-1 and GHS-R in rat GASMCs. (A) GASMCs
were treated with 10−10−10−7 mol/l ghrelin
for 24, 48 and 72 h. The Cell Counting Kit-8 assay was used to
determine cell viability. GASMCs were treated with
10−10−10−7 mol/l ghrelin for 48 h. (B) The
mRNA expression of MYL9, MLCK, RhoA, ROCK-1 and GHS-R were
determined using reverse transcription-quantitative PCR. ANOVA was
used to analyze the differences. *P<0.05 vs. Control. Β-HB,
β-hydroxybutyric acid; GASMCs, gastric antral smooth muscle cells;
MYL9, myosin regulatory light polypeptide 9; MLCK, myosin light
chain kinase; RhoA, transforming protein RhoA; ROCK-1,
Rho-associated protein kinase-1; GHS-R, growth hormone secretagogue
receptor; OD, optical density. |
Effects of ghrelin on cell viability
and ROS levels in GASMCs treated with β-HB
Ghrelin (10−8 mol/l) improved the
viability of GASMCs following β-HB treatment at 24, 48 and 72 h
(Fig. 3A). Ghrelin
(1×10−8 mol/l) decreased the level of ROS following
treatment with or without β-HB in GASMCs at 48 h (Fig. 3E and F).
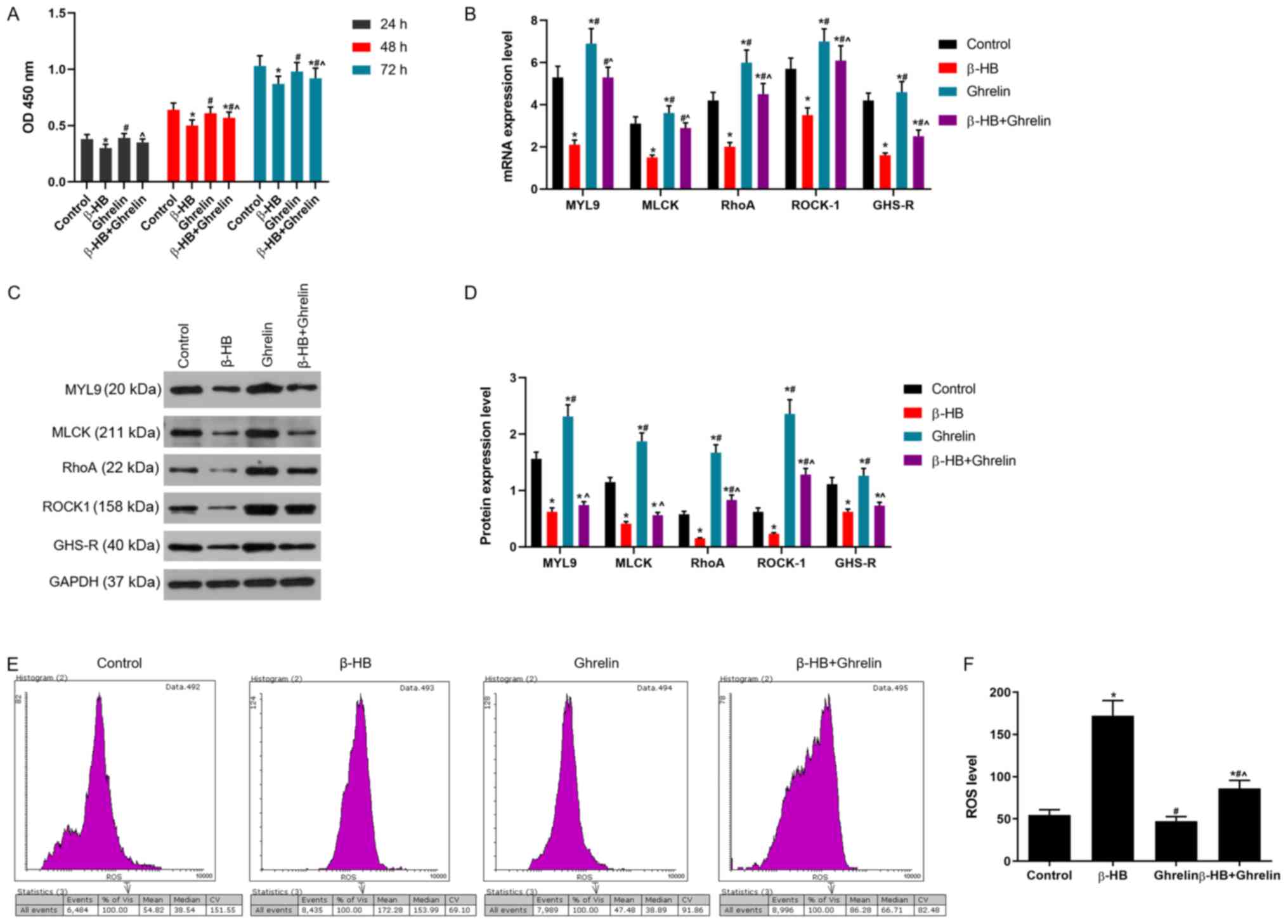 | Figure 3.Effects of ghrelin in combination
with β-HB on cell viability and the expression of MYL9, MLCK, RhoA,
ROCK-1, GHS-R and ROS in rat GASMCs. (A) GASMCs were treated with
10−8 mol/l ghrelin and 5 mmol/l β-HB for 24, 48 and 72
h. Cell viability was determined using the Cell Counting Kit-8
assay. (B) Following treatment of GASMCs for 48 h, the mRNA
expression of MYL9, MLCK, RhoA, ROCK-1 and GHS-R were evaluated
using reverse transcription-quantitative PCR. (C) Western blot
analysis was used to determine the protein levels of MYL9, MLCK,
RhoA, ROCK-1 and GHS-R. (D) Quantification of the levels of MYL9,
MLCK, RhoA, ROCK-1 and GHS-R. (E) ROS levels were analyzed using a
ROS assay and flow cytometry. (F) Quantification of ROS levels.
ANOVA was used to analyze the differences. *P<0.05 vs. Control
group; #P<0.05 vs. β-HB; ^P<0.05 vs.
10−8. β-HB, β-hydroxybutyric acid; GASMCs, gastric
antral smooth muscle cells; MYL9, myosin regulatory light
polypeptide 9; MLCK, myosin light chain kinase; RhoA, transforming
protein RhoA; ROCK-1, Rho-associated protein kinase-1; GHS-R,
growth hormone secretagogue receptor; ROS, reactive oxygen species;
OD, optical density. |
Effects of ghrelin on the expression
of MYL9, MLCK, RhoA, ROCK-1, GHS-R, catalase, manganese (Mn)
superoxide dismutase (SOD), copper/zinc (Cu/Zn)SOD, TNF-α and IL-6
in GASMCs treated with β-HB
After 48 h, a low concentration of ghrelin
(1×10−8 mol/l) led to an increase in the expression of
MYL9, MLCK, RhoA, ROCK-1 and GHS-R following the treatment of
GASMCs with β-HB (Fig. 3B-D).
Treatment of GASMCs with β-HB or ghrelin inhibited the expression
of catalase. β-HB decreased the expression of Cu/ZnSOD and MnSOD,
whereas ghrelin increased the levels of Cu/ZnSOD and MnSOD after 48
h cultured (Fig. 4A-C). Ghrelin
and β-HB co-treatment reduced the expression of Cu/ZnSOD and MnSOD
and increased the expression of catalase compared to ghrelin
treatment alone at 48 h. A low concentration of ghrelin
(1×10−8 mol/l) had a modest inhibitory effect on the
expression of TNF-α and IL-6 (Fig.
4D), whereas a low concentration of ghrelin (1×10−8
mol/l) significantly decreased the β-HB-induced expression of TNF-α
and IL-6 (Fig. 4D).
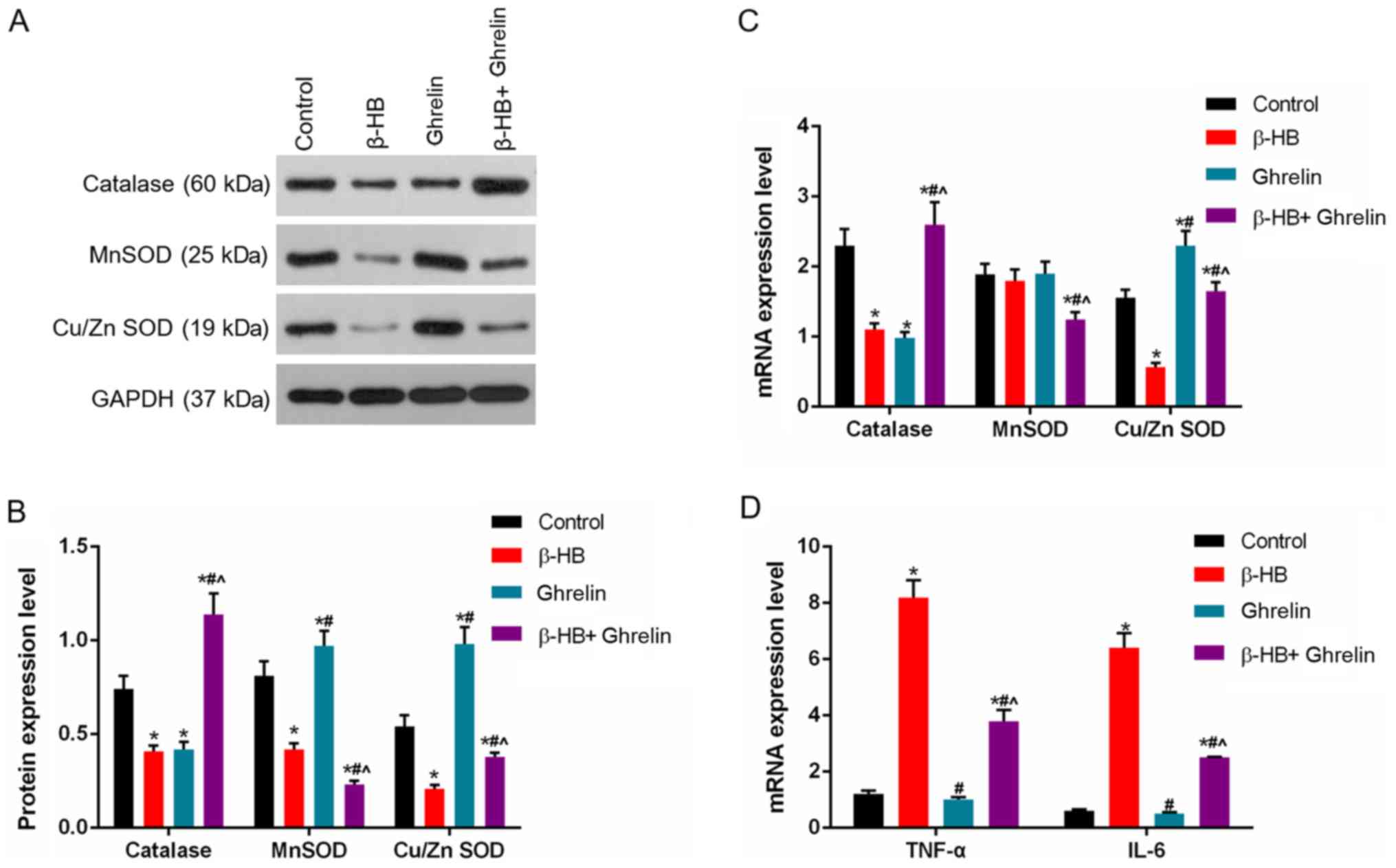 | Figure 4.Effects of ghrelin and β-HB on the
expression of catalase, MnSOD, Cu/ZnSOD, TNF-α and IL-6 in rat
GASMCs. GASMCs were treated with 10−8 mol/l ghrelin and
5 mmol/l β-HB for 48 h. (A) The protein levels of catalase, MnSOD
and Cu/ZnSOD were determined using western blot analysis and (B)
quantified. Reverse transcription-quantitative PCR was used to
determine the mRNA levels of (C) catalase, MnSOD, Cu/ZnSOD, (D)
TNF-α and IL-6. ANOVA was used to analyze the differences.
*P<0.05 vs. Control; #P<0.05 vs. β-HB;
^P<0.05 vs. 10−8. β-HB, β-hydroxybutyric
acid; GASMCs, gastric antral smooth muscle cells; TNF-α, tumor
necrosis factor-α; IL-6, interleukin-6; MnSOD, manganese superoxide
dismutase; Cu/ZnSOD, copper/zinc superoxide dismutase. |
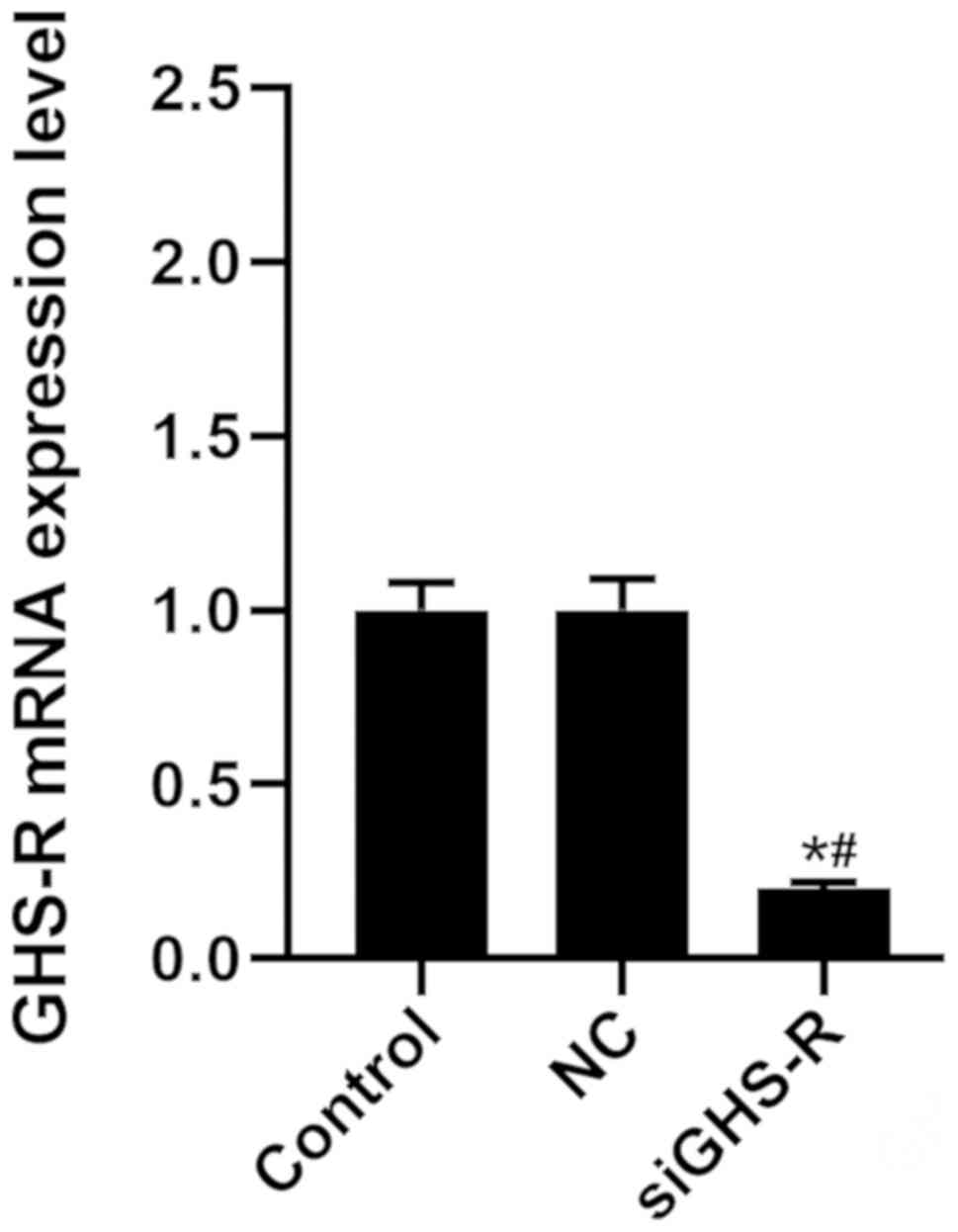
Effects of siGHS-R on the levels of
ROS and the expression of MYL9, MLCK, RhoA, ROCK-1, GHS-R, TNF-α
and IL-6 in GASMCs
As shown in Fig. 5,
transfection of cells with siGHS-R led to the significant depletion
of GHS-R in GASMCs compared with the control (Fig. 5). β-HB inhibited the expression of
MYL9, MLCK, RhoA, ROCK-1 and GHS-R in GASMCs. By contrast, β-HB
promoted the expression of TNF-α and IL-6 after 48 h of culture
(Fig. 6A-C). β-HB promoted the
production of ROS (Fig. 6D and E).
siGHS-R significantly inhibited the expression of MYL9, MLCK, RhoA,
ROCK-1 and GHS-R following treatment with β-HB, ghrelin or their
combination (Fig. 6A and B).
siGHS-R significantly increased the levels of ROS, TNF-α and IL-6
following treatment with β-HB, ghrelin or their combination in
GASMCs (Fig. 6C-E).
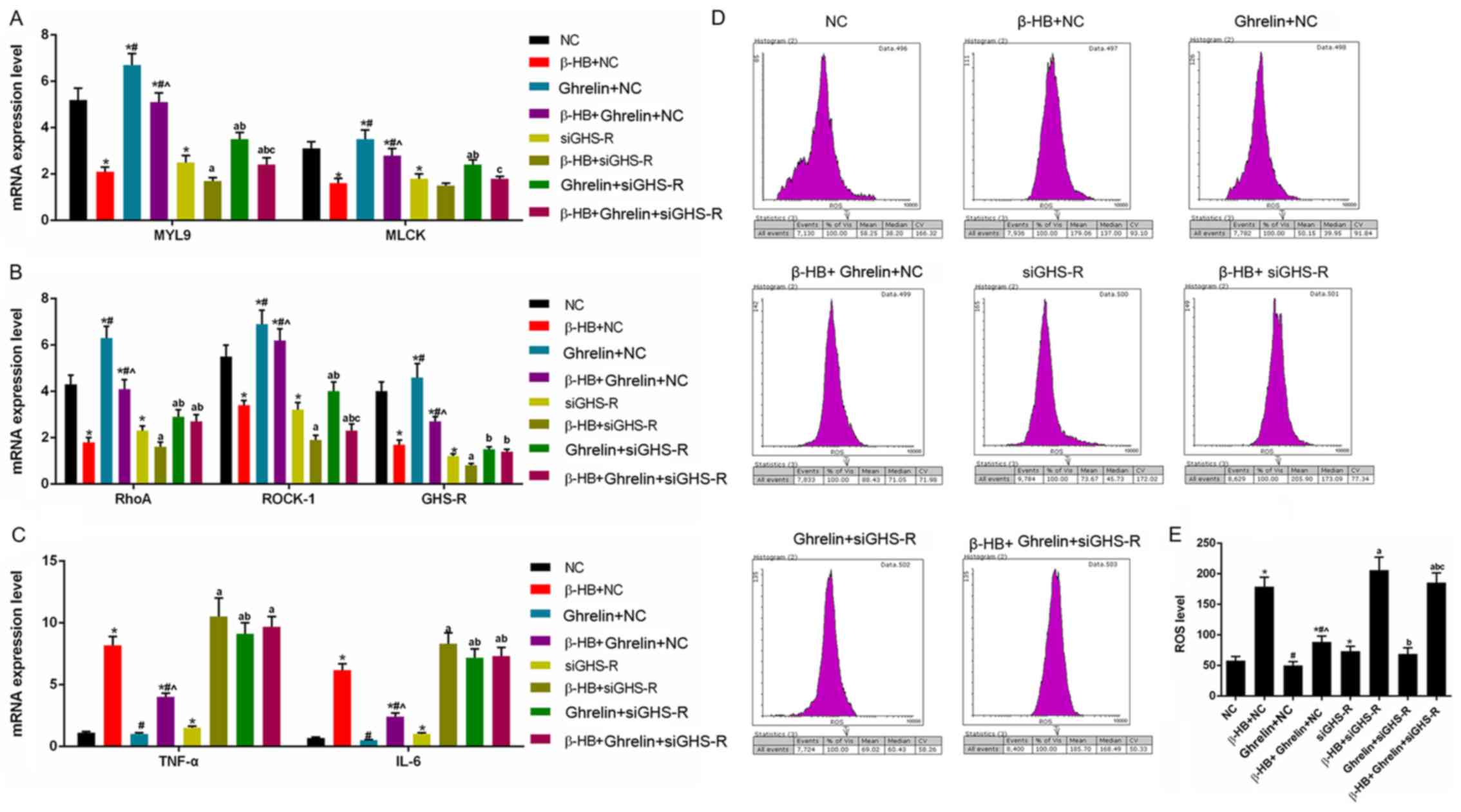 | Figure 6.Effects of siGHS-R on the expression
of MYL9, MLCK, RhoA, ROCK-1, GHS-R, TNF-α and IL-6, and ROS levels
in rat GASMCs. GASMCs were transfected with siGHS-R and treated for
48 h, as indicated. The mRNA expression of (A) MYL9, MLCK, (B)
RhoA, ROCK-1, GHS-R, (C) TNF-α and IL-6 were determined using
reverse transcription-quantitative PCR. (D) ROS levels were
analyzed using a ROS assay kit and flow cytometry. (E)
Quantification of the ROS levels. ANOVA was used to analyze the
differences. *P<0.05. vs. NC; #P<0.05 vs. β-HB +
NC; ^P<0.05 vs. 10−8 + NC;
aP<0.05 vs. siGHS-R; bP<0.05 vs. β-HB +
siGHS-R; cP<0.05 vs. 10−8 + siGHS-R. β-HB,
β-hydroxybutyric acid; si, short interfering RNA; GASMCs, gastric
antral smooth muscle cells; MYL9, myosin regulatory light
polypeptide 9; MLCK, myosin light chain kinase; RhoA, transforming
protein RhoA; ROCK-1, Rho-associated protein kinase-1; GHS-R,
growth hormone secretagogue receptor; ROS, reactive oxygen species;
TNF-α, tumor necrosis factor-α; IL-6, interleukin-6; NC, negative
control. |
Discussion
MLCK is a skeletal and smooth muscle enzyme that is
encoded by two different genes in higher organisms: The mylk-1 and
mylk-2 genes (16,17). The mylk-2 gene encodes an MLCK
isoform that is only expressed in skeletal muscle cells. The mylk-1
gene encodes a 220 kDa MLCK, a 130 kDa MLCK and telokin, and is
widely expressed in a diverse range of tissues and cells (18,19).
MLCK is involved in adhesion and migration, which are basic
characteristics of cells (20,21).
A previous pharmacological study revealed that the inhibition of
MLCK changed cell motility and wound contraction (22). There are three types of myosin
regulatory light chains: MYL12B, MYL12A and MYL9 (23). The MYL9 gene is highly expressed in
vascular smooth muscle cells (23). It has been reported that MYL9
impacts cell motility and contractility, and that is an important
component of the contractile apparatus of cells (24). In the present study, β-HB inhibited
the expression of MLCK and MYL9, while 10−8 mol/l
ghrelin promoted the expression of MLCK and MYL9 in GASMCs.
There are three Ras homolog gene family members:
RhoA, RhoB and RhoC in human and rat (25). Rho isoforms have GTPase activity
and impact the levels of GDP and GTP. The sequences of RhoA, RhoB
and RhoC share ~85% homology (26). Rho plays an important role in the
regulation of cell shape and locomotion through actin and Rho is
required for lamellipodia (27).
RhoA directly promotes the polymerization of actin, and RhoA is
considered to regulate actomyosin contractility, which is important
for the ability of migrating cells to detach from the rear of the
cell (26). ROCK and its two
isoforms (ROCK-1 and ROCK-2) have an important role in cell
motility and migration (28).
ROCK-1 and ROCK-2 contain different Rho-binding regions, and ROCK
has a higher affinity with RhoC, which is important for cell
locomotion (26). Previous studies
reported that the inhibition of ROCK-1 decreased cell migration and
motility (29,30). The present study identified that
β-HB inhibited the expression of RhoA and ROCK1, whereas ghrelin
increased the levels of RhoA and ROCK1 in GASMCs.
MnSOD is encoded by the SOD2 gene and the protein is
located in the mitochondrial matrix. Cu/ZnSOD is encoded by the
SOD1 gene, and the protein is located in the cytoplasm and the
mitochondrial intermembrane space of cells (31). MnSOD and Cu/ZnSOD protect cells
from oxidative damage (31);
inflammation changes the antioxidative system, and increases
inflammatory cytokines, which decreases the activities of the SOD
proteins (32). Hydrogen peroxide
can be neutralized by catalase during the process of antioxidation
(33), and the overexpression of
catalase reduces levels of DNA damage (34). ROS, TNF-α and IL-6 are involved in
inflammation and immune responses; the inhibition of TNF-α, ROS and
IL-6 expression reduces inflammation (35–37).
A limitation of the present study may be the fact that the protein
levels of TNF-α and IL-6 in GASMCs were not analyzed. However,
changes in the mRNA expression level may reflect changes in the
protein level. In the present study, β-HB promoted the expression
of TNF-α and IL-6 in GASMCs, while ghrelin inhibited the
β-HB-induced expression of TNF-α and IL-6. In addition, β-HB
promoted inflammation in GASMCs, whereas ghrelin inhibited
β-HB-induced inflammation in the GASMCs.
Ghrelin is a 28-amino-acid peptide, and is the
endogenous ligand for GHS-R (38).
In the present study, it was found that siGHS-R significantly
inhibited the expression of MYL9, MLCK, RhoA and ROCK-1. By
contrast, siGHS-R significantly increased the levels of ROS, TNF-α
and IL-6 following treatment with β-HB, ghrelin or their
combination in GASMCs. This indicated that silencing of GHS-R
inhibited the motility of GASMCs, and promoted inflammation in
GASMCs. The silencing GHS-R increased inflammation and the
inhibition of GASMCs motility induced by the β-HB, ghrelin or their
combination, which suggested that GHS-R may be a regulator of
motility and inflammation in GASMCs. A limitation of the present
study was that Transwell or wound healing assays were not used to
determine the motility of GASMCs.
In conclusion, the present study has provided
preliminary data to suggest that β-HB inhibits the motility of
GASMCs and promotes inflammation, whereas ghrelin decreases these
effects. GHS-R acted as a regulator of motility and inflammation in
GASMCs treated with β-HB and ghrelin. Not analyzing the expression
of classical markers of smooth muscle cells, such as osteopontin
and calponin, may be a limitation of the present study, which
should be addressed in future studies. Further research in
vivo is also required.
Acknowledgements
Not applicable.
Funding
The present study was supported by the Fundamental
Research Funds for the Central University for the Southwest
University (grant no. XDJK2018C083).
Availability of data and materials
The datasets used and/or analyzed during the study
are available from the corresponding author on reasonable
request.
Authors' contributions
XHu and LY provided substantial contributions to the
concept and design of the study. CH, MA, JW, WH, XHe and ZW were
involved in data acquisition, data analysis and interpretation. XHu
and LY were involved in drafting the article or critically revising
it for important intellectual content. All authors gave final
approval of the version to be published. All authors agree to be
accountable for all aspects of the work in ensuring that questions
related to the accuracy or integrity of the work are appropriately
investigated and resolved.
Ethics approval and consent to
participate
Animal experiments were approved by the
Institutional Animal Care and Use Committee of Southwest University
Hospital (no. 2017110853n).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Svintsitskyi AS and Soloviova HA:
Disturbances of gastrointestinal motility of the stomach in
patients with chronic gastric erosions and biliary tract disease.
Lik Sprava. 47–53. 2012.(In Ukrainian). PubMed/NCBI
|
|
2
|
Wang F, Meng W, Wang B and Qiao L:
Helicobacter pylori-induced gastric inflammation and gastric
cancer. Cancer Lett. 345:196–202. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kim SS, Lee HS, Cho YS, Lee YS, Bhang CS,
Chae HS, Han SW, Chung IS and Park DH: The effect of the repeated
subcultures of Helicobacter pylori on adhesion, motility,
cytotoxicity, and gastric inflammation. J Korean Med Sci.
17:302–306. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Marignani M, Delle Fave G, Mecarocci S,
Bordi C, Angeletti S, D'Ambra G, Aprile MR, Corleto VD, Monarca B
and Annibale B: High prevalence of atrophic body gastritis in
patients with unexplained microcytic and macrocytic anemia: A
prospective screening study. Am J Gastroenterol. 94:766–772. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Miralles B, Del Barrio R, Cueva C, Recio I
and Amigo L: Dynamic gastric digestion of a commercial whey protein
concentrate†. J Sci Food Agric. 98:1873–1879. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hunt RH, Camilleri M, Crowe SE, El-Omar
EM, Fox JG, Kuipers EJ, Malfertheiner P, McColl KE, Pritchard DM,
Rugge M, et al: The stomach in health and disease. Gut.
64:1650–1668. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Krueger T and Melendez P: Effect of
ghrelin on feed intake and metabolites in lambs. Appetite.
58:758–759. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Klok MD, Jakobsdottir S and Drent ML: The
role of leptin and ghrelin in the regulation of food intake and
body weight in humans: A review. Obes Rev. 8:21–34. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Patterson M, Bloom SR and Gardiner JV:
Ghrelin and appetite control in humans-potential application in the
treatment of obesity. Peptides. 32:2290–2294. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fu SP, Liu BR, Wang JF, Xue WJ, Liu HM,
Zeng YL, Huang BX, Li SN, Lv QK, Wang W and Liu JX:
β-Hydroxybutyric acid inhibits growth hormone-releasing hormone
synthesis and secretion through the GPR109A/extracellular
signal-regulated 1/2 signalling pathway in the hypothalamus. J
Neuroendocrinol. 27:212–222. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun M, Martin RJ and Edwards GL: ICV
beta-hydroxybutyrate: Effects on food intake, body composition, and
body weight in rats. Physiol Behav. 61:433–436. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hawkins RA, Williamson DH and Krebs HA:
Ketone-body utilization by adult and suckling rat brain in vivo.
Biochem J. 122:13–18. 1971.PubMed/NCBI
|
|
13
|
Nowroozi-Asl A, Aarabi N and
Rowshan-Ghasrodashti A: Ghrelin and its correlation with leptin,
energy related metabolites and thyroidal hormones in dairy cows in
transitional period. Pol J Vet Sci. 19:197–204. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Poggioli R, Vitale G, Colombo G, Ottani A
and Bertolini A: Gamma-hydroxybutyrate increases gastric emptying
in rats. Life Sci. 64:2149–2154. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xiong Y, Wang C, Shi L, Wang L, Zhou Z,
Chen D, Wang J and Guo H: Myosin light chain kinase: A potential
target for treatment of inflammatory diseases. Front Pharmacol.
8:2922017. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Pires E, Perry SV and Thomas MA: Myosin
light-chain kinase, a new enzyme from striated muscle. FEBS Lett.
41:292–296. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Herring BP, El-Mounayri O, Gallagher PJ,
Yin F and Zhou J: Regulation of myosin light chain kinase and
telokin expression in smooth muscle tissues. Am J Physiol Cell
Physiol. 291:C817–C827. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Herring BP, Dixon S and Gallagher PJ:
Smooth muscle myosin light chain kinase expression in cardiac and
skeletal muscle. Am J Physiol Cell Physiol. 279:C1656–C1664. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Webb DJ, Donais K, Whitmore LA, Thomas SM,
Turner CE, Parsons JT and Horwitz AF: FAK-Src signalling through
paxillin, ERK and MLCK regulates adhesion disassembly. Nat Cell
Biol. 6:154–161. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
21
|
Khapchaev AY and Shirinsky VP: Myosin
Light Chain Kinase MYLK1: Anatomy, interactions, functions, and
regulation. Biochemistry (Mosc). 81:1676–1697. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Levinson H, Moyer KE, Saggers GC and
Ehrlich HP: Calmodulin-myosin light chain kinase inhibition changes
fibroblast-populated collagen lattice contraction, cell migration,
focal adhesion formation, and wound contraction. Wound Repair
Regen. 12:505–511. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Aoki T, Miyazaki K, Katayama T, Watanabe
M, Horie R, Danbara M and Higashihara M: Surface CD3 expression
proceeds through both myosin regulatory light chain 9
(MYL9)-dependent and MYL9-independent pathways in Jurkat cells. J
Smooth Muscle Res. 48:137–147. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Betapudi V, Licate LS and Egelhoff TT:
Distinct roles of nonmuscle myosin II isoforms in the regulation of
MDA-MB-231 breast cancer cell spreading and migration. Cancer Res.
66:4725–4733. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cannizzaro LA, Madaule P, Hecht F, Axel R,
Croce CM and Huebner K: Chromosome localization of human ARH genes,
a ras-related gene family. Genomics. 6:197–203. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wheeler AP and Ridley AJ: Why three Rho
proteins? RhoA, RhoB, RhoC, and cell motility. Exp Cell Res.
301:43–49. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Ridley AJ, Schwartz MA, Burridge K, Firtel
RA, Ginsberg MH, Borisy G, Parsons JT and Horwitz AR: Cell
migration: Integrating signals from front to back. Science.
302:1704–1709. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Borin TF, Arbab AS, Gelaleti GB, Ferreira
LC, Moschetta MG, Jardim-Perassi BV, Iskander AS, Varma NR, Shankar
A, Coimbra VB, et al: Melatonin decreases breast cancer metastasis
by modulating Rho-associated kinase protein-1 expression. J Pineal
Res. 60:3–15. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li CH, Yu TB, Qiu HW, Zhao X, Zhou CL and
Qi C: miR-150 is downregulated in osteosarcoma and suppresses cell
proliferation, migration and invasion by targeting ROCK1. Oncol
Lett. 13:2191–2197. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Schofield AV, Steel R and Bernard O:
Rho-associated coiled-coil kinase (ROCK) protein controls
microtubule dynamics in a novel signaling pathway that regulates
cell migration. J Biol Chem. 287:43620–43629. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
O'Brien KM, Dirmeier R, Engle M and Poyton
RO: Mitochondrial protein oxidation in yeast mutants lacking
manganese-(MnSOD) or copper- and zinc-containing superoxide
dismutase (CuZnSOD): Evidence that MnSOD and CuZnSOD have both
unique and overlapping functions in protecting mitochondrial
proteins from oxidative damage. J Biol Chem. 279:51817–51827. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Al-Asmari AK and Khan MW: Inflammation and
schizophrenia: Alterations in cytokine levels and perturbation in
antioxidative defense systems. Hum Exp Toxicol. 33:115–122. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kirkman HN and Gaetani GF: Catalase: A
tetrameric enzyme with four tightly bound molecules of NADPH. Proc
Natl Acad Sci USA. 81:4343–4347. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Selvaratnam J and Robaire B:
Overexpression of catalase in mice reduces age-related oxidative
stress and maintains sperm production. Exp Gerontol. 84:12–20.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Blaser H, Dostert C, Mak TW and Brenner D:
TNF and ROS Crosstalk in Inflammation. Trends Cell Biol.
26:249–261. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Tanaka T, Narazaki M and Kishimoto T: IL-6
in inflammation, immunity, and disease. Cold Spring Harb Perspect
Biol. 6:a0162952014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Yao X, Huang J, Zhong H, Shen N, Faggioni
R, Fung M and Yao Y: Targeting interleukin-6 in inflammatory
autoimmune diseases and cancers. Pharmacol Ther. 141:125–139. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Okada Y, Sugita Y, Ohshima K, Morioka M,
Komaki S, Miyoshi J and Abe H: Signaling of ghrelin and its
functional receptor, the growth hormone secretagogue receptor,
promote tumor growth in glioblastomas. Neuropathology. 36:535–543.
2016. View Article : Google Scholar : PubMed/NCBI
|