Introduction
Pulmonary arterial hypertension (PAH) is a
multifactorial and devastating cardiopulmonary disorder
characterized by the progressive sustained increase in pulmonary
arterial pressure, which leads to right ventricular hypertrophy and
eventually heart failure (1–4).
Pulmonary vascular remodeling caused by excessive proliferation and
apoptosis resistance of vascular smooth muscle cells is a prominent
feature of PAH (5–7). The majority of existing medications
(endothelin antagonists, prostacyclins and phosphodiesterase type 5
inhibitors) alleviate PAH by mainly dilating partially occluded
vessels, rather than inhibiting proliferation and promoting the
apoptosis of the pulmonary artery smooth muscle cells (PASMCs)
(8,9). Thus, the mortality of PAH is high,
≤15% a year, as are the costs of clinical patient care (5,8,10).
Furthermore, numerous studies have validated that the
phosphoinositide 3-kinase (PI3K)/protein kinase B (AKT) and
extracellular signal-regulated kinase (ERK) pathways are involved
in the survival and apoptosis of PASMCs in PAH (11–13),
and are therefore considered therapeutic targets for the treatment
of PAH.
In recent years, traditional Chinese medicine has
received increasing attention due to its potential advantages,
including abundant natural resources, strong targeting potentials,
and reduced costs (14).
Formononetin (FMN) is an active isoflavone extracted from the
traditional Chinese herb Trifolium pratense L. (15,16),
which has been widely used to treat cardiovascular diseases
(17,18). FMN also exhibits strong antitumor
activity in human prostate cancer cells and nasopharyngeal
carcinoma cells (19,20). This novel compound has been
reported to inhibit proliferation and induce the apoptosis of tumor
cells by increasing the B-cell lymphoma 2 (Bcl-2)-associated X
protein (Bax)/Bcl-2 ratio and activating caspases (21–23).
In addition, the induction of FMN-mediated apoptosis and the
inhibition of proliferation are associated with the inactivation of
AKT and ERK signaling in various cell types (24,25);
however, the therapeutic effects of FMN on PAH and its possible
mechanisms remain unknown.
Based on these previous findings, we proposed that
FMN could attenuate PAH by inhibiting pulmonary vascular
remodeling. In the present study, we explored the protective
effects of FMN on the progression of PAH induced by monocrotaline
(MCT). Furthermore, the effects of FMN on the apoptosis and
proliferation of PASMCs, and underlying molecular mechanisms in
vivo were also investigated.
Materials and methods
Chemicals and reagents
FMN (purity >98.0%) was purchased from MedChem
Express, LLC, (New Jersey, USA). MCT, dimethyl sulfoxide (DMSO),
bovine serum albumin (BSA) and anti-α-smooth muscle actin (α-SMA)
antibody were purchased from Sigma-Aldrich; Merck KGaA (Darmstadt,
Germany). FMN was dissolved in DMSO and diluted with olive oil (45
mg/ml). MCT was dissolved in 1 M HCL neutralized with 1 M NaOH, and
diluted with normal saline. Then, the pH was adjusted to 7.2–7.4.
Anti-cleaved caspase-3, anti-GAPDH, anti-phosphorylated-AKT,
anti-AKT, anti-P-ERK, anti-ERK, anti-rabbit IgG horseradish
peroxidase (HRP)-conjugated and anti-mouse IgG HRP-conjugated
antibodies were obtained from Cell Signaling Technology, Inc.,
Danvers, MA, USA. Anti-Bax, anti-Bcl-2, and anti-proliferating cell
nuclear antigen (PCNA) antibodies were purchased from Abcam,
Cambridge, UK. An H&E assay kit was obtained from Beijing
Solarbio Science & Technology Co., Ltd., Beijing, China. BCA
Protein and Colorimetric TUNEL Apoptosis assay kits were purchased
from Beyotime Institute of Biotechnology.
Animals
Male Sprague-Dawley (7-weeks-old) rats weighing
230–250 g were obtained from the Experimental Animal Center of
Zhejiang. The experimental protocol was approved by the Ethics
Review of Animal Use Application of The Fifth Affiliated Hospital
of Wenzhou Medical University (Wenzhou, China) in accordance with
the National Institutes of Health Guidelines For the Care and Use
of Experimental Animals (26). All
rats were housed in an environmentally controlled room at 20–26°C,
50±5% humidity under a 12 h light/dark cycle, and had free access
to food and water. After 1 week of acclimation, 68 rats were
randomly divided into five groups: i) The control group (n=8) which
received normal saline; ii) the MCT group (n=15) received MCT at 60
mg/kg; iii) the FMN-low group (n=15) received MCT + FMN at 10
mg/kg/day; iv) the FMN-medium group (n=15) received MCT + FMN at 30
mg/kg/day; and v) the FMN-high group (n=15) received MCT + FMN at
60 mg/kg/day. PAH was induced as described previously (27). MCT was administered from at day 0;
after 2 weeks, the rats in each FMN group were intraperitoneally
administered with different doses of FMN and maintained daily for 2
weeks.
Humane endpoints were set according to the
Organisation for Economic Co-operation and Development Guidance
document on the Recognition, Assessment, and Use of Clinical Signs
as Humane End points for Experimental Animals Used in Safety
Evaluation (https://www.aaalac.org/accreditation/RefResources/RR_HumaneEndpoints.pdf).
Specifically, as 1 rat demonstrated a reduction in body
temperature, dyspnea, cyanosis, appeared hunched with decreased
activity and no response to touch, and abrupt weight loss with a
reduction in body weight of >10% per day for 2 days, the rat was
euthanized. On the 22nd day of the experiment, a rat in the MCT
group was humanely sacrificed. On day 24, a rat of the FMN-Low
group was euthanized. On day 25, two rats of the MCT group and one
rat of the FMN-medium group were sacrificed. On day 26, an FMN-low
group rat was sacrificed. On day 27, one rat in the FMN-medium and
the FMN-High groups were euthanized. All of the animals that
survived were weighed weekly for 4 weeks, and were used for
hemodynamic and histological analysis. The animals treated with MCT
that succumbed during the experimental procedure due to PAH or
heart failure were used only for survival analysis.
Hemodynamic analysis
After weighing, the rats were anesthetized with
sodium pentobarbital (30 mg/kg) intraperitoneally. Then, the right
ventricle (RV) systolic pressure (RVSP) was measured as previously
reported (27). Once the
hemodynamic data had been collected, animals were sacrificed via an
intraperitoneal injection of 150 mg/kg sodium pentobarbital. The RV
was separated and weighed. The left lung tissues were excised for
histological and immunohistochemical analyses, while the right lung
tissues were stored at −80°C for western blot analysis.
Evaluation of right heart
hypertrophy
Following hemodynamic analysis, the hearts
were separated, dissected into the RV, left ventricle (LV) and
septum (S), and weighed, respectively. The weight ratio of RV to LV
plus S, and the weight ratio of RV to body weight (BW) were
calculated as indexes to reflect RV hypertrophy.
Assessment of pulmonary vascular
remodeling
The isolated left lungs were fixed in 4%
paraformaldehyde at room temperature for 48 h, embedded in paraffin
and sectioned at 4-µm thickness. H&E staining was performed on
the lung tissue sections according to the manufacturer's protocols,
the structural remodeling of pulmonary arteries was analyzed via
microscopy. In pulmonary arteries with diameters from 50–150 µm,
the wall thickness (WT) was measured under a light microscope
(Nikon Corporation) at a magnification of ×400. For each artery,
the degree of WT was calculated as follows: The ratio of vascular
WT%=100% × WT/outer diameter and the ratio of the vascular wall
area (WA%)=100% × transection area of the wall/cross-sectional
area. A random selection of five fields of six sections were
selected for analysis per each group.
Immunohistochemistry
Lung tissue sections (4-µm) were dewaxed, rehydrated
and then washed with PBS (pH 7.2–7.4). Following antigen retrieval
at 100°C and blocking with 5% BSA at room temperature for 1 h, the
sections were incubated with anti-α-SMA antibody (1:500, ab2547) or
anti-PCNA antibody (1:200, ab18197) overnight at 4°C, followed by
the anti-rabbit (1:50, 7074S) or anti-mouse (1:50, 7076S)
horseradish peroxidase (HRP)-conjugated secondary antibody.
Following washing with PBS, the sections were visualized with
3′3′-diaminobenzidene (DAB) at room temperature for 5 min and
counterstained with hematoxylin at room temperature for 2 min.
Apoptosis was analyzed via a terminal
deoxynucleotidyl-transferase-mediated dUTP nick end (TUNEL) assay
in accordance with the manufacturer's instructions. DAB was used as
the chromogen and counterstained with hematoxylin at room
temperature for 2 min. Subsequently, the stained sections were
observed with a light microscope (magnification, ×400; Nikon
Corporation, Tokyo, Japan). The integrated optical density (IOD) of
α-SMA in the pulmonary arteriole was d using Image-Pro Plus
software (Media Cybernetics, Inc., Rockville, MD, USA), and then
the ratio of the IOD to the area of the arteriole was calculated to
reflect the expression of α-SMA. In addition, the percentages of
PCNA-positive and TUNEL-positive PASMCs were evaluated in each
group.
Western blotting
Proteins were extracted from the lung tissues using
lysis buffer containing protease (Beyotime Institute of
Biotechnology, Haimen, China) and phosphatase inhibitors (Cell
Signaling Technology, Inc.) under homogenization. The protein
concentration was measured with the BCA Protein Assay kit. An
equivalent amount (50 µg) of protein lysate was separated with
10–12% SDS-PAGE and transferred to a polyvinylidene difluoride
membrane. Subsequently, the membranes were blocked with 5% BSA at
room temperature for 2 h and incubated with anti-PCNA antibody
(1:1,000, cat. no. ab18197), anti-Bax antibody (1:1,000, cat. no.
ab32503), anti-Bcl-2 antibody (1:1,000, cat. no. ab59348),
anti-Cleaved caspase-3 antibody (1:1,000, cat. no. 9661S),
anti-Caspase-3 antibody (1:1,000, cat. no. 9662S), anti-P-ERK
antibody (1:1,000, cat. no. 9101S), anti- ERK antibody (1:1,000,
cat. no. 9102S), anti-P-AKT antibody (1:1,000, cat. no. 4060S),
anti-AKT antibody (1:1,000, cat. no. 9272S), overnight at 4°C.
Then, the membranes were washed with TBST and incubated with
anti-rabbit IgG HRP-conjugated antibody (1:1,000, cat. no. 7074S)
or anti-mouse IgG HRP-conjugated antibody (1:1,000, cat. no. 7076S)
at room temperature for 1 h. The protein bands were visualized
using enhanced chemiluminescence (EMD Millipore, Billerica, MA,
USA). The content of protein was analysis by densitometric
quantification using AlphaView software (ProteinSimple, San Jose,
CA, USA). GAPDH (1:1,000, cat. no. 5174S) was used as an internal
control.
Statistical analysis
The data were presented as the mean ± standard error
of the mean, and analysis was performed with GraphPad Prism 5
software (GraphPad Software, Inc., La Jolla, CA, USA). Differences
between groups were analyzed by one-way analysis of variance and a
Student-Newman-Keuls post-hoc test for multiple comparisons. Kaplan
Meier analysis was used to analyze the survival rate of rats.
P<0.05 was considered to indicate a statistically significant
difference.
Results
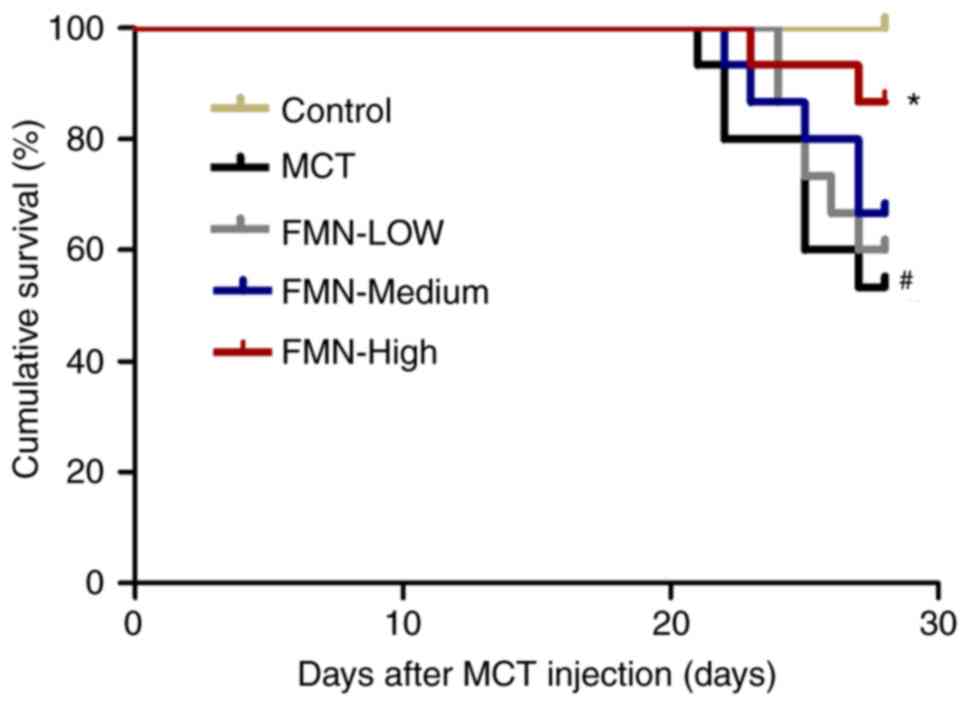
FMN improves the survival rate of rats
with PAH
No rats succumbed after 28 days in the control
group; however, ~50% rats in the MCT group succumbed. On the
contrary, treatment with FMN (60 mg/kg) increased the survival of
rats with PAH (P<0.05; Fig.
1).
FMN alleviates MCT-induced hemodynamic
alterations and RV hypertrophy
MCT induced a significant increase in RVSP compared
with the control (P<0.01; Fig.
2A). Additionally, there were no significant differences in
RVSP following low- and medium-dose FMN treatments (10 and 30
mg/kg) compared with the MCT group; however, high-dose FMN (60
mg/kg) administration significantly decreased the RVSP (P<0.01).
The right ventricular hypertrophy index (RVHI) and RV/BW ratio were
calculated to evaluate the extent of RV hypertrophy. MCT
significantly increased the RVHI compared with the control group,
but was significantly reduced following treatment with 60 mg/kg FMN
(P<0.01; Fig. 2B). In addition,
MCT-treated rats exhibited a significantly increased RV/BW ratio
than the control group. FMN treatment reduced this ratio in a
dose-dependent manner (P<0.05; Fig.
2C).
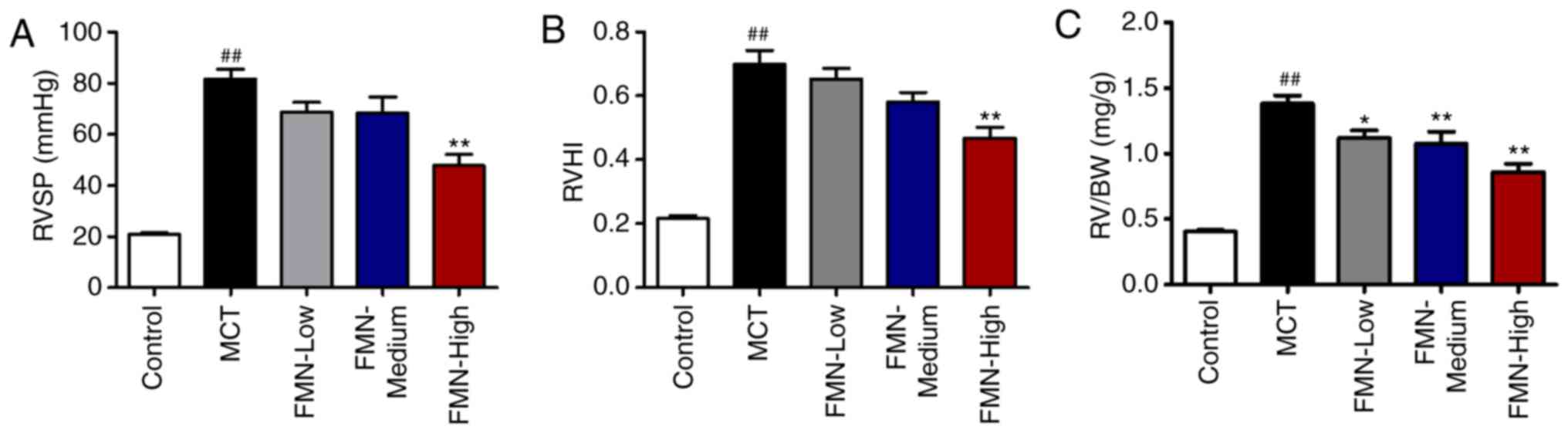 | Figure 2.Effects of FMN on hemodynamic
alterations and right ventricular hypertrophy. Effect of FMN on (A)
RVSP, (B) RVHI and (C) the RV/BW ratio. ##P<0.01 vs.
the control group. *P<0.05, **P<0.01 vs. the MCT group.
n=8–13 per group. FMN, formononetin; MCT, monocrotaline; FMN-Low,
10 mg/kg FMN; FMN-Medium, 30 mg/kg FMN; FMN-High, 60 mg/kg FMN;
RVSP, right ventricular systolic pressure; RVHI, right ventricular
hypertrophy index; RV/BW, right ventricle/body weight ratio. |
FMN suppresses MCT-induced pulmonary
vascular remodeling
To evaluate pulmonary vascular remodeling, we
measured the WT in small pulmonary arteries (external diameters of
50–150 µm) by H&E staining. After MCT treatment, the indices of
the WT of pulmonary arterioles and the WA were significantly
increased compared with the control (P<0.01). FMN (30 and 60
mg/kg) treatment significantly inhibited these pathological
alternations in the lungs compared with the MCT group (P<0.05;
Fig. 3).
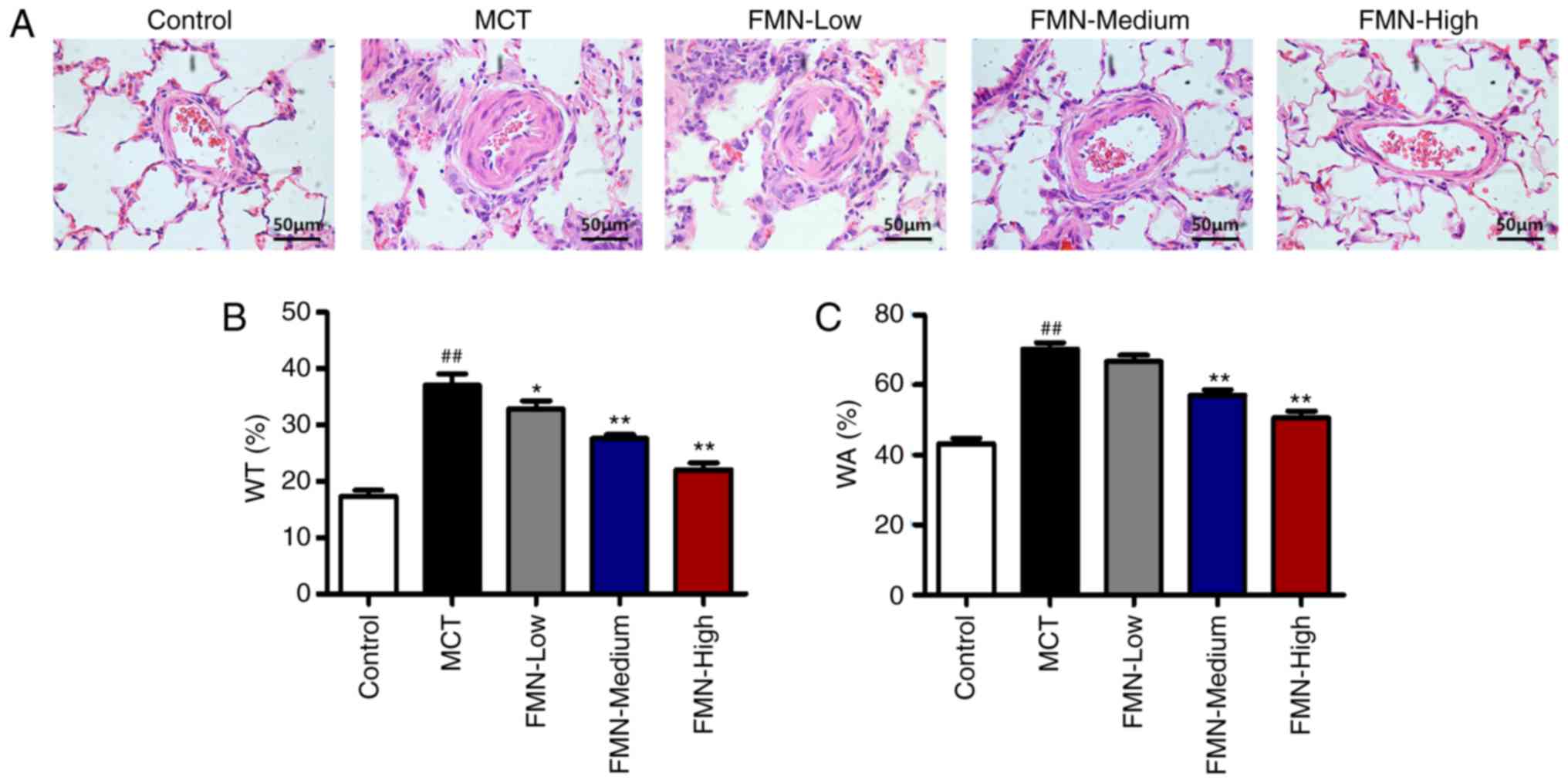 | Figure 3.Effects of FMN on pulmonary vascular
remodeling. (A) Representative image of pulmonary vascular
remodeling as detected by H&E staining in the lung tissues of
rats. Magnification, ×400, scale bar, 50 µm. Quantification
analysis of (B) the WT and (C) WA of the pulmonary arterioles.
##P<0.01 vs. the control group. *P<0.05,
**P<0.01 vs. MCT group. n=6 per group. FMN, formononetin; MCT,
monocrotaline; FMN-Low, 10 mg/kg FMN; FMN-Medium, 30 mg/kg FMN;
FMN-High, 60 mg/kg FMN; WT, vascular wall thickness; WA, vascular
wall area. |
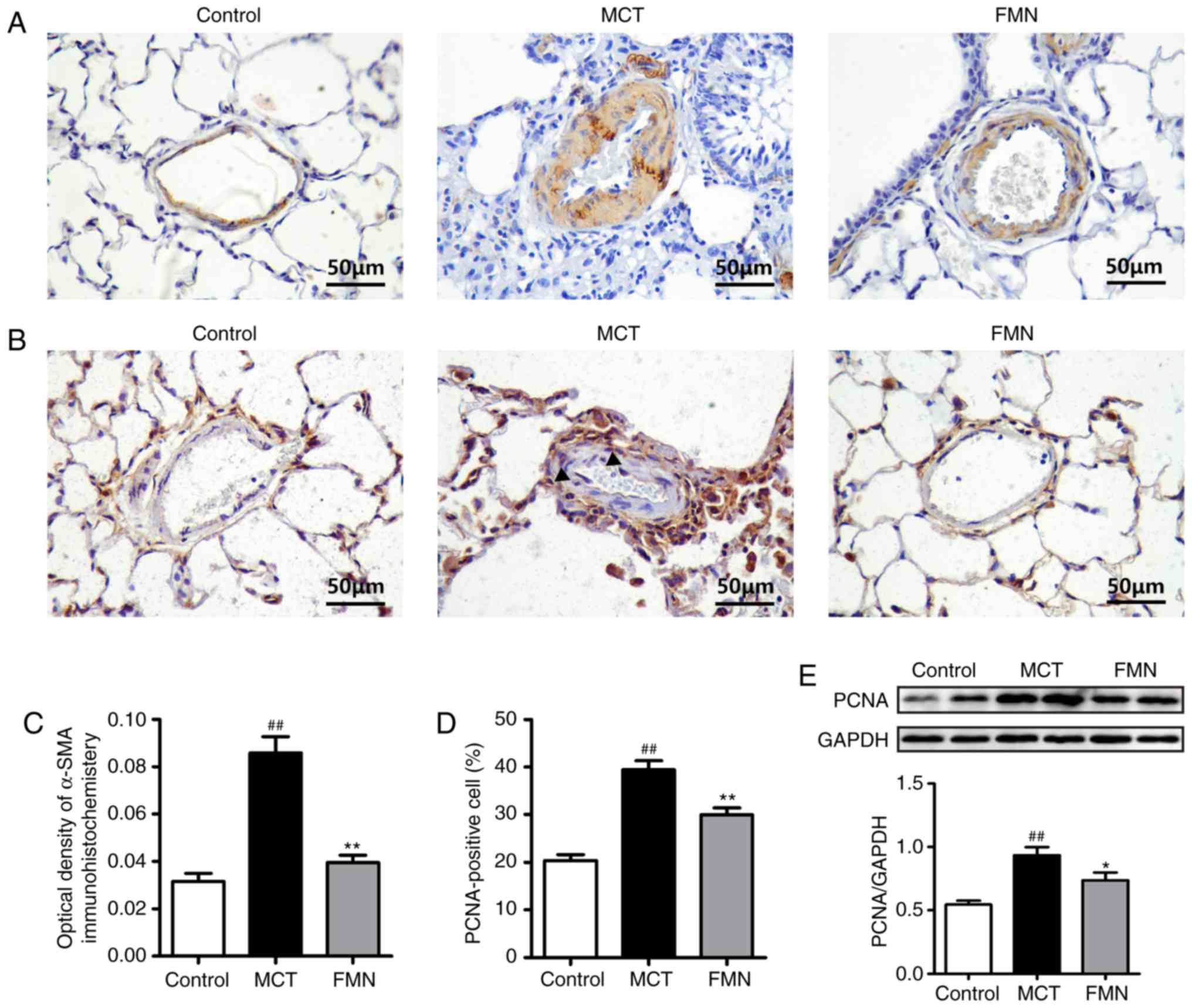
FMN attenuates the excessive
proliferation of PASMCs induced by MCT
Immunohistological staining was used to examine the
expression of α-SMA in rat lung tissues. The results revealed that
α-SMA was significantly upregulated in MCT-treated rats relative to
the control group, but was inhibited following high-dose FMN
treatment (P<0.01; Fig. 4A and
C). The proliferative ability of PASMCs was analyzed by
immunostaining and western blot analysis for PCNA. There was a
significant increase in PCNA-positive cells following MCT
treatment; however, high-dose FMN treatment FMN significantly
abrogated this effect (P<0.01; Fig.
4B and D). In addition, the results of western blot analysis
for PCNA were consistent with the observations from
immunobiological staining (P<0.05; Fig. 4B and E).
FMN inhibits the apoptotic resistance
of PASMCs induced by MCT
To evaluate the effects of FMN on apoptosis in rats
with PAH, PASMC apoptosis was examined by a TUNEL assay. MCT
injection induced a significant decrease in TUNEL-positive cells
compared with the control; however, this was reduced following
treatment with FMN (P<0.01; Fig. 5A
and B). Then, we detected the expression of several
apoptosis-associated markers in lung tissues. The expression of Bax
and cleaved caspase-3 in lung tissues were significantly
downregulated, while Bcl-2 was upregulated in the MCT group
compared with the control. High-dose FMN treatment attenuated
decreases in Bax and cleaved caspase-3 expression, and the increase
in Bcl-2 expression induced by MCT (P<0.05; Fig. 5C-E, G and H). In addition, the
relative Bax/Bcl-2 ratio was significantly decreased by MCT
compared with the control, but increased following high-dose FMN
treatment (P<0.05; Fig.
5F).
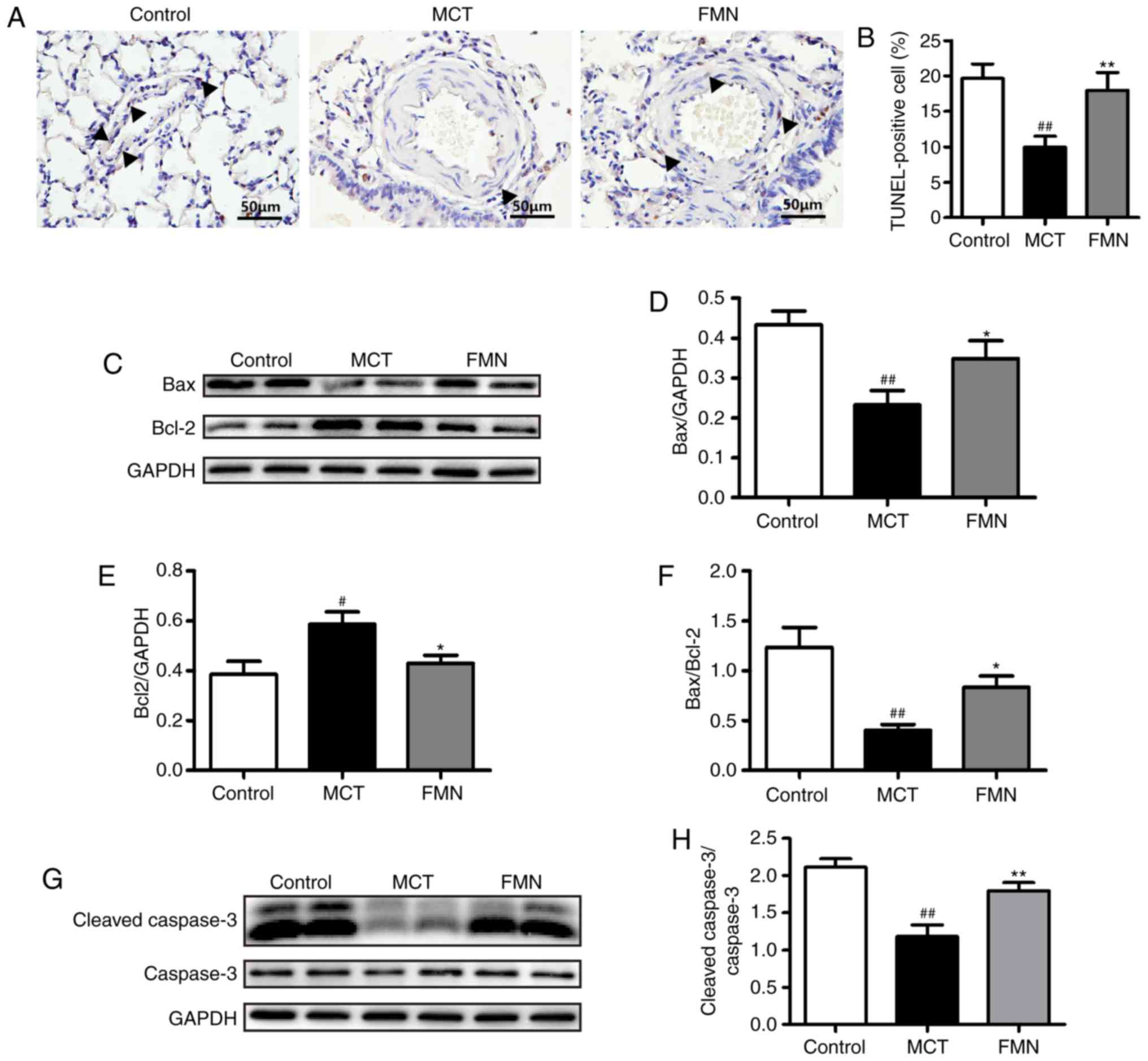 | Figure 5.Effects of FMN on apoptosis of
PASMCs. (A) Representative images of serial lung sections were
analyzed by a TUNEL assay. Magnification, ×400, scale bars, 50 µm.
The black arrows indicate positive cells. (B) Percentage of
TUNEL-positive cells. The expression of (C and D) Bax, (C and E)
Bcl-2 and (G and H) cleaved caspase-3 in lung tissues was analyzed
by western blotting. (F) The relative expression levels of Bax and
Bcl-2 were evaluated. #P<0.05, ##P<0.01
vs. the control group, *P<0.05, **P<0.01 vs. the MCT group.
n=6. per group. Bcl-2, B-cell lymphoma 2; Bcl-2-associated X
protein; FMN, 60 mg/kg formononetin; MCT, monocrotaline; TUNEL,
terminal deoxynucleotidyl-transferase-mediated dUTP nick end. |
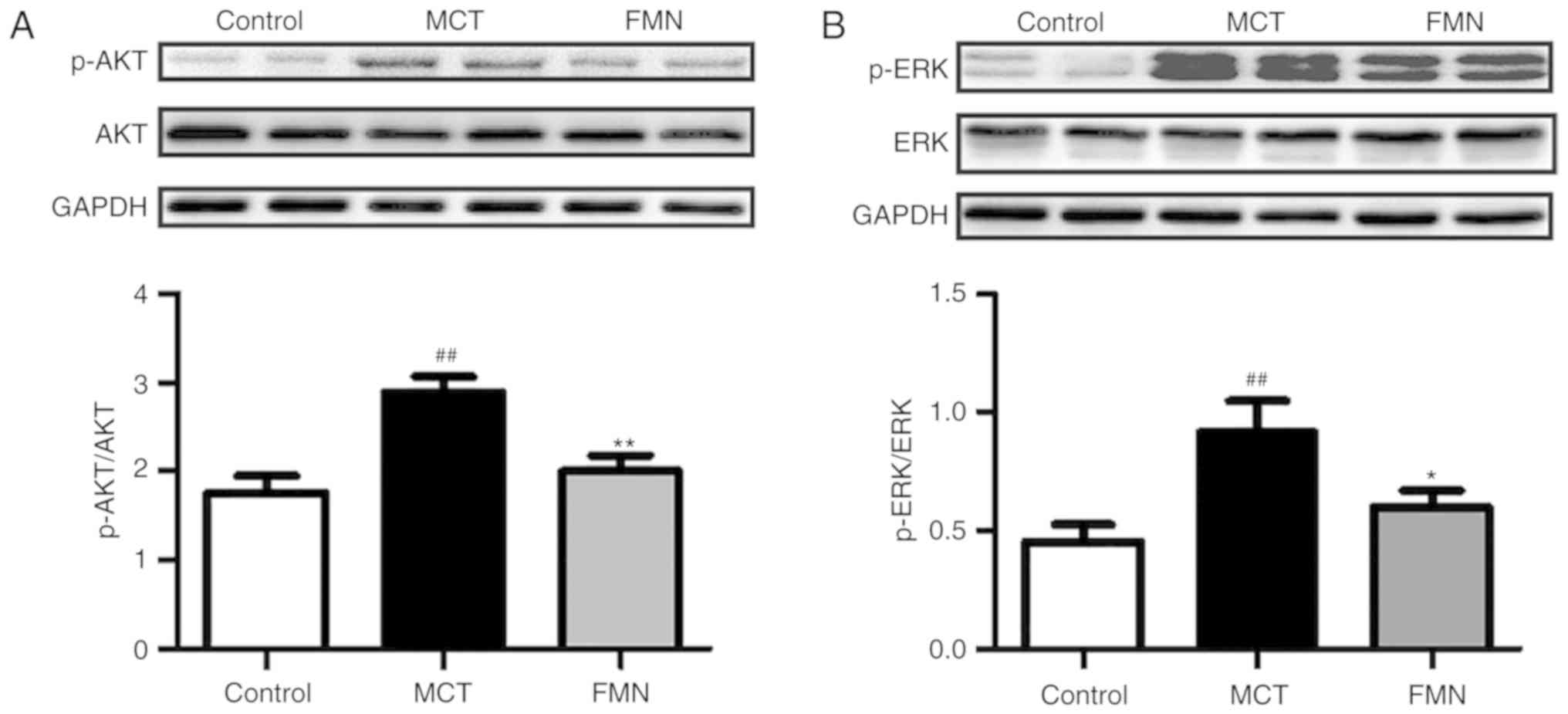
FMN inhibits the activation of AKT and
ERK induced by MCT
To further explore the effects of FMN on the
regulation of the PI3K/AKT signal pathway, we examined the
expression of AKT, which serves a crucial role in the PI3K/AKT
signaling pathway. It was demonstrated that MCT significantly
increased the levels of P-AKT phosphorylation of AKT in lung
tissues compared with the control (P<0.01; Fig. 6A). The increased phosphorylation of
AKT was significantly reduced following high-dose treatment with
FMN (P<0.01). In addition, we determined the expression of ERK,
which also serves a pivotal role in the pathology of hypoxia and
MCT-induced PAH (28,29). The results of the present study
revealed that the expression of P-ERK was significantly promoted in
the MCT group compared with the control; P-ERK was significantly
downregulated following high-dose FMN treatment (P<0.05;
Fig. 6B).
Discussion
PAH is characterized by pulmonary vascular
remodeling, leading to the narrowing or occlusion of pulmonary
vessels, resulting in elevated pulmonary vascular resistance. It is
well reported that pulmonary vascular remodeling is primarily due
to the uncontrolled growth and apoptosis resistance of PASMCs
(5–7). Therefore, effective therapeutic
strategies for treating pulmonary vascular remodeling should be
developed. FMN, a major component of isoflavones, exerts a variety
of pharmacological effects, which have been associated with the
prevention of cardiovascular diseases (17,18).
In addition, accumulating evidence suggests that FMN inhibits the
proliferation and promotes the apoptosis of tumor cells to exert an
antitumor effect. Data from Liang et al (30) revealed that treatment with 50 mg/kg
FMN mediated neuroprotection against cerebral ischemia/reperfusion
in rats. For improved efficacy, we decided to increase the dose to
60 mg/kg; following preliminary experiments, we reported that 60
mg/kg FMN treatment could effectively improve PAH in rats.
Therefore, 60 mg/kg was selected as the treatment dose in the
present study. Furthermore, we employed two additional treatment
groups, 10 and 30 mg/kg FMN, to observe whether the effects of FMN
on PAH in rats occur in a dose-dependent manner. The results of the
present study demonstrated that FMN exerted protective effects on
MCT-induced PAH in rats and participated in the regulation of
pulmonary vascular remodeling, and PASMC proliferation and
apoptosis. Additionally, it was proposed that inhibiting PI3K/AKT
and ERK pathways may be involved in mediating the effects of FMN on
MCT-induced PAH.
Previous studies found that rats developed severe
PAH with high mortality after treatment with MCT (14,31).
Furthermore, providing that abnormal hemodynamic changes and right
ventricular hypertrophy are representative symptoms of PAH
(32–35), long-term survival, RVSP and right
ventricular hypertrophy in rats induced by MCT were examined in the
present study. Our findings revealed that RVSP and RVHI in the MCT
group were significantly elevated, and were associated with a high
mortality rate. This indicated that the rat model of MCT-induced
PAH was successfully established. Furthermore, high-dose FMN
treatment significantly improved these adverse phenomena,
suggesting the protective effects of PAH.
Several studies reported that the progressive
thickening of the pulmonary vascular wall contributes to the
development of PAH (36,37). Additionally, FMN was observed to
normalize the pulmonary vascular morphology following the induction
of PAH via H&E staining of rat lung tissues in the present
study. FMN treatment decreased WT and WA in a dose-dependent
manner. Collectively, these findings indicate that FMN inhibited
pulmonary vascular remodeling as a protective mechanism against
PAH, at least in the rat model of MCT-induced PAH. To further
elucidate the possible mechanisms underlying the protective effects
on pulmonary vascular remodeling, we selected the high-dose
treatment (60 mg/kg) for subsequent analyses.
FMN has been reported to exert anticancer effects by
inhibiting the proliferation and promoting the apoptosis of tumor
cells, suggesting that FMN may, by regulating the proliferation and
apoptosis of PASMCs, exert protective effects against PAH. The key
pathological alteration responsible for increased pulmonary
vascular resistance is pulmonary arteriole remodeling, which is
primarily attributed to the excessive proliferation of PASMCs
(38). In the present study, we
detected the expression of α-SMA (as a marker of PASMCs) in rat
lung tissues. The results revealed that the MCT-induced expression
of α-SMA was suppressed by FMN. Immunostaining and western blot
analyses of PCNA expression also suggested that FMN reduced the
proliferation of PASMCs induced by MCT. In addition, studies in
related fields have demonstrated that the pro-apoptotic factor Bax
and activated caspase-3 execute the apoptotic program, while Bcl-2,
an inhibitor of apoptosis, suppresses the activation of Bax,
thereby inhibiting apoptosis (25,39).
Under normal circumstances, there is a balance between Bax and
Bcl-2, which is dysregulated when pulmonary artery remodeling
occurs (40). Multiple studies
into the effects of FMN on tumors have revealed that the expression
of Bax and cleaved caspase-3 was downregulated, while that of Bcl-2
was increased (20–23). As we expected, TUNEL staining and
western blot analysis of apoptosis-related factors suggested that
FMN reversed the suppression of apoptosis induced by MCT. The
findings of the present study indicated that FMN ameliorated
pulmonary vascular remodeling by regulating the proliferation and
apoptosis of PASMCs.
Like many cardiovascular diseases, the development
of PAH is closely associated with the aberrant transduction of the
PI3K/AKT and ERK signaling pathways (10). Activation of the PI3K/AKT signaling
pathway contributes to pulmonary vascular medial thickening, and
the proliferation and apoptosis of PASMCs under hypoxic conditions
or in rats with MCT-induced PAH (11). AKT serves a major role in the
PI3K/AKT signaling pathway, and its activation favors cell survival
by regulating apoptotic proteins, including caspases and the Bcl-2
family of proteins (41). ERK is a
major factor of the MAPK family, and its activity is considerably
increased in various animal models of PAH (37). In addition, decreased ERK
activation leads to caspase-3-dependent apoptosis (42). It has been reported that FMN
treatment leads to the significant inactivation of AKT and ERK,
followed by the upregulation of Bax and downregulation of Bcl-2,
which eventually increases the expression of caspase-3 (25). Consistent with previous reports,
our results indicate that FMN induced PASMC apoptosis and inhibits
its proliferation, possibly by inactivating the AKT and ERK
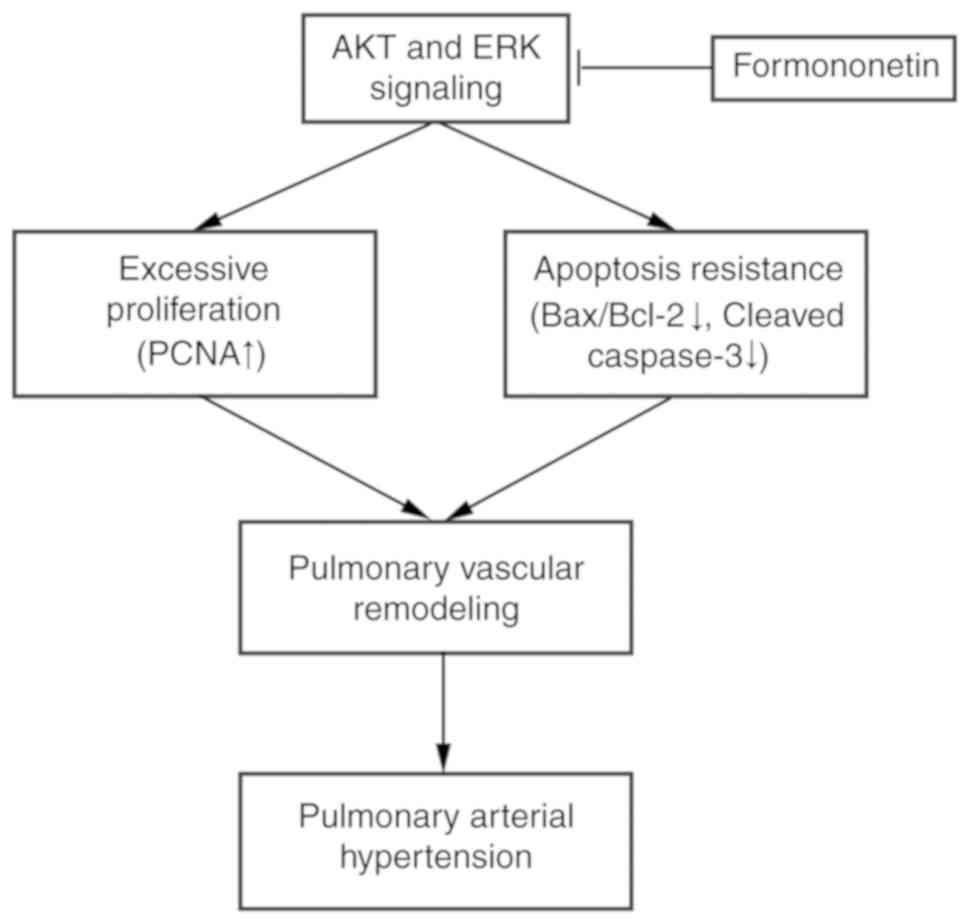
signaling pathways (Fig. 7).
As previously reported, FMN promotes the apoptosis
of tumor cells (21–23). After observing the lung tissue
sections of rats by TUNEL staining, we revealed that FMN could
promote the apoptosis of PASMCs in vivo. In order to further
elucidate the mechanism of FMN pro-apoptosis in vitro,
relevant cell experiments must be conducted in the future.
In conclusion, FMN was observed to exert a
significant protective effect against MCT-induced PAH and pulmonary
vascular remodeling, partially via inhibiting the activation of the
PI3K/AKT and ERK pathways. The findings of the present study may
provide a theoretical basis for the clinical treatment of PAH.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
CC participated in the design of this study and
wrote the manuscript. YX, YW and NZ were responsible for data
acquisition and statistical analysis. HZ, JX and WL performed the
experiments and provided technological assistance. CZ assisted with
data analysis and approved the final version of the manuscript. All
authors have read and approved the content of manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics Review
of Animal Use Application of The Fifth Affiliated Hospital of
Wenzhou Medical University (Wenzhou, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Maron BA and Leopold JA: Emerging concepts
in the molecular basis of pulmonary arterial hypertension: Part II:
Neurohormonal signaling contributes to the pulmonary vascular and
right ventricular pathophenotype of pulmonary arterial
hypertension. Circulation. 131:2079–2091. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Simonneau G, Gatzoulis MA, Adatia I,
Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, Krishna Kumar
R, Landzberg M, Machado RF, et al: Updated clinical classification
of pulmonary hypertension. J Am Coll Cardiol. 62 (25
Suppl):D34–D41. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lai YC, Potoka KC, Champion HC, Mora AL
and Gladwin MT: Pulmonary arterial hypertension: The clinical
syndrome. Cir Res. 115:115–130. 2014. View Article : Google Scholar
|
|
4
|
Guignabert C, Tu L, Girerd B, Ricard N,
Huertas A, Montani D and Humbert M: New molecular targets of
pulmonary vascular remodeling in pulmonary arterial hypertension:
Importance of endothelial communication. Chest. 147:529–537. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schermuly RT, Ghofrani HA, Wilkins MR and
Grimminger F: Mechanisms of disease: Pulmonary arterial
hypertension. Nat Rev Cardiol. 8:443–455. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Sakao S and Tatsumi K: Vascular remodeling
in pulmonary arterial hypertension: Multiple cancer-like pathways
and possible treatment modalities. Int J Cardiol. 147:4–12. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dumas de la Roque E, Savineau JP and
Bonnet S: Dehydroepiandrosterone: A new treatment for vascular
remodeling diseases including pulmonary arterial hypertension.
Pharmacol Ther. 126:186–199. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Michelakis ED: Pulmonary arterial
hypertension: Yesterday, today, tomorrow. Circ Res. 115:109–114.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Montani D, Chaumais MC, Guignabert C,
Günther S, Girerd B, Jaïs X, Algalarrondo V, Price LC, Savale L,
Sitbon O, et al: Targeted therapies in pulmonary arterial
hypertension. Pharmacol Ther. 141:172–191. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Archer SL, Weir EK and Wilkins MR: Basic
science of pulmonary arterial hypertension for clinicians: New
concepts and experimental therapies. Circulation. 121:2045–2066.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang Q, Cao Y, Luo Q, Wang P, Shi P, Song
C, E M, Ren J, Fu B and Sun H: The transient receptor potential
vanilloid-3 regulates hypoxia-mediated pulmonary artery smooth
muscle cells proliferation via PI3K/AKT signaling pathway. Cell
Prolif. 51:e124362018. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wu J, Yu Z and Su D: BMP4 protects rat
pulmonary arterial smooth muscle cells from apoptosis by
PI3K/AKT/Smad1/5/8 signaling. Int J Mol Sci. 15:13738–13754. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang W, Prince CZ, Mou Y and Pollman MJ:
Notch3 signaling in vascular smooth muscle cells induces c-FLIP
expression via ERK/MAPK activation. J Biol Chem. 277:21723–21729.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Wu F, Hao Y, Yang J, Yao W, Xu Y, Yan L,
Niu Y, Sun T, Yu J and Zhou R: Protective effects of aloperine on
monocrotaline-induced pulmonary hypertension in rats. Biomed
Pharmacother. 89:632–641. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Krenn L and Paper DH: Inhibition of
angiogenesis and inflammation by an extract of red clover
(Trifolium pratense L.). Phytomedicine. 16:1083–1088. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mu H, Bai YH, Wang ST, Zhu ZM and Zhang
YW: Research on antioxidant effects and estrogenic effect of
formononetin from Trifolium pratense (red clover). Phytomedicine.
16:314–319. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang S, Tang X, Tian J, Li C, Zhang G,
Jiang W and Zhang Z: Cardioprotective effect of sulphonated
formononetin on acute myocardial infarction in rats. Basic Clin
Pharmacol Toxicol. 108:390–395. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sun T, Wang J, Huang LH and Cao YX:
Antihypertensive effect of formononetin through regulating the
expressions of eNOS, 5-HT2A/1B receptors and α1-adrenoceptors in
spontaneously rat arteries. Eur J Pharmacol. 699:241–249. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li T, Zhao X, Mo Z, Huang W, Yan H, Ling Z
and Ye Y: Formononetin promotes cell cycle arrest via
downregulation of Akt/Cyclin D1/CDK4 in human prostate cancer
cells. Cell Physiol Biochem. 34:1351–1358. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Qi C, Xie M, Liang J, Li H, Li Z, Shi S,
Yang X, Wang Z, Tang J and Tang A: Formononetin targets the MAPK
and PI3K/AKT pathways to induce apoptosis in human nasopharyngeal
carcinoma cells in vitro and in vivo. Int J Clin Exp Med.
9:1180–1189. 2016.
|
|
21
|
Jin YM, Xu TM, Zhao YH, Wang YC and Cui
MH: In vitro and in vivo anti-cancer activity of formononetin on
human cervical cancer cell line HeLa. Tumor Biol. 35:2279–2284.
2014. View Article : Google Scholar
|
|
22
|
Zhang X, Bi L, Ye Y and Chen J:
Formononetin induces apoptosis in PC-3 prostate cancer cells
through enhancing the Bax/Bcl-2 ratios and regulating the p38/Akt
pathway. Nutr Cancer. 66:656–661. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Yang Y, Zhao Y, Ai X, Cheng B and Lu S:
Formononetin suppresses the proliferation of human non-small cell
lung cancer through induction of cell cycle arrest and apoptosis.
Int J Clin Exp Pathol. 7:8453–8461. 2014.PubMed/NCBI
|
|
24
|
Chen Z, Liu S, Cai Y, Xie K, Zhang W, Dong
L, Liu Y, Zheng F, Dun Y and Li N: Suppressive effect of
formononetin on platelet-derived growth factor-BB-stimulated
proliferation and migration of vascular smooth muscle cells. Exp
Ther Med. 12:1901–1907. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liu Y, He J, Chen X, Li J, Shen M, Yu W,
Yang Y and Xiao Z: The proapoptotic effect of formononetin in human
osteosarcoma cells: Involvement of inactivation of ERK and Akt
pathways. Cell Physiol Biochem. 34:637–645. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Isoda K, Akita K, Kitamura K,
Sato-Okabayashi Y, Kadoguchi T, Isobe S, Ohtomo F, Sano M, Shimada
K, Iwakura Y and Daida H: Inhibition of interleukin-1 suppresses
angiotensin II-induced aortic inflammation and aneurysm formation.
Int J Cardiol. 270:221–227. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhu N, Zhao X, Xiang Y, Ye S, Huang J, Hu
W, Lv L and Zeng C: Thymoquinone attenuates monocrotaline-induced
pulmonary artery hypertension via inhibiting pulmonary arterial
remodeling in rats. Int J Cardiol. 221:587–596. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Huang Z, Liu Z, Luo Q, Zhao Z, Zhao Q,
Zheng Y, Xi Q and Tang Y: Glycoprotein 130 inhibitor ameliorates
monocrotaline-induced pulmonary hypertension in rats. Can J
Cardiol. 32:1356.e1–1356.e10. 2016. View Article : Google Scholar
|
|
29
|
Xia S, Tai X, Wang Y, An X, Qian G, Dong
J, Wang X, Sha B, Wang D, Murthi P, et al: Involvement of Gax gene
in hypoxia-induced pulmonary hypertension, proliferation, and
apoptosis of arterial smooth muscle cells. Am J Respir Cell Mol
Biol. 44:66–73. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Liang K, Ye Y, Wang Y, Zhang J and Li C:
Formononetin mediates neuroprotection against cerebral
ischemia/reperfusion in rats via downregulation of the Bax/Bcl-2
ratio and upregulation PI3K/Akt signaling pathway. J Neurol Sci.
344:100–104. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li XL, Guan RJ and Li JJ: Attenuation of
monocrotaline-induced pulmonary arterial hypertension in rats by
rosuvastatin. J Cardiovasc Pharmacol. 60:219–226. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wilcox SR, Kabrhel C and Channick RN:
Pulmonary hypertension and right ventricular failure in emergency
medicine. Ann Emerg Med. 66:619–628. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tang B, Chen GX, Liang MY, Yao JP and Wu
ZK: Ellagic acid prevents monocrotaline-induced pulmonary artery
hypertension via inhibiting NLRP3 inflammasome activation in rats.
Int J Cardiol. 180:134–141. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Thenappan T, Ormiston ML, Ryan JJ and
Archer SL: Pulmonary arterial hypertension: Pathogenesis and
clinical management. BMJ. 360:j54922018. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ryan JJ and Archer SL: The right ventricle
in pulmonary arterial hypertension: Disorders of metabolism,
angiogenesis and adrenergic signaling in right ventricular failure.
Circ Res. 115:176–188. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Guignabert C, Tu L, Le Hiress M, Ricard N,
Sattler C, Seferian A, Huertas A, Humbert M and Montani D:
Pathogenesis of pulmonary arterial hypertension: Lessons from
cancer. Eur Respir Rev. 22:543–551. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Rabinovitch M: Molecular pathogenesis of
pulmonary arterial hypertension. J Clin Invest. 122:4306–4313.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
38
|
Falcetti E, Hall SM, Phillips PG, Patel J,
Morrell NW, Haworth SG and Clapp LH: Smooth muscle proliferation
and role of the prostacyclin (IP) receptor in idiopathic pulmonary
arterial hypertension. Am J Respir Crit Care Med. 182:1161–1170.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Bishopric NH, Andreka P, Slepak T and
Webster KA: Molecular mechanisms of apoptosis in the cardiac
myocyte. Curr Opin Pharmacol. 1:141–150. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huang X, Zou L, Yu X, Chen M, Guo R, Cai
H, Yao D, Xu X, Chen Y, Ding C, et al: Salidroside attenuates
chronic hypoxia-induced pulmonary hypertension via adenosine A 2a
receptor related mitochondria-dependent apoptosis pathway. J Mol
Cell Cardiol. 82:153–166. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Rahmani M, Aust MM, Attkisson E, Williams
DC Jr, Ferreira-Gonzalez A and Grant S: Dual inhibition of Bcl-2
and Bcl-xL strikingly enhances PI3K inhibition-induced apoptosis in
human myeloid leukemia cells through a GSK3- and Bim-dependent
mechanism. Cancer Res. 73:1340–1351. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Diab S, Kumarasiri M, Yu M, Teo T, Proud
C, Milne R and Wang S: MAP kinase-interacting kinases-emerging
targets against cancer. Chem Biol. 21:441–452. 2014. View Article : Google Scholar : PubMed/NCBI
|