Introduction
Hepatocellular carcinoma (HCC) is a primary liver
cancer with high mortality (1,2) and
is one of the most common malignancies worldwide, particularly in
Asia, Africa and Southern Europe (3). Numerous advanced methods and
techniques have been recently developed and applied for the
treatment of cancer, including chemotherapy, targeted therapy and
immunotherapy from which therapeutic outcomes have been observed;
however, the majority of HCC cases remain incurable once metastatic
and poor prognosis is exhibited (4). To develop an effective treatment
against HCC, understanding the underlying molecular mechanisms of
the occurrence, proliferation, metastasis and carcinogenesis
associated with HCC is required.
Epithelial-mesenchymal transition (EMT) is a
biological process in which epithelial cells transform and exhibit
a mesenchymal phenotype via a specific program (5). The EMT process serves an essential
role in embryonic development, chronic inflammation, tissue
reconstruction, cancer metastasis and a variety of fibrotic
diseases; EMT also possesses unique characteristics of gene
regulation, such as that for the downregulation of E-cadherin
(6). In addition, it has been
demonstrated that EMT exhibits a significant effect on malignant
tumors, whereby epithelial cells transform to obtain migration and
invasion abilities associated with malignancy (7). Thus, it is important to investigate
the molecular mechanism underlying the EMT process and understand
its pathological significance in tumor occurrence, development and
metastasis.
A previous study demonstrated that Up-frameshift 1
(UPF1), a potential tumor suppressor, not only serves a key role in
RNA degradation pathways, but exhibits significant effects on cell
proliferation and differentiation by promoting these potentials of
undifferentiated cells (8). UPF1
also promotes the decay of mRNAs encoding several other proteins
that oppose the proliferative and undifferentiated cell state
(9). Our previous study reported
that UPF1 was downregulated and its gene was commonly mutated in
pancreatic adenosquamous carcinoma (ASC), and to the best of our
knowledge, it was the first to report UPF1 gene mutations in
nonsense-mediated mRNA decay in ASC (10). However, a limited number of studies
have reported the mechanism by which the expression levels of UPF1
affect HCC.
In the present study, it was demonstrated that UPF1
was significantly downregulated in HCC cell lines compared with in
normal hepatocytes. Based on this finding, the expression levels of
19 key proteins associated with numerous signaling pathways were
investigated in the presence of UPF1 overexpression (OE). In
addition, the effects of UPF1 expression on the EMT process were
analyzed by targeting the protein expression of E-cadherin,
N-cadherin, Vimentin and Twist-related protein 1 (Twist). The
findings of the present study may contribute to the development of
HCC treatment in the future.
Materials and methods
Cell culture
The Huh-7 HCC cell line, HL-7702 normal hepatocytes
and 293T normal cells were purchased from the Cell Bank of Type
Culture Collection of the Chinese Academy of Sciences (Shanghai,
China) and cultured in Minimum Essential medium with 10% fetal
bovine serum (both Gibco; Thermo Fisher Scientific, Inc., Waltham,
MA, USA) at 37°C. HL-7702 normal hepatocytes and 293T normal cells
were cryopreserved and thawed prior to analysis.
Polymerase chain reaction (PCR)
DNA was extracted from 293T cells following
transfection with the UPF1 lentiviral vector. The UPF1 gene was
amplified using primers designed by Primer Premier 5.0 software
(PREMIER Biosoft International, Palo Alto, CA, USA) and synthesized
by Sangon Biotech Co., Ltd. (Shanghai, China). The primer sequences
were as follows: UPF1 forward, 5′-AACACTATCAACCCGGGAGC-3′ and
reverse, 5′-GCTAGCTCATTTGTCGTCATC-3′; GAPDH forward,
5′-TGACTTCAACAGCGACACCCA-3′ and reverse,
5′-CACCCTGTTGCTGTAGCCAAA-3′. The PCR reaction system contained
13.25 µl double-distilled water, 5 µl 10X PCR buffer
(Mg2+ Plus), 2 µl deoxynucleotide triphosphates, 2 µl
DNA template, 1 µl forward primer, 1 µl reverse primer and 0.25 µl
rTaq DNA polymerase (all from Takara Bio, Inc., Otsu, Japan), in a
total volume of 25 µl. The PCR thermocycling conditions were as
follows: 95°C for 5 min, followed by 35 cycles of 95°C for 30 sec,
55°C for 30 sec and 72°C for 1 min; and 72°C for 10 min. The PCR
products were separated by 1.5% agarose gel electrophoresis. The
positive control was GAPDH.
Reverse transcription-quantitative
(RT-q)PCR
Total RNA was extracted from cells using SuperfecTRI
containing TRIzol (Takara Bio, Inc., Otsu, Japan). cDNA was
obtained following RT using the M-MLV kit (Promega Corporation),
according to the manufacturer's protocols. qPCR was performed on
Step-one Plus system (Applied Biosystems; Thermo Fisher Scientific,
Inc.) according to the manufacturer's protocols of the SYBR-Green
kit (Takara Biotechnology Co., Ltd., Dalian, China). The
thermocycling conditions were as follows: Denaturation at 95°C for
10 sec, followed by 40 cycles of 95°C for 5 sec and 60°C for 20
sec. GAPDH was used as an internal control, and the
2−ΔΔCq method (11) was
used to normalize expression levels against GAPDH. Each sample was
analyzed in triplicate. The primers used in the present study were
presented in Table I.
 | Table I.Primer sequences employed in the
present study. |
Table I.
Primer sequences employed in the
present study.
| Genes | Forward primer
(5′-3′) | Reverse primer
(5′-3′) | Length (bp) |
|---|
| UPF1 |
CCTTGTGAGGGCAAAATGCAA |
TGAAGCCGAGGAGGAAGACGT | 113 |
| GV358-UPF1 |
GAGGATCCCCGGGTACCGGTCGCCACCATGAGCGTGGAGGCGTACGGGCCCAGCTCGCAGACTCTC |
TCCTTGTAGTCCATACCATACTGGGACAGCCCCGTCACCCCGCC | 3398 |
| GAPDH |
TGACTTCAACAGCGACACCCA |
CACCCTGTTGCTGTAGCCAAA | 121 |
Lentiviral vector construction and
transduction
UPF1 (GenBank: NM_002911; http://www.ncbi.nlm.nih.gov/genbank/) was cloned into
GV358 vector (Ubi-MCS-3FLAG-SV40-EGFP-IRES-puromycin; Shanghai
Genechem Co., Ltd., Shanghai, China) at restriction site
AgeI. A total of 4×105 293T cells were
transfected with 1 µg plasmid using the FuGene HD transfection
reagent (Promega Corporation, Madison, WI, USA), according to the
manufacturer's protocols. Nontransduced cells (CON) and negative
control cells transfected with the empty vector (NC) were used as
the controls. Green fluorescent protein was used as a reporter, to
measure transfection efficiency by confocal microscopy
(magnification, ×100). Four randomly chosen microscopic fields were
analyzed. The lentiviral supernatant was harvested 48–72 h after
transfection and used to infect Huh-7 cells at a viral titer of
2×108 TU/ml. The expression levels of UPF1 were
confirmed by RT-qPCR and western blotting (described below).
Western blotting
Protein was extracted from cells using
radioimmunoprecipitation assay buffer (Beyotime Institute of
Biotechnology, Haimen, China) and protein concentration was
determined by bicinchoninic acid assay. Cellular proteins (20 µg)
from each sample were electrophoresed by SDS-PAGE (5% stacking and
10% separating gels). Then, the proteins were transferred onto
polyvinylidene fluoride membranes (Merck KGaA, Darmstadt, Germany)
and blocked with 5% skim milk in Tris-buffered saline and Tween-20
for 1 h at room temperature. The membranes were incubated with
primary antibodies overnight at 4°C. Subsequently, membranes were
incubated with horseradish peroxidase-conjugated secondary
antibodies for 1.5 h at room temperature. All antibodies used in
the present study were listed in Table II. Protein bands were visualized
using Pierce™ ECL Western Blotting Substrate (Thermo
Fisher Scientific, Inc.). The positive control was the standard
protein SURVIVIN-3FLAG-GFP (Shanghai Genechem Co., Ltd.).
 | Table II.Antibodies employed in the present
study. |
Table II.
Antibodies employed in the present
study.
| Antibody (cat.
no.) | Dilution | Host species | Supplier |
|---|
| UPF1 (ab109363) | 1:10,000–50,000 | Rabbit | Abcam (Cambridge,
UK) |
| P-UPF1
(07–1016) | 1: 500 | Rabbit | Merck KGaA |
| GAPDH
(sc-32233) | 1:2,000 | Mouse | Santa Cruz
Biotechnology, Inc. (Dallas, TX, USA) |
| FLAG (F1804) | 1:3,000 | Mouse | Sigma-Aldrich;
Merck KGaA |
| E-cadherin
(14472) | 1:1,000 | Mouse | Cell Signaling
Technology, Inc. |
| N-cadherin
(13116) | 1:1,000 | Rabbit | Cell Signaling
Technology, Inc. |
| Vimentin
(5741) | 1:1,000 | Rabbit | Cell Signaling
Technology, Inc. |
| Twist
(ab50581) | 0.5–1.0 µg/ml | Rabbit | Abcam |
| Goat anti-mouse
IgG-HRP (sc-2005) | 1:5,000 | Goat | Santa Cruz
Biotechnology, Inc. |
| Goat anti-rabbit
IgG-HRP (sc-2004) | 1:5,000 | Goat | Santa Cruz
Biotechnology, Inc. |
Protein antibody array assay
The PathScan® Cancer Phenotype Antibody
Array kit (Cell Signaling Technology, Inc., Danvers, MA, USA) was
utilized to investigate alterations in the expression of key
proteins associated with various signaling pathways in the presence
of UPF1 overexpression. A total of 2×105 cells were
lysed using cell lysis buffer (cat. no. 7018; Cell Signaling
Technology, Inc.) on ice, and then incubated with a slide for 2 h
at room temperature or 4°C overnight. Following washing, the slide
was incubated sequentially with detection antibody cocktail,
horseradish peroxidase-linked streptavidin, and LumiGLO®
and peroxide reagents. Images were obtained using a
chemiluminescence scanner (ChemiScope 5300; Clinx Science
Instruments Co., Ltd., Shanghai, China).
Statistical analysis
Data are presented as the mean ± standard deviation
from at least three independent experiments. A Student's t-test,
χ2 test or Fisher's exact test were used for the
comparisons between groups using SPSS 22.0 software (IBM Corp.,
Armonk, NY, USA). P<0.05 was considered to indicate a
statistically significant difference.
Results
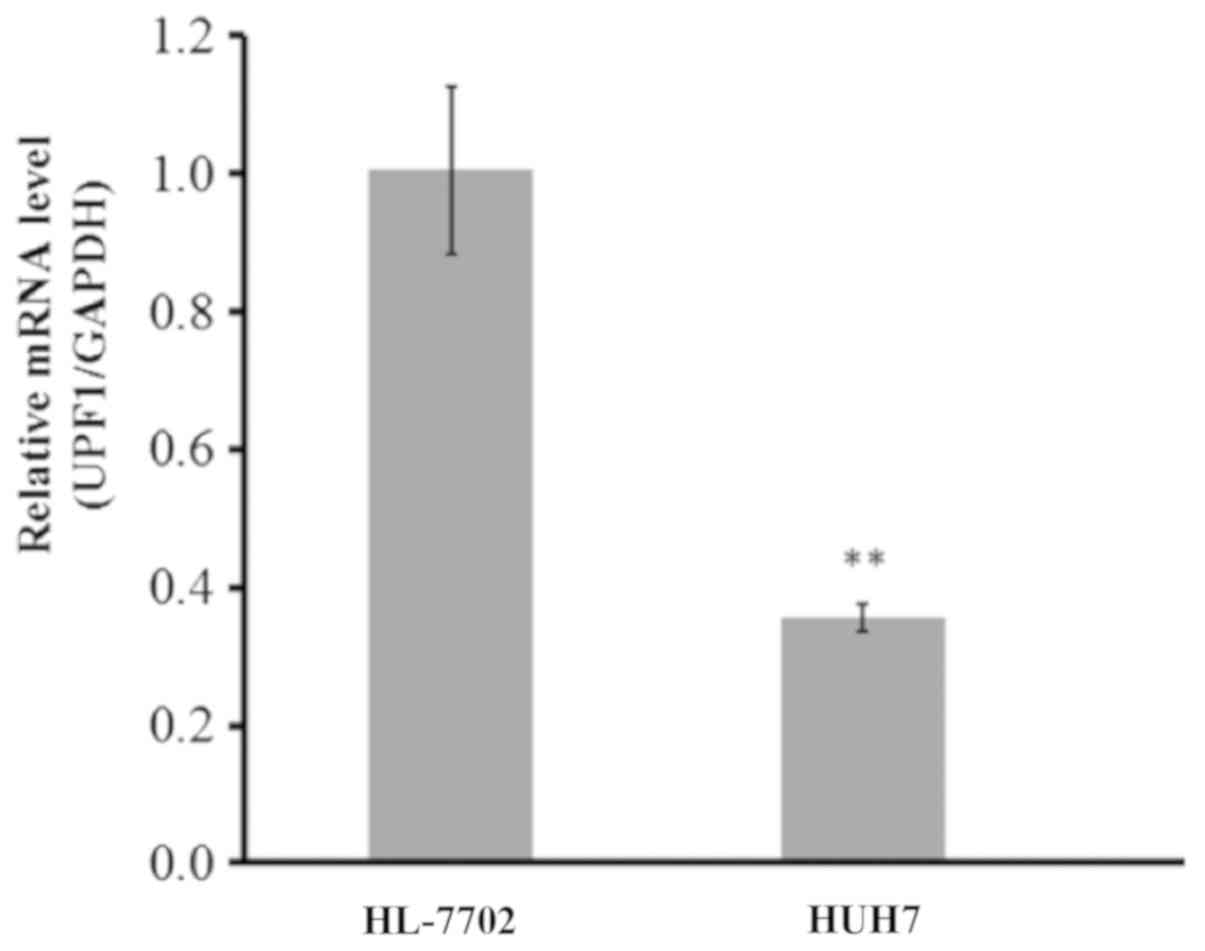
UPF1 is significantly downregulated in
an HCC cell line
The expression levels of UPF1 were determined within
HCC (Huh-7) and normal hepatocyte (HL-7702) cell lines. A
significant downregulation of UPF1 in the HCC cell line was
observed compared with in HL-7702 cells, as presented in Fig. 1. The expression levels of UPF1 in
the HCC cell line was ~60% lower than that of normal hepatocytes
(P<0.01).
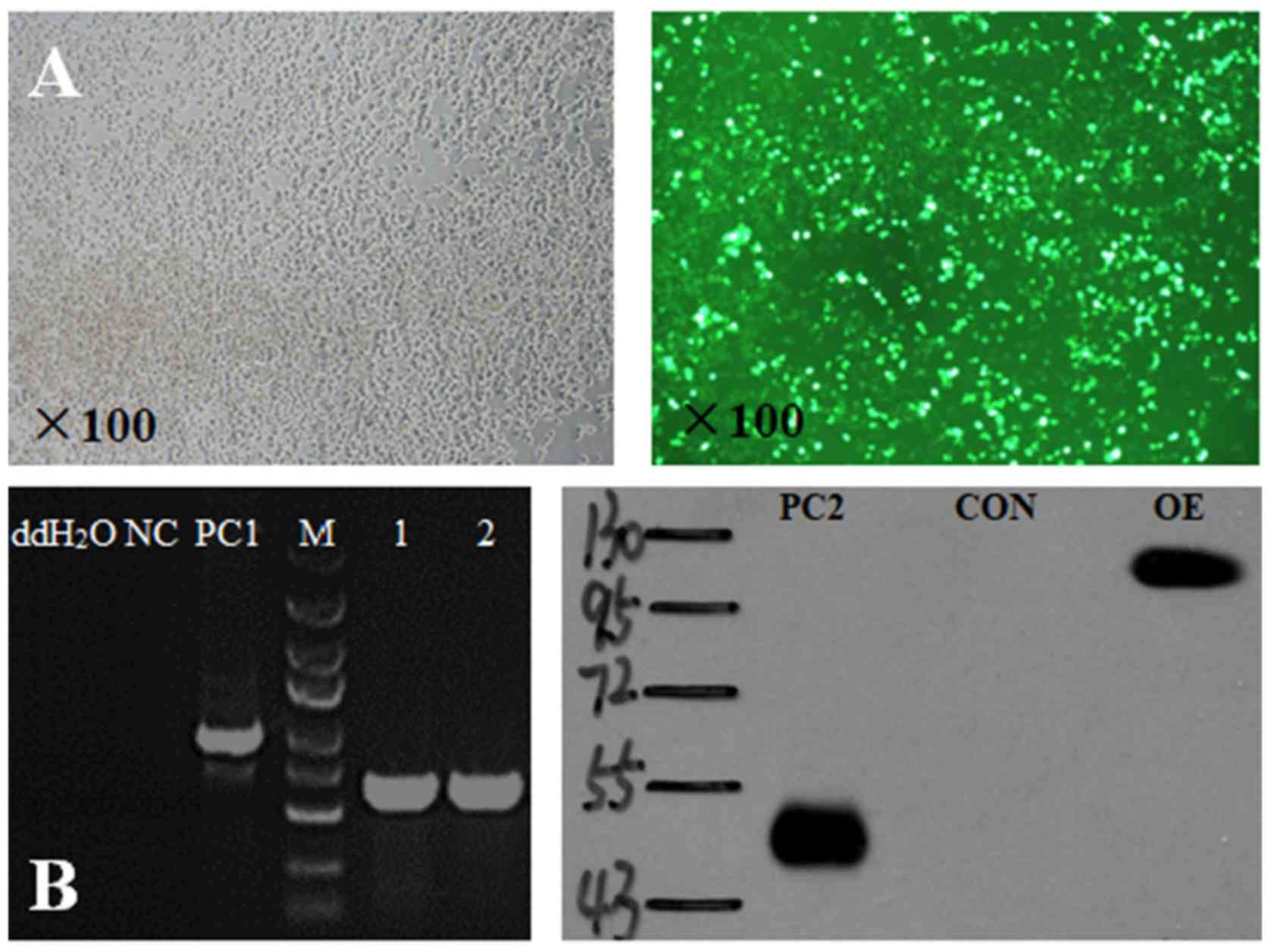
Successful transduction of the
lentiviral vector constructs and UPF1 overexpression
Following transduction, the packaging cell line,
293T, exhibited notable green fluorescence (Fig. 2A). This indicated that the
lentivirus was successfully transduced and that the UPF1 target
gene was overexpressed. Expression of the target sequence following
total DNA extraction and subsequent PCR was observed. Furthermore,
a band near 125 kDa was detected on the gel, whose location is
consistent with the target protein observed via western blotting
(Fig. 2B). The positive control
(PC) was encoded by a scrambled sequence, independent of the UPF1
sequence.
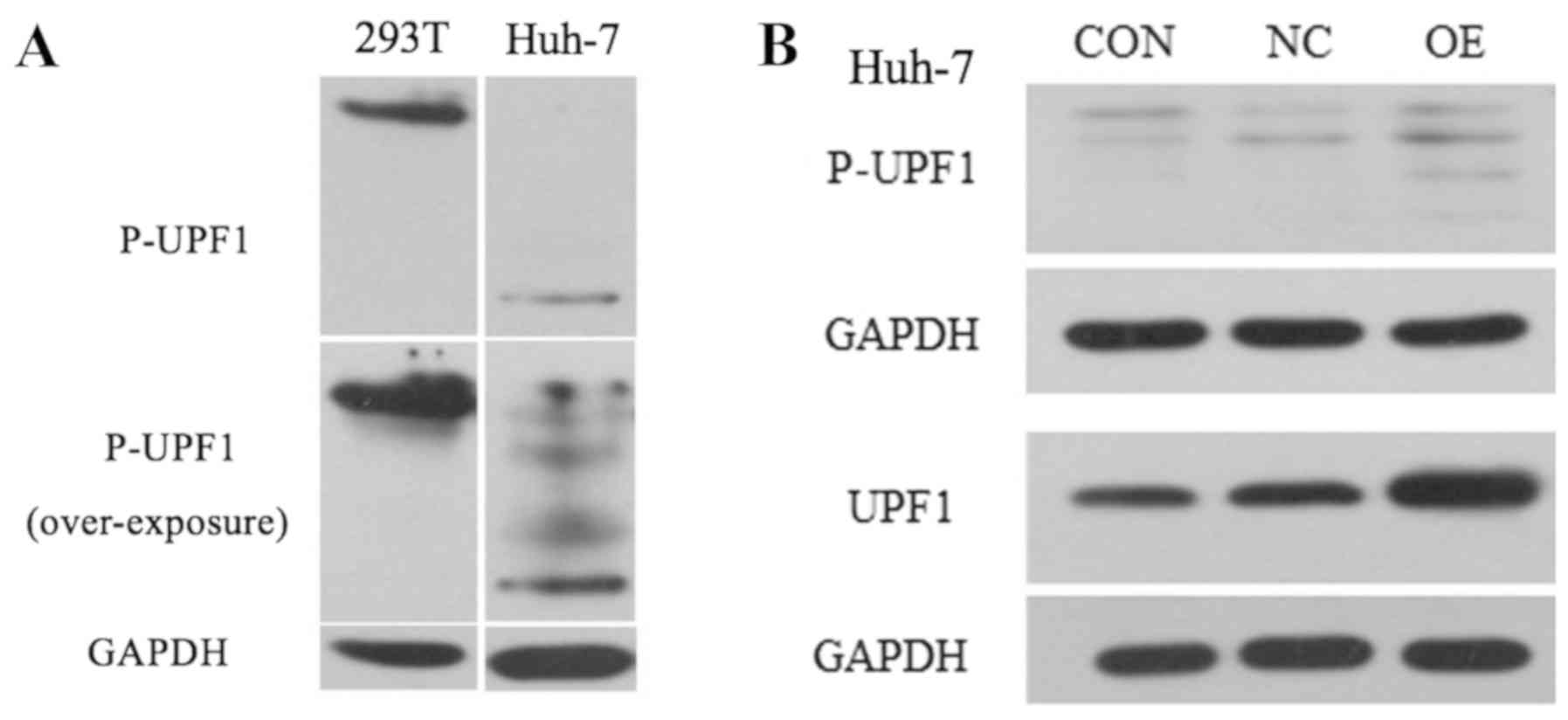
UPF1 OE exhibits no effect on the
levels of protein phosphorylation
Numerous studies have demonstrated that the
phosphorylation of key proteins is involved in tumorigenesis
(12,13). To investigate the association
between UPF1 OE and the extent of phosphorylation, the levels of
phosphorylated UPF1 protein (P-UPF1) following lentiviral
transduction of Huh-7 HCC and 293T normal cells were determined.
Prior to transduction, no target band was observed for P-UPF1 for
Huh-7 cells; however, a weak target band was observed under the
UPF1 OE condition in Huh-7 cells (Fig.
3A). Following over-exposure several nonspecific bands were
observed; therefore, the P-UPF1 band in Huh-7 cells may also be
considered nonspecific (Fig. 3A).
To investigate the effects of lentiviral transduction on the HCC
cell line, another set of experiments were conducted to transduce
the HCC cell line for UPF1 OE. As presented in Fig. 3B, UFP1 and P-UPF1 were detected in
Huh-7 cell. Compared with in the CON and NC cell groups, UPF1 was
notably upregulated in the OE group. However, the multiple bands
observed for P-UPF1 may be nonspecific.
UPF1 is involved in the regulation of
several key proteins of numerous signaling pathways
The PathScan Cancer Phenotype Antibody Array kit was
utilized to investigate the effects of UPF1 on the expression of
numerous signaling pathways in Huh-7 HCC cells. All analyzed genes
were listed in Table III; the
results of chemiluminescent detection and grayscale analysis were
presented in Fig. 4A. Compared
with in the NC groups, the expression levels of the majority of
proteins were upregulated when UPF1 was overexpressed. The protein
expression levels of cluster of differentiation (CD)31 (platelet
endothelial cell adhesion molecule-1, PECAM-1), Vimentin, CD44,
proliferating cell nuclear antigen (PCNA), Ki-67, N-Cadherin,
retinoblastoma (Rb), Survivin, P53 and Met were significantly
upregulated in their respective signal pathways (Fig. 4B; P<0.0.5). These findings
suggested that the expression levels of UPF1 exerted significant
effects on numerous signaling pathways.
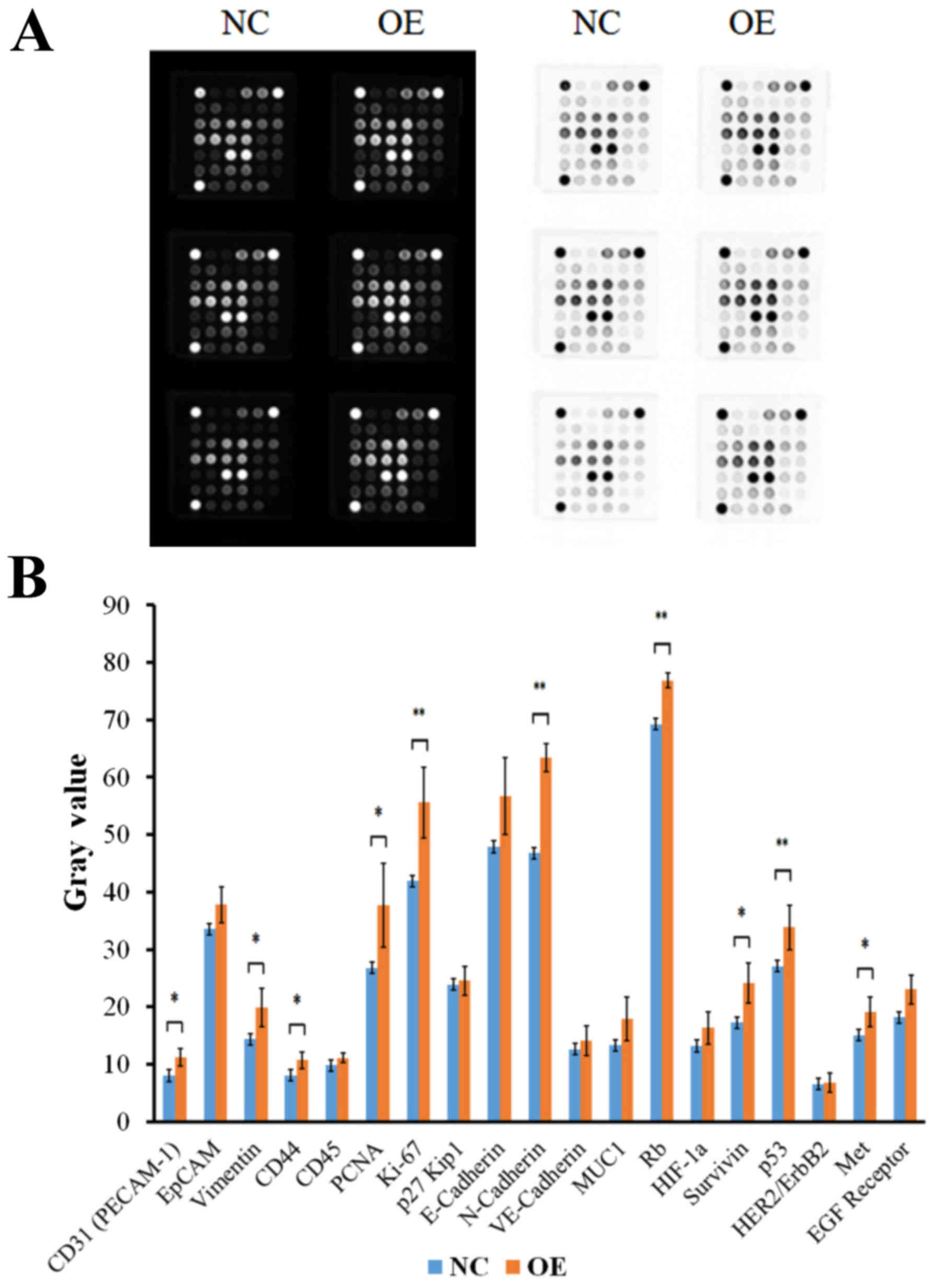 | Figure 4.UPF1 OE upregulates the expression of
numerous key proteins. (A) Results of chemiluminescent detection
and gray analysis. (B) Compared with in the NC group the expression
levels of the majority of proteins were upregulated when UPF1 was
overexpressed. *P<0.05, **P<0.01 vs. the NC group. CD,
cluster of differentiation; EGF, epidermal growth factor; EpCAM,
epithelial cell adhesion molecule; HER2/ErbB2, human epidermal
growth factor receptor 2; HIF-1α, hypoxia inducible factor-1α;
PECAM-1, platelet endothelial cell adhesion molecule-1; PCNA,
proliferating cell nuclear antigen; MUC1, mucin 1; NC, negative
control; OE, overexpression; Rb, retinoblastoma protein; UPF1,
Up-frameshift 1; VE-cadherin, vascular endothelial cadherin. |
 | Table III.Protein array analysis results. |
Table III.
Protein array analysis results.
|
|
|
| Average Gray
Value | Standard
deviation |
|
|
|---|
|
|
|
|
|
|
|
|
|---|
| Target | Site | Modification | NC | OE | NC | OE | P-value |
Upregulated/downregulated (%) |
|---|
| Positive
control | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| NC | N/A | N/A | N/A | N/A | N/A | N/A | N/A | N/A |
| CD31 (PECAM-1) | N/A | N/A | 8.00 | 11.18 | 2.27 | 1.57 | 0.0181 | 39.79 |
| EpCAM | N/A | N/A | 33.52 | 37.78 | 9.04 | 3.17 | 0.3159 | 12.73 |
| Vimentin | N/A | N/A | 14.38 | 19.92 | 3.21 | 3.37 | 0.0155 | 38.47 |
| CD44 | N/A | N/A | 8.05 | 10.70 | 1.99 | 1.44 | 0.0247 | 32.92 |
| CD45 | N/A | N/A | 9.80 | 11.08 | 2.23 | 0.84 | 0.2169 | 13.10 |
| PCNA | N/A | N/A | 26.75 | 37.73 | 8.83 | 7.33 | 0.0410 | 41.06 |
| Ki-67 | N/A | N/A | 41.90 | 55.62 | 5.60 | 6.16 | 0.0024 | 32.74 |
| p27 Kip1 | N/A | N/A | 23.88 | 24.57 | 3.47 | 2.51 | 0.7043 | 2.86 |
| E-Cadherin | N/A | N/A | 47.90 | 56.78 | 8.21 | 6.69 | 0.0670 | 18.55 |
| N-Cadherin | N/A | N/A | 46.78 | 63.45 | 6.81 | 2.41 | 0.0012 | 35.63 |
| VE-Cadherin | N/A | N/A | 12.58 | 14.13 | 2.62 | 2.55 | 0.3235 | 12.32 |
| MUC1 | N/A | N/A | 13.28 | 17.88 | 3.54 | 3.79 | 0.0550 | 34.63 |
| Rb | Ser807/811 |
Phosphorylation | 69.27 | 76.88 | 4.64 | 1.27 | 0.0089 | 11.00 |
| HIF-1a | Total | N/A | 13.18 | 16.32 | 2.93 | 2.83 | 0.0890 | 23.77 |
| Survivin | Total | N/A | 17.25 | 24.10 | 4.65 | 3.49 | 0.0163 | 39.71 |
| p53 | Total | N/A | 27.05 | 33.82 | 3.48 | 3.86 | 0.0097 | 25.02 |
| HER2/ErbB2 | Total | N/A | 6.50 | 6.75 | 1.73 | 1.69 | 0.8051 | 3.85 |
| Met | Total | N/A | 15.07 | 19.08 | 3.52 | 2.55 | 0.0473 | 26.66 |
| EGF Receptor | Total | N/A | 18.18 | 23.02 | 5.32 | 2.48 | 0.0716 | 26.58 |
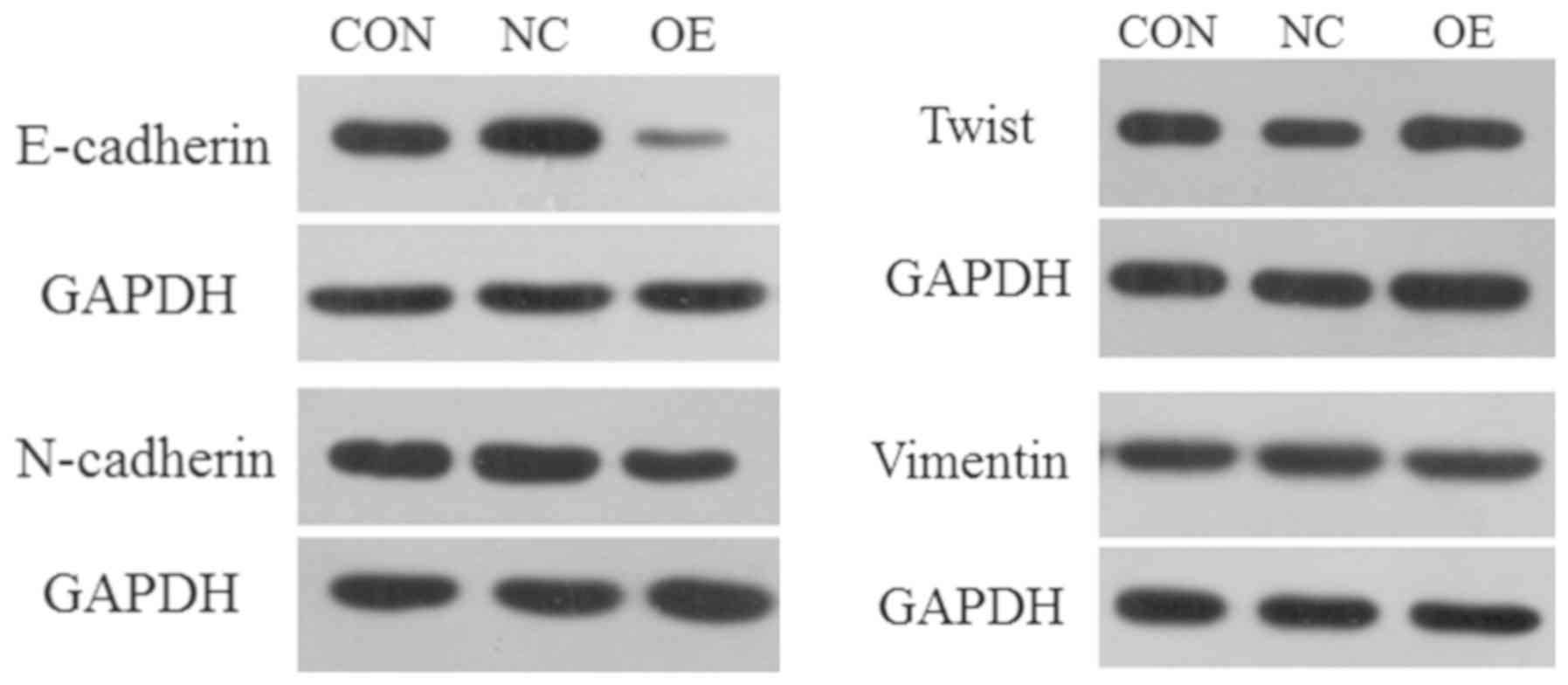
UPF1 serves an important role in EMT
process
EMT is an important biological process for the
development and progression of malignant tumors, in which
epithelial cells are transformed to obtain malignant migration and
invasive abilities (14,15). In the present study, 4 proteins
(E-cadherin, N-cadherin, Vimentin and Twist) were selected as
targets to investigate whether the expression levels of UPF1 can
affect the EMT process in Huh-7 HCC cells. Western blotting
revealed that compared with in the CON and NC groups, E-cadherin
and N-cadherin were downregulated when UPF1 was overexpressed;
however, that of Vimentin and Twist were notably upregulated
(Fig. 5).
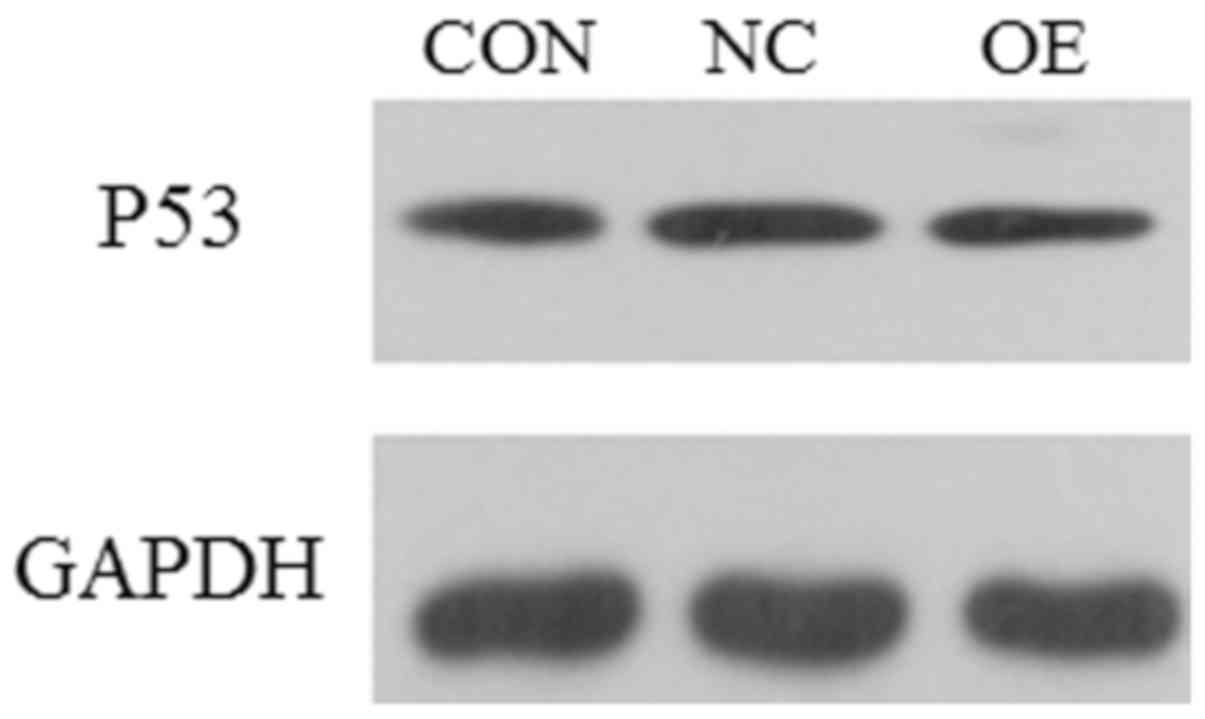
Overexpression of UPF1 affects the
expression of antioncogene P53
P53 gene is a tumor suppressor gene (16,17).
In the present study, the expression levels of P53 were
investigated in the presence of UPF1 OE in Huh-7 HCC cell (Fig. 6). The results revealed that the
expression of P53 was notably unaffected in the OE experimental
group when UPF1 was overexpressed.
Discussion
HCC is the third leading cause of cancer-associated
mortality worldwide (18). Several
studies have demonstrated that tumorigenesis is a process
associated with numerous factors (19–21).
In the present study, the association between UPF1 expression and
the EMT process underlying the tumorigenesis of HCC was
investigated.
The expression levels of UPF1 in two cell lines were
determined in the present study. RT-qPCR revealed that the
expression levels of UPF1 in HCC cell lines were lower than that in
normal hepatocytes, which was consistent with a recent report
(22). This finding suggested a
potentially important role of UPF1 in tumorigenesis. In addition,
the phosphorylation posttranslational modification level of UPF1
was also evaluated in the presence and absence of UPF1 OE.
Interestingly, no notable difference was observed in the OE group
when UPF1 was overexpressed. Studies have reported that
phosphorylation is one of the main factors associated with
tumorigenesis (23–25); the present study reported notable
association between the phosphorylation and overexpression of UPF1
in HCC.
To further understand the effects of UPF1 expression
on the EMT process, a protein assay was conducted to determine
whether UPF1 OE affected the expression of key proteins of several
signaling pathways. The majority of the proteins analyzed in the
present study were upregulated in the presence of UPF1 OE. Among
these proteins, CD31/PECAM-1, Vimentin, CD44, PCNA, Ki-67,
N-cadherin, Survivin, p53, Met and Rb exhibited a significant
association with UPF1 OE. Additionally, the expression levels of
E-cadherin, of N-Cadherin and Vimentin were upregulated, which has
been associated with the EMT process. Based on these findings, the
present study investigated how UPF1 affects the EMT process. As
aforementioned, the process of EMT serves an important role in
embryonic development, chronic inflammation, tissue reconstruction,
cancer metastasis and variety of fibrotic diseases, and possesses
unique characteristics of gene regulation, such as that for
E-cadherin downregulation (26,27).
Western blotting demonstrated the expression levels of N-cadherin,
Vimentin and Twist were upregulated under the UPF1 OE condition,
which was consistent with the findings of PathScan analysis of
Huh-7 HCC cells. In addition, when UPF1 was overexpressed, the
expression of E-cadherin was downregulated in the present study,
which was contradictory to the results of PathScan analysis. These
observations suggested that UPF1 did not serve its role to suppress
the expression of N-cadherin, Vimentin and Twist when
overexpressed; the expression of E-cadherin may be regulated by the
manifold. In a variety of human tumors, the role of E-cadherin may
be affected by gene mutations, resulting in mutant proteins,
abnormal post-translational modification (phosphorylation and
glycosylation) and increased protein hydrolysis (28). Conversely, the results of the
present study indicated that higher expression levels of Twist
associated with the overexpression of UPF1 may lead to the
downregulation of E-cadherin and upregulation of N-cadherin, which
is consistent with recent findings (29,30).
At present, the molecular mechanism of UPF1 underlying the EMT
process is notably complex and is not fully understood; however,
the results of the present study suggested the possibility that the
expression levels of UPF1 affect the EMT process by targeting
E-cadherin, N-cadherin, Vimentin and Twist proteins.
Furthermore, the expression levels of P53 were
determined when UPF1 was overexpressed in the present study. P53
has been reported as a tumor suppressor gene, and its inactivation
may promote tumor formation (31).
The results of the present study demonstrated that UPF1 OE did not
affect the expression levels of P53 in Huh-7 HCC cell.
Several studies have demonstrated low expression
levels of UPF1 in HCC cells and UPF1-suppressed tumorigenesis
(32). Conversely, the results of
RT-qPCR and western blotting in the present study revealed that
UPF1 OE promoted the expression of numerous key proteins in several
signaling pathways, including N-cadherin, Vimentin and Twist,
contrary to the characteristics of UPF1; the expression of
E-cadherin may be regulated by the manifold. The results of the
present study indicated that UPF1 may be a potential target for
regulating the EMT process (32).
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81702450), the
Natural Science Research Major Project of Education Office of Anhui
Province (grant no. KJ2016SD40) and Support Program for Outstanding
Young Talents in Colleges and Universities of Anhui Province (grant
no. gxyq2018038).
Availability of data and materials
The datasets used or analyzed during the present
study are available from the corresponding authors on reasonable
request.
Authors' contributions
YL, QW and FS designed the study and drafted the
manuscript. YL, TZ, YC and ZZ performed the experiments. SQ, RW and
YL analyzed and interpreted the data. All authors read and approved
the final manuscript.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Klingenberg M, Matsuda A, Diederichs S and
Patel T: Non-coding RNA in hepatocellular carcinoma: Mechanisms,
biomarkers and therapeutic targets. J Hepatol. 67:603–618. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gong C, Fang J, Li G, Liu HH and Liu ZS:
Effects of microRNA-126 on cell proliferation, apoptosis and tumor
angiogenesis via the down-regulating ERK signaling pathway by
targeting EGFL7 in hepatocellular carcinoma. Oncotarget.
8:52527–52542. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chen W, Zheng R, Baade PD, Zhang S, Zeng
H, Bray F, Jemal A, Yu XQ and He J: Cancer Statistics in China,
2015. CA Cancer J Clin. 66:115–132. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kuo TC, Chen CK, Hua KT, Yu P, Lee WJ,
Chen MW, Jeng YM, Chien MH, Kuo KT, Hsiao M and Kuo ML: Glutaminase
2 stabilizes Dicer to repress Snail and metastasis in
hepatocellular carcinoma cells. Cancer Lett. 383:282–294. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Luo W, Zhu X, Liu W, Ren Y, Bei C, Qin L,
Miao X, Tang F, Tang G and Tan S: MYC associated zinc finger
protein promotes the invasion and metastasis of hepatocellular
carcinoma by inducing epithelial mesenchymal transition.
Oncotarget. 7:86420–86432. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Yoshinaga T, Uwabe K, Naito S, Higashino
K, Nakano T, Numata Y and Kihara A: AM251 suppresses
epithelial-mesenchymal transition of renal tubular epithelial
cells. PLoS One. 11:e01678482016. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kim CW, Hwang KA and Choi KC:
Anti-metastatic potential of resveratrol and its metabolites by the
inhibition of epithelial-mesenchymal transition, migration, and
invasion of malignant cancer cells. Phytomedicine. 23:1787–1796.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Azzalin CM and Lingner J: The human RNA
surveillance factor UPF1 is required for S phase progression and
genome stability. Curr Biol. 16:433–439. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lou CH, Shao A, Shum EY, Espinoza JL,
Huang L, Karam R and Wilkinson MF: Posttranscriptional control of
the stem cell and neurogenic programs by the nonsense-mediated RNA
decay pathway. Cell Rep. 6:748–764. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu C, Karam R, Zhou Y, Su F, Ji Y, Li G,
Xu G, Lu L, Wang C, Song M, et al: The UPF1 RNA surveillance gene
is commonly mutated in pancreatic adenosquamous carcinoma. Nat Med.
20:596–598. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Carr MI, Roderick JE, Gannon HS, Kelliher
MA and Jones SN: Mdm2 phosphorylation regulates its stability and
has contrasting effects on oncogene and radiation-induced
tumorigenesis. Cell Rep. 16:2618–2629. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Takada M, Zhang W, Suzuki A, Kuroda TS, Yu
Z, Inuzuka H, Gao D, Wan L, Zhuang M, Hu L, et al: FBW7 loss
promotes chromosomal instability and tumorigenesis via cyclin
E1/CDK2-mediated phosphorylation of CENP-A. Cancer Res.
77:4881–4893. 2017.PubMed/NCBI
|
|
14
|
Ma F, Li W, Liu C, Li W, Yu H, Lei B, Ren
Y, Li Z, Pang D and Qian C: MiR-23a promotes TGF-β1-induced EMT and
tumor metastasis in breast cancer cells by directly targeting CDH1
and activating Wnt/β-catenin signaling. Oncotarget. 8:69538–69550.
2017.PubMed/NCBI
|
|
15
|
Natsuizaka M, Whelan KA, Kagawa S, Tanaka
K, Giroux V, Chandramouleeswaran PM, Long A, Sahu V, Darling DS,
Que J, et al: Interplay between Notch1 and Notch3 promotes EMT and
tumor initiation in squamous cell carcinoma. Nat Commun.
8:17582017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Solomon H, Brosh R, Buganim Y and Rotter
V: Inactivation of the p53 tumor suppressor gene and activation of
the Ras oncogene: Cooperative events in tumorigenesis. Discov Med.
9:448–454. 2010.PubMed/NCBI
|
|
17
|
Rivlin N, Brosh R, Oren M and Rotter V:
Mutations in the p53 tumor suppressor gene: Important milestones at
the various steps of tumorigenesis. Gene Cancer. 2:466–474. 2011.
View Article : Google Scholar
|
|
18
|
Venook AP, Papandreou C, Furuse J and de
Guevara LL: The incidence and epidemiology of hepatocellular
carcinoma: A global and regional perspective. Oncologist. 15 Suppl
4:5–13. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jin H, Li Q, Cao F, Wang SN, Wang RT, Wang
Y, Tan QY, Li CR, Zou H, Wang D and Xu CX: miR-124 inhibits lung
tumorigenesis induced by K-ras mutation and NNK. Mol Ther Nucleic
Acids. 9:145–154. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Furic L, Rong L, Larsson O, Koumakpayi IH,
Yoshida K, Brueschke A, Petroulakis E, Robichaud N, Pollak M,
Gaboury LA, et al: eIF4E phosphorylation promotes tumorigenesis and
is associated with prostate cancer progression. Proc Natl Acad Sci
USA. 107:14134–14139. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhang X, Qiao Y, Wu Q, Chen Y, Zou S, Liu
X, Zhu G, Zhao Y, Chen Y, Yu Y, et al: The essential role of YAP
O-GlcNAcylation in high-glucose-stimulated liver tumorigenesis. Nat
Commun. 8:152802017. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chang L, Li C, Guo T, Wang H, Ma W, Yuan
Y, Liu Q, Ye Q and Liu Z: The human RNA surveillance factor UPF1
regulates tumorigenesis by targeting Smad7 in hepatocellular
carcinoma. J Exp Clin Cancer Res. 35:82016. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Darini C, Wang S, Krishnamoorthy J and
Koromilas A: Translation initiation factor eIF2 serine 51
phosphorylation suppresses HER2-mediated tumorigenesis and
increases sensitivity of HER2+ breast cancer to
trastuzumab therapy. Mol Cancer Res. 16:82–83. 2018.
|
|
24
|
Zhang Y, Yu G, Chu H, Wang X, Xiong L, Cai
G, Liu R, Gao H, Tao B, Li W, et al: Macrophage-associated PGK1
phosphorylation promotes aerobic glycolysis and tumorigenesis. Mol
Cell. 71:201–215.e7. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Han T, Zhan W, Gan M, Liu F, Yu B, Chin YE
and Wang JB: Phosphorylation of glutaminase by PKCε is essential
for its enzymatic activity and critically contributes to
tumorigenesis. Cell Res. 28:655–669. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dai X, Ahn KS, Wang LZ, Kim C,
Deivasigamni A, Arfuso F, Um JY, Kumar AP, Chang YC, Kumar D, et
al: Ascochlorin enhances the sensitivity of doxorubicin leading to
the reversal of epithelial-to-mesenchymal transition in
hepatocellular carcinoma. Mol Cancer Ther. 15:2966–2976. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang WN, Li W, Wang XL, Hu Z, Zhu D, Ding
WC, Liu D, Li KZ, Ma D and Wang H: CLDN1 expression in cervical
cancer cells is related to tumor invasion and metastasis.
Oncotarget. 7:87449–87461. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ross JS, Wang K, Sheehan CE, Boguniewicz
AB, Otto G, Downing SR, Sun J, He J, Curran JA, Ali S, et al:
Relapsed classic E-cadherin (CDH1)-mutated invasive lobular breast
cancer shows a high frequency of HER2 (ERBB2) gene mutations. Clin
Cancer Res. 19:2668–2676. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Balakrishnan S, Bhat FA, Raja Singh P,
Mukherjee S, Elumalai P, Das S, Patra CR and Arunakaran J: Gold
nanoparticle-conjugated quercetin inhibits epithelial-mesenchymal
transition, angiogenesis and invasiveness via EGFR/VEGFR-2-mediated
pathway in breast cancer. Cell Prolif. 49:678–697. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Bokhari AA, Baker TM, Dorjbal B, Waheed S,
Zahn CM, Hamilton CA, Maxwell GL and Syed V: Nestin suppression
attenuates invasive potential of endometrial cancer cells by
downregulating TGF-β signaling pathway. Oncotarget. 7:69733–69748.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Du J, Wang Y, Chen D, Ji G, Ma Q, Liao S,
Zheng Y, Zhang J and Hou Y: BAY61-3606 potentiates the anti-tumor
effects of TRAIL against colon cancer through up-regulating DR4 and
down-regulating NF-κB. Cancer Lett. 383:145–153. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhang H, You Y and Zhu Z: The human RNA
surveillance factor Up-frameshift 1 inhibits hepatic cancer
progression by targeting MRP2/ABCC2. Biomed Pharmacother.
92:365–372. 2017. View Article : Google Scholar : PubMed/NCBI
|