Introduction
Alzheimer's disease (AD) is one of the most
debilitating neurodegenerative diseases (1); it is characterized by the excessive
accumulation of β-amyloid (Aβ) and severe neuronal loss in the
brains of patients with AD (1).
The microtubule-associated protein tau is an axonal phosphoprotein.
It has been shown that total tau, phosphorylated (p)-tau and
Aβ42 are key biomarkers for AD pathophysiology, and the
deposition of peptides into plaques in patients with AD is closely
associated with neuronal degeneration, p-tau and Aβ aggregation
(2). Additionally, β-secretase is
a key protease that controls the formation of the Aβ peptide, which
is hypothesized to be a key mediator of the amyloid-driven
pathology of AD (3).
In vitro and in vivo studies have
suggested that protocatechuic acid (PA), a phenolic compound from
Radix Salviae miltiorrhizae, may be an effective
neuroprotective agent for AD therapy. PA has a protective effect in
cultured rat cortical neurons against Aβ25–35-induced
cytotoxicity, and can inhibit the mRNA expression of amyloid
precursor protein (APP) in double-transfected [human APP
gene and presenilin-1 (PS1) gene] Chinese hamster ovary
cells (M146L) (4,5). Moreover, PA has also been shown to
improve cognitive deficits and increase the level of brain-derived
neurotrophic factor, as well as attenuating amyloid deposits and
the inflammatory response in aged APP/PS1 double transgenic mice
(6). Previous studies have shown
that PA is an efficient and safe substance in the prevention of AD
progression (4–6).
Autophagy is a cellular process that degrades
proteins and recycles cellular components (7). There are three main types of
autophagy in mammals: Macroautophagy; microautophagy; and
chaperone-mediated autophagy (CMA) (7). Beclin-1, an autophagy-related
protein, is involved in the initiation of autophagy (8). Previous report has shown that
autophagy may be related to the pathogenesis of AD (9). Autophagy contributes to the clearance
of Aβ aggregates and helps to preserve neuronal function in AD
(9). The PI3K-Akt-mTOR signaling
pathway is generally considered to be important for autophagy. Akt,
which is located downstream of class I PI3K, is a serine threonine
kinase that suppresses autophagy (10). A previous study has indicated that
CMA plays a role in the direct degradation of the neuronal
transcription factor myocyte enhancer factor 2D (MEF2D), a protein
commonly known to promote neuronal survival (11). Recent studies have suggested that
the administration of methylene blue also promotes the
phosphorylation of Akt and glycogen synthase kinase (GSK)-3β, which
leads to an increased concentration of MEF2D in the nucleus
(12–14). In the present study, an okadaic
acid (OA)-induced PC12 cell injury model was used to further
understand the molecular mechanisms underlying the neuroprotective
effects of PA on the biomarkers of AD, and to study its effects on
the signaling pathways in autophagy. The effects of PA on cell
viability, p-tau, Aβ42 and β-secretase levels, as well
as its effect on the expression levels of p-Akt, p-GSK-3β, MEF2D
and Beclin-1 channel proteins in OA-treated PC12 cells were
examined.
Materials and methods
Cell culture
The PC12 cell line was obtained from The Cell Bank
of Type Culture Collection of the Chinese Academy of Sciences.
Cells were cultured in high glucose DMEM containing 10% FBS (both
purchased from Gibco; Thermo Fisher Scientific, Inc.). The cells
were seeded in 25-cm2 polystyrene flasks (Costar;
Corning Inc.) at 37°C in a humidified atmosphere of 5%
CO2.
OA and PA preparation
OA (cat. no. P112508; Adamas Reagent, Ltd.) was
dissolved in PBS containing 0.001% DMSO (15) at a concentration of 10 µg/ml, and
stored in sterile Eppendorf Tubes® at −20°C for 7 days
to obtain the aggregated form. The aggregated OA was then diluted
to the desired concentration (0–250 nM) in high-glucose DMEM
containing 10% FBS.
PA (cat. no. A0617-1, LOT: Cat. no. 110809-201605;
National Institutes for Food and Drug Control) was diluted to 1
µg/ml in PBS containing 0.001% DMSO (15), and stored in sterile Eppendorf
Tubes® that were incubated at −20°C. Upon use, it was
diluted to different concentrations (25, 50 or 100 µg/ml) in
high-glucose DMEM medium containing 10% FBS. In addition, PC12
cells were incubated with the autophagy inhibitor 3-methyladenine
(3-MA; 100 mM; cat no. M9281; Sigma-Aldrich; Merck KGaA) and the
autophagy activator rapamycin (0.2 mg/ml; cat no. R0395;
Sigma-Aldrich; Merck KGaA) at 37°C for 24 h, which were used as
controls to ensure that the neuroprotective effects of PA on
autophagy were regulated by the Akt/GSK-3β/MEF2D pathway.
Determination of cell viability
PC12 cells were cultured at a density of
1×104 cells/well in 96-well culture plates at 37°C in an
incubator with a humidified atmosphere of 5% CO2. After
48 h incubation, the PC12 cells were pre-incubated with 50 µl PA at
different concentrations (25, 50 or 100 µg/ml) or without PA
(16) for 0.5 h at 37°C, followed
by incubation with OA (175 nM) for 24 h at 37°C. Cell Counting
Kit-8 (CCK-8; cat. no. CK04; Dojindo Molecular Technologies, Inc.)
solution (10 µl/well; concentration of 0.5 g/l) was added and the
cells were incubated at 37°C for 2 h. The number of viable cells in
each well was determined at 450 nm on a microplate reader
(Multiskan MK3; Thermo Fisher Scientific, Inc.). The cell viability
rate of the PC12 cells was calculated as follows: Cell viability
rate (%)=(ODtest group-ODblank of test
group)/(ODcontrol group-ODblank of control
group) ×100%.
Enzymatic assay analyzing p-tau,
Aβ42 and β-secretase activity
PC12 cells were seeded into a 6-well culture plate
at a density of 1×105 cells/well. The seeded cells were
then subjected to various treatments as described above for the
CCK-8 cell viability assay. Briefly, the media from the treated
PC12 cells was collected and centrifuged at 10,000 × g at 4°C for
10 min. Then, the supernatant was collected and measured for p-tau
(cat. no. P261FC), Aβ42 (cat. no. A227FC) and
β-secretase (cat. no. B096FC) using ELISA according to the
manufacturer's protocol (Elixir Medical Corporation).
Western blot analysis
Western blotting was performed to detect p-Akt,
p-GSK-3β, Akt, GSK-3β, MEF2D and Beclin-1. These proteins of
similar molecular weight were detected by the membrane regeneration
method; the corresponding internal reference was used for
quantitative analysis (17). PC12
cells were harvested and lysed using a phenylmethanesulfonyl
fluoride lysis buffer (Sigma-Aldrich; Merck KGaA). The lysates were
incubated for 30 min at 4°C, centrifuged at 13,000 × g for 15 min
at 4°C, and the total proteins were extracted and quantified using
a bicinchoninic acid kit (Wuhan Boster Biological Technology,
Ltd.). Electrophoresis was performed with 40 µg of the total
proteins using 10% SDS-PAGE gels and transferred to PVDF membranes.
The membranes were blocked in 5% BSA (cat. no. 810652; EMD
Millipore) for 1 h at 4°C and incubated with primary antibodies
against GAPDH (cat. no. ab8245; Abcam), p-Akt (cat. no. 9275S; Cell
Signaling Technology, Inc.), p-GSK-3β (cat. no. ab75745; Abcam),
Akt (cat. no. 9272S; Cell Signaling Technology, Inc.), GSK-3β (cat.
no. ab131356; Abcam), MEF2D (cat. no. ab32845; Abcam) and Beclin-1
(cat. no. ab62557; Abcam) proteins overnight at 4°C (all 1:1,000
dilution). The blots were subsequently washed and incubated with
their respective horseradish peroxidase (HRP)-conjugated
immunoglobulin G (IgG; anti-mouse and anti-rabbit) secondary
antibodies (1:2,000; cat. nos.7076S and 7074S; Cell Signaling
Technology, Inc.) at 37°C for 1 h. GAPDH was used as an internal
control. The bound secondary antibodies were visualized using an
enhanced chemiluminescence kit (Bio-Rad Laboratories, Inc.) with
the ChemiDoc XRS™ with Quantity One® 1-D analysis
software (Bio-Rad Laboratories, Inc.). Blots were repeated at least
three times for each condition. After development, the band
intensities were quantified with Image-Pro Plus 6.0 analysis
software (Media Cybernetics, Inc.).
Immunofluorescence
The cells were treated with cell culture medium
containing OA (175 nM) and PA (25, 50 or 100 µg/ml) for 24 h.
Following a 60-min fixation in 4% paraformaldehyde at 4°C, the
cells were washed with PBS and treated with 300 µl of BSA (1:100)
for 20 min at 37°C. The cells were then incubated with Rabbit
anti-Beclin-1 primary antibodies (1:50; Abcam) for 1 h at 37°C.
After washing with PBS, the cells were then incubated with Alexa
488-conjugated anti-rabbit IgG (1:200; cat no. 4412S; Cell
Signaling Technology, Inc.) for 30 min at 37°C. Thereafter, the
cells were washed and treated with 300 µl of DAPI (Wuhan Boster
Biological Technology, Ltd.) for 5 min at 37°C to stain the cell
nuclei. Finally, the cells were imaged using a light microscope
(U-SPT; Olympus Corporation) at a magnification ×200, in five
random fields of view. Data analyses were performed using the
ImageJ software v1.48 (National Institutes of Health).
Immunocytochemistry
The methods used to fix the cells for the
immunocytochemistry experiments were the same as those performed
for the immunofluorescence experiments. Following these steps, the
cells were incubated with mouse anti-Beclin-1 primary antibodies
(1:50 dilution; Abcam) for 1 h at 37°C. After washing with PBS, the
cells were incubated with HRP-conjugated anti-Rabbit IgG for 20 min
at 37°C (1:200; cat no. 7074S; Cell Signaling Technology, Inc.).
The cells were subsequently washed with PBS and amplified via
avidin biotin-peroxidase complex labeling by adding 300 µl of
streptavidin-biotin complex (cat no. SA1021; Wuhan Boster
Biological Technology, Ltd.) for 20 min overnight. The cells were
then washed and treated with 300 µl of 3,3′-diaminobenzidine (cat
no. AR1022; Wuhan Boster Biological Technology, Ltd.) for 5 min at
37°C to stain the cell nuclei. Finally, cell imaging was performed
using a light microscope (U-SPT; Olympus Corporation) at a
magnification ×200, in five random fields of view. Data were
analyzed using the ImageJ software v1.48 (National Institutes of
Health).
Statistical analysis
Data were expressed as the mean ± SD; significant
differences between different groups were determined by one-way or
two-way ANOVA followed by post hoc testing with Bonferroni
correction for multiple comparisons, with a significance threshold
of P<0.05. The experiments were repeated ≥3 times. Correlations
between p-tau, Aβ42, β-secretase, p-Akt, p-GSK-3β, MEF2D
and Beclin-1 expression were identified by Pearson correlation
analysis. P<0.05 was considered statistically significant. All
statistical analyses were performed using the SPSS 13.0 (SPSS,
Inc.) statistical software package.
Results
CCK-8 assay
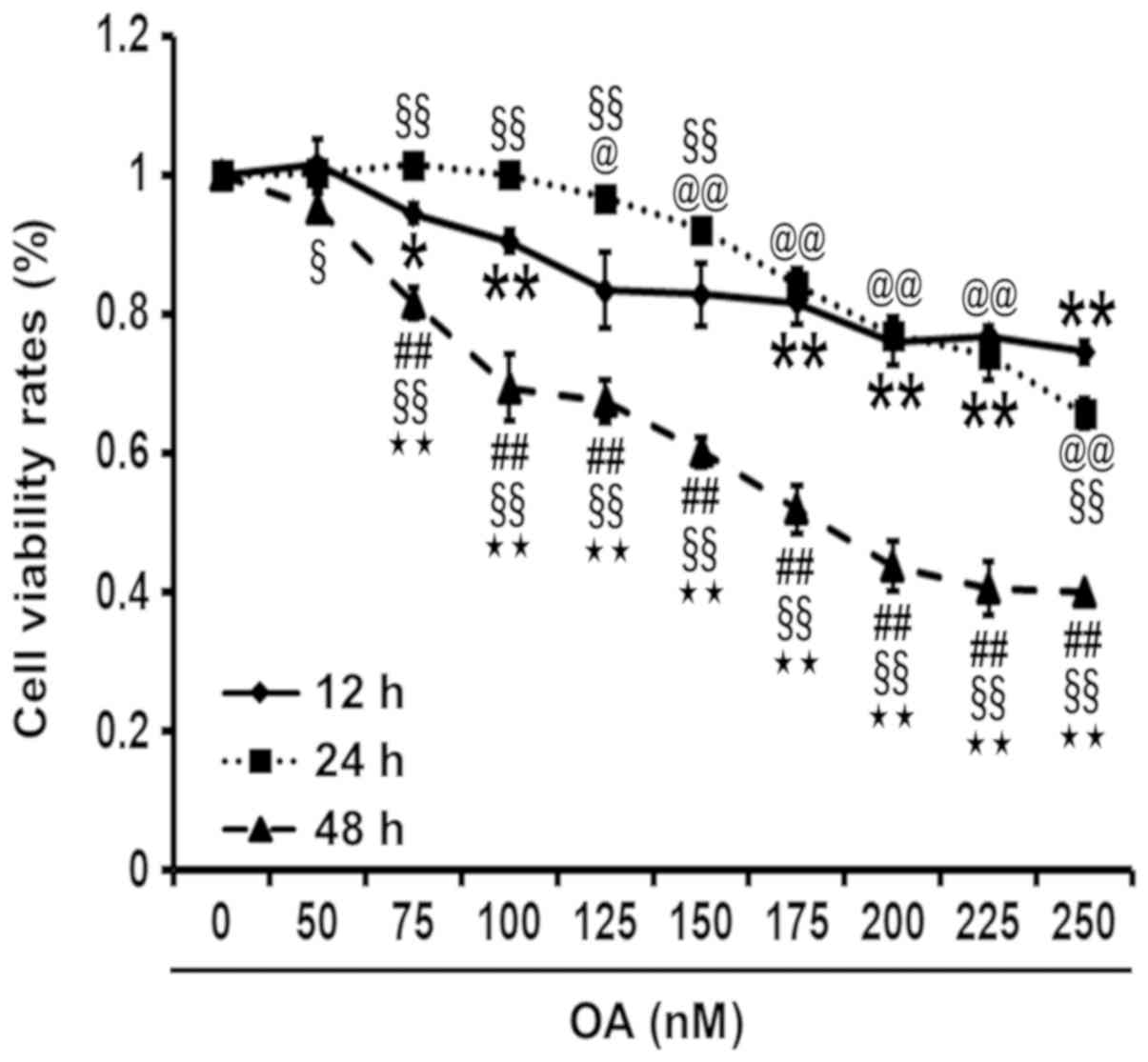
The CCK-8 assay was used to profile cell viability
and determine the optimal concentration and length of time for OA
treatment in PC12 cells. OA can lead to a reduction in cell
viability, as indicated by the negative association between the
cell viability rates, and the concentration and duration of OA
treatment. The cell viability rate of OA-induced PC12 cells was
significantly reduced at 48 h compared to 12 and 24 h. The 50%
inhibitory concentration of OA was determined to be 175 nM at 48 h
(Fig. 1).
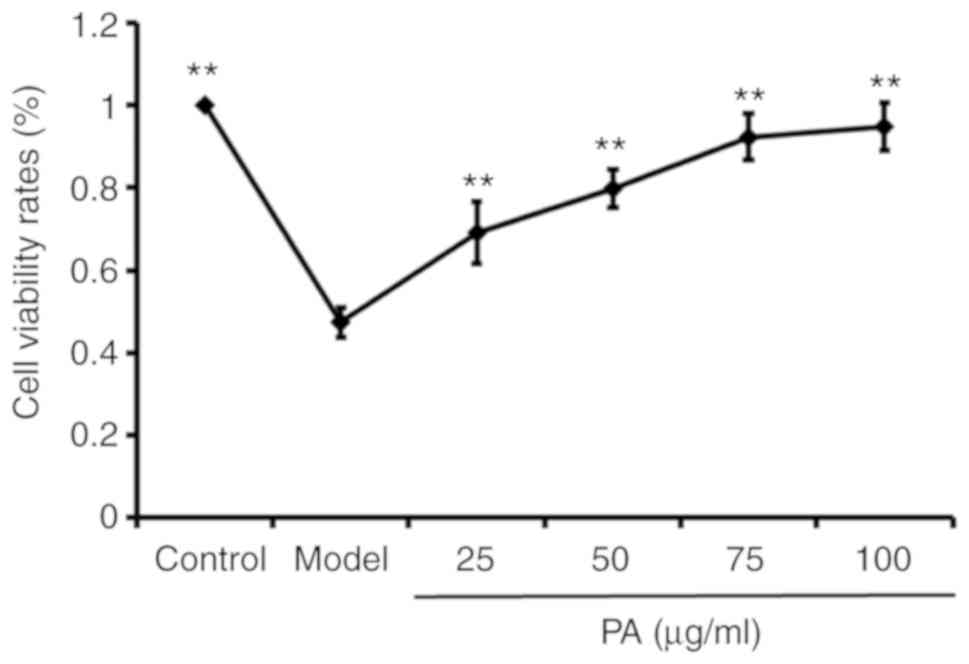
OA treatment alone decreased the cell viability rate
of PC12 cells in the model group (OA treated group without PA
pretreatment) when compared to the control group (PC12 cells
without OA and PA treatment). The PC12 cells were then pre-treated
with PA at different concentrations for 0.5 h, followed by the
addition of 175 nM OA in a combined cultured for 48 h. It was found
that PA could increase the viability rate of the OA-treated PC12
cells in a dose-dependent manner when compared to the model group
(P<0.01; Fig. 2).
p-tau, Aβ42 and β-secretase
levels by ELISA assay
Treatment with OA yielded a significant increase in
p-tau, Aβ42 and β-secretase levels in the model group
when compared to the control group (P<0.01). However, cells
exposed to pretreatment with PA followed by the addition of OA
showed a decrease in p-tau (Fig.
3A), Aβ42 (Fig. 3B)
and β-secretase (Fig. 3C) levels
at all doses for the PA-treated groups when compared to the model
group (P<0.05).
p-Akt, p-GSK-3β, MEF2D and Beclin-1
expression in PA-treated PC12 cells
As shown in Fig.
4A, there was a significant decrease in the levels of p-Akt
(Fig. 4B) and MEF2D (Fig. 4C) expression, and a significant
increase in p-GSK-3β (Fig. 4D) and
Beclin-1 (Fig. 4E) expression in
the model group (group B) compared with the control group (group A;
P<0.01). In addition, the expression of p-Akt (Fig. 4B) and MEF2D (Fig. 4C) increased, while the expression
of p-GSK-3β (Fig. 4D) and Beclin-1
(Fig. 4E) decreased in the
autophagy-inhibiting 3-MA-treated (group C) and 100 µg/ml
PA-treated (group E) PC12 groups when compared to the model group
(group B) (P<0.01 or P<0.05). It was found that 100 µg/ml PA
decreased he expression levels of p-tau, Aβ42 and
β-secretase to a greater extend compared with 25 and 50 µg/ml PA;
therefore, 100 µg/ml PA was used for the mechanism experiments. In
contrast, p-Akt (Fig. 4B) and
MEF2D (Fig. 4C) expression
decreased, and GSK-3β (Fig. 4D)
and Beclin-1 (Fig. 4E) expression
increased, in the autophagy-activating rapamycin-treated group
(group D) when compared to the model group (group B; P<0.01 or
P<0.05). Furthermore, the expression of p-Akt (Fig. 4B) and MEF2D (Fig. 4C) was significantly upregulated,
whereas the expression of p-GSK-3β (Fig. 4D) and Beclin-1 (Fig. 4E) was significantly downregulated,
in the rapamycin-treated group (group D) when compared to the 3-MA
treated group (group C; P<0.01 or P<0.05, respectively).
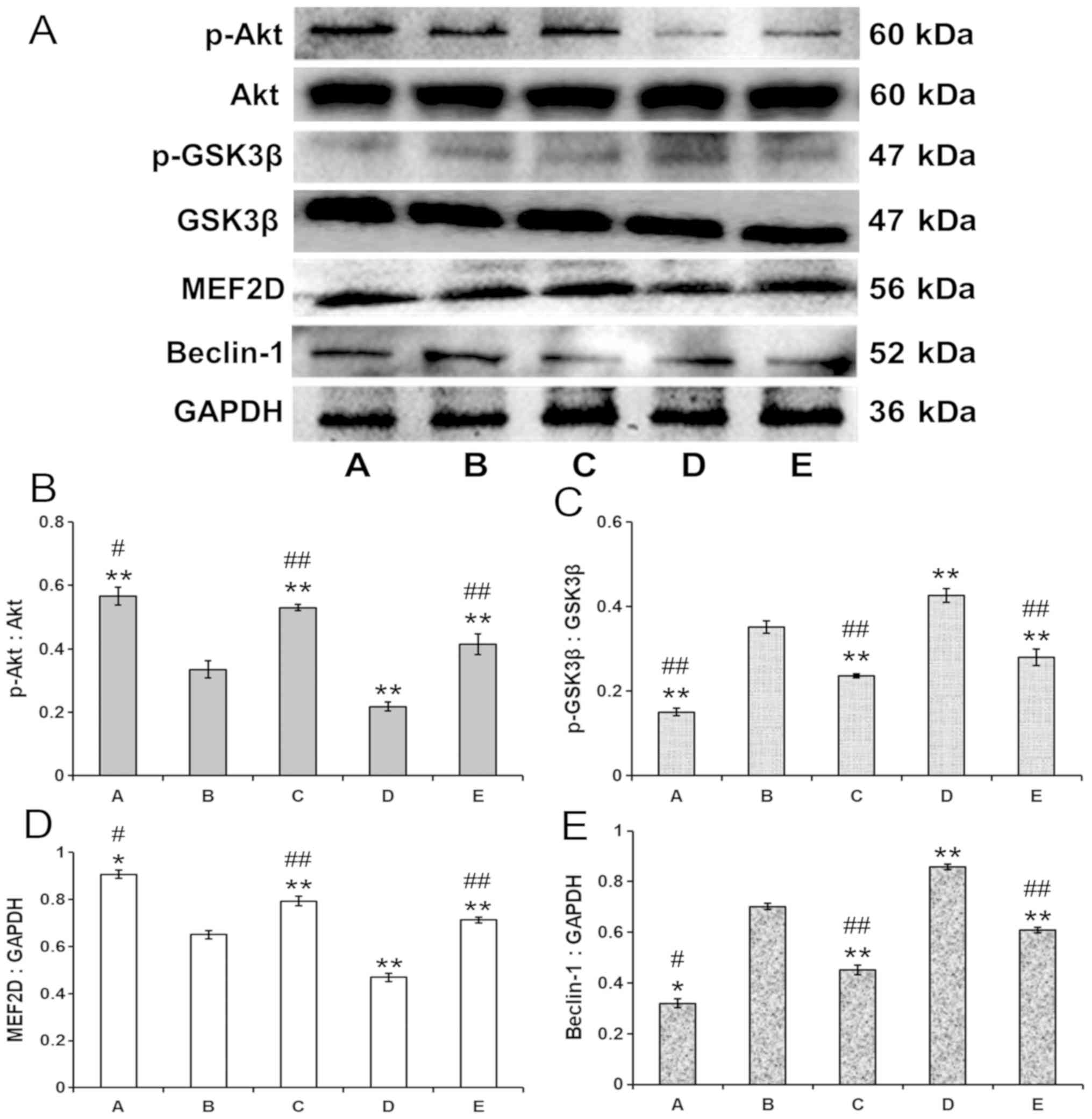 | Figure 4.Effects of PA on the expression of
p-Akt, p-GSK-3β, MEF2D and Beclin-1 proteins in the OA-induced PC12
cells. (A) Western blotting results. PA treatment in OA-induced
PC12 cells enhanced the expression of (B) p-Akt and (C) MEF2D, and
reduced the expression of (D) p-GSK-3β and (E) Beclin-1 when
compared to the model group. Values are expressed as the mean ± SD
(n=3). *P<0.05, **P<0.01 vs. model group;
#P<0.05, ##P<0.01 vs. rapamycin group.
A, control group; B, model group; C, 3-methyladenine group; D,
rapamycin group; E, PA group. Each blot was repeated at least three
times for every condition. PA, protocatechuic acid; p,
phosphorylated; OA, okadaic acid; MEF2D, myocyte enhancer factor
2D; GSK, glycogen synthase kinase. |
Beclin-1-positive staining in
PA-treated PC12 cells
To investigate the effects of PA on OA-induced
autophagy, Beclin-1 expression was detected in the PC12 cells using
immunocytochemistry and immunofluorescence staining methods
(Fig. 5A). Beclin-1-positive
expression was increased in the model group (group b) when compared
to the control group (group a; P<0.05; Fig. 5B and C). In contrast, pretreatment
with 3-MA (group c) or 100 µg/ml PA (group e) led to a clear
reduction in Beclin-1-positive expression when compared to the
model group (group B) or the rapamycin-treated group (group d;
P<0.01).
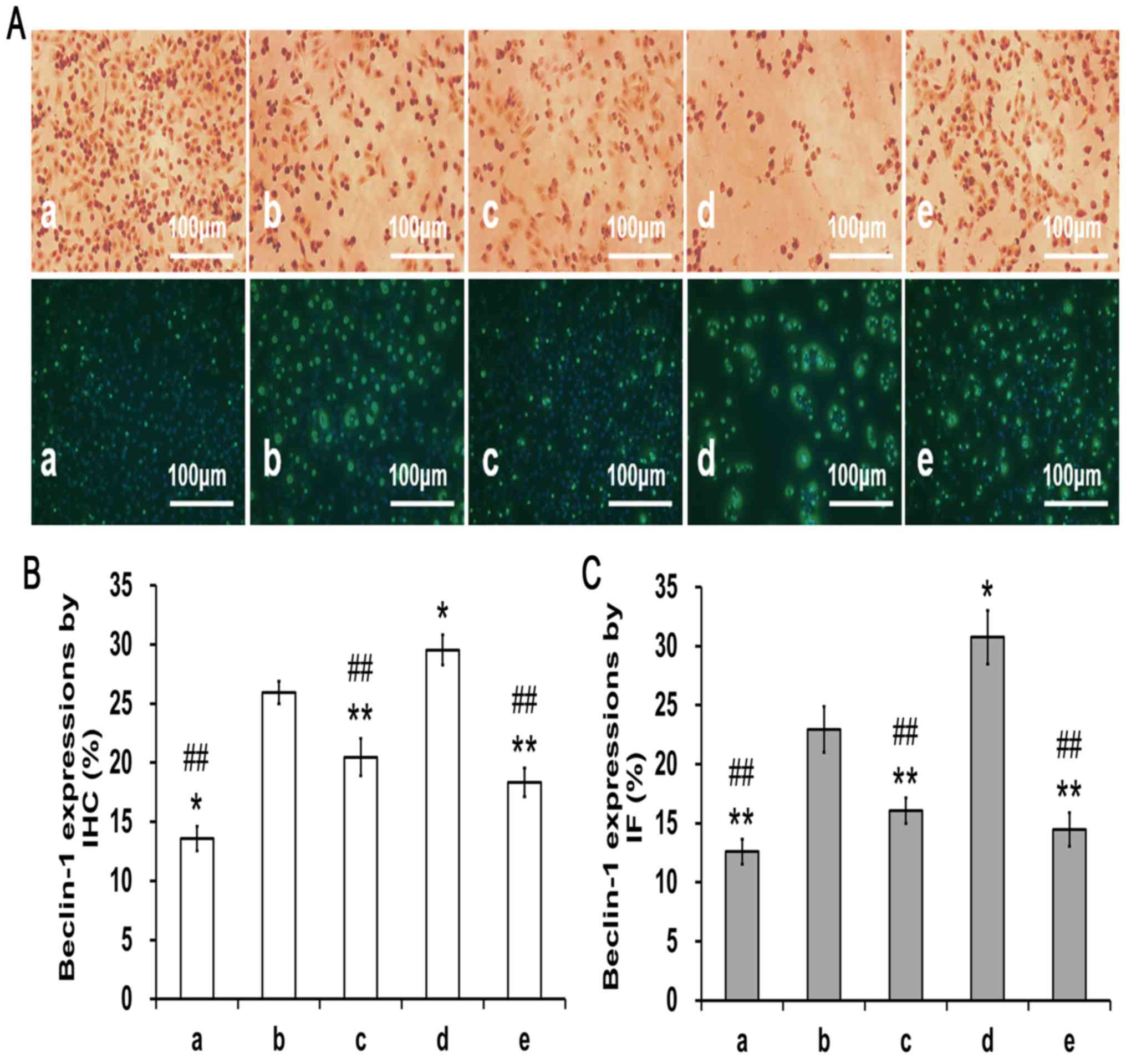 | Figure 5.Effects of PA on Beclin-1 expression
in the OA induced PC12 cells (magnification, ×200). Cells were
pre-treated with PA and then exposed to OA (175 nM) for 48 h,
during which the same treatments were applied. (A)
Immunohistochemistry and immunofluorescent results. (B)
Quantification of Beclin-1 expression from immunohistochemistry.
(C) Quantification of Beclin-1 expression from immunofluorescence
experiment. PA treatment in OA-induced PC12 cells reduced the
expression of Beclin-1 compared with the model group. Data are
presented as the mean ± SD. n=3. *P<0.05, **P<0.01 vs. model
group; ##P<0.01 vs. rapamycin group. a, control
group; b, model group; c, 3-methyladenine group; d, rapamycin
group; e, PA group; PA, protocatechuic acid; OA, okadaic acid. |
Correlation analysis
Pearson correlation analysis was performed to
investigate the relationships between the reduction of p-tau,
Aβ42 and β-secretase, and the expression of pathway
proteins (including p-Akt, p-GSK-3β, MEF2D and Beclin-1 proteins)
in the PC12 cells that were pre-treated with PA followed by the
addition of OA. The data are shown in Table I. The Pearson analysis showed that
in the PA-treated cells there were significant positive
correlations between: p-tau and p-GSK-3β, Aβ42 and
β-secretase; Aβ42 and β-secretase, p-GSK-3β and
Beclin-1; and β-secretase and Beclin-1 (P<0.01 or P<0.05).
Conversely, there were significant negative correlations between:
Aβ42 and p-Akt; β-secretase and p-Akt; p-Akt and
Beclin-1; p-GSK-3β and MEF2D; and MEF2D and Beclin-1 (P<0.01 or
P<0.05).
 | Table I.Correlation analysis. |
Table I.
Correlation analysis.
| Protein | p-tau |
Aβ42 | β-secretase | p-Akt | p-GSK3β | MEF2D | Beclin-1 |
|---|
| p-tau | 1.000 | 0.839b | 0.733b | −0.231 | 0.789b | −0.282 | 0.240 |
|
Aβ42 |
| 1.000 | 0.978b | −0.622a | 0.507a | −0.188 | 0.618a |
| β-secretase |
|
| 1.000 | −0.692a | 0.434 | −0.215 | 0.701a |
| p-Akt |
|
|
| 1.000 | −0.171 | 0.426 | −0.990b |
| p-GSK3β |
|
|
|
| 1.000 | −0.761b | 0.242 |
| MEF2D |
|
|
|
|
| 1.000 | −0.518a |
| Beclin-1 |
|
|
|
|
|
| 1.000 |
Discussion
OA is a potent polyether marine toxin obtained from
the black sponge Halichondria okadai that effectively and
selectively inhibits serine/threonine residues of protein
phosphatase 1 and protein phosphatase 2A (PP2A) (18). A previous study has shown that in
the brains of patients with AD there is decreased activity of PP2A
(an important tau dephosphorylating enzyme) and increased
phosphorylation of tau (19).
Intracerebral injections of OA can lead to tau
hyperphosphorylation, the formation of neurofibrillary tangles and
Aβ deposition, as well as memory loss and neurodegeneration
(20), which could be used to
produce a useful model of AD. In the present study, the aim was to
develop OA-induced PC12 cells as a research model. Cell viability
testing by CCK-8 showed that different concentrations of OA were
negatively associated with the cell viability rate of PC12 cells
from 12 to 48 h. However, pretreatment with 25–100 µg/ml of PA in
the OA-induced PC12 cells was sufficient to increase the cell
viability rate in a dose-dependent manner. This suggested that PA
could attenuate the OA-induced damage to PC12 cells.
A growing body of evidence indicates that PA has
beneficial effects on improving the cognitive deficits of patients
with AD. PA has neuroprotective effects against oxidative stress,
nitrosative stress and excitotoxicity, as well as
anti-neuroinflammatory and anti-apoptotic effects in cerebellar
granule neurons (21–23). PA has also been shown to alleviate
oxidative stress, and can be used as an effective agent to treat
renal ischemia and reperfusion injuries (24). Additionally, it has been shown that
PA can enhance learning and memory performance, and alleviate
apoptosis and glial proliferation following exposure to chronic
intermittent hypoxia in rats (25). PA also improves cognitive deficits
and decreases Aβ deposition, as well as decreasing the expression
levels of APP in aged APP/PS1 double transgenic mice (6). In the present study, it was found
that the expression levels of p-tau, Aβ42 and
β-secretase decreased in the OA-induced PC12 cells that were
incubated with different concentrations of PA. These results
suggested a negative association between cell viability and p-tau,
Aβ42 and β-secretase.
Moreover, the possible mechanisms via which PA
affected cell autophagy induced by OA were examined. Autophagy
regulates the pathological markers of AD in a bidirectional manner,
and disorders of autophagy may lead to abnormal protein deposition
in the nervous system (26).
Therefore, autophagy is considered to be closely related to AD; it
is not only involved in the generation and clearance of Aβ, but
also plays a key role in the metabolism of tau proteins (27). A key protein involved in the
initiation of autophagy is Beclin-1, which is a 60-kDa coiled-coil
protein that is expressed in neurons and glia (28). A recent study found reduced levels
of Beclin-1, disruptions to autophagy and accumulations of Aβ in a
transgenic AD mouse model (28).
Additionally, it was found that β-asarone has neuroprotective
effects against 6-hydroxydopamine-induced Parkinson's disease via
the JNK/Bcl-2/Beclin-1 pathway, and it can increase Bcl-2
expression and inhibit Beclin-1 expression (29). MEF2D is also strongly associated
with autophagy (30). APP
processing and Aβ generation are associated with the autophagic
pathway (31), which suggests that
the upregulation of Beclin-1-dependent autophagy may contribute to
the elimination of aggregated Aβ. In the present study, it was
shown that OA could induce autophagy and increase the expression of
Beclin-1. In contrast, PA significantly decreased the expression of
Beclin-1, which suggested that PA can attenuate OA-induced
autophagy. In comparison, pretreatment with the autophagy inhibitor
3-MA or the autophagy activator rapamycin significantly inhibited
or enhanced the expression of Beclin-1, respectively. Beclin-1 was
used to assess autophagy; however, autophagy morphological
detection may be more effective in determining the occurrence of
autophagy. Due to the limitation of experimental conditions,
autophagy morphological detection was not carried out in this
study.
Activated Akt plays important roles in cellular
growth, cell cycle progression and cell survival (32). Akt/GSK-3β is the classical Akt
pathway; GSK-3β is a downstream signaling molecule of Akt, and Akt
can be directly suppressed by GSK-3β (33). It has been shown that osthol can
decrease p-tau levels via the PI3K/Akt/GSK-3β signaling pathway in
AD, which suggests that the phosphorylation of tau is closely
related to this signaling pathway (34). Moreover, the phosphorylation of Akt
and GSK-3β can lead to an increased concentration of MEF2D in the
nucleus (12). In the present
study, proteins from the autophagic pathway were detected, which
were similarly controlled by 3-MA and rapamycin. A limitation of
this study is that the levels of MEF2D were detected in total cell
lysates instead of analyzing the MEF2D content in the nucleus. It
was found that levels of p-Akt and MEF2D were downregulated
significantly, while the levels of p-GSK-3β were upregulated
significantly in the OA treatment group when compared to the normal
control group. However, PA could significantly upregulate the
levels of p-Akt and MEF2D, and reduce the levels of p-GSK-3β, when
compared to the OA treatment group. According to previous research,
suppressing the activity of GSK-3β can increase the expression of
MEF2D (30,35). The results of the present study are
consistent with previous studies; overall, it was shown that PA
could attenuate the autophagy responses induced by OA, and its
neuroprotective effects may be exerted via the activation of the
Akt/GSK-3β/MEF2D signaling pathway.
Finally, significant positive correlations,
including p-tau/p-GSK-3β, Aβ42 and p-GSK-3β/Beclin-1 and
β-secretase/Beclin-1, and significant negative correlations,
including Aβ42/p-Akt, β-secretase/p-Akt, MEF2D and
p-GSK-3β/Beclin-1, and p-Akt/Beclin-1, were found. These findings
indicated that the downregulation of β-secretase, Aβ42
and p-tau levels, in OA-induced PC12 cells after treatment with PA,
may be caused by the effect of PA on autophagy via the regulation
of the Akt/GSK-3β/MEF2D pathway. Further experiments are required
to identify the mechanisms by which PA regulates autophagy.
In conclusion, these results indicated that PA
attenuates OA-induced autophagy via the Akt/GSK-3β/MEF2D pathway.
PA enhanced Akt phosphorylation and increased levels of MEF2D, but
suppressed GSK-3β phosphorylation, which is known to inhibit
autophagy. These findings suggested that PA could be a potential
drug candidate for the prevention of AD.
Acknowledgements
Not applicable.
Funding
This work was supported by the Lingnan Normal
University-level talent project (grant no. ZL1801), the Natural
Science Foundation of Guangdong province of China (grant no.
2018A030307037), the Hainan Natural Science Foundation of China
(grant no. 20168266), the Program of Hainan Association for Science
and Technology Plans to Youth R&D Innovation (grant no.
HAST201635), the Scientific Research Cultivating Fund of Hainan
Medical University (grant no. HY2015-01), the National Natural
Science Foundation of China (grant nos. 81904104 and 31900297) and
the Administration of Traditional Chinese Medicine of Guangdong
Province, China (grant no. 20181114).
Availability of data and materials
The datasets used and/or analyzed during the present
study are available from the corresponding author on reasonable
request.
Authors' contributions
LH and MD conceived the study, designed the
experiments, analyzed the data and prepared the manuscript. LH, XZ,
SQ and MD contributed to conception and obtained samples for the
present study. XZ, MD and LH performed the experiments. All authors
have read and approved the manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Reas ET, Hagler DJ Jr, White NS, Kuperman
JM, Bartsch H, Cross K, Loi RQ, Balachandra AR, Meloy MJ, Wierenga
CE, et al: Sensitivity of restriction spectrum imaging to memory
and neuropathology in Alzheimer's disease. Alzheimers Res Ther.
9:552017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lue LF, Guerra A and Walker DG: Amyloid
beta and tau as Alzheimer's disease blood biomarkers: Promise from
new technologies. Neurol Ther. 6 (Suppl 1):S25–S36. 2017.
View Article : Google Scholar
|
|
3
|
Mamada N, Tanokashira D, Ishii K, Tamaoka
A and Araki W: Mitochondria are devoid of amyloid β-protein
(Aβ)-producing secretases: Evidence for unlikely occurrence within
mitochondria of Aβ generation from amyloid precursor protein.
Biochem Biophys Res Commun. 486:321–328. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ban JY, Cho SO, Jeon SY, Bae K, Song KS
and Seong YH: 3,4-dihydroxybenzoic acid from Smilacis chinae
rhizome protects amyloid beta protein (25–35)-induced neurotoxicity
in cultured rat cortical neurons. Neurosci Lett. 420:184–188. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Xue XY, Zhang Z, Zhou XW, Zhou X and Luo
HM: The inhibition effect of protocatechuic acid on the mRNA
expression of APP on M146L cell. Zhong Yao Cai. 35:1813–1816.
2012.(In Chinese). PubMed/NCBI
|
|
6
|
Song Y, Cui T, Xie N, Zhang X, Qian Z and
Liu J: Protocatechuic acid improves cognitive deficits and
attenuates amyloid deposits, inflammatory response in aged AβPP/PS1
double transgenic mice. Int Immunopharmacol. 20:276–281. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Todde V, Veenhuis M and van der Klei IJ:
Autophagy: Principles and significance in health and disease.
Biochim Biophys Acta. 1792:3–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Qian X, Li X, Cai Q, Zhang C, Yu Q, Jiang
Y, Lee JH, Hawke D, Wang Y, Xia Y, et al: Phosphoglycerate kinase 1
phosphorylates beclin1 to induce autophagy. Mol Cell.
65:917–931.e6. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bustos V, Pulina MV, Bispo A, Lam A,
Flajolet M, Gorelick FS and Greengard P: Phosphorylated Presenilin
1 decreases β-amyloid by facilitating autophagosome-lysosome
fusion. Proc Natl Acad Sci USA. 114:7148–7153. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gu X, Guo W, Zhao Y, Liu G, Wu J and Chang
C: Deoxynivalenol-induced cytotoxicity and apoptosis in IPEC-J2
cells through the activation of autophagy by inhibiting
PI3K-AKT-mTOR signaling pathway. ACS Omega. 4:18478–18486. 2019.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang Q, She H, Gearing M, Colla E, Lee M,
Shacka JJ and Mao Z: Regulation of neuronal survival factor MEF2D
by chaperone-mediated autophagy. Science. 323:124–127. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xu H, Li J, Wang Z, Feng M, Shen Y, Cao S,
Li T, Peng Y, Fan L, Chen J, et al: Methylene blue attenuates
neuroinflammation after subarachnoid hemorrhage in rats through the
Akt/GSK-3β/MEF2D signaling pathway. Brain Behav Immun. 65:125–139.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Song Z, Feng C, Lu Y, Gao Y, Lin Y and
Dong C: Overexpression and biological function of MEF2D in human
pancreatic cancer. Am J Transl Res. 9:4836–4847. 2017.PubMed/NCBI
|
|
14
|
Chen ZW, Liu A, Liu Q, Chen J, Li WM, Chao
XJ, Yang Q, Liu PQ, Mao ZX and Pi RB: MEF2D mediates the
neuroprotective effect of methylene blue against glutamate-induced
oxidative damage in HT22 hippocampal cells. Mol Neurobiol.
54:2209–2222. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Brayton CF: Dimethyl sulfoxide (DMSO): A
review. Cornell Vet. 76:61–90. 1986.PubMed/NCBI
|
|
16
|
Usman MS, Hussein MZ, Kura AU, Fakurazi S,
Masarudin MJ and Ahmad Saad FF: Graphene oxide as a nanocarrier for
a theranostics delivery system of protocatechuic acid and
gadolinium/gold nanoparticles. Molecules. 23(pii): E5002018.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang L, Deng M, He Y, Lu S, Ma R and Fang
Y: β-asarone and levodopa co-administration increase striatal
dopamine level in 6-hydroxydopamine induced rats by modulating
P-glycoprotein and tight junction proteins at the blood-brain
barrier and promoting levodopa into the brain. Clin Exp Pharmacol
Physiol. 43:634–643. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Koehler D, Shah ZA and Williams FE: The
GSK3β inhibitor, TDZD-8, rescues cognition in a zebrafish model of
okadaic acid-induced Alzheimer's disease. Neurochem Int. 122:31–37.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Theendakara V, Bredesen DE and Rao RV:
Downregulation of protein phosphatase 2A by apolipoprotein E:
Implications for Alzheimer's disease. Mol Cell Neurosci. 83:83–91.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang Y, Song X, Liu D, Lou YX, Luo P, Zhu
T, Wang Q and Chen N: IMM-H004 reduced okadaic acid-induced
neurotoxicity by inhibiting Tau pathology in vitro and in vivo.
Neurotoxicology. 75:221–232. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Krzysztoforska K, Mirowska-Guzel D and
Widy-Tyszkiewicz E: Pharmacological effects of protocatechuic acid
and its therapeutic potential in neurodegenerative diseases: Review
on the basis of in vitro and in vivo studies in rodents and humans.
Nutr Neurosci. 22:72–82. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Winter AN, Brenner MC, Punessen N,
Snodgrass M, Byars C, Arora Y and Linseman DA: Comparison of the
neuroprotective and anti-inflammatory effects of the anthocyanin
metabolites, protocatechuic acid and 4-hydroxybenzoic acid. Oxid
Med Cell Longev. 2017:62970802017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Adedara IA, Okpara ES, Busari EO, Omole O,
Owumi SE and Farombi EO: Dietary protocatechuic acid abrogates male
reproductive dysfunction in streptozotocin-induced diabetic rats
via suppression of oxidative damage, inflammation and caspase-3
activity. Eur J Pharmacol. 849:30–42. 2019. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tang XL, Liu JX, Dong W, Li P, Li L, Lin
CR, Zheng YQ, Cong WH and Hou JC: Cardioprotective effect of
protocatechuic acid on myocardial ischemia/reperfusion injury. J
Pharmacol Sci. 125:176–183. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Yin X, Zhang X, Lv C, Li C, Yu Y, Wang X
and Han F: Protocatechuic acid ameliorates neurocognitive functions
impairment induced by chronic intermittent hypoxia. Sci Rep.
5:145072015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zeng Q, Siu W, Li L, Jin Y, Liang S, Cao
M, Ma M and Wu Z: Autophagy in Alzheimer's disease and promising
modulatory effects of herbal medicine. Exp Gerontol. 119:100–110.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Huang L, Lin M, Zhong X, Yang H and Deng
M: Galangin decreases p-tau, Aβ42 and β-secretase levels, and
suppresses autophagy in okadaic acid-induced PC12 cells via an
Akt/GSK3β/mTOR signaling-dependent mechanism. Mol Med Rep.
19:1767–1774. 2019.PubMed/NCBI
|
|
28
|
Deng M, Huang L, Ning B, Wang N, Zhang Q,
Zhu C and Fang Y: β-asarone improves learning and memory and
reduces Acetyl Cholinesterase and Beta-amyloid 42 levels in APP/PS1
transgenic mice by regulating Beclin-1-dependent autophagy. Brain
Res. 1652:188–194. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zhang S, Gui XH, Huang LP, Deng MZ, Fang
RM, Ke XH, He YP, Li L and Fang YQ: Neuroprotective effects of
β-asarone against 6-hydroxy dopamine-induced parkinsonism via
JNK/Bcl-2/Beclin-1 pathway. Mol Neurobiol. 53:83–94. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Huang L, Deng M, He Y, Lu S, Liu S and
Fang Y: β-asarone increases MEF2D and TH levels and reduces
α-synuclein level in 6-OHDA-induced rats via regulating the
HSP70/MAPK/MEF2D/Beclin-1 pathway: Chaperone-mediated autophagy
activation, macroautophagy inhibition and HSP70 up-expression.
Behav Brain Res. 313:370–379. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Reddy PH, Yin X, Manczak M, Kumar S,
Pradeepkiran JA, Vijayan M and Reddy AP: Mutant APP and amyloid
beta-induced defective autophagy, mitophagy, mitochondrial
structural and functional changes and synaptic damage in
hippocampal neurons from Alzheimer's disease. Hum Mol Genet.
27:2502–2516. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Song J, Ma SJ, Luo JH, Zhang H, Wang RX,
Liu H, Li L, Zhang ZG and Zhou RX: Melatonin induces the apoptosis
and inhibits the proliferation of human gastric cancer cells via
blockade of the AKT/MDM2 pathway. Oncol Rep. 39:1975–1983.
2018.PubMed/NCBI
|
|
33
|
Zhang LY, Liu ZH, Zhu Q, Wen S, Yang CX,
Fu ZJ and Sun T: Resolvin D2 relieving radicular pain is associated
with regulation of inflammatory mediators, Akt/GSK-3β signal
pathway and GPR18. Neurochem Res. 43:2384–2392. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Yao Y, Wang Y, Kong L, Chen Y and Yang J:
Osthole decreases tau protein phosphorylation via PI3K/AKT/GSK-3β
signaling pathway in Alzheimer's disease. Life Sci. 217:16–24.
2019. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Hu S, Cui W, Zhang Z, Mak S, Xu D, Li G,
Hu Y, Wang Y, Lee M, Tsim KW and Han Y: Indirubin-3-oxime
effectively prevents 6OHDA-induced neurotoxicity in PC12 cells via
activating MEF2D through the inhibition of GSK3β. J Mol Neurosci.
57:561–570. 2015. View Article : Google Scholar : PubMed/NCBI
|