Introduction
Idiopathic pulmonary fibrosis (IPF) is a chronic,
progressive, fibrosing and interstitial lung disease. The median
survival of patients with IPF is 2 to 3 years, with clinical
symptoms of progressively aggravated cough and dyspnea (1). The pathogenesis of IPF is still
unknown, and the state of existing therapeutic treatments is poor,
barring lung transplantation. Further studies are therefore
required to ascertain the mechanism of the disease, in order to
explore new therapeutic targets.
Epithelial-mesenchymal transition (EMT) refers to
the process through which fully differentiated epithelial cells
transform into mesenchymal cells with specific phenotypes under
particular pathological and physiological conditions, which is the
key step in fibrosis progression. EMT plays an important role in
malignant tumor formation and infiltration, tissue remodeling, and
fibrosis in multiple organs (2–4). It
may play a key role in IPF (5,6). Its
controversial role makes this study meaningful as yet.
The initial stage of IPF is the formation of lesions
of alveolar epithelial cells, which release multiple cytokines such
as transforming growth factor-β1 (TGF-β1). This promotes cell
apoptosis, migration and extracellular matrix (ECM) formation,
which has been widely used to induce EMT in vitro. The
Wnt/β-catenin signaling pathway is a classic Wnt pathway, which is
highly conserved and plays meaningful roles in embryo and organ
development, as well as the maintenance of homeostasis. However,
abnormalities in the Wnt/β-catenin pathway can lead to various lung
diseases, including lung cancer and IPF (7,8). One
study has demonstrated the close relationship between Wnt/β-catenin
signaling and fibrosis-related factors and EMT during lung fibrosis
(9). Tribbles homologue 3 (TRB3)
is a member of the putative protein kinase family with a
kinase-like domain. TRB3 can react with transcription factors, the
type II bone morphogenetic protein (BMP) receptor on the plasma
membrane, and members of signaling pathways such as the
Wnt/β-catenin pathway to inhibit mitosis and modulate cell
proliferation, apoptosis, migration and morphological alterations.
Studies have demonstrated that TRB3 overexpression is involved in
renal fibrosis by increasing apoptosis rates in renal tubular
epithelial (RTE) cells and by inhibiting cell proliferation
(10–12). Nonetheless, the role of TRB3 in
lung fibrosis remains to be fully elucidated. In the present study,
a new target for IPF treatment was explored by uncovering the role
of TRB3 in IPF pathogenesis.
Materials and methods
Cell culture and grouping
MLE-12 cells [mouse type II alveolar epithelial
cells, purchased from American Type Culture Collection (ATCC)] were
cultured in DMEM/F12 (Gibco; Thermo Fisher Scientific, Inc.)
supplemented with 2% fetal bovine serum (FBS; Gibco; Thermo Fisher
Scientific, Inc.) in 5% atmospheric CO2 at 37°C. Cells
with 80 to 90% confluency were passaged using 0.25% trypsin-EDTA
(Gibco; Thermo Fisher Scientific, Inc.) and split 1:4 to 1:6 and
transferred to fresh culture flasks.
Cells were divided into eight groups: i) control
group; ii) Ad-GFP transduction group (GFP group); iii) Ad-TRB3
transduction group (TRB3 group); iv) Ad-TRB3-shRNA transduction
group (shR-TRB3 group); v) group with TGF-β1 stimulation only
(TGF-β1 group); vi) TGF-β1 and Ad-GFP transduction group (T+GFP
group); vii) TGF-β1 and Ad-TRB3 transduction group (T+TRB3 group);
viii) TGF-β1 and Ad-TRB3-shRNA transduction group (T+shR-TRB3
group).
Primers were designed based on the TRB3 gene
sequence (MGI 3562334) (NCBI Gene-Bank) and the sequences of the
primers are listed in Table I. PCR
gene (SYBR Green) amplification was performed to construct the
Ad-TRB3 recombinant plasmid and Ad-TRB3-shRNA recombinant plasmid.
The plasmid carrier was pacAd5 CMV–IRES-GFP, 7.5 kb; MCS:
PmeI, ClaI, EcoRV, EcoRI, BamHI.
The recombinant plasmids were then amplified, cultured, purified
and packaged with adenovirus.
 | Table I.Primer sequences for reverse
transcription-PCR. |
Table I.
Primer sequences for reverse
transcription-PCR.
| Name | Primer sequence | Product (bp) |
|---|
| TRB3 | Forward
5′-GGAACCTTCAGAGCGACTT-3′ | 341 |
|
| Reverse
5′-TGGCACTCAGGGAGCATC-3′ |
|
| β-catenin | Forward
5′-AATCCGAGGACTCAATACC-3′ | 636 |
|
| Reverse
5′-AGAGTAAAGTATTCACCCA-3′ |
|
| α-SMA | Forward
5′-GTACCCAGGCATTGCTGACA-3′ | 271 |
|
| Reverse
5′-GAGGCGCTGATCCACAAAAC-3′ |
|
| Collagen I | Forward
5′-GAGACAGGCGAACAAGGTGA-3′ | 399 |
|
| Reverse
5′-CTCAAGGTCACGGTCACGAA-3′ |
|
| Collagen III | Forward
5′-AGTGGGCATCCAGGTCCTAT-3′ | 480 |
|
| Reverse
5′-GTGCTTACGTGGGACAGTCA-3′ |
|
| β-actin | Forward
5′-CCACCATGTACCCAGGCATT-3′ | 189 |
|
| Reverse
5′-CGGACTCATCGTACTCCTGC-3′ |
|
|
TRIB3-EcoRI-S |
5′-CCGGAATTCGCCACCATGCGAGCTACACCTCTGGC-3′ |
|
|
TRIB3-BamHI-AS |
5′-CGCGGATCCGCCGTACAGCCCCACCTCCC-3′ |
|
Six independent parallel experiments were performed
for each group. Preliminary results indicated that the optimal
concentration of TGF-β1 stimulation was 10 ng/ml, and the optimal
multiplicity of infection (MOI) for adenovirus vector Ad-GFP,
Ad-TRB3, Ad-TRB3-shRNA was 100, 800 and 200, respectively, with an
optimal transduction time of 48 h.
Cell Counting Kit-8 (CCK-8)
MLE-12 cells were cultured in 96-well plates at a
concentration of 103−104/well, and
pre-incubated in a cell culture incubator with 5% atmospheric
CO2 at 37°C for 24 h, and then incubated for 12 h in
serum-free medium. After the cells attached to the plate
completely, three virus vectors and TGF-β1 recombinant protein were
loaded into the wells according to the aforementioned groupings,
and CO2 incubation was resumed. CCK-8 (Dojindo, China)
solution was pipetted into each well (10 µl/well) after 48 h of
cultivation, and then incubation was continued. After 2 h,
absorbance was measured at 450 nm using a plate reader (Bio-Tek,
USA). The experiments were conducted in triplicate.
Flow cytometry
MLE-12 cells were plated into 6-well plates at a
concentration of (1–5)×105 cells/well and cultured in a
CO2 incubator overnight. After the cells attached to the
plate completely, the corresponding virus vectors and TGF-β1
recombinant protein were loaded into the wells according to the
aforementioned grouping. After 48 h, the cells were harvested, and
the cell suspension was prepared. In a 1.5-ml centrifuge tube, 100
µl of the cell suspension from each well was mixed with 10 µl
Annexin V-R-PE (Southern Biotech, USA) by clicking the tube gently.
The tube was bathed in ice in a dark chamber for 10 min. Next, 380
µl 1X binding buffer was pipetted into the tube and mixed by
clicking the tube gently, before loading the samples for flow
cytometry to evaluate the early-stage apoptotic rates for each
group.
Reverse transcription-quantitative PCR
(RT-qPCR)
Total RNA was extracted using TRIzol®
(Invitrogen; Thermo Fisher Scientific, Inc.) 48 h after the cell
vectors or recombinant protein treatments. Total RNA (3 µl) was
pipetted into a 33.5 µl reaction master mix for reverse
transcription of cDNA. TRB3, β-catenin, α-SMA (fibrosis-related
protein), collagen I/III and β-actin genes were amplified using
fluorogenic RT-qPCR (Takara), along with the housekeeping gene.
Relative mRNA expression levels of target genes compared to the
housekeeping gene were calculated using the ΔΔCt calculation
method. Primers for all target genes are listed in Table I.
Western blotting
Total cell protein content was extracted using cell
lysis buffer (Gibco; Thermo Fisher Scientific, Inc.), and sample
protein concentrations were determined for each group using a BCA
protein assay kit (Wuhan Boster Biological, Co., Ltd.), 48 h after
vector or recombinant protein treatment. SDS-PAGE was performed for
each group on 10% gels, with 30–50 µg protein sample per lane, and
proteins were transferred from gel to the membrane via the wet
method. After 2 h blocking using 5% skim milk powder at 4°C, the
membranes were incubated with a dilution of 1:1,000 rabbit
anti-mouse TRB3 polyclonal antibody (PcAb; cat. no. ab73547)
(anti-mouse TRB3 antibody prepared from rabbit plasma), a dilution
of 1:1,000 rabbit anti-mouse β-catenin (PcAb; cat. no. ab16051), a
dilution of 1:500 rabbit anti-mouse E-cadherin (PcAb; cat. no.
ab202413), a dilution of 1:500 rabbit anti-mouse vimentin (PcAb;
cat. no. ab45939), and a dilution of 1:1,000 rabbit anti-mouse
fibronectin (PcAb; cat. no. ab2413), respectively, on a 4°C shaker
overnight. Membranes were then incubated statically with 1:2,000
goat anti-rabbit IgG (PcAb; cat. no. ab21058) labeled with HRP at
room temperature for 1 h. All antibodies mentioned above were
obtained from Abcam, USA. Lastly, membranes were photographed using
a visible/ultraviolet gel scanning analysis system (UVP, LLC) and
LabWorks™ 4.5 Analysis software (UVP, LLC), and the calibrated band
brightness of each group was calculated by dividing the real
brightness by the brightness of β-tubulin or GAPDH (internal
reference).
Immunofluorescence (IF) assay
Cell coverslips were prepared 48 h after vector or
recombinant protein treatment and were fixed using paraformaldehyde
for 15 min and washed using PBS. Next, 200 µl of the corresponding
primary antibody (1:500; cat. no. sc-390242; Santa Cruz
Biotechnology, Inc.) diluted with 1% donkey serum (Sigma-Aldrich;
Merck KGaA) was dripped onto each coverslip, which was then
incubated at 4°C overnight. After incubation, the coverslips were
washed with PBS and incubated at 4°C for 1.5 to 2 h, after
pipetting 200 µl secondary antibody (1:500; cat. no. sab4600003;
Sigma-Aldrich; Merck KGaA) diluted with 1% donkey serum. Then, the
coverslips were washed with PBS, stained with DAPI combined with
FITC (Sigma-Aldrich; Merck KGaA) and incubated at 4°C for 5 min.
The treated cells were moved from the multi-well plate onto
microscopic slides and spun. Next, the coverslip was placed onto an
object slide, 5 µl fluorescence protection solution was pipetted
onto each slide, and the whole system was sealed with cover glass.
Finally, the sample was examined under confocal laser scanning
microscopy (magnification, ×100; FV1000; Olympus Corporation) and
imaged.
Statistical analysis
SPSS 19.0 (IBM, Corp.) was utilized for database
establishment using data from this study. All data are consistent
with a normal distribution and are presented as the mean ± SD.
Differences of means between two groups were compared using t-test,
while those among multiple groups were compared using ANOVA, with
the Least Significant Difference test LSD-t and SNK as post hoc
tests. P<0.05 was considered to indicate a statistically
significant difference.
Results
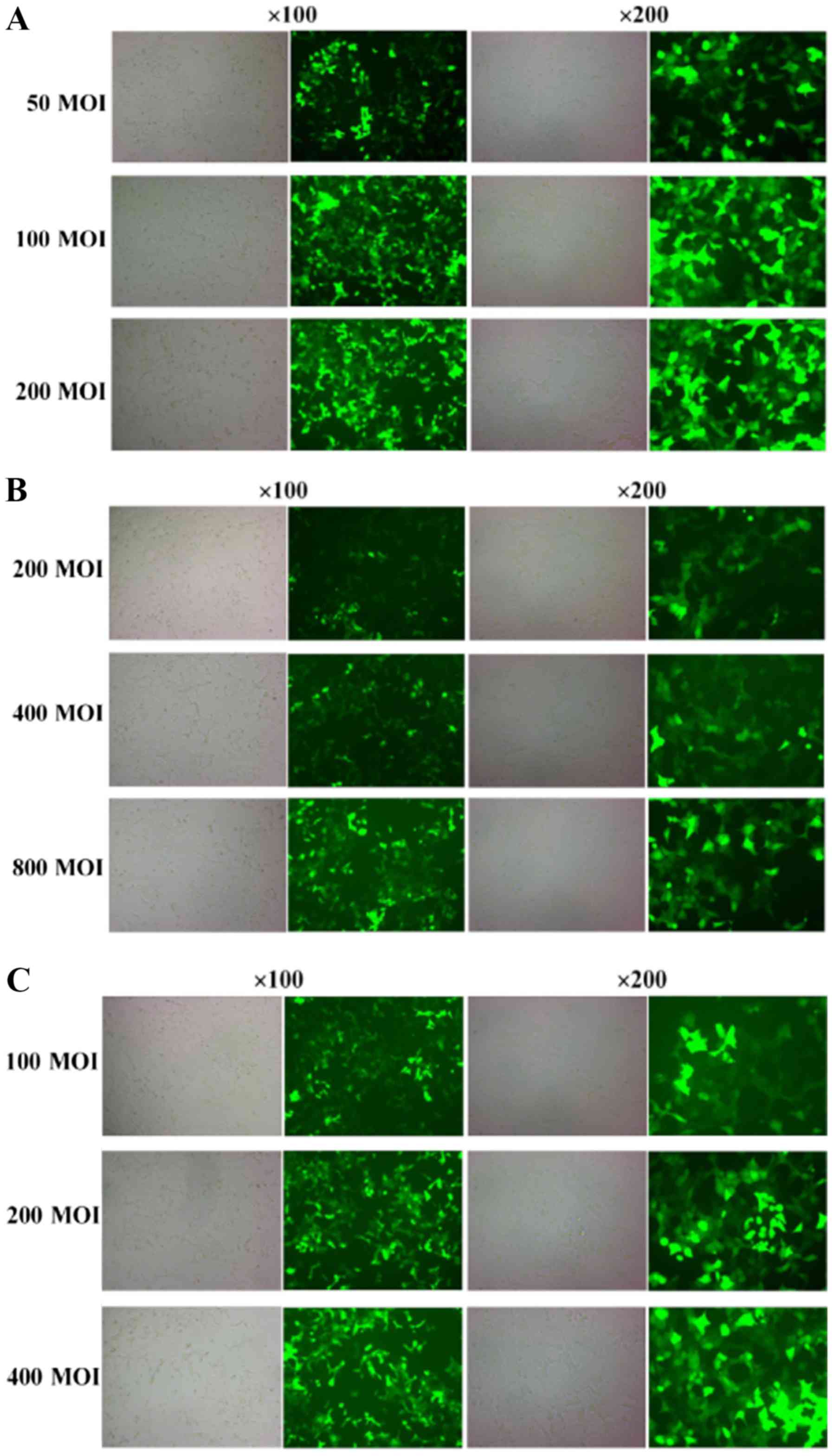
Fluorescence detection of viral
infection of MLE-12 cells
Twenty-four hours after viral infection, GFP
expression could be detected and the fluorescence signal was
gradually increased with the prolongation of observation time in
the same multiplicity of infection (MOI) value group, which
exhibited the best infection efficiency at 48 h, approximately
>90%. At the same time, the fluorescence signal increased with
the increase in MOI value (Fig.
1). The optimal MOI values of GFP (Fig. 1A), TRB3 (Fig. 1B) and shR-TRB3 (Fig. 1C) groups were 100, 800 and 200,
respectively, and they expressed exogenous genes stably and
efficiently in target cells. The intensity of the fluorescence
signal of TRB3-transfected cells was significantly decreased, while
that of TRB3-transfected cells was increased after silencing.
Effect of the alteration of TRB3
expression on proliferation activity and early-stage apoptosis
Proliferation activity assay indicated that the
OD450 values of MLE-12 cells in the TRB3 overexpression groups
(TRB3 group, T+TRB3 group) were significantly lower than those in
the TRB3 downregulated groups (shR-TRB3 group, T+shR-TRB3 group)
(P<0.05). After TGF-β1 administration, the results demonstrated
an enhancing effect of TRB3 overexpression on the role of TGF-β1 in
inhibiting MLE-12 proliferation activity; while downregulation of
TRB3 expression led to the opposite effect (P<0.05). Early-stage
apoptotic rates monitored by flow cytometric assay revealed that
with the adenovirus vector only, TRB3 overexpression significantly
promoted early-stage apoptosis in MLE-12 cells; while
downregulation of TRB3 expression exerted the opposite effect
(P<0.05). After TGF-β1 administration, the results demonstrated
a positive effect of TRB3 overexpression on MLE-12 early-stage
apoptosis induced by TGF-β1; while downregulation of TRB3
expression inhibited early-stage apoptosis (P<0.05; Table II).
 | Table II.Effect of TRB3 expression on MLE-12
cell proliferation and apoptosis induced by TGF-β1 (mean ± SD,
n=3). |
Table II.
Effect of TRB3 expression on MLE-12
cell proliferation and apoptosis induced by TGF-β1 (mean ± SD,
n=3).
| Treatment group | OD value | Apoptotic rate
(%) |
|---|
| Control |
0.4387±0.0025a |
1.56±0.71a |
| GFP |
0.4227±0.0060c |
1.55±0.70c |
| TRB3 |
0.2487±0.0330b |
10.16±0.5b |
| shR-TRB3 |
0.5480±0.0291b |
1.15±0.07b |
| TGF-β1 |
0.3537±0.0189d |
3.87±0.13d |
| T+GFP | 0.3600±0.0240 | 3.84±0.23 |
| T+TRB3 |
0.2340±0.0101e |
10.21±0.10e |
| T+shR-TRB3 |
0.4213±0.0114e |
6.52±0.10e |
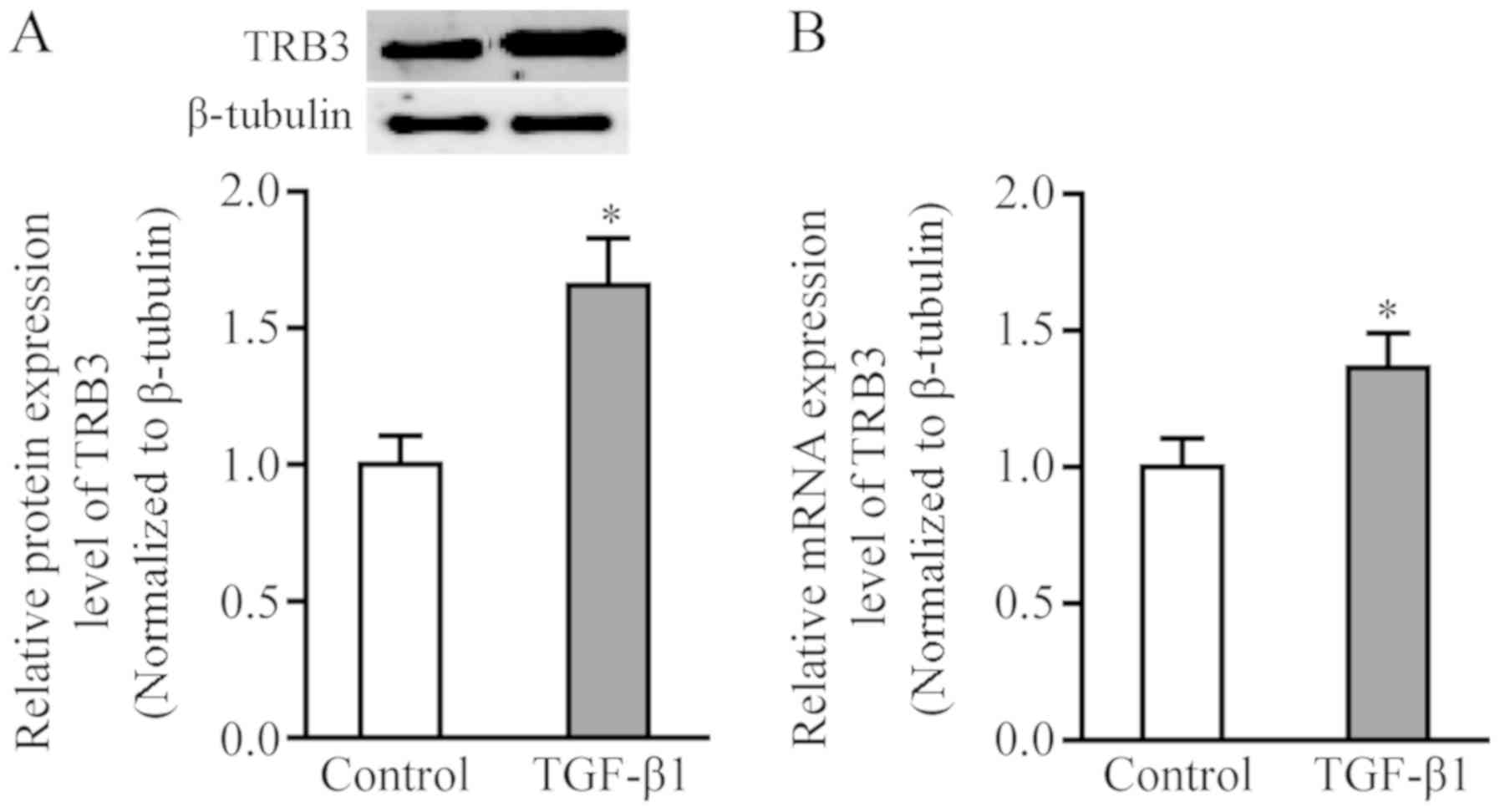
TRB3, β-catenin, α-SMA and collagen I/III
mRNA
TRB3 mRNA expression during EMT
induced by TGF-β1
TRB3 mRNA expression was significantly increased in
the TGF-β1-stimulated group relative to the control group
(P<0.05; Fig. 2B).
Impact of TRB3 on EMT-related protein
and β-catenin expression
The mRNA expression of β-catenin, α-SMA and collagen
I/III was significantly increased in the TRB3 overexpression group
(P<0.05); whereas expression levels of all three were reduced in
the TRB3 downregulated (shR-TRB3) group (Table III).
 | Table III.TRB3, β-catenin, α-SMA and collage
I/III mRNA in MLE-12 cells. |
Table III.
TRB3, β-catenin, α-SMA and collage
I/III mRNA in MLE-12 cells.
| Treatment
group | β-catenin | α-SMA | Collagen I | Collagen III |
|---|
| Control |
20.34±0.379a |
22.80±0.178a |
21.11±0.449a |
20.56±0.371a |
| GFP | 20.27±0.405 | 22.68±0.280 | 21.10±0.794 | 20.57±0.694 |
| TRB3 |
23.36±0.319b |
24.80±0.983b |
23.71±0.193b |
23.08±0.489b |
| shR-TRB3 |
18.53±0.285b |
21.16±0.118b |
19.61±0.193b |
18.50±0.145b |
| TGF-β1 |
24.41±0.128c |
25.81±0.783c |
24.67±0.101c |
24.10±0.554c |
| T+GFP | 24.40±0.143 | 25.84±0.716 | 24.63±0.525 | 24.10±0.299 |
| T+TRB3 |
27.43±0.155d |
29.34±0.124d |
26.83±0.724d |
26.12±0.199d |
| T+shR-TRB3 |
22.57±0.162d |
23.17±0.423d |
22.25±0.657d |
22.85±0.637d |
TRB3 is able to affect EMT induced by
TGF-β1 and the Wnt/β-catenin signaling pathway
The mRNA expression of β-catenin, α-SMA and collagen
I/III was significantly increased in the TRB3 overexpression
combined with the TGF-β1 stimulated (T+TRB3) group (P<0.05). The
results were the opposite in the TRB3 downregulated combined with
TGF-β1 stimulated (T+shR-TRB3) group (Table III).
Protein expression levels of TRB3,
β-catenin, E-cadherin, vimentin, fibronectin and α-SMA
TRB3 expression during EMT induced by
TGF-β1
TRB3 expression was significantly increased in the
TGF-β1-stimulated group compared to the control group (P<0.05,
Fig. 2A).
Impact of TRB3 on EMT and β-catenin
expression
The expression levels of both β-catenin and EMT
mesenchymal protein markers were significantly increased in the
TRB3 overexpression (TRB3) group, while expression of the
epithelial marker, E-cadherin, was decreased dramatically
(P<0.05). The results were the opposite in the TRB3
downregulated (shR-TRB3) group (Table
IV).
 | Table IV.Effect of TRB3 on β-catenin and EMT
mesenchymal marker protein expression in TGF-β1-stimulated MLE-12
cells. |
Table IV.
Effect of TRB3 on β-catenin and EMT
mesenchymal marker protein expression in TGF-β1-stimulated MLE-12
cells.
| Group | β-catenin | E-cadherin | Vimentin | Fibronectin | α-SMA |
|---|
| Control |
350.46±1.324a |
127.23±1.134a |
108.22±0.542a |
113.54±0.832a |
208.35±0.956a |
| GFP | 351.05±0.751 | 127.64±1.272 | 108.33±0.767 | 115.09±0.772 | 208.20±1.063 |
| TRB3 |
463.86±3.663b |
51.22±0.605b |
164.66±0.557b |
186.71±0.930b |
359.57±0.645b |
| shR-TRB3 |
166.79±0.960b |
284.61±0.532b |
80.59±0.536b |
40.36±0.940b |
112.61±0.419b |
| TGF-β1 |
426.82±2.450c |
72.56±0.550c |
145.48±0.586c |
179.46±2.030c |
303.08±0.697c |
| T+GFP | 397.12±5.629 | 72.22±0.844 | 145.63±0.416 | 182.46±1.017 | 303.32±0.382 |
| T+TRB3 |
567.28±1.609d |
35.52±1.147d |
216.39±0.435d |
321.55±1.374d |
427.65±0.432d |
| T+shR-TRB3 |
186.02±1.276d |
123.51±0.532d |
90.33±0.444d |
58.50±0.886d |
154.01±0.719d |
TRB3 affects EMT induced by TGF-β1 and
the Wnt/β-catenin signaling pathway
The expression levels of both β-catenin and EMT
mesenchymal protein markers were significantly increased in the
TGF-β1 stimulation group compared to the control group, while
E-cadherin expression was decreased dramatically (P<0.05).
Compared to the control group, the expression levels of both
β-catenin and EMT mesenchymal protein markers were found to be
significantly increased in the TRB3 overexpression combined with
TGF-β1 stimulated group (T+TRB3); while E-cadherin expression was
decreased dramatically (P<0.05). The results were the opposite
in the TRB3 downregulated combined with TGF-β1 stimulated
(T+shR-TRB3) group (Table
IV).
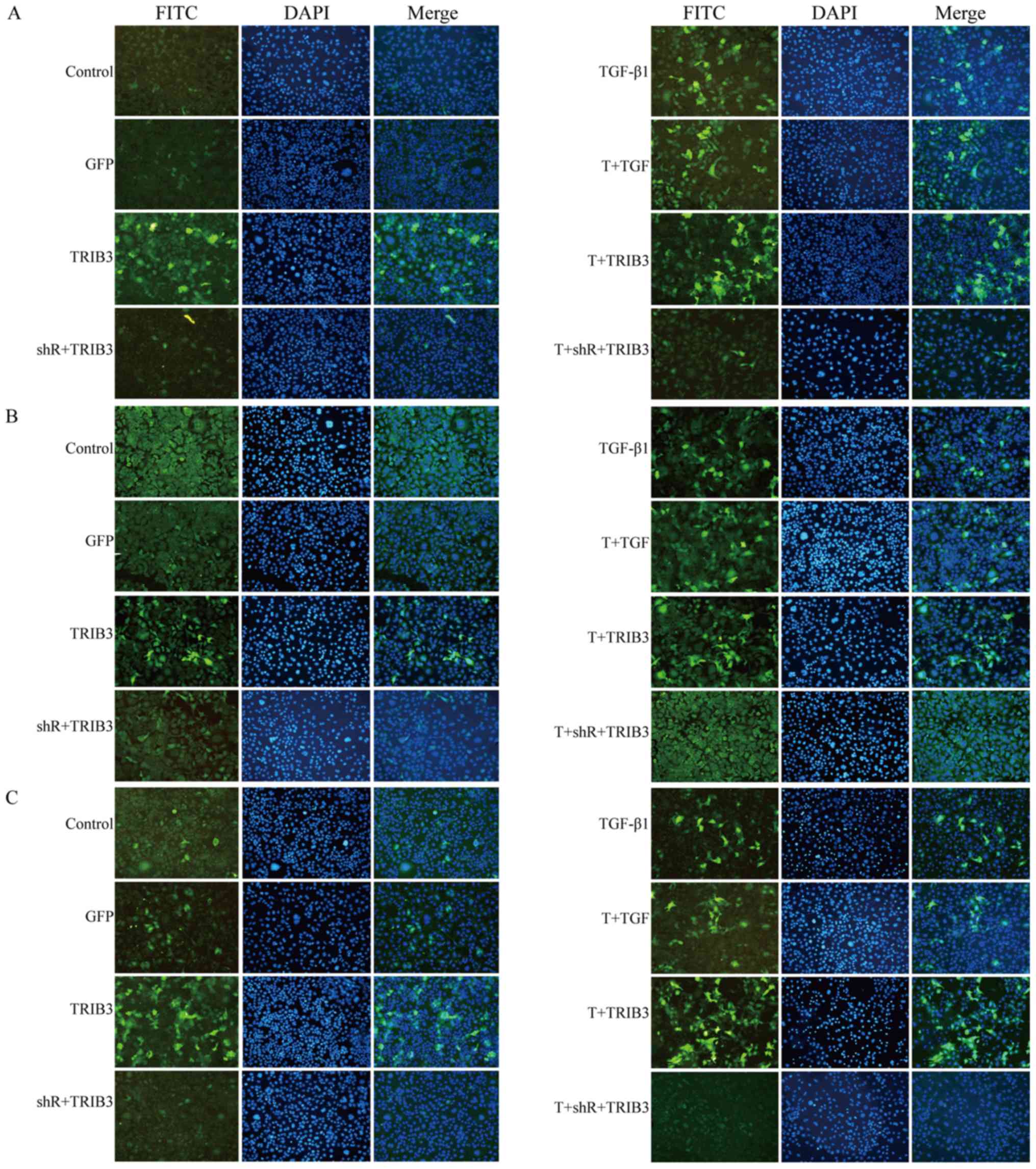
Visualizing expression of TRB3,
β-catenin, E-cadherin, fibronectin and α-SMA
Since DAPI revealed the MLE-12 cells, and FITC
revealed the different antibodies, Fig. 3A displays the expression of TRB3 in
the different groups. The figure shows that the overexpression of
TRB3, whether in the control group (left panels) or the TGF-β1
administration group (right panels), coincides with a significant
increase in the expression of β-catenin (Fig. 3B), fibronectin (Fig. 3C), α-SMA (Fig. 3D) and E-cadherin (Fig. 3E) in both the adenovirus vector
transduction group (TRB3) and the adenovirus transduction combined
with TGF-β1 stimulated group (T+TRB3) P<0.05. The TRB3
downregulated group had opposing results.
Discussion
The pathogenesis of idiopathic pulmonary fibrosis
(IPF) remains ambiguous. Its pathological features are a loss of
alveolar epithelial cell membrane integrity and abnormal cell wound
healing, resulting in a large amount of extracellular matrix (ECM)
deposition. This disrupts normal physiological tissue structure and
eventually leads to irreversible pulmonary structural remodeling
(1). The transdifferentiation of
fibroblasts to myofibroblast afters activation, which produces an
excessive amount of ECM, is one of the key steps in the progression
of fibrotic disease. Transforming growth factor β1 (TGF-β1) is the
key factor regulating fibroblast activation during the occurrence
of fibrotic disease, and it is regulated by intricate intracellular
signaling networks (13,14). Research has demonstrated that cells
that have undergone EMT exhibit increased proliferation, while
NSCLC cell lines with high EMT gene signature scores (mesenchymal
cell lines) were more sensitive to PLK1 (polo-like kinase 1)
inhibition than epithelial lines (15).
The Wnt/β-catenin signaling pathway plays an
important role in the pathogenesis of various human diseases.
Studies have demonstrated the underlying relationship between
TGF-β1 and the Wnt/β-catenin signaling pathway. β-catenin and TGF-β
pathway signals were found to co-regulate EMT formation, and exert
a regulatory effect through transcriptional activation of cAMP
response element binding protein (CREB) (16). Akhmetshina et al (17) indicated that blocking the
Wnt/β-catenin signaling pathway could serve as a new therapeutic
method against fibrosis mediated by TGF-β. TRB3 has been proven to
inhibit mitosis of renal tubular epithelial cells, induce cell
apoptosis, and suppress cell proliferation activity. TRB3
overexpression could stimulate the classic TGF-β1 signaling pathway
and induce phenotypic transition of static fibroblasts; however,
TGF-β also induces TRB3 expression. While TRB3 gene knockout led to
significantly reduced TGF-β1-induced fibrosis and collagen
synthesis, previous studies demonstrated that in the fibroblasts of
systemic scleroderma patients, TRB3 expression was increased in a
TGF-β/Smad-dependent manner (18,19).
Furthermore, Zhang et al (20) revealed that high TRB3 expression
was observed in diabetic nephropathy mouse renal tissue, which
showed a positive correlation between TGF-β1 expression and kidney
interstitial fibrosis level. A recent study demonstrated the role
of TRB3 in regulating fibroblast activation and the onset and
development of tissue or organ fibrosis, by stimulating the classic
TGF-β signaling pathway (21).
Based on the evidence above, we propose the following hypothesis.
During fibrosis, TGF-β1 is involved in a positive feedback loop,
where it can induce upregulation of TRB3 expression and activate
the Wnt/β-catenin signaling pathway. However, TRB3 can in turn
affect TGF-β1 and activate the classic TGF-β/Smad signaling
pathway, leading to stimulation of collagen synthesis, and finally
the abnormal activation of the TGF-β signaling pathway and the
onset of fibrosis.
Our study found low TRB3 gene and protein expression
in normal MLE-12 cells, whereas during TGF-β1-induced EMT, TRB3
gene and protein expression was significantly upregulated. TGF-β1
enhances all EMT hallmarks. TGF-β1 administration along with the
TRB3 vector promoted EMT to a greater extent; however, TGF-β1 with
shTRB3 altered all values to the levels of the control group. This
suggests that inhibition of TRB3 may interdict the entire pathway
of TGF-β1. In addition, when the results of the Ad-GFP group were
compared with those of the control group, no significant expression
changes in EMT-related proteins and genes were observed in the
Ad-GFP group. This suggests that the adenovirus vector and GFP gene
did not affect EMT, while significant upregulation or
downregulation of EMT-related genes and proteins was found in the
TRB3 group and the shR-TRB3 group, respectively. This indicates
that EMT is impacted by overexpression or downregulation of TRB3.
One study reported that TGF-β1 is a key cytokine in the promotion
of EMT through the TGF-β1/Smad signaling pathway, by interacting
with Smad signaling protein, and subsequently further promoting
related gene and protein expression (17). In our research, when the results of
the TGF-β1 group were compared with those of the control group, the
expression levels of EMT-related genes and proteins were
significantly increased, and fibrosis-related cytokines in the
supernatant also increased, confirming the promotive effect of
TGF-β1 on EMT. This is consistent with the results of the
aforementioned study (17). No
significant alteration in the expression of EMT-related genes and
proteins or fibrosis-related proteins and cytokines was discovered
when the results of the T+Ad-GFP group were compared with those of
the TGF-β1 group. These results indicate that the adenovirus vector
and GFP gene expression did not affect EMT. However, significant
upregulation and downregulation of EMT-related genes and proteins
and fibrosis-related proteins and cytokines were found in the
T+TRB3 group and the T+shR-TRB3 group, respectively, when compared
with the TGF-β1 stimulation only group. These results indicate that
EMT induced by TGF-β1 could be promoted by TRB3 expression; while
downregulated TRB3 impaired TGF-β1-induced EMT, with a probable
mechanism of TRB3 influencing EMT through the TGF-β/Smad signaling
pathway.
Various signaling pathways have been demonstrated to
be involved in pulmonary fibrosis, including the Smad-dependent
signaling pathway and the Wnt/β-catenin signaling pathway (22). These pathways are interconnected
with one other and form an intricate network, modulating the onset
of fibrosis, and the Wnt/β-catenin pathway is an important part of
this network. Our research demonstrated that expression of
β-catenin, a key protein in the Wnt/β-catenin signaling pathway,
increased significantly during EMT induced by TGF-β1. Furthermore,
TRB3 overexpression or downregulation affected expression of
Wnt/β-catenin signaling pathway-related proteins. Therefore, we
conclude that TRB3 may influence TGF-β1-induced EMT through the
Wnt/β-catenin signaling pathway, and fibrosis can be modulated
through the Wnt/β-catenin signaling pathway by altering TRB3
expression. Only one cell line was used in this study, and this was
a limitation. siRNA or shRNA targeting β-catenin or chemicals that
exhibit action on GSK3β should be used in future research. We will
also verify our experimental results using animal models as well.
Further study is required regarding the components within the
regulatory network of fibrosis development, as well as the
differences between in vitro and in vivo studies.
Acknowledgements
Not applicable.
Funding
The present study was funded by The Natural Science
Fund of Shandong Province (grant no. ZR2014HM079).
Availability of data and materials
The datasets used during the present study are
available from the corresponding author upon reasonable
request.
Authors' contributions
WY, FW and LM made substantial contributions to the
conception and design of the present study. WY and FW collected,
analyzed and interpreted the data. FW and LM drafted the
manuscript. WY critically revised the manuscript. WY has given
final approval of the version to be published and agreed to be
accountable for all aspects of the work in ensuring that questions
related to the accuracy or integrity of any part of the work are
appropriately investigated and resolved. All authors read and
approved the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Sgalla G, Biffi A and Richeldi L:
Idiopathic pulmonary fibrosis: Diagnosis, epidemiology and natural
history. Respirology. 21:427–437. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Su Y, Yu L, Liu N, Guo Z, Wang G, Zheng J,
Wei M, Wang H, Yang AG, Qin W and Wen W: PSMA specific single chain
antibody-mediated targeted knockdown of Notch1 inhibits human
prostate cancer cell proliferation and tumor growth. Cancer Lett.
338:282–291. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Carew RM, Wang B and Kantbaridis P: The
role of EMT in renal fibrosis. Cell Tissue Res. 347:103–116. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Qi X, Zhang L and Lu X: New insights into
the epithelial-to-mesenchymal transition in cancer. Trends
Pharmacol Sci. 37:246–248. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kretzschmar K and Watt FM: Lineage
tracing. Cell. 148:33–45. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kim KK, Kugler MC, Wolters PJ, Robillard
L, Galvez MG, Brumwell AN, Sheppard D and Chapman HA: Alveolar
epithelial cell mesenchymal transition develops in vivo during
pulmonary fibrosis and is regulated by the extracellular matrix.
Proc Natl Acad Sci USA. 103:13180–13185. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Huang C, Ma R, Xu Y, Li N, Li Z, Yue J, Li
H, Guo Y and Qi D: Wnt2 promotes non-small cell lung cancer
progression by activating WNT/β-catenin pathway. Am J Cancer Res.
5:1032–1046. 2015.PubMed/NCBI
|
|
8
|
Kim TH, Kim SH, Seo JY, Chung H, Kwak HJ,
Lee SK, Yoon HJ, Shin DH, Park SS and Sohn JW: Blockade of the
Wnt/β-catenin pathway attenuates bleomycin-induced pulmonary
fibrosis. Tohoku J Exp Med. 223:45–54. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Meuten T, Hickey A, Franklin K, Grossi B,
Tobias J, Newman DR, Jennings SH, Correa M and Sannes PL: WNT7B in
fibroblastic fociof idiopathic pulmonary fibrosis. Respir Res.
13:622012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang W, Cheng J, Sun A, Lv S, Liu H, Liu
X, Guan G and Liu G: TRB3 mediates renal tubular cell apoptosis
associated with proteinuria. Clin Exp Med. 15:167–177. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ti Y, Xie GL, Wang ZH, Bi XL, Ding WY,
Wang J, Jiang GH, Bu PL, Zhang Y, Zhong M and Zhang W: TRB3 gene
silencing alleviates diabetic cardiomyopathy in a type 2 diabetic
rat model. Diabetes. 60:2963–2974. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lampiasi N, Azzolina A, Umezawa K,
Montalto G, McCubrey JA and Cervello M: The novel NF-κB inhibitor
DHMEQ synergizes with celecoxib to exert antitumor effects on human
liver cancer cells by a ROS-dependent mechanism. Cancer Lett.
322:35–44. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Pardo A and Selman M: Role of matrix
metaloproteases in idiopathic pulmonary fibrosis. Fibrogenesis
Tissue Repair. 5 (Suppl 1):S92012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sakai N and Tager AM: Fibrosis of Two:
Epithelial cell-fibmblast interactions inpulmonary fibrosis.
Biochim Biophys Acta. 1832:911–921. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ferrarotto R, Goonatilake R, Yoo SY, Tong
P, Giri U, Peng S, Minna J, Girard L, Wang Y, Wang L, et al:
Epithelial-mesenchymal transition predicts polo-like kinase 1
inhibitor-mediated apoptosis in non-small cell lung cancer. Clin
Cancer Res. 22:1674–1686. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhou B, Liu Y, Kahn M, Ann DK, Han A, Wang
H, Nguyen C, Flodby P, Zhong Q, Krishnaveni MS, et al: Interactions
between β-catenin and transforming growth factor-β signaling
pathways mediate epithelial-mesenchymal transition and are
dependent on the transcriptional co-activator cAMP-response
element-binding protein (CREB)-binding protein (CBP). J Biol Chem.
287:7026–7038. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Akhmetshina A, Palumbo K, Dees C, Bergmann
C, Venalis P, Zerr P, Horn A, Kireva T, Beyer C, Zwerina J, et al:
Activation of canonical Wnt signalling is required for
TGF-β-mediated fibrosis. Nat Commun. 3:7352012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ohoka N, Yoshii S, Hattori T, Onozaki K
and Hayashi H: TRB3, a novel ER stress-inducible gene, is induced
via ATF4-CHOP pathway and is involved in cell death. EMBO J.
24:1243–1255. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Tomcik M, Palumbo-Zerr K, Zerr P, Sumova
B, Avouac J, Dees C, Distler A, Becvar R, Distler O, Schett G, et
al: Tribbles homologue 3 stimulates canonical TGF-β signalling to
regulate fibroblast activation and tissue fibrosis. Ann Rheum Dis.
75:609–616. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang L, Zhang J, Liu X, Liu S and Tian J:
Tribbles 3 regulates the fibrosis cytokine TGF-β1 through
ERK1/2-MAPK signaling pathway in diabetic nephropathy. J Immunol
Res. 2014:2403962014. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Xu L, Cui WH, Zhou WC, Li DL, Li LC, Zhao
P, Mo XT, Zhang Z and Gao J: Activation of Wnt/β-catenin signalling
is required for TGF-β/Smad2/3 signalling during myofibroblast
proliferation. J Cell Mol Med. 21:1545–1554. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim MK, Maeng YI, Sung WJ, Oh HK, Park JB,
Yoon GS, Cho CH and Park KK: The differential expression of TGF-β1,
ILK and wnt signaling inducing epithelial to mesenchymal transition
in human renal fibrogenesis: An immunohistochemical study. Int J
Clin Exp Pathol. 6:1747–1758. 2013.PubMed/NCBI
|