Introduction
Melanoma, also known as malignant melanoma, is the
most dangerous type of skin cancer, with a high degree of
malignancy and poor patient prognosis. It is commonly observed on
the skin and mucosa, originating mainly from epithelial melanocytes
(1,2). Although melanoma accounts for
approximately 10% of all skin cancers, it accounts for
approximately 80% of the all skin cancer-related deaths (1). The etiopathogenesis of melanoma is
complex, and most common etiologies involve an endocrine
abnormality, genetic variation or ultraviolet irradiation exposure
(3). The extent of malignant
melanoma has a positive relationship with the number of tumor
metastatic sites. Studies have shown that the main reason for the
death of patients with malignant melanoma is tumor metastases to
the liver, lung, bone and brain (4–7). The
authors conclude there are many means of melanoma metastasis, e.g.,
lymphatic metastasis, blood metastasis and implantation metastasis.
Melanoma is characterized by malignant proliferation, immune
escape, metastasis and angiogenesis (8). Accordingly, the options for melanoma
treatment are limited and are ineffective to improve the quality of
life or survival of patients.
Targeted therapy for mutated genes, abnormal genes
and their signal transduction systems has been applied to clinical
therapy gradually, and it may benefit patients with melanoma;
however, there are still many issues associated with melanoma
treatment, e.g, drug resistance and drug insensitivity (9). Taken together, there exists an urgent
need to perform in-depth research on the pathogenesis of melanoma
to further explore new targets of drug action to be used in the
clinic.
According to a previous report, PLCB2 is a
critical regulator of platelet responses upon activation and takes
part in biological behaviors, e.g., tumorigenesis, cell growth and
activation of neutrophils by pro-inflammatory mediators (10,11).
In addition, bioinformatic analysis has revealed that PLCB2
is highly expressed in melanoma cells with high ABCB5
expression. Further reports have shown that PLCB2 is
significantly expressed in kidney renal clear cell carcinoma and
bladder cancer (12,13). Thus, PLCB2 may be a novel
gene involved in the incidence and development of melanoma.
The Ras/Raf/MAPK signaling pathway is closely
associated with cell proliferation and differentiation and takes
part in the development and progression of skin malignant tumors
(14,15). Ras, as an oncogene, plays a pivotal
role in cell life activities and mediates tumorigenesis by the
Raf/MEK/ERK and PI3K/Akt signaling pathways (16). Raf leads to the cascade of the
Ras/Raf/MEK1/2/-ERK1/2 signaling pathway. ERK is a
MAPK kinase and plays an important role in cellular signal
transduction in the Ras/Raf/MAPK signaling pathway (17). Combined with our previous studies,
our results suggest that the MAPK signaling pathway is
closely associated with melanoma progression. Therefore, the
present study was conducted to ascertain whether PLCB2
influences cell viability and apoptosis by activation of the
Ras/Raf/MAPK signaling pathway.
Materials and methods
Bioinformatic analysis
To assess the expression of PLCB2 in human
melanoma cells, the online microarray profiling data set, GEO
accession no. GSE38290 (18),
which includes 12 samples, was used. Each of the following 4 groups
had three replicates: A375 pSUPER-retro-puro-Vector vs. A375
pSUPER-retro-puro-ABCB5-KD; and G3361 pSUPER-retro-puro-shCNTRL vs.
G3361 pSUPER-retro-puro-ABCB5-KD. To renormalize the Affymetrix
array data, the authors averaged the intensity values of 3 probes
per sample in each data set as these probes adopted the same
internal control. For each data set, the average expression level
of PLCB2 in the A375 pSUPER-retro-puro-Vector and G3361
pSUPER-retro-puro-shCNTRL groups was set to 1, and the expression
of PLCB2 in the A375 pSUPER-retro-puro-ABCB5-KD and G3361
pSUPER-retro-puro-ABCB5-KD groups was adjusted accordingly. Using
the ‘gplots’ package of R (https://cran.r-project.org/web/packages/gplots/),
heatmap visualization of data matrices was performed. Using the
variance stabilizing transformation methods, the authors conducted
principal component analysis of RNA-Seq results in the
‘wilcoxTest’ package of Bioconductor (http://bioconductor.org/), and then plotted the first
two principal components. Volcano plots were derived from
‘wilcoxTest’-based differential gene expression
analysis.
Gene set enrichment analysis
(GSEA)
The Broad Institute website (http://www.broadinstitute.org/gsea/index.jsp) was
used to conduct the gene set enrichment analysis (19). This analysis covers versions
compatible with Java, R or Gene Pattern. The authors conducted all
GSEAs presented by implanting the Java GSEA. No experiments
(covering human material, humans, or animals) were conducted.
Accordingly, no ethical approval was required.
Protein-protein interaction (PPI)
network analysis
The STRING database (www.string-db.org) (20) was used to collect and integrate
known and predicted protein-protein association data for 13 genes.
In the present study, STRING was used to construct the PPI network
of 13 genes with a minimum required interaction score of 0.7. In
the present study, the crucial genes were identified based on four
different centrality measures, e.g. betweenness centrality (BC),
degree centrality (DC), eigenvector centrality (EGC), and closeness
centrality (CC). According to the centrality values of genes in the
PPI network, the top 3 genes were identified as crucial genes.
Cell lines and cell culture
The normal human epidermal melanocyte (NHEM) cell
line was provided by Cell Applications Inc. and cultured in M2
medium. The China Center for Type Culture Collection (Wuhan, China)
provided human melanoma cell lines (451Lu, Mel Ju, Mel 928,
SK-MEL-28, SK-MEL-2, Hs294T, A375 and WM35) which were cultivated
in Dulbecco's modified Eagle's medium (DMEM) (Gibco; Thermo Fisher
Scientific, Inc.) supplemented with 10% fetal bovine serum. All
cell lines were incubated at 37°C with 5% CO2 in a
humidified environment.
RNA interference
The authors adopted negative control small
interfering RNA (siRNA) (5′-UUCUCCGAACGUGUCACGUTT-3) and an siRNA
against PLCB2 (5′-CCCTGCCCAGCCCTCAGGGGATCAGT-3′)
(GenePharma). The siRNAs were transfected into A375 cells using
Lipofectamine™ 2000 transfection reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) (21). After
transfection, the cells underwent 48 h of culture before treatment
according to the manufacturer's instructions.
Transfection of cells with the
PLCB2-overexpressing plasmid vector
A PLCB2 overexpression vector
(pcDNA3.1/PLCB2-HisB) was used for the transfection studies. Using
Lipofectamine™ 2000 transfection reagent (Invitrogen; Thermo Fisher
Scientific, Inc.) following the manufacturer's instructions
(22), all transfection
experiments were performed. Cells (1×103) were plated in
24-well plates and allowed to grow until reaching 80–90%
confluency. The plasmid DNA and Lipofectamine (4 µl/ml of
transfection medium) were diluted in Opti-MEM reduced serum medium
(Thermo Fisher Scientific, Inc.) separately and incubated for 10
min at room temperature. The diluted solutions were then mixed and
incubated for 30 min. Subsequently, 4 µg plasmid and 10 µl reagent
were added to each well containing cells, mixed gently, and the
cells were then stored in a CO2 incubator for 48 h at
37°C.
Cell viability assays
Cell viability was assessed using the Cell Counting
Kit-8 assay (Sigma-Aldrich; KGaA), following the manufacturer's
instructions. Briefly, cells were seeded in 96-well plates at a
density of 4×103 cells per well in 100 µl of growth
medium, and allowed to attach overnight. After achieving attachment
for 24 h, the cells were treated with oxaliplatin (µM) at different
concentrations for different periods. At the end of the treatment,
10 µl of tetrazolium substrate was added to each well of the plate.
The plates were then stored at 37°C for 1 h and the optical density
(OD) was measured at 450 nm with a Bio-Rad ELISA microplate reader
(Bio-Rad 680; Bio-Rad Laboratories, Inc.).
Colony formation assay
For the colony formation assay, cells were seeded at
300 cells/well on 6-well plates in triplicate, and maintained in
complete culture medium for 12 days until obvious colonies formed.
Colonies were then fixed with 70% ethanol for 5 min at room
temperature and stained with Coomassie blue dye for 5 min as well.
A colony containing more than 50 cells was considered a colony and
was counted. Colony number was determined under an Olympus
fluorescence microscope equipped with MicroPublisher 3.3 RTV CCD
camera.
Analysis of apoptosis by flow
cytometry
With the use of Annexin V-FITC apoptosis detection
reagent (BD Biosciences) following the manufacturer's instructions,
cell apoptosis was detected. Briefly, cells were washed twice with
cell staining buffer and resuspended in binding buffer at a density
of 1×105 cells/100 µl. To 100 µl of the cell suspension,
5 µl of Annexin V-FITC was added, and the cells were incubated at
ambient temperature for 15 min, followed by addition of another 400
µl of binding buffer. Subsequently, the cell apoptosis was detected
with the use of flow cytometry (LSR II, BD Biosciences), and the
data were analyzed using FlowJo software (FlowJo LLC, USA).
RNA extraction and quantitative
real-time PCR
Total RNA was extracted from A375 cells
overexpressing or not overexpressing PLCB2 using TRIzol reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) following the
manufacturer's instructions. The RNA concentration was ascertained
using UV spectrophotometry. Total RNA (0.5 µg) was employed to
yield cDNA with SuperScript II reverse transcriptase (Invitrogen;
Thermo Fisher Scientific, Inc.) following the specifications of the
manufacturer (23,24); overall RNA (0.5 µg) was employed.
Genes of interest were amplified by real-time PCR using a
LightCycler TaqMan Master kit (Roche Diagnostics) following the
specifications of the manufacturer and primers designed by Roche
Diagnostics. The following primers were employed: p53
forward, 5′- CTGGCATTTGCACCTACCTC-3′ and reverse,
5′-TAACCAGCTGCCCACTGTA-3′ (product 176 bp); Bax forward,
5′-TTTGCTTCAGGGTTTCATCC-3′ and reverse, 5′-AGACCTGCCGTTGAAGTTGAC-3′
(product 120 bp); Bcl-2 forward, 5′-CGCCAACATTCTCTCCACAG-3′
and reverse, 5′-CTGGGCCAGAGCTACATCTT-3′ (product 119 bp); and
GAPDH forward, 5′-ACCCAGAAGACTGTGGATGG-3′ and reverse,
5′-TCAGCTCAGGGATGACCTTG-3′ (product 124 bp). The final reaction
volume reached 10 µl, and for PCR (Roche Diagnostics), a
LightCycler instrument was utilized. The following PCR conditions
were used: 15 min at 95°C, followed by 40 cycles for 10 sec at
95°C, 30 sec at 60°C, and 1 sec at 72°C, and 1 cycle of cooling for
30 sec at 50°C. The authors normalized the samples to the
GAPDH gene. The authors obtained the corresponding
expression using the 2−ΔΔCq formula (25).
Protein extraction and western
blotting
At 48 h post transfection, the cells were lysed
using NP-40 lysis buffer (50 mM Tris, pH 7.5, 150 mM NaCl, 1.0%
NP-40, 2 mM EDTA, 10 mM NaF, 1 mM Na3VO4 and
2 mM PMSF). As previously described (26), the concentration of protein samples
underwent measurement with the use of a BCA kit (Solarbio) in line
with the instructions of the manufacturer. An amount of 50 µl of
cell lysates was loaded on 8% SDS-PAGE gel and then transferred
onto a polyvinylidene difluoride (PVDF) membrane (Millipore). After
being blocked using 5% non-fat dry milk powder in PBS with 0.1%
Tween-20 for 1 h, the membrane underwent incubation using the
indicated antibodies at 4°C overnight. After being washed using
Tris-buffered saline-Tween-20 (TBST) 3 times, the membranes
underwent incubation using horseradish peroxidase (HRP)-conjugated
goat anti-rabbit (cat. no. 6721, dilution of 1:5,000; Abcam) or
goat anti-mouse (cat. no. 6789, dilution of 1:2,000; Abcam)
secondary antibodies at ambient temperature for 1 h and washed with
TBST 3 times. Finally, the proteins underwent visualization with an
ECL Western blotting detection kit (Amersham Biosciences) in line
with the manufacturer's instructions. Quantification of the bands
was performed via densitometry and the ImageJ Pro 6.0 analyses
systems (National Institutes of Health, NIH, Bethesda, MD, USA).
The following antibodies were employed: p53 (cat. no. 32389,
dilution 1:500; Abcam), c-caspase-3 (cat. no. 49822, dilution
1:800; Abcam), Bax (cat. no. 77566, dilution 1:800; Abcam), Bcl-2
(cat. no. 32124, dilution 1:1,000; Abcam), Ras (cat. no. 52939,
dilution 1:800; Abcam), Raf (cat. no. 200653, dilution 1:800;
Abcam), p-ERK1/2 (CST cat. no. 4370, dilution 1:500; Cell Signaling
Technology, Inc.), ERK1/2 (CST cat. no. 46955, dilution 1:1,000;
Cell Signaling Technology, Inc.) and GAPDH (cat. no. 181602,
dilution 1:2,000; Abcam).
Statistical analysis
Statistical analysis was conducted with the use of
SPSS 18.0 software (SPSS, Inc., Chicago, IL, USA). Data are shown
as the mean ± standard deviation (SD). Statistical significance was
ascertained by Dunnett's -test or Tukey's test. A P-value <0.05
was regarded as indicative of statistical significance.
Results
High expression of PLCB2 in human
melanoma cell lines
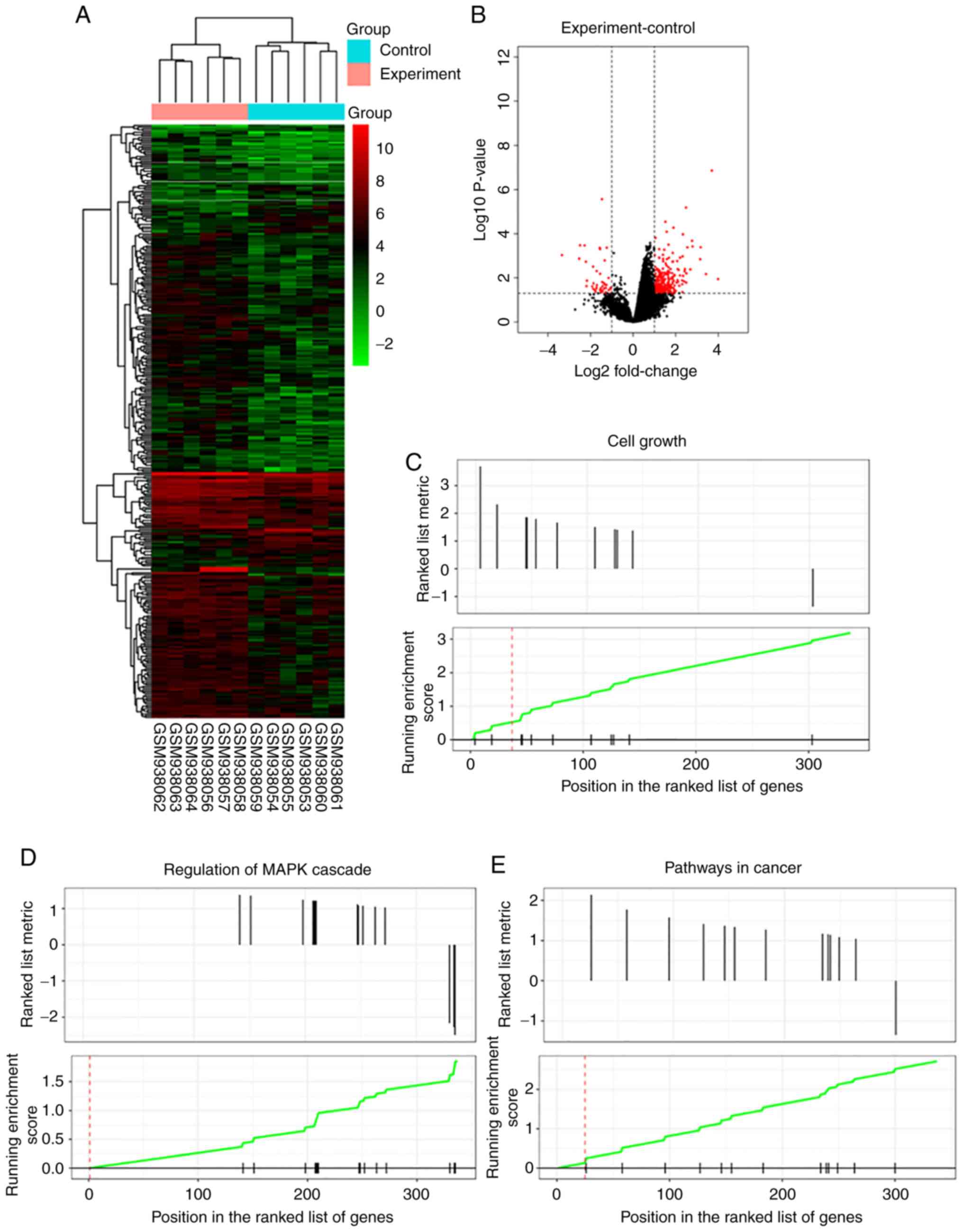
The authors performed heatmap analysis of the
expression profiles in ABCB5 highly expressing human melanoma cell
lines (A375 and G3361 cells) in the GEO database (GSE38290).
Amplified aRNA from each sample was subjected to microarray
hybridization, and 14,209 extracted genes were analyzed by
hierarchical clustering, which revealed distinct expression
patterns in human melanoma cells with high ABCB5 expression
(experimental group) and human melanoma cells with empty vectors
(control group) (Fig. 1A). A
volcano plot of the identified quality-controlled genes (P<0.05,
fold change >2) is presented in Fig. 1B. Our data identified 289
upregulated and 56 downregulated mRNAs in human melanoma cells with
high ABCB5 expression compared with matched human melanoma
cells with empty vectors (Fig.
1B). GSEA showed that GNG13, PLCB2, EDNRA, LPAR4, PIK3R3,
PRKCG, MMP2, PAX8, PPARG, FGF7 and PTGER4 were
significantly enriched in Cell growth, MAPK cascade and
Cancer-related signaling pathways (Fig. 1C-E). Thus, network analysis of the
13 genes identified in the present study was performed to predict
functional gene-gene interactions with the use of the STRING
database (www.string-db.org) (Fig. 1F). PLCB2 was identified as a
crucial gene in the PPI network (Fig.
1G). In addition, PLCB2 was found to be significantly
highly expressed in human melanoma cell lines, as determined by
RT-PCR analysis (Fig. 1H).
PLCB2 affects cell viability and
apoptosis
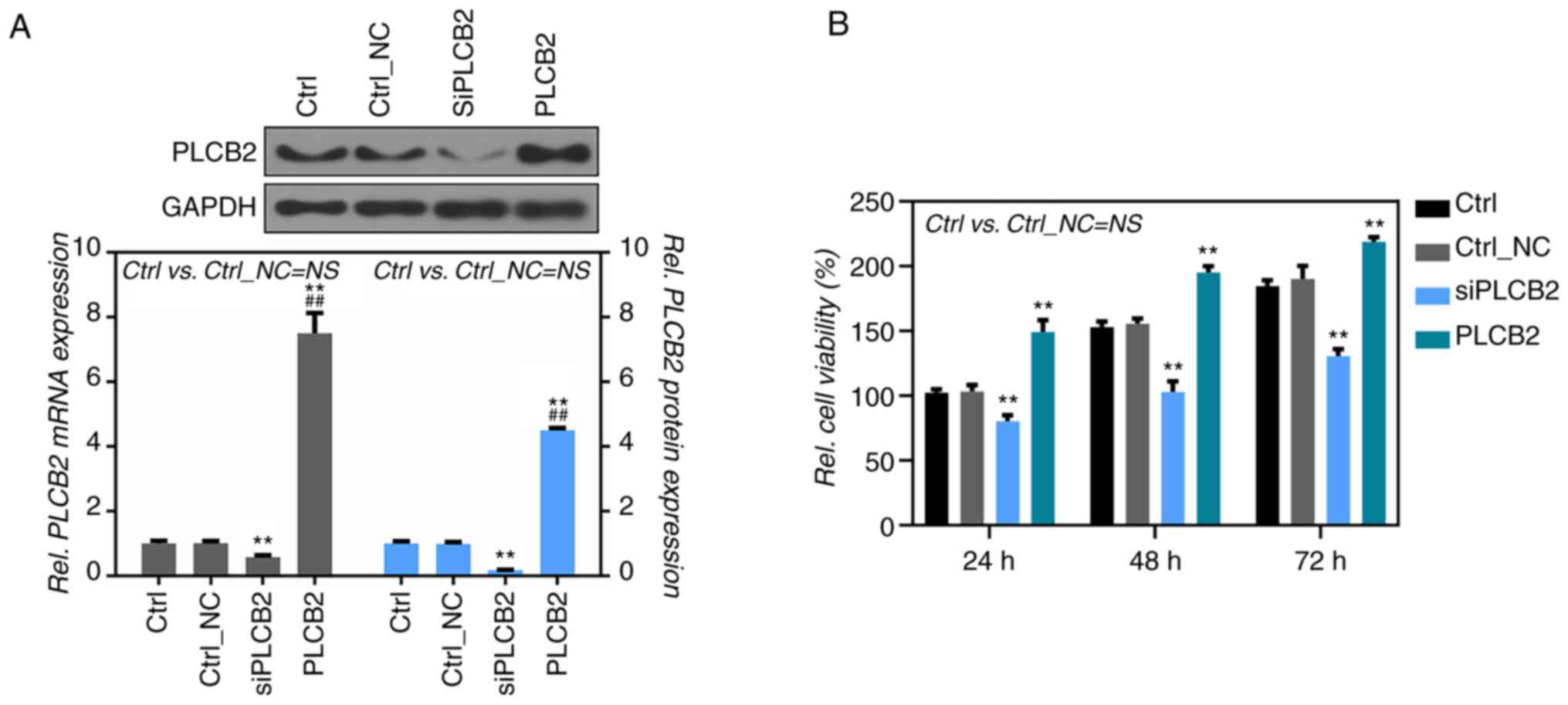
To further confirm the effect of PLCB2 on the
proliferation and apoptosis of human melanoma cells, A375 cells
were transfected with overexpressing or not overexpressing
PLCB2 plasmids. Efficiency of the PLCB2 siRNA and the
overexpressing plasmid was examined by RT-PCR and western blot
analysis (Fig. 2A). A significant
decrease in the cell viability was noted in the A375 cells treated
with the PLCB2-siRNAs (siPLCB2) when compared to the
controls at 24, 48 and 72 h after transfection (100, 79.3 and
58.2%, respectively) (Fig. 2B),
while cell viability in the A375 cells treated with the
PLCB2 plasmid (PLCB2) was significantly increased
(100, 178.5 and 207.4%, respectively) (Fig. 2B). The role of PLCB2 in the
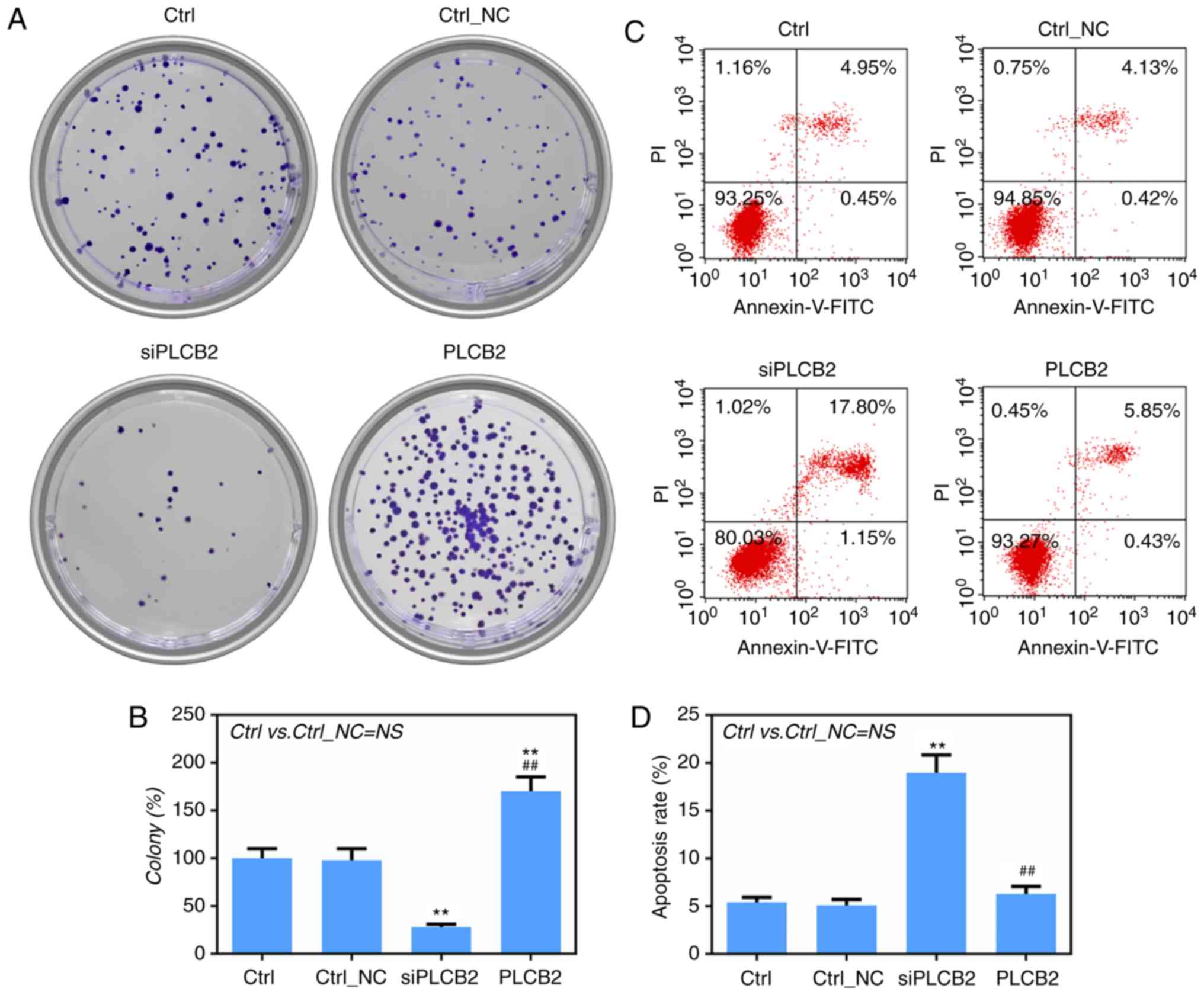
regulation of the colony-formation ability of A375 cells was
investigated. The results showed that low expression of
PLCB2 significantly suppressed cell growth and that high
expression of PLCB2 significantly promoted cell growth
(Fig. 3A and B). Flow cytometric
analysis indicated that siPLCB2 significantly enhanced the
apoptotic rate of the A375 cells (Fig.
3C and D).
PLCB2 regulates cell viability and the
expression levels of apoptosis-related proteins
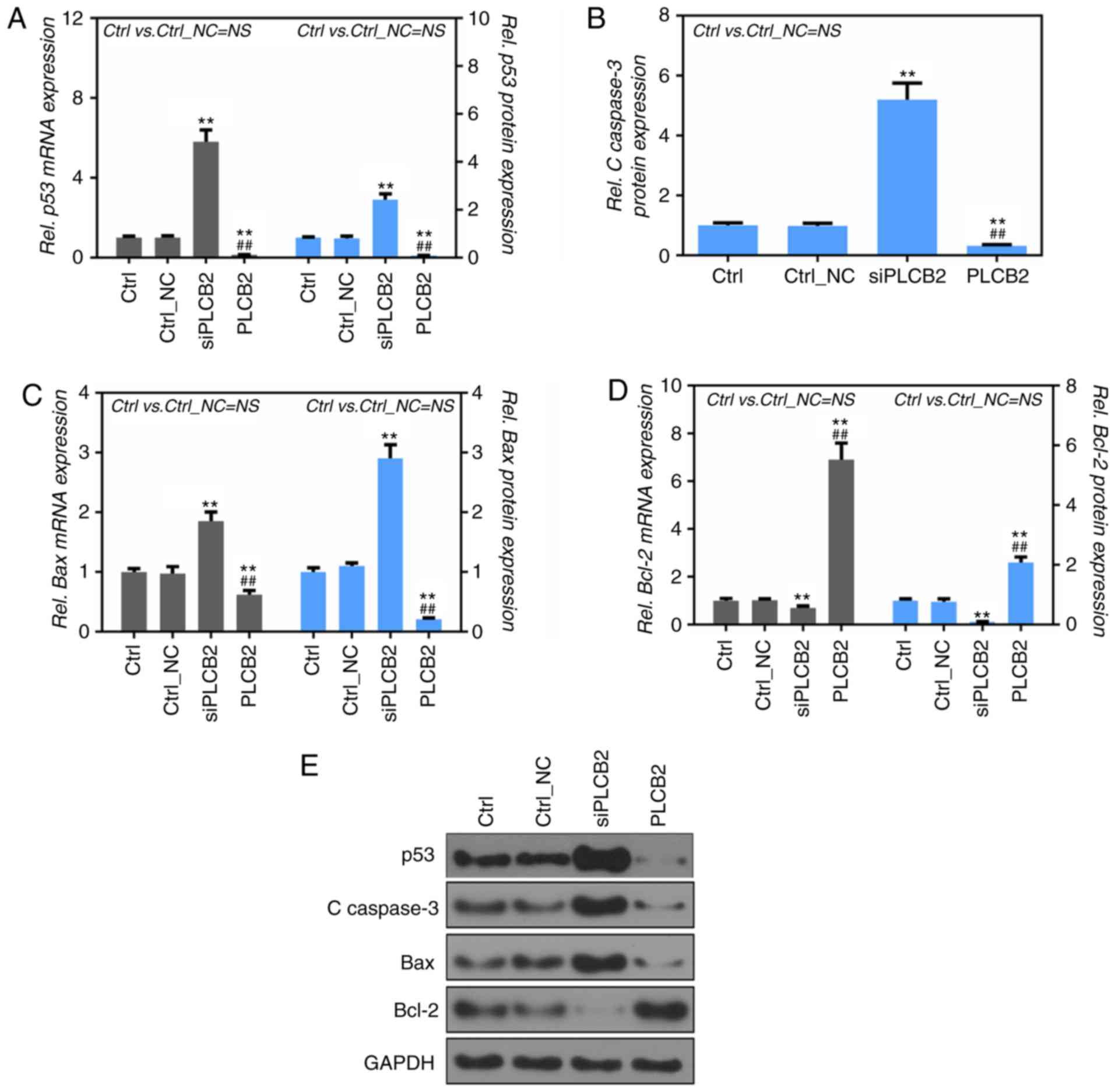
To detect the mRNA and protein expression levels of
cleaved caspase-3, Bax and Bcl-2, RT-PCR and western
blot analysis were performed. p53, cleaved caspase-3
and Bax mRNA and protein expression levels were
significantly increased in the siPLCB2 group, compared to the Ctrl
and Ctrl_NC groups (P<0.01, Fig.
4A-C and E). It was also found that the Bcl-2 protein
expression level was significantly upregulated in the PLCB2 group
compared with the Ctrl and Ctrl_NC groups (P<0.01, Fig. 4D), while the p53, cleaved
caspase-3, Bax and Bcl-2 protein levels showed a
reverse trend in the PLCB2 group (Fig.
4A-D).
PLCB2 affects the activation of the
Ras/Raf/MAPK signaling pathway
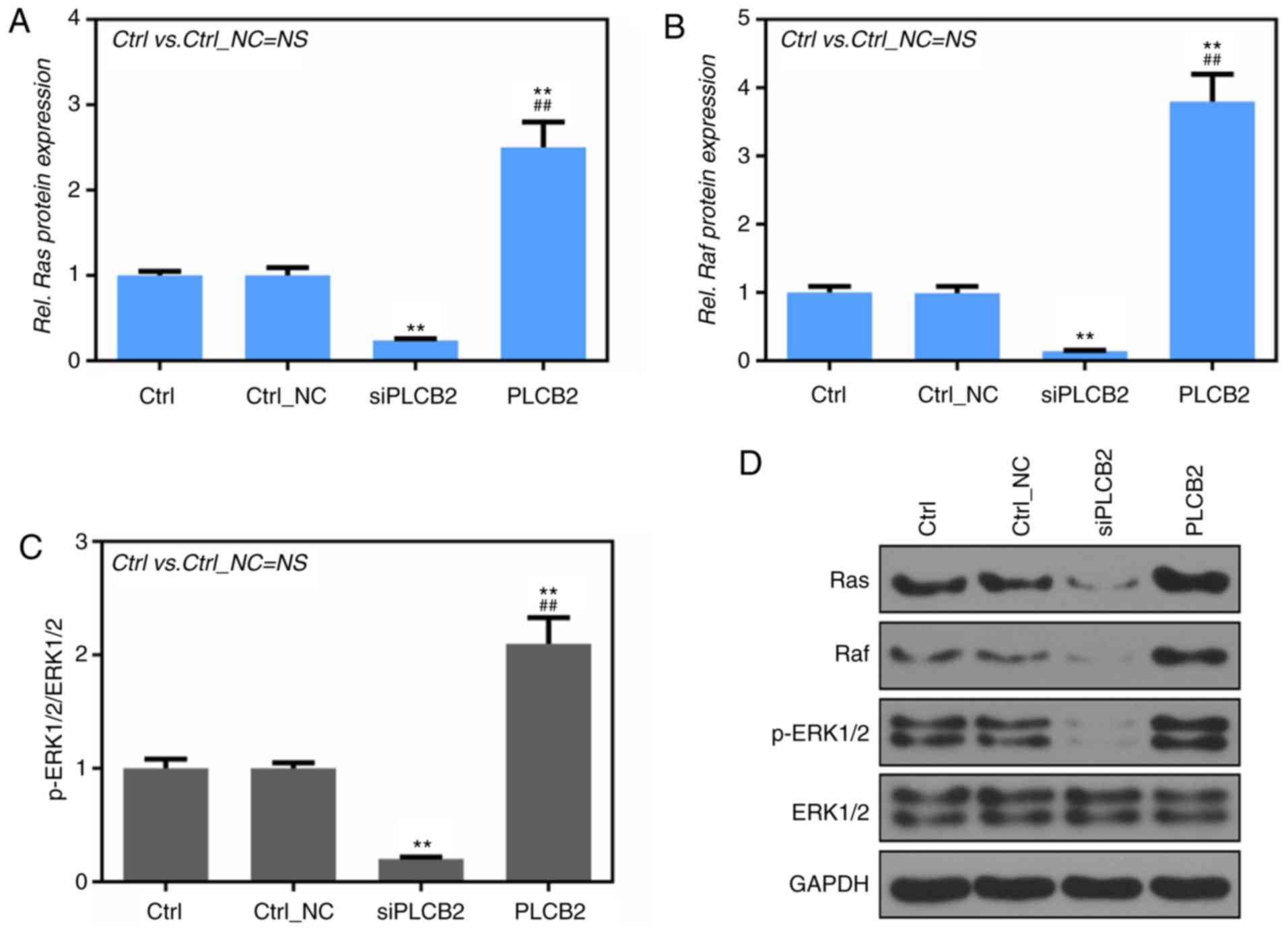
Fig. 5A, B and D
shows that the protein levels of Ras and Raf were significantly
lower in the siPLCB2 group than that noted in the Ctrl and Ctrl_NC
groups and that the protein levels of Ras and Raf were
significantly higher in the PLCB2 group than in the Ctrl and
Ctrl_NC groups, as determined by western blot analysis (P<0.01).
Furthermore, PLCB2 silencing significantly suppressed the ratio of
phosphorylated (p)-ERK1/2/total ERK1/2, and PLCB2 overexpression
significantly upregulated the phosphorylation levels of ERK1/2
(P<0.01, Fig. 5C and D).
Discussion
Due to a deeper understanding and an increasing
number of studies on gene variations in cancer, gene therapy is
gradually being used to treat cancer. In recent years, some
targeted drug therapies have significantly affected the treatment
of melanoma (27). However, some
patients with melanoma are insensitive to targeted therapy drugs,
further causing preexisting resistance (28). Accordingly, it is a key task for
melanoma researchers to search for novel prognostic markers, to
improve early diagnostic rates, and to identify new targets of
molecular-targeted therapy for melanoma. ATP-binding cassette
subfamily B member 5 (ABCB5) plays a key role in cellular
biological functions in melanoma (29), breast cancer (30), liver cancer (31), oral cancer (32) and colorectal cancer (33). A previous report showed that ABCB5
could regulate melanoma growth and metastasis. These results
suggest that ABCB5-related genes might also play a role in cell
viability, apoptosis and metastasis in melanoma (29). Bioinformatics analysis could
suggest the possible functions of many different novel genes. The
GEO database (GSE38290) (18) was
analyzed by bioinformatics. The authors identified 345
differentially expressed genes (DEGs), 289 of which were
significantly upregulated and 56 of which were significantly
downregulated (pP<0.05, fold-change >2) in ABCB5
highly expressing human melanoma cell lines (A375 and G3361)
(Fig. 1A and B). After gene
integration, 13 differentially expressed genes (DEGs) were
determined to be closely associated with cancer and to regulate the
MAPK cascade (Fig. 1C-E).
In addition, further study revealed that PLCB2 was the
crucial gene in the PPI network (Fig.
1F and G). In other words, the expression level of PLCB2
was significantly increased after melanoma cells were transfected
with the ABCB5 gene. Thus, the authors conclude that
PLCB2 may play an important role in cell viability and
apoptosis. PLCB2 was significantly upregulated in human
melanoma cell lines (Fig. 1H).
Given the above results, the expression of PLCB2 was higher
in 451-Lu and SK-Mel-28 cells than that noted in the other cell
lines and the expression of PLCB2 was lower in Mel Ju and
Mel 928 cells than that noted in the other melanoma cell lines, and
the expression of PLCB2 in A375 cells was between.
Therefore, A375 cells were used in the following experiments.
In the present study, the cell viability ability was
determined by CCK-8 and colony-forming assays and then the cell
apoptosis rate was detected by flow cytometry. It was found that
PLCB2 interference significantly suppressed cell viability
and promoted cell apoptosis (Figs.
2B and 3). As previously
shown, PLCB2 affects the associated biological functions in
melanoma (29). Thus, the
following experiments were performed to preliminarily explore the
relevant molecular mechanisms in human melanoma cells
overexpressing or not overexpressing PLCB2. According to the
above results, PLCB2 has a close relation with the
MAPK cascade in melanoma, and the Ras/Raf/MAPK signaling
pathway, which belongs to the MAPK signaling transduction pathway,
has a close association with skin malignant tumors (15,17).
Therefore, it is important to investigate variations in proteins in
the Ras/Raf/MAPK signaling pathway. Ras and p53 are
crucial genes involved in cell proliferation, cell apoptosis, cell
movement, the inflammatory response and angiogenesis (34). There is cooperation between the
Ras and p53 genes. Accordingly, the expression level
of p53 was detected by RT-PCR and western blot analysis.
PLCB2 interference significantly increased the mRNA and
protein levels of p53, and the overexpression of
PLCB2 significantly decreased the mRNA and protein levels of
p53 (Fig. 4A). Ras
expression changes in A375 cells overexpressing or not
overexpressing PLCB2 were the same as those changes noted
for p53 expression (Fig.
5A). This result is consistent with those that have been well
documented. The MAPK signaling pathway regulates Bax
and Bcl-2 gene expression and actives caspase-3
expression (35). In the present
study, PLCB2 interference significantly enhanced cleaved
caspase-3 and Bax gene expression and suppressed
Bcl-2 gene expression (Fig.
4). Guanosine triphosphate (GTP)-Ras was found to
activate Raf gene expression to activate MEK/ERK
signaling (36). According to a
previous report, Ras can activate the phosphorylation level
of ERK1/2 in the MAPK signaling pathway to regulate cell apoptosis
(36,37). The results described here revealed
that PLCB2 interference suppressed the activation of the
Ras/Raf/MAPK signaling pathway, as demonstrated by western blot
analysis (Fig. 5).
In summary, PLCB2 may be a potential target
gene in the treatment of melanoma and could affect the activation
of the Ras/Raf/MAPK signaling pathway to regulate the expression
levels of Bcl-2, Bax, caspase-3 and p53, thereby
affecting cell viability and apoptosis.
Acknowledgements
Not applicable.
Funding
The present study was funded by The Medical and
Health Science and Technology Project of Zhejiang Province to HZ
(grant no. 2017KY070).
Availability of data and materials
GSE38290 dataset (18) was used to perform our study. The
datasets generated and/or analyzed during the current study are
available from the Gene Expression Omnibus, https://www.ncbi.nlm.nih.gov/geo/.
Authors' contributions
HZ wrote the main manuscript and analyzed the data.
TX and YS performed all the experiments and collected the data. HZ
and YQ designed the study. All authors read and approved the final
manuscript and agree to be accountable for all aspects of the
research in ensuring that the accuracy or integrity of any part of
the work are appropriately investigated and resolved.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Little EG and Eide MJ: Update on the
current state of melanoma incidence. Dermatol Clin. 30:355–361.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Eggen CAM, Durgaram VVL, van Doorn R, Mooi
WJ, Pardo LM, Pasmans SGMA and Hollestein LM: Incidence and
relative survival of melanoma in children and adolescents in the
Netherlands, 1989–2013. J Eur Acad Dermatol Venereol. 32:956–961.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Aung PP, Nagarajan P and Prieto VG:
Regression in primary cutaneous melanoma: Etiopathogenesis and
clinical significance. Lab Invest. Feb 27–2017.(Epub ahead of
print). doi: 10.1038/labinvest.201. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Lorigan JG, Wallace S and Mavligit GM: The
prevalence and location of metastases from ocular melanoma: Imaging
study in 110 patients. AJR Am J Roentgenol. 157:1279–1281. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yang M, Jiang P, An Z, Baranov E, Li L,
Hasegawa S, Al-Tuwaijri M, Chishima T, Shimada H, Moossa AR and
Hoffman RM: Genetically fluorescent melanoma bone and organ
metastasis models. Clin Cancer Res. 5:3549–3559. 1999.PubMed/NCBI
|
|
6
|
Seifert H, Hirata E, Gore M, Khabra K,
Messiou C, Larkin J and Sahai E: Extrinsic factors can mediate
resistance to BRAF inhibition in central nervous system melanoma
metastases. Pigment Cell Melanoma Res. 29:92–100. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bedikian AY, Papadopoulos NE, Kim KB,
Vardeleon A, Smith T, Lu B and Deitcher SR: A pilot study with
vincristine sulfate liposome infusion in patients with metastatic
melanoma. Melanoma Res. 18:400–404. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Elder DE: Pathology of melanoma. Surg
Oncol Clin N Am. 24:229–237. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Amann VC, Ramelyte E, Thurneysen S,
Pitocco R, Bentele-Jaberg N, Goldinger SM, Dummer R and Mangana J:
Developments in targeted therapy in melanoma. Eur J Surg Oncol.
43:581–593. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mao G, Jin J, Kunapuli SP and Rao AK:
Nuclear factor-κB regulates expression of platelet phospholipase
C-beta2 (PLCB2). Thromb Haemost. 116:931–940. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Suire S, Lecureuil C, Anderson KE,
Damoulakis G, Niewczas I, Davidson K, Guillou H, Pan D, Jonathan
Clark, Phillip T Hawkins and Stephens L: GPCR activation of Ras and
PI3Kc in neutrophils depends on PLCb2/b3 and the RasGEF RasGRP4.
EMBO J. 31:3118–3129. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen G, Wang Y, Wang L and Xu W:
Identifying prognostic biomarkers based on aberrant DNA methylation
in kidney renal clear cell carcinoma. Oncotarget. 8:5268–5280.
2017.PubMed/NCBI
|
|
13
|
Zekri AR, Hassan ZK, Bahnassy AA, Khaled
HM, El-Rouby MN, Haggag RM and Abu-Taleb FM: Differentially
expressed genes in metastatic advanced Egyptian bladder cancer.
Asian Pac J Cancer Prev. 16:3543–3549. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Collisson EA, De A, Suzuki H, Gambhir SS
and Kolodney MS: Treatment of metastatic melanoma with an orally
available inhibitor of the Ras-Raf-MAPK cascade. Cancer Res.
63:5669–5673. 2003.PubMed/NCBI
|
|
15
|
Poenitzsch Strong AM, Setaluri V and
Spiegelman VS: MicroRNA-340 as a modulator of RAS-RAF-MAPK
signaling in melanoma. Arch Biochem Biophys. 563:118–124. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Barault L, Veyrie N, Jooste V, Lecorre D,
Chapusot C, Ferraz JM, Lièvre A, Cortet M, Bouvier AM, Rat P, et
al: Mutations in the RAS-MAPK, PI(3)K (phosphatidylinositol-3-OH
kinase) signaling network correlate with poor survival in a
population-based series of colon cancers. Int J Cancer.
122:2255–2259. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li XJ, Li B, Huang JS, Shi JM, Wang P, Fan
W and Zhou YL: Effects of acrylonitrile on lymphocyte lipid rafts
and RAS/RAF/MAPK/ERK signaling pathways. Genet Mol Res.
13:7747–7756. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wilson BJ, Saab KR, Ma J, Schatton T, Pütz
P, Zhan Q, Murphy GF, Gasser M, Waaga-Gasser AM, Frank NY and Frank
MH: ABCB5 maintains melanoma-initiating cells through a
proinflammatory cytokine signaling circuit. Cancer Res.
74:4196–207. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Subramanian A, Tamayo P, Mootha VK,
Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub
TR, Lander ES and Mesirov JP: Gene set enrichment analysis: A
knowledge-based approach for interpreting genome-wide expression
profiles. Proc Natl Acad Sci USA. 102:15545–15550. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gao X, Wang X and Zhang S: Bioinformatics
identification of crucial genes and pathways associated with
hepatocellular carcinoma. Biosci Rep. 38:2018. View Article : Google Scholar
|
|
21
|
Qin J, Fang N, Lou J, Zhang W, Xu S, Liu
H, Fang Q, Wang Z, Liu J, Men X, et al: TRB3 is involved in free
fatty acid-induced INS-1-derived cell apoptosis via the protein
kinase C delta pathway. PLoS One. 9:e960892014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mohan N, Ai W, Chakrabarti M, Banik NL and
Ray SK: KLF4 overexpression and apigenin treatment down regulated
anti-apoptotic Bcl-2 proteins and matrix metalloproteinases to
control growth of human malignant neuroblastoma SK-N-DZ and IMR-32
cells. Mol Oncol. 7:464–474. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Flores-Fernandez R, Blanco-Favela F,
Fuentes-Panana EM, Chavez-Sanchez L, Gorocica-Rosete P and
Chávez-Rueda AK: Prolactin rescues immature B-cells from apoptosis
induced by B-cell receptor cross-linking. J Immunol Res.
2016:32190172016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang XD, Wu Q and Yang SH: Ferulic acid
promoting apoptosis in human osteosarcoma cell lines. Pak J Med
Sci. 33:127–131. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhao L, Gu H, Chang J, Wu J, Wang D, Chen
S, Yang X and Qian B: MicroRNA-383 regulates the apoptosis of tumor
cells through targeting Gadd45g. PLoS One. 9:e1104722014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Queirolo P and Spagnolo F: BRAF plus
MEK-targeted drugs: a new standard of treatment for BRAF-mutant
advanced melanoma. Cancer Metastasis Rev. 36:35–42. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ahn A, Chatterjee A and Eccles MR: The
slow cycling phenotype: A growing problem for treatment resistance
in melanoma. Mol Cancer Ther. 16:1002–1009. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wang S, Tang L, Lin J, Shen Z, Yao Y, Wang
W, Tao S, Gu C, Ma J, Xie Y and Liu Y: ABCB5 promotes melanoma
metastasis through enhancing NF-κB p65 protein stability. Biochem
Biophys Res Commun. 492:18–26. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Yao J, Yao X, Tian T, Fu X, Wang W, Li S,
Shi T, Suo A, Ruan Z, Guo H, et al: ABCB5-ZEB1 axis promotes
invasion and metastasis in breast cancer cells. Oncol Res.
25:305–316. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Chong CC, Cheung ST, Cheung YS, Chan AW,
Chan SL, Yu SC and Lai PB: Novel biomarkers GEP/ABCB5 regulate
response to adjuvant transarterial chemoembolization after curative
hepatectomy for hepatocellular carcinoma. Hepatobiliary Pancreat
Dis Int. 17:524–530. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Grimm M, Krimmel M, Polligkeit J,
Alexander D, Munz A, Kluba S, Keutel C, Hoffmann J, Reinert S and
Hoefert S: ABCB5 expression and cancer stem cell hypothesis in oral
squamous cell carcinoma. Eur J Cancer. 48:3186–3197. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Guo Q, Grimmig T, Gonzalez G,
Giobbie-Hurder A, Berg G, Carr N, Wilson BJ, Banerjee P, Ma J, Gold
JS, et al: ATP-binding cassette member B5 (ABCB5) promotes tumor
cell invasiveness in human colorectal cancer. J Biol Chem.
293:11166–11178. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Zhang X, Cheng Q, Yin H and Yang G:
Regulation of autophagy and EMT by the interplay between p53 and
RAS during cancer progression (Review). Int J Oncol. 51:18–24.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Yousefi H, Karimi P, Alihemmati A, Alipour
MR, Habibi P and Ahmadiasl N: Therapeutic potential of genistein in
ovariectomy-induced pancreatic injury in diabetic rats: The
regulation of MAPK pathway and apoptosis. Iran J Basic Med Sci.
20:1009–1015. 2017.PubMed/NCBI
|
|
36
|
Ritt DA, Abreu-Blanco MT, Bindu L, Durrant
DE, Zhou M, Specht SI, Stephen AG, Holderfield M and Morrison DK:
Inhibition of Ras/Raf/MEK/ERK pathway signaling by a stress-induced
phospho-regulatory circuit. Mol Cell. 64:875–887. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Han YF, Ji LH, Feng TT, Liu F, Cui S and
Su J: Effect of ERK1/2 signaling pathway inhibitor PD98059 on the
expression of ras, BRaf, MEK, ERK1/2 in marrow nucleated red blood
cells of CMS Patients. Zhongguo Shi Yan Xue Ye Xue Za Zhi.
25:1571–1575, (In Chinese). PubMed/NCBI
|