Introduction
Oral cancer refers to the malignant tumors that
occur in the oral cavity, of which 80% are squamous cell carcinomas
including tongue, buccal, gingival, palatal, lip, maxillary and
mandibular, mouth and oropharyngeal cancers, salivary gland
adenocarcinoma and maxillary sinus cancer, as well as cancers that
occur in the facial skin (1,2). The
incidence of oral cancer accounts for ~5% of the incidence of
systemic malignancies (1,2). Overall, 49,670 oral cavity and
pharynx cancer cases were estimated in 2017 in United States, which
accounts for ~3% of the incidence of systemic malignancies
(2). Oral cancer has the
characteristics of rapid progression, extensive invasion and poor
prognosis (1). In early oral
cancer, if there is no cervical lymph node metastasis, the use of
surgery alone or radiation therapy has a good effect (1). In advanced oral cancer, surgical
treatment combined with postoperative radiotherapy would be more
suitable (1). However, the
deficiency is that our understanding of oral cancer is not fully
understood. The complexity of oral cancer made it hard to achieve
significant results in the biological treatment of oral cancer.
Therefore, biomarkers for oral cancer have not yet been fully
investigated.
Kinesin superfamily proteins (KIFs) serve important
roles in intracellular transport system and are essential for
cellular morphology (3–12). KIFs are motors for transport of
mitochondria during cell division (13), and function as a biomacromolecule
transporter, such as protein complexes and mRNAs to microtubules
and hydrolyzing adenosine triphosphatase for energy (14–16).
These proteins are also involved in chromosome condensation and are
essential in maintaining chromosomal integrity during mitosis
(17–23). Kinesin-6 superfamily is a
sub-family of KIFs, which function during cytokinesis, establishing
telophase spindle structure and mobilizing mitotic regulator
proteins (24,25). Kinesin-6 are also involved in
microtubule transport (26).
KIF20B, which is also known as membrane palmitoylated protein 1
(MPP-1) or MPHOSPH1, is a member of the Kinesin-6 family (3,4).
KIF20B was first identified as a plus-end-directed
kinesin-associated protein and was phosphorylated at the G2/M
transition (3,4). KIF20B could also influence
microtubule-binding, microtubule-bundling properties and
microtubules-stimulated adenosine triphosphatase activity in
vitro, and is essential for cytokinesis in cell cycle (5). KIF20B was recently found to serve
important roles in multiple types of cancer, which could function
as cancer-testis antigen specific to human bladder cancer (4,6), and
KIF20B was associated with cell proliferation, apoptosis and tumor
growth in hepatocellular carcinoma targeting p53 (7). Several studies have indicated that
KIF20B serves a role in tumor progression, including colorectal
cancer, hepatocellular carcinoma, pancreatic cancer and bladder
cancer (4,6–10).
In addition, KIF20B predicts poor prognosis, and is associated with
cell proliferation, apoptosis and tumor growth in hepatocellular
carcinoma through targeting p53 (7,10).
However, the function and mechanism of KIF20B in different tumors
has not been fully elucidated, and further discussions are still
needed.
To explore the function and the role of KIF20B in
tongue cancer, a total of 82 patients were recruited and KIF20B
expression levels were investigated by immunohistochemistry. Next,
the clinicopathological features and survival-associated data of
the two groups were analyzed and the results were provided as a
table and by a Kaplan-Meier plot, respectively. In addition, the
function of KIF20B in tongue cancer in vitro and in
vivo was examined. Therefore, through the above results, KIF20B
might be a potential biomarker for tongue cancer and could become a
novel target for biotherapy.
Materials and methods
Specimen collection
A total of 82 surgical tongue cancer specimens from
January 2005 to March 2009 were collected from the Fifth Central
Hospital of Tianjin. Age, sex, tumor stage, and pathologic
diagnosis including lymphatic and venous invasion data were
retrospectively collected. Participants provided written informed
consent, whose preoperative diagnosis was clear, without other
systemic serious diseases, with indications of surgical resection,
with clear pathological diagnosis following surgical resection and
with no serious systemic complications following surgery. No
evidence of tumor metastasis was confirmed by cross-sectional
imaging in all patients. The present study was approved by the
Ethics Committee of the Fifth Central Hospital of Tianjin.
Immunohistochemistry (IHC) and
scoring
IHC staining was performed on paraffin-embedded
tissue sections (4 µm thickness) by using the polymer peroxidase
method, with tongue cancer and adjacent non-cancerous samples fixed
overnight in 20% phosphate buffered formalin (pH 7.4) at room
temperature. After deparaffinization with xylene and rehydration
with an alcohol-water mixture, the sections were treated with 0.3%
hydrogen peroxide in methanol at room temperature for 30 min before
being heat-treated with 10 mM citric acid (pH 6.0) for antigen
retrieval and in order to disrupt endogenous peroxidase activity.
After washing with PBS, the sections were incubated with rabbit
polyclonal anti-KIF20B antibody (cat. no. NBP1-88042; 1:100; Novus
Biologicals, Ltd., Cambridge, UK) at 4°C overnight. After washing
with PBS, slides were treated with horseradish
peroxidase-conjugated goat anti-rabbit secondary antibody (cat. no.
PV-6001; 75 µl; Zhongshan Jinqiao Biotechnology Co., Ltd., Beijing,
China) for 30 min at room temperature. Staining with
diaminobenzidine (cat. no. ZLI-9018; OriGene Technologies, Inc.,
Beijing, China), and then stained with 0.5% hematoxylin for 20 sec
at room temperature.
KIF20B expression scoring was based on the staining
area and staining intensity. Semi-quantitative results were
obtained to judge the percentage of microscopically positive cells
and staining intensity. Five high-power fields (magnification,
×200) were observed per slice, the percentage of positive cells was
counted as follows: If the number of positive cells was <5% this
corresponded to 0 point, if it was 5–25% this corresponded to 1
point, if it was 26–50% this corresponded to 2 points, if it was
51–75% this corresponded to 3 points, and if it was 76–100% this
corresponded to 4 points. Positive staining intensity: 0 for no
color, 1 for light yellow, 2 for brown and 3 for dark brown. The
two scores were multiplied by the positive rating: 0 was negative
(−), 1–4 was weakly positive (+), 5–8 was positive (++), and 9–12
was strongly positive (+++).
Cell culture
CAL-27 and TCA8113 tongue cancer cell lines used in
the present study, were obtained from the American Type Culture
Collection (Manassas, VA, USA). CAL-27 was cultured in Dulbecco's
Modified Eagle's Medium (DMEM; Gibco; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) with 10% fetal bovine serum and TCA8113
cells were cultured in RPMI-1640 (both Gibco; Thermo Fisher
Scientific, Inc.) with 20% fetal bovine serum. The cells were kept
in a 37°C incubator with 5% CO2 and 40–70% humidity.
Cells were regularly monitored using a light microscope, and
subcultured as soon as they reached 80–90% confluency.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) and primers
Total RNA was extracted from cells using TRIzol
reagent (Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. cDNA was synthesized from 2 µg of total
RNA with a random hexamer using the Roche-Transcriptor First Strand
cDNA Synthesis kit (Roche Diagnostics, Basel, Switzerland). These
cDNAs were used for the measurement of gene expression. The
expression of KIF20B was quantified using RT-qPCR using the SYBR
Green PCR kit (Applied Biosystems; Thermo Fisher Scientific, Inc.)
The following thermocycling conditions were used: 95°C for 2 min,
followed by 35 cycles of 95°C for 5 sec, 55°C for 30 sec and 72°C
for 30 sec, and final extension at 72°C for 6 min. GAPDH was used
as the internal reference gene. The primers sequences were: KIF20B
forward, 5′-CCGGGAAAGTAAACTGACTCAC-3′ and reverse,
5′-TTCTAGCTCCTCAACCAAATCCT-3′; GAPDH forward,
5′-CGACCACTTTGTCAAGCTCA-3′ and reverse,
5′-GGTTGAGCACAGGGTACTTTATT-3′. Relative KIF20B gene expression was
calculated using the 2−ΔΔCq method (11).
RNA interference and transfection
A total of four small hairpin (sh)RNAs were
purchased from Vigene Biosciences (Rockville, MA, USA). However,
shRNA 1 (NM_016195) was the most effective one and was therefore
used in subsequent experiments (NM_016195,
AATAAATTTCGATGGCATTAAGC). KIF20B was silenced using the following
specific short hairpin RNA (shRNA) with sequences. A scrambled
sequence was used as a negative control. The shRNAs was synthesized
by ViGene Bioscience (SH836784). Lipofectamine® 3000
(Invitrogen; Thermo Fisher Scientific, Inc.) was used for the shRNA
transfection (500 ng/µl), according to the manufacturer's protocol.
A total of 10×105 cells per well in a 6-well plate were
provided in four groups including: Sh-KIF20B group transfected with
shRNA targeting KIF20B, negative control group transfected with
promiscuous sequences without transfection. Silencing efficiency
was measured by RT-PCR and western blotting after 48 h
transfection.
Western blotting
A total of 50 µg of protein was extracted by protein
lysate buffer (cat. no. M329-10ML; VWR International, Radnor, PA,
USA) from CAL27 or TCA8113 cells, and the concentration of protein
was measured with a bicinchoninic acid protein assay kit (Beyotime
Institute of Biotechnology, Haimen, China). Then the protein was
transferred to nitrocellulose membranes after 10% SDS-PAGE. The
membranes were blocked and incubated with primary antibodies,
including anti-KIF20B antibody (cat. no. NBP1-88042; 1:100; Novus
Biologicals), mouse anti-β-actin (cat. no. 3700; 1:500; CST
Biological Reagents Co., Ltd., Shanghai, China), rabbit anti-Ki67
(1:1,000; cat. no. ab16667; Abcam, Cambridge, UK), mouse
anti-proliferating cell nuclear antigen (PCNA; 1:1,000; cat. no.
2586; CST Biological Reagents Co., Ltd.) monoclonal antibodies
overnight at 4°C, and then incubation with the goat anti-rabbit
(1:10,000; cat. no. 711–1122; Rockland Immunochemicals, Inc.,
Limerick, PA, USA) or goat anti-mouse (1:10,000; cat. no. 110–1103;
Rockland Immunochemicals, Inc., Limerick, PA, USA) secondary
antibodies at 37°C for 1 h. Western blot data were quantified by
densitometric analysis with ImageJ software version 1.8.0 (National
Institutes of Health, Bethesda, MD, USA), and the relative
expression level was normalized by the internal standard
β-actin.
Colony formation assay
Each group of cells in the logarithmic growth phase
was selected and digested with 0.25% trypsin and then splashed into
single cells. The cell suspensions were diluted and each group had
a gradient density of 50, 100, 200 cells per dish and were
inoculated with 10 ml at 37°C culture medium in the dish, and cells
were shaken gently to be evenly dispersed. The cells were incubated
in a 37°C, 5% CO2 and saturated cell culture incubator
for 7 days. When macroscopic colonies appeared in the culture dish,
the culture was terminated. The supernatant was discarded and cells
were carefully washed twice with PBS. Cells were fixed with 4%
paraformaldehyde at room temperature for 15 min. Subsequently, the
fixative was removed, and an appropriate amount of GIMSA staining
solution was used at room temperature for 10–30 min. Subsequently,
cells were washed with running water and left to dry in the air.
Colonies were then counted with a light microscope.
MTT assay for cell viability
Cells were cultured in the aforementioned culture
solutions with 10% fetal bovine serum and 5,000 cells per well were
seeded into 96-well plates. After 3–5 days of culture, 20 µl of MTT
solution was added (5 mg/ml in PBS) to each well for 4 h at 37°C.
The culture supernatant was carefully discarded. Subsequently,
cells were centrifuged 560 × g at 4°C. A total of 150 µl dimethyl
sulfoxide was added to each well and was shaken for 10 min at room
temperature to allow the pellet to fully dissolve. Optical density
was obtained at a wavelength of 490 nm.
In vivo xenograft assay
BALB/c nude mice (age, 5 weeks; female, 20–25 g;
n=10) were provided by Shanghai SLAC Laboratory Animal Co., Ltd.
(Shanghai, China) and mice were housed in pathogen-free animal
facilities at 20–25°C and 40–70% humidity in a 12 h light/dark
cycle, with free access to food and water. A total of
2×106 cells were injected subcutaneously into nude mice
and the tumor volume was measured. Mice (8 per group) were
randomized into two groups and treated with CAL-27 control cells
and CAL-27 KIF20B shRNA cells. After injection, the animals were
housed for three weeks. The volume of the tumor was measured every
three days from the third week using a Vernier caliper. The tumor
volume was calculated as follows: Tumor volume
(mm3)=Tumor length (mm) × Tumor width
(mm)2/2. The present study was approved by the
Laboratory Animal Ethics Committee of the Fifth Central Hospital of
Tianjin (SYXK 2017–0210).
Statistical analysis
The data were analyzed by IBM SPSS version 22.0
software (IBM Corp., Armonk, NY, USA). For immunohistochemistry
experiments, the association between KIF20B expression and
clinicopathological features was assessed using the χ2
test. Association of survival and tumor progression with KIF20B
expression was estimated by the Kaplan-Meier method and the
log-rank test. Data are shown as the mean ± standard deviation.
Student's t-test was used for statistical comparisons. P<0.05
was considered to indicate a statistically significant
difference.
Results
KIF20B is highly expressed in tongue
cancer and is associated with poor clinicopathological
features
KIF20B antibody was used on the cancer tissue and
corresponding paraneoplastic tissue for the immunohistochemistry
experiments, and it was found that there was significantly higher
expression levels of KIF20B in cancer tissues, compared with the
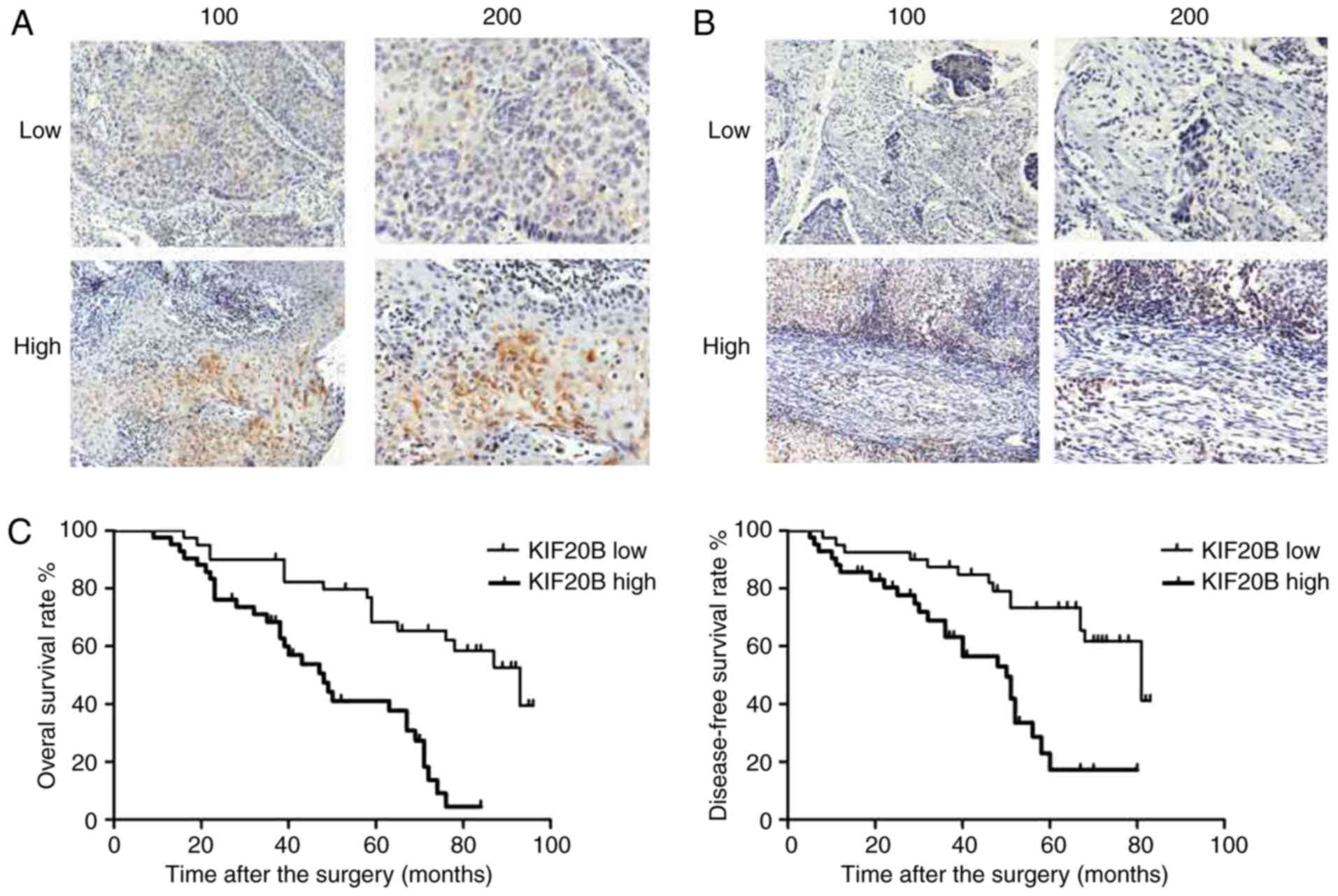
noncancerous ones. Tongue cancer (Fig.
1A) and paired para-carcinoma tissue samples (Fig. 1B) were collected from 82 patients,
and were divided into high and low groups according to the
expression of KIF20B. High KIF20B expression was found in 40/82
cases and low KIF20B was found in 42/82 cancerous tissues. The
results showed that the expression levels of KIF20B were different
between carcinoma and adjacent normal tissues. High KIF20B
expression was found in 25/82 cases and low expression in 57/82
cases. High KIF20B expression was relatively lower in adjacent
normal tissues (high-positive rate, 40/82 vs. 25/82;
χ2=7.293; P<0.05). By analyzing the
clinicopathological data of the two groups, the expression level of
KIF20B was closely associated with clinical stage (P<0.05) and
lymph node metastasis (P<0.05) in tongue cancer, but not with
age, sex, tumor size and cell differentiation (Table I). The differences in tumor
differentiation refers to a comparison of well-differentiated and
moderately-poorly differentiated tumors. Clinical stage refers to
the phase I, II, III and IV comparison, as seen in Table I. From the above results, it seems
that patients with high expression of KIF20B had significantly poor
clinical and pathological features, compared with the
low-expression group.
 | Table I.Association between KIF20B expression
and clinicopathological characteristics in 82 patients with tongue
cancer. |
Table I.
Association between KIF20B expression
and clinicopathological characteristics in 82 patients with tongue
cancer.
|
|
| KIF20B
expression |
|
|
|---|
|
|
|
|
|
|
|---|
| Feature | All n=82 | Low n=40 | High n=42 | χ2 | P-value |
|---|
| Age (years) |
|
|
| 0.597 | 0.440 |
|
<65 | 54 | 28 | 26 |
|
|
|
≥65 | 28 | 12 | 16 |
|
|
| Sex |
|
|
| 1.179 | 0.278 |
|
Male | 46 | 20 | 26 |
|
|
|
Female | 36 | 20 | 16 |
|
|
| Clinical stage |
|
|
| 6.057 | 0.014a |
|
I–II | 30 | 20 | 10 |
|
|
|
III–IV | 52 | 20 | 32 |
|
|
|
Differentiation |
|
|
| 3.241 | 0.072 |
|
Low | 24 | 8 | 16 |
|
|
|
High | 58 | 32 | 26 |
|
|
| Tumor size |
|
|
| 0.532 | 0.466 |
|
<3 | 50 | 26 | 24 |
|
|
| ≥3 | 32 | 14 | 18 |
|
|
| Lymph node
metastasis |
|
|
| 6.129 | 0.013a |
|
Yes | 36 | 12 | 24 |
|
|
| No | 46 | 28 | 18 |
|
|
High KIF20B expression leads to poor
clinical outcomes
Kaplan-Meier survival curve was used to analyze the
clinical outcomes of these two groups of patients. A 5-year overall
survival (OS) and disease-free survival (DFS) was performed with
these 82 patients. As shown in Fig.
1C, postoperative OS and DFS for KIF20B high expression group
were significantly worse than the low expression group (P<0.05).
It can be seen that the KIF20B high expression group was
significantly poorer both in OS and in DFS. These results indicate
that the expression of KIF20B could affect the overall
postoperative survival time and recurrence-free survival time in
patients with tongue cancer, and was closely associated with the
clinical outcomes of tongue cancer.
Knockdown of KIF20B suppresses cancer
cell proliferation
Based on the above findings, the role of KIF20B in
tongue cancer cell lines was investigated. Two tongue cancer cell
lines, CAL-27 and TCA8113 were used for the in vitro
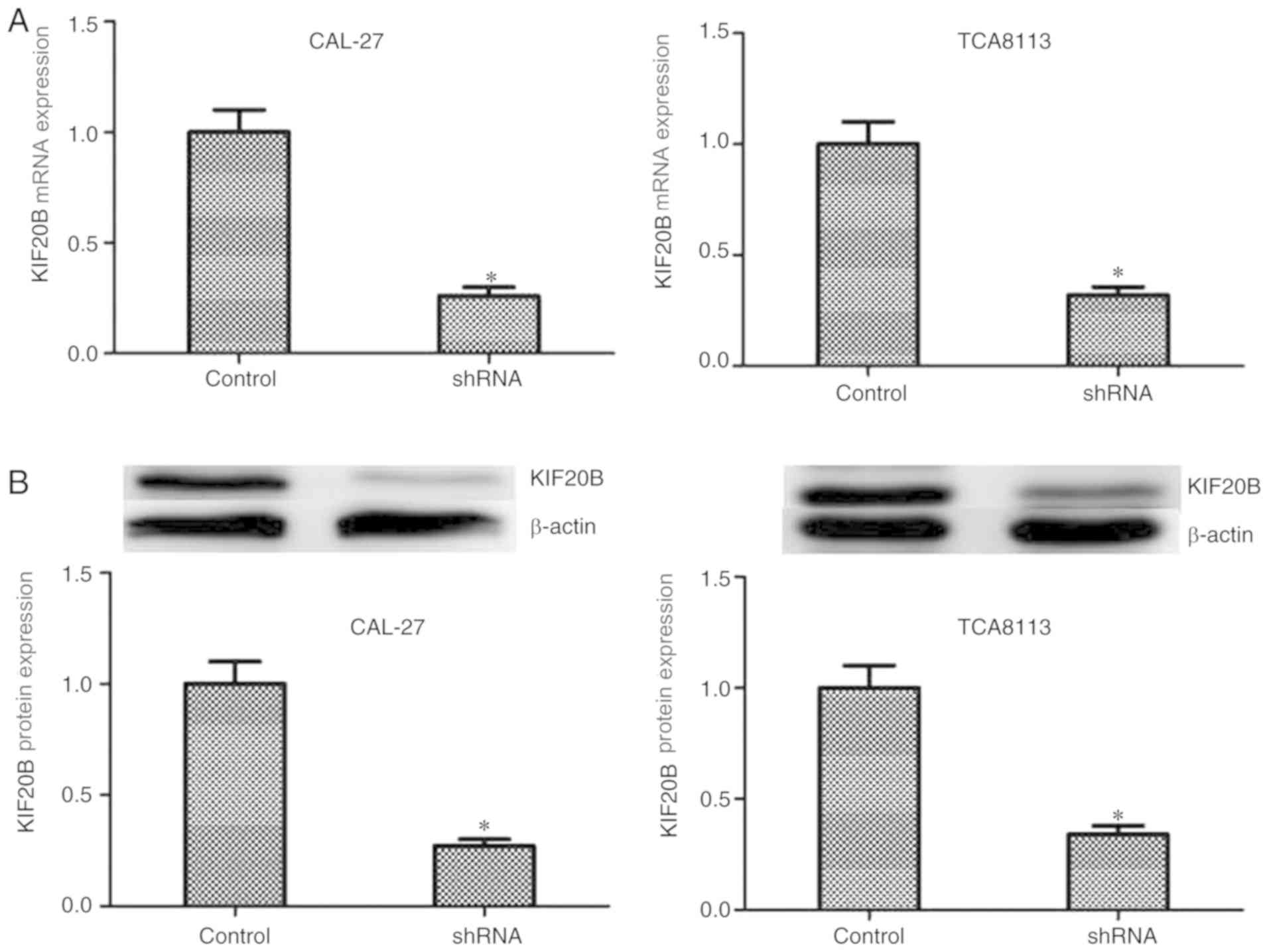
experiment. The expression of KIF20B in both cell lines was
investigated by RT-qPCR and western blotting. KIF20B was knocked
down in both cell lines using shRNA and the knockdown efficiency
was tested by RT-qPCR and western blotting (Fig. 2). The results showed that KIF20B
was expressed in both cell lines and shRNA knocked down the
expression of KIF20B in both cell lines successfully.
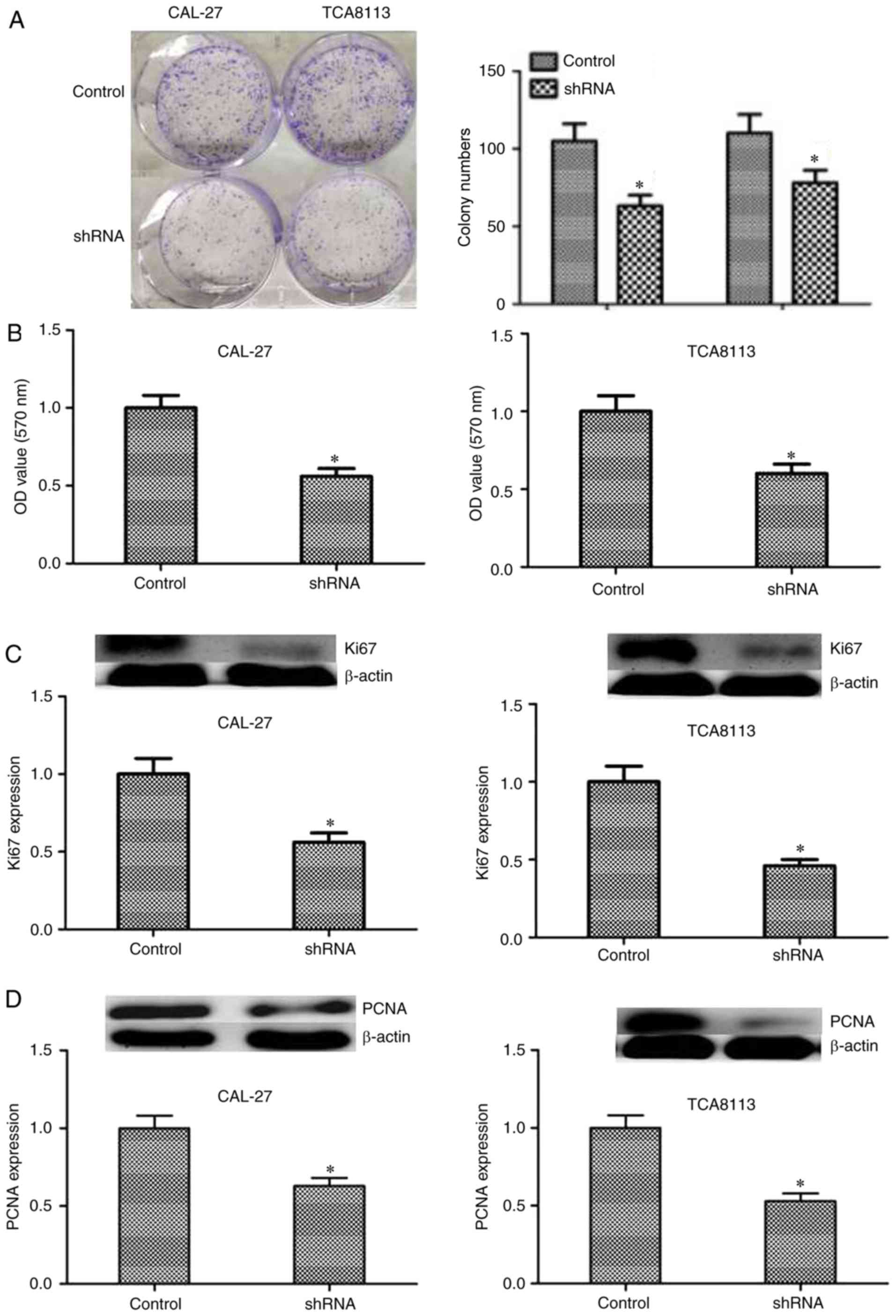
MTT and colony formation assay were used to detect
the impact of KIF20B knockdown in the viability of these two cell
lines. Following knockdown of KIF20B, proliferative ability of
CAL-27 and TCA8113 cells was significantly inhibited (Fig. 3A and B), indicating that the
knockdown of KIF20B might inhibit cell proliferation. Furthermore,
expression levels of proliferation-associated proteins PCNA and
Ki67 were investigated by western blotting (Fig. 3C and D). The results showed that
the expression levels of Ki67 and PCNA were significantly decreased
after knockdown of KIF20B (P<0.05), suggesting that KIF20B may
regulate the proliferation of tongue cancer cells by affecting the
expression of PCNA and Ki67, but this needs to be validated with
further experiments.
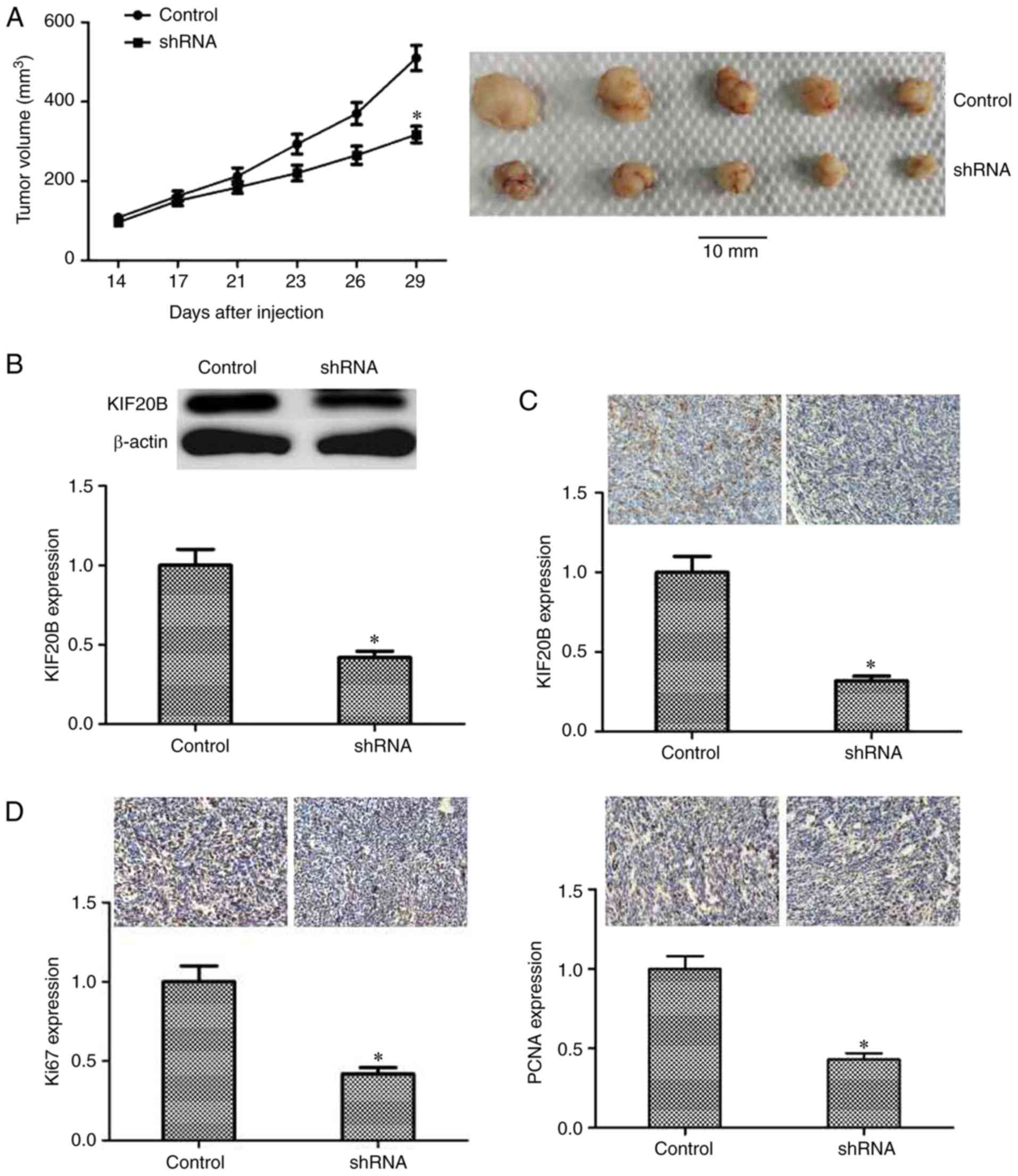
Knocking down KIF20B in vivo results
in smaller tumor
Through the above experiments and analysis, it seems
that knockdown of KIF20B could inhibit the proliferation of tumor
cells in vitro, but it was not known whether it had the
similar effect in vivo. Therefore, a subcutaneous tumor
formation experiment in nude mice was performed to explore the
impact of knocking down KIF20B on tumor formation in vivo.
Nude mice were divided randomly into two groups, one was
subcutaneously implanted with shRNA-KIF20B cells, which lacked
KIF20B expression, and the other group was subcutaneously implanted
with normal control cells. The volume of the tumors were analyzed
after 4 weeks, and the results showed that, the tumor volume of
shRNA group was significantly smaller than that of the control
(P<0.05; Fig. 4A). KIF20B was
indeed significantly knocked down in shRNA group, as detected by
western blotting (Fig. 4B) and
immunohistochemistry (Fig. 4C).
Immunohistochemistry results also showed that Ki67 and PCNA protein
expression levels were significantly decreased after knockdown of
KIF20B (P<0.05; Fig. 4D). The
above results showed that knocking down KIF20B could reduce tumor
growth in vivo.
Discussion
KIF20B was first identified as a plus-end-directed
kinesin- associated protein and is phosphorylated at the G2/M
transition (3). KIF20B could also
influence microtubule-binding, microtubule-bundling properties and
microtubules-stimulated ATPase activity in vitro, and is
essential for cytokinesis in cell cycle (5). KIF20B is reported to be required for
efficient cytokinetic furrowing and timely abscission in human
cells, which could regulate late steps of maturation including
ESCRT-III recruitment and the microtubule constriction sites
formation (27). Due to the
important function of KIF family, abnormal KIFs expression often
leads to a series of cell dysfunction. Overexpression of KIFs might
result in a series of errors including excessive spindle
separation, premature separation of sister chromatids, advanced
leads, and eventually bipolar or unipolar spindles, while lost
expression of KIFs might lead to the opposite result (28). Abnormalities in these functions
might eventually lead to the development of different diseases,
including tumors (3–10). KIF20A was reported to promote the
invasiveness of pancreatic cancer by regulating the transport of
insulin like growth-binding protein 3-containing stress granules
(29–32). Downregulation of KIF20A could cause
genistein-induced mitotic arrest in gastric cancer cells (33), and KIF20A also had a role in breast
cancer (34). Shear variants of
KIF23 can lead to shortened survival in patients with
hepatocellular carcinoma (35),
and high expression of KIF23 is closely associated with the
survival of patients with lung cancer (36). KIF23 could also promote glioma
progression and being regulated by transcription factor 4 (37,38).
As a member of kinesin-6 family, KIF20B functions as cancer-testis
antigen specific to human bladder cancer (4,5),
which is associated with cell proliferation and tumor growth in
hepatocellular carcinoma through targeting p53, serving a crucial
role in cancer (6–10). However, unlike KIF20A, there are
few studies on the function and mechanism of KIF20B in tumors
(4,6–10),
especially in tongue cancer. In the present study, it was found
that KIF20B was significantly overexpressed in tongue cancer
patients. These results were consistent with previous studies in
other types of cancer (4,6–10).
Furthermore, high expression of KIF20B was associated with advanced
tumor stage, lymph node metastasis and poor prognosis, suggesting
that KIF20B may serve an important role in the development process
of tongue cancer. Additionally, consistent with previous reports
demonstrating high KIF20B expression is associated with poor
prognosis in hepatocellular carcinoma (10), the results of the present study
showed that high KIF20B expression may be closely associated with
worse clinical prognosis of tongue cancer.
Most studies targeting KIF20B remained focused on
its normal function (3,5,27).
KIF20B was previously demonstrated to function as an ‘onco-protein’
in lung, hepatocellular, pancreatic, colorectal and bladder cancer
(4,6–10).
However, there is no study of tongue cancer about KIF20B.
Therefore, cell assays were performed using two oral squamous cell
carcinoma cell lines CAL-27 and TCA8113. The expression of KIF20B
was measured in both cell lines and KIF20B was subsequently knocked
down in both cell lines using shRNA. Consistent with previous
studies (4,6–10),
the results of the present study demonstrated that decreased KIF20B
significantly inhibited tumor cell proliferation by mediating
proliferation-associated protein expression, such as PCNA and
Ki67.
By cell experiments, KIF20B is likely to serve an
important role in the proliferation and metastasis of tongue cancer
cells. To further verify if KIF20B has similar effects in
vivo, a subcutaneous tumorigenic experiment was performed in
nude mice using stable knockdown cell lines. The results showed
that knockdown of KIF20B could inhibit tumor volume, thereby
inhibiting tumor progression, in vivo. Perhaps owing to the
small number of the cells and the low malignancy of the tumor,
there was no significant difference between the control and the
shRNA group on day 14, although it seemed that the tumor was a
little smaller in the shRNA group than that in the control group.
And the significant difference between the control and the shRNA
group on the day 29. Based on the above, it seems that KIF20B
serves a very important role both in vivo and in
vitro. Therefore, KIF20B can affect tongue cancer progression,
but this needs to be verified. tongue cancer is a very complex
disease, many factors may lead to its occurrence and development
(1). There are not many reports on
biomarkers for tongue cancer, so this needs to be investigated
further.
KIF20B may be a potential tumor marker for tongue
cancer and its expression levels may be able to reflect the
progression status of patients with tongue cancer, to some extent,
making it a potential factor to predict the prognosis, but this
needs to be validated further.
In conclusion, in the present study, KIF20B was
found to be closely associated with tumor differentiation, lymph
node metastasis and clinical stage through clinical analysis, as
well as with prognostic indicators such as survival and recurrence.
Subsequently, knockdown of KIF20B could inhibit cell proliferation,
in vitro. Additionally, knockdown of KIF20B could reduce
tumor volume, in vivo. KIF20B could promote tongue cancer
progression in vitro and in vivo, therefore KIF20B
might be a potential biomarker for tongue cancer, and might be a
novel target for biotherapy.
Acknowledgements
Not applicable.
Funding
The present study was supported by Tianjin Binhai
new district health planning commission project (Grant no.
2015BWKY003).
Availability of data and materials
The datasets used and/or analyzed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
Z-YL, Z-XW and C-CL conceived and designed the
experiments. Z-YL and Z-XW performed the experiments. C-CL analyzed
the data. Z-YL, Z-XW and C-CL contributed reagents, materials and
analytical tools. Z-YL and C-CL wrote the manuscript.
Ethics approval and consent to
participate
The present study was approved by the Ethics
Committee of the Fifth Central Hospital of Tianjin. Participants
provided written informed consent. The study on animals was
approved by the Laboratory Animal Ethics Committee of the Fifth
Central Hospital of Tianjin (SYXK 2017–0210).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Lingen MW, Kalmar JR, Karrison T and
Speight PM: Critical evaluation of diagnostic aids for the
detection of oral cancer. Oral Oncol. 44:10–22. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2017. CA Cancer J Clin. 67:7–30. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Matsumoto-Taniura N, Pirollet F, Monroe R,
Gerace L and Westendorf JM: Identification of novel M phase
phosphoproteins by expression cloning. Mol Biol Cell. 7:1455–1469.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kanehira M, Katagiri T, Shimo A, Takata R,
Shuin T, Miki T, Fujioka T and Nakamura Y: Oncogenic role of
MPHOSPH1, a cancer-testis antigen specific to human bladder cancer.
Cancer Res. 67:3276–3285. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Abaza A, Soleilhac JM, Westendorf J, Piel
M, Crevel I, Roux A and Pirollet F: M phase phosphoprotein 1 is a
human plus-end-directed kinesin-related protein required for
cytokinesis. J Biol Chem. 278:27844–27852. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Obara W, Ohsawa R, Kanehira M, Takata R,
Tsunoda T, Yoshida K, Takeda K, Katagiri T, Nakamura Y and Fujioka
T: Cancer peptide vaccine therapy developed from oncoantigens
identified through genome-wide expression profile analysis for
bladder cancer. Jpn J Clin Oncol. 42:591–600. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu X, Zhou Y, Liu X, Peng A, Gong H,
Huang L, Ji K, Petersen RB, Zheng L and Huang K: MPHOSPH1: A
potential therapeutic target for hepatocellular carcinoma. Cancer
Res. 74:6623–6634. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lin WF, Lin XL, Fu SW, Yang L, Tang CT,
Gao YJ, Chen HY and Ge ZZ: Pseudopod-associated protein KIF20B
promotes Gli1-induced epithelial-mesenchymal transition modulated
by pseudopodial actin dynamic in human colorectal cancer. Mol
Carcinog. 57:911–925. 2018. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ansari D, Andersson R, Bauden MP,
Andersson B, Connolly JB, Welinder C, Sasor A and Marko-Varga G:
Protein deep sequencing applied to biobank samples from patients
with pancreatic cancer. J Cancer Res Clin Oncol. 141:369–380. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Liu X, Li Y, Zhang X, Liu XY, Peng A, Chen
Y, Meng L, Chen H, Zhang Y, Miao X, et al: Inhibition of kinesin
family member 20B sensitizes hepatocellular carcinoma cell to
microtubule-targeting agents by blocking cytokinesis. Cancer Sci.
109:3450–3460. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Miki H, Okada Y and Hirokawa N: Analysis
of the kinesin superfamily: Insights into structure and function.
Trends Cell Biol. 15:467–476. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Schlisio S, Kenchappa RS, Vredeveld LC,
George RE, Stewart R, Greulich H, Shahriari K, Nguyen NV, Pigny P,
Dahia PL, et al: The kinesin KIF1Bbeta acts downstream from EglN3
to induce apoptosis and is a potential 1p36 tumor suppressor. Genes
Dev. 22:884–893. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hirokawa N: Organelle transport along
microtubules-the role of KIFs. Trends Cell Biol. 6:135–141. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hirokawa N: Kinesin and dynein superfamily
proteins and the mechanism of organelle transport. Science.
279:519–526. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Hirokawa N and Takemura R: Molecular
motors and mechanisms of directional transport in neurons. Nat Rev
Neurosci. 6:201–214. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tipton AR, Wren JD, Daum JR, Siefert JC
and Gorbsky GJ: GTSE1 regulates spindle microtubule dynamics to
control Aurora B kinase and Kif4A chromokinesin on chromosome arms.
J Cell Biol. 216:3117–3132. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Li QR, Yan XM, Guo L, Li J and Zang Y:
AMPK regulates anaphase central spindle length by phosphorylation
of KIF4A. J Mol Cell Biol. 10:2–17. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gu Y, Lu L, Wu L, Chen H, Zhu W and He Y:
Identification of prognostic genes in kidney renal clear cell
carcinoma by RNAseq data analysis. Mol Med Rep. 15:1661–1667. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hu CK, Coughlin M, Field CM and Mitchison
TJ: KIF4 regulates midzone length during cytokinesis. Curr Biol.
21:815–824. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lee YM, Lee S, Lee E, Shin H, Hahn H, Choi
W and Kim W: Human kinesin superfamily member 4 is dominantly
localized in the nuclear matrix and is associated with chromosomes
during mitosis. Biochem J. 360:549–556. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu G and Chen PL: Structural requirements
of chromokinesin Kif4A for its proper function in mitosis. Biochem
Biophys Res Commun. 372:454–458. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wandke C, Barisic M, Sigl R, Rauch V, Wolf
F, Amaro AC, Tan CH, Pereira AJ, Kutay U, Maiato H, et al: Human
chromokinesins promote chromosome congression and spindle
microtubule dynamics during mitosis. J Cell Biol. 198:847–863.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Adams RR, Tavares AA, Salzberg A, Bellen
HJ and Glover DM: Pavarotti encodes a kinesin-like protein required
to organize the central spindle and contractile ring for
cytokinesis. Genes Dev. 12:1483–1494. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hill E, Clarke M and Barr FA: The
Rab6-binding kinesin, Rab6-KIFL, is required for cytokinesis. EMBO
J. 19:5711–5719. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Nislow C, Lombillo VA, Kuriyama R and
McIntosh JR: A plus-end-directed motor enzyme that moves
antiparallel microtubules in vitro localizes to the interzone of
mitotic spindles. Nature. 359:543–547. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Janisch KM, McNeely KC, Dardick JM, Lim SH
and Dwyer ND: Kinesin-6 KIF20B is required for efficient
cytokinetic furrowing and timely abscission in human cells. Mol
Biol Cell. 29:166–179. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lucanus AJ and Yip GW: Kinesin
superfamily: Roles in breast cancer, patient prognosis and
therapeutics. Oncogene. 37:833–838. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Taniuchi K, Furihata M and Saibara T:
KIF20A-mediated RNA granule transport system promotes the
invasiveness of pancreatic cancercells. Neoplasia. 16:1082–1093.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stangel D, Erkan M, Buchholz M, Gress T,
Michalski C, Raulefs S, Friess H and Kleeff J: Kif20a inhibition
reduces migration and invasion of pancreatic cancer cells. J Surg
Res. 197:91–100. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Taniuchi K, Nakagawa H, Nakamura T, Eguchi
H, Ohigashi H, Ishikawa O, Katagiri T and Nakamura Y:
Down-regulation of RAB6KIFL/KIF20A, a kinesin involved with
membrane trafficking of discs large homologue 5, can attenuate
growth of pancreatic cancer cell. Cancer Res. 65:105–112.
2005.PubMed/NCBI
|
|
32
|
Asahara S, Takeda K, Yamao K, Maguchi H
and Yamaue H: Phase I/II clinical trial using HLA-A24-restricted
peptide vaccine derived from KIF20A for patients with advanced
pancreatic cancer. J Transl Med. 11:2912013. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Yan GR, Zou FY, Dang BL, Zhang Y, Yu G,
Liu X and He QY: Genistein-induced mitotic arrest of gastric cancer
cells by downregulating KIF20A, a proteomics study. Proteomics.
12:2391–2399. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Khongkow P, Gomes AR, Gong C, Man EP,
Tsang JW, Zhao F, Monteiro LJ, Coombes RC, Medema RH, Khoo US and
Lam EW: Paclitaxel targets FOXM1 to regulate KIF20A in mitotic
catastrophe and breast cancer paclitaxel resistance. Oncogene.
35:990–1002. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sun X, Jin Z, Song X, Wang J, Li Y, Qian
X, zhang Y and Yin Y: Evaluation of KIF23 variant 1 expression and
relevance as a novel prognostic factor in patients with
hepatocellular carcinoma. BMC Cancer. 15:9612015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kato T, Wada H, Patel P, Hu HP, Lee D,
Ujiie H, Hirohashi K, Nakajima T, Sato M, Kaji M, et al:
Overexpression of KIF23 predicts clinical outcome in primary lung
cancer patients. Lung Cancer. 92:53–61. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Takahashi S, Fusaki N, Ohta S, Iwahori Y,
Iizuka Y, Inagawa K, Kawakami Y, Yoshida K and Toda M:
Downregulation of KIF23 suppresses glioma proliferation. J
Neurooncol. 106:519–529. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Sun L, Zhang C, Yang Z, Wu Y, Wang H, Bao
Z and Jiang T: KIF23 is an independent prognostic biomarker in
glioma, transcriptionally regulated by TCF-4. Oncotarget.
7:24646–24655. 2016.PubMed/NCBI
|