Introduction
Renal cell carcinoma (RCC) is the most common solid
lesion located within the kidney and represents ~90% of all renal
malignancies (1). The prevalence
of the classic triad of hematuria, flank pain and palpable
abdominal mass in RCC patients is actually lower than 10%, even 6%
(2). Therefore, approximately
20–30% of patients present with metastatic RCC at initial
diagnosis, and the recurrence rate of post-surgical treatment cases
is 20–30% (3). RCC is typically
resistant to radiation therapy and chemotherapy, and therefore
surgery remains the only curative treatment for RCC (1,4).
Recently, much attention has shifted to targeted therapies for
which either sunitinib or pazopanib is the standard first-line
treatment for RCC (5). As the
clinical effects of these therapies are not effective, it is
crucial to develop more effective therapeutic strategies against
RCC.
MicroRNAs (miRNAs, miRs) are a class of non-coding
RNAs of 17–24 nucleotides, which mediate cell differentiation,
proliferation, migration, apoptosis and invasion by targeting the
3′-untranslated region (UTR) of mRNA post-transcriptionally
(6–8). Aberrant expression of several miRNAs
has been implicated in the tumorigenesis of RCC, including as
miR-21 (9), miR-23a (10), miR-566 (11) and miR-425-5p (12). Since its discovery in the human
fetal liver (13), miR-486 has
been reported in several malignancies, including esophageal cancer
(14), non-small cell lung cancer
(NSCLC) (15), colorectal cancer
(CRC) (16) and osteosarcoma
(17). However, miR-486 and its
role in RCC remain largely unknown.
In the present study, miR-486 expression was
evaluated in RCC tissues and RCC cell lines. The effects of miR-486
on kidney cancer cell proliferation, invasion and apoptosis were
also investigated. Finally, the association between miR-486
expression and outcome in RCC patients was explored.
Materials and methods
Specimens
Samples including 47 paired RCC tissue samples and
adjacent normal tissue samples (extracted at a distance of 5-cm
from the RCC tissue) were obtained from the Department of Urology,
Affiliated Hospital of Jiangnan University (Wuxi, China) from May
2013 to April 2015. These patients consisted of 29 males and 18
females, ranging in age from 21 to 73 years old. Immediately after
resection, the specimens were maintained in RNAlater®
(Qiagen, Inc.) and stored at −80°C until further use. All
participants provided written informed consent, and the study was
approved by the Ethics Committee of the Affiliated Hospital of
Jiangnan University. Table I lists
the clinicopathological features of the 47 patients enrolled in the
study. In addition, 96 formalin-fixed paraffin-embedded (FFPE) RCC
tissues were supplied by the Department of Pathology, Affiliated
Hospital of Jiangnan University between February 2011 and January
2014. These patients consisted of 63 males and 33 females, ranging
in age from 21 to 78 years old. Clinicopathological characteristics
of the FFPE samples are presented in Table II. These are two different batches
of specimens. Table I lists fresh
tissue samples from 47 patients with renal carcinoma (including
renal carcinoma and adjacent normal tissue). Table II documents the paraffin tissue
samples from 96 patients with renal cancer.
 | Table I.Clinicopathological features of the
RCC patients (N=47). |
Table I.
Clinicopathological features of the
RCC patients (N=47).
| Characteristics | Data |
|---|
| Age, mean (range) in
years | 50 (21–73) |
| Sex, n |
| Male/female | 29/18 |
| Tumor stage, n |
| T1/T2/T3 + T4 | 22/15/10 |
| Fuhrman grade,
n |
| I/II/III/IV | 17/20/7/3 |
| AJCC clinical
stage, n |
| I/II/III + IV | 19/11/17 |
 | Table II.Association between miR-486
expression levela and
the clinical characteristics of the FFPE renal cancer samples
(N=96). |
Table II.
Association between miR-486
expression levela and
the clinical characteristics of the FFPE renal cancer samples
(N=96).
|
|
| No. of
patients |
|
|---|
|
|
|
|
|
|---|
| Variable | Total | High | Low |
P-valueb |
|---|
| Sex |
|
Male | 63 | 33 | 30 | 0.519 |
|
Female | 33 | 15 | 18 |
|
| Age (years) |
|
≤60 | 69 | 33 | 36 | 0.496 |
|
>60 | 27 | 15 | 12 |
|
| Tumor size
(cm) |
|
≤4.0 | 37 | 16 | 21 | 0.294 |
|
>4.0 | 59 | 32 | 27 |
|
| Tumor stage |
| I +
II | 71 | 35 | 36 | 0.816 |
| III +
IV | 25 | 13 | 12 |
|
RNA extraction, cDNA synthesis and
reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted by using TRIzol (Invitrogen;
Thermo Fisher Scientific, Inc.) according to the manufacturer's
instructions, purified by using the RNeasy Maxi kit (Qiagen, Inc.)
and quantified on a NanoDrop 2000c (Thermo Fisher Scientific,
Inc.). cDNA was synthesized by reverse transcription according to
the instructions of an miScript II RT kit (Qiagen, Inc.). The
expression of miR-486 was detected using an miScript
SYBR® Green PCR kit (Qiagen, Inc.) on the Roche
Lightcycler® 480 Real-Time PCR System (Roche
Diagnostics). The thermocycling steps were: 95°C for 15 min,
followed by 40 cycles of 94°C for 15 sec, 55°C for 30 sec and 72°C
for 30 sec. The primer sequences used were: miR-486-5p forward,
5′-ACACTCCAGCTGGGTCCTGTACTGAGCTGCCC-3′ and reverse,
5′-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGCCCCGAG-3′; U6 forward,
5′-CTCGCTTCGGCAGCACA-3′ and reverse, 5′-AACGCTTCACGAATTTGCGT-3′. U6
was regarded as the internal control in this study. Relative
expression of miR-486 was analyzed by the 2−ΔΔCq method
(18).
Cell culture and transfection
Immortalized normal human kidney cells (HK2) and RCC
cell lines (ACHN, 786-O, Caki-1) were supplied by the Key
Laboratory of Carbohydrate Chemistry and Biotechnology (Wuxi,
China). Cell lines were cultured and maintained according to the
instructions provided by the American Type Culture Collection.
Cells were cultured in at 37°C in a 5% CO2 humidified
incubator. ACHN and 786-O cells were transiently transfected with
100 pmol miR-486 mimic (forward,
5′-ACACUCCAGCUGGGUCCUGUACUGAGCUGCCC-3′ and reverse,
5′-GGCCACCGCCGAGCGGACUU-3′) or miR-negative control (NC; forward,
5′-UUCUCCGAACGUGUCACGUTT-3′ and reverse,
5′-ACGUGACACGUUCGGAGAATT-3′) using Lipofectamine 2000 (Invitrogen;
Thermo Fisher Scientific, Inc.) and Opti-MEM® (Gibco;
Thermo Fisher Scientific, Inc.) following the manufacturer's
instructions. Following transfection, ACHN or 786-O cells were
starved for 6 h before being transferred to DMEM (for ACHN cells)
or RPMI-1640 (for 786-O cells; both Gibco; Thermo Fisher
Scientific, Inc.) with 10% FBS (Gibco; Thermo Fisher Scientific,
Inc.) and 1% glutamine (Gibco; Thermo Fisher Scientific, Inc.).
Transfection efficiency was measured via RT-qPCR analysis. miR-486
mimic and NC were synthesized by Shanghai GenePharma Co., Ltd.
CCK-8 assay
Proliferation of ACHN and 786-O cells was assessed
using a Cell Counting Kit-8 (CCK-8; Beyotime Institute of
Biotechnology). Briefly, ~10,000 cells seeded in 96-well plates
were transfected with miR-486 mimic or NC. CCK-8 (10 µl) was added
into each well and incubation was carried out for 30 min.
Absorbance was then assessed at 450 nm (with 620 nm as the
reference wavelength) at 0, 24, 48 and 72 h on an ELISA microplate
reader (Bio-Rad Laboratories, Inc.).
Transwell assays
Transwell inserts were used to assess migration and
invasion of the ACHN and 786-O cells. For the migration assay,
~3×104 cells were seeded in a 24-well plate and
transfected with miR-486 mimic or NC as described above. At 24 h
later, ~3×104 cells were seeded into each upper
Transwell chamber (BD Biosciences). A volume of 600 µl DMEM with
10% FBS and 1% glutamine was added to the bottom Transwell chamber.
For the invasion assay, cells were seeded into a Matrigel-coated
upper chamber of the Transwell inserts. After incubation for 48 h,
the cells in the lower chamber were fixed in 0.1% paraformaldehyde
and stained with 4% crystal violet (both for 25 min at room
temperature), and observed by a light microscope at magnification,
×100.
Wound healing assay
Migration of the ACHN and 786-O cells was also
determined by the wound healing assay. Cells (~105) were
seeded in 6-well plates and after 24 h were respectively
transfected according to the manufacturer's instructions. A sterile
1-ml pipette tip was used to scratch a vertical horizontal line.
Serum-free medium was added after the cells were scratched for
excluding cell proliferation. Images of the scratches at 0, 12 and
24 h were captured by a light microscope at magnification, ×100.
The assay was repeated at least three times.
Flow cytometry
Apoptosis analysis of the ACHN and 786-O cells was
carried out by flow cytometry. Cells (~105) were seeded
in each well of a 6-well plate and transfected as described above.
After 48 h, the cells were harvested and washed twice in cold PBS,
resuspended in 100 µl 1X binding buffer with 5 µl Annexin V-FITC
(Invitrogen; Thermo Fisher Scientific, Inc.) and 5 µl propidium
iodide (PI, Invitrogen; Thermo Fisher Scientific, Inc.) and stained
for 15 min at room temperature in a dark place. Thereafter, 400 µl
of cold 1X binding buffer was added into each tube and apoptosis
was assessed by flow cytometry (EPICS XL-4; Beckman Coulter, Inc.).
FlowJo 7.6.1 (FlowJo LLC) was used to analyze the apoptotic
rate.
Statistical analysis
All assays were repeated at least three times. All
the statistical analyses were performed using SPSS 23.0 (IBM
Corp.). Data are presented as the mean ± standard deviation (SD).
Expression of miR-486 in clinical specimens and different cell
lines was analyzed using paired t-tests and ANOVA followed by
Dunnett's test, respectively. Student's t-test, Fisher's exact test
or Pearson χ2 test were used to analyze the association
between miR-486 expression and clinicopathological variables. The
correlation between miR-486 expression and clinicopathological
variables or survival was analyzed using Cox proportional hazard
regression analysis. Kaplan-Meier curves were used to plot overall
survival. A value of P<0.05 was considered to be indicative of a
statistically significant result.
Results
miR-486 is upregulated in RCC tissues
and RCC cell lines
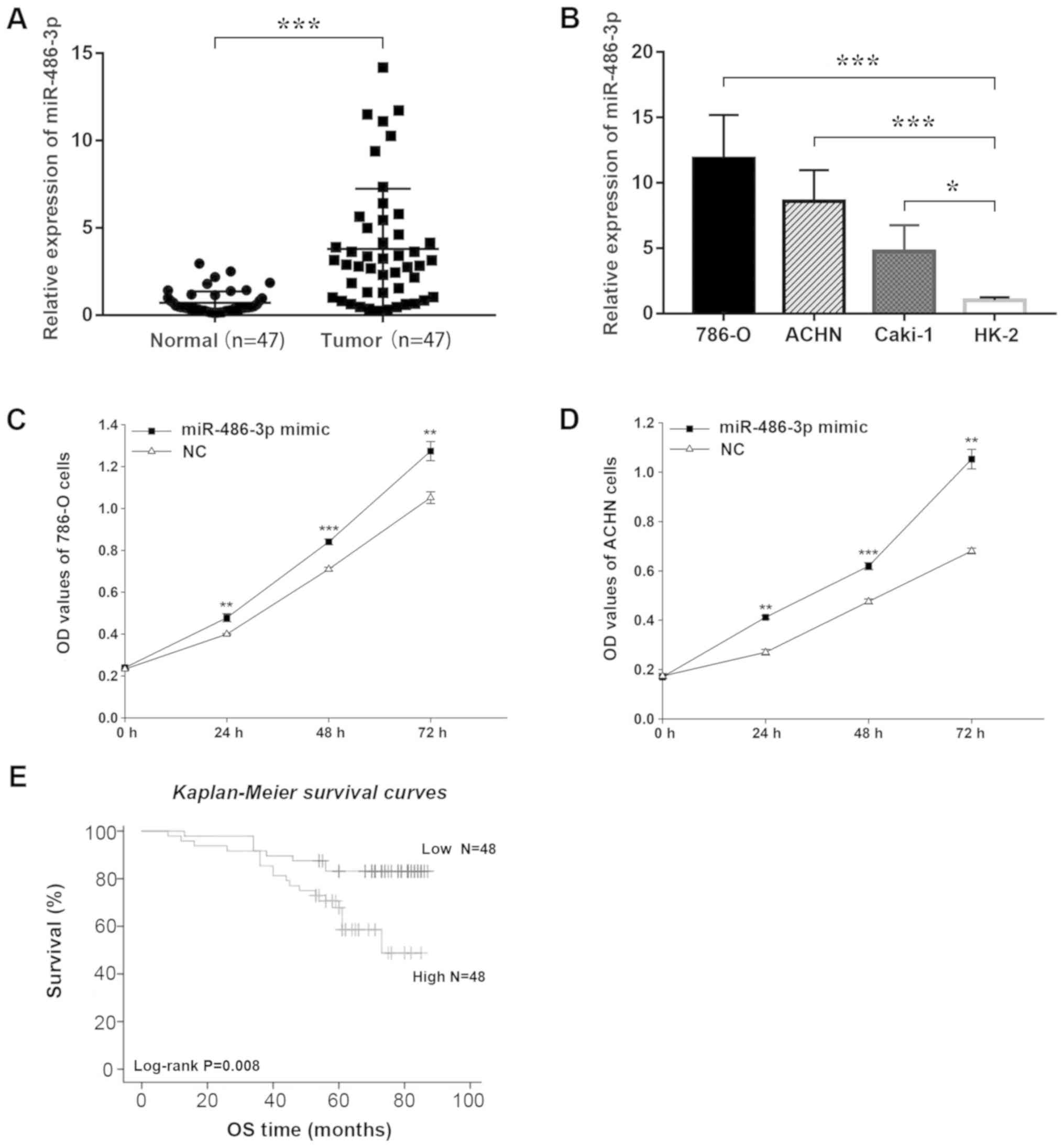
RT-qPCR analysis revealed higher transcript
abundance of miR-486 in RCC tissue samples when compared with that
in the adjacent normal tissues (P<0.001; Fig. 1A). Similarly, relative expression
of miR-486 in RCC cell lines was significantly higher than that
noted in the normal HK2 cells (Fig.
1B).
miR-486 promotes cell
proliferation
The CCK-8 assay was used to assess proliferation
in vitro. Data showed that overexpression of miR-486
significantly increased the proliferation of the 786-O and ACHN
cell lines compared with cells in the NC groups (Fig. 1C and D). The results indicated that
miR-486 promotes cell proliferation.
miR-486 is a potential prognostic
marker for RCC
The association between miR-486 expression and
clinicopathological characteristics was analyzed in 96 FFPE renal
cancer samples. As presented in Table
II, there were no significant assocations between miR-486
expression and any clinical characteristics (P>0.05); however,
it was revealed that the survival of patients with RCC was
significantly associated with tumor stage (P<0.001) and miR-486
expression (P<0.05), but not gender, age or tumor size (Table III). Univariate and multivariate
analyses showed that patients with low expression of miR-486 had a
longer overall survival (OS; P<0.05). Similarly, Kaplan-Meier
survival analysis showed that patients with low miR-486 expression
had longer OS than those with high expression (P=0.008; Fig. 1E).
 | Table III.miR-486 expression and RCC patient
survival. |
Table III.
miR-486 expression and RCC patient
survival.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Sex (female vs.
male) | 0.625
(0.264–1.477) | 0.284 | 0.661
(0.271–1.613) | 0.363 |
| Age (years) (≤60
vs. >60) | 0.934
(0.409–2.135) | 0.872 | 1.269
(0.545–2.954) | 0.581 |
| Tumor size (cm)
(≤4.0 vs. >4.0) | 0.721
(0.324–1.607) | 0.424 | 0.728
(0.323–1.638) | 0.442 |
| Tumor stage (I + II
vs. III + IV) | 0.055
(0.022–0.139) | <0.001 | 0.057
(0.022–0.143) | <0.001 |
| miR-486-3p (low vs.
high) | 0.342
(0.148–0.790) | 0.012 | 0.398
(0.170–0.929) | 0.033 |
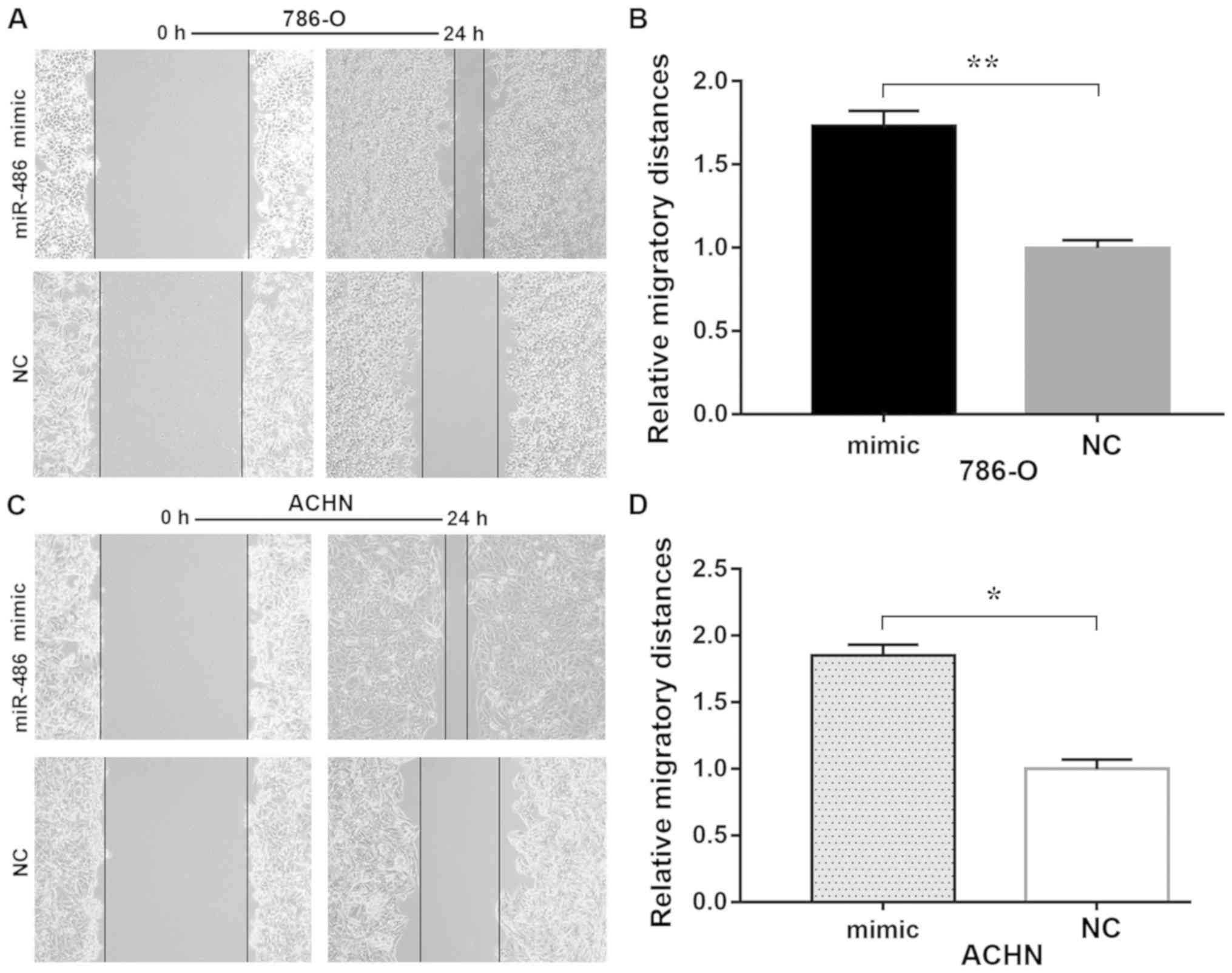
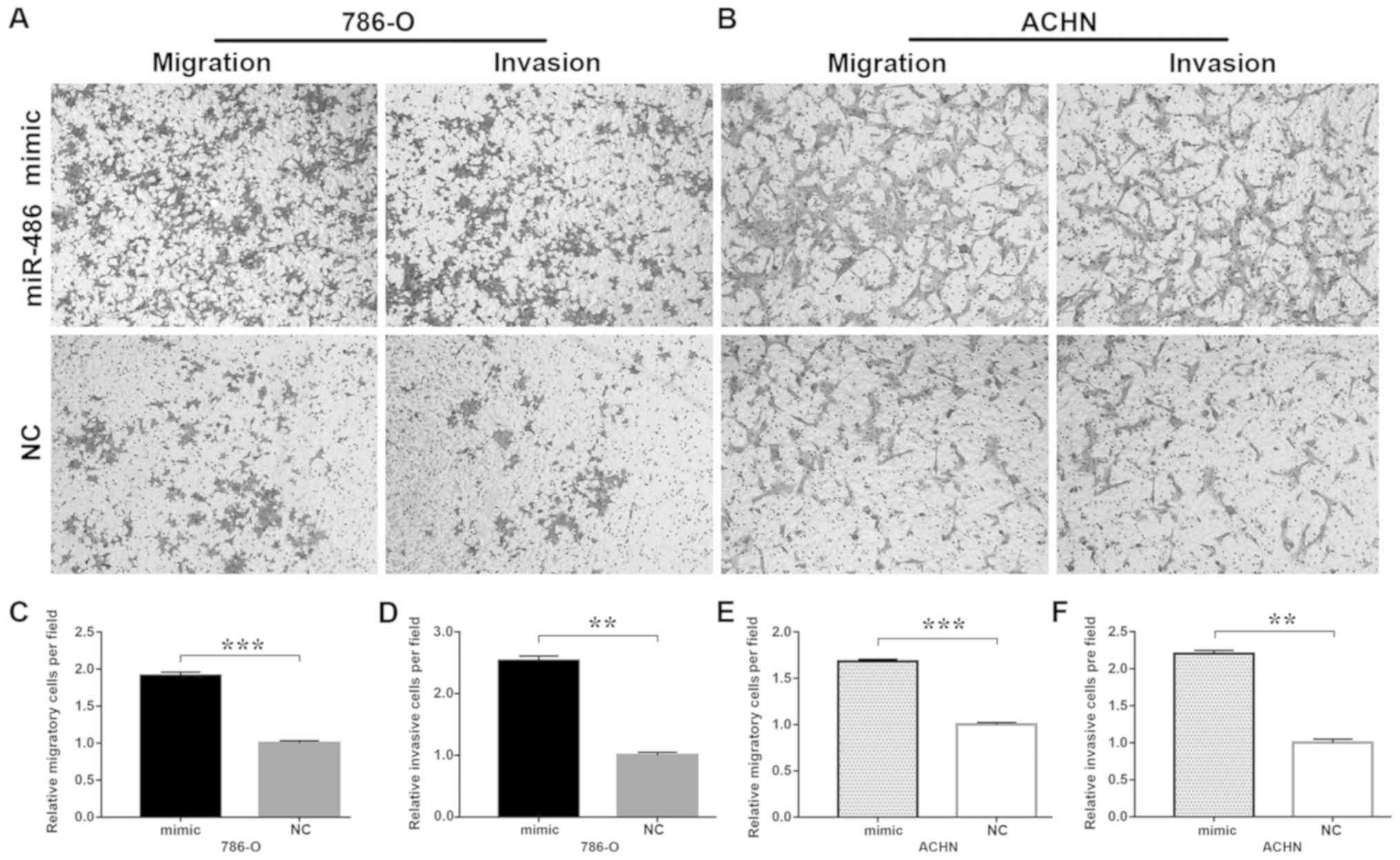
miR-486 promotes cell mobility
In vitro assessment of migration by wound
healing and Transwell migration assays revealed that ectopic
expression of miR-486 significantly increased migration of the
786-O and ACHN cells compared with that noted in the corresponding
controls (Figs. 2 and 3). Overexpression of miR-486 resulted in
increased invasiveness of 786-O (P<0.01) and ACHN (P<0.01)
cells when compared with the NC group. These results indicated that
miR-486 promotes cell mobility.
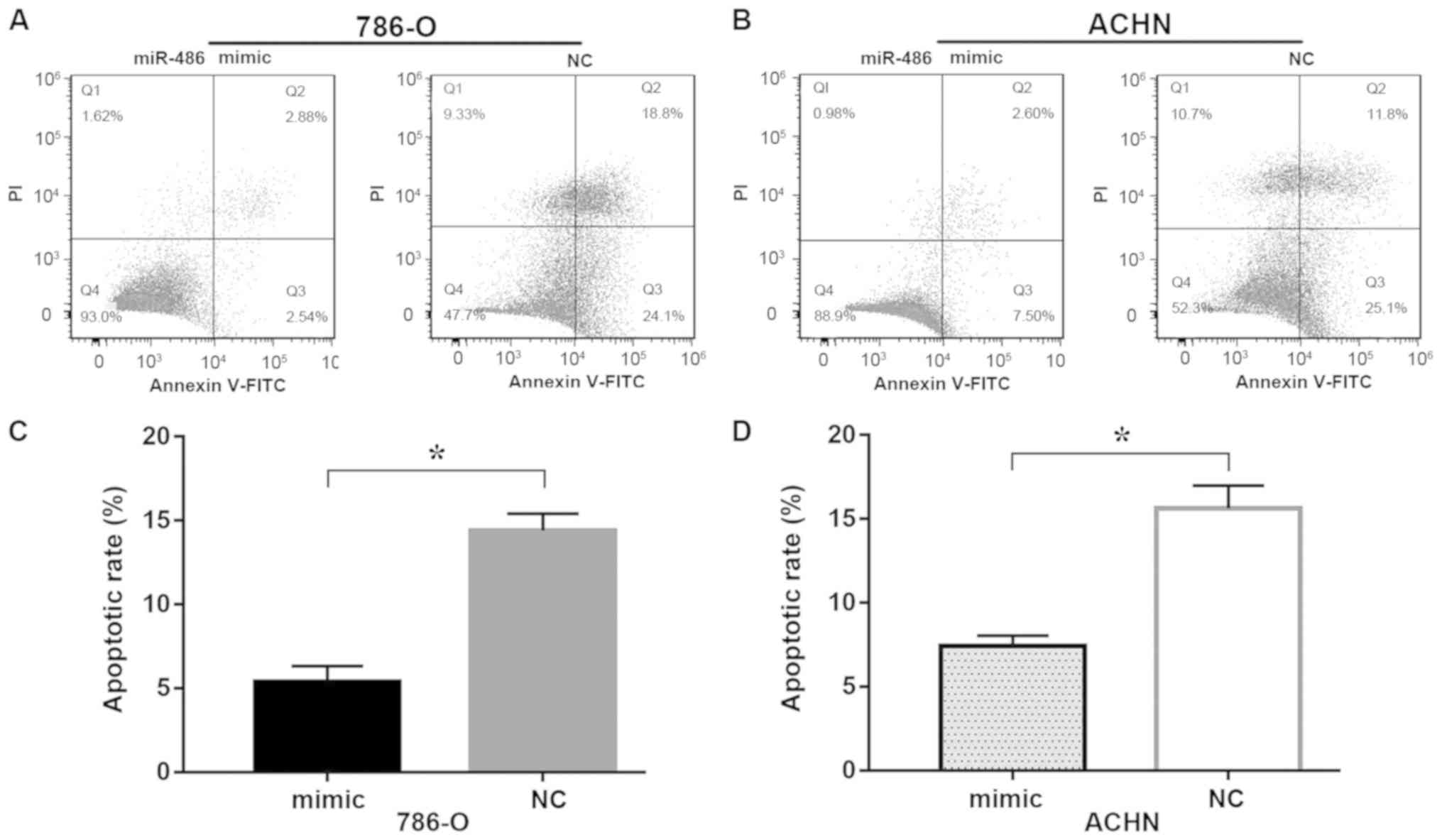
miR-486 inhibits cell apoptosis
Flow cytometry was used to detect apoptosis in
vitro. In the present study, early apoptosis (the Q3 region)
was regarded as an indicator of apoptosis and was used to calculate
the apoptotic rate. As shown in Fig.
4, the apoptotic rate of 786-O (Fig. 4C) cells in the group transfected
with miR-486 mimic was significantly decreased compared with the NC
group (5.377±0.950 vs. 14.406±1.000%; P<0.05), as was the
apoptotic rate of ACHN (Fig. 4D)
cells in the group transfected with miR-486 mimic compared with the
NC (7.403±0.637 vs. 15.633±1.365%; P<0.05). Thus, the results
indicated that overexpression of miR-486 decreased the apoptosis
rate in 786-O and ACHN cells.
Discussion
Renal cell carcinoma (RCC) is a common malignant
tumor and accounts for 3–5% of all malignant diseases (19). Due to its late diagnosis and
insensitivity to radiotherapy and chemotherapy, the prognosis of
RCC is extremely poor (4).
Although studies have identified many of the genetic and epigenetic
changes associated with tumorigenesis of RCC, the complete
understanding of the molecular mechanisms of RCC pathogenesis is
still unclear. Playing an important role in tumorigenesis, miRNAs
suppress gene expression by binding to the 3′-UTR of target mRNAs
(20) and as a result can modulate
various cellular processes including cell viability (21), proliferation (10), migration (12), invasion (22), apoptosis (23), epithelial-mesenchymal transition
(EMT) (24) and self-renewal of
tumor stem cells (25).
Studies have demonstrated miR-486 as a driver of
various types of cancer. Zhang et al (16), found that miR-486-5p inhibited the
migration and invasion of colorectal cancer (CRC) cells by binding
to PIK3R1. As a tumor suppressor, miR-486 suppressed cell invasion
and EMT by targeting PIM in osteosarcoma (26) and mitigated prostate cancer
metastasis by targeting Snail and regulating EMT (27). However, there are few reports
concerning the oncogenic functions of miR-486. One such study by
Gao et al (15), reported
the oncogenic ability of miR-486-5p in the progression of non-small
cell lung cancer (NSCLC) by targeting the tumor-suppressor PTEN. In
contrast, Shao et al (28)
reported that miR-486-5p functions as a tumor suppressor in NSCLC
by directly targeting the oncogene CDK1. This disparity in findings
warrants further investigation on the function of miR-486 in
NSCLC.
In the present study, it was demonstrated that
miR-486 is upregulated in RCC tissues and cell lines by RT-qPCR,
which is in line with the findings of Goto et al (29). Moreover, ectopic expression of
miR-486 promoted proliferation, migration, and invasion and
suppressed apoptosis of the ACHN and 786-O cells.
Accumulating evidence suggests that miR-486 can be
an ideal prognostic and diagnostic biomarker in various types of
cancer. Jiang et al (30)
revealed that low miR-486-3p and miR-103a-3p expression was
respectively correlated with shorter overall survival (OS) of
patients with muscle-invasive bladder cancer (MIBC). Similarly, Ren
et al (31) revealed that
low or unchanged miR-486-5p predicted poor prognosis in patients
with esophageal squamous cell carcinoma and gastric cancer, which
was contrary to our finding that elevated miR-486 indicated a
remarkably shorter OS in RCC patients, which also suggested its
potential as a prognostic marker for RCC. Li et al (32), found that serum miR-486-5p serves
as a diagnostic biomarker for cervical cancer with an AUC=0.90. It
was reported that compared to traditional urine cytology, the
four-miRNA signature (miR-422a-3p, miR-486-3p, miR-103a-3p and
miR-27a-3p) displayed higher accuracy in predicting MIBC (30). Furthermore, miR-486 proved to be a
potential blood-based biomarker for early diagnosis and recurrence
of NSCLC (33).
However, there are several limitations to our study.
Firstly, to better verify our conclusion, we must complete
functional experiments to determine how an miR-486 inhibitor
regulates RCC cell proliferation and migration. Secondly, in the
present study, miR-486 expression was found to be associated with
patient survival; however, miR-486 expression was not associated
with tumor stage. This may be due to the lack of tumor specimens of
T3 and T4, especially T4. Further investigation of the relationship
between miR-486 and renal cancer tumor staging using a higher
number of T3 and T4 tumor samples will be carried out.
In conclusion, it was demonstrated that miR-486 was
upregulated in RCC tissues and cell lines, and overexpression of
miR-486 was associated with a poor prognosis of RCC patients. In
the present study, miR-486 acted as an oncogene and promoted
proliferation, migration, invasion and suppressed apoptosis. With a
better understanding of its function, miR-486 may emerge as a
potential prognostic marker and therapeutic target in the treatment
of RCC.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed datasets generated during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
ChS and CaS carried out the molecular genetic
studies and drafted the manuscript. JS and YZ participated in the
design of the study. GY and GL performed the statistical analysis.
CX and XD participated in the research design and coordination, and
helped to draft the manuscript. All authors read and approved the
final manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
All procedures performed in studies involving human
participants were in accordance with the ethical standards of the
institutional and/or national research committee and with the 1964
Helsinki declaration and its later amendments or comparable ethical
standards. All participants provided written informed consent, and
the study was approved by the Ethics Committee of the Affiliated
Hospital of Jiangnan University (Wuxi, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Ljungberg B, Bensalah K, Canfield S,
Dabestani S, Hofmann F, Hora M, Kuczyk MA, Lam T, Marconi L,
Merseburger AS, et al: EAU guidelines on renal cell carcinoma: 2014
update. Eur Urol. 67:913–924. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Patard JJ, Leray E, Rodriguez A,
Rioux-Leclercq N, Guillé F and Lobel B: Correlation between symptom
graduation, tumor characteristics and survival in renal cell
carcinoma. Eur Urol. 44:226–232. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang M, Gao H, Qu H, Li J, Liu K and Han
Z: MiR-137 suppresses tumor growth and metastasis in clear cell
renal cell carcinoma. Pharmacol Rep. 70:963–971. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Keegan KA, Schupp CW, Chamie K, Hellenthal
NJ, Evans CP and Koppie TM: Histopathology of surgically treated
renal cell carcinoma: Survival differences by subtype and stage. J
Urol. 188:391–397. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Powles T, Staehler M, Ljungberg B,
Bensalah K, Canfield SE, Dabestani S, Giles R, Hofmann F, Hora M,
Kuczyk MA, et al: Updated EAU guidelines for clear cell renal
cancer patients who fail VEGF targeted therapy. Eur Urol. 69:4–6.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Berezikov E, Guryev V, van de Belt J,
Wienholds E, Plasterk RH and Cuppen E: Phylogenetic shadowing and
computational identification of human microRNA genes. Cell.
120:21–24. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Zamore PD and Haley B: Ribo-gnome: The big
world of small RNAs. Science. 309:1519–1524. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ying SY, Chang DC and Lin SL: The microRNA
(miRNA): Overview of the RNA genes that modulate gene function. Mol
Biotechnol. 38:257–268. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Pfeffer SR, Yang CH and Pfeffer LM: The
Role of miR-21 in cancer. Drug Dev Res. 76:270–277. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Quan J, Jin L, Pan X, He T and Lai Y, Chen
P, Lin C, Yang S, Zeng H and Lai Y: Oncogenic miR-23a-5p is
associated with cellular function in RCC. Mol Med Rep.
16:2309–2317. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pan X, Quan J, Li Z, Zhao L, Zhou L,
Jinling X, Weijie X, Guan X, Li H, Yang S, et al: miR-566 functions
as an oncogene and a potential biomarker for prognosis in renal
cell carcinoma. Biomed Pharmacother. 102:718–727. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Quan J, Li Y, Pan X, Lai Y, He T, Lin C,
Zhou L, Zhao L, Sun S, Ding Y, et al: Oncogenic miR-425-5p is
associated with cellular migration, proliferation and apoptosis in
renal cell carcinoma. Oncol Lett. 16:2175–2184. 2018.PubMed/NCBI
|
|
13
|
Fu H, Tie Y, Xu C, Zhang Z, Zhu J, Shi Y,
Jiang H, Sun Z and Zheng X: Identification of human fetal liver
miRNAs by a novel method. FEBS Lett. 579:3849–3854. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lang B and Zhao S: miR-486 functions as a
tumor suppressor in esophageal cancer by targeting CDK4/BCAS2.
Oncol Rep. 39:71–80. 2018.PubMed/NCBI
|
|
15
|
Gao ZJ, Yuan WD, Yuan JQ, Yuan K and Wang
Y: miR-486-5p functions as an oncogene by targeting PTEN in
non-small cell lung cancer. Pathol Res Pract. 214:700–705. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhang Y, Fu J, Zhang Z and Qin H:
miR-486-5p regulates the migration and invasion of colorectal
cancer cells through targeting PIK3R1. Oncol Lett. 15:7243–7248.
2018.PubMed/NCBI
|
|
17
|
He M, Wang G, Jiang L, Qiu C, Li B, Wang J
and Fu Y: miR-486 suppresses the development of osteosarcoma by
regulating PKC-δ pathway. Int J Oncol. 50:1590–1600. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Behm-Ansmant I, Rehwinkel J and Izaurralde
E: MicroRNAs silence gene expression by repressing protein
expression and/or by promoting mRNA decay. Cold Spring Harb Symp
Quant Biol. 71:523–530. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Liu F, Sang M, Meng L, Gu L, Liu S, Li J
and Geng C: miR-92b promotes autophagy and suppresses viability and
invasion in breast cancer by targeting EZH2. Int J Oncol.
53:1505–1515. 2018.PubMed/NCBI
|
|
22
|
Luo L, Xia L, Zha B, Zuo C, Deng D, Chen
M, Hu L, He Y, Dai F, Wu J, et al: miR-335-5p targeting ICAM-1
inhibits invasion and metastasis of thyroid cancer cells. Biomed
Pharmacother. 106:983–990. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang X, Yang Y and Feng Z: Suppression of
microRNA-495 alleviates high-glucose-induced retinal ganglion cell
apoptosis by regulating Notch/PTEN/Akt signaling. Biomed
Pharmacother. 106:923–929. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cao L, Wan Q, Li F and Tang CE: MiR-363
inhibits cisplatin chemoresistance of epithelial ovarian cancer by
regulating snail-induced epithelial-mesenchymal transition. BMB
Rep. 51:456–461. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Bitarte N, Bandres E, Boni V, Zarate R,
Rodriguez J, Gonzalez-Huarriz M, Lopez I, Javier Sola J, Alonso MM,
Fortes P and Garcia-Foncillas J: MicroRNA-451 is involved in the
self-renewal, tumorigenicity, and chemoresistance of colorectal
cancer stem cells. Stem Cells. 29:1661–1671. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Liu Y, Zhang J, Xing C, Wei S, Guo N and
Wang Y: miR-486 inhibited osteosarcoma cells invasion and
epithelial-mesenchymal transition by targeting PIM1. Cancer
Biomark. 23:269–277. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang X, Zhang T, Yang K, Zhang M and Wang
K: miR-486-5p suppresses prostate cancer metastasis by targeting
Snail and regulating epithelial-mesenchymal transition. OncoTargets
Ther. 9:6909–6914. 2016. View Article : Google Scholar
|
|
28
|
Shao Y, Shen YQ, Li YL, Liang C, Zhang BJ,
Lu SD, He YY, Wang P, Sun QL, Jin YX and Ma ZL: Direct repression
of the oncogene CDK4 by the tumor suppressor miR-486-5p in
non-small cell lung cancer. Oncotarget. 7:34011–34021. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Goto K, Oue N, Shinmei S, Sentani K,
Sakamoto N, Naito Y, Hayashi T, Teishima J, Matsubara A and Yasui
W: Expression of miR-486 is a potential prognostic factor after
nephrectomy in advanced renal cell carcinoma. Mol Clin Oncol.
1:235–240. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Jiang X, Du L, Duan W, Wang R, Yan K, Wang
L, Li J, Zheng G, Zhang X, Yang Y and Wang C: Serum microRNA
expression signatures as novel noninvasive biomarkers for
prediction and prognosis of muscle-invasive bladder cancer.
Oncotarget. 7:36733–36742. 2016.PubMed/NCBI
|
|
31
|
Ren C, Chen H, Han C, Fu D, Zhou L, Jin C,
Wang F, Wang D, Chen Y, Ma L, et al: miR-486-5p expression pattern
in esophageal squamous cell carcinoma, gastric cancer and its
prognostic value. Oncotarget. 7:15840–15853. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Li C, Zheng X, Li W, Bai F, Lyu J and Meng
QH: Serum miR-486-5p as a diagnostic marker in cervical cancer:
With investigation of potential mechanisms. BMC Cancer. 18:612018.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li W, Wang Y, Zhang Q, Tang L, Liu X, Dai
Y, Xiao L, Huang S, Chen L, Guo Z, et al: MicroRNA-486 as a
biomarker for early diagnosis and recurrence of non-small cell lung
cancer. PLoS One. 10:e01342202015. View Article : Google Scholar : PubMed/NCBI
|