Introduction
Ovarian cancer poses a serious threat to female
health due to its high morbidity and high mortality (1,2).
Cisplatin is widely used in chemotherapy as the first-line agent
for treating ovarian cancer (3);
however, patients are prone to developing drug resistance, which
may lead to the failure of cancer treatment (4,5). Our
previous study reported that the expression of connexin (Cx)32 was
gradually increased during the development of acquired cisplatin
resistance in the human ovarian cancer cell line A2780 and that
upregulated Cx32 could contribute to cisplatin resistance (6); however, the mechanism underlying
Cx32-induced acquired cisplatin resistance requires further
investigation.
Gap junctions (GJs) are regarded as tumour
suppressors, while Cx, a subunit of GJ, can modulate a series of
cellular processes, including proliferation, differentiation and
apoptosis, in a GJ-dependent or GJ-independent manner (7–9).
Recently, numerous studies have indicated that Cx contributes to
chemoresistance in several types of tumour. Upregulated Cx26
contributed to gefitinib resistance in non-small cell lung cancer
cells (10), while increased Cx26
expression was able to reverse oxaliplatin resistance in
hepatocellular carcinoma (11).
Upregulation of Cx43 led to the insensitivity to temozolomide in
glioblastoma cells (12), while
increased Cx43 expression enhanced the cytotoxicity of oxaliplatin
in colorectal cancer cell lines (13). Our previous study also indicated
that Cx32 promoted cisplatin resistance in human ovarian cancer
cells (6); Cx32 inhibited
apoptosis in cervical cancer and (14,15).
At present, alterations in drug transporter
expression, abnormal activation of the DNA repair system and
activation of the anti-apoptotic signalling pathway are believed to
be the few main causes of chemoresistance (16–18).
Studies on the mechanism of Cx-mediated chemoresistance have mainly
focused on Cx43. For example, Cx43 expression in SH-SY5Y
neuroblastoma cells could inhibit the mitochondrial apoptosis
pathway and led to resistance to
1-methyl-4-phenylpyridinium-induced apoptosis (19). Nevertheless, the association
between Cx32 and chemoresistance, and the mechanism by which Cx32
mediates cancer chemoresistance, particularly cisplatin resistance,
remains unknown.
The present study aimed to determine the possible
mechanisms by which Cx32 mediates acquired cisplatin resistance in
ovarian cancer. Cx32 was knocked down or overexpressed in the
ovarian cancer cell line A2780. These cells and a
cisplatin-resistant ovarian cancer cell line (A2780/CDDP) were used
for the experimentation. The findings of the present study provide
insight as to how Cx32 is involved in cisplatin resistance and
proposed Cx32 as a potential target to effectively reverse
cisplatin resistance in human ovarian cancer.
Materials and methods
Materials
Primary antibodies against Cx32 (cat. no. sc-59948),
ATPase copper transporting α (ATP7A; cat. no. sc-376467), and
ATPase copper transporting β (ATP7B; cat. no. sc-373964) for
western blot analysis were purchased from Santa Cruz Biotechnology,
Inc. (Dallas, TX, USA). Primary antibodies against multi-drug
resistance protein 2 (MRP-2; cat. no. ab3373) and copper uptake
protein 1 (CTR1; cat. no. ab129067) for western blotting were
purchased from Abcam (Cambridge, UK). Primary antibodies against
excision repair cross-complementation group 1 (ERCC1; cat. no.
5437), epidermal growth factor receptor (EGFR; cat. no. 4267),
phosphorylated-EGFR (p-EGFR; cat. no. 2236), protein kinase B (Akt;
cat. no. 4685), p-Akt (cat. no. 4060), cleaved caspase-3 (cat. no.
9664), B-cell lymphoma 2 (Bcl-2; cat. no. 4223), Bcl-2 associated X
(Bax; cat. no. 5023), Bcl-2 antagonist/killer 1 (Bak; cat. no.
12105) and the cell lysis buffer (cat. no. 9803) were purchased
from Cell Signaling Technology, Inc. (Danvers, MA, USA). Primary
antibodies against tubulin (cat. no. T4026) for western blot
analysis and cisplatin were purchased from Sigma-Aldrich (Merck
KGaA, Darmstadt, Germany). Secondary antibodies, including goat
anti-mouse IgG (cat. no. 115-005-003) and goat anti-rabbit IgG
(cat. no. 111-035-003) for western blot analysis were purchased
from Jackson ImmunoResearch Laboratories, Inc. (West Grove, PA,
USA). Cell culture-associated reagents and
Lipofectamine™ 3000 were purchased from Invitrogen
(Thermo Fisher Scientific, Inc., Waltham, MA, USA). The fluorescein
isothiocyanate (FITC) Annexin V Apoptosis Detection kit was
purchased from BD Biosciences (San Jose, CA, USA). Cx32 small
interfering (si)RNA was constructed by Guangzhou RiboBio Co., Ltd.
(Guangzhou, China). The empty plasmid (cat. no. EX-EGFP-M02) and
the plasmid overexpressing Cx32 (cat. no. EX-A0514-M02-5) were
constructed by Genecopoeia, Inc. (Rockville, MD, USA). All other
reagents were purchased from Sigma-Aldrich (Merck KGaA) unless
otherwise stated.
Cell culture
The A2780 cell line was originally purchased from
the American Type Culture and Collection (Manassas, VA, USA). The
cisplatin-resistant cell line A2780/CDDP was generated in our
previous study (6). A2780 and
A2780/CDDP cells were routinely cultured in Dulbecco's Modified
Eagle's medium (DMEM; Thermo Fisher Scientific, Inc.) containing
10% foetal bovine serum (Thermo Fisher Scientific, Inc.) and 1%
penicillin-streptomycin (Thermo Fisher Scientific, Inc.) at 37°C in
a 5% CO2 atmosphere.
To induce apoptosis, A2780 and A2780/CDDP cells were
exposed to 5 and 15 µg/ml cisplatin for 24 h, respectively.
Assessment of cell viability
A2780 or A2780/CDDP cells were plated at
5×103 cells/well in 96-well plates. Cells were treated
with a concentration series (0, 0.3, 1, 3, 10, 30 and 100 µg/ml) of
cisplatin at 37°C for 48 h, and the untreated cells served as the
negative control. Cell viability was assessed using a Cell Counting
Kit-8 assay (CCK-8; Dojindo Molecular Technologies, Inc., Kumamoto,
Japan). After a 1-h incubation with CCK-8 reagent, the plate was
placed in a microplate reader (BioTek Instruments, Inc., Winooski,
VT, USA) and the absorbance was determined at a wavelength of 450
nm.
The resistance index (RI) was used to test the
degree of cisplatin resistance, and it was determined by the ratio
of the IC50 of drug-resistant cells to the
IC50 of sensitive cells.
Western blot analysis
For western blot analysis, the cells were washed
with ice-cold PBS, lysed by cell lysis buffer (Cell Signaling
Technology, Inc.) and centrifuged at 4°C and 12,000 × g for 30 min.
Total protein concentration was quantified using a BCA assay kit
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). Subsequently, a
total of 20 µg protein was loaded in each lane. Proteins were
separated by 10% SDS-PAGE or, for the detection of cleaved
caspase-3, by 12% SDS-PAGE. Proteins were transferred onto a
polyvinylidene difluoride membrane. The membranes were blocked with
TBS containing 5% dry milk and 0.1% Tween-20 at 25°C for 2 h.
Following blocking, specific primary antibodies were applied to the
membranes at 4°C for 16 h. The monoclonal antibodies were diluted
with TBS containing 0.1% Tween-20 as follows: Anti-Cx32 (1:2,000),
anti-MRP-2 (1:500), anti-ATP7A (1:1,000), anti-ATP7B (1:1,000),
anti-CTR1 (1:1,000), anti-ERCC1 (1:1,000), anti-EGFR (1:1,000),
anti-p-EGFR (1:1,000), anti-Akt (1:1,000), anti-p-Akt (1:1,000),
anti-cleaved caspase-3 (1:1,000), anti-Bcl-2 (1:1,000), anti-Bak
(1:1,000), anti-Bax (1:1,000) and anti-tubulin (1:10,000). The
membranes were incubated with the relevant horseradish
peroxidase-conjugated secondary antibodies (diluted in TBS
containing 0.1% Tween-20; 1:2,000) at room temperature for 1 h. The
membranes were subsequently incubated with Immobilon™
Western Chemiluminescent HRP Substrate (EMD Millipore, Billerica,
MA, USA) and immunoreactive bands were detected by ImageQuant LAS
4000 (GE Healthcare Life Sciences, Little Chalfont, UK). Band
density was analysed by ImageJ software (version 1.46r; National
Institutes of Health, Bethesda, MD, USA). Each experiment was
repeated at least three times.
Plasmid transfection and siRNA
interference experiments
For Cx32 overexpression (Plasmid-Cx32), A2780 cells
were plated at 3×105 cells/well in 6-well plates. A
total of 4 µg Plasmid-Cx32 was mixed with Lipofectamine®
3 000 reagent. The mix was added to the cells in DMEM. After 48 h,
expression of Cx32 was detected by western blot analysis. A2780
cells transfected with 4 µg empty vector served as the control
group.
Cx32 siRNA interference (knockdown Cx32) was
performed in a similar manner as plasmid transfection. A2780/CDDP
cells were plated at 1.5×105 cells/well in 6-well
plates. Lipofectamine 3000 was mixed with the siRNAs at a
concentration of 50 nM and added to the cell medium. After 48 h,
the levels of Cx32 expression were assessed by western blot
analysis. The sequences for the negative control (NC) siRNA and
siRNAs targeting Cx32 (siRNA-Cx32) were as follows: siRNA-NC:
5′-UUCUCCGAACGUGUCACGUTT-3′; siRNA-Cx32_1:
5′-CCGGCATTCTACTGCCATT-3′; siRNA-Cx32_2: 5′-GGCTCACCAGCAACACATA-3′
and siRNA-Cx32_3: 5′-GCAACAGCGTTTGCTATGA-3′. A2780/CDDP cells
transfected with siRNA_NC at a concentration of 50 nM served as the
control group. The siRNA-Cx32_3 was used for subsequent knockdown
Cx32 experiments.
Flow cytometry for apoptosis
analysis
A2780 and A2780/CDDP cells were seeded in six-well
plates and cultured with 5 µg/ml cisplatin at 37°C for 36 h.
Untreated A2780 and A2780/CDDP cells served as the control for
treated A2780 and treated A2780/CDDP, respectively. Cells were
washed with PBS and trypsinised with 0.05% trypsin at 37°C for 2
min. Subsequently, harvested cells were centrifuged at 2,000 × g
for 5 min and resuspended in binding buffer (provided in the FITC
Annexin V Apoptosis Detection kit) mixed with FITC-Annexin V and
propidium iodide (PI) according to the manufacturer's protocols.
The data were detected and analyzed using the flow cytometer
BriCyte E6 and the software MRFlow (Shenzhen Mindray Bio-Medical
Electronics Co., Ltd., Shenzhen, China) within 1 h after cells were
stained.
Statistical analysis
All experiments were repeated at least three times.
Parametric data were analysed using an unpaired Student's t-test or
two-way analysis of variance using GraphPad Prism 6.0 software
(GraphPad Software, Inc., La Jolla, CA, USA). A Bonferroni post hoc
test was applied for multiple comparisons. The results are
expressed as the mean ± standard error of the mean. P<0.05 was
considered to indicate a statistically significant difference.
Results
Drug resistance analysis in an
acquired cisplatin-resistant human ovarian cancer cell line
(A2780/CDDP)
The A2780/CDDP cells were established by the
stepwise selection of A2780 cells cultured in growth media with
increasing cisplatin concentrations (6). Several experiments were performed to
determine the drug resistance of A2780/CDDP cells.
The CCK-8 assay revealed that the RI of A2780/CDDP
cells was 5.105 (Fig. 1A). A2780
and A2780/CDDP cells were treated with 5 µg/ml cisplatin to induce
apoptosis. Western blot analysis revealed that the expression
levels of cleaved caspase-3 in A2780/CDDP cells were lower than
that of A2780 cells (Fig. 1B and
C). In addition, flow cytometry analysis demonstrated the
significantly enhanced resistance of A2780/CDDP cells to
cisplatin-induced apoptosis compared with in the control and A2780
cells (Fig. 1D and E).
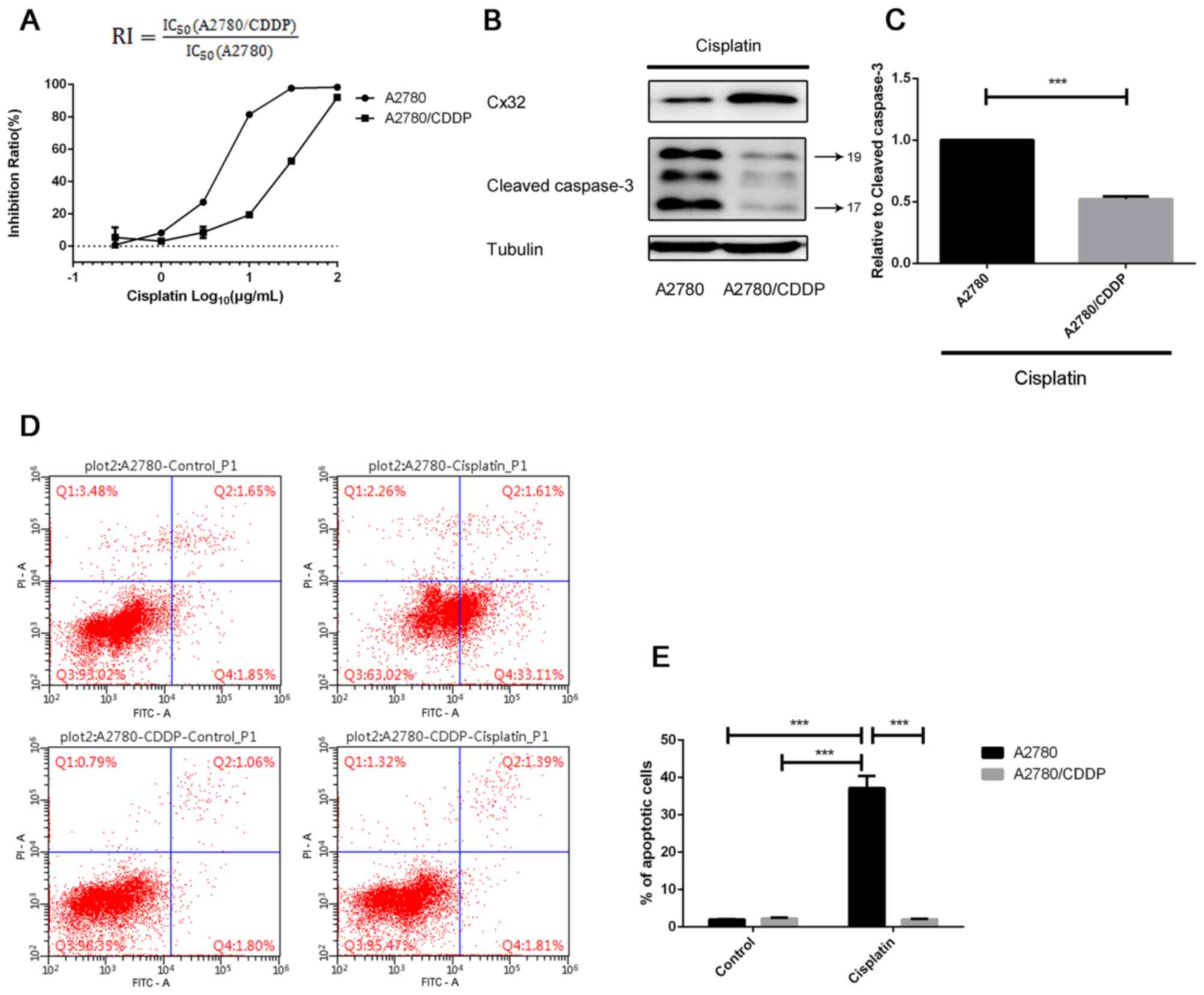 | Figure 1.Drug resistance analysis in an
acquired CDDP-resistant human ovarian cancer cells, A2780/CDDP. (A)
A2780 cells and A2780/CDDP cells were treated with a series of
concentrations of cisplatin for 48 h, and the inhibition ratio of
cell growth was determined by a Cell Counting Kit-8 assay (n=3).
(B) Following treatment with 5 µg/ml cisplatin for 24 h, the
expression levels of cleaved caspase-3 was detected by western
blotting (n=3). (C) Densitometric analysis. (D) A2780 and
A2780/CDDP cells were exposed to 5 µg/ml cisplatin for 36 h, and
the (E) percentage of apoptotic cells was detected by flow
cytometry (n=3); quantitative results are presented. ***P<0.001.
Error bar, standard error. CDDP, cisplatin; Cx32, connexin 32;
IC50, half-maximal inhibitory concentration; FITC,
fluorescein isothiocyanate; RI, resistance index. |
Cx32 participates in cisplatin
resistance by affecting the expression of drug efflux
transporters
To demonstrate the effects of Cx32 on cisplatin
resistance, the association between Cx32 and three factors
associated with drug resistance was investigated. We compared the
expression levels of several drug resistance-associated proteins
between A2780 cells and A2780/CDDP cells. After altering the
expression of Cx32, the expression levels of numerous drug
resistance-associated proteins were detected. In siRNA experiments,
interference fragment S3 (siRNA-Cx32_3) significantly decreased the
expression of Cx32 in A2780/CDDP cells compared with the negative
control (Fig. 2A and B).
Therefore, in subsequent siRNA interference experiments,
siRNA-Cx32_3 (siRNA-Cx32) was selected to target Cx32
expression.
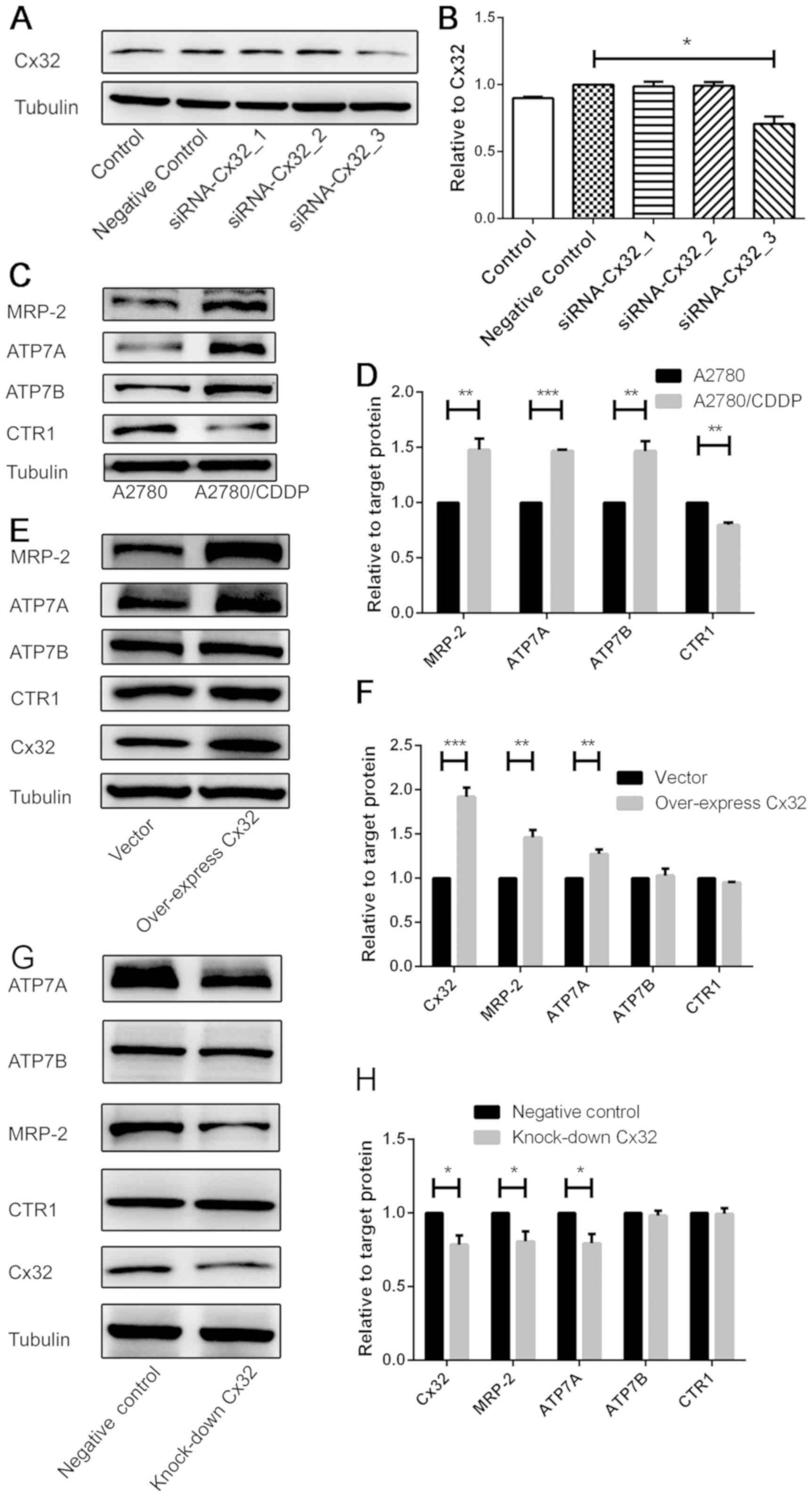 | Figure 2.Cx32 participates in CDDP resistance
by affecting the expression of drug efflux transporters. (A and B)
A2780/CDDP cells were transfected with siRNA targeting Cx32, and
the expression levels of Cx32 were detected by western blotting
(n=3). (C and D) Numerous CDDP transport-associated proteins were
detected by western blotting (n=3). A2780/CDDP cells exhibited
increased expression of MRP-2, ATP7A and ATP7B, but decreased
expression of CTR1. (E and F) Cx32 was overexpressed in A2780
cells; cisplatin transporters were detected by western blotting
(n=3). (G and H) Cx32 knockdown by siRNA-Cx32 in A2780/CDDP cells;
cisplatin transport-associated proteins were detected by western
blotting (n=3). *P<0.05, **P<0.01, ***P<0.001 Error bar,
standard error. ATP7A, ATPase copper transporting α; ATP7B, ATPase
copper transporting β; CDDP, cisplatin; Cx32, connexin 32; CTR1,
copper uptake protein 1; MRP-2, multi-drug resistance protein 2;
siRNA, small interfering RNA. |
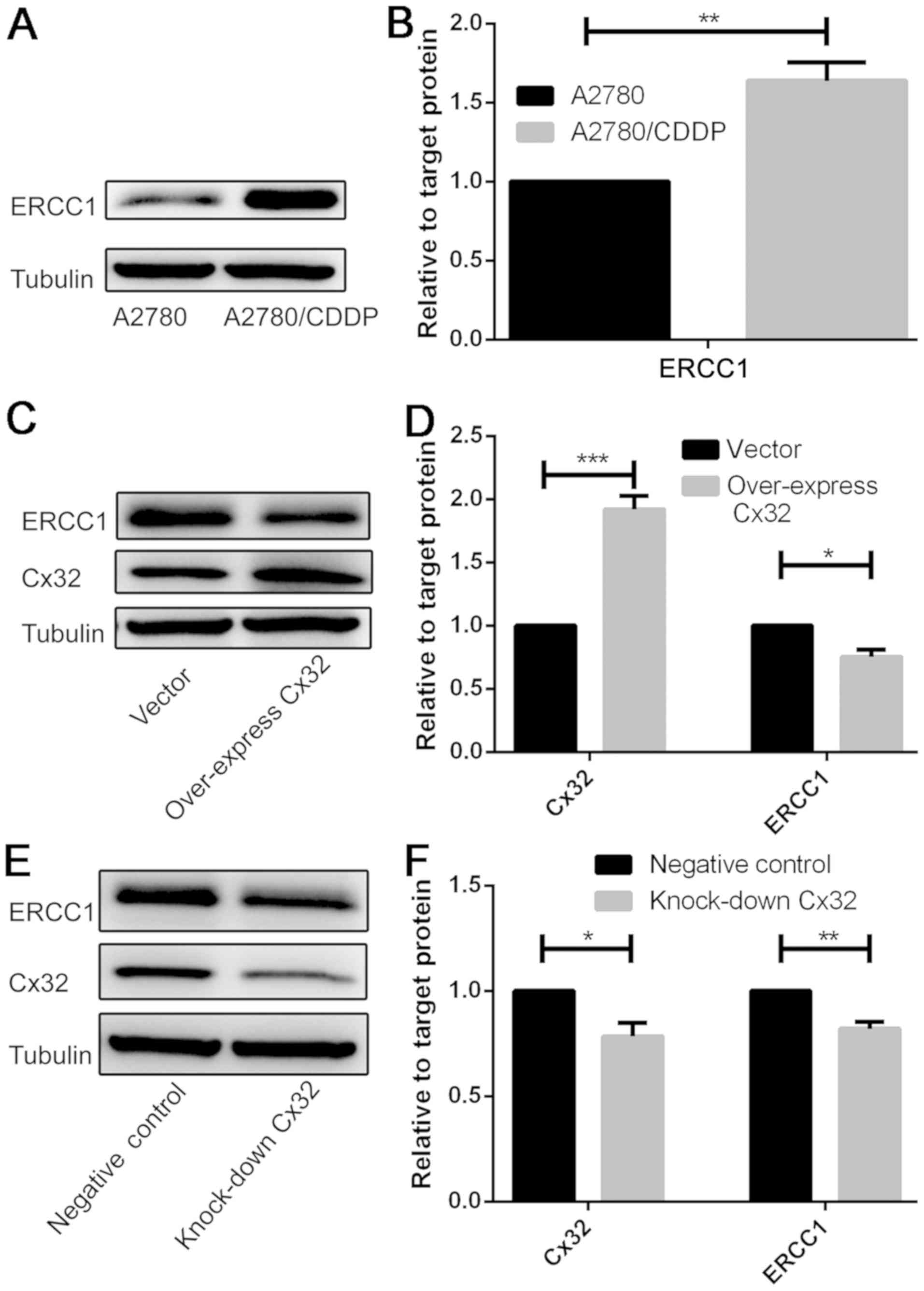
A statistically significant increased expression of
efflux transporters was observed, including MRP-2, ATP7A and ATP7B,
in A2780/CDDP cells, while the expression of CTR1 (an influx
transporter) was significantly lower in A2780/CDDP cells than in
A2780 cells (Fig. 2C and D).
Following overexpression of Cx32 in A2780 cells, the
expression levels of MRP-2 and ATP7A were significantly increased,
while the levels of ATP7B and CTR1 exhibited no significant
difference compared with the control (Fig. 2E and F). However, following Cx32
knockdown in A2780/CDDP cells, the expression levels of MRP-2 and
ATP7A were significantly decreased, while the levels of ATP7B and
CTR1 exhibited no significant difference compared with the control
(Fig. 2G and H).
Activation of the DNA repair system
does not involve the mechanism of Cx32-mediated cisplatin
resistance
The results revealed a statistically significant
increase in ERCC1 expression in A2780/CDDP cells compared with in
A2780 cells (Fig. 3A and B).
Overexpression of Cx32 in A2780 cells and knockdown of Cx32 in
A2780/CDDP cells significantly decreased the protein expression
levels of ERCC1 compared with the control (Fig. 3C-F). The present results suggested
that the activation of the DNA repair system may not be the
principal mechanism of Cx32-mediated chemoresistance to cisplatin
in ovarian cancer.
Cx32 can protect against cell
apoptosis induced by cisplatin
Following Cx32 overexpression using plasmid-Cx32 in
A2780 cells, these cells were exposed to cisplatin for 48 h. The
expression levels of cleaved caspase-3 were detected by western
blot analysis and the cell inhibition ratio was determined by a
CCK-8 assay. The results revealed that the sensitivity to cisplatin
and the expression levels of cleaved caspase-3 significantly
decreased upon Cx32 overexpression compared with the control
(Fig. 4A-C). Furthermore, the
protein expression levels of three members of the Bcl-2 family
(Bcl-2, Bak and Bax) were analysed. Following transfection of
plasmid-Cx32, the expression of anti-apoptotic Bcl-2 was
significantly increased, while that of the pro-apoptotic members
Bak and Bax was decreased compared with the control (Fig. 4D and E). On the contrary, Cx32
knockdown was performed in A2780/CDDP cells via siRNA-Cx32; the
cells were then treated with cisplatin for 48 h. Cx32 knockdown
significantly increased cisplatin cytotoxicity in response to 0.3,
1, 3 and 10 µg/ml cisplatin; the expression of cleaved caspase-3
was significantly upregulated compared with the control (Fig. 4F-H). When the expression of Cx32
was reduced, alterations in the expression of the three Bcl-2
protein family members revealed opposing results to that of Cx32
overexpression (Fig. 4I and
J).
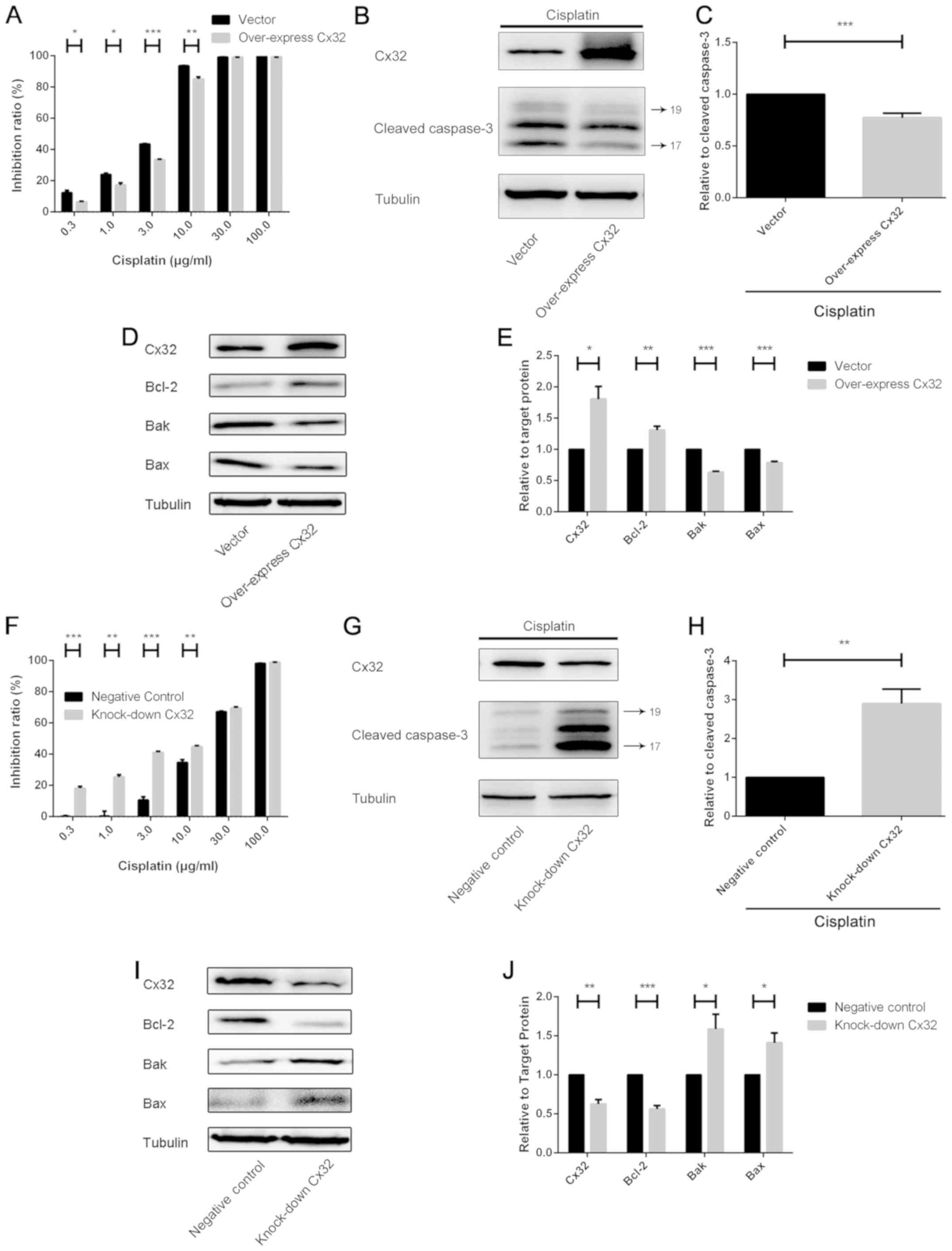 | Figure 4.Cx32 can protect against cell
apoptosis induced by CDDP. (A) Following overexpressing Cx32, A2780
cells were treated with a concentration series of CDDP. After 48 h,
cell viability was determined by the CCK-8 assay (n=3). (B and C)
Cx32 was overexpressed via plasmid-Cx32 in A2780 cells. The cells
were treated with CDDP (5 µg/ml) for 24 h. Cleaved caspase-3
expression was detected by western blotting (n=3). (D and E)
Following Cx32 overexpression, Bcl-2 family members (Bcl-2, Bak and
Bax) were analysed by western blotting (n=3). (F) Following Cx32
knockdown, A2780/CDDP cells were treated with a concentration
series of CDDP. After 48 h, cell viability was determined by a
CCK-8 assay. (G and H) Cx32 was downregulated by siRNA-Cx32 in
A2780/CDDP cells. The cells were treated with CDDP (15 µg/ml) for
24 h. Cleaved caspase-3 was detected by western blotting (n=3). (I
and J) Cx32 was downregulated by siRNA-Cx32; the aforementioned
Bcl-2 family members were detected by western blotting (n=3).
*P<0.05, **P<0.01, ***P<0.001. Error bar, standard error.
Bcl-2, B-cell lymphoma 2; Bak, Bcl-2 antagonist/killer 1; Bax,
Bcl-2 associated X; Cx32, connexin 32; CCK-8, Cell Counting Kit-8;
CDDP, cisplatin; siRNA, small interfering RNA. |
Cx32 promotes cisplatin resistance by
activating the EGFR-Akt signalling pathway
Activation of the EGFR signalling pathway may
inhibit cell apoptosis (14). In
the present study, a statistically significant increase in EGFR,
p-EGFR, and p-Akt expression in A2780/CDDP cells compared with in
A2780 cells was observed (Fig. 5A and
B). To further investigate the anti-apoptotic effects of Cx32
in cisplatin resistance, it was proposed that Cx32 may activate the
EGFR signalling pathways. Thus, we overexpressed Cx32 in A2780
cells not treated with cisplatin; the expression of EGFR and
downstream Akt was analysed by western blot analysis. The results
revealed that the expression levels of p-EGFR and p-Akt were
upregulated, and the ratios of p-EGFR/EGFR and p-Akt/Akt were
significantly increased compared with the control (Fig. 5C-E). This suggested that the
EFGR-Akt signalling pathway was activated. Additionally, Cx32 was
knocked down in A2780/CDDP cells and the EFGR-Akt pathway was
investigated. The results demonstrated that the expression levels
of EGFR and p-Akt were significantly downregulated, and the ratio
of p-Akt/Akt was significantly decreased compared with the control
(Fig. 5F-H). This indicated that
activation of the EFGR-Akt signalling pathway was suppressed.
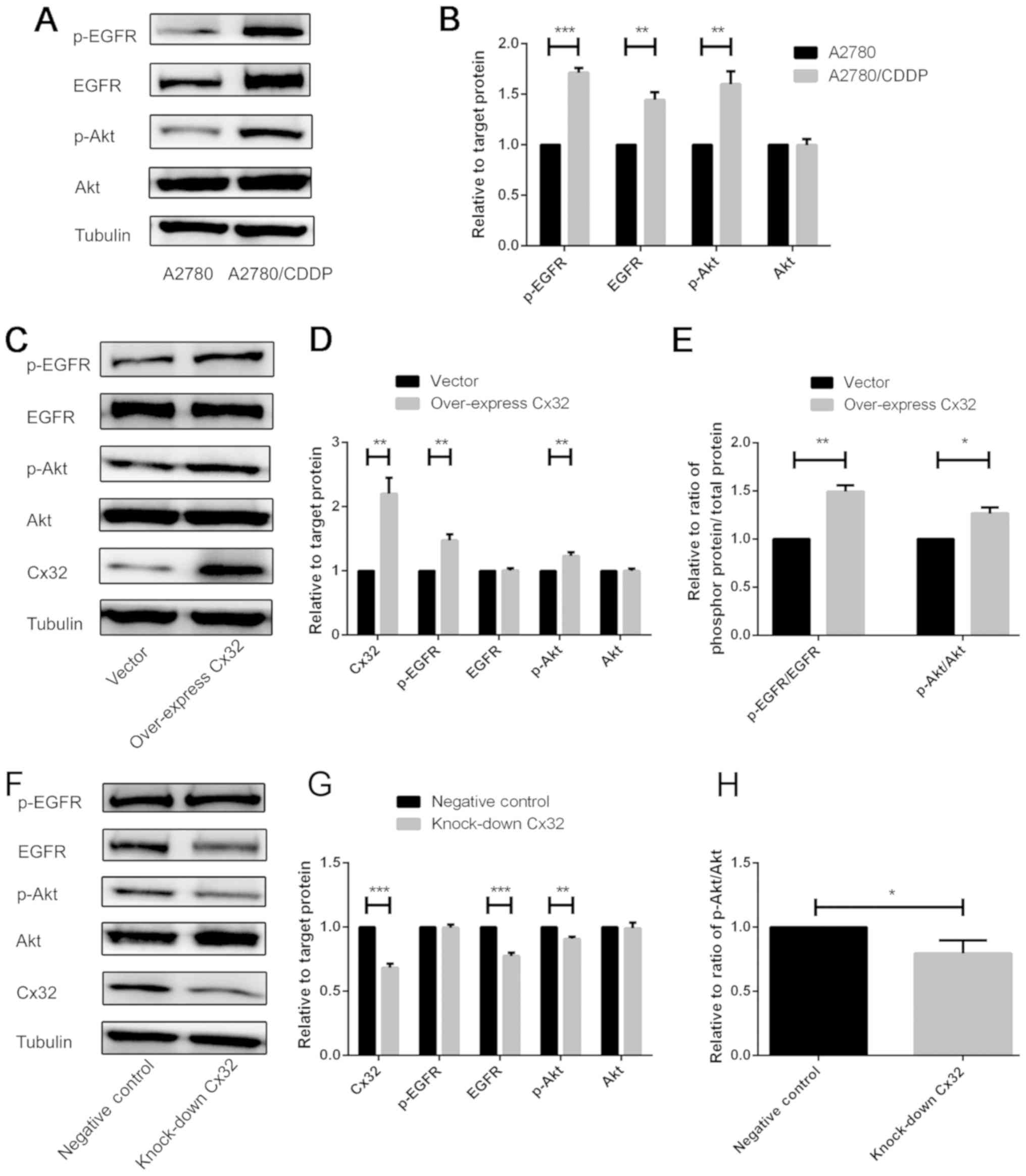 | Figure 5.Cx32 promotes cisplatin resistance by
activating the EGFR-Akt signalling pathway. (A and B) Expression of
numerous proteins associated with the EGFR signalling pathway were
compared between the A2780 cells and A2780/CDDP cells (n=3).
A2780/CDDP cells exhibited increased expression levels of p-EGFR,
EGFR and p-Akt. (C-E) Following Cx32 overexpression in A2780 cells,
numerous proteins associated with the EGFR-Akt signalling pathway
were detected by western blotting (n=3). (F-H) Cx32 was knocked
down by small interfering RNA-Cx32 in A2780/CDDP cells; several
proteins associated with the EGFR-Akt signalling pathway were
detected by western blotting (n=3). *P<0.05, **P<0.01,
***P<0.001. Error bar, standard error. Akt, protein kinase B;
Cx32, connexin 32; CDDP, cisplatin; EGFR, epidermal growth factor
receptor; p, phosphorylated. |
Discussion
In clinical practice, cisplatin is a first-line
chemotherapeutic drug for the treatment of ovarian cancer, which
threatens women's health due to its high rate of morbidity and
mortality (2,3); however, during the course of
treatment, drug resistance may be acquired, which leads to
treatment failure. Our prior research has determined that Cx32
could serve an anti-apoptotic role independent of GJ in human
cervical cancer cells, which suggests that Cx32 may contribute to
chemoresistance (14). However,
our previous study on non-drug-resistant cells did not demonstrate
that Cx32 was involved in chemoresistance (14). Additionally, a two-fold increase in
the expression of Cx32 and the functional inhibition of GJ was
observed, which occurred when A2780 cells acquired cisplatin
resistance (RI=7.1) (6). Thus,
studies on cisplatin-resistant cells (A2780/CDDP) may further
demonstrate how Cx32 is involved in cisplatin resistance.
At present, numerous factors have been proposed to
affect cisplatin resistance, including drug transporters, the DNA
repair system and the anti-apoptotic signalling pathway (18,20).
The resistance of A2780/CDDP cells (RI=5.105) was identified by
CCK-8 assay, western blot analysis and flow cytometry in the
present study. Thus, the association between Cx32 expression and
the three aforementioned factors involved in cisplatin resistance
was investigated.
Alterations in the expression levels of drug
transporters lead to cisplatin resistance by affecting the
intracellular drug concentration (21,22).
Compared with the levels in A2780 cells, high expression levels of
efflux transporters (MRP-2, ATP7A and ATP7B) and low expression of
an influx transporter (CTR1) in A2780/CDDP cells were observed.
Following the dysregulation of Cx32 expression, the expression
levels of two cisplatin-associated efflux transporters (MRP-2 and
ATP7A) were affected. Cx-controlled expression of drug transporters
has been reported in several studies. For example, Liu et al
(23) reported that increasing
Cx43 expression enhanced chemotherapy sensitivity in BGC-823 cells
by downregulating the levels of P-glycoprotein; however, few
studies have reported that Cx32 has such a function. The results of
the present study suggested that Cx32-regulated the expression of
drug efflux transporters, which may underlie cisplatin
resistance.
Activation of the DNA repair system can counteract
the fatal DNA damage caused by cisplatin (22). Compared with in A2780 cells, the
expression of ERCC1 in A2780/CDDP cells was significantly higher in
the present study. Regardless of whether Cx32 was overexpressed in
A2780 cells or downregulated in A2780/CDDP cells, the expression of
ERCC1 was reduced. The expression levels of ERCC1, a key enzyme of
nucleotide excision repair, participates in the DNA repair system
(24,25). Our previous study suggested that
GJs reduced the function of the DNA repair system in cancer cells
(26). Accordingly, it was
proposed that overexpressing Cx32 in A2780 cells may enhance the
function of GJs and weaken the activity of the DNA repair system,
leading to reduced ERCC1 expression. Thus, the activation of the
DNA repair system may not comprise the mechanism of Cx32-mediated
cisplatin resistance.
Activation of anti-apoptotic pathways promotes the
survival of cells following cisplatin treatment (27,28).
In the present study, the expression of cleaved caspase-3
increased, and cisplatin cytotoxicity was enhanced following Cx32
knockdown. Conversely, Cx32 overexpression significantly increased
cell chemoresistance to cisplatin and decreased the protein
expression levels of cleaved caspase-3. The expression of Bcl-2
family members was also detected in the present study. Cx32
overexpression increased the expression of Bcl-2 (an anti-apoptotic
member) and decreased the expression of pro-apoptotic members (Bak
and Bax), and vice versa. Based on these findings, Cx32 may
participate in cisplatin resistance via its anti-apoptotic effect.
In addition, the EGFR-Akt signalling pathway, known for its
anti-apoptotic effect (29),
exhibited variations in protein expression between A2780 and
A2780/CDDP cells; expression was upregulated or downregulated via
alterations in Cx32 expression. Therefore, it was hypothesized that
Cx32 serves an anti-apoptotic role in cisplatin resistance by
activating the EGFR-Akt signalling pathway.
Although numerous potential mechanisms of
Cx32-mediated cisplatin resistance in human ovarian cancer were
investigated, the most upstream mechanism of Cx32-mediated
cisplatin resistance remains unclear. The present study was
conducted using only one ovarian cancer cell line (A2780), which
was of endometrioid ovarian cancer origin. Future studies on a
variety of ovarian cancer cell lines, particularly the most
clinically representative cell lines, may be of greater value. In
addition, relevant clinical research data and xenograft animal
model experiments are required for further investigation into the
mechanisms underlying cisplatin resistance in ovarian cancer in the
future.
Collectively, the findings of the present study
indicated that Cx32 contributes to cisplatin resistance in human
ovarian cancer cells via two mechanisms. Cx32 may be involved in
cisplatin resistance by modulating the expression of drug efflux
transporters and activating the EGFR-Akt anti-apoptosis axis. Thus,
Cx32 may be a considered as a potential therapeutic target for
overcoming cisplatin resistance in the treatment of human ovarian
cancer.
Acknowledgements
Not applicable.
Funding
The present study was supported by the National
Natural Science Foundation of China (grant no. 81473234), the
Fundamental Research Funds for the Central Universities (grant no.
16ykjc01), the grant from Department of Science and Technology of
Guangdong Province (grant no. 20160908), and the Joint Fund of the
National Natural Science Foundation of China (grant no.
U1303221).
Availability of data and materials
The datasets used and/or analysed during the current
study are available from the corresponding author on reasonable
request.
Authors' contributions
YZ performed western blotting, flow cytometry,
plasmid transfection and siRNA interference experiments, and
collected and analysed data, and wrote the manuscript. LT was
involved in analysing the results and performing flow cytometry;
LXF made substantial contributions in the acquisition and
interpretation of the data. KH acquired the reagents and materials
and was involved in performing western blotting and collecting
data. HML performed the CCK-8 assay and cell culture; HG
contributed to the detection of protein expression; XW and QW made
substantial contributions to the design of the present study. All
authors read the paper, discussed the results and approved the
final version.
Ethics approval and consent to
participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing
interests.
Glossary
Abbreviations
Abbreviations:
|
GJ
|
gap junction
|
|
Cx
|
connexin
|
|
MRP-2
|
multi-drug resistance protein 2
|
|
ATP7A
|
ATPase copper transporting α
|
|
ATP7B
|
ATPase copper transporting β
|
|
CTR1
|
copper uptake protein 1
|
|
ERCC1
|
excision repair cross-complementation
group 1
|
|
EGFR
|
epidermal growth factor receptor
|
|
p-EGFR
|
phosphorylated-epidermal growth factor
receptor
|
|
Akt
|
protein kinase B
|
|
p-Akt
|
phosphorylated-protein kinase B
|
|
Bcl-2
|
B-cell lymphoma 2
|
|
Bak
|
Bcl-2 antagonist/killer 1
|
|
Bax
|
Bcl-2 associated X
|
References
|
1
|
Cho KR and Shih IeM: Ovarian cancer. Annu
Rev Pathol. 4:287–313. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2018. CA Cancer J Clin. 68:7–30. 2018. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Armstrong DK, Bundy B, Wenzel L, Huang HQ,
Baergen R, Lele S, Copeland LJ, Walker JL and Burger RA;
Gynecologic Oncology Group, : Intraperitoneal cisplatin and
paclitaxel in ovarian cancer. N Engl J Med. 354:34–43. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ai Z, Lu Y, Qiu S and Fan Z: Overcoming
cisplatin resistance of ovarian cancer cells by targeting
HIF-1-regulated cancer metabolism. Cancer Lett. 373:36–44. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Agarwal R and Kaye SB: Ovarian cancer:
Strategies for overcoming resistance to chemotherapy. Nat Rev
Cancer. 3:502–516. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wu W, Fan L, Bao Z, Zhang Y, Peng Y, Shao
M, Xiang Y, Zhang X, Wang Q and Tao L: The cytoplasmic
translocation of Cx32 mediates cisplatin resistance in ovarian
cancer cells. Biochem Biophys Res Commun. 487:292–299. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kanczuga-Koda L, Koda M, Sulkowski S,
Wincewicz A, Zalewski B and Sulkowska M: Gradual loss of functional
gap junction within progression of colorectal cancer-a shift from
membranous CX32 and CX43 expression to cytoplasmic pattern during
colorectal carcinogenesis. In Vivo. 24:101–107. 2010.PubMed/NCBI
|
|
8
|
Kar R, Batra N, Riquelme MA and Jiang JX:
Biological role of connexin intercellular channels and
hemichannels. Arch Biochem Biophys. 524:2–15. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kumar NM and Gilula NB: The gap junction
communication channel. Cell. 84:381–388. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang J, Qin G, Luo M, Chen J, Zhang Q, Li
L, Pan L and Qin S: Reciprocal positive regulation between Cx26 and
PI3K/Akt pathway confers acquired gefitinib resistance in NSCLC
cells via GJIC-independent induction of EMT. Cell Death Dis.
6:e18292015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yang Y, Zhu J, Zhang N, Zhao Y, Li WY,
Zhao FY, Ou YR, Qin SK and Wu Q: Impaired gap junctions in human
hepatocellular carcinoma limit intrinsic oxaliplatin
chemosensitivity: A key role of connexin 26. Int J Oncol.
48:703–713. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Murphy SF, Varghese RT, Lamouille S, Guo
S, Pridham KJ, Kanabur P, Osimani AM, Sharma S, Jourdan J, Rodgers
CM, et al: Connexin 43 inhibition sensitizes chemoresistant
glioblastoma Cells to Temozolomide. Cancer Res. 76:139–149. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang SQ, Zhang SW, Zhang CZ, Zhao ZY and
Wang YJ: Connexin 43 enhances oxaliplatin cytotoxicity in
colorectal cancer cell lines. Cell Mol Biol. 63:53–58. 2017.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhao Y, Lai Y, Ge H, Guo Y, Feng X, Song
J, Wang Q, Fan L, Peng Y, Cao M, et al: Non-junctional Cx32
mediates anti-apoptotic and pro-tumor effects via epidermal growth
factor receptor in human cervical cancer cells. Cell Death Dis.
8:e27732017. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lai Y, Fan L, Zhao Y, Ge H, Feng X, Wang
Q, Zhang X, Peng Y, Wang X and Tao L: Cx32 suppresses extrinsic
apoptosis in human cervical cancer cells via the NF-κB signalling
pathway. Int J Oncol. 51:1159–1168. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wu Q, Yang Z, Nie Y, Shi Y and Fan D:
Multi-drug resistance in cancer chemotherapeutics: Mechanisms and
lab approaches. Cancer Lett. 347:159–166. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Kathawala RJ, Gupta P, Ashby CR Jr and
Chen ZS: The modulation of ABC transporter-mediated multidrug
resistance in cancer: A review of the past decade. Drug Resist
Updat. 18:1–17. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
O'Grady S, Finn SP, Cuffe S, Richard DJ,
O'Byrne KJ and Barr MP: The role of DNA repair pathways in
cisplatin resistant lung cancer. Cancer Treat Rev. 40:1161–1170.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim IS, Ganesan P and Choi DK: Cx43
mediates resistance against MPP+-induced apoptosis in
SH-SY5Y neuroblastoma cells via modulating the mitochondrial
apoptosis pathway. Int J Mol Sci. 17(pii): E18192016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Galluzzi L, Senovilla L, Vitale I, Michels
J, Martins I, Kepp O, Castedo M and Kroemer G: Molecular mechanisms
of cisplatin resistance. Oncogene. 31:1869–1883. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Amable L: Cisplatin resistance and
opportunities for precision medicine. Pharmacol Res. 106:27–36.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Holohan C, Van Schaeybroeck S, Longley DB
and Johnston PG: Cancer drug resistance: An evolving paradigm. Nat
Rev Cancer. 13:714–726. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Liu D, Zhou H, Wu J, Liu W, Li Y, Shi G,
Yue X, Sun X, Zhao Y, Hu X, et al: Infection by Cx43 adenovirus
increased chemotherapy sensitivity in human gastric cancer BGC-823
cells: Not involving in induction of cell apoptosis. Gene.
574:217–224. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Takahata C, Masuda Y, Takedachi A, Tanaka
K, Iwai S and Kuraoka I: Repair synthesis step involving ERCC1-XPF
participates in DNA repair of the Top1-DNA damage complex.
Carcinogenesis. 36:841–851. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
McNeil EM and Melton DW: DNA repair
endonuclease ERCC1-XPF as a novel therapeutic target to overcome
chemoresistance in cancer therapy. Nucleic Acids Res.
40:9990–10004. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Zhang Y, Tao L, Fan L, Peng Y, Yang K,
Zhao Y, Song Q and Wang Q: Different gap junction-propagated
effects on cisplatin transfer result in opposite responses to
cisplatin in normal cells versus tumor cells. Sci Rep.
5:25632015.
|
|
27
|
Norouzi-Barough L, Sarookhani MR, Sharifi
M, Moghbelinejad S, Jangjoo S and Salehi R: Molecular mechanisms of
drug resistance in ovarian cancer. J Cell Physiol. 233:4546–4562.
2018. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Mohammad RM, Muqbil I, Lowe L, Yedjou C,
Hsu HY, Lin LT, Siegelin MD, Fimognari C, Kumar NB, Dou QP, et al:
Broad targeting of resistance to apoptosis in cancer. Semin Cancer
Biol. 35 (Suppl):S78–S103. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Seshacharyulu P, Ponnusamy MP, Haridas D,
Jain M, Ganti AK and Batra SK: Targeting the EGFR signaling pathway
in cancer therapy. Expert Opin Ther Targets. 16:15–31. 2012.
View Article : Google Scholar : PubMed/NCBI
|