Introduction
Bronchopulmonary dysplasia (BPD) is an acquired form
of chronic lung disease mainly induced by hyperoxia. The general
pathogenesis of BPD is attributed to oxidative stress and
inflammation injury, and the oxygen free radicals and inflammatory
factors are important reasons for hyperoxia-induced lung injury
(1). The mechanisms of
hyperoxia-induced lung injury in BPD involved pulmonary endothelial
cell barrier disruption, increased vascular permeability,
neutrophil invasion, alveolar hypoplasia and reduced capillary
development (2). Previous study
reported that overexpression of heme oxygenase-1 (HO-1) in lung
epithelial cells of neonatal mice model of BPD attenuated lung
inflammation, pulmonary arterial remodeling and vascular leak,
markedly reduced thickening of alveolar septa and reserved vessel
density, attenuated the histological injury of BPD (3). In a rat model of BPD by
lipopolysaccharide (LPS), the expression of interleukin 6 (IL-6) in
lung tissues increased (4).
Inflammatory marker monocyte chemotactic protein 1 (MCP-1) in
newborn mouse exposed to 85% O2 was higher than those
exposed to room air (5). Although
pathogenesis of BPD is widely discussed, but the effective
treatment is still intractable. Application of glucocorticoids,
especially inhaled glucocorticoids, showed some therapeutic effect
for BPD, but was not recommended as the preferred treatment due to
its potential systemic damage (6–8).
Lipoxin A4 (LXA4) is a
metabolites of arachidonic acid and has dual powerful
anti-inflammatory and proresolution activities (9). Lipoxins have protective effects on
many inflammatory organ models, such as colitis (10), brain ischemia reperfusion injury
(11), asthma (12), acute pancreatitis (13). BML-111, an agonist of
LXA4 receptor, attenuated LPS-induced lung injury via
inhibition of expression of IL-6 and of activation of the protein
kinase B (Akt), extracellular signal-regulated kinase 1/2 (ERK1/2),
and p38 mitogen-activated protein kinase (p38 MAPK) signaling
pathways (14).
LXA4-imparted inhibition of IL-6 and IL-1β was related
to blockage of p38 MAPK and ERK1/2 (15). HO-1 is an important component of
the cellular defense enzyme that is induced by and acts against
oxidant-induced tissue injury (16). HO-1 overexpression mainly preserved
vascular growth and barrier function via antioxidant,
anti-inflammatory and iron-independent pathways to meliorate the
histological injury of BPD (3).
Our previous studies confirmed that LXA4 may protect
oxidative stress-induced injury of cardiomyocytes via HO-1
overexpression (17).
LXA4 amplified HO-1 gene expression in human corneal
epithelial cells (18).
LXA4 has glucocorticoids-like anti-inflammatory and
anti-oxidant effects without the side effects like glucocorticoids,
therefore becomes a promising therapeutic for BPD (19), since studies have shown that the
main causes of BPD were hyperoxia injury and inflammation (20). Up to now, it remains unclear
whether LXA4-imparted therapeutic effect for BPD is
mediated by upregulation of HO-1 and downregulation of IL-6 and
MCP-1. Given this background of above mentioned studies, we
hypothesized that LXA4 may protect hyperoxia-induced
lung epithelial cells injury via regulation of HO-1, IL-6 and
MCP-1, and LXA4-imparted regulation of IL-6 and MCP-1
may be related to modulation of p38 MAPK, ERK1/2 and Akt signaling
pathways. In the present studies, a classical hyperoxia-induced
cellular model of BPD in vitro was used to investigate the
protective effect of LXA4 on murine lung epithelial
cells against hyperoxia-induced injury.
Materials and methods
Reagents
Fetal bovine serum (FBS) was purchased from Gibco;
Thermo Fisher Scientific, Inc., Waltham, MA, USA. TRIzol reagents
were purchased from Invitrogen; Thermo Fisher Scientific, Inc..
LXA4 was obtained from Calbiochem (San Diego, CA, USA).
Rabbit anti-mouse threonine/tyrosine-dephosphorylated ERK1/2
(P-ERK1/2), total-ERK1/2, threonine/tyrosine-diphosphorylated p38
MAPK (P-p38 MAPK), total-P38 MAPK, serine-phosphorylated Akt
(P-Akt), total-Akt, β-actin antibodies were purchased from Cell
Signaling Technologies (Danvers, MA, USA). LY294002, an inhibitor
of the phosphotransferase activity of Akt, PD98059, an inhibitor of
ERK1/2 phosphorylation and SB203580, an inhibitor of p38 MAPK
phosphorylation, were obtained from Selleck Chemicals (Boston, MA,
USA). IL-6, MCP-1, rabbit anti-mouse IL-6 and anti-mouse MCP-1
antibodies were purchased from Pepro Tech (Rocky Hill, NJ, USA).
Rabbit anti-mouse HO-1 antibodies were obtained from Santa Cruz
Biotechnology (Dallas, TX, USA). Enzyme-linked immunosorbent assay
kits (ELISA) for IL-6, MCP-1 assessment were purchased from Assay
Designs (Ann Arbor, MI, USA). Prime Script™ RT reagent kit and SYBR
premix Ex Taq™ were obtained from Takara Bio Inc (Shiga, Japan).
Trypan blue, cell counting kit-8 (CCK-8) and superoxide dismutase
(SOD) kits were purchased from Nanjing Jiancheng Bioengineering
Institute (Nanjing, China). Zinc protoporphyrin-IX (ZnPP-IX), a
specific inhibitor of HO-1 activity, was obtained from
Sigma-Aldrich; Merck KGaA, Darmstadt, Germany.
Cell culture
Murine lung epithelial cells (MLE-12) cells were
obtained from Shanghai Institutes of Biological Sciences, Chinese
Academy of Medical Sciences. MLE-12 cells were cultured in
RPMI-1640 supplemented with 10% FBS, 100 µg/ml streptomycin and 100
U/ml penicillin in a 5% CO2 incubator at 37°C, The
medium was changed every 2–3 days. The cells were seeded into 6,
12, or 96-well cell culture plates for different experiments.
Logarithmic growth phase cells were prepared for experiment. The
cells were then cultured under 21% O2 or 85%
O2 for 12 h after pretreatment with or without
LXA4 (10 nmol/l), ZnPP-IX (10 µmol/l), IL-6 (10 ng/ml),
MCP-1 (10 ng/ml), anti-IL-6 (10 ng/ml) and anti-MCP-1 (10 ng/ml)
for 12 h, SB203580 (30 µmol/l), LY294002 (10 µmol/l) or PD98059 (40
µmol/l) for 30 min. For all cell stimulations, three independent
experiments were performed.
Measurement of cell survival rates and
viability
The cells were seeded into 12 and 96-well plates for
cell stimulation experiment, and then collected for determination
of cell survival rates and viability by Trypan blue exclusion and
CCK-8 respectively following the manufacturer's instructions.
SOD assay
MLE-12 cells were seeded into 12-well plates for 24
h, the supernatants were collected for determination of SOD using
SOD kits following the manufacturer's instructions.
Flow cytometry assay for
apoptosis
Identification of apoptotic cells was performed by
using allophycocyanin conjugated Annexin V labeled with fluorescein
isothiocyanate (FITC), following the recommendations of the
manufacturer. Necrotic cells were excluded by counter-staining with
2 µg/ml propidium iodide. Data were collected by using a
fluorescence activated cell sorter (FACS) Canto flow cytometer and
analyzed by using a FACS Diva software package.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) analysis
Expression of lipoxin receptor (ALX) mRNA was
determined by semi-quantitative PCR analysis using 10% gels.
Expressions of HO-1, IL-6 and MCP-1 mRNA were determined by RT-qPCR
analysis. Total RNA was isolated by using TRIzol reagent. The RNA
was reverse transcribed by the Prime Script™ RT reagent kit
following the manufacturer's instructions. The sets of ALX, HO-1,
IL-6, MCP-1 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH)
primers were selected by software-aided analysis (Primer Premier
5.0). The following sets of primers were used for ALX sense,
5′-GTTGAACACAGCTATCACGTTTGT-3′ and antisense,
5′-ACAACTCCTGTAAGAACTCGGAAA-3′ generating a 171-bp fragment, for
HO-1 sense, 5′-ACAGATGGCGTCACTTCG-3′ and antisense,
5′-TGAGGACCCACTGGAGGA-3′ generating a 128-bp fragment, for IL-6
sense, 5′-CGGAGAGGAGACTTCACAGAG-3′ and antisense,
5′-CATTTCCACGATTTCCCAGA-3′ amplifying a 105-bp fragment, for MCP-1
sense, 5′-CAACGAGATGCTCTGGGTAGA-3′ and antisense,
5′-TACCTCTTGGGACCCTCCT-3′ amplifying a 585-bp fragment, for GAPDH
sense, 5′-TGACAAACGGGACCTAAT-3′ and antisense,
5′-CTGGCACTGCACAAGAAG-3′ generating a 101-bp fragment. RT-qPCR was
performed by using StepOne™ Real-Time PCR System machine (Applied
Biosystems, Foster City, CA, USA). A typical cycling protocol was
consisted of three stages: 15 sec at 95°C for denaturation, 1 min
at 60°C for annealing, 15 sec at 95°C for extension, and an
additional 20 s for fluorescent signal acquisition. A total of 40
cycles were performed. The results were analyzed by computing the
Cq values for target gene in samples using the 2−∆∆Cq
method (21).
Immunofluorescence assay
Cellular HO-1 was determined by using
immunofluorescence assay. The cells were grown on glass coverslips
over 24-well plates, and then fixed with 4% paraformaldehyde,
washed and incubated with 5% BSA for 30 min at 37°C. The cells were
then incubated with the antibodies against HO-1 at 1:100 dilution
over night at 4°C. Subsequently, the cells were washed and
incubated with biotin-conjugated anti-rabbit IgG at 1:500 dilution,
followed by incubation with FITC-conjugated streptavidin for 1 h at
room temperature. Coverslips were flipped on slides, and images of
labeled cells were visualized by fluorescence microscopy (Axiovert
200 M; Carl Zeiss, Jena, Germany).
Western blot analysis
MLE-12 cells were collected, total proteins of the
cells were abstracted by using protein extraction kits following
the manufacturer instructions. Protein concentration was estimated
by using the BCA kit. The 50 µg of the protein was loaded for
SDS-polyacrylamide gel for 2 h before transferred onto PVDF
membranes. Nonspecific sites on the membranes were blocked for 1 h
in Tris buffered saline with Tween-20 (TBST) containing 5% nonfat
milk. The membranes were incubated with antibodies against HO-1 at
1:1,000 dilution, p38 MAPK, P-p38 MAPK, Akt, P-Akt, ERK1/2,
P-EKR1/2 at 1:1,000 dilution at 4°C overnight and washed with TBST.
The membranes were then incubated with horseradish
peroxidase-conjugated secondary antibodies for 1 h at 37°C. After
washing with TBST, signals were visualized by chemiluminescent
horseradish peroxidase substrate and normalized to β-actin.
ELISA of IL-6 and MCP-1
The levels of IL-6 and MCP-1 in cellular
supernatants were determined by using ELISA kits according to the
manufacturer's instructions.
Statistical analysis
Results are expressed as the mean ± standard error
of the mean. Experimental data were analyzed using one-way analysis
of variance followed by the Least Significant Difference post hoc
test and SPSS version 19.0 software (IBM Corp., Armonk, NY, USA).
P<0.05 was considered to indicate a statistically significant
difference.
Results
LXA4 alleviated
hyperoxia-induced cell injury
Normal MLE-12 cells were showed as polygonal-shaped
with full and integral appearance accompanied by good adhesion,
hyperoxia exposure led to alterations in cell morphology including
cell shrinkage, nonfullness and pyknosis/necrosis accompanied by
adhesion disability, however, pretreatment with LXA4
significantly protected the cells from the morphological changes
induced by hyperoxia exposure (Fig.
1A). Above results are consistent with the cell viability
analysis assessed by using CCK-8 assay (Fig. 1E) and the cell survival rates
(Fig. 1D) assessed by using trypan
blue exclusion (Fig. 1B). As shown
in Fig. 1F, the SOD levels were
decreased in the cells exposed to hyperoxia as compared to the
cells treated with air alone. However, pretreatment of the cells
exposed to hyperoxia with LXA4 increased the SOD levels
as compared to the cells undergoing hyperoxia alone. As elucidated
in Fig. 1C, the ALX was existed in
MLE-12 cells.
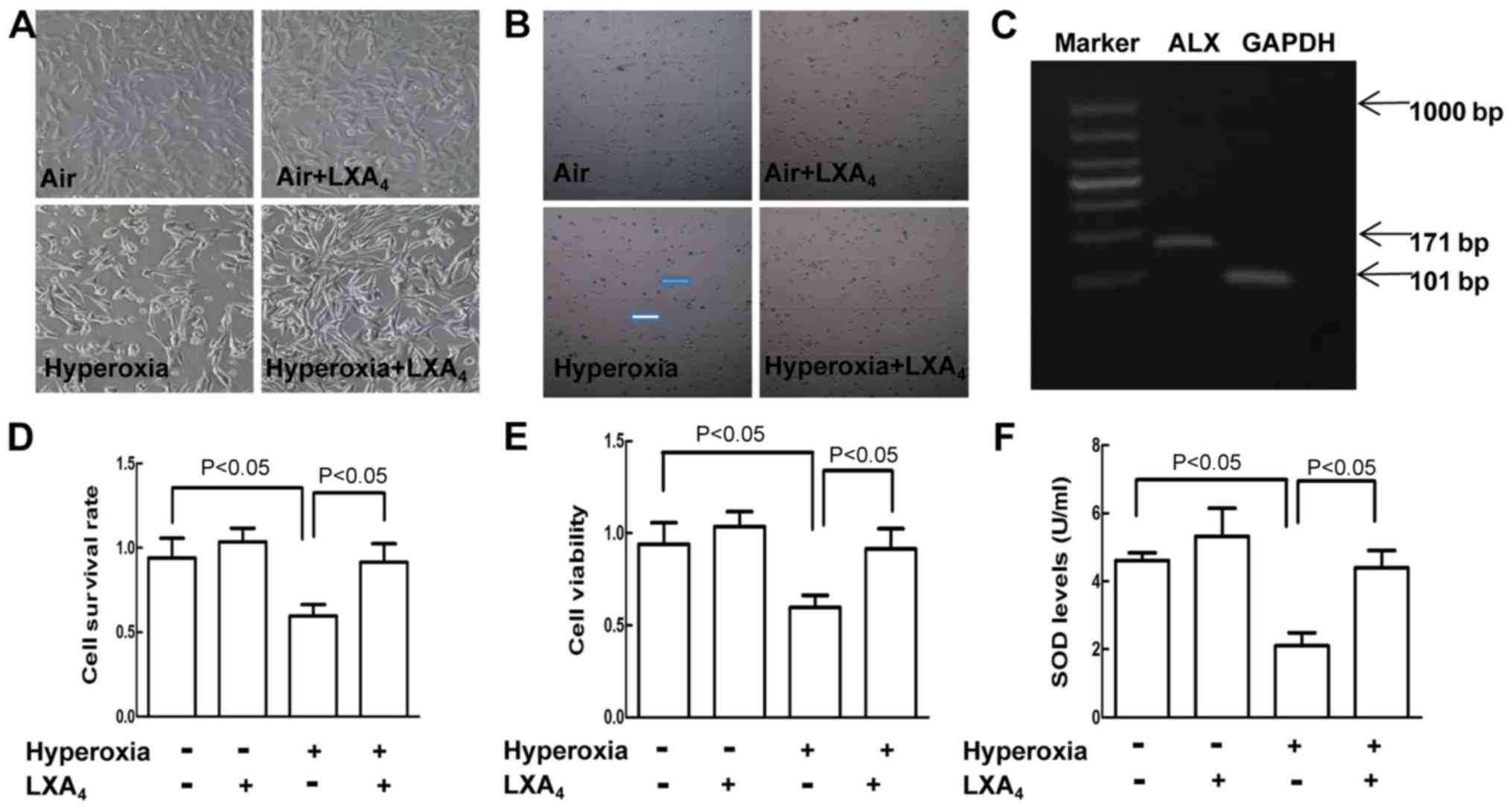 | Figure 1.Cellular morphology, survival rate,
viability and SOD release in MLE-12 cells. (A) The morphology of
the murine lung epithelial cell monolayer (magnification, ×100).
(B) Trypan blue staining of cells. The cells exposed to room air or
hyperoxia for 12 h were pretreated with or without LXA4
for 12 h. Blue arrow indicate the necrotic cells dyed blue, and the
white arrow denotes the living cells dyed transparent
(magnification, ×100). (C) The expression of ALX was evaluated by
semi-quantitative polymerase chain reaction in MLE-12 cells, and
the expression of GAPDH was used as the internal control. (D) Cell
survival rates were calculated by using the formula: Living
cells/(living cells + dead cells), based on the results of Trypan
blue cell staining. (E) Cell viability was measured by Cell
Counting kit-8 assay. (F) SOD levels in the cell supernatants were
measured using a SOD kit. Results are representative of three
independent experiments and are presented as the mean ± standard
deviation. P<0.05, as indicated. LXA4, lipoxin
A4; ALX, lipoxin receptor; SOD, superoxide dismutase;
GAPDH, glyceraldehyde-3-phosphate dehydrogenase; MLE-12, murine
lung epithelial cells. |
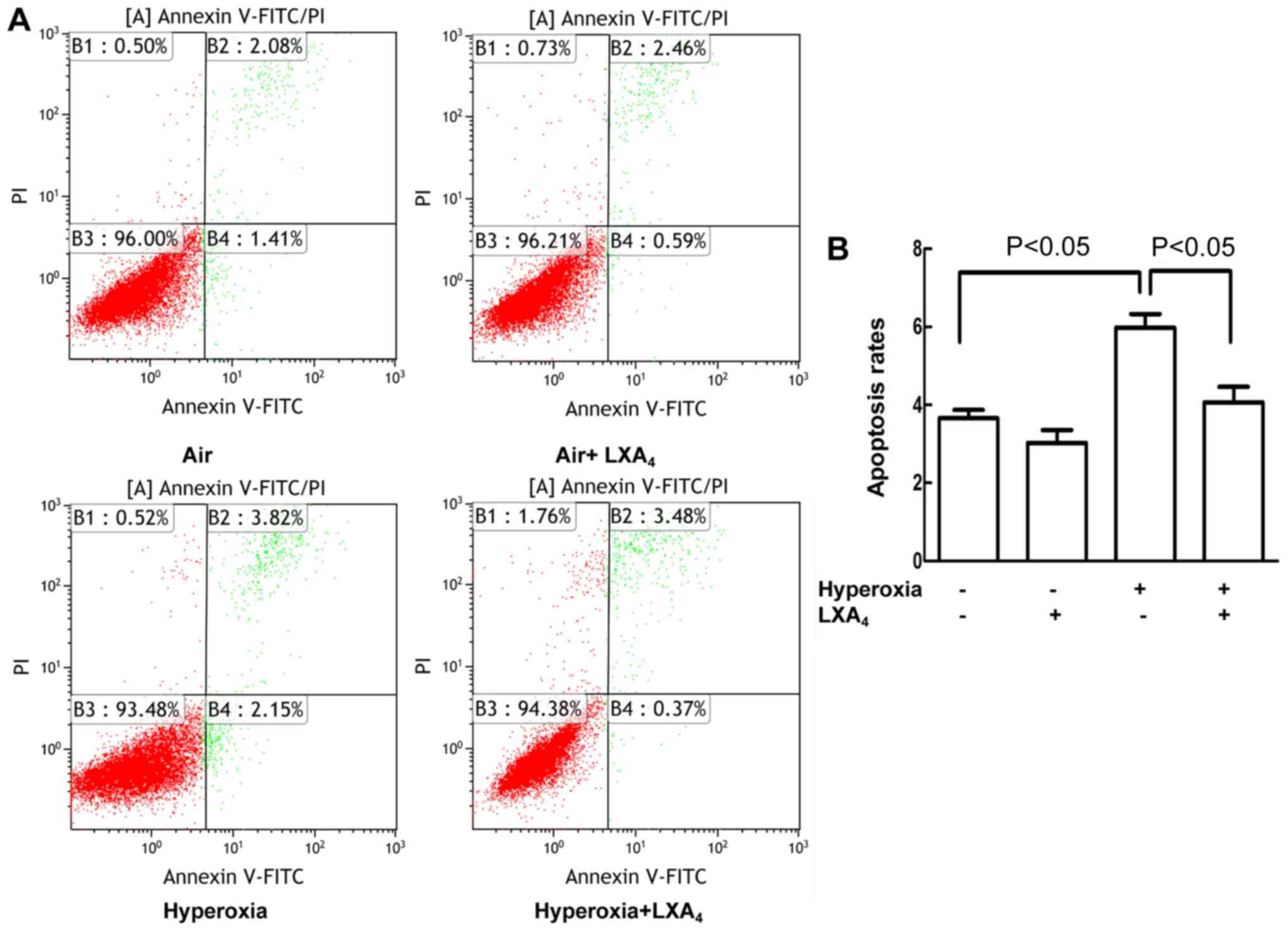
LXA4 reduced apoptosis
caused by hyperoxia
As presented in Fig. 2A
and B, the cell apoptosis rates were increased in the cells
exposed to hyperoxia as compared to the cells treated with air
alone. LXA4 reduced the hyperoxia-induced cell apoptosis
rates as compared to the cells undergoing hyperoxia alone.
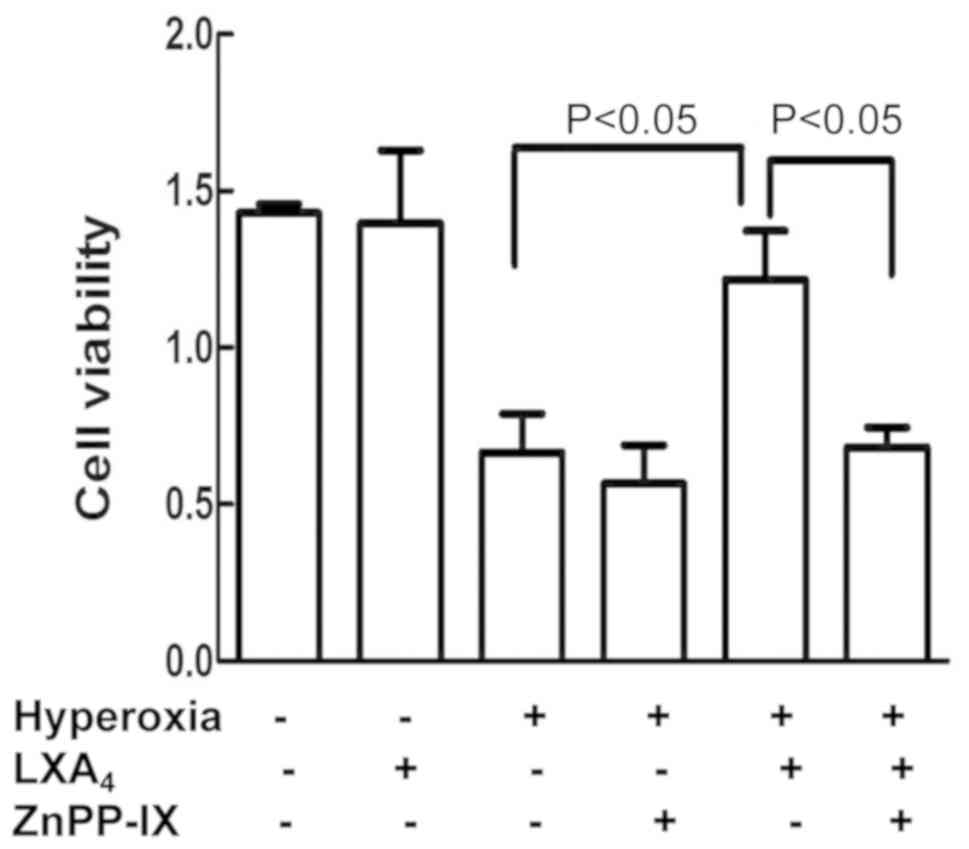
ZnPP-IX reversed
LXA4-imparted protection
As indicated in Fig.
3, pretreatment of the cells with ZnPP-IX abolished the
LXA4-imparted protection cell viability which was
reduced by hyperoxia.
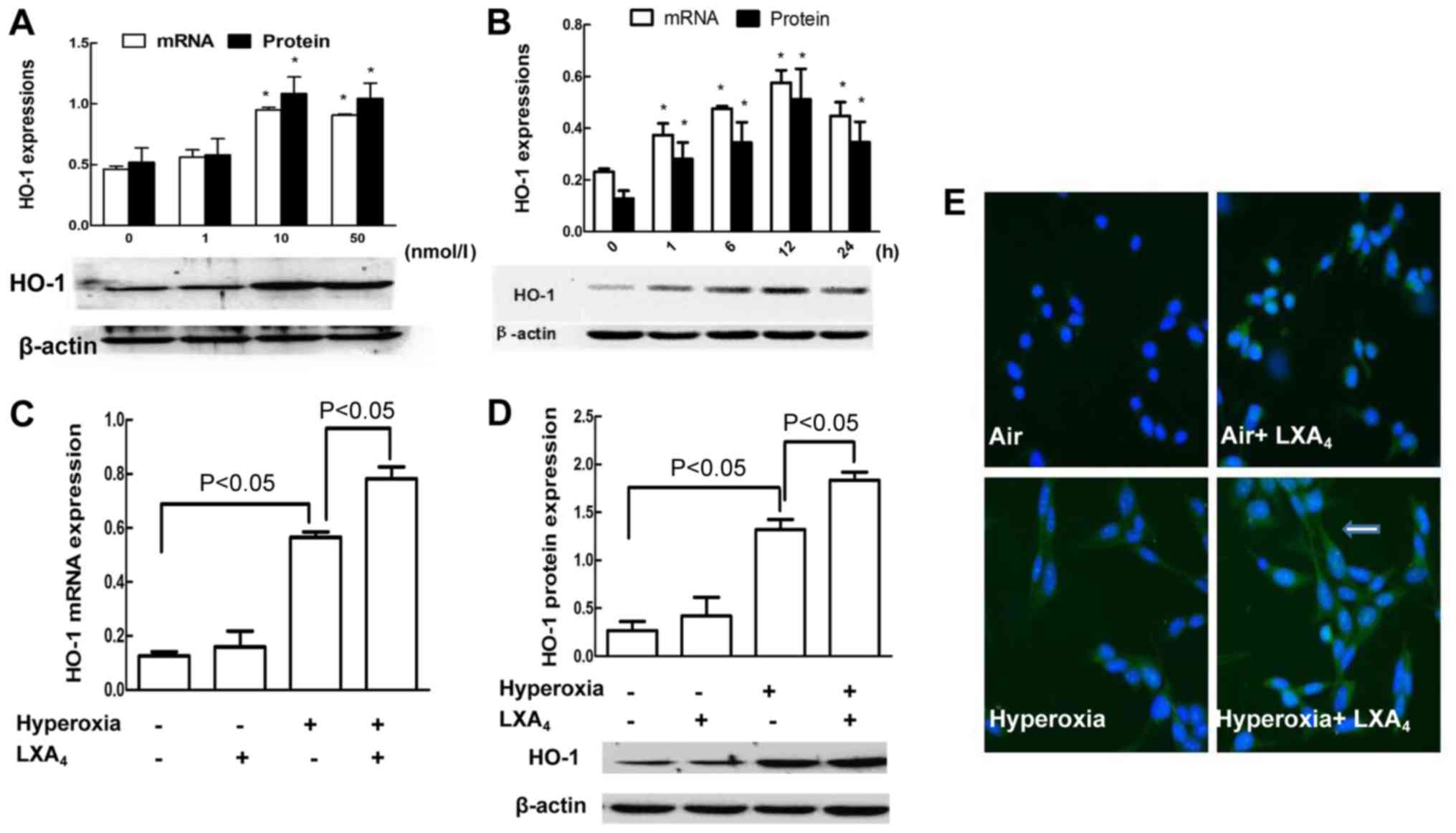
LXA4 induced HO-1
expression
HO-1 mRNA and protein expressions were measured in
the cells exposed to hyperoxia and pretreated with 0, 1, 10 and 50
nmol/l LXA4 for 1, 6, 12, 24 h. LXA4
upregulated the levels of HO-1 in a dose-dependent manner, and the
peak expression of HO-1 was induced by 10 nmol/l LXA4 at
12 h (Fig. 4A and B). Accordingly,
10 nmol/l LXA4 was used for measurement of HO-1
expressions in the cells after treatment with LXA4 for
12 h. LXA4 (10 nmol/l) slightly increased the
expressions of HO-1 mRNA and protein in the cells exposed to air
alone (Fig. 4C and D) but not
reach to statistical significance. Consistently, LXA4
upregulated the levels of HO-1 in the cells exposed to hyperoxia
(Fig. 4C and D). The localization
of HO-1 in the MLE-12 cells in response to LXA4 and
hyperoxia was also assessed by using fluorescence microscope
(Fig. 4E). There was no HO-1
expression in the cells exposed to air alone. LXA4
slightly promotes HO-1 expression in the cells exposed to air
alone. A stronger expression of HO-1 in the cytoplasm of the cells
undergoing hyperoxia was induced at 12 h after the stimulation with
LXA4 as compared to the cells exposed to hyperoxia
alone.
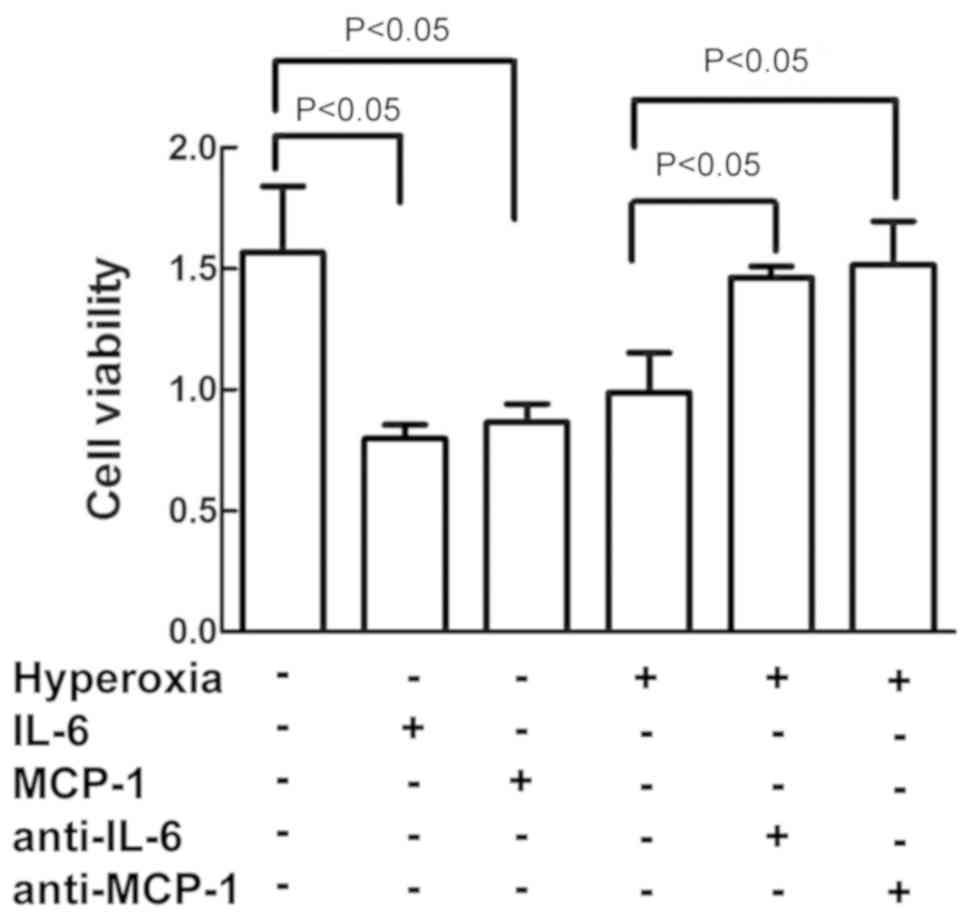
IL-6 and MCP-1 increased cell
damage
As depicted in Fig.
5, treatment of the cells with IL-6 and MCP-1 decreased the
cell viability in the cells exposed to air alone, similar to the
effect of hyperoxia on cell viability. On the contrary,
pretreatment of cells with anti-IL-6 and anti-MCP-1 antibodies
reversed the hyperoxia-induced inhibition on cell vitality as
compared to cells exposed to hyperoxia alone.
LXA4 inhibited IL-6 and
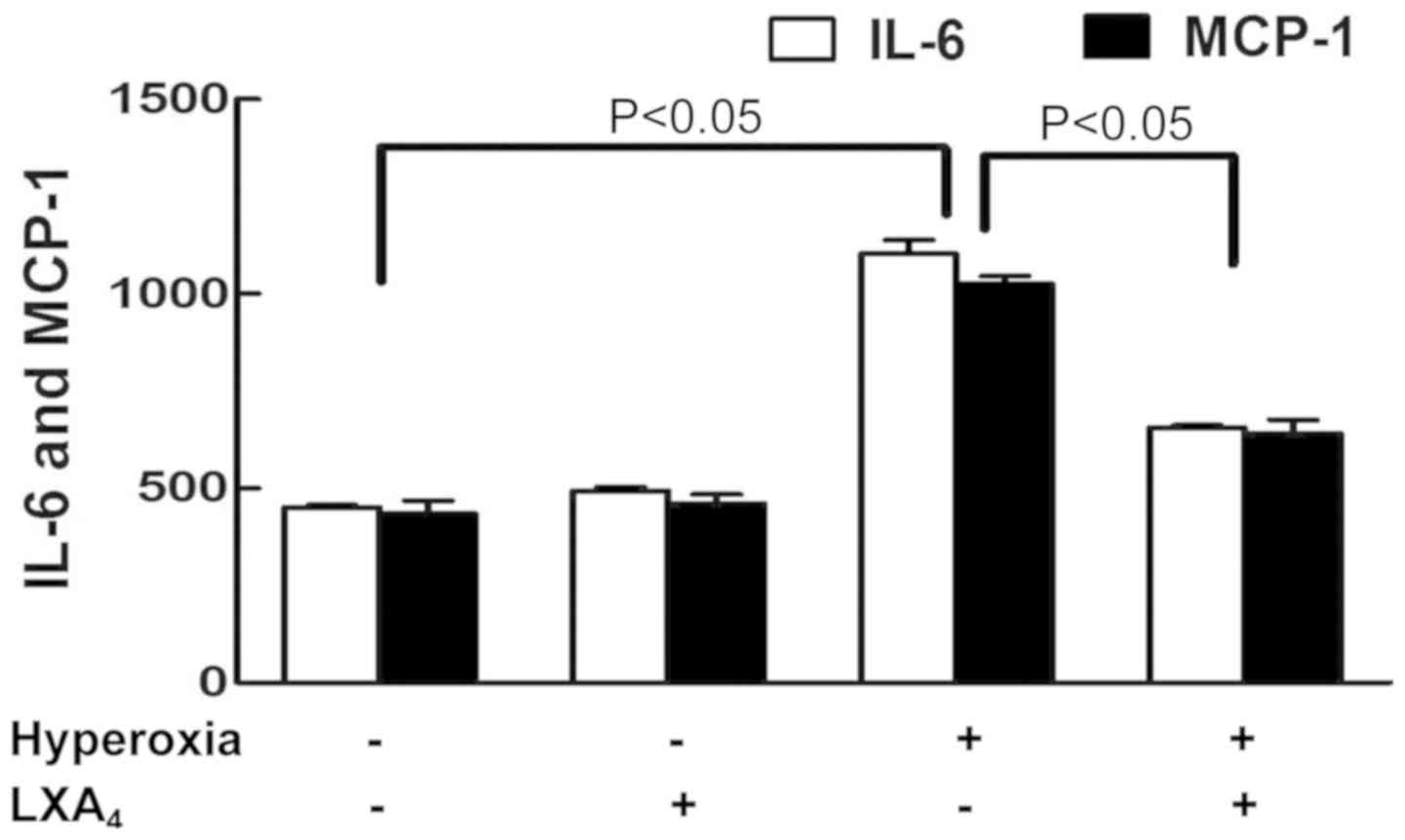
MCP-1 induced by hyperoxia
There were higher levels of IL-6 and MCP-1 in the
cells undergoing hyperoxia as compared to cells treated with air
alone. However, pretreatment of the cells with LXA4
inhibited the IL-6 and MCP-1 levels induced by hyperoxia as
compared to cells exposed to hyperoxia alone (Fig. 6).
Role of p38 MAPK, ERK1/2 and Akt in
IL-6 and MCP-1 expression
The effects of p38 MAPK, ERK1/2 and Akt inhibition
on expression of IL-6 and MCP-1 were illustrated in Fig. 7A and B. The p38 MAPK pathway
inhibitor SB203580 and ERK1/2 pathway inhibitors PD98059
significantly inhibited the hyperoxia-induced IL-6 and MCP-1
expressions respectively, and LXA4 also significantly
inhibited the hyperoxia-induced IL-6 and MCP-1 expressions, whereas
the Akt inhibitor LY294002 did not. As revealed in Fig. 7C-E, LXA4 alone slightly
decreased P-p38 MAPK and P-ERK1/2 expressions but increased P-Akt
expression in the cells treated with air alone. Hyperoxia exposure
significantly increased the P-p38 MAPK and P-ERK1/2 expressions but
decreased the P-Akt expression in the cells without LXA4
pretreatment. LXA4 significantly decreased P-p38 MAPK
and P-ERK1/2 expressions but increased P-Akt expression in the
cells treated with hyperoxia. LXA4 upregulated Akt
signaling pathways which was inhibited by hyperoxia.
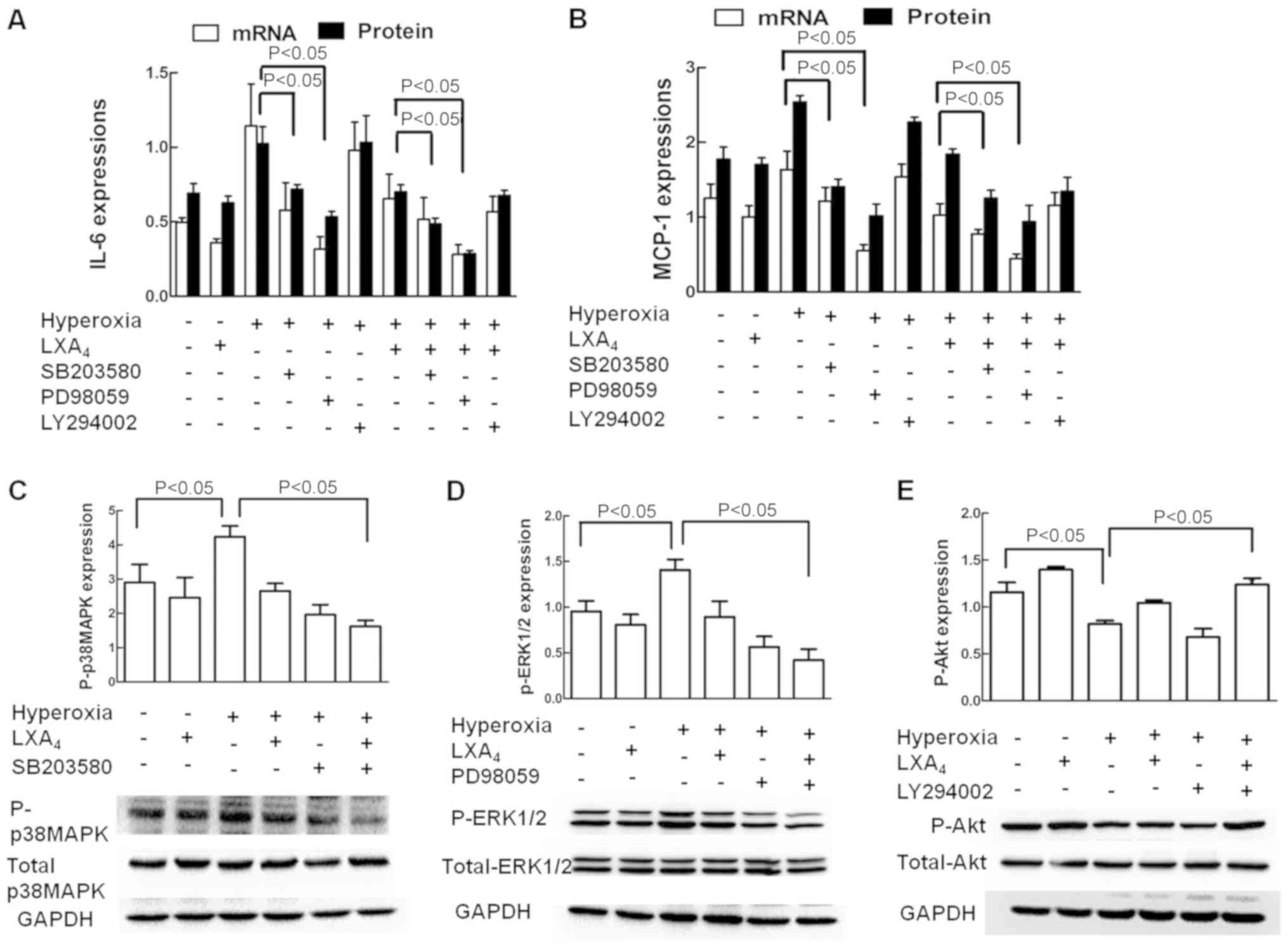 | Figure 7.Involvement of ERK1/2 and p38 MAPK in
the expression of IL-6 and MCP-1. The cells exposed to room air or
hyperoxia were treated with or without LXA4, SB203580,
PD98059 or LY294002. The mRNA and protein expressions of (A) IL-6
and (B) MCP-1 were measured by reverse transcription-quantitative
polymerase chain reaction and ELISA, respectively. (C) Total and
P-p38 MAPK, (D) total and P-ERK1/2, and (E) total and P-Akt
expressions were measured by western blot analysis. Values are
presented as the mean ± standard deviation of three independent
experiments. P<0.05, as indicated. LXA4, lipoxin
A4; IL-6, interleukin 6; MCP-1, monocyte chemotactic
protein 1; ERK1/2, extracellular signal-regulated kinase 1/2; p38
MAPK, p38 mitogen-activated protein kinase; P-, phosphorylated;
Akt, protein kinase B; GAPDH, glyceraldehyde-3-phosphate
dehydrogenase;. |
Discussion
BPD is one of the most serious lung complication in
premature infant caused by hyperoxia and inflammation.
Unfortunately, few effective therapies are known for BPD until now.
LXA4 play a unique protection on endothelial cells,
epithelial cells, lung and other tissues, has anti-oxidative
stress, anti-inflammation and anti-proliferation effect (22–24),
may be the good candidate therapy for BPD (19). In the present study, we identified
that LXA4 play a protective role on hyperoxia-induced
injury in murine lung epithelial cell model of BPD. First,
treatment of MLE-12 cells with LXA4 ameliorated the
morphological injury induced by hyperoxia. Second, treatment of
MLE-12 cells with LXA4 improved the cell survival rates,
cell viability, and the SOD level in the cells exposed to
hyperoxia. Furthermore, treatment of MLE-12 cells with
LXA4 suppressed apoptosis rates induced by hyperoxia.
Our results are in accordance with previous reports which
demonstrated that LXA4 improved alveolarization, reduced
mucosal inflammation, promoted resolution in a neonatal murine BPD
model induced by hyperoxia (19).
Oxygen radicals are direct and important causes of
oxidative stress injury, and HO-1 is the powerful antioxidant
enzyme to reduce the oxygen free radicals in vivo (25,26).
HO-1 and its metabolites carbon monoxide, biliverdin and bilirubin
have anti-inflammatory, antioxidant, and cytoprotective functions
(27,28). HO-1 can be induced by a variety of
factors including inflammatory cytokines, oxidative stress, LPS and
endotoxin. LPS induces HO-1 overexpression in monocytes to modulate
the expressions of inflammatory cytokines including IL-6 and MCP-1
(29,30). In the present study, we offered the
evidence for the first time that overexpression of HO-1 induced by
LXA4 attenuated hyperoxia-induced cell injury. First,
LXA4 upregulated the expressions of HO-1 in the cells
exposed to air alone or hyperoxia. Moreover, ZnPP-IX, a HO-1
inhibitor, reversed the LXA4-imparted protection on cell
viability which was reduced by hyperoxia. These results are
supported by previous investigations which demonstrated that
LXA4 and LXA4 receptor agonist can protect
the heart and renal oxidative stress damage via HO-1 overexpression
(17,31), and HO-1 play an important role in
protecting the developing pulmonary vasculature from
hyperoxia-induced injury in a murine model of BPD (3).
In the developing lung, extreme hyperoxia exposure
produces a sustained inflammation (32,33),
and inflammatory cytokines lead to structural abnormalities and
remodeling of the vessels of the neonatal lung (34–36).
However, it remains unclear whether LXA4 inhibits IL-6
and MCP-1 expressions in hyperoxia-induced cell injury. In the
present study, our results offered the evidence for the first time
that LXA4-imparted suppression on hyperoxia-induced
inflammatory injury is related to downregulation of IL-6 and MCP-1.
First, treatment of the cells with LXA4 inhibited the
expressions of IL-6 and MCP-1 induced by hyperoxia in parallel to
the inhibition on hyperoxia-induced cell injury. Second, IL-6 and
MCP-1 decreased cell viability, increased cell damage in cells
exposed to air alone. MLE-12 is murine Type II alveolar epithelial
cells, can secrete alveolar surfactant to maintain the normal
morphology of alveoli, but also can secrete IL-6, MCP-1, MIP-2 and
other chemokines involved in inflammation. These inflammatory
mediators are involved in the process of alveolar cell injury,
which involves a number of signaling pathways that I have already
supplemented. IL-6 is a multi-directional cytokine of inflammatory
response and immune system. It is involved in the pathological
process of many diseases such as immune response and acute phase
reaction. IL-6 regulates cell proliferation, differentiation and
apoptosis in different tissues through complex cell signaling
pathways Death and so on (37,38).
Inflammatory reaction can activate many kinds of different stress
signal pathways and change the balance between them. JAK-STAT
signal pathway is the main signal transduction pathway downstream
of IL-6 family and is involved in the induction of various
cytokines Of signal transduction, involving a variety of
neurological functions such as cell growth, differentiation,
inflammation, mutation and apoptosis. Inflammatory reaction can
activate many kinds of different stress signal pathways and change
the balance between them. JAK-STAT signal pathway is the main
signal transduction pathway downstream of IL-6 family and is
involved in the induction of various cytokines Of signal
transduction, involving a variety of neurological functions such as
cell growth, differentiation, inflammation, mutation and apoptosis.
Upregulation of IL-6 activates phosphorylation of STAT3 (39). Among the predisposing factors of
apoptosis, inflammatory factors play an important role (40,41).
MAPKs are intracellular serine/threonine protein kinases. MAPKs
signal transduction pathway exists in most cells, and plays an
important role in cell proliferation, differentiation,
transformation and apoptosis. At present, four parallel MAPKs
signaling pathways have been found in higher mammalian cells. Among
them, P38MAPK and JNK/SAPK can be stimulated by inflammatory
stimuli (IL-6, IL-1 and TNF-α). Growth factor (EGF) and some G
protein-coupled receptors are activated by three-layer activated
enzyme systems, MAPKs, MAP2Ks, and MAP3Ks, which act through
cascade of protein phosphorylation-induced cascades effect.
Activated P38MAPK and JNK/SAPK Pathways Regulate Gene Transcription
by Phosphorylation of Transcription Factors and Other Targets
thereby Inducing Apoptosis (42–44).
Monocyte chemoattractant protein-1 (MCP-1) is the first human CC
chemokine that has been discovered and is currently a member of
more research in the MCP family. It is associated with CC
chemokines CCR2 can exert different physiological functions, such
as inducing homing, migration, activation, differentiation and
development of lymphocytes and natural killer cells, inflammation,
angiogenesis and the like. The main mechanisms CCR2 and MCP 1 can
induce calcium influx. NF-κB is the main regulator of inflammatory
response, NF-κB can activate the transcription of MCP-1 gene,
upregulated MCP-1 level expression (45). The signaling pathways involved in
the damage of alveolar epithelial cells by these inflammatory
factors need to be further studied. Furthermore, anti-IL-6 and
anti-MCP-1 antibodies reversed the hyperoxia-reduced cell
viability, decreased cell injury induced by hyperoxia. These
results suggest that effect of LXA4 on hyperoxia-induced
cell injury is similar to that of anti-IL-6 and anti-MCP-1. Our
results are consistent with previous reports which demonstrated
that hyperoxia induced long-term airway reactivity with persistent
lung inflammation associated with a marked increase in inflammatory
cytokines in newborn mice (5), and
LXA4 receptor agonist BML-111 and LXA4
attenuated LPS-induced IL-6 expression (14,15),
and LXA4 inhibited IL-6 expression in rat mesangial
cells (46), and LXA4
attenuated MCP-1 release in human intestinal mucosa (47).
The phosphorylation of p38 MAPK and ERK1/2 is
necessary for IL-6 secretion in endothelial cells exposed to LPS
(48,49). In the present study, we offered the
evidence that LXA4-imparted suppression of IL-6 and
MCP-1 is mediated by p38 MAPK and ERK1/2-dependent signaling
pathways in the cells exposed to hyperoxia. First, hyperoxia
activated the phosphorylation of p38 MAPK and ERK1/2 in parallel to
stimulation of IL-6 and MCP-1 expressions, and LXA4
inhibited the phosphorylation of p38 MAPK and ERK1/2 induced by
hyperoxia in parallel to inhibition of IL-6 and MCP-1 expressions
induced by hyperoxia. Moreover, treatment of the cells with
SB203580 and PD98059 reduced IL-6 and MCP-1 levels induced by
hyperoxia, and SB203580, PD98059 plus LXA4 further
reduced IL-6 and MCP-1 levels in cells exposed to hyperoxia. These
results are supported by previous reports which showed that
LXA4 receptor agonist inhibited the phosphorylation of
ERK1/2, p38 MAPK in endothelial cells exposed to LPS (14), and LXA4-imparted
inhibition of IL-6 and IL-1β was related to blockage of p38 MAPK,
p42/44 MAPK (ERK1/2) (15). In
addition, we found that the phosphorylation of Akt was inhibited by
hyperoxia exposure in MLE-12 cells, and LXA4 reversed
the expression of phosphorylation of Akt inhibited by hyperoxia,
this result is consistent with the previous study which showed that
activation of Akt protected alveoli from neonatal oxygen-induced
lung injury (50). However, the
precise mechanism needs to be explored in further study.
In conclusion, our present studies demonstrate
following findings, first, LXA4 attenuates
hyperoxia-induced cell injury via upregulation of HO-1, second,
LXA4 decreased the expressions of IL-6 and MCP-1 induced
by hyperoxia injury, third, LXA4-imparted inhibition of
IL-6 and MCP-1 may be mediated by downregulation of p38 MAPK and
ERK1/2 signaling pathways. Overall, our data indicate that
LXA4 could be a useful chemical for the choice of a new
therapy in treatment of BPD.
Acknowledgements
The authors would like to thank Dr Chao Gao and Dr
Yu-Gui Cui for their technical assistance (First Affiliated
Hospital with Nanjing Medical University, Jiangsu, China). The
abstract was submitted to the American Academy of Pediatrics to be
presented on 15th September 2018 at the Marriott Marquis Chicago,
Great Lakes, Chicago, IL, USA; however, the abstract was not
presented, only published in Pediatrics, Neonatal-Perinatal
Medicine Program: Day 1, 142 (1 Meeting Abstract), 191–191:
2018.
Funding
The present study was supported by National Natural
Scientific Fund of China (grant no. 81871195 and 81741052) and
Construction Program of Jiangsu Provincial Clinical Research Center
Support System (grant no. BL2014084).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
SHW, XQC, RJ and HYL conceived and designed the
experiments. YYL, BJL, SJL and ZYS performed the experiments, and
analyzed the data. RJ and HYL revised the manuscript critically for
important intellectual content. YYL, XQC and SHW wrote the
paper.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Balany J and Bhandari V: Understanding the
impact of infection, inflammation, and their persistence in the
pathogenesis of bronchopulmonary dysplasia. Front Med (Lausanne).
2:902015.PubMed/NCBI
|
|
2
|
Bhandari V: Hyperoxia-derived lung damage
in preterm infants. Semin Fetal Neonatal Med. 15:223–229. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fernandez-Gonzalez A, Alex Mitsialis S,
Liu X and Kourembanas S: Vasculoprotective effects of heme
oxygenase-1 in a murine model of hyperoxia-induced bronchopulmonary
dysplasia. Am J Physiol Lung Cell Mol Physiol. 302:L775–L784. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Muramatsu Y, Ito M, Oshima T, Kojima S and
Ohno K: Hydrogen-rich water ameliorates bronchopulmonary dysplasia
(BPD) in newborn rats. Pediatr Pulmonol. 51:928–935. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kumar VH, Lakshminrusimha S, Kishkurno S,
Paturi BS, Gugino SF, Nielsen L, Wang H and Ryan RM: Neonatal
hyperoxia increases airway reactivity and inflammation in adult
mice. Pediatr Pulmonol. 51:1131–1141. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Schreiner C, Schreiner F, Härtel C,
Heckmann M, Heep A, Bartmann P, Woelfle J, Müller A, Herting E and
Göpel W; German Neonatal Network GNN, : Glucocorticoid receptor
gene variants and neonatal outcome in very-low-birth-weight preterm
infants. Neonatol. 111:22–29. 2016. View Article : Google Scholar
|
|
7
|
Lakshminrusimha S, Mathew B and Leach CL:
Pharmacologic strategies in neonatal pulmonary hypertension other
than nitric oxide. Semin Perinatol. 40:160–173. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Slaughter JL, Stenger MR, Reagan PB and
Jadcherla SR: Utilization of inhaled corticosteroids for infants
with bronchopulmonary dysplasia. PLoS One. 9:e1068382014.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Serhan CN, Chiang N and Van Dyke TE:
Resolving inflammation: Dual anti-inflammatory and pro-resolution
lipid mediators. Nat Rev Immunol. 8:349–361. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fiorucci S, Wallace JL, Mencarelli A,
Distrutti E, Rizzo G, Farneti S, Morelli A, Tseng JL, Suramanyam B,
Guilford WJ and Parkinson JF: A beta-oxidation-resistant lipoxin
A4 analog treats hapten-induced colitis by attenuating
inflammation and immune dysfunction. Proc Natl Acad Sci USA.
101:15736–15741. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ye XH, Wu Y, Guo PP, Wang J, Yuan SY,
Shang Y and Yao SL: Lipoxin A4 analogue protects brain
and reduces inflammation in a rat model of focal cerebral ischemia
reperfusion. Brain Res. 1323:174–183. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Levy BD, De Sanctis GT, Devchand PR, Kim
E, Ackerman K, Schmidt BA, Szczeklik W, Drazen JM and Serhan CN:
Multi-pronged inhibition of airway hyper-responsiveness and
inflammation by lipoxin A(4). Nat Med. 8:1018–1023. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yang F, Xie J, Wang W, Xie Y, Sun H, Jin
Y, Xu D, Chen B, Andersson R and Zhou M: Regional arterial infusion
with lipoxin A4 attenuates experimental severe acute
pancreatitis. PLoS One. 9:e1085252014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Tang M, Chen L, Li B, Wang Y, Li S, Wen A,
Yao S and Shang Y: BML-111 attenuates acute lung injury in
endotoxemic mice. J Surg Res. 200:619–630. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu SH, Liao PY, Dong L and Chen ZQ: Signal
pathway involved in inhibition by lipoxin A(4) of production of
interleukins induced in endothelial cells by lipopolysaccharide.
Inflamm Res. 57:430–437. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hangaishi M, Ishizaka N, Aizawa T,
Kurihara Y, Taguchi J, Nagai R, Kimura S and Ohno M: Induction of
heme oxygenase-1 can act protectively against cardiac
ischemia/reperfusion in vivo. Biochem Biophys Res Commun.
279:582–588. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chen XQ, Wu SH, Zhou Y and Tang YR:
Lipoxin A4-induced heme oxygenase-1 protects
cardiomyocytes against hypoxia/reoxygenation injury via p38 MAPK
activation and Nrf2/ARE complex. PLoS One. 8:e671202013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Biteman B, Hassan IR, Walker E, Leedom AJ,
Dunn M, Seta F, Laniado-Schwartzman M and Gronert K:
Interdependence of lipoxin A4 and heme-oxygenase in
counter-regulating inflammation during corneal wound healing. FASEB
J. 21:2257–2266. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Martin CR, Zaman MM, Gilkey C, Salguero
MV, Hasturk H, Kantarci A, Van Dyke TE and Freedman SD: Resolvin D1
and lipoxin A4 improve alveolarization and normalize
septal wall thickness in a neonatal murine model of
hyperoxia-induced lung injury. PLoS One. 9:e987732014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kair LR, Leonard DT and Anderson JM:
Bronchopulmonary dysplasia. Pediatr Rev. 33:255–264. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu L, Liu ZJ, Miao S, Zou LB, Cai L, Wu P,
Ye du Y, Wu Q and Li HH: Lipoxin A4 ameliorates cerebral
ischaemia/reperfusion injury through upregulation of nuclear factor
erythroid 2-related factor 2. Neurol Res. 35:968–975. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen XQ, Wu SH, Zhou Y and Tang YR:
Involvement of K+ channel-dependant pathways in lipoxin
A4-induced protective effects on hypoxia/reoxygenation
injury of cardiomyocytes. Prostaglandins Leukot Essent Fatty Acids.
88:391–397. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Yang F, Xie J, Wang W, Xie Y, Sun H, Jin
Y, Xu D, Chen B, Andersson R and Zhou M: Regional arterial infusion
with lipoxin A4 attenuates experimental severe acute pancreatitis.
PLoS One. 9:e108525. 2014.
|
|
25
|
Masini E, Vannacci A, Marzocca C,
Pierpaoli S, Giannini L, Fantappié O, Mazzanti R and Mannaioni PF:
Heme oxygenase-1 and the ischemia-reperfusion injury in the rat
heart. Exp Biol Med (Maywood). 228:546–549. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chok MK, Ferlicot S, Conti M, Almolki A,
Dürrbach A, Loric S, Benoît G, Droupy S and Eschwège P:
Renoprotective potency of heme oxygenase-1 induction in rat renal
ischemia-reperfusion. Inflamm Allergy Drug Targets. 8:252–259.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Paine A, Eiz-Vesper B, Blasczyk R and
Immenschuh S: Signaling to heme oxygenase-1and its
anti-inflammatory therapeutic potential. Biochem Pharmacol.
80:1895–1903. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ryter SW and Choi AM: Heme
oxygenase-1/carbon monoxide: From metabolism to molecular therapy.
Am J Respir Cell Mol Biol. 41:251–260. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Rushworth SA, MacEwan DJ and O'Connell MA:
Lipopolysaccharide-induced expression of NAD (P)H: Quinone
oxidoreductase 1 and heme oxygenase-1 protects against excessive
inflammatory responses in human monocytes. J Immunol.
181:6730–6737. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
So Y, Lee SY, Han AR, Kim JB, Jeong HG and
Jin CH: Rosmarinic acid methyl ester inhibits LPS-induced NO
production via suppression of MyD88-dependent and -independent
pathways and induction of HO-1 in RAW 264.7 cells. Molecules.
21(pii): E10832016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Wu SH, Chen XQ, Lü J and Wang MJ: BML-111
attenuates renal ischemia/reperfusion injury via peroxisome
proliferator-activated receptor-α-regulated heme oxygenase-1.
Inflammation. 39:611–624. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wu J, Hafner C, Schramel JP, Kaun C,
Krychtiuk KA, Wojta J, Boehme S, Ullrich R, Tretter EV, Markstaller
K and Klein KU: Cyclic and constant hyperoxia cause inflammation,
apoptosis and cell death in human umbilical vein endothelial cells.
Acta Anaesthesiol Scand. 60:492–501. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Deng H, Mason SN and Auten RL Jr: Lung
inflammation in hyperoxia can be prevented by antichemokine
treatment in newborn rats. Am J Respir Crit Care Med.
162:2316–2323. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Aslam M, Baveja R, Liang OD,
Fernandez-Gonzalez A, Lee C, Mitsialis SA and Kourembanas S: Bone
marrow stromal cells attenuate lung injury in a murine model of
neonatal chronic lung disease. Am J Respir Crit Care Med.
180:1122–1130. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Bry K, Hogmalm A and Bäckström E:
Mechanisms of inflammatory lung injury in the neonate: Lessons from
a transgenic mouse model of bronchopulmonary dysplasia. Semin
Perinatol. 34:211–221. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ryan RM, Ahmed Q and Lakshminrusimha S:
Inflammatory mediators in the immunobiology of bronchopulmonary
dysplasia. Clin Rev Allergy Immunol. 34:174–190. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ishihara K and Hirano T: IL-6 in
autoimmune disease and chronic inflammatory proliferative disease.
Cytokine Growth Factor Rev. 13:357–368. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Donegan JJ, Girotti M, Weinberg MS and
Morilak DA: A novel role for brain interleukin-6: Facilitation of
cognitive flexibility in rat orbitofrontal cortex. J Neurosci.
34:953–962. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Hao Y, Jing H, Bi Q, Zhang J, Qin L and
Yang P: Intra-amygdala microinfusion of IL-6 impairs the auditory
fear conditioning of rats via JAK/STAT activation. Behav Brain Res.
275:88–95. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wong LM, Myers SJ, Tsou CL, Gosling J,
Arai H and Charo IF: Organization and differential expression of
the human monocyte chemoattractant protein 1 receptor gene.
Evidence for the role of the carboxyl-terminal tail in receptor
trafficking. J Biol Chem. 272:1038–1045. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Cho ML, Yoon BY, Ju JH, Jung YO, Jhun JY,
Park MK, Park SH, Cho CS and Kim HY: Expression of CCR2A, an
isoform of MCP-1 receptor, is increased by MCP-1, CD40 ligand and
TGF-beta in fibroblast like synoviocytes of patients with RA. Exp
Mol Med. 39:499–507. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Han J and Sun P: The pathways to tumor
suppression via route p38. Trends Biochem Sci. 32:364–371. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hui L, Bakiri L, Stepniak E and Wagner EF:
p38alpha: A suppressor of cell proliferation and tumorigenesis.
Cell Cycle. 6:2429–2433. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Mishra S, Mishra JP and Kumar A:
Activation of JNK-dependent pathway is required for HIV viral
protein R-induced apoptosis in human monocytic cells: Involvement
of antiapoptotic BCL2 and c-IAP1 genes. J Biol Chem. 282:4288–4300.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Zhao Y, Wang CL, Li RM, Hui TQ, Su YY,
Yuan Q, Zhou XD and Ye L: Wnt5a promotes inflammatory responses via
nuclear factor κB (NF-κB) and mitogen-activated protein kinase
(MAPK) pathways in human dental pulp cells. J Biol Chem.
292:43582017. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Wu SH, Lu C, Dong L, Zhou GP, He ZG and
Chen ZQ: Lipoxin A4 inhibits TNF-α-induced production of
interleukins and proliferation of rat mesangial cells. Kidney Int.
68:35–46. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Goh J, Baird AW, O'Keane C, Watson RW,
Cottell D, Bernasconi G, Petasis NA, Godson C, Brady HR and
MacMathuna P: Lipoxin A4 and aspirin-triggered
15-epi-lipoxin A4 antagonize TNF-alpha-stimulated
neutrophil-enterocyte interactions in vitro and attenuate
TNF-alpha-induced chemokine release and colonocyte apoptosis in
human intestinal mucosa ex vivo. J Immunol. 167:2772–2780. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Arditi M, Zhou J, Torres M, Durden DL,
Stins M and Kim KS: Lipopolysaccharide stimulates the tyrosine
phosphorylation of mitogen-activated protein kinases p44, p42, and
p41 in vascular endothelial cells in a soluble CD14-dependent
manner. Role of protein tyrosine phosphorylation in
lipopolysaccharide-induced stimulation of endothelial cells. J
Immunol. 155:3994–4003. 1995.PubMed/NCBI
|
|
49
|
Matsuda T, Omori K, Vuong T, Pascual M,
Valiente L, Ferreri K, Todorov I, Kuroda Y, Smith CV, Kandeel F and
Mullen Y: Inhibition of p38 pathway suppresses human islet
production of pro-inflammatory cytokines and improves islet graft
function. Am J Transplant. 5:484–493. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Alphonse RS, Vadivel A, Coltan L, Eaton F,
Barr AJ, Dyck JR and Thébaud B: Activation of Akt protects alveoli
from neonatal oxygen-induced lung injury. Am J Respir Cell Mol
Biol. 44:146–154. 2011. View Article : Google Scholar : PubMed/NCBI
|