Introduction
Myoblast stem cells are characterized by ability to
self-renew and the potential to regenerate muscle. Myoblasts are
mainly distributed in skeletal muscle tissues, but not in mature
cardiac and smooth muscle tissues. Normally, myoblasts are present
as muscle satellite cells. When muscles are damaged by external
stimuli, myoblasts are activated and undergo proliferation and
differentiation, finally becoming new muscle fibers. They therefore
serve critical roles in muscle regeneration.
The PI3K/AKT signaling pathway participates in
biological processes, including proliferation and differentiation.
It is also involved in pathological processes of cancers and
inflammations. PI3K activation phosphorylates and activates the
plasma membrane located AKT molecule (1). By modulating expression of proteins,
such as Bad, caspase-9 and runt-related transcription factor 2, the
PI3K/AKT signaling pathway inhibits cell apoptosis and triggers
cell proliferation. The PI3K/AKT signaling pathway serves a
significant role in the suppression of apoptosis and promotion of
cell proliferation. AKT demonstrates a number of downregulatory
effects by activating mTOR (2),
which can affect transcription of p70 or 4E-binding protein 1
(4EBP1). mTOR, as a member of serine/threonine protein kinase
family, tends to be affected by internal and external stimuli and
can regulate the physiological activities of cells (2). In addition, the PI3K/AKT/mTOR
signaling pathway is also considered to be an intracellular
signaling pathway that can regulate cell cycles (1,2).
mTOR can regulate cell proliferation, cell motility,
protein synthesis, autophagy and transcription (3). The mTOR complex includes two
sub-types, mTORc1 and mTORc2, both of which can be activated by
molecules in the PI3K signaling pathway. mTORC1 activation is
required for myofiber synthesis and skeletal muscle hypertrophy
(4,5). mTOR can transmit intracellular
signals to downstream proteins and regulate cell proliferation. The
most important downstream target of mTOR is p70S6K, which is a key
molecule promoting protein synthesis and cell proliferation.
PI3K/AKT/mTOR-S6K has been proven to be a classical signaling
pathway in promoting cell differentiation and proliferation
(3–5).
Mechanical stress can directly influence muscle
growth by stretching myoblasts. In response to continuous force and
injuries, myoblasts always shift to the influenced regions in order
to repair the injuries. There are several molecules involving in
mechanical stress, such as PI3K, NF-κB and p38MAPK, all of which
are associated with cell differentiation, proliferation and
apoptosis (4,5). Overloading and continuous stress
usually induces apoptosis of myoblasts through activating the NF-κB
signaling pathway (4,5). Therefore, it was hypothesized that
there may be an association between mechanical stress
loading-activated PI3K expression and cell proliferation. However,
to the best of our knowledge, there are no studies investigating
the roles of the PI3K/AKT/mTOR signaling pathway in the
proliferation of myoblasts undergoing mechanical stress.
Therefore, the present study first investigated the
effects of mechanical stress on the proliferation of myoblasts.
Then, the signaling pathways that mediate stress-loading triggered
proliferation of myoblast were explored by evaluating the
expression of S6K, mTOR and AKT. Finally, the present study
addressed whether mechanical stress involves the cell cycle by
evaluating mTOR signaling pathway associated molecules.
Materials and methods
Cell culture and characterization of
C2C12 myoblasts
C2C12 myoblasts were purchased from American Type
Culture Collection. C2C12 cells were warmed in a 37°C water bath
and cultured in 5 ml DMEM (Gibco; Thermo Fisher Scientific, Inc.)
containing 10% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.) at 37°C. C2C12 cells were centrifuged at 1,000 ×
g for 5 min at 37°C. The supernatant was removed and 1 ml culture
medium was added. Cells were diluted with medium containing 20% FBS
and cultured at 37°C with appropriate humidity and 5%
CO2 for6, 12, 24, 48 or 72 h, according to the different
experimental protocols. In the indicated experiments, the C2C12
cells were also treated with the PI3K inhibitor LY294002 (cat. no.
A8250; APExBio Technology LLC).
Cells in culture bottles were washed with PBS2-3
times (10 min each time). Then, cells were digested using
pancreatic enzyme (Beyotime Institute of Biotechnology) with
shaking at 37°C for 2–5 min. When cells had shrunk and fallen off
the walls of the culture bottles, the cells were collected (2–3 ml)
and added to centrifugal tubes for following experiments or
tests.
Establishment of cyclic mechanical
stress model of C2C12 myoblasts
When adherent cells achieved a confluence of 70–80%,
they were washed with PBS2-3 times (10 min each time). The cells
were then digested using pancreatic enzyme at 37°C for 2–5 min.
When cells had shrunk and fallen off the walls of the culture
bottles, 2–3 ml of cells were collected, added to centrifuge tubes
and adjusted to a density of 5×104 cells/ml. Then, the
cells were added into 24-well plates (Corning, Inc.) for subsequent
experiments. C2C12 myoblasts were administrated with cyclic
mechanical stress at 5% (stress 1 group), 10% (stress 2 group), 15%
(stress 3 group) deformation and 0.5 Hz (30 cycles/min) for 1, 6,
12 and 24 h, using FX-4000 strain unit (Flexcell International
Corp.). Cyclic mechanical stress loading was conducted at room
temperature. Non-loaded control cells were cultured on a 24-well
plate and kept in the same incubator. The mechanical stress loading
experiment was conducted at least 3 times (10 min each time) and
the data at each time-point were collected.
Reverse transcription-quantitative
(RT-q) PCR
Total RNA of cultured cells was extracted using
TRIzol® reagent (Invitrogen; Thermo Fisher Scientific,
Inc.), according to manufacturer's instruction. cDNA was
synthesized using the QuantiTect reverse transcription kit (cat.
no. 205311; Qiagen GmbH), following the supplier's protocol. The
BeyoFast SYBR-Green qPCR Kit (cat. no. D7265; Beyotime Institute of
Biotechnology) was used to amplify the target genes. The
gene-specific primers used in the present study were synthesized
based on the gene sequences listed in GenBank (https://www.ncbi.nlm.nih.gov/genbank/).
Primer sequences for PCR are listed in Table I. The gene expression was
quantified by normalizing to the GAPDH gene expression using the
optimized comparative cycle threshold (2−ΔΔCq) method
(6).
 | Table I.Primer sequences for reverse
transcription-quantitative PCR. |
Table I.
Primer sequences for reverse
transcription-quantitative PCR.
| Target gene | Gene ID | Primer | Primer sequence
(5′-3′) | Fragment size
(bp) |
|---|
| PI3K | 18708 | Forward |
AAGCCATTGAGAAGAAAGGACTG | 176 |
|
|
| Reverse |
ATTTGGTAAGTCGGCGAGATAG |
|
| 4EBP1 | 13685 | Forward |
GGGAGGAACCAGGATTATCTATG | 121 |
|
|
| Reverse |
ATCGCTGGTAGGGCTAGTGAC |
|
| GAPDH | 14433 | Forward |
GAGACCTTCAACACCCCAGC | 263 |
|
|
| Reverse |
ATGTCACGCACGATTTCCC |
|
Western blotting
Cells were digested and harvested in RIPA lysis
buffer (Beyotime Institute of Biotechnology). The lysates were
centrifuged at 15,000 × g and 4°C for 30 sec. The concentrations of
the lysates were evaluated using thebicinchoninic acid kit (cat.
no. P0010S; Beyotime Institute of Biotechnology). Then, a total of
2 µg proteins were separated using 8–15% SDS-PAGE, and
electrotransferred onto polyvinylidene fluoride membranes (PVDF).
The PVDF membranes were blocked with 5% lipid milk in Tris-buffered
saline containing 0.05% Tween-20 (TBST) for 2 h at room
temperature. PVDF membranes were also incubated using primary
antibodies (1:1,000) at 4°C overnight. Subsequently, PVDF membranes
were incubated with secondary antibodies (1:1,000) at room
temperature for 1.5 h. Following washing with TBST four times (10
min each), protein signals were detected using an enhanced
chemiluminescence kit (cat. no. 32109; Pierce; Thermo Fisher
Scientific, Inc.). Primary antibodies were as follows: Rabbit
anti-p-mTOR (cat. no. ab109268; Abcam), rabbit anti-p-AKT (cat. no.
ab38448; Abcam), rabbit anti-4EBP1 (cat. no. ab32024; Abcam),
rabbit internal anti-β-actin (cat. no. ab5694, Abcam). The
secondary antibody sheep anti-rabbit IgG (cat. no. SAB3700918;
Sigma-Aldrich; Merck KGaA) was used. When evaluating cyclin D
expression, cells were separated into normal cells group and stress
loading cells group. The primary antibodies, including rabbit
anti-p-S6 (cat. no. ab109393; Abcam), rabbit anti-cyclin D (cat.
no. ab16663; Abcam), rabbit internal GAPDH (cat. no. ab9485; Abcam)
were used. The sheep anti-rabbit IgG (cat. no. SAB3700918;
Sigma-Aldrich; Merck KGaA) was employed as the secondary antibody.
Finally, the western blotting images were captured and analyzed
using Labworks Analysis Software (version 3.0; Labworks LLC).
Cell counting kit-8 (CCK-8) assay
C2C12 cells were separated into normal group and
normal + stress group, and cultured in 10% FBS solution. After
inoculating in 96-well plates for 1–4 h, C2C12 cells were analyzed
using the CCK-8 assay (cat. no. C0037; Beyotime Institute of
Biotechnology), following the protocol of the manufacturer. The 450
nm absorbance was read using a microplate reader. A blank plate and
control plate were also set up at the same time. Blank groups were
treated with CCK-8 solution without cells, and control plate groups
were treated with normal cells without stress administration.
following the above tests, cell vitality was calculated and the
cell inhibition rate evaluated.
Immunofluorescence assay
Cultured C2C12 cells were washed using PBS three
times (10 min each time), and then fixed with 4% paraformaldehyde
(Sigma-Aldrich; Merck KGaA) for 15 min at room temperature. After
treating cells with 0.5% Triton X-100 (Sigma-Aldrich; Merck KGaA)
for 15 min, 6% goat serum (Gibco; Thermo Fisher Scientific, Inc.)
was added for 30 min at room temperature. Then, cells were
incubated with rabbit anti-PI3K monoclonal antibody (cat. no.
ab32089; Abcam) overnight at 4°C. After washing with PBS, cells
were incubated with goat anti-rabbit Alexa Fluor 647-conjugated
antibody (cat. no. ab150083;Abcam) for 30 min in the dark. Finally,
cells were counted on a glass slide and observed using a confocal
laser scanning microscope (FluoView FV1200; Olympus
Corporation).
Flow cytometry assay
Cells at 60–70% confluence were harvested and
treated with mechanical stress. Cells were washed twice with PBS
(10 min each time) and centrifuged at 5,000 × g for 5 min.
Apoptosis was evaluated using the Annexin V-PE/7-AAD Apoptosis
Detection Kit (cat. no. CA1030; Beijing Solarbio Science &
Technology Co., Ltd.). Briefly, 5 µl 7-AAD dye solution in binding
buffer was added to the cells at room temperature in the dark for
5–15 min, and then mixed with 450 µl binding buffer. Following
mixing with 1 µl Annexin V-PE for 5–15 min, cells were analyzed
using a flow cytometer (Beckman Coulter, Inc.). The data was
analyzed using the Flow Cytometer System II software (version 3.0;
Beckman Coulter, Inc.).
Statistical analysis
Data were presented as mean ± standard deviation and
analyzed using GraphPad Prism 7 software (GraphPad Software, Inc.).
Differences between treatment group and normal group were conducted
using Student's t-test. Tukey's post-hoc test was used to validate
the two-way analysis of variance for comparing differences among
multiple groups. All of the experiments or tests were repeated ≥3
times. P<0.05 was considered to indicate a statistically
significant difference.
Results
C2C12 myoblasts culture and
characterization
Representative images of the features of C2C12 cells
are shown in Fig. 1. Regular cells
were spread and distributed in Fig.
1A. After 6 h stress, cells were arranged regularly when
compared with the normal group (Fig.
1B). Following 12 and 24 h cyclic stress, cells conforming to
loading direction are shown in Fig. 1C
and D.
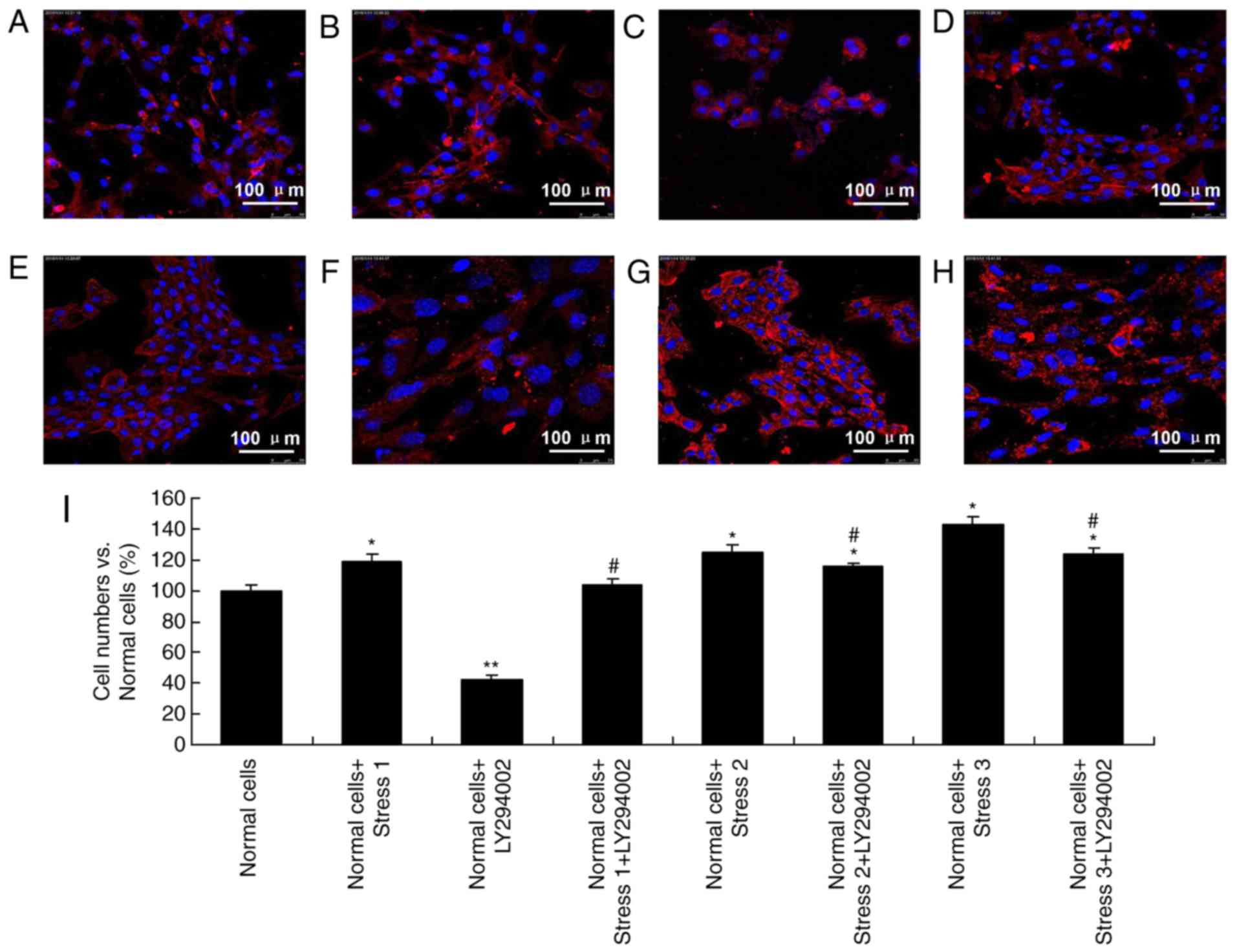
Immunofluorescence
Due to the important roles of cycle stress in
influencing the PI3K signaling pathway, immunofluorescence was used
to observe the status of C2C12 myoblasts. Cell counts of each group
were compared with cell counts in the normal group (Fig. 2A). Cells after treatment with
LY294002 and loading stress (stress 1), demonstrated obvious
changes of morphology (Fig. 2).
Comparing with the normal group, loading stress 1-treated cells
were distributed following the tension direction and oriented in
the direction of the stress source (Fig. 2A and B). Following the
administration of inhibitors, the quantity of normal cells
decreased (Fig. 2C). When cells
were treated with cyclic tension and inhibitors together, cell
numbers were slightly increased (Fig.
2D). Following exposure to increased stress (stress 2), the
number of cells increased, and cell morphology changed to a shuttle
shape (Fig. 2E). Nuclear
fragmentation and lysates were barely detected. Following inhibitor
treatment, cells were scattered compared with cells in the normal
group (Fig. 2E-G). In addition,
following further stress (stress 3), the cells became apoptotic
(Fig. 2G and H). A quantification
of these changes is presented in Fig.
2I.
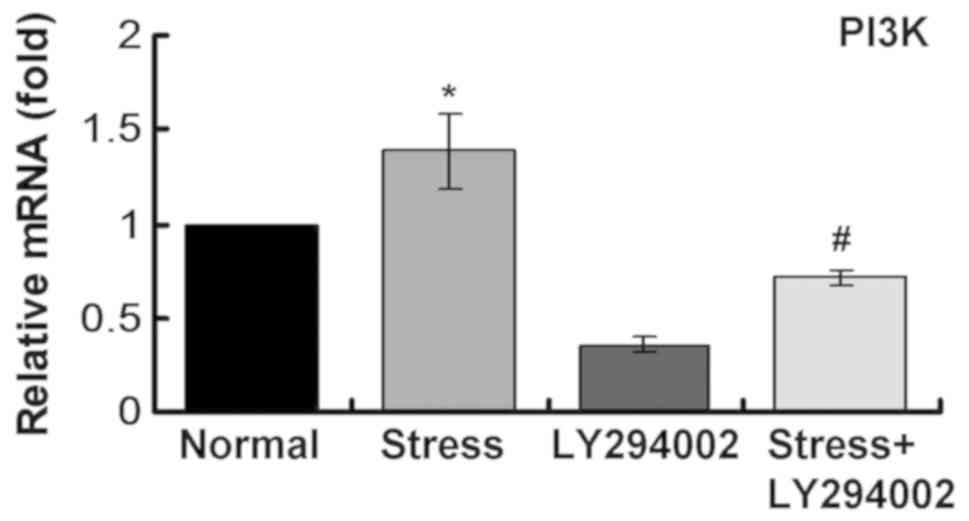
Mechanical stress upregulates PI3K in
C2C12 cells
The mRNA expression levels of PI3K were determined
using RT-qPCR. The results demonstrated that mechanical stress
upregulated PI3K mRNA expression (Fig.
3). mRNA expression levels of PI3K decreased significantly
following inhibitor treatment (Fig.
3). Following mechanical stress and inhibitor administration to
C2C12 cells, PI3K mRNA expression levels were clearly reduced
compared with the single stress loading group (Fig. 3). These results suggest that the
PI3K signaling pathway may be activated following mechanical stress
loading.
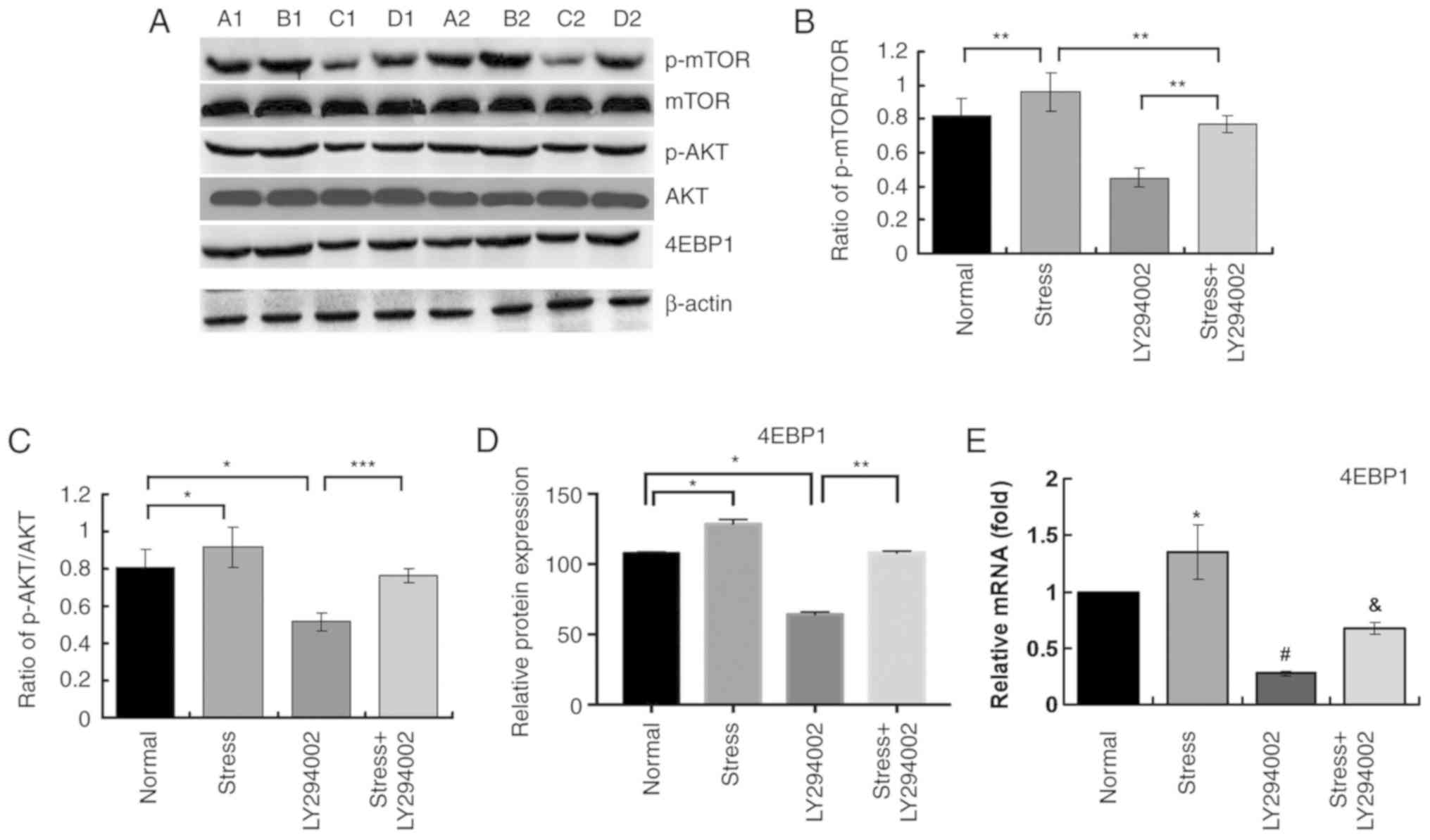
Mechanical stress promotes
phosphorylation of AKT and mTOR
To further examine whether the PI3K signaling
pathway is activated by mechanical stress, western blotting was
used to evaluate the phosphorylation of AKT and mTOR. First, 24 h
loading stress was selected as the peak expression time-point and
β-actin was used as the internal control. Following exposure to
stress, p-AKT and p-mTOR reached their peak levels. Then, C2C12
cells were treated with LY294002, and p-AKT/AKT and p-mTOR/mTOR
ratios determined. The ratios of p-AKT/AKT and p-mTOR/mTOR declined
significantly. With cells treated with both inhibitor and
mechanical stress, expressions of p-mTOR and p-AKT significantly
increased compared with inhibitor alone, as demonstrated in
Fig. 4A-C. It should be noted that
ratios of p-AKT/AKT and p-mTOR/mTOR (phosphorylation of AKT and
mTOR) were lower in inhibitor-treated cells undergoing loading
stress compared with the normal cells undergoing loading stress.
Collectively, these results confirmed the hypothesis that cyclic
stress not only activates PI3K signaling pathway but also increases
the expression of mTOR.
Mechanical stress directly promotes
4EBP1 expression in C2C12 myoblasts by activating the PI3K
pathway
As demonstrated in Fig.
4A and D, it was found that protein levels of 4EBP1increased
when stress loading was administrated to cells. Meanwhile, when
blocking PI3K signaling pathway, 4EBP1 levels declined
significantly. However, when treating cells with both inhibitor and
stress, the protein expression levels of 4EBP1 were significantly
reduced compared with the Stress alone group (Fig. 4A and D). Similar results were
obtained for the mRNA expression levels of 4EBP1 by RT-qPCR
analysis (Fig. 4E). Taken
together, these results suggest that mechanical stress might
modulate proliferation of myoblasts via activating the PI3K
signaling pathway.
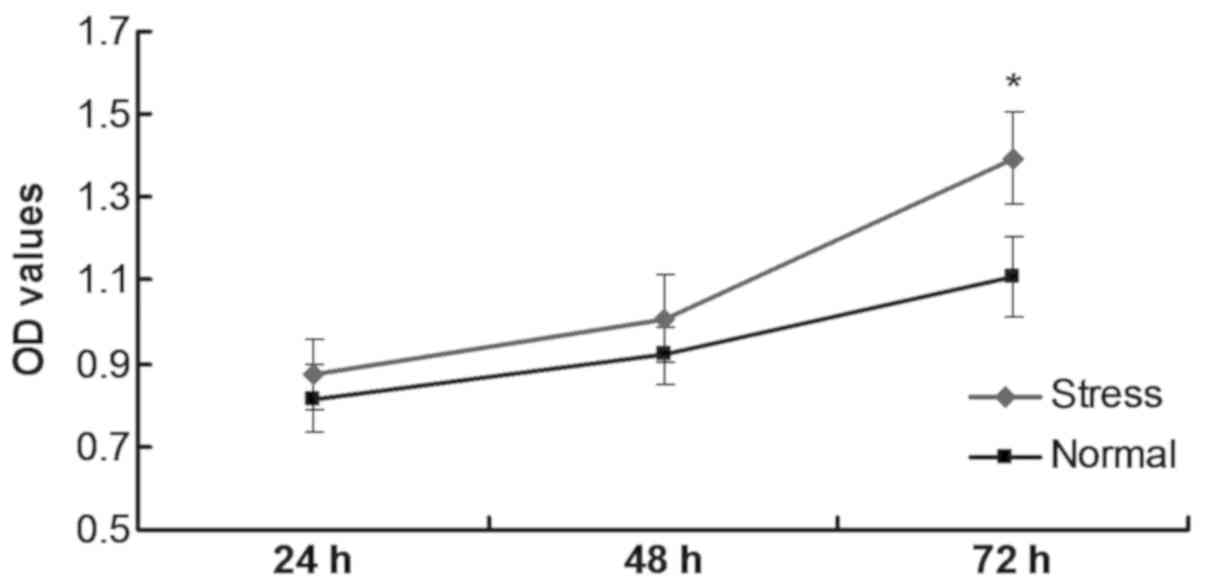
Mechanical stress promotes C2C12 cell
proliferation
C2C12 cells undergoing stress loading were separated
into the stress-loading group and the normal group. The
stress-loading group demonstrated increased proliferation compared
with the normal group, and this result was significant at 72 h
(P<0.05; Fig. 5). These data
indicated that mechanical stress promoted C2C12 proliferation.
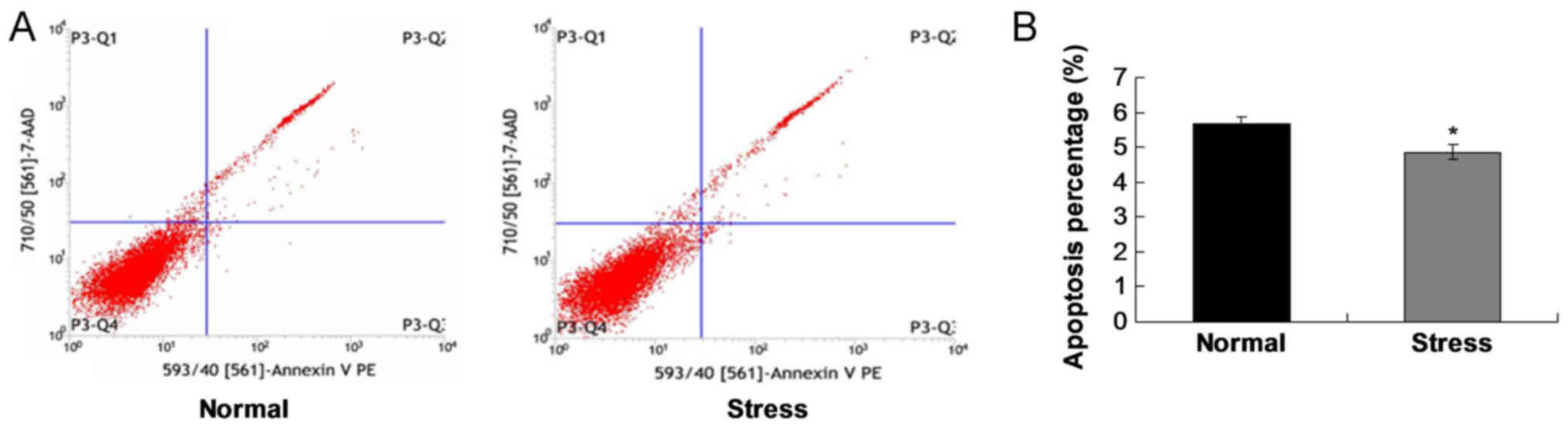
Mechanical stress inhibits C2C12 cell
apoptosis
To verify the effects of mechanical stress on cell
apoptosis, flow cytometry assay was conducted. The results
demonstrated that the apoptosis rate of the stress group was
significantly lower compared with the normal group (P<0.05;
Fig. 6). These results suggested
that stress loading inhibited apoptosis in C2C12 cells.
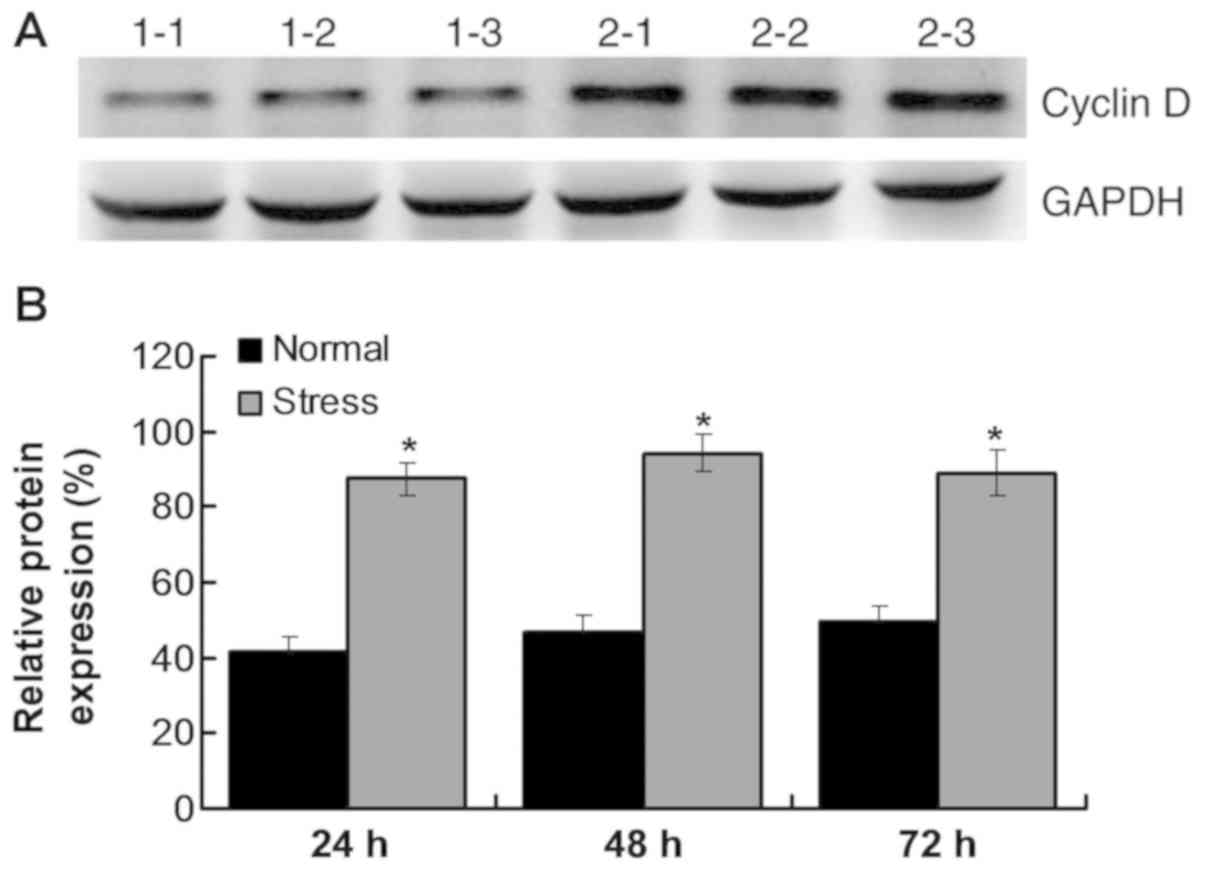
Mechanical stress regulates the cell
cycle by activating the PI3K/AKT/mTOR signaling pathway
An important cell cycle regulatory molecule, cyclin
D, was also detected. The results indicated that synthesis of
cyclin D was initiated during G1 phase. Cyclin D expression
increased after stress administration compared with the normal
group (P<0.05; Fig. 7A and B).
As a key downstream protein of the mTOR signaling pathway, cyclin D
can promote protein synthesis and trigger cell proliferation.
Therefore, the results suggest that mechanical stress can modulate
cell cycle and promote cell proliferation.
Discussion
A number of extracellular signaling pathways are
involved in regulating myoblasts proliferation. Mechanical stress
is considered to be a key factor in maintaining myoblast growth and
survival. Mechanical stress can regulate metabolism and gene
expression of myoblasts and serves important roles in skeletal
muscle formation and development (7–10).
In order to explore biological characteristics of
stress-loading in myoblasts, intermittent cyclic stress was
selected as an experimental strategy. Zhang et al (11) found that cyclic tensile stress
serves critical roles in activating downstream signaling molecules,
such as focal adhesion kinase (FAK) and Ras homolog gene family
member A. Charrasse et al (12) identified that M-cadherin is an
intermediate signaling pathway that is involved in the process of
myoblasts fusing into muscle tubes. Kumar et al (13) demonstrated that cyclic stress can
inhibit the differentiation of myoblasts by activating the
FAK/Rac-1/GTPase/NF-κB signaling pathway. Formigli et al
(14) identified that ion-channels
serve important roles in cyclic tensile processes. In addition,
several other studies (4,5,13)
confirm that the NF-κB signaling pathway is involved in the
apoptosis of myoblasts undergoing cyclic stress loading. Xiao et
al (15) argued that
stretch-induced connective tissue grow factor expression is
mediated and modulated by the PI3K/JNK-dependent signaling pathway.
However, few studies (4,5,14,15)
have clarified how the PI3K signaling pathway serves roles in the
proliferation of myoblasts undergoing mechanical stress. In the
present study, cyclic mechanical stress at 10% elongation with 0.5
Hz was used to simulate oral biomechanical stimulation on C2C12
myoblasts.
The mRNA expression levels of several genes,
including MyoD, Myogenin, MRF4 and Myf5, have been reported to be
associated with mechanical stress (16). As a conclusive gene in
differentiation of cells, MyoD promotes differentiation of
myoblasts into skeletal muscles. MyoD and Myf5are usually
over-expressed in myoblasts. In addition, recent researches
(13,15) have also demonstrated that 5%
stretch stress can promote the proliferation of myoblasts. Stretch
stress also inhibits Myogenin expression, which further regulates
cell differentiation. When stretch is administrated at >10%,
numbers of PARP shear content exist. Then, 15% stress with 10
cycles/min induces decreasing survival rates for myoblasts.
Therefore, stress can accelerate proliferation, but higher stresses
will promote apoptosis (17).
There are several factors involving in myoblasts
proliferation, such as insulin-like growth factor 1 (IGF-1), zinc
and follistatin. Mechanical stress constantly stimulates protein
and mRNA synthesis in first hours of stretching (18), which is consistent with the results
of the present study. Therefore, the relationship between
mechanical stress and PI3K signaling pathway is precise. Many
studies have demonstrated that stress activates proliferation, but
over-expressed PI3K leads to tumor growth (13–15).
PI3K/AKT is a classic pathway in connecting normal proliferating
cells and tumor cells. The present study investigated the levels of
p-AKT, p-mTOR and p-4EBP1. First, it was confirmed that stress can
activate AKT. Second, levels of p-AKT decreased after the
inhibition of the PI3K signaling pathway without stress. Third, it
was also demonstrated that when both of stress and LY294002 were
used on cells, levels of p-AKT only declined a little. These
results suggest that mechanical stress may promote proliferation of
myoblasts by activating the PI3K signaling pathway.
mTOR, an important downstream factor of the PI3K/AKT
signaling pathway in transduction process, was also examined in the
present study. Examination of mTOR may further demonstrate the
cooperative effects of mechanical stress on the proliferation of
cells via triggering PI3K signaling pathway. The present study
demonstrated that mTOR also participates in mechanical stress
induced cell proliferation. In addition, AKT promoted mTOR
transcription by triggering mTOR mRNA expression in stress
stimulated myoblasts. Blocking the PI3K signaling pathway resulted
in a significant reduction of mTOR expression in cells undergoing
stress loading. However, a number of other signaling pathways may
also participate in this process.
Although expression of 4EBP1 was decreased, the
levels were not stable. Therefore, unsteady expression of 4EBP1 was
an unexpected finding. When two factors were administrated to
myoblasts together, p-4EBP1 levels were unstable. This result
suggested that PI3K/AKT had no direct effects on 4EBP1
proliferation, while higher expression of mTOR resulted in PI3K
signaling pathway activation (17,18).
4EBP1 expression is not influenced by mechanical stress directly,
therefore there may be other mechanisms involving in 4EBP1
expression (19,20).
The PI3K/AKT signaling pathway was activated by
mechanical stress and resulted in increased p-mTOR expression,
which further enhanced 4EBP1 expression. LY294002 treatment
inhibited PI3K activation and reduced p-mTOR levels. In the present
study, mRNA expression of PI3K signaling pathway associated genes
was connected with p-mTOR expression, which suggested that
activated mTOR can regulate targeting genes. Therefore, it can be
concluded that the PI3K signaling pathway not only promoted cell
proliferation, but also phosphorylated mTOR in mechanical stress
loaded cells. mTOR serves an irreplaceable role in myoblasts
proliferation under mechanical stress loading.
In order to confirm the proliferative effects of
mechanical stress on C2C12 cells, a CCK-8 assay was performed. The
results demonstrated that C2C12 proliferation was significantly
increased following mechanical stress. It was hypothesized that
proliferation and apoptosis may not always be consistently altered.
Therefore, flow cytometry was conducted to examine the apoptosis
rate. The results indicated that apoptosis was suppressed in
mechanical stress-loaded cells. Therefore, stress inhibition may
promote apoptosis, while enhanced mechanical stress in loading
groups may result in increased proliferation by activating the PI3K
signaling pathway.
The effects of mechanical stress on C2C12
proliferation may be associated with PI3K signaling pathway in two
ways: i) PI3K significantly promotes cell proliferation of
stress-stimulated C2C12 cells (13,15),
while other signaling pathways may also participate in the
stress-mediated proliferation of cells; or ii) PI3K significantly
inhibits stress-caused cell apoptosis (13–15).
In the present study, when compared with normal cell groups, stress
groups demonstrated a lower expression of PI3K.
A previous study reported that mTOR can be activated
in distinct ways (21). mTOR can
always be activated via phosphorylating or activating p70S6K in a
mTOR-dependent pathway, triggering IGF-1/easing protein synthesis
(22,23). The present study also demonstrated
that mechanical stress activated mTOR, especially for mTORc1.
Cyclin D mainly includes cyclin D1,cyclin D2 and
cyclin D3 (24). Inhibition of
cyclin D leads to cell cycle arrest and cell differentiation
(25). Cyclin D is regulated by a
downstream pathway of mitogen receptors via activating PI3K and
glycogen synthase kinase three β (GSK3β) (25). GSK3β can also cause cyclin D
degradation by inhibiting phosphorylation of cyclin D molecules
(25). GSK3β is negatively
modulated by PI3K signaling pathway in form of phosphorylation, and
is associated with the expression of cyclin D (25). The present study established cyclin
D as a biomarker for C2C12 cells undergoing stress stimuli. Its
results suggested that mechanical stress can promote cell cycle of
C2C12 cells by activating proliferation and activating the PI3K
signaling pathway. However, the deeper mechanisms of this process
have not been elucidated in the present study.
A few published studies (26–28)
report that proliferation of C2C12 myoblasts is associated with the
PI3K/AKT/mTOR signaling pathway. However, the present study
investigated the effects of mechanical stress on C2C12
proliferation by exploring the PI3K/AKT signaling pathway and
evaluating the apoptosis process for the first time, to the best of
the authors' knowledge. The present study provided insight for
clarifying the molecular mechanism of intracellular stress in C2C12
cells and may be of benefit to studies investigating skeletal
muscle cell associated disorders.
Although the present study provided several notable
results, there were also a few limitations. First, it only used
CCK-8 assay to evaluate proliferation of C2C12 cells. It might be
more convincing for the conclusions if other methods had been used
to examine proliferation. Second, the specific mechanism for the
potential effects of stress loading on cell proliferation has not
been clarified. Future studies should examine the cell cycle phase
distribution of cells undergoing stress stimuli. Third, the
expression levels of the total and the phosphorylated PI3K were not
examined in the present study.
In conclusion, the present study demonstrated that
mechanical stress promotes C2C12 proliferation by activating the
PI3K/AKT signaling pathway and inhibiting the apoptosis process.
These results would contribute to a better understanding for
mechanism of functional appliance via PI3K/AKT signaling
pathway.
Acknowledgements
Not applicable.
Funding
The present study was supported by The Nanjing
Science and Technology Development Project (2016; grant no.
201605067).
Availability of data and materials
All data generated or analyzed during this study are
included in this published article.
Authors' contributions
YD, YM, MW and XY performed the experiments. YD and
YM designed the study and wrote the manuscript. FY and FZ conducted
the statistical analysis. WL conducted the literature review. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Zhang B, Liu Y, Li Y, Zhe X, Zhang S and
Zhang L: Neuroglobin promotes the proliferation and suppresses the
apoptosis of glioma cells by activating the PI3K/AKT pathway. Mol
Med Rep. 17:2757–2763. 2018.PubMed/NCBI
|
|
2
|
Rafalski VA and Brunet A: Energy
metabolism in adult neural stem cell fate. Prog Neurobiol.
93:182–203. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hay N and Sonenberg N: Upstream and
downstream of mTOR. Genes Dev. 18:1926–1945. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Brook MS, Wilkinson DJ, Phillips BE,
Perez-Schindler J, Philp A, Smith K and Atherton PJ: Skeletal
muscle homeostasis and plasticity in youth and ageing: Impact of
nutrition and exercise. Acta Physiol (Oxf). 216:15–41. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Brioche T, Pagano AF, Py G and Chopard A:
Muscle wasting and aging: Experimental models, fatty infiltrations,
and prevention. Mol Aspects Med. 50:56–87. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2 (-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fujita H, Hida M, Kanemoto K, Fukuda K,
Nagata M and Awazu M: Cyclic stretch induces proliferation and
TGF-beta1-mediated apoptosis via p38 and ERK in ureteric bud cells.
Am J Physiol Renal Physiol. 299:F648–F655. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nakai N, Kawano F, Oke Y, Nomura S, Ohira
T, Fujita R and Ohira Y: Mechanical stretch activates signaling
events for protein translation initiation and elongation in C2C12
myoblasts. Mol Cells. 30:513–518. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wang BW, Chang H and Shyu KG: Regulation
of resistin by cyclic mechanical stretch in cultured rat vascular
smooth muscle cells. Clin Sci (Lond). 118:221–230. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shyu KG, Wang BW, Lin CM and Chang H:
Cyclic stretch enhances the expression of toll-like receptor 4 gene
in cultured cardiomyocytes via p38 MAP kinase and NF-kappaB
pathway. J Biomed Sci. 17:152010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang SJ, Truskey GA and Kraus WE: Effect
of cyclic stretch on beta1D-integrin expression and activation of
FAK and RhoA. Am J Physiol Cell Physiol. 292:C2057–C2069. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Charrasse S, Comunale F, Fortier M,
Portales-Casamar E, Debant A and Gauthier-Rouviere C: M-cadherin
activates Rac1 GTPasethrough the Rho-GEF trio during myoblast
fusion. Mol Biol Cell. 18:1734–1743. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kumar A, Murphy R, Robinson P, Wei L and
Boriek AM: Cyclic mechanical strain inhibits skeletal myogenesis
through activation of focal adhesion kinase, Rac-1 GTPase, and
NF-kappaB transcription factor. FASEB J. 18:1524–1535. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Formigli L, Meacci E, Sassoli C, Squecco
R, Nosi D, Chellini F, Naro F, Francini F and Zecchi-Orlandini S:
Cytoskeleton/stretch-activated ion channel interaction regulates
myogenic differentiation of skeletal myoblasts. J Cell Physiol.
211:296–306. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xiao LW, Yang M, Dong J, Xie H, Sui GL, He
YL, Lei JX, Liao EY and Yuan X: Stretch-inducible expression of
connective tissue growth factor (CTGF) in human osteoblasts-like
cells is mediated by PI3K-JNK pathway. Cell Physiol Biochem.
28:297–304. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Abe S, Rhee S, Iwanuma O, Hiroki E,
Yanagisawa N, Sakiyama K and Ide Y: Effect of mechanical stretching
on expressions of muscle specific transcription factors MyoD,
Myf-5, myogenin and MRF4 in proliferated myoblasts. Anat Histol
Embryol. 38:305–310. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Peltier J, O'Neill A and Schaffer DV:
PI3K/Akt and CREB regulate adult neural hippocampal progenitor
proliferation and differentiation. Dev Neurobiol. 67:1348–1361.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sui L, Wang J and Li BM: Role of the
phosphoinositide 3-kinase-Akt-mammalian target of the rapamycin
signaling pathway in long-term potentiation and trace fear
conditioning memory in rat medial prefrontal cortex. Learn Mem.
15:762–776. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Datan E, Shirazian A, Benjamin S, Matassov
D, Tinari A, Malorni W, Lockshin RA, Garcia-Sastre A and Zakeri Z:
mTOR/p70S6K signaling distinguishes routine, maintenance-level
autophagy from autophagic cell death during influenza A infection.
Virology. 452-453:175–190. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ci Y, Shi K, An J, Yang Y, Hui K, Wu P,
Shi L and Xu C: ROS inhibit autophagy by downregulating ULK1
mediated by the phosphorylation of p53 in selenite-treated NB4
cells. Cell Death Dis. 5:e15422014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chung J, Kuo CJ, Crabtree GR and Blenis J:
Rapamycin-FKBP specifically blocks growth-dependent activation of
and signaling by the 70 kd S6 protein kinases. Cell. 69:1227–1236.
1992. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chiang GG and Abraham RT: Phosphorylation
of mammalian target of rapamycin (mTOR) at Ser-2448 is mediated by
p70S6 kinase. J Biol Chem. 280:25485–25490. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Rahman H, Qasim M, Oellerich M and Asif
AR: Identification of the novel interacting partners of the
mammalian target of rapamycin complex 1 in human CCRF-CEM and
HEK293 cells. Int J Mol Sci. 15:4823–4836. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Chang H, Liu YH, Wang LL, Wang J, Zhao ZH,
Qu JF and Wang SF: MiR-182 promotes cell proliferation by
suppressing FBXW7 and FBXW11 in non-small cell lung cancer. Am J
Transl Res. 10:1131–1142. 2018.PubMed/NCBI
|
|
25
|
Diehl JA, Cheng M, Roussel MF and Sherr
CJ: Glycogen synthase kinase-3beta regulates cyclin D1 proteolysis
and subcellular localization. Genes Dev. 12:3499–3511. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Matheny RW and Adamo ML: Effects of PI3K
catalytic subunit and Akt isoform deficiency on mTOR and p70S6K
activation in myoblasts. Biochem Biophys Res Commum. 390:252–257.
2009. View Article : Google Scholar
|
|
27
|
Hu SY, Tai CC, Li YH and WU JL:
Progranulin compensates for blocked IGF-1 signaling to promote
myotube hypertrophy in C2C12 myoblasts via the PI3K/Akt/mTOR
pathway. FEBS Lett. 586:3485–3492. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kitakaze T, Sakamoto T, Kitano T, Inoue N,
Sugihara F, Harada N and Yamaji R: The collagen derived dipeptide
hydroxyprolyl-glycine promotes C2C12 myoblast differentiation and
myotube hypertrophy. Biochem Biophys Res Commun. 478:1292–1297.
2016. View Article : Google Scholar : PubMed/NCBI
|