Introduction
Endothelial injury is the first step in the
development of atherosclerosis (1). Numerous factors are involved in this
endothelial injury process; for example, endothelial progenitor
cells (EPCs) serve an important function in the maintenance of
endothelium structural integrity and function (2,3).
Previous studies have revealed that shear stress promotes the
differentiation of EPCs to endothelial cells (ECs), and
participates in vascular re-endothelialization (4–6).
However, the transcriptional regulatory mechanisms involved in the
control of the differentiation of EPCs remain unclear.
Krüppel-like factor 2 (KLF2), a member of the zinc
finger transcription factor family, regulates cellular growth
during tissue development (7).
Previously, KLF2 has emerged as a primary regulator of endothelial
quiescence, anti-inflammatory responses, antithrombotic functions
and vascular tone by activating atheroprotective transcription and
inhibiting atherogenic transcription (8,9). In
addition, KLF2 is involved in the regulation of various immune
cells by inhibiting the proinflammatory activation of monocytes
(10). Das et al (11) revealed that KLF2 mediates the
transcriptional regulation of arthritis via the modulation of
monocyte differentiation and function. KLF2 expression is elevated
in the vascular endothelium and required for normal vessel
development (12,13). In addition, it is a prominent
anti-angiogenic factor, and modulates the expression of multiple
endothelial vasoprotective genes (14). In previous years, numerous studies
have revealed that KLF2 is also an important regulator for
cardiovascular cells, particularly cardiac ECs. For example,
Stroncek et al (15)
demonstrated that KLF2 expression was substantially higher in the
EPCs of patients with coronary artery disease compared with those
of healthy individuals. KLF2 also controls the phenotype and
metabolism of ECs (16). However,
the function of KLF2 in the differentiation from EPCs to ECs
remains unknown.
In the present study, the effects of KLF2 on the
differentiation of EPCs to ECs under shear stress were
investigated, in addition to the underlying mechanisms. One
previous study demonstrated that shear stress may induce
differentiation of EPCs to ECs in a magnitude-dependent manner
through its effects on the integrin-actin cytoskeleton system, and
may result in the increased expression of von Willebrand factor
(vWF) and cluster of differentiation 31 (CD31) under a shear stress
of 12 dyn/cm2 (5).
However, the underlying transcriptional mechanism remains unclear.
Therefore, the effects of KLF2 on the differentiation of EPCs to
ECs were investigated through the expression of two EC-specific
markers, including CD31 and vWF. These results may provide novel
evidence for the function of KLF2 in EPC differentiation, and may
illustrate the potential association between KLF2 and
atherosclerosis-associated diseases.
Materials and methods
EPC culture and identification
The present study was conducted in accordance with
the Guide for the Care and Use of Laboratory Animals published by
the National Institutes of Health (17), and the protocol was ethically
approved by the Local Animal Ethics Committee of Weifang Medical
College (Weifang, China). Late EPCs were isolated and cultured as
described previously (6). Briefly,
bone marrow mononuclear cells (MNCs) were fractionated at 4°C with
700 × g for 20 min by density gradient centrifugation with
Histopaque®−1083 (Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) from the whole bone marrow of Sprague-Dawley rats (n=10;
weight, 150–175 g; age 8 weeks; Weifang Medical College, Weifang,
China); rats were housed in controlled conditions:12-h light/dark
cycle, 22°C, 60% humidity with ad libitum access to food.
MNCs were plated on dishes precoated with 50 µg/ml fibronectin
(Sigma-Aldrich; Merck KGaA), and were maintained at 37°C in
complete EC growth medium-2 supplemented with 10% fetal calf serum
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Unattached cells were removed with sterile phosphate buffered
saline (PBS) after 4 days, and endothelial colonies appeared
subsequently. Late EPCs were used in the following experiments
subsequent to 3–5 passages.
Cell treatments
To verify whether integrin β1/β3 or F-actin
influences the expression of KLF2 under shear stress, late EPCs in
the experimental group were first pretreated with 50 µg/ml
anti-integrin β1 (clone MEM-101A; catalog no.
CSB-PA011880LA01MO-100; Dianova GmbH, Hamburg, Germany) and 10
µg/ml anti-integrin β3 (catalog no. MAB2023Z; Gibco; Merck KGaA)
antibodies for 30 min at 37°C, or incubated with 1 µmol/l
cytochalasin D (cytoD; catalog no. PHZ1063; Thermo Fisher
Scientific, Inc.) for 1 h at 37°C to disrupt actin filaments
(6), as described previously
(5). EPCs treated with dimethyl
sulfoxide (DMSO) were used as the control group. Then, the two
groups of EPCs were exposed to 12 dyn/cm2 shear stress
for 1 h. The KLF2 mRNA levels in the two groups were measured using
reverse transcription-quantitative polymerase chain reaction
(RT-qPCR) as described below.
Small interfering RNA (siRNA) was used to
demonstrate the function of KLF2 under shear stress. KLF2 siRNAs
(20 µmol/l) were transfected into EPCs (105 cells) using
Lipofectamine® 3000 (Invitrogen; Thermo Fisher
Scientific, Inc.) at room temperature, according to the
manufacturer's protocol; the medium contained was changed after 6 h
incubation. The cells were collected 72 h later, and the KLF2 mRNA
expression levels were assessed to verify the efficiency of
interference, while the expression levels of CD31 and vWF were
determined using flow cytometry. The sequences for KLF2 siRNA were
as follows: Sense, 5′-CAGGUGAGAAGCCUUAUCATT-3′; antisense,
5′-UGAUAAGGCUUCUCACCUGTT-3′ (Shanghai GenePharma Co., Ltd.,
Shanghai, China). In addition, a non-targeting control was used as
a negative control in the present study. The sequences of the
negative control were as follows: Sense,
5′-UUCUCCGAACGUGUCACGUTT-3′; antisense, 3′-ACGUGACACGUUCGGAGAATT-5′
(Shanghai GenePharma Co., Ltd.). Additionally, another group of
EPCs transfected with si-KLF2 for 60 h were exposed to 12
dyn/cm2 shear stress for an additional 12 h, and the
expression levels of CD31 and vWF were analyzed using flow
cytometry. The regulatory effects of KLF2 in the promoter region of
vWF were determined via a luciferase assay.
Shear stress experiments
EPCs were subjected to shear stress using a flow
chamber system as previously described (6). The following formula was used to
calculate the stress intensity: τω=6uh2b Q, where τω is the
shear stress, µ the medium viscosity (0.0077 g/cmNs), Q the
volumetric flow rate (2.05 cm3/s), h the chamber height
(0.03 cm) and b the chamber width (2.5 cm).
RT-qPCR
Total RNA was isolated from the aforementioned
treated cells with TRIzol® reagent (Invitrogen; Thermo
Fisher Scientific, Inc.), and the RNA was reverse-transcribed using
a SYBR PrimeScript RT-PCR kit (Takara Bio, Inc., Otsu, Japan) at
37°C for 15 min, according to the manufacture's protocol. RT-qPCR
was performed using SYBR Premix Ex Taq (Takara Bio, Inc.) to
determine gene expression levels. GAPDH was used as the internal
control. The primer sequences were as follows: GAPDH, forward
5′-GGCACAGTCAAGGCTGAGAATG-3′, reverse 5′-ATGGTGGTGAAGACGCCAGTA-3′;
KLF2, forward 5′-TCGCACCTAAAGGCGCATC-3′, reverse
5′-TAGTGGCGGGTAAGCTCGTC-3′; CD31, forward
5′-GACAGCCAAGGCAGATGCAC-3′, reverse 5′-ATTGGATGGCTTGGCCTGAA-3′;
vWF, forward 5′-GCGTGGCAGTGGTAGAGTA-3′, reverse
5′-GGAGATAGCGGGTGAAATA-3′. qPCR reactions were performed on a
LightCycler 480II instrument (Roche Diagnostics, Indianapolis, IN,
USA) with a final primer concentration of 0.4 µmol/l according to
the manufacturer's protocol. Thermocycling conditions were as
follows: 95°C for 30 sec; followed by 40 cycles at 95°C for 15 sec;
59°C for 1 min; and 72°C for 10 sec. The fold changes in gene
expression were calculated using the following formula: Fold
change=2−ΔΔCq (18).
Western blot analysis
EPCs were lysed at 4°C for 30 min in
Radioimmunoprecipitation Assay lysis buffer (catalog no. C1053;
Applygen Technologies Inc., Beijing, China), and protein
concentrations were determined with the Pierce Bicinchoninic Acid
Assay kit (Thermo Fisher Scientific, Inc.). An equal amount of
proteins (40 µg/lane) were separated by 12% (wt/vol) SDS-PAGE, and
the proteins were subsequently transferred to polyvinylidene
fluoride membranes and blocked with 5% bovine serum albumin
(catalog no. ST-023; Beyotime Institute of Biotechnology, Jiangsu,
China) in TBS with 0.05% Tween 20 (TBST) for 60 min at room
temperature, followed by a 4°C overnight incubation with the
primary antibodies against KLF2 (1:500; catalog no. SAB2108684;
Sigma-Aldrich; Merck KGaA). β-actin (1:1,000; catalog no. AF0003;
Beyotime Institute of Biotechnology) was used as a loading control
and for normalization. Membranes were then washed with TBST and
incubated with horseradish peroxidase (HRP)-conjugated mouse
anti-rabbit immunoglobulin (Ig)G (1:1,000; SC-2357; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) or HRP-conjugated goat
anti-mouse IgG(H+L) (1:2,000; catalog no. A0216; Beyotime Institute
of Biotechnology) at room temperature for 60 min. Immunoreactive
bands were visualized using enhanced chemiluminescence (GE
Healthcare, Chicago, IL, USA) and densitometric analysis was
conducted using ImageJ v1.8.0 software (National Institutes of
Health, Bethesda, MD, USA).
Flow cytometry
Flow cytometry was used to determine the expression
levels of CD31 and vWF. EPCs were trypsinized into a single cell
suspension (1×105 cells), fixed with 4% paraformaldehyde
for 15 min and washed three times with PBS for 5 min. Cells were
permeabilized with 0.1% TritonX-100 for 5 min, washed with PBS and
incubated with phycoerythrin-conjugated anti-CD31 (1:50; catalog
no. 25-0319-41; eBioscience; Thermo Fisher Scientific, Inc.) or
fluorescein isothiocyanate-conjugated anti-vWF (1:50; catalog no.
ab8822; Abcam) for 1 h at 4°C. Subsequent to washing with PBS to
remove unbound antibodies, cells were analyzed using a FACSCanto II
flow cytometer (BD Biosciences, San Jose, CA, USA) and the data
were analyzed utilizing a software FlowJo 10 (Tree Star, Inc.,
Ashland, OR, USA).
Luciferase assay
For the luciferase assay, various lengths (1/4, 2/4,
3/4 and full length) of the 5′untranslated (5′UTR) region of vWF,
including nucleotide regions 4851–5341 (490 bp), 4241–5341 (1,100
bp), 3848–5341 (1,493 bp) and 3341–5341 (2,000 bp), and KLF2 gene
were inserted into a psiCheck2 vector, respectively (Promega
Corporation, Madison, WI, USA). 293 cells (Type Culture Collection
of the Chinese Academy of Sciences, Shanghai, China) were
maintained in DMEM with 10% fetal bovine serum at 37°C. 293 cells,
which express vWF, but not KLF2, were co-transfected with the
KLF2-containing psiCheck2 plasmid using GeneJuice Transfection
Reagent (Merck KGaA) at 37°C for 48 h. Cells transfected with the
unmodified psiCheck2 empty vector were used as controls. Luciferase
reporter gene activity was measured 48 h following transfection
using the Promega Dual-luciferase reporter assay system (Promega
Corporation) an OPtocomp I luminometer (MGM Instruments, Inc.,
Hamden, CT, USA).
Statistical analysis
SPSS software version 16.0 (SPSS, Inc., Chicago, IL,
USA) was used to analyze all data. Unless otherwise indicated, the
data were expressed as the mean ± standard deviation. Statistical
analyses were performed using one-way analysis of variance,
followed by Tukey-Kramer multiple comparisons post-hoc tests.
P<0.05 was considered to indicate a statistically significant
difference.
Results
KLF2 expression increases in response
to shear stress
The KLF2 mRNA levels significantly increased in
response to 12 dyn/cm2 laminar shear stress compared
with the static group, peaking after 1 h (P<0.01), and
subsequently dropped, despite remaining significantly high compared
with the static group at 3 and 6 h (P<0.01; Fig. 1A). The KLF2 protein expression
level also demonstrated a significant increase in response to 3 h
or more of 12 dyn/cm2 laminar shear stress compared with
the static group (P<0.01), reaching a peak after 12 h, and
remaining comparatively high at 24 h (Fig. 1B). However, no significant
differences were observed between the 1 h group and the static
group. Furthermore, 5 dyn/cm2 shear stress inhibited
KLF2 mRNA expression in a time-dependent manner when treated for 1
h or more (Fig. 1C), with
significant differences identified between each group and the
static group (P<0.05, P<0.05 and P<0.01 for the 1, 3 and 6
h groups, respectively); however, the 0.5 h group exhibited a
significant increase in mRNA compared with the static group
(P<0.05). It was speculated that the short duration (<1 h) of
5 dyn/cm2 shear stress treatment may have promoted KLF2
mRNA expression, and only longer durations (>1 h) of 5
dyn/cm2 shear stress treatment inhibited KLF2 mRNA
expression in a time-dependent manner. Additionally, the KLF2
protein expression levels were significantly decreased at 12 h and
24 h of treatment, compared with the static group (P<0.01;
Fig. 1D).
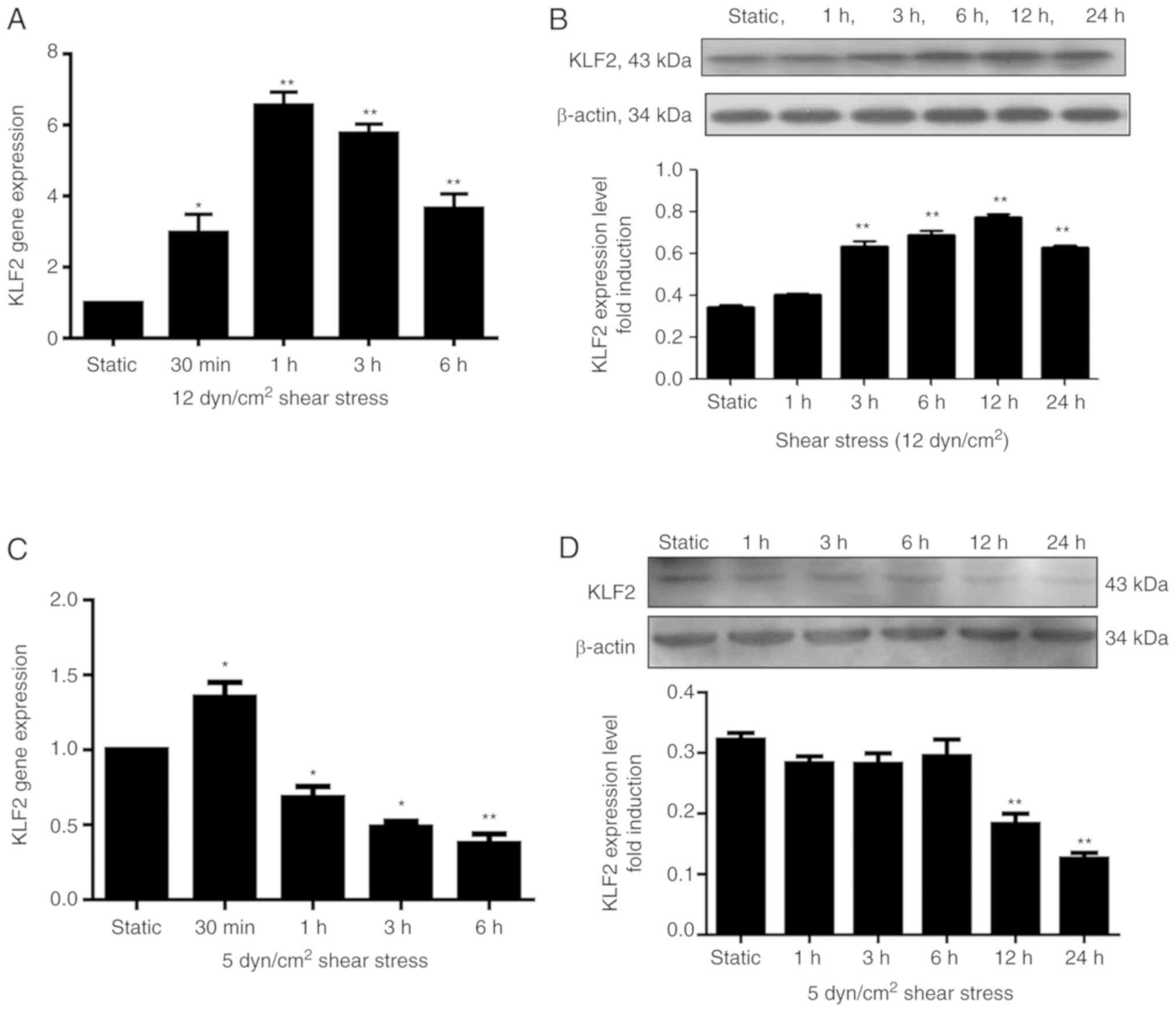 | Figure 1.mRNA and protein expression levels of
KLF2 in EPCs are affected by shear stress. (A and B) EPCs were
maintained in static conditions or exposed to 12 dyn/cm2
laminar shear stress for varying lengths of time; (A) RT-qPCR
analysis at static, 0.5, 1, 2, 3 and 6 h, and (B) western blot
analysis at static, 1, 3, 6, 12 and 24 h revealed that KLF2 mRNA
and protein expression levels, respectively, increased in EPCs
following exposure to 12 dyn/cm2 shear stress for
various time durations. (C and D) EPCs were also maintained in
static conditions or exposed to 5 dyn/cm2 laminar shear
stress for varying lengths of time; (C) RT-qPCR analysis at static,
0.5, 1, 3 and 6 h and (D) western blot analysis static, 1, 3, 6, 12
and 24 h revealed that KLF2 mRNA and protein expression levels,
respectively, were reduced in EPCs subsequent to exposure to 5
dyn/cm2 shear stress for various time durations. Data
were expressed as means ± standard deviation from 4 independent
experiments; n=4; *P<0.05 and **P<0.01 vs. Static group.
EPCs, endothelial progenitor cells; KLF2, Krüppel-like factor 2;
RT-qPCR, reverse transcription-quantitative polymerase chain
reaction. |
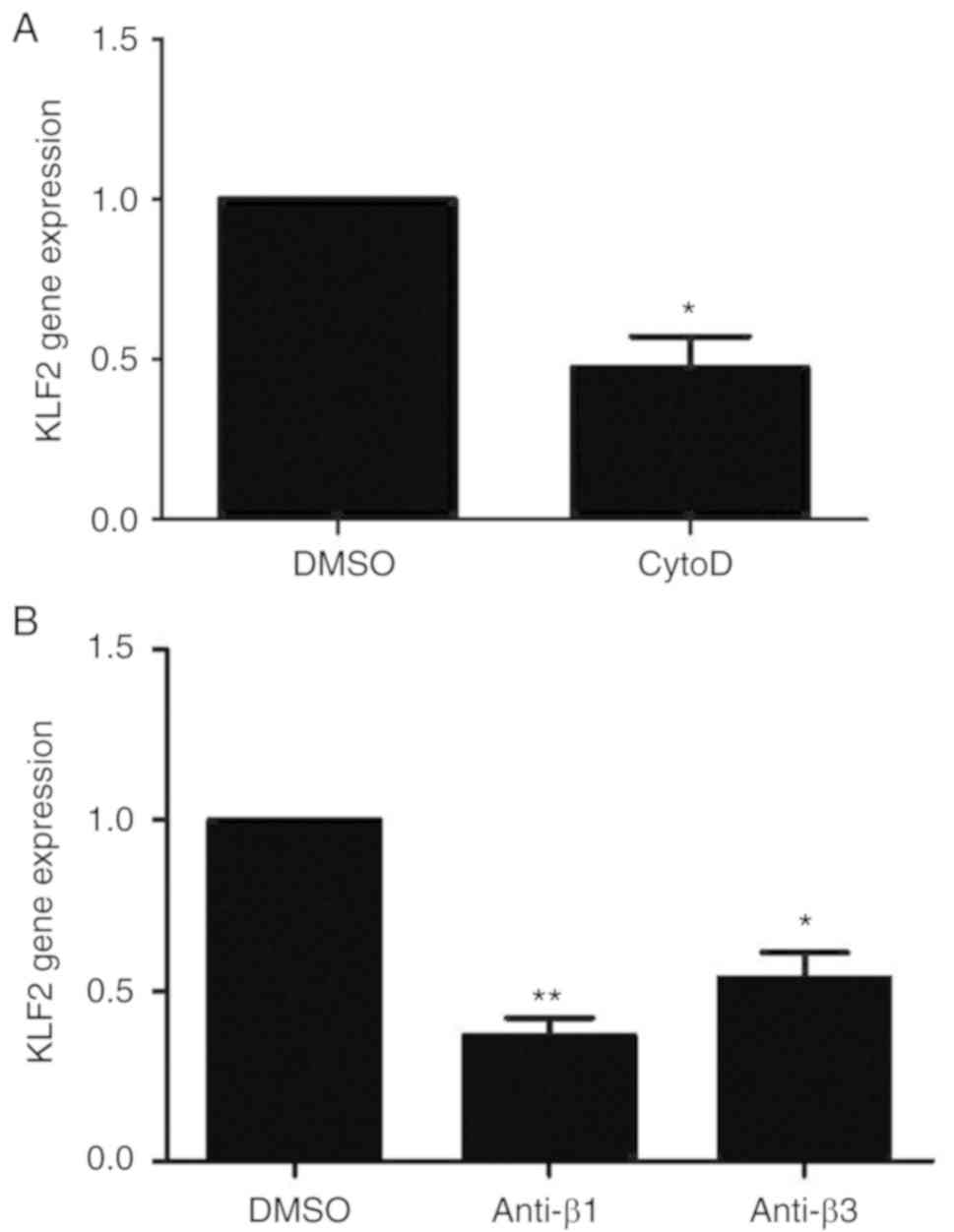
Expression of KLF2 decreases in
response to treatment with anti-integrin β1, β3 and cytoD
antibodies
KLF2 mRNA expression levels in the cytoD group were
significantly inhibited compared with the DMSO control group
(P<0.05; Fig. 2A). Similarly,
the KLF2 gene expression levels in the anti-integrin β1 (P<0.01)
and anti-integrin β3 (P<0.05) groups decreased significantly
compared with the DMSO control group (Fig. 2B).
Expression levels of CD31 and vWF
In cells transfected with siKLF2, KLF2 mRNA
expression levels decreased significantly compared with the control
group (P<0.01; Fig. 3A). The
mRNA levels of CD31 and vWF also decreased significantly compared
with the control group (mRNA levels of CD31 vs. the control group
P<0.05; mRNA levels of vWF vs. the control group, P<0.01;
Fig. 3B). Additionally, results
from flow cytometry revealed that the protein levels of CD31 and
vWF increased significantly in response to shear stress, compared
with the static group (P<0.01; Fig.
3C and D); however, siKLF2 pretreatment appeared to reverse
this effect (P<0.05; Fig. 3C and
D).
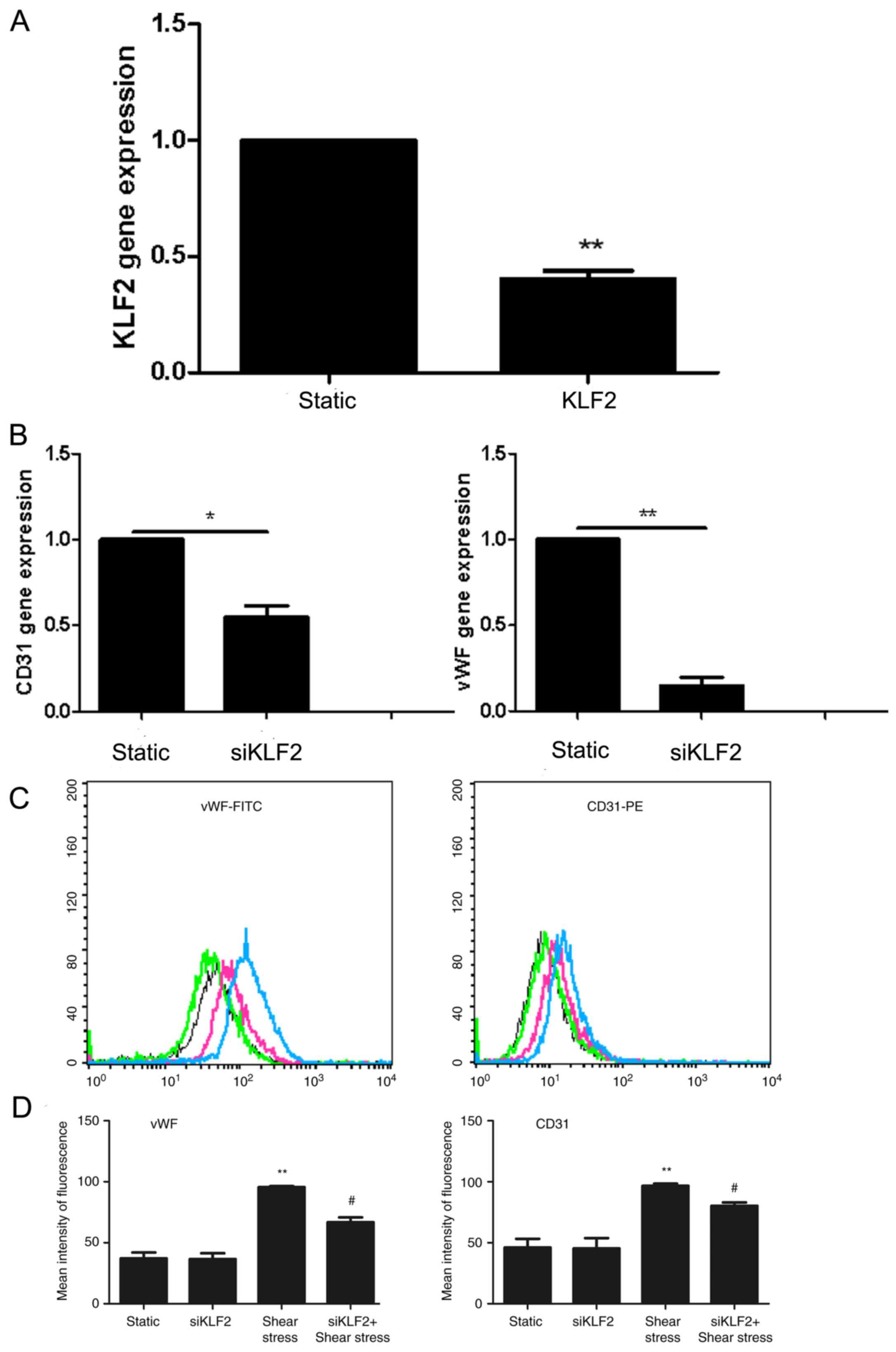 | Figure 3.Effects of siKLF2 on the expression
of EC markers in EPCs. (A-D) EPCs were treated with siKLF2 (20
µM/l) for 72 h under static conditions, and the expression levels
of KLF2 and EC markers were determined using reverse
transcription-quantitative polymerase chain reaction and flow
cytometry, respectively. (A) siKLF2 transfection successfully
decreased KLF2 mRNA expression levels; GAPDH was used to normalize
gene expression; n=4; **P<0.01 vs. (B) siKLF2 transfection
significantly decreased the mRNA expression levels of CD31 and vWF;
*P<0.05, **P<0.01. (C) Luciferase assay results revealed that
siKLF2 transfection decreased protein expression levels of the EC
makers vWF and CD31, under static and shear stress conditions.
Black and green lines represent the static and the
siKLF2-transfected groups, respectively; blue and red lines
represent the shear stress and the siKLF2 + shear stress groups,
respectively. (D) Quantification of luciferase assay results from
Part C. siKLF2 + shear stress decreased protein expression level of
EC marker. Values are expressed as the relative mean fluorescence
intensity ± standard deviation; n=3; **P<0.01 vs. Static group,
*P<0.05 vs. siKLF2 alone. EC, endothelial cells; EPCs,
endothelial progenitor cells; FITC, fluorescein isothiocyanate;
KLF2, Krüppel-like factor 2; PE, phycoerythrin; siRNA, small
interfering RNA; vWF, von Willebrand factor. |
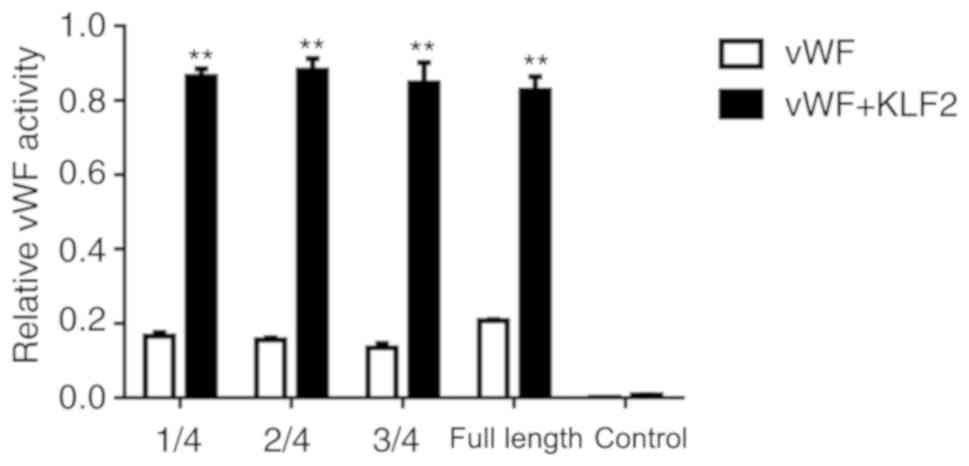
KLF2 binds to the promoter region of
vWF
Luciferase assay results revealed that various
lengths of the vWF 5′UTR may serve a function in inducing gene
expression; however, there were no statistical differences in the
activity in the different lengths of the vWF 5′UTR. The result
demonstrated that the length of the vWF 5′UTR was not related to
the gene expression. In 293 cells co-transfected with KLF2, the
expression level of vWF significantly increased compared with the
vWF alone group (P<0.01; Fig.
4). KLF2 effectively bound to the vWF promoter, thus increasing
the expression of vWF (as demonstrated by the increased
fluorescence intensity) (Fig.
4).
Discussion
Shear stress serves an important function in the
proliferation and differentiation of numerous types of cells,
including ECs, stem cells and EPCs (19,20).
Mechanotransduction, which induces changes in endothelial
metabolism, is a response to the extracellular stimuli of ECs and
EPCs (21). The results of the
present study revealed that shear stress upregulated the expression
of KLF2. Downregulation of KLF2 expression by siKLF2 significantly
inhibited the differentiation of EPCs to ECs under shear stress
conditions. Additionally, KLF2 was involved in the regulation of
vWF through binding to its promoter region.
Previous studies have demonstrated that KLF2, which
may be induced by shear stress, contributes to maintaining a
healthier endothelium by inducing a quiescent state, in addition to
having antithrombotic, vasorelaxing and anti-inflammatory effects
(8,9). The results of these previous studies
consistently revealed that shear stress increased KLF2 expression
in late EPCs, particularly when treated with 12 dyn/cm2
shear stress for 1 h, as applied in the present study. Results from
a previous study revealed that KLF2 is a central transcriptional
switch point, and is involved in the functional quiescent
differentiation of the endothelium (9). Thus, it was hypothesized that KLF2
may regulate the differentiation of EPCs to ECs. To the best of our
knowledge, there is no direct evidence regarding the effects of
KLF2 on EPCs. Therefore, the present study investigated the
potential mechanism underlying the effects of shear stress on KLF2
in EPCs. In the present study, KLF2 expression increased in
response to relatively high shear stress (12 dyn/cm2),
and decreased under relatively low shear stress (5
dyn/cm2), indicating that high shear stress may increase
KLF2 expression. One previous study has revealed that shear stress
of 6, 12 and 20 dyn/cm2 substantially increased the
expression of EC differentiation markers including vWF and CD31 in
late EPCs, while no effect was observed at a stress level of 2
dyn/cm2. These results suggest that shear stress-induced
EPC differentiation is associated with the intensity of shear
stress (22). This is may be an
explanation as to why atherosclerotic lesions occur predominantly
in regions of the vasculature exposed to low shear stress (23). KLF2 transcription factor was
revealed to be a protective mechanism for the endothelium against
inflammatory and thrombotic events (9,24).
Additionally, a 12 dyn/cm2 laminar shear stress was also
demonstrated to be a protective factor for the endothelium, while a
5 dyn/cm2 laminar shear stress may result in the
formation of atherosclerosis (25,26).
These results suggest that KLF2 function in EPCs differentiation
may be associated with the intensity of shear stress.
Boon et al (27) demonstrated that KLF2 was crucial to
shear stress-mediated actin cytoskeleton remodeling and shear fiber
assembly in the vascular endothelium. Furthermore, these fibers and
forces may be essential for the firm attachment of ECs to withstand
vascular mechanical forces. Additionally, microtubules may
contribute to the cytoskeletal-dependent regulation of KLF2
expression (28). A similar trend
in ECs, whose alignment was induced by fluid shear stress, further
suggests a potential cytoskeletal-dependent regulation of KLF2
(28). Furthermore, previous
studies have uncovered that shear stress may promote the
differentiation of EPCs to ECs via the integrin-actin cytoskeleton
system (5,6). Accordingly, it was hypothesized that
integrin β1/β3 or F-actin may influence the expression of KLF2
under shear stress conditions. The results of the present study
suggested that blocking integrin β1/β3 or destroying actin fibers
inhibited the expression of KLF2, suggesting that high shear stress
may increase KLF2 expression through the integrin-actin
cytoskeleton system. However, the inhibiting effects of low shear
stress on KLF2 expression should be given greater attention, and
further studies should be conducted on this topic.
EPCs participate in the process of endothelium
repair through differentiation to ECS (29). To date, numerous studies have
focused on the transcription factors active during EPC
differentiation under static conditions (30–32).
EPCs, which are located primarily in the bone marrow and peripheral
blood, are transported to the injury area through blood circulation
(33). Therefore, the effect of
fluid shear stress may also be important for EPC differentiation.
The regulatory effect of KLF2 on EC-specific markers, including
CD31 and vWF, was noted in the static state and in the shear stress
state in the present study. This suggests that KLF2 may serve an
important function in the differentiation of EPCs to ECs under
static and shear stress conditions, but particularly the latter.
The present research revealed that KLF2 influenced EPC
differentiation through binding to the promoter region of vWF. This
result is consistent with that of Hough et al (34) who argued that vWF promoter activity
was enhanced 3.4-fold when ECs were exposed to shear stress.
Furthermore, KLF2 bound to the vWF promoter effectively, thus
increasing the fluorescence value during a luciferase assay, and no
substantial differences were observed in vWF expression between the
one-quarter length shortened promoter and the full-length promoter.
This illustrated that there may be binding sites in this shortened
promoter, but the specific loci remain unknown. However, there was
a limitation in the present study, in that functional analysis of
the EPCs with KLF2 knockdown was not performed, and that further
studies are required to investigate the functional effects.
In conclusion, the present study provides potential
mechanistic insights into the promoting effects of KLF2 on EPC
differentiation under shear stress conditions.
Acknowledgements
Not applicable.
Funding
The present study was supported by The National
Natural Science Foundation of China (grant nos. 31570941, 31270993
and 81700406), The Program for New Century Excellent Talents in
University (grant no. NCET-10-0922), The Natural Science Foundation
of Shandong Province (grant nos. ZR2013CQ032, ZR2014JL018 and
KX2014015), The Traditional Chinese Medicine of Shandong Province
(grant nos. 2016WS0667 and 2015–239) and The Shandong Province
Higher Educational Science and Technology Program (grant nos.
J14LK59, J14LK12 and J15LK08).
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
HC and YS conceived and designed the research; YG,
XG and HL performed the experiments and acquired the data; HY, XC
and MC analyzed and interpreted the data; XZ and ZL performed
statistical analyses; HC and YS drafted the manuscript; HL and MC
revised the manuscript for important intellectual content. All
authors read and approved the final manuscript.
Ethics approval and consent to
participate
The present study was conducted in accordance with
The Guide for the Care and Use of Laboratory Animals published by
the National Institutes of Health (publication no. 85-23, revised
1996), and the protocol was approved by the Local Animal Ethics
Committee of Weifang Medical College (Weifang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Rafieian-Kopaei M, Setorki M, Doudi M,
Baradaran A and Nasri H: Atherosclerosis: Process, indicators, risk
factors and new hopes. Int J Prev Med. 5:927–946. 2014.PubMed/NCBI
|
|
2
|
Skrzypkowska M, Myśliwska J, Słomiński B,
Siebert J, Gutknecht P and Rybastanisławowska M: Quantitative and
functional characteristics of endothelial progenitor cells in newly
diagnosed hypertensive patients. J Hum Hypertens. 29:324–330. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Stancu CS, Toma L and Sima AV: Dual role
of lipoproteins in endothelial cell dysfunction in atherosclerosis.
Cell Tissue Res. 349:433–446. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhang X, Cui X, Cheng L, Guan X, Li H, Li
X and Cheng M: Actin stabilization by jasplakinolide affects the
function of bone marrow-derived late endothelial progenitor cells.
PLoS One. 7:e508992012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Cui X, Zhang X, Guan X, Li H, Li X, Lu H
and Cheng M: Shear stress augments the endothelial cell
differentiation marker expression in late EPCs by upregulating
integrins. Biochem Biophys Res Commun. 425:419–425. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Cheng M, Guan X, Li H, Cui X, Zhang X, Li
X, Jing X, Wu H and Avsar E: Shear stress regulates late EPC
differentiation via mechanosensitive molecule-mediated cytoskeletal
rearrangement. Plos One. 8:e676752013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bieker JJ: Kruppel-like factors: Three
fingers in many pies. J Biol Chem. 276:34355–34358. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Dekker RJ, van Thienen JV, Rohlena J, de
Jager SC, Elderkamp YW, Seppen J, de Vries CJ, Biessen EA, van
Berkel TJ, Pannekoek H and Horrevoets AJ: Endothelial KLF2 links
local arterial shear stress levels to the expression of vascular
tone-regulating genes. Am J Pathol. 167:609–618. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Dekker RJ, Boon RA, Rondaij MG, Kragt A,
Volger OL, Elderkamp YW, Meijers JC, Voorberg J, Pannekoek H and
Horrevoets AJ: KLF2 provokes a gene expression pattern that
establishes functional quiescent differentiation of the
endothelium. Blood. 107:4354–5363. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
He S, Yang L, Li D and Li M: Kruppel-like
factor 2-mediated suppression of MicroRNA-155 reduces the
proinflammatory activation of macrophages. Plos One.
10:e1390602015.
|
|
11
|
Das M, Lu J, Joseph M, Aggarwal R, Kanji
S, McMichael BK, Lee BS, Agarwal S, Ray-Chaudhury A, Iwenofu OH, et
al: Kruppel-like factor 2 (KLF2) regulates monocyte differentiation
and functions in mBSA and il-1β-induced arthritis. Curr Mol Med.
12:113–125. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Anderson KP, Kern CB, Crable SC and
Lingrel JB: Isolation of a gene encoding a functional zinc finger
protein homologous to erythroid Krüppel-like factor: Identification
of a new multigene family. Mol Cell Biol. 15:5957–5965. 1995.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kuo CT, Veselits ML, Barton KP, Lu MM,
Clendenin C and Leiden JM: The LKLF transcription factor is
required for normal tunica media formation and blood vessel
stabilization during murine embryogenesis. Genes Dev. 11:2996–3006.
1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Taniguchi H, Jacinto FV, Villanueva A,
Fernandez AF, Yamamoto H, Carmona FJ, Puertas S, Marquez VE,
Shinomura Y, Imai K and Esteller M: Silencing of Kruppel-like
factor 2 by the histone methyltransferase EZH2 in human cancer.
Oncogene. 31:1988–1994. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Stroncek JD, Grant BS, Brown MA, Povsic
TJ, Truskey GA and Reichert WM: Comparison of endothelial cell
phenotypic markers of late-outgrowth endothelial progenitor cells
isolated from patients with coronary artery disease and healthy
volunteers. Tissue Eng Part A. 15:3473–3486. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Doddaballapur A, Michalik KM, Manavski Y,
Lucas T, Houtkooper RH, You X, Chen W, Zeiher AM, Potente M,
Dimmeler S and Boon RA: Laminar shear stress inhibits endothelial
cell metabolism via KLF2-mediated repression of PFKFB3.
Arterioscler Thromb Vasc Biol. 35:137–145. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
National Research Council: Guide for the
Care and Use of Laboratory Animals. The National Academies Press.
(Washington, DC). 1996.
|
|
18
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Obi S, Masuda H, Shizuno T, Sato A,
Yamamoto K, Ando J, Abe Y and Asahara T: Fluid shear stress induces
differentiation of circulating phenotype endothelial progenitor
cells. Am J Physiol Cell Physiol. 303:C595–C606. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ahsan T and Nerem RM: Fluid shear stress
promotes an endothelial-like phenotype during the early
differentiation of embryonic stem cells. Tissue Eng Part A.
16:3547–3553. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chien S: Mechanotransduction and
endothelial cell homeostasis: The wisdom of the cell. Am J Physiol
Heart Circ Physiol. 292:H1209–H1224. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Ehsani Ardakani MJ, Safaei A, Arefi
Oskouie A, Haghparast H, Haghazali M, Mohaghegh Shalmani H,
Peyvandi H, Naderi N and Zali MR: Evaluation of liver cirrhosis and
hepatocellular carcinoma using protein-protein interaction
networks. Gastroenterol Hepatol Bed Bench. 9 Suppl 1:S14–S22.
2016.PubMed/NCBI
|
|
23
|
Cecchi E, Giglioli C, Valente S, Lazzeri
C, Gensini GF, Abbate R and Mannini L: Role of hemodynamic shear
stress in cardiovascular disease. Atherosclerosis. 214:249–256.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Doddaballapur A, Michalik KM, Manavski Y,
Lucas T, Houtkooper RH, You X, Chen W, Zeiher AM, Potente M,
Dimmeler S and Boon RA: Laminar shear stress inhibits endothelial
cell metabolism via KLF2-mediated repression of PFKFB3.
Arterioscler Thromb Vasc Biol. 35:137–145. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lehoux S and Jones EA: Shear stress,
arterial identity and atherosclerosis. Thromb Haemost. 115:467–473.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Olivon VC, Fraga-Silva RA, Segers D,
Demougeot C, de Oliveira AM, Savergnini SS, Berthelot A, de Crom R,
Krams R, Stergiopulos N and da Silva RF: Arginase inhibition
prevents the low shear stress-induced development of vulnerable
atherosclerotic plaques in ApoE-/- mice. Atherosclerosis.
227:236–243. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Boon RA, Leyen TA, Fontijn RD, Fledderus
JO, Baggen JM, Volger OL, van Nieuw Amerongen GP and Horrevoets AJ:
KLF2-induced actin shear fibers control both alignment to flow and
JNK signaling in vascular endothelium. Blood. 115:2533–2542. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Vartanian KB, Berny MA, Mccarty OJ, Hanson
SR and Hinds MT: Cytoskeletal structure regulates endothelial cell
immunogenicity independent of fluid shear stress. Am J Physiol Cell
Physiol. 298:C333–C341. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lu C, Zhang J, Zhang D, Uzan G and Li M:
EPCs in vascular repair: How can we clear the hurdles between bench
and bedside? Front Biosci (Landmark Ed). 19:34–48. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li X, Song Y, Wang D, Fu C, Zhu Z, Han Y,
Li C, Wang N and Zhu Y: LIF maintains progenitor phenotype of
endothelial progenitor cells via Krüppel-like factor 4. Microvasc
Res. 84:270–277. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cheng BB, Qu MJ, Wu LL, Shen Y, Yan ZQ,
Zhang P, Qi YX and Jiang ZL: MicroRNA-34a targets Forkhead box j2
to modulate differentiation of endothelial progenitor cells in
response to shear stress. J Mol Cell Cardiol. 74:4–12. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wei S, Huang J, Li Y, Zhao J, Luo Y, Meng
X, Sun H, Zhou X, Zhang M and Zhang W: Novel zinc finger
transcription factor ZFP580 promotes differentiation of bone
marrow-derived endothelial progenitor cells into endothelial cells
via eNOS/NO pathway. J Mol Cell Cardiol. 87:17–26. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Takahashi T, Kalka C, Masuda H, Chen D,
Silver M, Kearney M, Magner M, Isner JM and Asahara T: Ischemia-
and cytokine-induced mobilization of bone marrow-derived
endothelial progenitor cells for neovascularization. Nat Med.
5:434–438. 1999. View
Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hough C, Cameron CL, Notley CR, Brown C,
O'Brien L, Keightley AM, Berber E and Lillicrap D: Influence of a
GT repeat element on shear stress responsiveness of the VWF gene
promoter. J Thromb Haemost. 6:1183–1190. 2008. View Article : Google Scholar : PubMed/NCBI
|