Introduction
Intraocular neovascularization is an important
clinical manifestation which is the pathological basis for numerous
ocular disorders, such as proliferative diabetic retinopathy,
ischemic central retinal vein occlusion and retinopathy of
prematurity (1–3). No effective treatment for retinal
neovascularization (RNV) is available to date. Recent studies have
shown that retinal angiogenesis may due to complex interactions
among multiple angiogenic stimulators (4,5).
β-elemene, isolated from the Chinese medicinal herb
Zedoary (Curcuma zedoaria) exhibits substantial clinical
activity against various cancers in vitro and in vivo
(6–8). β-elemene reduces the proliferation of
blood vessel endothelial cells during tumor progression by
inhibiting the expression of angiogenic factors, such as vascular
endothelial growth factor (VEGF) and Notch-1 (9,10).
The previously reported effects of β-elemene in vascularization
prompted us to examine the therapeutic effects of β-elemene on RNV.
The present study investigated the suppressive effects of β-elemene
on RNV in a mouse model of oxygen-induced retinopathy (OIR). The
results showed that β-elemene inhibited RNV in OIR mice and
downregulated the expression of VEGF in the retina.
It is well known that VEGF is an important factor
that stimulates endothelial cell proliferation and tube formation,
and also mediates oxygen-induced RNV (11,12).
Therefore, understanding the abnormal regulation of VEGF may
contribute to the identification of effective RNV therapies. In
order to elucidate the underlying mechanisms of β-elemene-mediated
inhibition of VEGF expression, the present research focused on
microRNAs (miRNAs/miRs). Previous studies have shown a series of
miRNAs, including miR-9, miR-410 and miR-146a, inhibit retinal
neovascularization by targeting VEGF (13–15).
In the present study, miRNA microarrays were used to
screen the miRNAs with different expression levels between the OIR
and β-elemene-treated groups. Prediction websites TargetScan and
miRanda, as well as luciferase assays, were used to demonstrate
that miR-27a directly bound to the 3′-untranslated region (UTR) of
VEGF and inhibited its expression, suggesting that VEGF was a
direct target of miR-27a. In addition, it was demonstrated that
β-elemene upregulated miR-27a expression in vivo and in
vitro. Furthermore, miR-27a inhibitor transfection eliminated
the effects of β-elemene on RNV, suggesting that β-elemene reduced
RNV via miR-27a.
Taken together, it was concluded that β-elemene
inhibited RNV in OIR mouse models, by reducing VEGF expression via
miR-27a upregulation. This novel finding may encourage further
exploration of the role of β-elemene in the pathogenesis of these
diseases.
Materials and methods
Animals
Animal experiments were conducted following the
guidelines of the Animal Experiment Committee of The First
Affiliated Hospital of China Medical University, and the study was
approved by the ethics committee of The First Affiliated Hospital
of China Medical University (Shenyang, China). Mice were maintained
in a 12 h light/dark cycle at 23–25°C with 50–60% humidity.
C57BL/6J neonatal mice (23–28 g; routinely fed water and food) were
used to establish OIR models on postnatal day 7 (P7), according to
the Smith's method (16). A total
of 80 mice (37 male and 43 female) were randomly divided into 4
groups: i) Normoxia; ii) OIR; iii) OIR control; and iv) OIR
treated. Mice in the normoxia group were maintained under normoxic
conditions (oxygen concentration, 15±2%) for 17 days. The mice in
the OIR group were exposed to 75±5% oxygen for 5 days and returned
to a normal oxygen environment on P12. The OIR control and OIR
treated groups were intravitreally injected with 1 µl lipid
emulsion (0.25 mg/ml; Dalian Yuanda Pharmaceutical Co., Ltd.,
Dalian, China) and β-elemene (0.25 mg/ml, Dalian Yuanda
Pharmaceutical Co., Ltd.), respectively, on P12 and subsequently
returned to a normal oxygen environment for 5 days (P12-P17). Rats
were anesthetized with chloral hydrate (430 mg/kg; 4.3%) prior to
intravitreal injection.
Observation of RNV
On P17, all mice were sacrificed and the eyes were
harvested. The cornea, lens and vitreous humor were removed, and
the retinas were surgically dissected. The retinas were fixed with
4% paraformaldehyde for 8 h at 37°C, and retinal sections (1 mm)
were prepared for adenosine diphosphatase (ADPase) staining (10%
ammonium sulfide) and flat-mounted on microscope slides with a
gelatin-coated coverslip. Each retina was divided into 12 equal
segments and observed under an optical microscope (×200; Olympus
Corporation, Tokyo, Japan) and the proportion of neovascularization
areas were counted, as described previously (17). Three independent reviewers were
blinded to grouping to assess the severity of RNV.
Quantification of RNV
The mice were sacrificed, and the eyes were
enucleated, immersed in 40 g/l paraformaldehyde in PBS for at least
24 h at 37°C and embedded in paraffin. Serial 6-µm sections from
all eyes were cut sagittally, parallel to the optic nerve and
stained with hematoxylin and eosin (H&E) for 3 min at 37°C. The
preretinal neovascular cell nuclei, identified under a light
microscope (×200), were considered to be associated with new
vessels if they were found on the vitreal side of the internal
limiting membrane (ILM) (18). The
preretinal neovascular cells were counted to quantitatively assess
RNV (11,19). All retinas were examined in five
serial sections. Three independent reviewers were blinded to
grouping when counting the cells.
RNA preparation and reverse
transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from retinal tissues by
TRIzol reagent (Invitrogen; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) following the manufacturer's protocol. RT-qPCR
was performed using SYBR Premix Ex Taq (Takara Bio, Inc., Otsu,
Japan) on a Thermal Cycler Dice™ Real Time system (Takara Bio,
Inc.) using the following thermocycling conditions: 30 sec at 95°C
followed by two-step PCR for 40 cycles of 95°C for 5 sec and 64°C
for 30 sec. The primer sequences were as follows: VEGF forward,
5′-CAACTTCTGGGCTCTTCTCG-3′; VEGF reverse,
5′-CCTCTCCTCTTCCTTCTCTTCC-3′; miR-27a forward,
5′-CAACTTCTGGGCTCTTCTCG-3′; miR-27a reverse,
5′-GTCAGCGGACTCTGGATTCAG-3′; GAPDH forward,
5′-ATAGCACAGCCTGGATAGCAACGTAC-3′; GAPDH reverse,
5′-CACCTTCTACAATGAGCTGCGTGTG-3′; U6B forward,
5′-CTCGCTTCGGCAGCACATATACT-3′; U6B reverse,
5′-ACGCTTCACGAATTTGCGTGTC-3′. GADPH was used as a reference control
of VEGF. U6B was used as a reference control of miR-27a. All
primers were purchased from Sangon Biotech Co., Ltd. (Shanghai,
China). Relative gene expression data was analyzed using the
2-ΔΔCq method (20).
Immunohistochemistry (IHC)
Retinas were fixed in 4% paraformaldehyde for 48 h
at 37°C, embedded in paraffin and cut into 4-µm sections. Following
general deparaffinization, antigen retrieval was performed for 1
min with an autoclave using 0.01 mol/l sodium citrate buffer.
H2O2 (0.3%) was used to block endogenous
peroxidase activity. Goat serum (6%; Maixin-Bio, Fuzhou, China) was
used to block the nonspecific immunoglobulin-binding sites for 20
min at 37°C. The slices were incubated overnight with anti-VEGF
(1:200; cat. no. 1402390; Sigma-Aldrich, MO, USA) at 4°C, rinsed
with PBS and incubated with goat anti-rabbit immunoglobulin G
secondary antibody (1:200; Sigma-Aldrich; Merck KGaA, Darmstadt,
Germany) for 30 min at 37°C The peroxidase reaction was developed
with 3,3′-diaminobenzidine tetrahydrochloride (DAB). The sections
were counterstained with Mayer's hematoxylin for 1 min at 37°C,
dehydrated, cleared in xylene and mounted in Permount medium
(Thermo Fisher Scientific, Inc.).
In situ hybridization (ISH)
The ISH kit was purchased from Wuhan Boster
Biological Technology, Ltd. (Wuhan, China) and used according to
the manufacturer's protocol. In brief, tissue slides were
hybridized with 20 µl 5-digoxigenin LNA-modified-miR-27a-3p
(synthesized by Wuhan Boster Biological Technology, Ltd.). The
sequence was 5′-TTCAGCCCCATGTTTGCCTC-3′. The peroxidase reaction
was developed with DAB.
Quantitative assessment and
scoring
The expression of VEGF and miR-27a detected in the
immunohistochemical and ISH analysis was quantitatively assessed
using Image-Pro Plus Software 6.0 (Media Cybernetics, Inc.,
Rockville, MD, USA). The integrated optical density of retina
sections was calculated by two investigators in a double blinded
manner.
Western blot analysis
A total protein extraction kit (Beijing Solarbio
Science & Technology, Co., Ltd., Beijing, China) was used to
extract protein according to the instructions. Ultraviolet
absorption was the protein determination method. Proteins (40
µg/lane) were separated by 10% SDS-PAGE and transferred onto
polyvinylidene difluoride membranes. Next, 5% skimmed milk solution
in Tris-buffered saline with Tween 20 (0.05%) buffer was used to
block the membranes, which were then incubated with VEGF (1:2,000;
cat. no. 1402390; Sigma-Aldrich; Merck KGaA), platelet-derived
endothelial cell growth factor (PD-ECGF; 1:2,000; Abcam; Cambridge,
UK), transforming growth factor β (TGF-β; cat. no. ab186838;
1:1,500; Abcam), tumor necrosis factor α (TNF-α; cat. no. ab6671;
1:2,000; Abcam) and GADPH (cat. no. 60004-1-Ig; 1:2,000;
ProteinTech Group, Inc. Chicago, IL, USA), antibodies overnight at
4°C. Next, membranes were incubated with goat anti-rabbit IgG
secondary antibody (cat. no. TA130015; OriGene Technologies, Inc.,
Beijing, China) for 2 h at room temperature. Proteins were
visualized using an enhanced chemiluminescence system (Merck
KGaA).
miRNA PCR array
Total RNA was extracted from the retinal tissues of
the normoxia, OIR, and OIR-treated groups using TRIzol®
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and cleaned
with the RNeasy_MinElute Cleanup kit (Qiagen GmbH, Hilden,
Germany). SuperScript III Reverse Transcriptase (Qiagen GmbH) was
used to reverse transcribe the total RNA, and cDNA was amplified by
polymerase chain reaction using the 2_Super Array PCR Master mix
(Qiagen GmbH). The MicroRNA PCR array (SuperArray Bioscience
Corporation, Frederick, MD, USA) was performed in a Thermal Cycler
Dice Real-Time system (Takara Bio, Inc.) according to the
manufacturer's protocol. The results were normalized to U6B levels
using the 2−ΔΔCq method (21).
Luciferase assays
Cells (293T, Procell Life Technology Co., Ltd.,
Wuhan, China) were plated into 24-well plates at 80% confluence 24
h before transfection. The wild-type VEGF-3′UTR (WT) and mutant
VEGF-3′UTR (MUT) containing the binding site of miR-27a-3p were
established and cloned into a Firefly luciferase-expressing vector
(Obio Technology Corp., Ltd., Shanghai, China). A mixture of 200 ng
WT or MUT, 700 ng pGV214-miR-27a and 100 ng Renilla
luciferase plasmid were transfected into 293T cells using
Lipofectamine® 2000. A dual-luciferase reporter system
(Promega Corporation, Madison, WI, USA) was used to evaluate
Firefly and Renilla luciferase activity 36 h after
transfection.
In vitro cell culture
Mouse retinal microvascular endothelial cells (cat.
no. CM-2098) were obtained from Cmbio company (Shanghai, China,
www.biomart.cn/infosupply/63235021.htm). The cells
were cultured in Endothelial Cell Medium (Beijing Solarbio Science
& Technology, Co., Ltd.) with 10% fetal bovine serum (Absin
Bioscience, Inc., Shanghai, China) and 100 units/ml of penicillin
and streptomycin (Hyclone; GE Healthcare Life Sciences, Logan, UT,
USA) in an incubator with 5% CO2 at 37°C. Cells in
passage two were used for subsequent experiments.
Oligonucleotide transfection
miR-27a mimic, inhibitor and miR-27a control
oligonucleotides were purchased from Shanghai GenePharma Co., Ltd.
(Shanghai, China). Transfection was performed using
Lipofectamine® 2000 (Invitrogen; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. The
oligonucleotide sequences were as follows: miR-27a mimic, sense
5′-UAACUACAUGGUUAACCUCUUU-3′ and antisense
5′-AGAGGUUAACCAUGUAUUGUAA-3′; miR-27a inhibitor, sense
5′-AGGGCTTAGCTGCTTGTGAGCA-3′ and antisense
5′-TTCACAGTGGCTAAGTTCCGC-3′; negative control, sense
5′-AACUCCGAACGUGUCACGUGT-3′ and antisense
5′-AUGUGACACGUUCGGAGAAGT-3′. Mice in the OIR treated group were
administered injections of 1 µl miR-27a inhibitor or miR-27a
control with a 33-gauge needle attached to a Hamilton syringe on
P11. On P12, mice were injected with 1 µl β-elemene, and
subsequently returned to a normal oxygen environment for 6 days
(P12-P17).
Cell Counting Kit-8 (CCK-8) assay
Treated cells (2,000/well) were plated in a 96-well
plate and incubated for 24, 48, 72 and 96 h. Following each time
point, 10 µl CCK8 solution (Beijing Solarbio Science &
Technology, Co., Ltd.) was added to each well and incubated for
additional 1 h at 37°C. The results were quantified
spectrophotometrically at a wavelength of 450 nm.
Statistical analysis
SPSS version 17.0 (SPSS, Inc., Chicago, IL, USA) was
applied to complete data processing. All data were presented as the
mean ± standard deviation. Statistical significance was evaluated
by one-way analysis of variance with the Least Significant
Difference test for post-hoc analysis. P<0.05 was considered to
indicate a statistically significant difference.
Results
Quantification of RNV
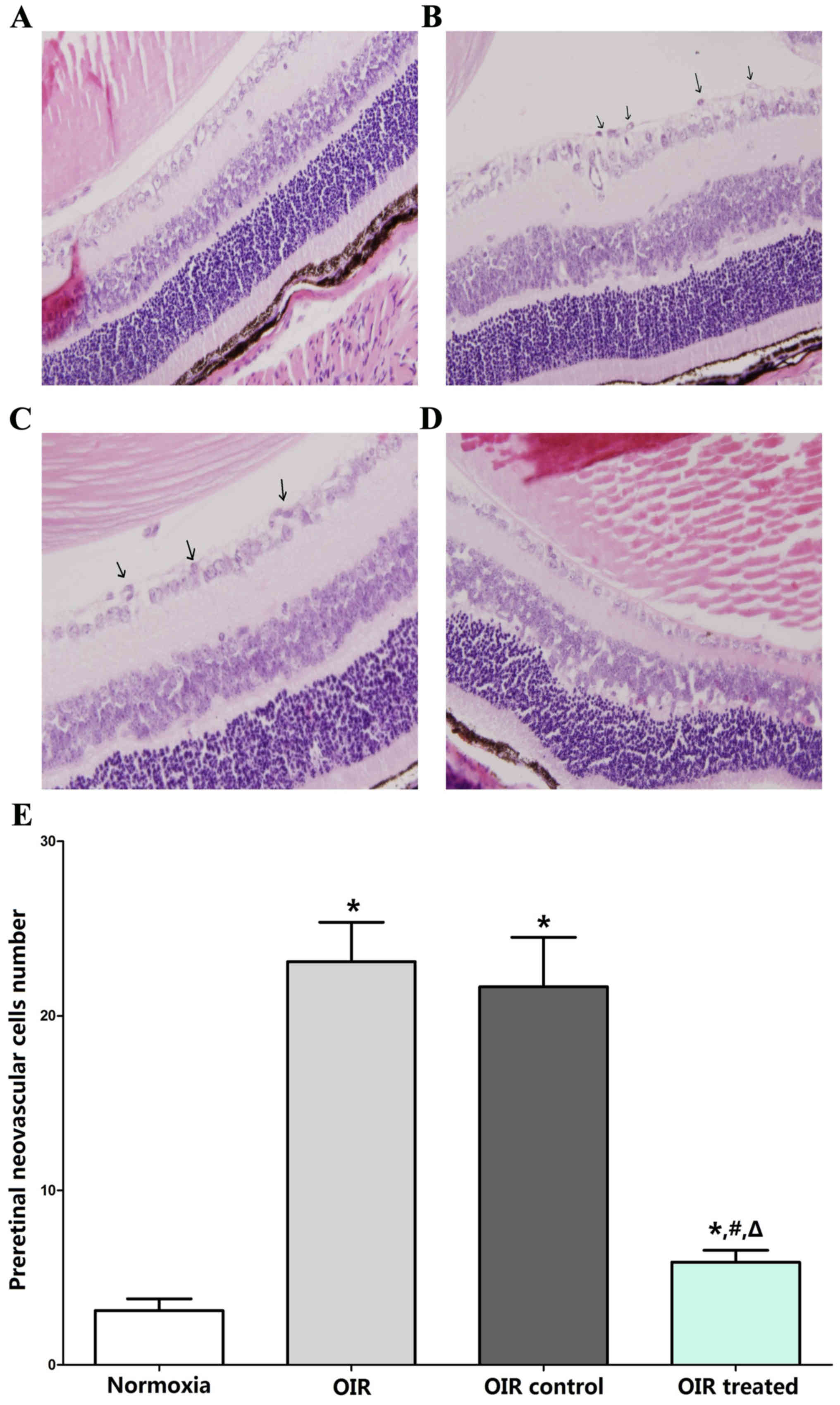
The results of H&E-stained retinal sections
showed an average of 3.11±2.03 nuclei per cross-section in the
normoxia group (Fig. 1A), compared
with 23.11±6.75 and 21.67±8.5 in the OIR (Fig. 1B) and OIR control (Fig. 1C) groups, respectively (P<0.05).
Furthermore, the average number of preretinal neovascular cells in
the OIR-treated group (5.89±2.03; Fig.
1D) decreased significantly compared with the OIR and OIR
control groups (P<0.05), confirming the anti-neovascularization
effects of β-elemene on RNV (Fig.
1E).
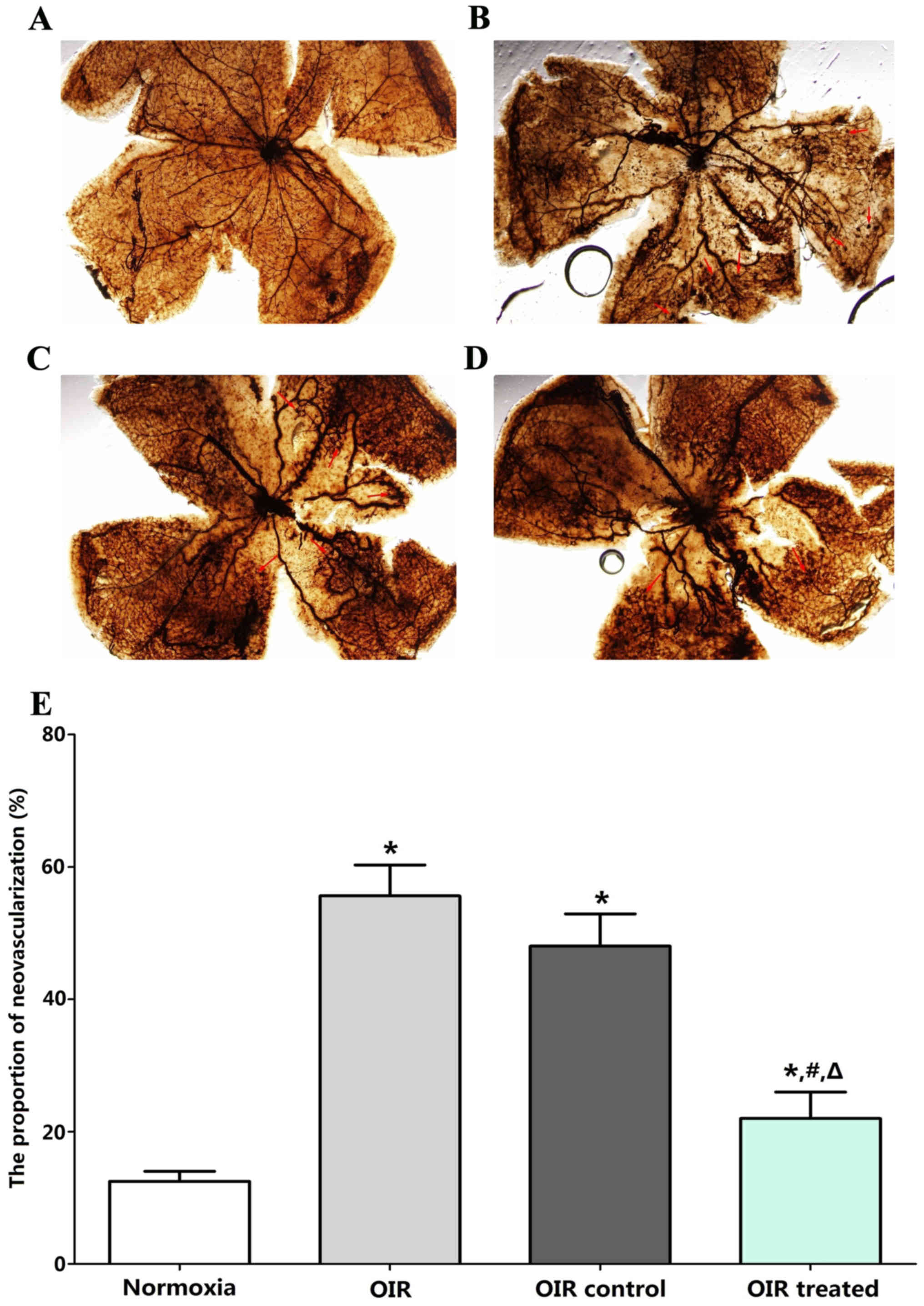
Qualitative analysis of RNV
Retinal vasculature was examined in the normoxia,
OIR, OIR control, and OIR treated groups by ADPase staining in
retinal flat mounts at P17. No abnormal blood vessels were observed
in the retinas of the normoxia group (Fig. 2A). Two layers of retinal vessels
were evenly distributed in the retina, the superficial blood
vessels were well formed, and the deep blood vessel formed a
polygon mesh pattern. The blood vessels in OIR (Fig. 2B) and OIR control groups (Fig. 2C) showed non-perfusion areas and
neovascularization. The ratio of new blood vessel area to total
retinal area was higher in the OIR treated (23±6%), OIR (56±8%),
and OIR control groups (47±10%) than in the normoxia group (12±4%;
all P<0.05; Fig. 2D and E). By
contrast, retinas in the OIR treated group (23±6%) developed less
severe neovascular tufts and regions of non-perfusion, compared
with the OIR (56±8%, P<0.05) and OIR control groups (47±10%,
P<0.05), which demonstrated a strong inhibitory effect of
β-elemene on RNV in the OIR treated group. No significant
difference was detected between the OIR and OIR control groups
(P>0.05).
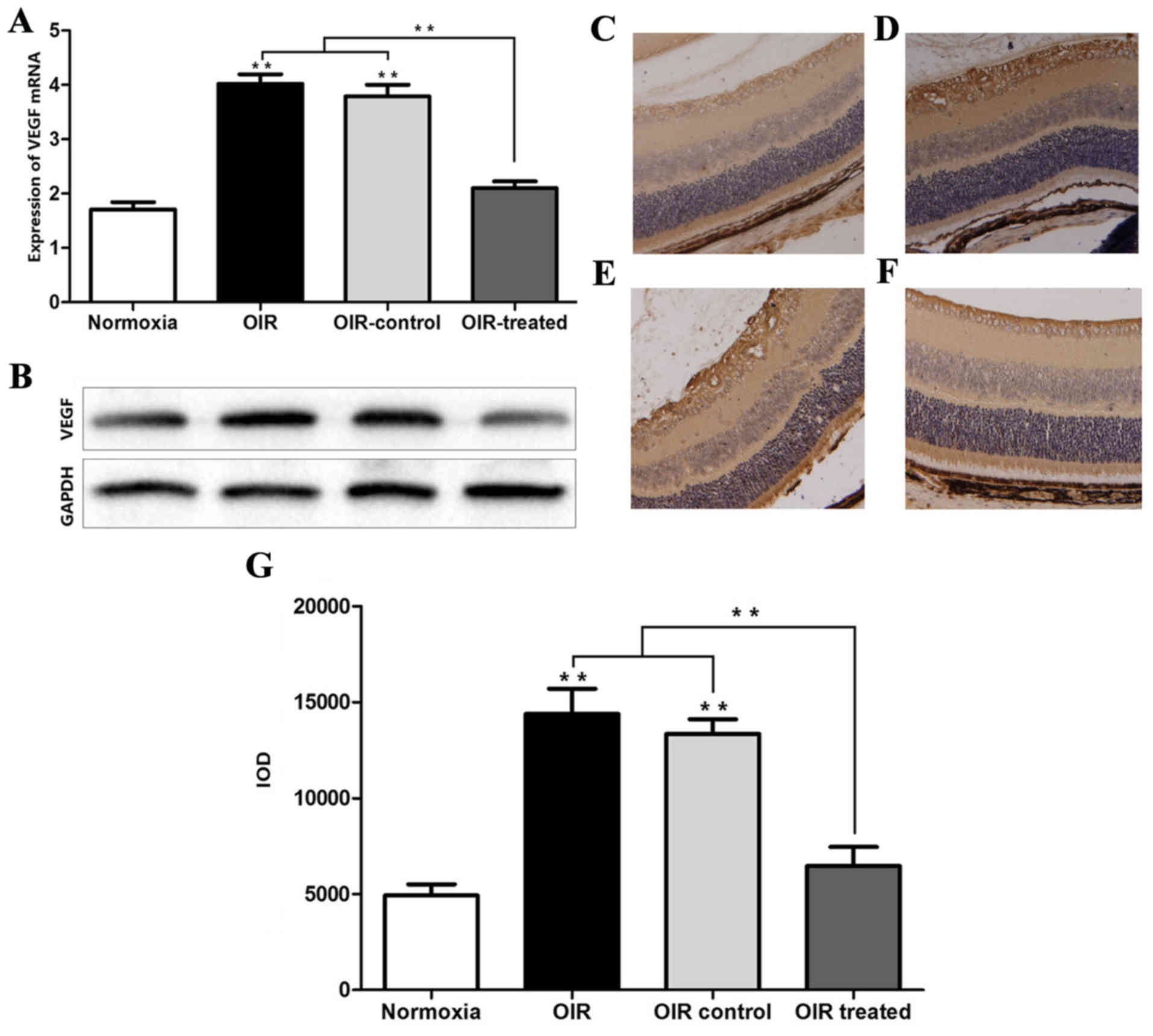
β-elemene inhibits VEGF
expression
The results of RT-qPCR and western blot analysis
showed that the expression levels of VEGF mRNA and protein in the
normoxia and OIR treated groups were low, compared with the OIR and
OIR control groups (Fig. 3A and
B). Consistently, the IHC results showed that VEGF expression
was higher in the OIR and OIR control groups, compared with the
normoxia and OIR-treated groups (Fig.
3C-G). In addition, the effect of β-elemene treatment on the
expression of other angiogenic factors, including PD-ECGF, TGF and
TNF was detected, and it was found that β-elemene had no effect
(data not shown). The aforementioned experimental results indicated
that high oxygen concentration upregulated VEGF expression to
promote retinal angiogenesis, and β-elemene suppressed this
effect.
miRNA microarray analysis
miRNA microarrays were used to screen out
differentially expressed miRNAs between the OIR and
β-elemene-treated groups, in order to examine the mechanisms
underlying the inhibitory effect of β-elemene on VEGF expression to
reduce RNV (Fig. 4A). The results
showed that the following miRNAs were upregulated in the
OIR-treated group and downregulated in the OIR group (Fig. 4B): miR-22 (3.17-fold), miR-181a-1
(21.36-fold), miR-335-5p (3.60-fold), miR-669n (2.99-fold),
miR-190b (3.68-fold), miR-27a (3.99-fold), and miR-93
(3.31-fold).
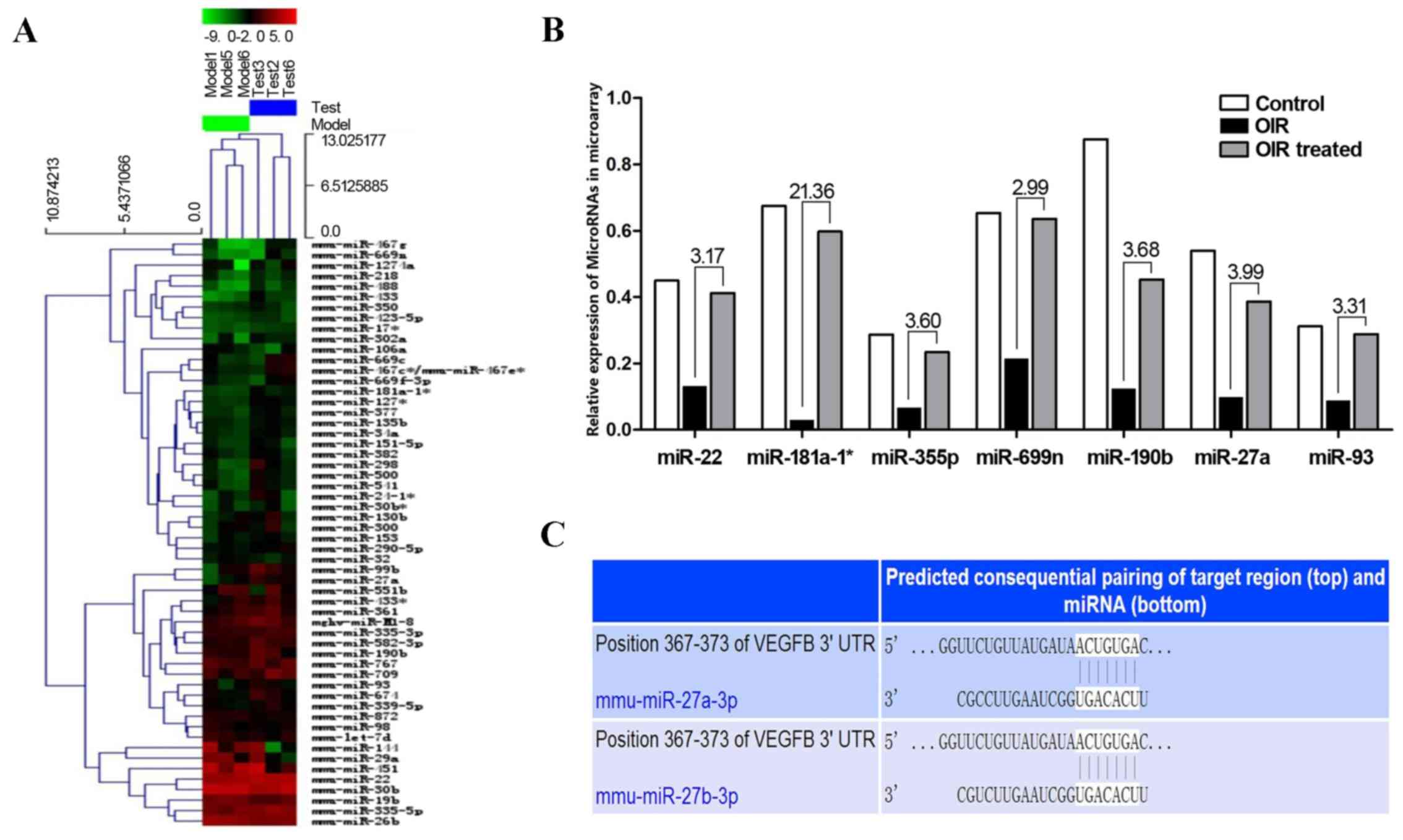 | Figure 4.Differentially expressed miRNAs in the
OIR and OIR treated groups. (A) Heat map of the miRNA microarray.
(B) miR-22, miR-181a-1, miR-335-5p, miR-669n, miR-190b, miR-27a,
miR-93 were upregulated in the OIR treated group and downregulated
in the OIR group. The numbers above the histogram indicate the
ratio of miRNA expression (OIR treated:OIR). (C) TargetScan and
miRanda predicted that VEGF contained one potential miR-27a binding
site in its 3′-UTR. miRNA/miR, microRNA; OIR, oxygen-induced
retinopathy; VEGF, vascular endothelial growth factor; UTR,
untranslated region. |
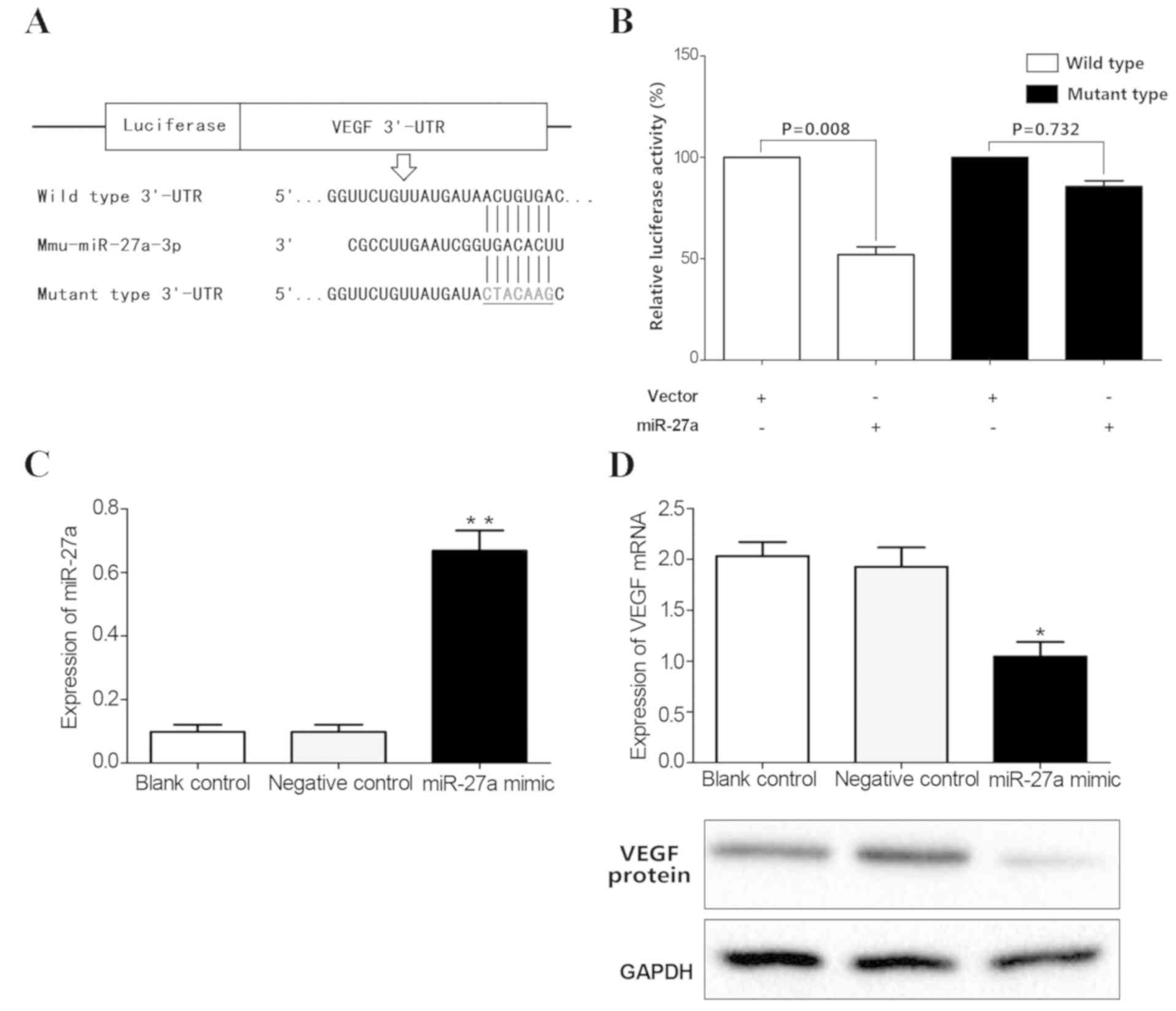
VEGF is a target gene of miR-27a
The potential target genes of the above miRNAs were
detected according to prediction websites TargetScan and miRanda.
The 3′-UTR of VEGF was found to contain one potential
miR-27a-binding site (Fig. 4C).
Next, luciferase assays were performed to test whether miR-27a
directly bound to VEGF. The vectors containing the wild-type and
mutant 3′-UTR of VEGF fused downstream of the Firefly luciferase
gene are shown in Fig. 5A. The
results indicated that miR-27a decreased the relative luciferase
activity of the wild-type VEGF 3′-UTR (45%), compared with the
mutant 3′-UTR (Fig. 5B),
suggesting that VEGF may be one of the target genes of miR-27a.
miR-27a mimics were subsequently transfected into mouse retinal
microvascular endothelial cells to upregulate miR-27a expression
(Fig. 5C). The results showed
overexpression of miR-27a decreased the expression of VEGF at both
the mRNA and protein level (P<0.05; Fig. 5D). Taken together, these results
confirmed that VEGF was a direct target of miR-27a.
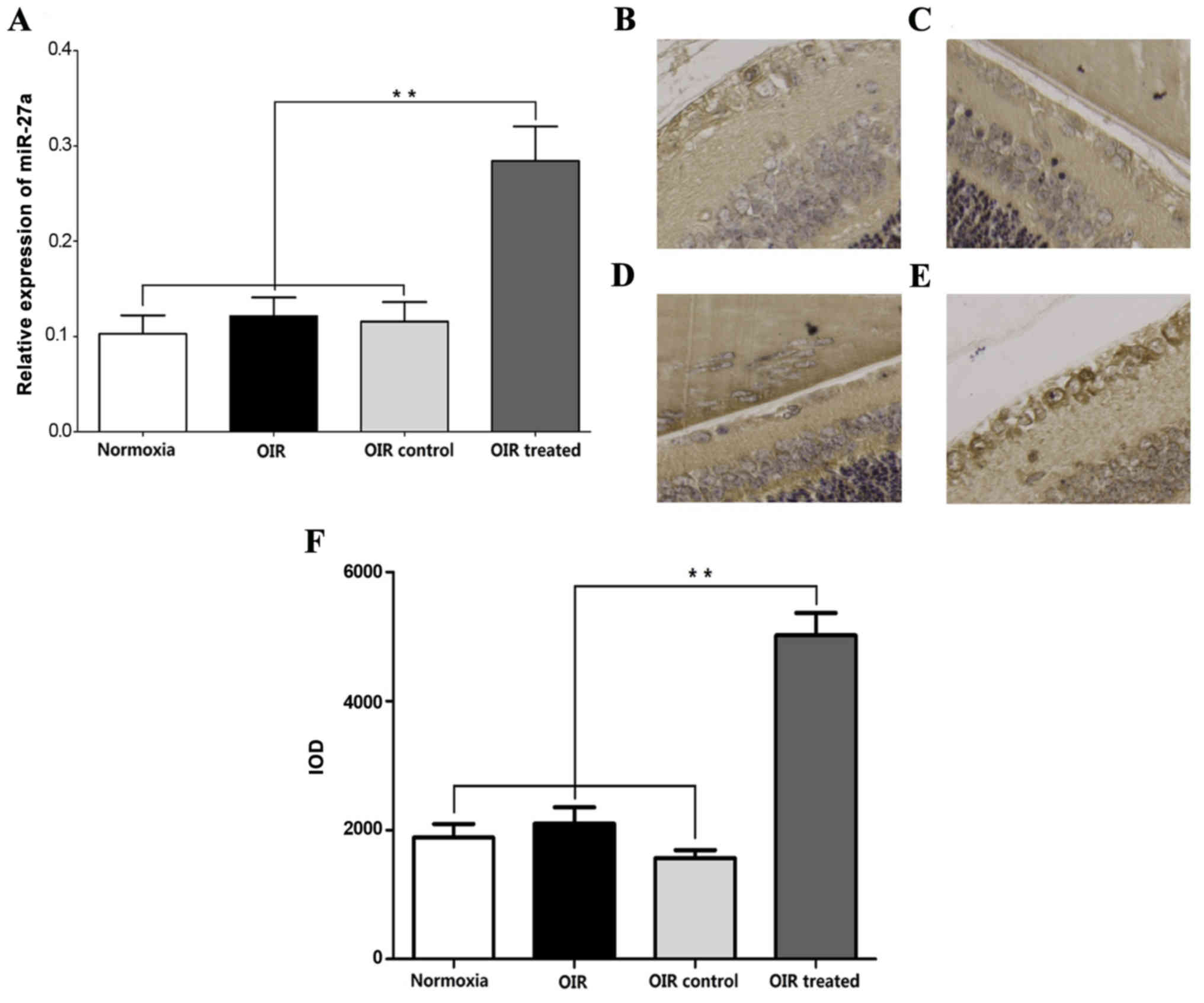
β-elemene promotes miR-27a
expression
RT-qPCR showed that the miR-27a expression in the
normoxia, OIR, and OIR control groups was low, compared with the
OIR-treated group (Fig. 6A).
Consistent with the miR-27a expression trends in RT-qPCR, ISH
experiments showed that miR-27a was predominantly expressed in
retinal endothelial cells and was higher in the OIR-treated group,
compared with the normoxia, OIR and OIR control groups (Fig. 6B-F). These results indicated that
β-elemene promoted miR-27a expression. In the in vitro
experiments, mouse retinal microvascular endothelial cells were
treated with different concentrations of β-elemene. The results
showed that β-elemene promoted miR-27a expression at concentrations
exceeding 50 µg/ml (Fig. 7A).
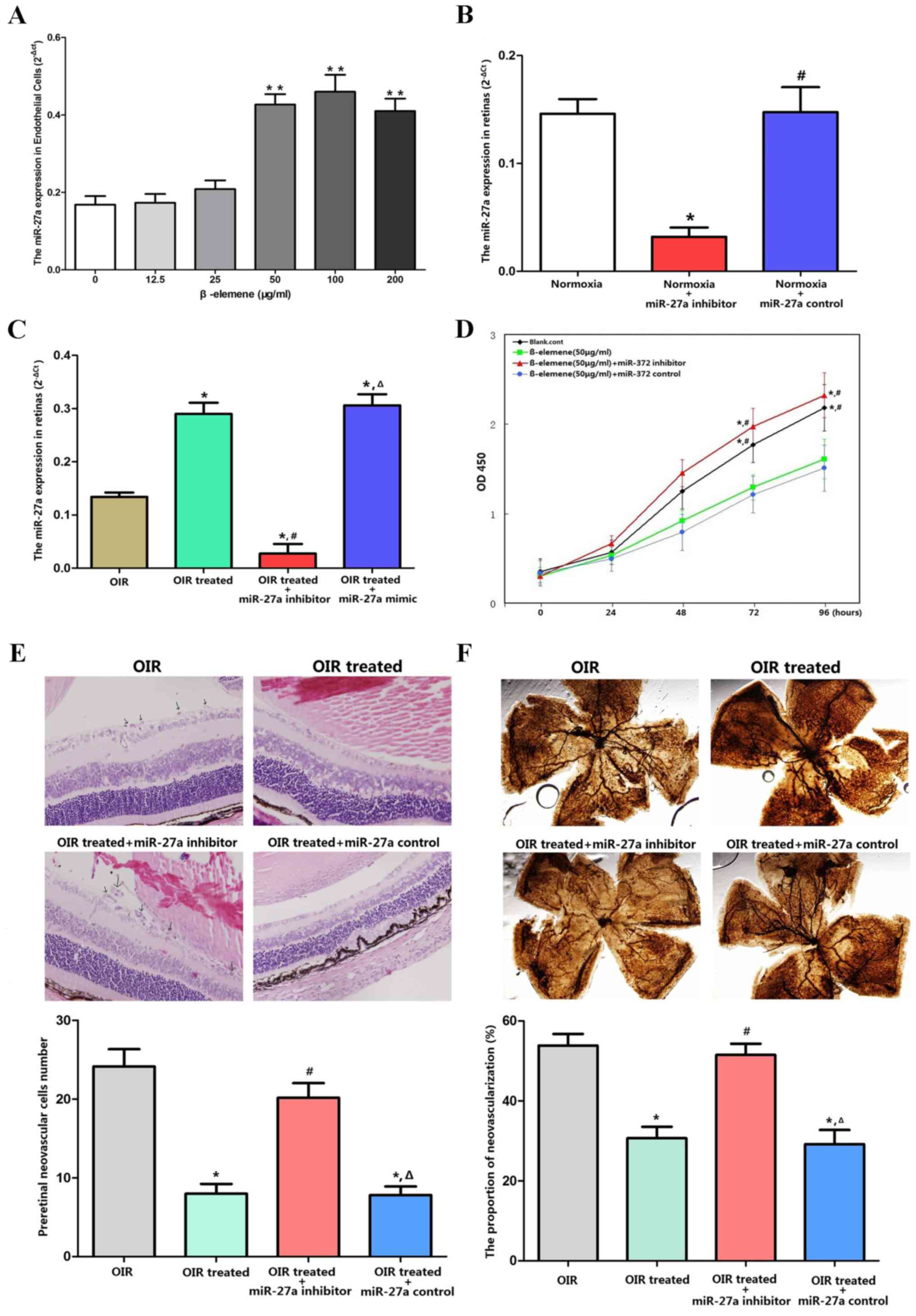
β-elemene reduces RNV via miR-27a in
vivo
The successful downregulation of miR-27a expression
by its inhibitor was confirmed in normoxia (Fig. 7B) and OIR conditions in vivo
(Fig. 7C). In vitro, CCK-8
assays showed that β-elemene inhibited cellular proliferation, and
miR-27a depletion eliminated β-elemene's efficacy (Fig. 7D). For the in vivo
experiments, miR-27a inhibitor was injected into the retina to
reduce miR-27a expression. The retinas were dissected and processed
for H&E and ADPase staining to assess RNV. H&E staining
revealed an average of 21.42±3.03 nuclei per cross-section in the
OIR treated + miR-27a inhibitor group, compared with 5.89±2.03 and
6.89±3.66 in the OIR treated and OIR treated + miR-27a control
groups, respectively (P<0.05; Fig.
7E). According to the ADPase staining results (Fig. 7F), the ratio of new blood vessel
area to total retinal area was higher in the OIR treated + miR-27a
inhibitor group (53±10%), compared with the OIR treated (28±8%,
P<0.05) and OIR treated + miR-27a control group (26±7%;
P<0.05). These results indicated that depletion of miR-27a
expression eliminated the protective effects of β-elemene on
oxygen-induced RNV.
Discussion
RNV is a key process in several types of
proliferative ischemic retinopathies (22,23).
It is stimulated by one or more angiogenic factors released by the
retina under ischemic or hypoxic conditions (24). VEGF has been demonstrated to be a
major pathogenic factor and therapeutic target in retinal
angiogenic diseases (11,12). It stimulates endothelial cell
proliferation and tube formation and mediates ischemia-induced RNV
(25,26). Previous studies have demonstrated
that intravitreal injection of anti-VEGF antibody (such as
bevacizumab or ranibizumab) is effective. However, neither of these
drugs provide sensitive and specific effects on RNV (2,27).
Therefore, finding a more effective method to suppress VEGF
expression to restrain RNV has become a research hotspot.
The present study showed that β-elemene, a prominent
component of traditional Chinese medicines, reduced the progression
of RNV in a mouse model of OIR. Furthermore, the results of
RT-qPCR, western blotting and IHC analyses indicated that
high-oxygen conditions upregulated VEGF expression. β-elemene
suppressed this effect and downregulated VEGF expression.
Therefore, it was speculated that β-elemene suppressed RNV
specifically by downregulating VEGF expression. This was also
supported by the observation that β-elemene did not influence the
protein expression of other angiogenic factors in mice retinas,
including PD-ECGF, TGF and TNF.
Studies focusing on the effects of β-elemene on VEGF
are extremely uncommon, with only one report demonstrating that
β-elemene inhibits melanoma growth and metastasis via VEGF
suppression, but they did not explore the mechanism (9). The present study aimed to explore the
mechanisms underlying the inhibitory effects of β-elemene on VEGF
expression and RNV. The study's focus was on miRNAs, which are
small, single stranded, non-coding RNAs that regulate gene
expression by directly degrading mRNA or suppressing
post-transcriptional protein translation by binding to the 3′-UTR
of their target mRNAs (28). It
has previously been shown that miR-218 and miR-410 inhibit
oxygen-induced RNV (14,29).
Therefore, microRNA microarrays were used to screen
out differentially expressed miRNAs in the normoxia, OIR and
OIR-treated groups. The results demonstrated that miR-22,
miR-181a-1, miR-335-5p, miR-669n, miR-190b, miR-27a, and miR-93
were upregulated in the OIR-treated group and downregulated in the
OIR group, compared with the control. The potential target genes of
miR-27a were identified with TargetScan and miRanda. The 3′-UTR of
VEGF was found to contain one potential miR-27a binding site.
Luciferase assays were used to test whether miR-27a could directly
bind to VEGF. The results showed that VEGF was a target gene of
miR-27a. In addition, overexpression of miR-27a decreased the
expression of VEGF, and β-elemene upregulated miR-27a expression
in vivo and in vitro. Furthermore, when miR-27a
expression was reduced by miR-27a inhibitor, the protective effect
of β-elemene on RNV was eliminated. Thus, it was hypothesized that
β-elemene reduced RNV via miR-27a regulation.
Taken together, it was concluded that β-elemene
inhibited RNV in OIR mouse models by reducing VEGF expression via
miR-27a. This novel finding may encourage further investigation of
the therapeutic role of β-elemene in the pathogenesis of these
diseases. The exact mechanisms underlying the regulation of miR-27a
expression by β-elemene remain unclear, and further experiments are
necessary to understand how β-elemene regulates miR-27a.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during the present
study are included in this published article.
Authors' contributions
LC designed the experiments. WZ conducted all
experiments and wrote the manuscript. JG, LL and LX analyzed the
data and discussed the results. All authors read and approved the
final manuscript.
Ethics approval and consent to
participate
Animal experiments were conducted following the
principles of the Animal Experiment Committee of The First
Affiliated Hospital of China Medical University, and the study was
approved by the ethics committee of The First Affiliated Hospital
of China Medical University, Shenyang, China.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
References
|
1
|
Yoshida S, Kobayashi Y, Nakao S, Sassa Y,
Hisatomi T, Ikeda Y, Oshima Y, Kono T, Ishibashi T and Sonoda KH:
Differential association of elevated inflammatory cytokines with
postoperative fibrous proliferation and neovascularization after
unsuccessful vitrectomy in eyes with proliferative diabetic
retinopathy. Clin Ophthalmol. 11:1697–1705. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jiang Y and Mieler WF: Update on the use
of anti-VEGF intravitreal therapies for retinal vein occulsions.
Asia Pac J Ophthalmol (Phila). 6:546–553. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fagerholm R and Vesti E: Retinopathy of
prematurity-from recognition of risk factors to treatment
recommendations. Duodecim. 133:337–344. 2017.PubMed/NCBI
|
|
4
|
Wu W, Duan Y, Ma G, Zhou G, Windhol C,
D'Amore PA and Lei H: AAV-CRISPR/Cas9-mediated depletion of VEGFR2
blocks angiogenesis in vitro. Invest Ophthalmol Vis Sci.
58:6082–6090. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Di Y, Nie QZ and Chen XL: Matrix
metalloproteinase-9 and vascular endothelial growth factor
expression change in experimental retinal neovascularization. Int J
Ophthalmol. 9:804–808. 2016.PubMed/NCBI
|
|
6
|
Chang Z, Gao M, Zhang W, Song L, Jia Y and
Qin Y: Beta-elemene treatment is associated with improved outcomes
of patients with esophageal squamous cell carcinoma. Surg Oncol.
26:333–337. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Liu Y, Jiang ZY, Zhou YL, Qiu HH, Wang G,
Luo Y, Liu JB, Liu XW, Bu WQ, Song J, et al: β-elemene regulates
endoplasmic reticulum stress to induce the apoptosis of NSCLC cells
through PERK/IRE1α/ATF6 pathway. Biomed Pharmacother. 93:490–497.
2017. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Wu J, Tang Q, Yang L, Chen Y, Zheng F and
Hann SS: Interplay of DNA methyltransferase 1 and EZH2 through
inactivation of Stat3 contributes to β-elemene-inhibited growth of
nasopharyngeal carcinoma cells. Sci Rep. 7:5092017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chen W, Lu Y, Wu J, Gao M, Wang A and Xu
B: Beta-elemene inhibits melanoma growth and metastasis via
suppressing vascular endothelial growth factor-mediated
angiogenesis. Cancer Chemother Pharmacol. 67:799–808. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yan B, Zhou Y, Feng S, Lv C, Xiu L, Zhang
Y, Shi J, Li Y, Wei P and Qin Z: β-Elemene-Attenuated tumor
angiogenesis by targeting notch-1 in gastric cancer stem-like
cells. Evid Based Complement Alternat Med 2013. 2684682013.
|
|
11
|
Di Y, Zhang Y, Yang H, Wang A and Chen X:
The mechanism of CCN1-enhanced retinal neovascularization in
oxygen-induced retinopathy through PI3K/Akt-VEGF signaling pathway.
Drug Des Devel Ther. 9:2463–2473. 2015.PubMed/NCBI
|
|
12
|
Sui A, Zhong Y, Demetriades AM, Lu Q, Cai
Y, Gao Y, Zhu Y, Shen X and Xie B: Inhibition of integrin α5β1
ameliorates VEGF-induced retinal neovascularization and leakage by
suppressing NLRP3 inflammasome signaling in a mouse model. Graefes
Arch Clin Exp Ophthalmol. 256:951–961. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Liu WL: MicroRNA-9 inhibits retinal
neovascularization in rats with diabetic retinopathy by targeting
vascular endothelial growth factor A. J Cell Biochem. Nov
28–2018.(Epub ahead of print) doi: 10.1002/jcb.28081.
|
|
14
|
Chen N, Wang J, Hu Y, Cui B, Li W, Xu G,
Liu L and Liu S: MicroRNA-410 reduces the expression of vascular
endothelial growth factor and inhibits oxygen-induced retinal
neovascularization. PLoS One. 9:e956652014. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ye EA and Steinle JJ: miR-146a suppresses
STAT3/VEGF pathways and reduces apoptosis through IL-6 signaling in
primary human retinal microvascular endothelial cells in high
glucose conditions. Vision Res. 139:15–22. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Smith LE, Wesolowski E, McLellan A, Kostyk
SK, D'Amato R, Sullivan R and D'Amore PA: Oxygen-induced
retinopathy in the mouse. Invest Ophthalmol Vis Sci. 35:101–111.
1994.PubMed/NCBI
|
|
17
|
Chikaraishi Y, Shimazawa M and Hara H: New
quantitative analysis, using high-resolution images, of
oxygen-induced retinal neovascularization mice. Exp Eye Res.
84:529–536. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park K, Chen Y, Hu Y, Mayo AS, Kompella
UB, Longeras R and Ma JX: Nanoparticle-mediated expression of an
angiogenic inhibitor ameliorates ischemia-induced retinal
neovascularization and diabetes-induced retinal vascular leakage.
Diabetes. 58:1902–1913. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yuan LH, Chen XL, Di Y and Liu ML:
CCR7/p-ERK1/2/VEGF signaling promotes retinal neovascularization in
a mouse model of oxygen-induced retinopathy. Int J Ophthalmol.
10:862–869. 2017.PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(-Delta Delta C(T)) method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Thomsen R, Sølvsten CA, Linnet TE,
Blechingberg J and Nielsen AL: Analysis of qPCR data by converting
exponentially related Ct values into linearly related X0 values. J
Bioinform Comput Biol. 8:885–900. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jiang N, Chen XL, Yang HW and Ma YR:
Effects of nuclear factor κB expression on retinal
neovascularization and apoptosis in a diabetic retinopathy rat
model. Int J Ophthalmol. 8:448–452. 2015.PubMed/NCBI
|
|
23
|
Nicholson L, Vazquez-Alfageme C, Patrao
NV, Triantafyllopolou I, Bainbridge JW, Hykin PG and Sivaprasad S:
Retinal nonperfusion in the posterior pole is associated with
increased risk of neovascularization in central retinal vein
occlusion. Am J Ophthalmol. 182:118–125. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Cabral T, Mello LGM, Lima LH, Polido J,
Regatieri CV, Belfort R Jr and Mahajan VB: Retinal and choroidal
angiogenesis: A review of new targets. Int J Retina Vitreous.
3:312017. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mi XS, Yuan TF, Ding Y, Zhong JX and So
XF: Choosing preclinical study models of diabetic retinopathy: Key
problems for consideration. Drug Des Devel Ther. 8:2311–2319. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sato T, Kusaka S, Shimojo H and Fujikado
T: Vitreous levels of erythropoietin and vascular endothelial
growth factor in eyes with retinopathy of prematurity.
Ophthalmology. 116:1599–1603. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hu J, Hoang QV, Chau FY, Blair MP and Lim
JI: Intravitreal anti-vascular endothelial growth factor for
choroidal neovascularization in ocular histoplasmosis. Retin CASES
Brief. 8:24–29. 2014. View Article : Google Scholar
|
|
28
|
Falcone G, Felsani A and D'Agnano I:
Signaling by exosomal microRNAs in cancer. J Exp Clin Cancer Res.
34:322015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Han S, Kong YC, Sun B, Han QH, Chen Y and
Wang YC: microRNA-218 inhibits oxygen-induced retinal
neovascularization via reducing the expression of roundabout 1.
Chin Med J (Engl). 129:709–715. 2016. View Article : Google Scholar : PubMed/NCBI
|