Introduction
Oxidative stress describes cellular damage caused by
reactive oxygen/nitrogen species (ROS/RNS), combined with a
biological inability to eliminate the oxidants or repair the
consequent damage (1,2). During the early stage of pregnancy,
extravillous cytotrophoblast cells occupy the uterine spiral
arterioles and create a low-oxygen environment (3). Thus, the placenta and embryo
experience hypoxia-induced oxidative stress as part of the normal
physiological process during early pregnancy (4–6).
However, excessive elevated oxidative stress leads to complications
such as preeclampsia (PE), spontaneous abortion and intrauterine
growth restriction (7–9). Ethical considerations restrict the
induction of oxidative stress directly onto the human embryo, so
knowledge concerning the consequences of excessive oxidative stress
during pregnancy has been solely acquired using cell and animal
models (10). There are five main
oxidative stress cell models used to investigate pregnancy
complications (11–15). Firstly, there is hypoxia or low
partial pressure of oxygen (<5% O2) (13,16,17).
Researchers usually culture cells in 1–2% O2 for 12–72 h
as a hypoxic condition to induce oxidative stress (13,16,17).
Hypoxia is perceived as the oxidative stress model for pregnancy
complications because trophoblast invasion and/or vascular
remodeling are compromised (18).
The persistent placental ischemia results in elevated ROS
production and provokes oxidative stress in the placenta (19). Conversely, hypoxia and
reoxygenation (HR) mimics the oxidative stress induced by ischemia
and reperfusion injury (14).
Researchers commonly cultivate cells in a low partial pressure of
oxygen (1–2%) for 4–8 h and subsequently enhance the oxygen
concentration to 20% to induce oxidative stress (20,21).
Alternatively, endogenous chemicals can be used to induce oxidative
stress, cobalt chloride (CoCl2) is known as a hypoxia
mimetic agent. The hypoxia-inducible factor (HIF) activates the
expression of genes that contain a hypoxia response element (HRE).
The α-subunits of the HIF transcription factors are degraded during
normoxia and stabilized under hypoxic conditions (22). The cobalt prevents the degradation
of HIF-1α and mimics the HIF-HRE cascade-induced hypoxia (15). Another oxidative stressor, sodium
nitroprusside (SNP) is a nitric oxide (NO) donor and induces a
state of oxidative stress via the generation of ROS and RNS
(5,23–25).
Researchers often treat villi explants or cells with various
concentrations of SNP to induce oxidative stress (11,26,27).
Finally, serum from patients with PE can be used; it is a common
practice that researchers create PE-like cell models via the
addition of PE serum/plasma into cell cultures (12,28–31).
This method mimics the second stage of the two-stage model of PE,
which is often associated with oxidative stress (32).
There are studies that have attempted to apply the
knowledge gained from these oxidative stress cell models to develop
new therapeutic interventions for pregnancy complications (33,34).
For instance, in vitro studies using oxidative stress cell
models have suggested that heparin may improve the uterine
environment for successful implantation by preventing apoptosis
caused by oxidative stress and modulating the decidual process
(33). Furthermore, the
antioxidant and cytoprotective mechanisms of aspirin have been
observed in a hydrogen peroxide-mediated oxidative stress cell
model, which may aid the understanding of the role of aspirin in
reducing the risks of preterm birth, PE and fetal growth
restriction (34). However, there
are no published studies that have evaluated the different types of
oxidative stress cell model used in pregnancy research. In this
study, the aim was to investigate the metabolite profiles of a
trophoblast cell line (HRT-8/SVneo) under five different models of
oxidative stress using gas chromatography-mass spectrometry
(GC-MS)-based metabolomic analysis. Identification of the metabolic
similarities and discordances among the cell models will further
the understanding of the mechanisms of oxidative stress and may
have useful therapeutic implications for pregnancy
complications.
Materials and methods
PE serum
All serum samples (n=6; age range, 26–34 years) from
participants with PE were collected from the Complex Lipids in
Mothers and Babies study that was conducted at The First Affiliated
Hospital of Chongqing Medical University in China between September
2015 and June 2017 (35). A total
of 3 ml of peripheral blood was collected from patients with PE,
transferred into coagulation-promoting tubes and centrifuged twice
at 2,400 × g for 10 min at 4°C. The supernatant serum was then
transferred into cryotubes (Dakewe Biotech Co., Ltd.) and stored at
−80°C in a freezer. All participants at enrollment signed written
informed consent and this study was approved by the Ethics
Committee of Chongqing Medical University (policy no. 2014034).
Culturing HTR8/SVneo cells
The immortalized human trophoblast cell line
HTR8/SVneo was kindly donated by Dr Charles Graham (Queen's
University, Kingston, Canada) (36). The cells were cultured in RPMI-1640
medium (Gibco; Thermo Fisher Scientific, Inc.) supplemented with
10% FBS (Gibco; Thermo Fisher Scientific, Inc.), penicillin (100
U/ml), and streptomycin (0.1 mg/ml) in 5% CO2 at 37°C.
The cells were passaged at a ratio of 1:4 every 4 days.
Cytotoxicity assays of
CoCl2 and SNP
To determine the most appropriate concentrations of
CoCl2 and SNP for the oxidative cell stress model, the
cytotoxicity of CoCl2 (Sigma-Aldrich; Merck KGaA) and
SNP (Sigma-Aldrich; Merck KGaA) were assessed using Cell Counting
Kit-8 (CCK-8) assays was used according to the manufacturer's
protocols (Dojindo Molecular Technologies, Inc.). Briefly,
HTR8/SVneo (1×104 cells/well) cells were seeded in a
96-well flat bottom plate and cultured overnight at 37°C.
Subsequently, cells were incubated with 100 µl of RPMI-1640 medium
with CoCl2 (0, 100, 200, 300, 400 and 500 µm) for 24 h
or SNP (0, 0.5, 1, 2, 3 and 4 mM) for 8 h at 37°C. A total of five
wells/group were tested at each concentration on the gradient.
After 10 µl of CCK-8 solution was added to each well, cells were
incubated at 37°C for 3 h, and the absorbance was measured at 450
nm, using a microplate reader.
Oxidative stress models
For subsequent experiments, the cells were seeded in
complete medium in 10-cm dishes at 5×105 cells/dish,
under standard culture conditions overnight (37°C in a
5%-humidified CO2 incubator). The cells were separated
randomly into seven groups with six replicates per treatment. There
were two control groups under normal levels of oxygenation (20%
O2, 5% CO2 and 75% N2); one group
was prepared for 8 h (control for SNP) and the second was prepared
for 24 h (control for hypoxia, HR, CoCl2 and PE serum).
A total of five oxidative stress groups were prepared: Hypoxia for
24 h (1% O2, 5% CO2 and 94% N2);
hypoxia followed by reoxygenation for 12 h; CoCl2 (300
µmol/l) under normal oxygenation for 24 h; serum of patients with
PE (10% v/v) under normal oxygenation for 24 h; and SNP (2.5
mmol/l) under normal oxygenation for 8 h.
Extracellular metabolite collection
from culture medium
After the oxidative stress conditions were
established, 1.5 ml of cell culture medium was transferred to a
centrifuge tube and cell debris was removed by centrifugation at
4°C and 2,000 × g for 10 min. The supernatants were isolated and 20
µl of 2,3,3,3-d4-alanine (10 mM; Thermo Fisher Scientific, Inc.)
internal standard was added. The blank media was treated exactly
the same way without any cell components. The isolated supernatants
were then concentrated by a Labconco CentriVap® SpeedVac
concentrator (Labconco Corporation) for 4–5 h and stored at −80°C
prior to chemical derivatization.
Intracellular metabolite extraction
from treated HTR8/SVneo cells
Treated HTR8/SVneo cells were washed with 10 ml of
PBS at 37°C. Cellular metabolism was terminated by the addition of
15 ml of liquid nitrogen into the cell culture dishes. After the
evaporation of liquid nitrogen, 1.5 ml of cold methanol-chloroform
extraction solvent at 4°C (9:1 ratio with 20 µl 2,3,3,3-d4-alanine;
10 mM; Thermo Fisher Scientific, Inc.) was added. Then, the cells
were scraped with a cell lifter and transferred to a 1.5-ml
centrifuge tube. After vortexing for 30 sec, samples were
centrifuged for 15 min at 4°C at 20,000 × g, and the supernatant
was isolated and dried using a Labconco CentriVap SpeedVac
concentrator for 4–5 h.
Methylchloroformate (MCF)
derivatization and GC-MS analysis
The dried pellets were resuspended in 200 µl of
sodium hydroxide (1 mol/l) and then transferred into silanized
glass tubes. The MCF derivatization was performed in accordance
with the protocol published in Smart et al (37). The derivatives of the MCF
metabolites were analyzed using an Agilent GC7890B system (Agilent
Technologies, Inc.) coupled to a MSD5977A mass spectrometer
(Agilent Technologies, Inc.) with the electron impact ionization
set at 70 eV. The gas chromatograph used to separate the
metabolites was a Zebron ZB-1701 Capillary GC column (30 mx250 µm
id ×0.15 µm with a 5 m guard column; Phenomenex); 1 µl of
derivatized sample was injected into the GC inlet that operated at
290°C in a split-less mode under 180 kPa for 1 min. Details of GC
and MS parameters were also set up according to the methodology
reported in Smart et al (37). Helium gas flow rate was constantly
controlled at 1 ml/min. The GC oven temperature was initiated at
45°C for 2 min and raised to 180°C at 9°C/min for 5 min.
Subsequently, the oven temperature was raised at 40°C/min to 220°C
and held for 5 min. The temperature was then increased again at
40°C/min to 240°C and held for 11.5 min. The last temperature
increase was at 40°C/min to 280°C and held for 2 min. The transfer
interface between GC and MS was setup at 250°C, the MS ion source
at 250°C and the quadrupole at 130°C.
Data mining and statistical
analyses
Metabolite identification and chromatographic
deconvolution were performed using AMDIS software (V2.1; National
Institute of Standards and Technology). The MS1 metabolite
identifications were achieved by comparing their fragmentation mass
spectrum to the in-house MCF mass spectral library (built using
chemical standards) (37) and
associated retention time within a 1-min window. The relative
concentration of identified metabolites was calculated using the
in-house XCMS-based R script (V 3.8.1) (38) by identifying the most abundant
fragment ion within an accurate retention time window. The
metabolite concentrations were normalized by 2,3,3,3-d4-alanine and
total protein level was used to correct for any dilution effect.
The extracellular metabolites were determined by subtracting the
levels of the corresponding metabolites detected in the control
medium without any cellular components. Principal component
analysis (PCA) was conducted via Metaboanalyst 3.0 package for R
(39). Tukey's honest significant
difference test was conducted to test for significant differences
between the treatment and the control groups. A false discovery
rate (Q-value) was used to account for multiple comparison testing.
Both P<0.05 and Q<0.05 were regarded as statistically
significant. Heatmap and line plots were illustrated via ggplot2 R
packages (40).
Results
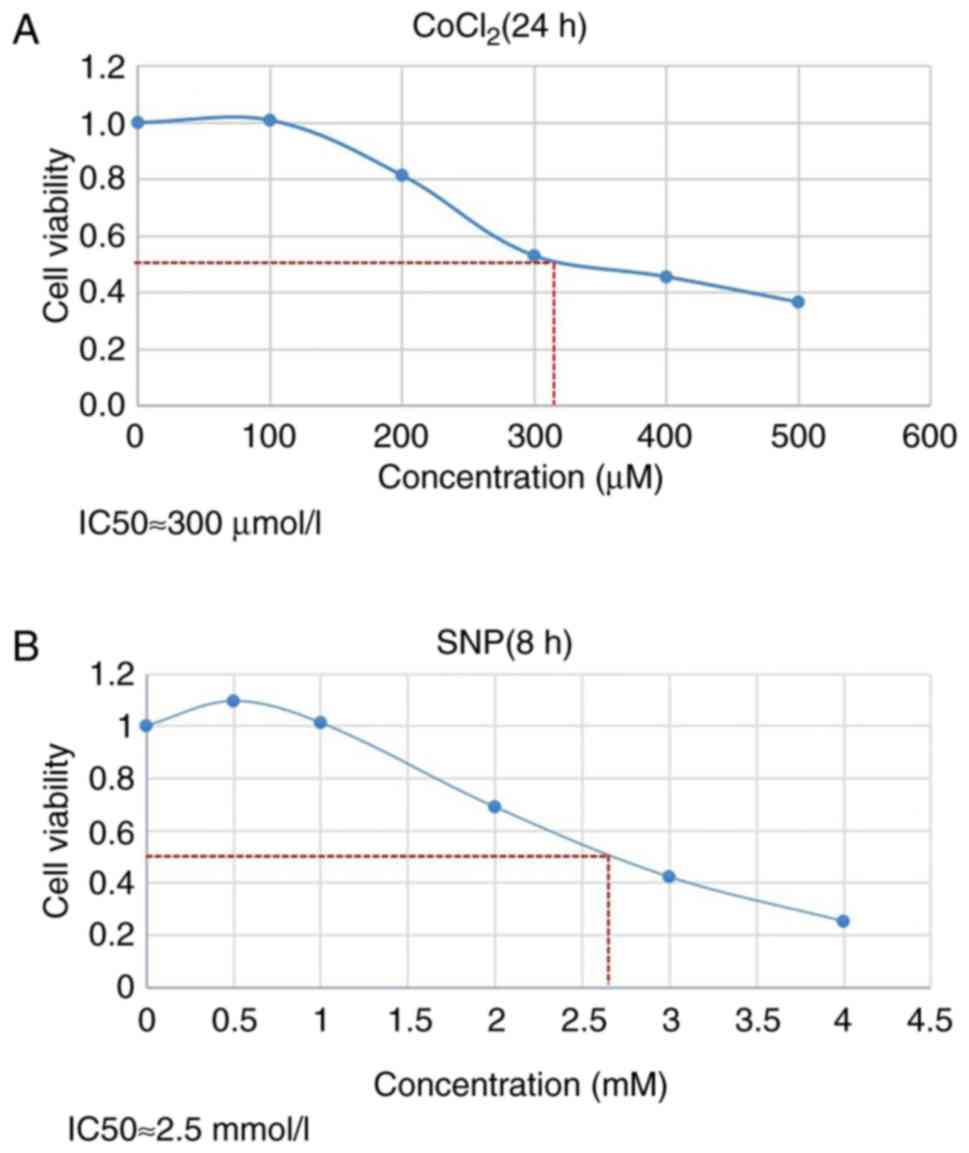
Cytotoxic effects of CoCl2
and SNP on cell viability
The viability of HTR8/SVneo cells was inhibited in a
dose-dependent manner by CoCl2 (Fig. 1A) and SNP (Fig. 1B). The inhibited concentration (IC)
of CoCl2 at which ~50% (IC50) of the cells
were viable was 300 µm (Fig. 1A),
while the IC50 of SNP on HTR8/SVneo cells was ~2.5 mM
(Fig. 1B). Therefore, 300 µm of
CoCl2 and 2.5 mM SNP were chosen for this
experiment.
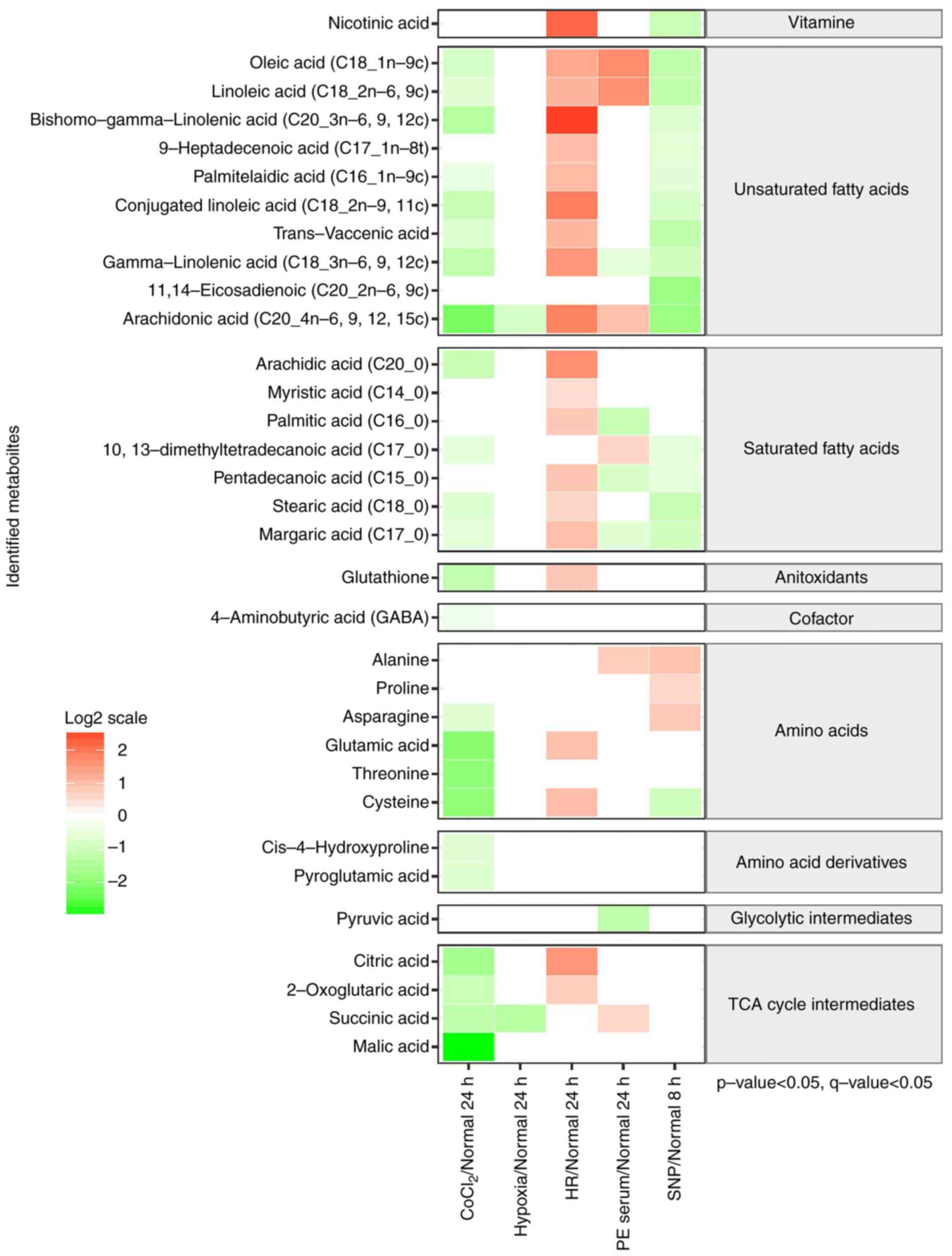
Intracellular metabolite profiling of
five oxidative stress cell models
The PCA scores plot (Fig. 2) of the intracellular metabolite
profiles of the five models revealed a clear separation between the
SNP group compared to the normal 8 h control group, as well as a
distinct disparity between the CoCl2 and HR groups, when
compared to the normal 24 h control group. Meanwhile, the hypoxia
and PE group didn't show a distinct disparity from the normal 24 h
group. The first two principal components explained 58.7 and 11.7%
of the variance in intracellular metabolites between all
experimental groups. There were a total of 103 metabolites
identified by the in-house mass spectral library, 33 of which were
found to significantly differ between the five oxidative stress
cell models and the corresponding normal control groups (P<0.05
and Q<0.05; Fig. 3). Levels of
saturated and unsaturated intracellular fatty acids were lower in
the models treated with CoCl2 and SNP. Lower levels of
glutathione, four amino acids, two amino acid derivatives and all
of the tricarboxylic acid (TCA) cycle intermediates were also
observed in the CoCl2-treated cells. In contrast, nine
unsaturated fatty acids, six saturated fatty acids, glutathione,
two amino acids and two TCA cycle intermediates were found to be
significantly elevated in the cells exposed to the HR treatment.
Metabolite levels were altered in various ways when treated with PE
serum (six metabolites increased and five decreased) and only two
metabolites, arachidonic acid and succinic acid, were reduced in
response to hypoxic conditions (Fig.
3). The overall intracellular metabolite profiles differed
greatly between the five oxidative stress cell models.
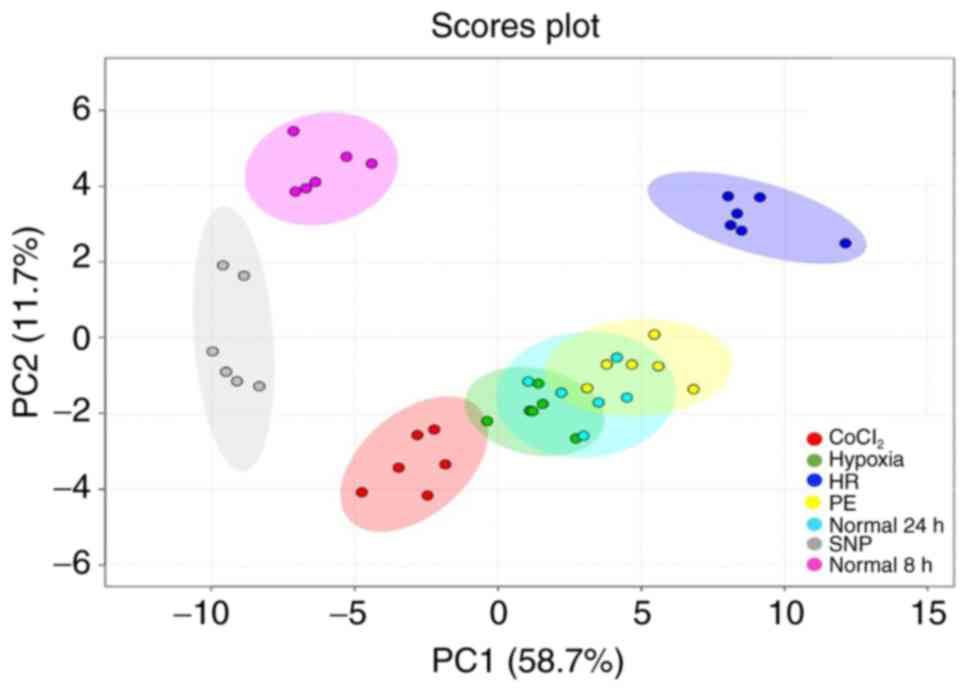 | Figure 2.PC analysis plot of the metabolic
profiles of HTR8/SVneo cells treated under seven different
conditions. Samples with similar metabolite profiles appear closer
together. Colored ellipses represent the 95% confidence intervals.
CoCl2 group (red dots), hypoxia group (green dots), HR
group (dark blue dots), normal 24 h group (bright blue dots), PE
group (yellow dots), SNP group (grey dots) and normal 8 h group
(purple dots). CoCl2, cobalt chloride; SNP, sodium
nitroprusside; PE, preeclampsia; HR, hypoxia and reoxygenation; PC,
principal component. |
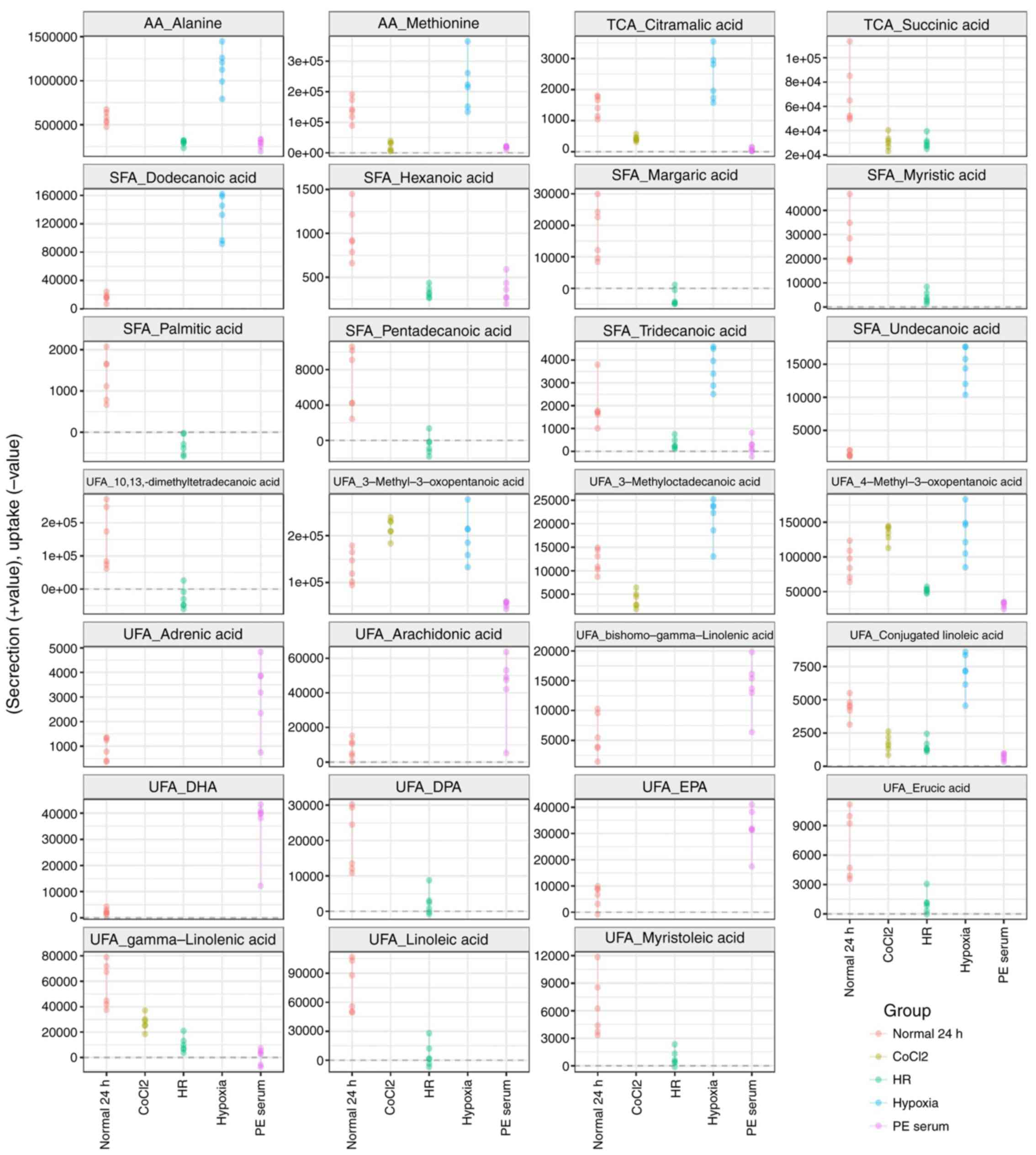
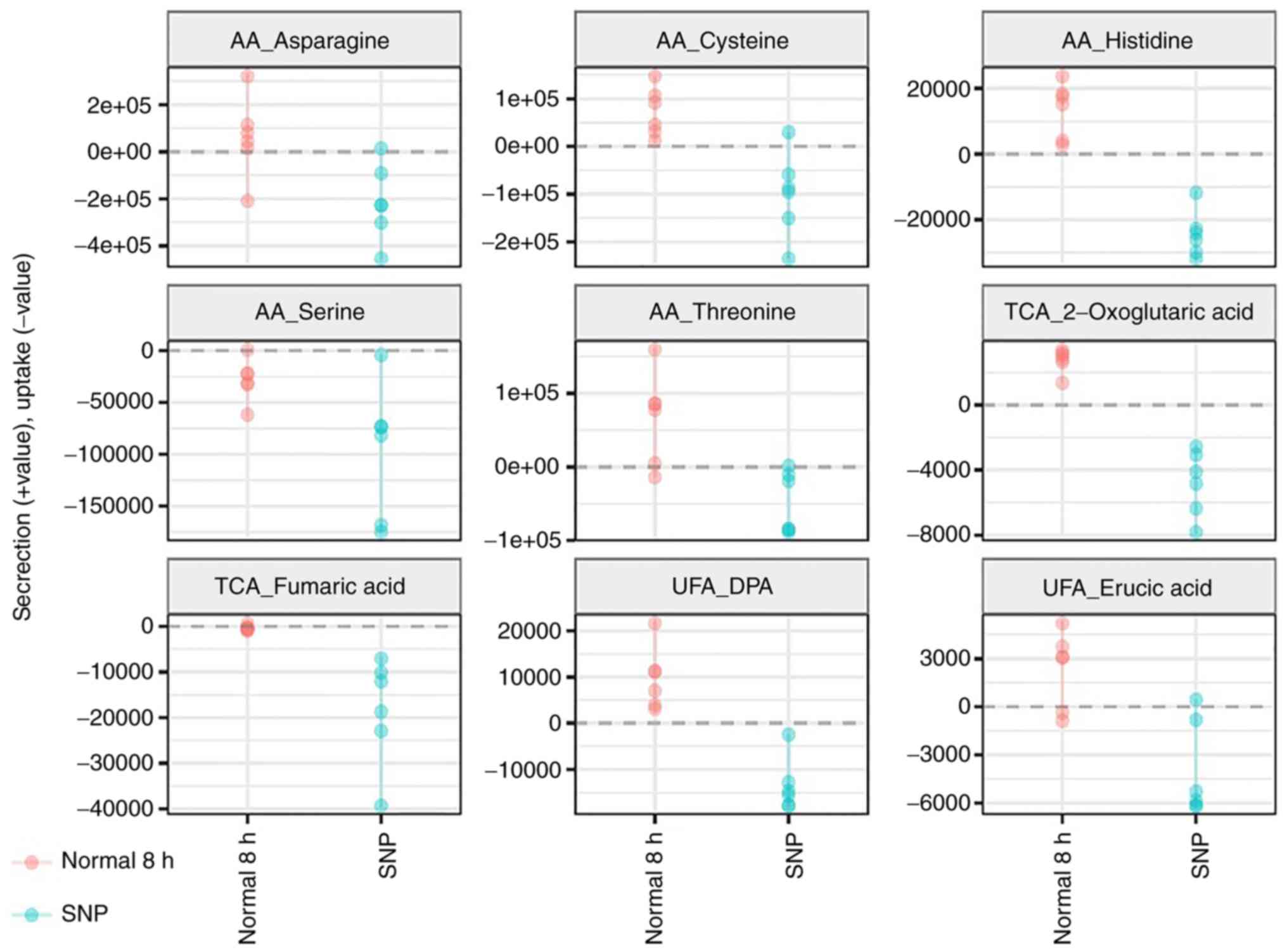
Extracellular metabolite profiling of
five oxidative stress cell models
A total of 174 metabolites were detected in the
spent media of the HTR8/SVneo culture; 36 of the extracellular
metabolites, including amino acids, unsaturated fatty acids,
saturated fatty acids and TCA cycle intermediates, were
significantly different (P<0.05 and Q<0.05) compared with the
normal and oxidative stress-treated groups (Figs. 4 and 5). A reduction in the secretion of
metabolites, including one amino acid (methionine), two TCA cycle
intermediates and two unsaturated fatty acids, was observed when
the cells were treated with CoCl2. When grown in hypoxic
conditions, most of the amino acids and unsaturated fatty acids
were secreted into the spent media. In contrast, all the
significant extracellular metabolites were found in lower levels
following exposure to the HR conditions. In the case of PE serum,
levels of the majority of the extracellular unsaturated fatty acids
were significantly increased. Interestingly, all significant
extracellular metabolites were absorbed from the culture medium
when cells were treated with SNP (Fig.
5).
Discussion
The present study is the first, to our knowledge, to
apply metabolomics to investigate the differences in the
intracellular and extracellular metabolite profiles of oxidative
stress cell models induced in vitro. Selecting an
appropriate cell model of oxidative stress applicable to obstetric
research is a challenging endeavor. One criterion that may be
useful in future selections is how well the model resembles the
metabolic etiology of the pregnancy complication under
investigation. Large metabolic disparities were observed between
the five different cell models investigated in the present study.
This raises concerns that the use of different models of oxidative
stress in prior studies may be responsible for the contradictory
findings observed.
The HR cell model demonstrated accumulation of all
intracellular metabolites and increased nutrient uptake. During
hypoxia, the oxygen deficiency resulted in the downregulation of
ATP production, and energy metabolism shifted towards
gluconeogenesis and fatty acid oxidation. Anaerobic respiration
converts glucose into lactate to sustain cellular viability
(41). Nevertheless, anaerobic
respiration is unable to meet the requirement of the aerobic cell,
as a result the electron transport chain is damaged and
intracellular pH levels are reduced (42). During the subsequent reoxygenation,
ROS are generated through the incomplete reduction of oxygen by the
damaged mitochondria (43). In
order to protect against free radicals, cells may uptake more
nutrients to feed into the TCA cycle for the generation of
additional NADPH required to maintain glutathione levels (43,44).
The results of the present study suggested that HTR8/SVneo cells
raise the levels of intracellular metabolites to protect from
oxidative stress induced by hypoxia followed by reoxygenation.
Interestingly, both CoCl2 and SNP-treated
cell models expressed the most similar metabolic profiles, in that
all intracellular and extracellular metabolites were reduced
following CoCl2 treatment, whereas all intracellular
fatty acids were decreased following SNP treatment. It is commonly
known that CoCl2 has the ability to stimulate an
intracellular hypoxia-like condition by regulating the stability of
HIF-1α, thus upregulating hypoxia-associated genes, reducing
antioxidant enzymes and rapidly increasing levels of intracellular
ROS (45). As a result,
CoCl2 may lead to cytotoxicity and downregulate the
global cellular metabolism, attenuating the biosynthesis of
intracellular metabolites and compromising extracellular excretion.
On the other hand, SNP is readily degraded into NO, which in turn
acts to induce oxidative degradation of unsaturated fatty acids,
thus resulting in the formation of unstable fatty acid radicals,
which can cause cell damage (44).
Moreover, in the presence of H2O2, NO can be
converted into peroxynitrite, a potent oxidant that reacts with
unsaturated fatty acids within liposomes to initiate lipid
peroxidation chain reactions and eliminate lipid-soluble
antioxidants (44,46,47).
Therefore, both exogenous chemicals CoCl2 and SNP induce
oxidative stress, and their cytotoxic effects on viable cells are
similar in terms of the overall reduction in metabolites, as
observed in the present study.
The oxidative stress cell model established using PE
serum did not share a similar metabolite profile with any of the
other four cell models. In the PE model, the extracellular
excretion of ω-6 unsaturated fatty acids (bihomo-γ-linoleate and
arachidonate) and ω-3 unsaturated fatty acids [eicosapentaenoate
(EPA) and docosahexaenoate (DHA)] was higher. These fatty acids are
involved in vasodilation, as well as having anti-inflammatory and
antioxidant properties. For instance, bihomo-γ-linoleate is
desaturated to arachidonate, which acts as the precursor for the
production of prostaglandins (48). EPA serves as another precursor for
the biosynthesis of prostaglandin and anti-inflammatory
leukotrienes-5 (49). Both EPA and
DHA, via the cyclooxygenase and lipoxygenase pathways, have potent
anti-inflammatory and pro-resolving effects, as well as the ability
to suppress the expression of pro-inflammatory cytokines such as
tumor necrosis factor-α, interleukin-6 and plasminogen activator
inhibitor-1 (50,51). EPA and DHA also facilitate
circulating glutathione peroxidase and superoxide dismutase, and
they exhibit vasodilating properties via the inhibition of
Na/Ca2+ exchange and α1-adrenoceptor activity (52). As these ω-unsaturated fatty acids
are involved in the protective cellular mechanisms against
inflammation and oxidative stress, it is hypothesized that PE
serum, rather than being a potent oxidative stress inducer like the
other models, initiates protective physiological responses in
HTR8/SVneo cells during the early stages of disease progression.
Therefore, the suitability of PE serum to study oxidative stress
underlying PE pathogenesis may need to be reconsidered.
In the present study, it was hypothesized that while
HTR8/SVneo cells are capable of maintaining their intracellular
metabolic homeostasis under 1% O2 hypoxia, they attempt
to modify their external environment via extracellular secretion.
In the hypoxia group, it was observed that only two intracellular
metabolites were reduced, meanwhile ten extracellular metabolites,
in particular fatty acids, were secreted into the spent media.
HTR8/SVneo cells are an extravillous trophoblast cell. Trophoblast
cells experience a physiological hypoxic environment (3%
O2) 8–10 weeks into pregnancy (53,54),
so it is unsurprising that these cells exhibited hypoxic tolerance
in 1% O2 for 24 h, with only minor intracellular
metabolic disturbances observed. In addition to withstanding
hypoxic conditions, trophoblast cells can also increase the
secretion of angiogenic factors in order to promote new blood
vessel formation to increase O2 supply (55). In the present study, cells in the
hypoxia group showed increased excretion of conjugated linoleic
acid; this is a polyunsaturated fatty acid that has been reported
to stimulate the angiogenesis of placental trophoblast cells in the
first trimester (56). In
addition, conjugated linoleic acid suppresses ROS production via
the upregulation of peroxisome proliferator-activated receptor γ
expression (57,58).
The present study had certain limitations that
should be considered when designing future studies. Firstly, it
would be useful to study primary cell lines isolated directly from
human placenta in order to investigate metabolic changes under
various oxidative perturbations. Secondly, genomic and proteomic
data should be investigated to further validate the metabolomic
findings and support conclusions from the present study.
In conclusion, the present study showed the
differences between the intracellular and extracellular metabolite
profiles of five different oxidative stress cell models commonly
used to study pregnancy complications. The findings suggested that
there is a need for the standardization of cell models used for
oxidative stress research in order to avoid contradictory findings,
particularly in the discipline of perinatal research.
Acknowledgements
The authors would like to thank Dr Charles Graham at
Queen's University, Canada, for providing HTR8/SVneo cells.
Funding
The present study was supported by The National
Natural Science Foundation of China (grant nos. 81571453, 81771607,
81871185, 81701477 and 81471473). The 111 Project [grant no.
Yuwaizhuan (2016)32], The National Key Research and Development
Program of Reproductive Health & Major Birth Defects Control
and Prevention (grant no. 2016YFC1000407), Chongqing Health
Commission (grant nos. 2017ZDXM008 and 2018ZDXM024), and Chongqing
Science & Technology Commission (grant no.
cstc2017jcyjBX0062).
Availability of data and materials
The datasets used and analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
JC performed the experiments. XZ designed the
experiment. JC and TLH conducted the statistical analysis of the
data and drafted the manuscript. HZ, YS and PB conceived the study,
participated in its design and coordination, and helped to draft
the manuscript.
Ethics approval and consent to
participate
All participants at enrollment signed written
informed consent and this study was approved by The Ethics
Committee of Chongqing Medical University (policy no. 2014034).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Aouache R, Biquard L, Vaiman D and
Miralles F: Oxidative stress in preeclampsia and placental
diseases. Int J Mol Sci. 19(pii): E14962018. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Espinosa-Diez C, Miguel V, Mennerich D,
Kietzmann T, Sánchez-Pérez P, Cadenas S and Lamas S: Antioxidant
responses and cellular adjustments to oxidative stress. Redox Biol.
6:183–197. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Burton GJ, Woods AW, Jauniaux E and
Kingdom JC: Rheological and physiological consequences of
conversion of the maternal spiral arteries for uteroplacental blood
flow during human pregnancy. Placenta. 30:473–482. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kalyanaraman B: Teaching the basics of
redox biology to medical and graduate students: Oxidants,
antioxidants and disease mechanisms. Redox Biol. 1:244–257. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mannaerts D, Faes E, Cos P, Briedé JJ,
Gyselaers W, Cornette J, Gorbanev Y, Bogaerts A, Spaanderman M, Van
Craenenbroeck E and Jacquemyn Y: Oxidative stress in healthy
pregnancy and preeclampsia is linked to chronic inflammation, iron
status and vascular function. PLoS One. 13:e02029192018. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yang X, Guo L, Li H, Chen X and Tong X:
Analysis of the original causes of placental oxidative stress in
normal pregnancy and pre-eclampsia: A hypothesis. J Matern Fetal
Neonatal Med. 25:884–888. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Burton GJ and Jauniaux E: Placental
oxidative stress: From miscarriage to preeclampsia. J Soc Gynecol
Investig. 11:342–352. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Mert I, Oruc AS, Yuksel S, Cakar ES,
Buyukkagnici U, Karaer A and Danisman N: Role of oxidative stress
in preeclampsia and intrauterine growth restriction. J Obstet
Gynaecol Res. 38:658–664. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wu F, Tian FJ, Lin Y and Xu WM: Oxidative
stress: Placenta function and dysfunction. Am J Reprod Immunol.
76:258–271. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
De Paepe C, Krivega M, Cauffman G, Geens M
and Van de Velde H: Totipotency and lineage segregation in the
human embryo. Mol Hum Reprod. 20:599–618. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alahari S, Post M and Caniggia I: Jumonji
domain containing protein 6: A novel oxygen sensor in the human
placenta. Endocrinology. 156:3012–3025. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Baker PN, Davidge ST, Barankiewicz J and
Roberts JM: Plasma of preeclamptic women stimulates and then
inhibits endothelial prostacyclin. Hypertension. 27:56–61. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
He G, Xu W, Chen Y, Liu X and Xi M:
Abnormal apoptosis of trophoblastic cells is related to the
up-regulation of CYP11A gene in placenta of preeclampsia patients.
PLoS One. 8:e596092013. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Luo X, Yao ZW, Qi HB, Liu DD, Chen GQ,
Huang S and Li QS: Gadd45a as an upstream signaling molecule of p38
MAPK triggers oxidative stress-induced sFlt-1 and sEng upregulation
in preeclampsia. Cell Tissue Res. 344:551–565. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yuan Y, Hilliard G, Ferguson T and
Millhorn DE: Cobalt inhibits the interaction between
hypoxia-inducible factor-alpha and von Hippel-Lindau protein by
direct binding to hypoxia-inducible factor-alpha. J Biol Chem.
278:15911–15916. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Luo R, Wang Y, Xu P, Cao G, Zhao Y, Shao
X, Li YX, Chang C, Peng C and Wang YL: Hypoxia-inducible miR-210
contributes to preeclampsia via targeting thrombospondin type I
domain containing 7A. Sci Rep. 6:195882016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zou Y, Zuo Q, Huang S, Yu X, Jiang Z, Zou
S, Fan M and Sun L: Resveratrol inhibits trophoblast apoptosis
through oxidative stress in preeclampsia-model rats. Molecules.
19:20570–20579. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Saito S and Nakashima A: A review of the
mechanism for poor placentation in early-onset preeclampsia: The
role of autophagy in trophoblast invasion and vascular remodeling.
J Reprod Immunol. 101-102:80–88. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Covarrubias AE, Lecarpentier E, Lo A,
Salahuddin S, Gray KJ, Karumanchi SA and Zsengellér ZK: AP39, a
modulator of mitochondrial bioenergetics, reduces antiangiogenic
response and oxidative stress in hypoxia-exposed trophoblasts:
Relevance for preeclampsia pathogenesis. Am J Pathol. 189:104–114.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Yang Z, Bai B, Luo X, Xiao X, Liu X, Ding
Y, Zhang H, Gao L, Li J and Qi H: Downregulated Kruppel-like factor
8 is involved in decreased trophoblast invasion under
hypoxia-reoxygenation conditions. Reprod Sci. 21:72–81. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhuang B, Luo X, Rao H, Li Q, Shan N, Liu
X and Qi H: Oxidative stress-induced C/EBPβ inhibits β-catenin
signaling molecule involving in the pathology of preeclampsia.
Placenta. 36:839–846. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nangaku M and Eckardt KU: Hypoxia and the
HIF system in kidney disease. J Mol Med (Berl). 85:1325–1330. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Myatt L and Cui X: Oxidative stress in the
placenta. Histochem Cell Biol. 122:369–382. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Silva JP, Proenca F and Coutinho OP:
Protective role of new nitrogen compounds on ROS/RNS-mediated
damage to PC12 cells. Free Radic Res. 42:57–69. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Williamson RD, McCarthy C, McCarthy FP and
Kenny LC: Oxidative stress in pre-eclampsia; Have we been looking
in the wrong place? Pregnancy Hypertension. 8:1–5. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Melland-Smith M, Ermini L, Chauvin S,
Craig-Barnes H, Tagliaferro A, Todros T, Post M and Caniggia I:
Disruption of sphingolipid metabolism augments ceramide-induced
autophagy in preeclampsia. Autophagy. 11:653–669. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Sugawara J, Suh DS, Faessen GH, Suen LF,
Shibata T, Kaper F, Giaccia AJ and Giudice LC: Regulation of
insulin-like growth factor-binding protein-1 by nitric oxide under
hypoxic conditions. J Clin Endocrinol Metab. 85:2714–2721. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
English FA, McCarthy FP, McSweeney CL,
Quon AL, Morton JS, Sawamura T, Davidge ST and Kenny LC: Inhibition
of lectin-like oxidized low-density lipoprotein-1 receptor protects
against plasma-mediated vascular dysfunction associated with
pre-eclampsia. Am J Hypertens. 26:279–286. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
McCarthy C and Kenny LC: Therapeutically
targeting mitochondrial redox signalling alleviates endothelial
dysfunction in preeclampsia. Sci Rep. 6:326832016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sokolov DI, Ovchinnikova OM, Korenkov DA,
Viknyanschuk AN, Benken KA, Onokhin KV and Selkov SA: Influence of
peripheral blood microparticles of pregnant women with preeclampsia
on the phenotype of monocytes. Transl Res. 170:112–123. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Xu Q, Du F, Zhang Y, Teng Y, Tao M, Chen
AF and Jiang R: Preeclampsia serum induces human glomerular
vascular endothelial cell hyperpermeability via the
HMGB1-Caveolin-1 pathway. J Reprod Immunol. 129:1–8. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Roberts JM and Hubel CA: The two stage
model of preeclampsia: Variations on the theme. Placenta. 30 (Suppl
A):S32–S37. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tamaru S, Kajihara T, Mizuno Y, Takano N,
Tochigi H, Sato T and Ishihara O: Heparin prevents oxidative
stress-induced apoptosis in human decidualized endometrial stromal
cells. Med Mol Morphol. Mar 16–2019.(Epub ahead of print). doi:
10.1007/s00795-019-00220-x. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Grosser N, Abate A, Oberle S, Vreman HJ,
Dennery PA, Becker JC, Pohle T, Seidman DS and Schröder H: Heme
oxygenase-1 induction may explain the antioxidant profile of
aspirin. Biochem Biophys Res Commun. 308:956–960. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huang S, Mo TT, Norris T, Sun S, Zhang T,
Han TL, Rowan A, Xia YY, Zhang H, Qi HB and Baker PN: The CLIMB
(Complex Lipids In Mothers and Babies) study: Protocol for a
multicentre, three-group, parallel randomised controlled trial to
investigate the effect of supplementation of complex lipids in
pregnancy, on maternal ganglioside status and subsequent cognitive
outcomes in the offspring. BMJ Open. 7:e0166372017.PubMed/NCBI
|
|
36
|
Graham CH, Hawley TS, Hawley RG,
MacDougall JR, Kerbel RS, Khoo N and Lala PK: Establishment and
characterization of first trimester human trophoblast cells with
extended lifespan. Exp Cell Res. 206:204–211. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Smart KF, Aggio RBM, Van Houtte JR and
Villas-Bôas SG: Analytical platform for metabolome analysis of
microbial cells using methyl chloroformate derivatization followed
by gas chromatography-mass spectrometry. Nat Protoc. 5:1709–1729.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Smith CA, Want EJ, O'Maille G, Abagyan R
and Siuzdak G: XCMS: Processing mass spectrometry data for
metabolite profiling using nonlinear peak alignment, matching, and
identification. Anal Chem. 78:779–787. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Xia J, Sinelnikov IV, Han B and Wishart
DS: MetaboAnalyst 3.0-making metabolomics more meaningful. Nucleic
Acids Res. 43:W251–W257. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Wickham H: Ggplot2: Elegant graphics for
data analysis. Springer; New York: 2009
|
|
41
|
Ferguson BS, Rogatzki MJ, Goodwin ML, Kane
DA, Rightmire Z and Gladden LB: Lactate metabolism: Historical
context, prior misinterpretations, and current understanding. Eur J
Appl Physiol. 118:691–728. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Hess ML and Manson NH: Molecular oxygen:
Friend and foe. The role of the oxygen free radical system in the
calcium paradox, the oxygen paradox and ischemia/reperfusion
injury. J Mol Cell Cardiol. 16:969–985. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Liu J, Litt L, Segal MR, Kelly MJ, Pelton
JG and Kim M: Metabolomics of oxidative stress in recent studies of
endogenous and exogenously administered intermediate metabolites.
Int J Mol Sci. 12:6469–6501. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hogg N and Kalyanaraman B: Nitric oxide
and lipid peroxidation. Biochim Biophys Acta. 1411:378–384. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Guan D, Su Y, Li Y, Wu C, Meng Y, Peng X
and Cui Y: Tetramethylpyrazine inhibits CoCl2-induced neurotoxicity
through enhancement of Nrf2/GCLc/GSH and suppression of
HIF1a/NOX2/ROS pathways. J Neurochem. 134:551–565. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Chun HS and Low WC: Ursodeoxycholic acid
suppresses mitochondria-dependent programmed cell death induced by
sodium nitroprusside in SH-SY5Y cells. Toxicology. 292:105–112.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Dominiak A, Wilkaniec A, Wroczynski P,
Jęśko H and Adamczyk A: Protective effects of selol against sodium
nitroprusside-induced cell death and oxidative stress in PC12
Cells. Neurochem Res. 41:3215–3226. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Innes JK and Calder PC: Omega-6 fatty
acids and inflammation. Prostaglandins Leukot Essent Fatty Acids.
132:41–48. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Pollard JK and Mitchell MD: Intrauterine
infection and the effects of inflammatory mediators on
prostaglandin production by myometrial cells from pregnant women.
Am J Obstet Gynecol. 174:682–686. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Geleijnse JM, Giltay EJ, Grobbee DE,
Donders AR and Kok FJ: Blood pressure response to fish oil
supplementation: Metaregression analysis of randomized trials. J
Hypertens. 20:1493–1499. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Poniedzialek-Czajkowska E, Mierzynski R,
Kimber-Trojnar Z, Leszczynska-Gorzelak B and Oleszczuk J:
Polyunsaturated fatty acids in pregnancy and metabolic syndrome: A
review. Curr Pharm Biotechnol. 15:84–99. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Hallaq H, Smith TW and Leaf A: Modulation
of dihydropyridine-sensitive calcium channels in heart cells by
fish oil fatty acids. Proc Natl Acad Sci USA. 89:1760–1764. 1992.
View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Bilodeau JF: Review: Maternal and
placental antioxidant response to preeclampsia-impact on vasoactive
eicosanoids. Placenta. 35 (Suppl):S32–S38. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Zhang Z, Li P, Wang Y and Yan H:
Hypoxiainduced expression of CXCR4 favors trophoblast cell
migration and invasion via the activation of HIF1a. Int J Mol Med.
42:1508–1516. 2018.PubMed/NCBI
|
|
55
|
Xu C, Li X, Guo P and Wang J:
Hypoxia-Induced Activation of JAK/STAT3 signaling pathway promotes
trophoblast cell viability and angiogenesis in preeclampsia. Med
Sci Monit. 23:4909–4917. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
56
|
Basak S, Das MK and Duttaroy AK: Fatty
acid-induced angiogenesis in first trimester placental trophoblast
cells: Possible roles of cellular fatty acid-binding proteins. Life
Sci. 93:755–762. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
57
|
Dipasquale D, Basiricò L, Morera P, Primi
R, Tröscher A and Bernabucci U: Anti-inflammatory effects of
conjugated linoleic acid isomers and essential fatty acids in
bovine mammary epithelial cells. Animal. 12:2108–2114. 2018.
View Article : Google Scholar : PubMed/NCBI
|
|
58
|
Li K, Sinclair AJ, Zhao F and Li D:
Uncommon fatty acids and cardiometabolic health. Nutrients.
10:15592018. View Article : Google Scholar :
|